Abstract
Background:
Symptom assessment is a critical component of concussion diagnosis and management, with item selection primarily driven by clinical judgment or expert consensus. We recently demonstrated that concussion symptoms assessed by the Sport Concussion Assessment Tool (SCAT) are essentially unidimensional, implying that overall symptom severity may be accurately estimated with relatively few questions. Briefer, evidence-based forms for symptom assessment would provide clinicians flexibility.
Purpose:
Using item response theory (IRT) analyses, we developed and validated an abbreviated assessment of general concussion symptom severity.
Study Design:
Prospective, longitudinal cohort study
Methods:
Broad clinical assessments (SCAT3/5, Immediate Post-Concussion and Cognitive Testing, Balance Error Scoring System, and Brief Symptom Inventory-18 Global Severity Index) were completed by 265 injured athletes and 235 matched teammate controls at 24–48 hours and 8, 15, and 45 days post-concussion. Symptom checklist short forms (3–14 items, from the original 22) were selected using IRT item information curves. Internal consistency reliability (Cronbach’s alpha), correlation with criterion measures assessed concurrently (i.e., acute neurocognitive performance, balance, and emotional symptoms), predictive validity (correlations with symptom duration), and differences between concussed and control groups (Cohen’s d) were examined across forms. Sensitivity and false positive rates of the forms were estimated and compared using reliable change indices derived from controls.
Results:
Across the 3- to 22-item forms, internal consistency was excellent (Cronbach’s alphas were .90–.94). Clinical correlations were significant (P ≤ .017) and to similar degrees for all short forms. Group difference confidence intervals overlapped across forms at 24–48-hour (Cohen’s ds 1.27–1.51) and 8-day follow-up (Cohen’s ds 0.31–0.44). Sensitivity remained similar across short forms, with a low false positive rate in controls.
Conclusions:
Our findings suggest that even an ultra-short (3-item) inventory provides sufficiently reliable and valid estimates of overall concussion symptom severity 24–48 hours post-injury. Future revisions of the SCAT could eliminate inefficient items, though replication in larger samples and extension to other post-injury time points is warranted.
Clinical Relevance:
Short forms can quickly screen global concussion symptom severity in settings requiring rapid evaluation and triage (e.g., sport sideline). Although, it not intended to replace comprehensive clinical evaluation during follow-up.
Keywords: mild traumatic brain injury, sports concussion, athletes, psychometrics, item response theory, symptom checklist
INTRODUCTION
A recent surveillance study revealed that 278,300 Americans with sport-related concussion were evaluated annually in an Emergency Department (ED).5 This figure represents a small proportion of the total incidence, as a subset of concussed athletes do not seek health care,17, 19 an unknown proportion receive care from within athletic departments; further, 88% of those who are identified with a concussion present to outpatient settings rather than the ED,2 and a high percentage who appear to EDs are not formally diagnosed with concussion.26 Estimates of the overall incidence of sport-related concussion range from 0.1 to 21.5 per 1,000 athletic exposures.4
Identification and management of sport-related concussion relies heavily on symptom assessment, in accordance with international consensus guidelines.20 Postconcussive symptom evaluations aid clinicians in diagnosing, monitoring, and returning athletes to activity following injury. A key method of symptom assessment is through graded rating scales such as the widely used Sport Concussion Assessment Tool (SCAT), a 22-item inventory comprising diverse cognitive, emotional, and physical symptoms.1, 7, 14, 15, 22, 23, 25 There are a number of similar concussion symptom scales available, which have evolved based on clinical judgment and expert consensus. However, these inventories have largely not been subjected to rigorous psychometric analysis, which limits their ability to advance evidence-based practice.1
Concussion symptoms are routinely aggregated into a total symptom severity score. Amongst the increasing variety of clinical assessment tools for concussion, the symptom severity score remains the cornerstone of the clinician’s toolkit given its robust sensitivity to concussion and prognostic value.12, 18, 21, 24 We recently validated this approach based on data that concussion symptoms assessed 24–48 hours post-injury by the SCAT are essentially unidimensional, with a single underlying dimension (the General factor) explaining 96% of the reliable variance in ratings.23 The high degree of unidimensionality of the SCAT implies that general concussion symptom severity is a coherent construct and could be estimated precisely using a subset of items from the full instrument. Prior work using a 1-parameter Item Response Theory (IRT; i.e., Rasch) model demonstrated that an abbreviated (12-item) symptom checklist yielded similarly strong sensitivity and specificity to concussion as compared to a historical 27-item checklist.27 We aimed to replicate and expand upon this work within a more contemporary athlete sample using a more realistic 2-parameter IRT model to develop and subsequently validate abbreviated forms of the SCAT symptom evaluation. We hypothesized that general concussion symptom severity could be reliably assessed in student athletes with short forms of the SCAT3/5 symptom evaluation acutely post-injury.
MATERIALS AND METHODS
Participants
Participants were concussed (n = 265)* and non-injured control (n = 235) athletes participating in the Project Head to Head I and II studies, which prospectively recruited from 9 high schools and 4 colleges in Southeastern Wisconsin from July 2012 through February 2018. The Medical College of Wisconsin institutional review board approved the Project Head to Head I and II studies. Concussion was diagnosed with a definition adopted from the study sponsor, the U.S. Department of Defense: “an injury to the brain resulting from an external force and/or acceleration/deceleration mechanism from an event, such as a blast, fall, direct impact, or motor vehicle accident which causes an alteration in mental status typically resulting in the temporally related onset of symptoms such as headache, nausea, vomiting, dizziness/balance problems, fatigue, insomnia/sleep disturbances, drowsiness, sensitivity to light/noise, blurred vision, difficulty remembering, and/or difficulty concentrating.”11 In 2015, the definition was modified to more closely resemble the U.S. Centers for Disease Control’s HEADS UP concussion initiative: “an injury resulting from a forceful bump, blow or jolt to the head that results in rapid movement of the head and causes a change in the athlete’s behavior, thinking, physical functioning, or the following symptoms: headache, nausea, vomiting, dizziness/balance problems, fatigue, difficulty sleeping, drowsiness, sensitivity to light/noise, blurred vision, memory difficulty, and difficulty concentrating.”28 A total of 3,561 athletes underwent baseline assessment. By study design, only participants with subsequent injury and their matched controls were followed with reassessments (n = 500). Figure 1 depicts a flow chart of participation identification and the broader study sample.
Figure 1.
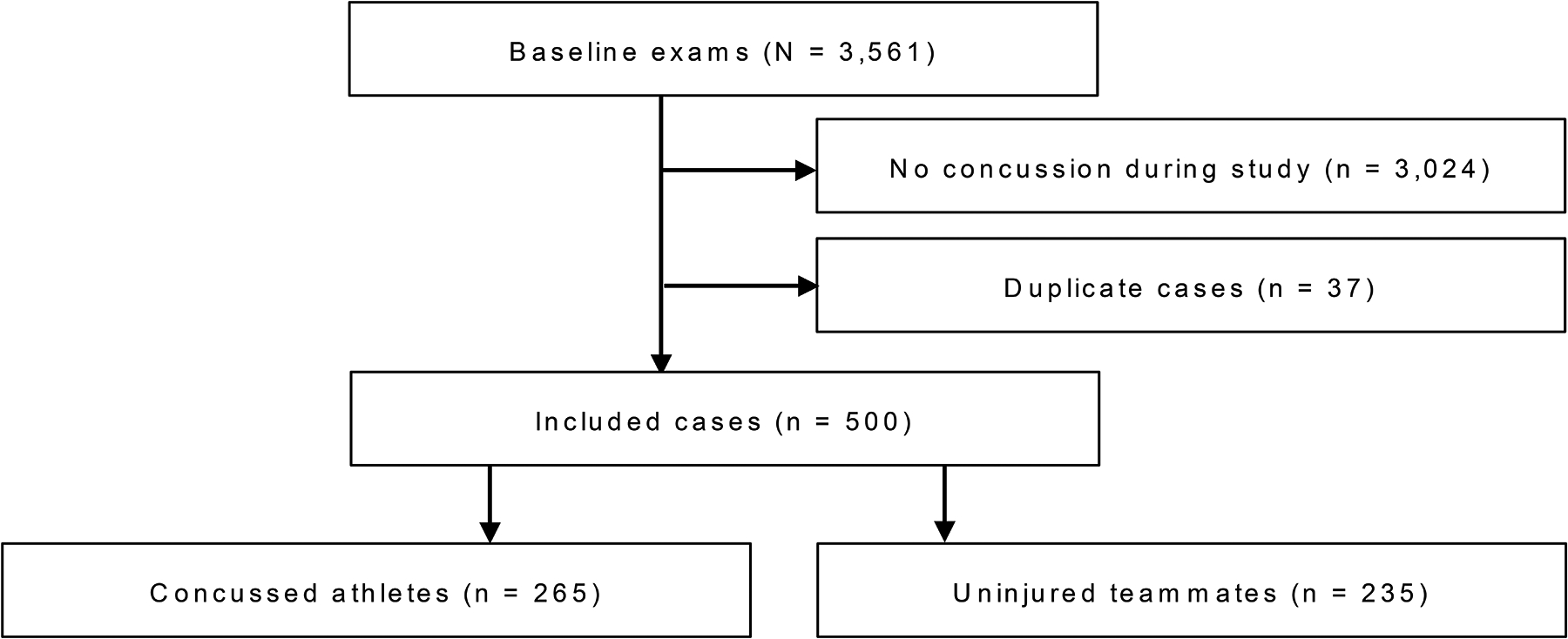
Flow diagram of case removal and final study sample.
Measures
Athletes completed a battery of clinical assessments comprised of concussion symptoms (SCAT3/5 22-item symptom evaluation),6, 8 cognitive performance (Immediate Post-Concussion and Cognitive Testing battery [ImPACT]; online version, ImPACT Applications, Inc.), postural stability (Balance Error Scoring System),9 and psychiatric symptoms (Brief Symptom Inventory-18).16 The SCAT symptom assessment encompasses common physical (e.g., dizziness, insomnia), emotional (e.g., irritability, sadness), and cognitive (e.g., confusion, memory problems) concussion sequelae. Participants rated symptoms on a graded scale (0–6). Ratings were summed to produce a symptom severity score, with higher scores indicating increased symptoms. Because the SCAT versions 2, 3, and 5 are identical in items and post-injury examinee instructions, we refer to the instrument as the SCAT in this manuscript.6, 8 Higher ImPACT verbal memory, visual memory, and visual motor speed composite scores indicated better performance, while lower reaction time composite scores indicated better performance. Lower BESS scores indicated better performance. The BSI-18 is a graded symptom checklist, with a greater global severity index score reflecting increased psychiatric symptoms.
Concussed athletes completed measures approximately 24–48 hours post-injury (mean = 24 hours, range: 1–91) and during 8-, 15- and 45-day post-injury follow-ups. The 24–48-hour and 8-day assessments were the focus of this manuscript given that concussion symptoms are typically resolved beyond these time points in athlete samples.18 Uninjured teammate controls were identified as soon after injuries as possible and followed at the same time intervals. Among those injured, 14% had loss of consciousness and 20% post-traumatic amnesia. Recovery time was assessed using self-reported duration of concussion symptoms (median = 6 days, IQR = 4–10 days) and was unknown for 16 athletes (6%) who were either lost to follow-up before they recovered (n = 12) or remained symptomatic at the end of the study (n = 4). Among all subjects, 3 (0.6%) had a psychiatric disorder and 6 (1.2%) endorsed a sleep disorder at pre-season baseline.
Statistical Analyses
Chi-square or independent samples t-tests compared concussed and control groups on demographic characteristics. Item Response Theory for Patient-Reported Outcomes (IRTPRO) software (version 4.2 for Windows) was used to model SCAT item information functions using a graded response model (GRM).3 The GRM enables estimation of both item difficulty and discrimination parameters in the presence of ordered polytomous items such as the 0–6 scale on the SCAT. These parameters can then be used to estimate item information, which reflects how well a test item estimates each level of a latent dimension (for the SCAT, concussion symptom severity). By plotting functions of item information, we can visualize where on the continuum of the domain (low to high) the item contributes information. Higher information can be interpreted as better precision to estimate an individual’s level of the latent dimension.
SCAT short forms with 14, 10, 5, and 3 items were developed based on item information curves for concussed athletes at 48-hours post-injury (Figure 2). For each SCAT form, summed symptom severity scores were computed and compared in terms of internal consistency reliability (Cronbach’s alpha), associations with criterion clinical measures assessed concurrently (Pearson correlations), predictive validity (Spearman correlation with symptom duration in days), and sensitivity to concussion (Cohen’s d comparing concussed and control groups). Criterion clinical measures included ImPACT verbal memory, visual memory, visual-motor speed, and reaction time composites as well as the BESS total score and BSI-18 global severity index. Spearman correlations were selected for association between symptom severity and recovery time, as proportional hazards assumptions for survival modeling were violated and symptom duration was highly skewed. For the remaining clinical measures, which were not highly skewed, Pearson correlations were used.
Figure 2.
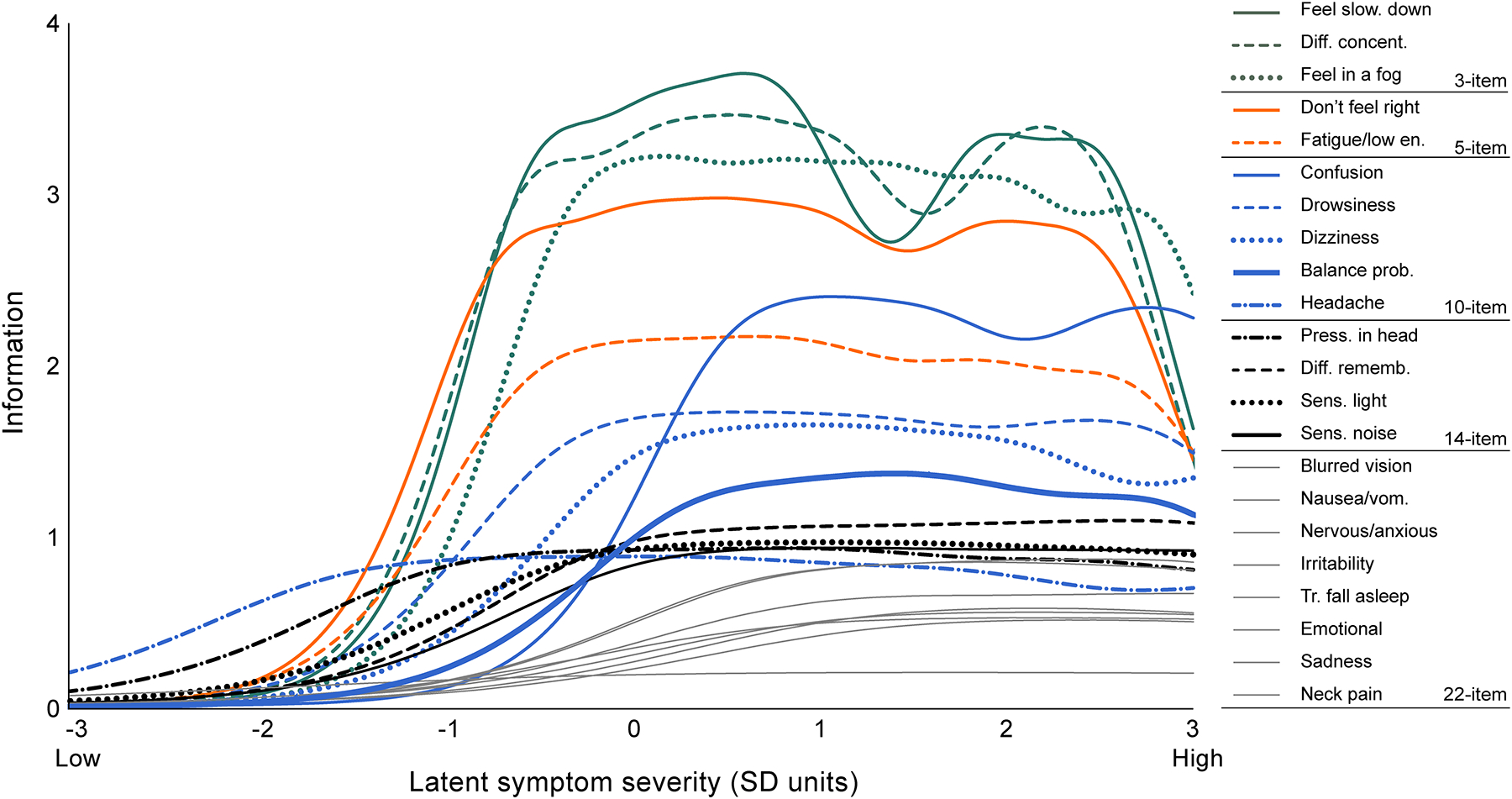
SCAT item information functions plotting how much item information each item contributes to estimation of individuals’ symptom severity levels across the continuum of the latent dimension (concussion symptom severity).
Test-retest reliability was assessed using Pearson’s correlation coefficients in control subjects for SCAT symptom severity from pre-injury baseline to both the 24–48-hour assessment (mean test-retest interval = 178 days, SD = 191, range: 1–842) and the 8-day follow-up (mean test-retest interval = 182 days, SD = 190, range: 8–849). Reliable change indices (RCIs) were established based on these data to estimate the sensitivity of the SCAT forms to concussion using a within-subjects design. RCIs were calculated using a standard error of the difference computed separately from baseline to each follow-up.13 We calculated the sensitivity of SCAT forms to concussion (percentage of all concussed athletes who exceeded RCIs) and the corresponding false positive rate in uninjured athletes. We also examined the clinical utility of SCAT forms to detect clinical impairment by calculating sensitivity, false positive rate, and positive predictive value in the concussed group based on recovery status. Our gold standard metric of recovery was self-reported symptom impairment at each time point (based on a recovery interview).
RESULTS
Sample characteristics
The sample contained 265 concussed athletes and 235 matched controls aged 14–22 years (M = 17.8, SD = 1.8). Participants were predominantly White (81%), male (89%), and football players (74%). There were no significant differences between concussed athletes and controls on age, race, history of ADHD, history of learning disorder, sport, or years of sport participation. However, the concussed group had a higher prevalence of previous concussion (56%) than the control group (34%), P < .001. Sample characteristics are described in Table 1.
Table 1.
Sample Characteristics Expressed as M (SD) or N (%)
| Concussed n = 265 |
Control n = 235 |
P | |
|---|---|---|---|
| Age in years | 17.7 (1.8) | 17.9 (1.8) | .197 |
| Male gender | 238 (89.8%) | 207 (88.1%) | .538 |
| Years of sport participation | 8.1 (3.9) | 8.4 (3.4) | .324 |
| Number of prior concussionsa | 1.03 (1.29) | 0.54 (0.96) | .001 |
| 0 prior concussions | 115 (43.4%) | 153 (65.1%) | |
| 1 prior concussion | 82 (30.9%) | 52 (22.1%) | |
| 2 prior concussions | 34 (12.8%) | 15 (6.4%) | |
| 3 or more prior concussions | 31 (11.7%) | 12 (5.1%) | |
| Race | .715 | ||
| White | 214 (80.8%) | 190 (80.9%) | |
| Black or African American | 43 (16.2%) | 35 (14.9%) | |
| Other/Unknown | 8 (3.0%) | 10 (4.3%) | |
| Sport played at study enrollment | .845 | ||
| Football | 209 (78.9%) | 178 (75.7%) | |
| Soccer | 38 (14.3%) | 39 (16.6%) | |
| Lacrosse | 7 (2.6%) | 6 (2.6%) | |
| Other | 11 (4.2%) | 12 (5.1%) | |
| ADHDa | 26 (9.8%) | 15 (6.4%) | .164 |
| Learning disordera | 8 (3.0%) | 5 (2.1%) | .534 |
Abbreviation: ADHD, Attention deficit/hyperactivity disorder.
Number of prior concussions, history of ADHD, and history of learning disorder was unknown for 3 concussed and 3 control athletes.
Sensitivity of SCAT items to levels of concussion symptom severity
Item information functions revealed the best performing items (Figure 2). Feeling slowed down, difficulty concentrating, and feeling in a fog comprised the 3-item short form. The 5-item form additionally included don’t feel right and fatigue/low energy. Items added for the 10-item form were confusion, drowsiness, dizziness, balance problems, and headache. The 14-item form added pressure in head, difficulty remembering, sensitivity to light, and sensitivity to noise. For extremely low-severity latent concussion symptoms (more than 1.5 SD below the mean), headache was the best performing item, followed by pressure in head. Across the continuum of concussion symptom severity, items pertaining to emotions provided the weakest estimates.
Comparison of concussion checklist short forms
Table 2 summarizes descriptive statistics and psychometric performance of each SCAT form’s symptom severity index in concussed athletes at 24–48 hours post-injury. Internal consistency was excellent, with Cronbach’s alpha ranging from .90 to .94 for the 3- to 22-item forms. All forms correlated with neurocognitive performance, balance, and emotional symptoms to similar degrees. Spearman correlations for each SCAT form and recovery time also yielded similarly sized effects ranging from .40 to .47. As shown in Table 2, statistical significance was reached (P < .05) for all correlation coefficients examined.
Table 2.
Descriptive Statistics, Internal Consistency Reliability, and Clinical Correlations of SCAT Forms Completed at 24 to 48 Hours Post-injury
| 22-item | 14-item | 10-item | 5-item | 3-item | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N (concussed) | 254 | 259 | 261 | 261 | 261 | |||||
| Total symptom severity score, mean (SD) | 24.25 (19.59) | 19.33 (15.10) | 14.37 (11.44) | 8.33 (6.97) | 4.75 (4.26) | |||||
| Cronbach’s alpha | .94 | .94 | .93 | .93 | .90 | |||||
| Concurrent association with criterion variables (Pearson correlations) | r | P | r | P | r | P | r | P | r | P |
| ImPACT verbal memory composite | −.30 | <.001 | −.31 | <.001 | −.30 | <.001 | −.29 | <.001 | −.27 | <.001 |
| ImPACT visual memory composite | −.35 | <.001 | −.36 | <.001 | −.35 | <.001 | −.32 | <.001 | −.28 | <.001 |
| ImPACT visual-motor speed composite | −.20 | .005 | −.22 | .001 | −.23 | .001 | −.21 | .002 | −.20 | .003 |
| ImPACT reaction time composite | .21 | .002 | .23 | .001 | .24 | .001 | .24 | <.001 | .25 | <.001 |
| BESS total score | .17 | .007 | .18 | .004 | .19 | .003 | .16 | .013 | .15 | .017 |
| BSI-18 global severity index | .69 | <.001 | .64 | <.001 | .65 | <.001 | .60 | <.001 | .59 | <.001 |
| Predictive validity (Spearman’s rho) | ρ | P | ρ | P | ρ | P | ρ | P | ρ | P |
| Days to self-reported recovery | .47 | <.001 | .47 | <.001 | .45 | <.001 | .41 | <.001 | .40 | <.001 |
Abbreviations: ImPACT, Immediate Post-Concussion Assessment and Cognitive Testing; BESS, Balance Error Scoring System; BSI-18, Brief Symptom Inventory-18.
Table 3 depicts the sensitivity of each SCAT form to concussion using both between- and within-subjects analyses. Between-subjects comparisons of symptom severity for concussed and control athletes revealed similarly sized group differences (i.e., all with overlapping confidence intervals) for the 22- to 3-item forms at 24–48 hours (Cohen’s d range: 1.27–1.51) and 8 days post-injury (Cohen’s d range: 0.31–0.44). Test-retest reliability of SCAT forms was relatively modest across forms and follow-up period (range: .36–.53). Sensitivity to concussion using the derived RCI cut scores was similar across forms. At the 95% confidence level, the 14-item short form performed best, with 66% of the concussed group at 24–48 hours and 14% at day 8 demonstrating significantly higher symptom severity than their pre-injury baselines. RCI cutoffs in controls yielded low rates of false positives (range: 0.9%–5.7%). Across all SCAT forms, sensitivity of the RCI cut scores declined at day 8, ranging from 9.0% to 17.9% in all concussed athletes. Examining RCI cut scores in athletes who had not yet recovered by day-8 post-injury assessment revealed 17.8% to 41.1% exceeding cutoffs, establishing concordance with self-reported symptom duration. Based on self-reported symptom duration, 96% of concussed athletes remained symptomatic at 24–48-hour assessment and 34% at day-8 assessment. Positive predictive value calculated using these base rates ranged from 84.2% to 100% (see Table 3).
Table 3.
Test-retest reliability, sensitivity, and group differences
| 24- to 48-hour follow-up | 8-day follow-up | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 22-item | 14-item | 10-item | 5-item | 3-item | 22-item | 14-item | 10-item | 5-item | 3-item | |
| Test-retest reliability (controls)a | .53 | .54 | .53 | .49 | .42 | .51 | .42 | .42 | .40 | .36 |
| Reliable change cutoffs | ||||||||||
| 80th percentile | 7.3 | 5.2 | 4.2 | 3.1 | 2.1 | 7.3 | 5.8 | 4.7 | 3.2 | 2.1 |
| 90th percentile | 9.4 | 6.6 | 5.5 | 3.9 | 2.7 | 9.4 | 7.4 | 6.0 | 4.1 | 2.7 |
| 95th percentile | 11.2 | 7.9 | 6.5 | 4.7 | 3.2 | 11.3 | 8.9 | 7.1 | 4.9 | 3.2 |
| Sensitivity | ||||||||||
| 80% CI | 68.5% | 70.0% | 65.5% | 54.6% | 51.5% | 15.4% | 17.5% | 17.9% | 13.0% | 12.6% |
| 90% CI | 64.0% | 67.8% | 61.1% | 54.6% | 51.5% | 13.1% | 14.3% | 14.3% | 10.8% | 12.6% |
| 95% CI | 57.2% | 65.6% | 58.5% | 51.1% | 41.5% | 11.8% | 13.9% | 12.1% | 10.8% | 9.0% |
| False positive rate in controls | ||||||||||
| 80% CI | 5.7% | 4.3% | 4.8% | 3.4% | 3.9% | 1.8% | 3.1% | 3.6% | 2.2% | 1.8% |
| 90% CI | 3.5% | 3.5% | 3.5% | 3.4% | 3.9% | 1.8% | 1.8% | 2.7% | 2.2% | 1.8% |
| 95% CI | 1.8% | 2.2% | 1.7% | 2.6% | 0.9% | 1.4% | 1.3% | 1.3% | 2.2% | 1.3% |
| Concussed vs. control difference | ||||||||||
| d | 1.45 | 1.51 | 1.46 | 1.35 | 1.27 | 0.40 | 0.44 | 0.39 | 0.31 | 0.33 |
| (95% CI) | (1.24–1.64) | (1.31–1.71) | (1.26–1.66) | (1.16–1.55) | (1.08–1.47) | (0.22–0.58) | (0.26–0.62) | (0.21–0.57) | (0.13–0.49) | (0.15–0.51) |
| Sensitivity (symptomatic) | ||||||||||
| 80% CI | 69.9% | 71.5% | 66.7% | 55.1% | 51.9% | 33.3% | 39.7% | 41.1% | 28.8% | 26.0% |
| 90% CI | 65.1% | 69.2% | 62.0% | 55.1% | 51.9% | 26.4% | 30.1% | 31.5% | 21.9% | 26.0% |
| 95% CI | 57.9% | 66.8% | 59.3% | 51.4% | 41.7% | 22.2% | 28.8% | 26.0% | 21.9% | 17.8% |
| False positive rate (asymptomatic) | ||||||||||
| 80% CI | 12.5% | 12.5% | 12.5% | 12.5% | 12.5% | 2.2% | 2.2% | 2.9% | 2.2% | 2.2% |
| 90% CI | 12.5% | 12.5% | 12.5% | 12.5% | 12.5% | 2.2% | 2.2% | 2.2% | 2.2% | 2.2% |
| 95% CI | 12.5% | 12.5% | 12.5% | 12.5% | 0.0% | 2.2% | 2.2% | 2.2% | 2.2% | 1.4% |
| Positive Predictive Valueb | ||||||||||
| 80% CI | 99.3% | 99.4% | 99.3% | 99.2% | 99.1% | 88.9% | 90.6% | 88.2% | 87.5% | 86.4% |
| 90% CI | 99.3% | 99.3% | 99.3% | 99.2% | 99.1% | 86.4% | 88.0% | 88.5% | 84.2% | 86.4% |
| 95% CI | 99.2% | 99.3% | 99.2% | 99.1% | 100% | 84.2% | 87.5% | 86.4% | 84.2% | 86.7% |
Test-retest reliability (r) computed for non-injured controls from preseason baseline to the 24–48-hour or 8-day follow-up.
Positive predictive value were calculated using the base rate of concussed athletes remaining symptomatic at each time point (96% at 24–48 hours, 34% at day 8).
DISCUSSION
We demonstrated that even an ultra-short (i.e., 3-item) form of the SCAT symptom evaluation was a sufficiently reliable and valid assessment of general concussion symptom severity at 24–48 hours and 8 days post-injury. Across the 3- and 14-item forms derived with IRT and the original 22-item form, there was excellent internal consistency and similarly robust correlations with other widely used clinical assessment tools and with symptom duration. Acutely post-injury, total symptom severity scores for all forms significantly discriminated concussed athletes from controls. Reduction in discrimination effect sizes for shorter forms of the SCAT was modest, with overlapping confidence intervals. Further, sensitivity to concussion across the forms was similar when applying RCI-based cut scores, derived using data on controls, to evaluate the significance of within-subjects changes in symptoms from pre- to post-injury.
That assessing relatively few symptoms can be used to estimate general concussion symptom severity is not surprising in light of our recent work demonstrating good fit of a bifactor model of the SCAT.23 In particular, the model implies a high level of concordance between ratings of diverse acute concussion symptoms, a consequence of which is that general concussion symptoms can be assessed with high precision using a subset of items. Nevertheless, this work provides compelling, practical data to support the clinical use of abbreviated symptom checklists when rapid screening of general concussion symptom severity is desired. The data provided through IRT techniques also inform understanding of at what levels of concussion symptom severity different items provide the most information. Interestingly, IRT information functions identified that items representing general torpor and decreased attention provided the best estimates of overall concussion symptom severity across a wide spectrum of severity. Headache and pressure in head, arguably amongst the most recognized and specific symptoms of concussion, only performed better than other symptoms at extremely low levels of the latent dimension (concussion symptom severity). In contrast, items comprising emotional and other somatic complaints provided the weakest estimates of overall general symptom severity at 24–48 hours. That elimination of these symptoms from the SCAT appeared to have little impact on discriminability suggests they are inefficient and possibly redundant. It is possible that athletes’ interpreted meaning of these symptoms is varied or problematic and contributing to the relatively lower item information. Given the derivation of short forms using the acute time period, it could also be that these symptoms tend to present later.
The relative superiority of general symptoms such as feeling slow, foggy, and not right is consistent with our work finding the highest loadings of such symptoms on the general factor of the SCAT, whereas emotional symptoms manifested the smallest loadings. Furthermore, that headache was the best performing item for low-severity symptoms supports the important role of this item in concussion assessment, particularly in populations likely to have very mild concussions. These findings also align with those of Randolph and colleagues whose 12-item Concussion Symptom Inventory, derived through Rasch modeling on athletes assessed from 1999–2003, closely resembled the 10-item inventory defined in the current study through a GRM IRT model.27 The relative consistency across this and the present study is striking in light of the numerous differences between the era, item pools, samples, and methods used, further supporting the robustness of these items as amongst the most sensitive to concussion.
Limitations
Our study was conducted in a fairly homogeneous sample of athletes from Southeastern Wisconsin. Replication in larger, national samples would be beneficial to further examine psychometric properties of SCAT short forms. Additionally, SCAT short forms and RCI cut scores were derived and validated predominantly at 24–48 hours post-injury, with some validation data presented at 8 days post-injury. We have not evaluated its use outside these time points.
Another important distinction in terms of limitations is to acknowledge the screening nature of these short forms. Use of any one clinical tool in isolation is not a sufficient assessment practice, and consensus guidelines recommend clinical correlation with evaluation in other domains.20 Based on evidence that concussion symptom severity is a unidimensional construct, SCAT abbreviated forms were derived to estimate overall symptom severity and, consequently, do not address specific domains of postconcussive symptoms (e.g., cognitive, emotional, physical). Furthermore, we did not explore possible confounds to the reporting of symptoms in the present investigation, a topic that could be explored with future study as assessment of symptoms via questionnaires (versus interview methods, for instance) can potentially introduce forms of bias.
Conclusions
These findings support the utility of more targeted assessment of acute concussion symptom severity. While the full-length symptom checklist may be preferred by clinicians in common care delivery, our findings also support the use of the short-form in settings that require rapid evaluation and triage of concussed athletes (e.g., sport sideline). The availability of multiple validated forms for symptom assessment ultimately provide clinicians with flexibility to meet the constraints common to all sports medicine settings. Future revisions of the SCAT could eliminate inefficient items, though additional study is first warranted. IRT analyses may be useful to this end. Though not intended to replace comprehensive clinical evaluation, short forms could assess general postconcussive symptom severity when full assessment is limited by time or cost.
What is known about the subject:
General concussion symptom severity is a coherent construct that is typically assessed using symptom checklists. The SCAT is arguably one of the most widely used instruments to this end. Though concussion can produce a constellation of heterogeneous symptoms, prior work found that a single underlying dimension (General factor) explains 96% of the reliable variance in SCAT symptom scores (Nelson et al., 2018), which indicates that total symptoms provide a useful assessment of injury severity. An abbreviated (12-item) symptom evaluation has previously been empirically derived with 1-parameter IRT analyses (i.e., Rasch) in athletes assessed 1999–2003 (Randolph et al. 2009).
What this study adds to existing knowledge:
We aimed to replicate and expand upon this prior work with 1) more advanced analyses, 2) a contemporary athlete sample, and 3) comparison of even shorter forms. Abbreviated forms of a concussion symptom scale (SCAT 3/5) were developed using IRT-methods and subsequently examined in concussed and control athletes undergoing broad clinical assessment. The 3- (feeling slowed down, difficulty concentrating, feeling like “in a fog”), 5- (“Don’t feel right”, fatigue or low energy), 10- (confusion, drowsiness, dizziness, balance problems, headache), and 14-item (“Pressure in head”, difficulty remembering, sensitivity to light, sensitivity to noise) short forms derived with a more realistic 2-parameter IRT model and the original 22-item checklist provided sufficiently reliable and valid estimates of overall concussion symptom severity.
ACKNOWLEDGEMENTS
We thank Ashley LaRoche and Alexa Wild for coordinating the Head to Head study as well as the many participants, families, school personnel, and research staff who contributed to the study. This secondary data analysis project was funded by National Institutes of Health (NIH) grants R03NS100691 and R01NS110856. The studies from which data were obtained were funded by U.S. Army Medical Research and Materiel Command (Department of Defense) under award numbers W81XWH-12-1-0004 and W81XWH-14-1-0561. Study data were collected and managed using REDCap electronic data capture tools,10 hosted at the Medical College of Wisconsin’s Clinical and Translational Science Institute, supported by NIH grant 1UL1-RR031973 (−01). The manuscript’s contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH and are not necessarily endorsed by the U.S. Army. The authors have no conflicts of interest to disclose.
Footnotes
Investigation performed at the Medical College of Wisconsin, Milwaukee, WI, USA
This sample included the 219 athletes used in the aforementioned study to establish the underlying unidimensional structure of the SCAT acutely post-injury.23
REFERENCES
- 1.Alla S, Sullivan SJ, Hale L, and McCrory P. Self-report scales/checklists for the measurement of concussion symptoms: a systematic review. Br J Sports Med 43(Suppl 1): i3–12, 2009. [DOI] [PubMed] [Google Scholar]
- 2.Arbogast KB, Curry AE, Pfeiffer MR, et al. Point of health care entry for youth with concussion within a large pediatric care network. JAMA Pediatr 170(7): e160294, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cai L, Du Toit S, and Thissen D. IRTPRO: Flexible, multidimensional, multiple categorical IRT modeling [Computer software]. Chicago, IL: Scientific Software International; 2011. [Google Scholar]
- 4.Clay MB, Glover KL, and Lowe DT. Epidemiology of concussion in sport: A literature review. J Chiropr Med 12(4): 230–251, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coronado VG, Haileyesus T, Cheng TA, et al. Trends in sports- and recreation-related traumatic brain injuries treated in US emergency departments: The National Electronic Injury Surveillance System-All Injury Program (NEISS-AIP) 2001–2012. J Head Trauma Rehabil 30(3): 185–197, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Echemendia RJ, Meeuwisse W, McCrory P, et al. The Sport Concussion Assessment Tool 5th Edition (SCAT5): Background and rationale. Br J Sports Med 51(11): 848–850, 2017. [DOI] [PubMed] [Google Scholar]
- 7.Gioia GA, Schneider JC, Vaughan CG, and Isquith PK. Which symptom assessments and approaches are uniquely appropriate for paediatric concussion? Br J Sports Med 43(Suppl 1): i13–22, 2009. [DOI] [PubMed] [Google Scholar]
- 8.Guskiewicz KM, Register-Mihalik J, McCrory P, et al. Evidence-based approach to revising the SCAT2: Introducing the SCAT3. Br J Sports Med 47(5): 289–293, 2013. [DOI] [PubMed] [Google Scholar]
- 9.Guskiewicz KM, Ross SE, and Marshall SW. Postural Stability and Neuropsychological Deficits After Concussion in Collegiate Athletes. J Athl Train 36(3): 263–273, 2001. [PMC free article] [PubMed] [Google Scholar]
- 10.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, and Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 42(2): 377–381, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Helmick K, Guskiewicz K, Barth J, et al. Defense and Veterans Brain Injury Center Working Group on the acute management of mild traumatic brain injury in military operational settings: Clinical practice guideline and recommendations. Washington, D.C.: Defense and Veteran Brain Injury Center, 2006. [Google Scholar]
- 12.Iverson GL, Gardner AJ, Terry DP, et al. Predictors of clinical recovery from concussion: a systematic review. Br J Sports Med 51(12): 941–948, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iverson GL, and Schatz P. Advanced topics in neuropsychological assessment following sport-related concussion. Brain Inj 29(2): 263–275, 2015. [DOI] [PubMed] [Google Scholar]
- 14.Joyce AS, Labella CR, Carl RL, Lai JS, and Zelko FA. The Postconcussion Symptom Scale: Utility of a three-factor structure. Med Sci Sports Exerc 47(6): 1119–1123, 2015. [DOI] [PubMed] [Google Scholar]
- 15.Kontos AP, Elbin RJ, Schatz P, et al. A revised factor structure for the post-concussion symptom scale: Baseline and postconcussion factors. Am J Sports Med 40(10): 2375–2384, 2012. [DOI] [PubMed] [Google Scholar]
- 16.Lancaster MA, McCrea MA, and Nelson LD. Psychometric properties and normative data for the Brief Symptom Inventory-18 (BSI-18) in high school and collegiate athletes. Clin Neuropsychol 30(2): 338–350, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Llewellyn T, Burdette GT, Joyner AB, and Buckley TA. Concussion reporting rates at the conclusion of an intercollegiate athletic career. Clin J Sport Med 24(1): 76–79, 2014. [DOI] [PubMed] [Google Scholar]
- 18.McCrea M, Guskiewicz K, Randolph C, et al. Incidence, clinical course, and predictors of prolonged recovery time following sport-related concussion in high school and college athletes. J Int Neuropsychol Soc 19(1): 22–33, 2013. [DOI] [PubMed] [Google Scholar]
- 19.McCrea M, Hammeke T, Olsen G, Leo P, and Guskiewicz K. Unreported concussion in high school football players: implications for prevention. Clin J Sport Med 14(1): 13–17, 2004. [DOI] [PubMed] [Google Scholar]
- 20.McCrory P, Meeuwisse W, Dvorak J, et al. Consensus statement on concussion in sport-The 5th international conference on concussion in sport held in Berlin, October 2016. Br J Sports Med 51(11): 838–847, 2017. [DOI] [PubMed] [Google Scholar]
- 21.Meehan WP 3rd, Mannix R, Monuteaux MC, Stein CJ, and Bachur RG. Early symptom burden predicts recovery after sport-related concussion. Neurology 83(24): 2204–2210, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Merritt VC, and Arnett PA. Premorbid predictors of postconcussion symptoms in collegiate athletes. J Clin Exp Neuropsychol 36(10): 1098–1111, 2014. [DOI] [PubMed] [Google Scholar]
- 23.Nelson LD, Kramer MD, Patrick CJ, and McCrea MA. Modeling the structure of acute sport-related concussion symptoms: A bifactor approach. J Int Neuropsychol Soc 24(8): 793–804, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patricios J, Fuller GW, Ellenbogen R, et al. What are the critical elements of sideline screening that can be used to establish the diagnosis of concussion? A systematic review. Br J Sports Med 51(11): 888–894, 2017. [DOI] [PubMed] [Google Scholar]
- 25.Piland SG, Motl RW, Ferrara MS, and Peterson CL. Evidence for the factorial and construct validity of a self-report concussion symptoms scale. J Athl Train 38(2): 104–112, 2003. [PMC free article] [PubMed] [Google Scholar]
- 26.Powell JM, Ferraro JV, Dikmen SS, Temkin NR, and Bell KR. Accuracy of mild traumatic brain injury diagnosis. Arch Phys Med Rehabil 89(8): 1550–1555, 2008. [DOI] [PubMed] [Google Scholar]
- 27.Randolph C, Millis S, Barr WB, et al. Concussion symptom inventory: an empirically derived scale for monitoring resolution of symptoms following sport-related concussion. Arch Clin Neuropsychol 24(3): 219–229, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.U.S. Department of Health and Human Services & Centers for Disease Control and Prevention. Heads Up: Concussion in High School Sports http://www.cdc.gov/headsup/pdfs/providers/facts_about_concussion_tbi-a.pdf.


