Abstract
Cocaine abuse remains a public health threat around the world. There are no pharmacological treatments approved for cocaine use disorder. Cannabis has received growing attention as a treatment for many conditions, including addiction. Most cannabis-based medication development has focused on cannabinoid CB1 receptor (CB1R) antagonists (and also inverse agonists) such as rimonabant, but clinical trials with rimonabant have failed due to its significant side-effects. Here we sought to determine whether a novel and selective CB2R inverse agonist, Xie2-64, has similar therapeutic potential for cocaine use disorder. Computational modeling indicated that Xie2-64 binds to CB2R in a way similar to SR144528, another well-characterized but less selective CB2R antagonist/inverse agonist, suggesting that Xie2-64 may also have CB2R antagonist profiles. Unexpectedly, systemic administration of Xie2-64 or SR144528 dose-dependently inhibited intravenous cocaine self-administration and shifted cocaine dose-response curves downward in rats and wild-type, but not in CB2R-knockout, mice. Xie2-64 also dose-dependently attenuated cocaine-enhanced brain-stimulation reward maintained by optical stimulation of ventral tegmental area dopamine neurons in DAT-Cre mice, while Xie2-64 or SR144528 alone inhibited optical brain-stimulation reward. In vivo microdialysis revealed that systemic or local administration of Xie2-64 into the nucleus accumbens reduced extracellular dopamine levels in a dose-dependent manner in rats. Together, these results suggest that Xie2-64 has significant anti-cocaine reward effects likely through a dopamine-dependent mechanism, and therefore, deserves further study as a new pharmacotherapy for cocaine use disorder.
Keywords: Cannabinoid, CB2 receptor, cocaine, Xie2-64, dopamine, self-administration, optogenetics
1. Introduction
Cocaine abuse remains a major public health burden around the world. In the United States alone, the number of young adults aged 12 and older trying cocaine for the first time increased by 61% from 2013 to 2015, reaching 766,000 new users (Hughes, 2016). Although overshadowed in recent years by the opioid crisis, cocaine abuse continues to rise in prevalence. Currently, there are no approved pharmacological medications for the treatment of cocaine use disorder. Recently, cannabis has received growing public attention as putative therapy for myriad physiological and neurological conditions, including glaucoma, cancer, epilepsy, dementia and even drug addiction (Gonzalez-Cuevas et al., 2018; Jordan and Xi, 2019; Solimini et al., 2017). Early strategies to develop cannabis-based medications have focused on cannabinoid CB1 receptor (CB1R) antagonists and inverse agonists (Sloan et al., 2017). However, clinical studies with such a compound, rimonabant, have been terminated worldwide due to significant adverse effects such as depression and suicidal tendencies (Le Foll et al., 2009). Therefore, recent studies have shifted their focus to other targets in the endocannabinoid systems, such as the CB2 receptor (CB2R) (Jordan and Xi, 2019), fatty acid amide hydroxylase (Deutsch, 2016), monoacylglycerol lipase (Gil-Ordonez et al., 2018), or non-psychoactive phytocannabinoids (Galaj et al., 2020; He et al., 2020; Sloan et al., 2017; see review by Galaj and Xi, 2020).
While the CB2R was initially thought to be expressed exclusively in the periphery (Griffin et al., 1997; Howlett, 1998; Munro et al., 1993; see review by Atwood and Mackie, 2010), over the last decade, growing evidence has revealed its expression in the brain and in neurons, albeit at lower levels than brain CB1R or peripheral CB2R (Lanciego et al., 2011; Van Sickle et al., 2005; Zhang et al., 2014; Zhang et al., 2015; see review by Jordan and Xi, 2019). Our lab and others have shown that CB2R is detectable in multiple phenotypes of neurons, including glutamate neurons in the prefrontal cortex and hippocampus (Stempel et al., 2016; Stumpf et al., 2018) and DA neurons in the ventral tegmental area (VTA) (Zhang et al., 2015; Zhang et al., 2014; Foster et al., 2016). Furthermore, repeated cocaine administration or prolonged cocaine self-administration can up-regulate CB2R mRNA expression in the VTA, PFC and NAc in rats and mice (Zhang et al., 2015), suggesting that CB2R could be a valuable therapeutic target for cocaine use disorder.
We have previously reported that the CB2R-selective agonist JWH-133 was able to inhibit cocaine action, including intravenous cocaine self-administration, cocaine-conditioned place preference, cocaine-induced increases in locomotion, cocaine-enhanced extracellular DA and C-fos expression (Canseco-Alba et al., 2019; Delis et al., 2017; Gobira et al., 2019; Xi et al., 2011; Zhang et al., 2014). In the present study, we further explored the potential utility of Xie2-64, a highly selective human CB2R inverse agonist (Yang et al., 2013), on cocaine self-administration and DA-dependent brain-stimulation reward, based on the findings that CB1R antagonists/inverse agonists such as rimonabant and AM251 have potent anti-cocaine potential in experimental animals (Xi et al., 2006; Xi et al., 2008; Galaj and Xi, 2019).
Xie2-64 exhibits a high affinity for CB2R (Ki=0.5 nM) and a >2500-fold selectivity for human CB2R over CB1R (Yang et al., 2013). In this study, we first evaluated Xie2-64 in computational models to determine its CB2R binding profile. We then used intravenous (i.v.) cocaine self-administration in rats and wild-type and CB2-knockout (KO) mice to evaluate the efficacy and selectivity of Xie2-64 or SR144528, another well-characterized CB2R antagonist/inverse agonist, in attenuating cocaine reward. Next, we used optogenetics to determine whether Xie2-64 or SR144528 reduces DA-mediated optical intracranial self-stimulation (oICSS) produced by selective stimulation of VTA DA neurons in DAT-Cre mice. Lastly, we used in vivo brain microdialysis to observe the effects of Xie2-64 on brain DA release in the NAc. We found unexpectedly that this novel CB2R compound is effective in reducing the rewarding effects of cocaine in both rats and mice, likely via a DA-dependent mechanism, supporting further study of Xie2-64 or other similar CB2R antagonists/inverse agonists as potential therapeutics for cocaine use disorder.
2. Methods
2.1. Animals and housing
Male Long-Evans rats (275-325 g; Charles-River Laboratories, Raleigh, NC, USA) and male CB2-KO, heterozygous DAT-Cre+/− or wild-type mice (bred at the NIDA IRP) were used in these studies. All animals were housed in the animal facility at the NIDA IRP under a reverse 12-hr light-dark cycle (lights on at 7:00 PM) with ad libitum access to food and water. All procedures were approved by the Animal Care and Use Committee (ACUC) of NIDA and were consistent with the Guide for the Care and Use of Laboratory Animals, 8th edition (National Research Council, 2011).
2.2. Drugs and solutions
Cocaine (NIDA Drug Supply Program, MD, USA) was dissolved in saline and diluted to unit doses appropriate for behavioral studies (see below). Xie2-64 was synthesized and provided by Xiang-Qun Xie, according to previously published methods (Yang et al., 2013). For in vivo systemic or intracranial local administration, Xie2-64 was dissolved in 10% DMSO, 15% Tween-80 and saline. For intra-NAc local perfusion, we first dissolved Xie2-64 in 10% DMSO + 15% Tween-80 in saline and then diluted it with artificial cerebral spinal fluid (aCSF) by 4 times to lower the DMSO and Tween-80 concentrations. This drug solution was then further diluted with aCSF to the desired drug concentrations (i.e., 1, 10, 30, 100, 300 μM Xie2-64) for intracranial perfusion.
2.3. CB2R structure preparation for molecular docking
The human CB2R crystal structure (PDB: 5ZTY, Resolution: 2.8 Å; Li et al., 2019) complexed with antagonist (AM10257) was retrieved and downloaded from the Protein Data Bank (http://www.pdb.org/pdb/). SYBYL-X 1.3 (Informer Technologies, Inc) was used to repair all the residues and perform the energy minimizations for molecular docking. Briefly, the parameters defined in SYBYL were as follows: gradient set to 0.5 kcal/mol, max iterations set to 5000, force field set to MMFF94s, and charge methods set to MMFF94. ProSA-web Z-scores and PROCHECK Ramachandran plots were used to further validate the model.
2.4. Docking study of CB2R selective inverse agonists
The MOLCAD module in SYBYL-X 1.3 was used to explore the reported orthosteric binding pocket on CB2R. The docking program Surflex-Dock GeomX (SFXC) in SYBYL-X 1.3 was applied to construct receptor-ligand complexes in which the docking scores were expressed in −log10 (Kd). Briefly, the docking parameters used were (a) number of starting conformations per ligand set to 10, max conformations per fragment set to 20, (b) maximum number of rotatable bonds per molecule set to 100, (c) flags were turned on at pre-dock minimization, post-dock minimization, molecule fragmentation, and soft grid treatment, (d) activate spin alignment method with density of search set to 9.0, and (e) number of spins per alignment set to 12.
In the present work, we docked Xie2-64 and SR144528 into the orthosteric binding pocket of the CB2R crystal structure using our reported protocol (Hu et al., 2016; Li et al., 2019; Zhou et al., 2018). Similar to endogenous CB2R ligands and other synthesized agonists (such as CP55940, HU210, and JWH-133), both SR144528 and Xie2-64 bound to the same orthosteric binding pocket of CB2R. In addition, SR144528 is a potent and highly selective CB2R inverse agonist with a Ki of 0.6 nM for CB2R and 400 nM for CB1R. Xie2-64, our in-house compound, is a selective CB2R inverse agonist and exhibited very high affinity for CB2R (Ki=0.5 nM) and great CB2R > CB1R selectivity (>2500-fold; Yang et al., 2013).
2.5. Cocaine self-administration
Rat self-administration.
Intravenous (i.v.) jugular catheter surgery and cocaine self-administration procedures were conducted as reported previously (Li et al., 2019). After implantation, catheters were flushed daily with a gentamicin–heparin–saline solution (0.1 mg/ml gentamicin and 30 IU/ml heparin) to prevent clogging and infection. Cocaine self-administration training began 5 days following surgery in an operant conditioning chamber equipped with two response levers, a house light, and a cue light mounted above the active lever (Med Associates Inc., Georgia, VT, USA). Rats were allowed to press the active lever to earn an i.v. infusion of cocaine (1 mg/kg) at a rate of 0.08 ml/4.6 sec under a fixed ratio 1 (FR1) schedule of reinforcement in daily, three-hour sessions. During the infusion period, the house light was extinguished while the cue light was illuminated. Each infusion was also paired with an auditory tone cue. During infusion intervals active lever responses were recorded but had no additional consequences. Rats were transitioned to an FR2 schedule after 2 weeks of training.
After stable self-administration was achieved under FR2 (defined as earning ≥ 20 cocaine infusions or active lever responses during a three hour session, ≤ 20% variability in infusions earned, and an active/inactive lever press ratio ≥2:1 for at least 5 consecutive sessions), rats received either vehicle, Xie2-64 (10 or 20 mg/kg, i.p.), or SR144528 (1, 3 mg/kg, i.p.; doses chosen based on prior studies; Adamczyk et al., 2012) 30-min prior to the self-administration session (counterbalanced) to determine whether Xie2-64 alters cocaine self-administration maintained by a high dose (1 mg/kg/infusion) of cocaine.
2.6. Multiple-dose cocaine self-administration
After the completion of the above cocaine self-administration, we evaluated the effects of Xie2-64 or SR144528 on self-administration maintained by a full range of cocaine doses (0.031, 0.0625, 0.125, 0.25, 0.5 and 1 mg/kg/infusion). Within each daily session rats self-administered multiple doses of cocaine across three hours in an ascending dose sequence (20 minutes/dose, with 10 minute intervals between doses; Keck et al., 2013). The dose was adjusted by changing the infusion volume via the duration of pump activation. After stable cocaine self-administration was achieved for at least 2-3 days, the animals received vehicle, Xie2-64 (10 or 20 mg/kg, i.p.), or SR144528 (1, 3 mg/kg, i.p.) 30-min prior to sessions. After each drug test, animals were allowed to self-administer cocaine under the same conditions until the behavior was re-stabilized for at least 2-3 days. Each animal was tested 3 times with intervals of 2-3 days.
2.7. Cocaine self-administration under a PR schedule of reinforcement
We then evaluated the effects of Xie2-64 on the rewarding value of cocaine and/or motivation to earn cocaine. Rats with stable responding were allowed to self-administer cocaine (first for 1.0 mg/kg/infusion, and then for 0.5 mg/kg/infusion) under a progressive ratio (PR) schedule of reinforcement. The number of lever presses required to receive a single infusion of cocaine was increased according to the following schedule: 1, 2, 4, 6, 9, 12, 15, 20, 25, 32, 40, 50 etc. Breakpoints were operationally defined as a number of lever presses for the last cocaine infusion before a one-hour period during which no cocaine reward was obtained. Once responding stabilized with breakpoints varying by <1-2 increments for 3 consecutive days, animals were divided into 3 dose groups. Thirty min prior to a test session, rats were injected with vehicle or Xie2-64 (10 or 20 mg/kg).
2.8. Cocaine self-administration in wild-type and CB2-KO mice
To determine whether the effects of Xie2-64 on cocaine intake were due to selective effects at CB2R, additional cohorts of wild-type and CB2-KO mice were trained to self-administer cocaine. Surgery and cocaine self-administration procedures were identical to those described above for rats and previously in mice (Jordan et al., 2019b; Xi et al., 2011) . After recovery from surgery, mice were allowed to lever press to earn 0.5 mg/kg/infusion cocaine under a FR1 schedule in operant chambers sized for mice (Med Associates Inc.). Once stable baselines were established as described above, mice received either vehicle or Xie2-64 (10 or 20 mg/kg), 30-min prior to their self-administration session.
2.9. Optogenetic intracranial self-stimulation (oICSS)
Optogenetic experiments were designed to determine whether Xie2-64 alters DA-dependent rewarding-seeking behaviors (Han et al., 2017; Jordan et al., 2019). Briefly, AAV-EF1α-DIO-ChR2-EGFP (150 nl, ~2 × 1012 genomes/ml, University of North Carolina Gene Therapy Center) was microinjected into the VTA of adult DAT-Cre mice (AP −3.28; ML 0.43; DV −4.41 mm relative to Bregma) under anesthesia, as we reported previously (Jordan et al., 2019b). After 4 weeks of recovery, mice were allowed to lever press in mouse operant chambers (Med Associates Inc.) to earn a one second pulse train of laser stimulation (473 nm, 20 mW, 5 ms duration, 25 Hz), paired with one second illumination of a cue light above the active lever during daily, one hour sessions. Inactive responses were recorded but had no scheduled consequences. Once reliable responding was established for 25 Hz, mice were transitioned to a rate-frequency program in which 6 different stimulation frequencies (100, 50, 25, 10, 5, and 1 Hz) were presented in descending order for 10 minutes each. After stable oICSS baselines were established, mice were treated i.p. with vehicle, cocaine alone (2 or 10 mg/kg, 5-min. prior to oICSS sessions), Xie2-64 (10 or 20 mg/kg) or SR144528 (1, 3 mg/kg, 30-min prior to oICSS sessions), either alone or 30-min prior to cocaine (10 mg/kg) and oICSS sessions.
2.10. Microdialysis
Microdialysis procedures in rats were conducted as described previously (Gao et al., 2018). Briefly, intracranial guide cannulae (CMA Microdialysis AB, Solna, Sweden) were implanted bilaterally into the NAc (AP +1.7; ML ±1.7; DV −5.8 mm with 6° angled away from the midline). After 1-week recovery, microdialysis probes were inserted into the NAc. Twelve hours later, dialysis buffer was perfused at a rate of 2.0 μL/min. Dialysate was collected in 20-min time blocks into separate aliquots containing 10 μL of 0.1 M perchloric acid to prevent DA degradation. Baseline samples were collected for two hours, followed by i.p. injection of Xie2-64 (10 or 20 mg/kg) or intra-NAc local perfusion of Xie2-64. Samples were then collected for 3 additional hours. All samples were frozen at −80°C until analysis. DA was measured using high-performance liquid chromatography, as described previously (Gao et al., 2018; Xi et al., 2006).
2.11. Data analysis for in vivo experiments
Dependent measures for drug self-administration and oICSS included active and inactive lever responses and reinforcers earned. Dependent measures for microdialysis were DA levels in each dialysate sample, expressed as a percentage of pre-injection baseline (Xi et al., 2006). All behavioral data were expressed as Mean ± SEM and analyzed using one- or two-way ANOVAs, followed by post-hoc Student-Newman-Keuls tests for individual group comparisons.
2.12. Chemogenomics Knowledgebase and Systems Pharmacology Analysis
We have constructed a drug abuse-related G-protein coupled receptors knowledgebase (DAKB-GPCRs) (https://www.cbligand.org/dakb-gpcrs/) (Chen et al., 2019) for use in target, off-target, or additional identification and systems pharmacology analyses. As of this writing, DAKB-GPCRs has already archived 86 drug abuse-related GPCRs with ~90,000 associated chemicals having various reported bioactivities with different bioassays and references. Moreover, several in-house chemoinformatics tools were also used, including TargetHunter, HTDocking, Blood-Brain Barrier (BBB) Predictor, Spider Plot, and more. In this work, we applied DAKB-GPCRs (https://www.cbligand.org/dakb-gpcrs/) and our established chemoinformatics tools to perform systems pharmacological analysis on Xie2-64. First, Xie2-64 was docked into the collected proteins from our database. Predicted proteins were then ranked based on their docking scores and similarity score (targets with higher docking scores and similarity scores may have higher binding affinities and therefore a greater chance of interacting with ligands). We next mapped out a pharmacological network of interactions between drug compounds and target proteins at the molecular level. Cytoscape 3.4.0 (Shannon et al., 2003) was used to generate, analyze, and visualize the network of targets and drugs/compounds, as described previously (Chen et al., 2019).
3. Results
3.1. Interactions between Xie2-64 and the CB2R structure
Similar to the endogenous cannabinoid ligand 2-Arachidonoylglycerol (2-AG) and the synthesized agonist JWH133, both of the selective inverse agonists, Xie2-64 and SR144528, are reported to bind to the orthosteric binding pocket of CB2Rs. As described in the CB2R crystal structure, the reported orthosteric ligand-binding site is formed by hydrophobic residues from TM2, TM3, TM5, TM6, and TM7, as well as the extracellular loop 2 (ECL2), including Phe87, Phe91, Phe94, His95, Ile110, Val113, Thr114, Phe117, Phe183, Trp194, Trp258, and Ser285 (Li et al., 2019; Xing et al., 2020).
Next, Xie2-64 and SR144528 were docked into the CB2R crystal structure complexed with antagonist (AM10257) using the same protocol. Fig. 1A shows that Xie2-64 and SR144528 highly overlapped in the CB2R crystal structure. The detailed interactions of SR144528 and Xie2-64 in the CB2R are shown in Figs. 1B and 1C, respectively. Our results indicate that both Xie2-64 and SR144528 approached the “toggle switch” residue Trp2586.48 with distances of 3.2 and 3.6 Å, respectively, consistent with both small molecules being inverse agonists. Moreover, we found that these two ligands interacted with Phe87, Phe91, Phe94, Ile110, Val113, Phe117, Phe183, and Trp194 through similar hydrophobic interactions. Although our results showed that Xie2-64 shared a high similarity with SR144528 in its binding to CB2R, subtle differences also existed. For example, we observed a single strong hydrogen bond formed between Ser285 and SR144528, with a distance of 2.8 Å (Fig. 1B). However, there were two hydrogen bonds formed between Xie2-64 and residues in CB2R, including His95 (2.9 Å) and Thr114 (3.9 Å). Moreover, Xie2-64 shows a more favorable CB2R selectivity profile compared with SR144528 (Table 1). Oral administration of Xie2-64 affords modest bioavailability (12% F) and demonstrates a half-life (T1/2) of 10 h, shorter than that observed from intravenous administration (17 h). In addition, the in vivo acute toxicity of Xie2-64 is LD50 > 2560 mg/kg. These results, taken together with results from our previous reports with JWH133, led us to predict that Xie2-64 may have therapeutic potential for the treatment of cocaine use disorder by interactions at CB2R. This was explored in the subsequent behavioral studies.
Fig. 1:
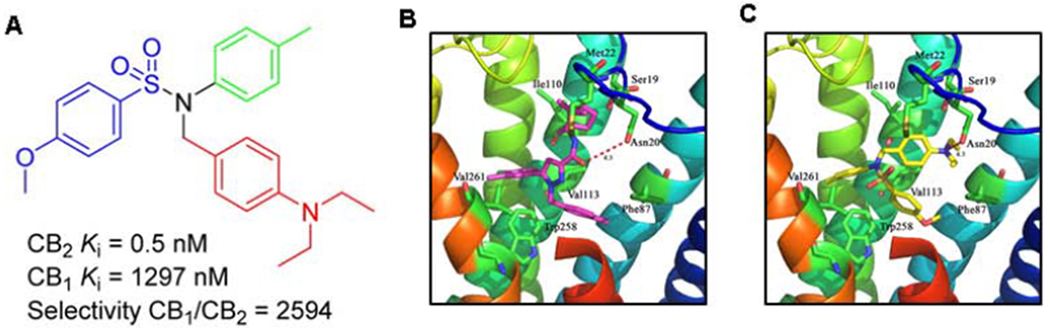
Schematic of Xie2-64 structure, receptor binding profiles, and computational modeling of Xie2-64 or SR144528 binding in the crystal structure of CB2R. A, Molecular structure of Xie2-64 and alignment of Xie2-64 or SR144528 in the CB2R crystal structure. Xie2-64 is a novel CB2R inverse agonist recently synthesized (Yang et al., 2013; compound #57). B, Detailed interactions between SR144528 and CB2R. C, Detailed interactions between Xie2-64 and CB2R.
Table 1:
Receptor binding affinities of CB2R Ligands in vitro cell lines
| Compound | CB2R Ki | CB1R Ki | CB1R/CB2R |
|---|---|---|---|
| JWH133 (Huffman et al., 1999) | 3.4 nM | 677 nM | 200 |
| SR144528 (Rinaldi-Carmona et al., 1998) | 0.6 nM | 400 nM | 667 |
| Xie2-64 (Yang et al., 2013) | 0.5 nM | 1,297 nM | 2,594 |
3.2. Xie2-64 inhibits cocaine self-administration in rats
After validating Xie2-64 binding to CB2R, we sought to determine whether Xie2-64 could inhibit cocaine self-administration and cocaine intake. Fig. 2A shows that systemic administration of Xie2-64, at 10 or 20 mg/kg (i.p.), failed to alter self-administration maintained by 1 mg/kg/infusion cocaine under FR2 reinforcement conditions (F2,14 = 0.86, p>0.05). We speculated that higher doses of Xie2-64 may be required to antagonize cocaine’s action, or the cocaine dose was too high to reveal the therapeutic efficacy of Xie2-64. To test this hypothesis, we next investigated the effects of Xie2-64 on cocaine self-administration under a PR schedule of reinforcement, in which the cost or work load (i.e., active lever presses) for receiving each subsequent cocaine infusion is progressively increased, and thus, the total cocaine intake during the self-administration session is much lower than that under the FR2 reinforcement schedule as described above. Fig. 2 (B–C) shows that systemic administration of the same doses of Xie2-64 produced a dose-dependent reduction in either the PR cocaine infusions earned or the breakpoint level for cocaine self-administration. Two-way repeated measures ANOVA of breakpoints revealed a significant main effect of treatment (Fig. 2B, F2,18 = 9.14, p<0.01; Fig. 2C, F2,18 = 7.88, p<0.01). Post-hoc testing indicated that 20 mg/kg Xie2-64 significantly reduced cocaine infusions or break-point levels compared to vehicle treatment (p<0.01).
Fig. 2:
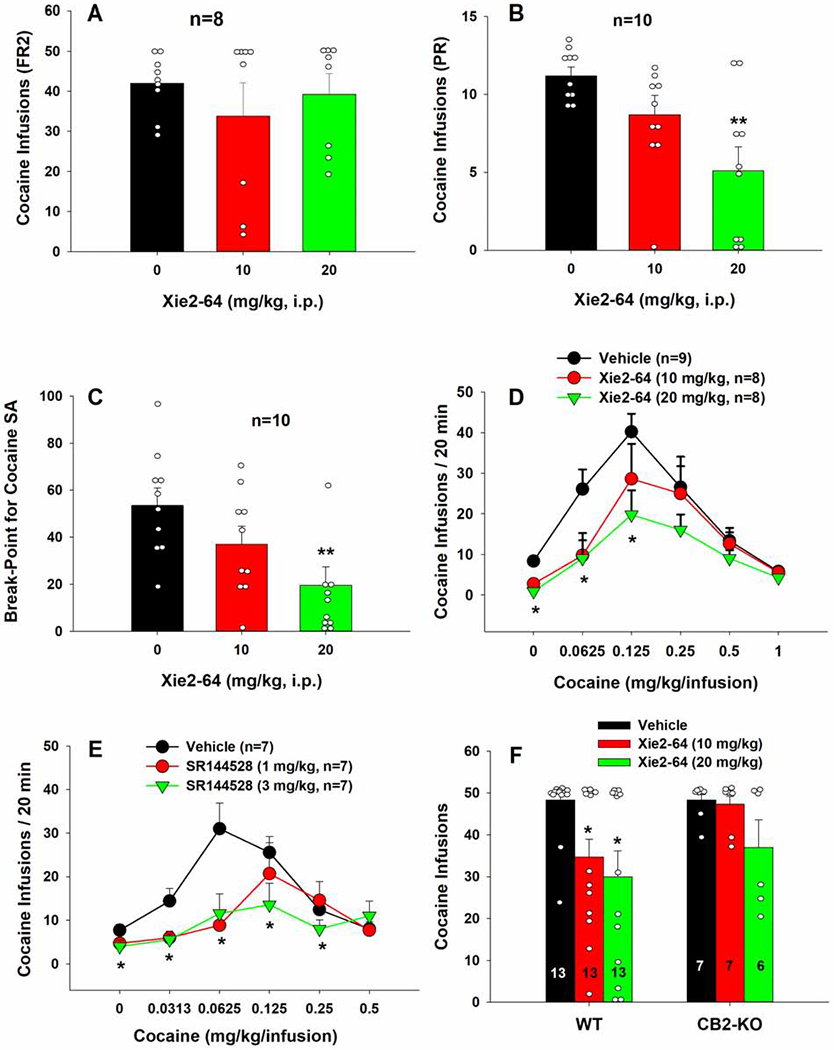
Effects of Xie2-64 on cocaine self-administration. A, Xie2-64, at the doses of 10 or 20 mg/kg, i.p., failed to alter cocaine self-administration maintained by 1 mg/kg/infusion under a low fixed-ratio (FR2) reinforcement schedule. B, Xie2-64 decreased cocaine self-administration in rats under a PR schedule of reinforcement. C, Xie2-64 reduced PR breakpoints for cocaine self-administration, indicating a reduction in motivation to earn cocaine reward. D, Xie2-64, at the same doses, inhibited cocaine self-administration maintained by lower doses of cocaine under an FR2 schedule of reinforcement and shifted the cocaine dose-response curves downward. E, SR144528, another CB2R inverse agonist, also inhibited cocaine self-administration and shifted the cocaine dose-response curves downward. F, Xie2-64 reduced cocaine self-administration in wild-type, but not CB2-KO mice, under a FR1 schedule of reinforcement. **p < 0.01, *p < 0.05 compared to vehicle treatment.
To further confirm the above finding, we observed the effects of Xie2-64 on multiple doses of cocaine self-administration. Fig. 2D shows that systemic administration of the same doses of Xie2-64 produced dose-dependent downward shifts in cocaine dose-response functions under a FR2 schedule. Two-way repeated measures ANOVA for infusions revealed a cocaine treatment main effect (F5,110 = 20.1, p<0.001). Post-hoc testing revealed that both 10 and 20 mg/kg Xie2-64 significantly reduced cocaine self-administration maintained by lower doses of cocaine, compared to vehicle treatment (p<0.05).
To determine whether the effects of Xie2-64 are mediated by its inverse agonist profile, we examined the effects of SR144528, another CB2R ligand with similar inverse agonist profile, on cocaine self-administration. Fig. 2E shows that SR144528 produced a similar inhibitory effect on cocaine self-administration. Two-way repeated measures ANOVA for infusions revealed a cocaine dose main effect (F5,90=8.79, p<0.001), a Xi2-64 treatment main effect (F2,18=4.26, p<0.05), and a cocaine dose × treatment interaction (Fig. 2E, F10,90=2.47, p<0.05). Post-hoc testing revealed that both 1 and 3 mg/kg SR144528 reduced cocaine self-administration maintained by lower doses of cocaine, compared to vehicle treatment (p<0.05).
3.3. Xie2-64 inhibits cocaine self-administration in wild-type, but not in CB2-KO mice
We next sought to determine whether the effects of Xie2-64 on cocaine self-administration were specific to action at CB2R. Fig. 2F shows a comparison of Xie2-64 treatment on cocaine self-administration by wild-type and CB2-KO mice. Two-way repeated measures ANOVA indicated a significant treatment main effect in wild-type mice (F2,24 = 4.4, p<0.05), but not in CB2-KO mice. Post-hoc testing revealed that 10 and 20 mg/kg Xie 2-64 significantly reduced cocaine self-administration in wild-type mice (p<0.05) but not in CB2-KO mice.
3.4. Xie2-64 attenuates cocaine-enhanced oICSS in mice
We have previously reported that CB2Rs are expressed in the brain, and particularly, in VTA DA neurons (Jordan et al., 2019a), suggesting that a DA-dependent mechanism may underlie the pharmacological action of Xie2-64 on cocaine reward. To test this hypothesis, we next examined the effects of cocaine and Xie2-64 on oICSS maintained by photoactivation of DA neurons in the VTA using DAT-Cre mice (Fig. 3 – A, B, C). As we reported previously (Jordan et al., 2019b; Newman et al., 2019), optical stimulation of VTA DA neurons produces robust, stimulation frequency-dependent lever responding (Fig. 3D). As anticipated with drugs of abuse, cocaine alone produced a significant leftward or upward shift in oICSS responding (Fig. 3E). Two-way ANOVA of active lever presses revealed main effects of laser frequency (F5,143=33.1, p<0.001) and treatment (F2,134=4.3, p<0.01). Post-hoc testing indicated that 10 mg/kg cocaine significantly increased responding at the 10 Hz frequency compared to vehicle (p<0.01).
Fig. 3:
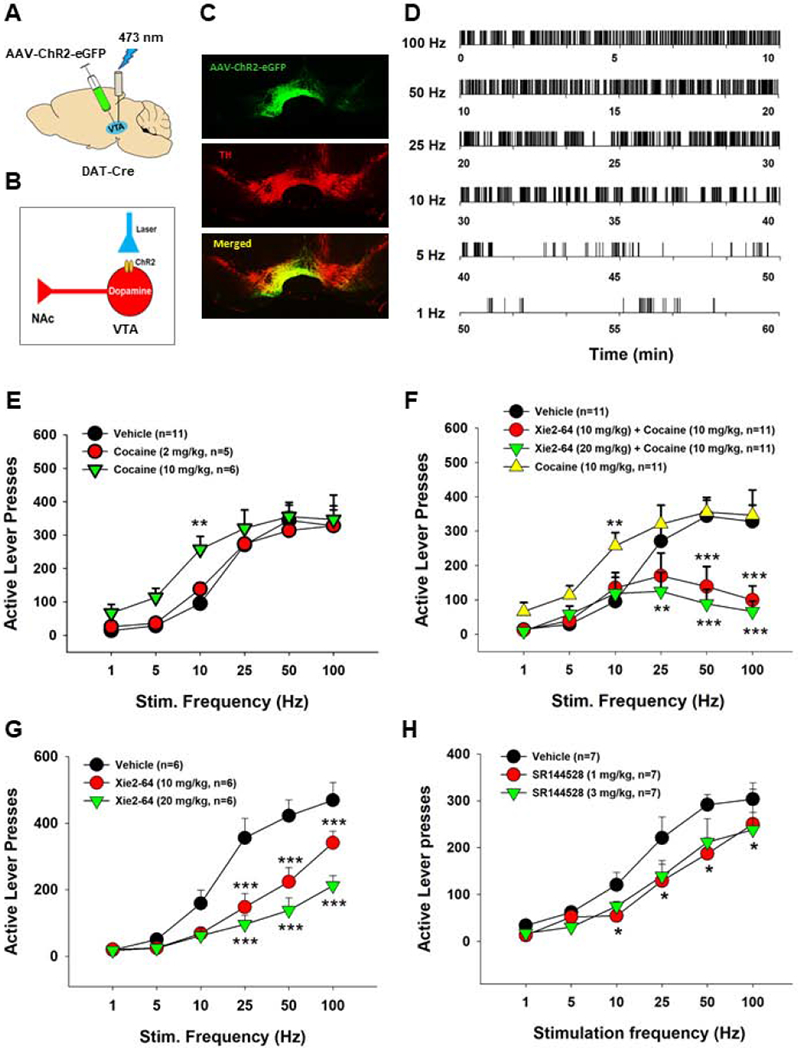
Effects of Xie2-64, SR144528, and cocaine on optogenetic brain-stimulation reward. A, Schematic of experimental model, illustrating AAV-DIO-ChR2-eGFP was microinjected into the VTA and an optical fiber was implanted into the VTA in DAT-Cre mice. B, Schematic diagram showing ChR2 expression in VTA DA neurons that can be activated by 473 nm laser activation. C, Representative images, showing that AAV-DIO-ChR2-eGFP is selectively expressed in VTA DA neurons (labeled with the enzyme tyrosine hydroxylase [TH]) in DAT-Cre mice. D, Representative oICSS (active lever responses) maintained by different frequencies of laser stimulation in a DAT-Cre mouse. Activation of ChR2 via a 473 nm laser sustained robust, frequency-dependent lever responding in the oICSS paradigm. E, Cocaine dose-dependently increased oICSS responding and shifted the rate-frequency curve to the left. F, Pretreatment with Xie2-64 (10 or 20 mg/kg, i.p. 30 min prior to test) dose-dependently attenuated cocaine-enhanced oICSS. G, Xie2-64 alone lowered oICSS responding. H, SR144528 alone also lowered oICSS responding. ***p < 0.001, **p < 0.01, *p < 0.05 compared to vehicle treatment.
Fig. 3F shows that pretreatment with Xie-264 significantly attenuated cocaine-induced increases in oICSS responding in a dose-dependent manner. Two-way ANOVA revealed main effects of frequency (F5,218=13.9, p<0.001) and treatment (F3,20=14.4, p<0.001), and a frequency X treatment interaction (F15,218=3.1, p<0.001). Post-hoc testing revealed that 10 and 20 mg/kg Xie2-64, in combination with cocaine, significantly attenuated active lever presses at 100 and 50 Hz compared to vehicle or cocaine alone (p<0.01). The 20 mg/kg Xie2-64 dose also reduced responding in combination with cocaine at 25 Hz compared to cocaine alone (p<0.01).
We next examined the impact of Xie2-64 or SR144528 alone on oICSS responding. We found that both CB2R inverse agonists produced a significant reduction in oICSS, with Xie2-64 being more potent (Fig. 3 – G, H). Two-way repeated measures ANOVA for the Xie2-64 data shown in Fig. 3G revealed main effects of frequency (F5,75=111, p<0.001) and treatment (F2,10=12.3, p<0.001) and a frequency × treatment interaction (F10,75=9.8, p<0.001). Two-way repeated measures ANOVA for the SR144528 data shown in Fig. 3F revealed main effects of frequency (F5,30=71.59, p<0.001) and treatment (F2,12=5.25, p<0.05). Post-hoc tests revealed that 10 and 20 mg/kg Xie2-64 or 1 and 3 mg/kg SR144528 significantly attenuated oICSS responding at 100, 50, and 25 Hz compared to vehicle (p<0.05).
3.5. Xie2-64 reduces extracellular DA levels in the nucleus accumbens (NAc)
Next, we sought to determine whether the attenuation of cocaine reward by Xie2-64 could be attributed to a suppressed DA response to cocaine in the NAc, using in vivo brain microdialysis. Fig. 4A shows that systemic administration of Xie2-64 produced a significant reduction in NAc extracellular DA in a dose-dependent manner. Two-way repeated measures ANOVA revealed significant main effects of treatment (F1,13=10.1, p<0.01) and time (F9,117=8.,1 p<0.001), and a treatment X time interaction (F9,117=2.2, p<0.05). Post-hoc testing revealed that 10 mg/kg Xie2-64 significantly reduced NAc extracellular DA at 20 and 140-min post-injection (p<0.05) compared to the pre-injection baseline. The 20 mg/kg Xie2-64 dose significantly reduced NAc extracellular DA at all post-injection timepoints sampled (p<0.001, except for 20-min post-injection where p<0.05). Fig. 4B shows that intra-NAc local administration of Xie2-64 also produced a significant reduction in NAc extracellular DA. One-way repeated measures ANOVA revealed a significant main effect of treatment (F5,14=2.02, p<0.05).
Fig. 4:
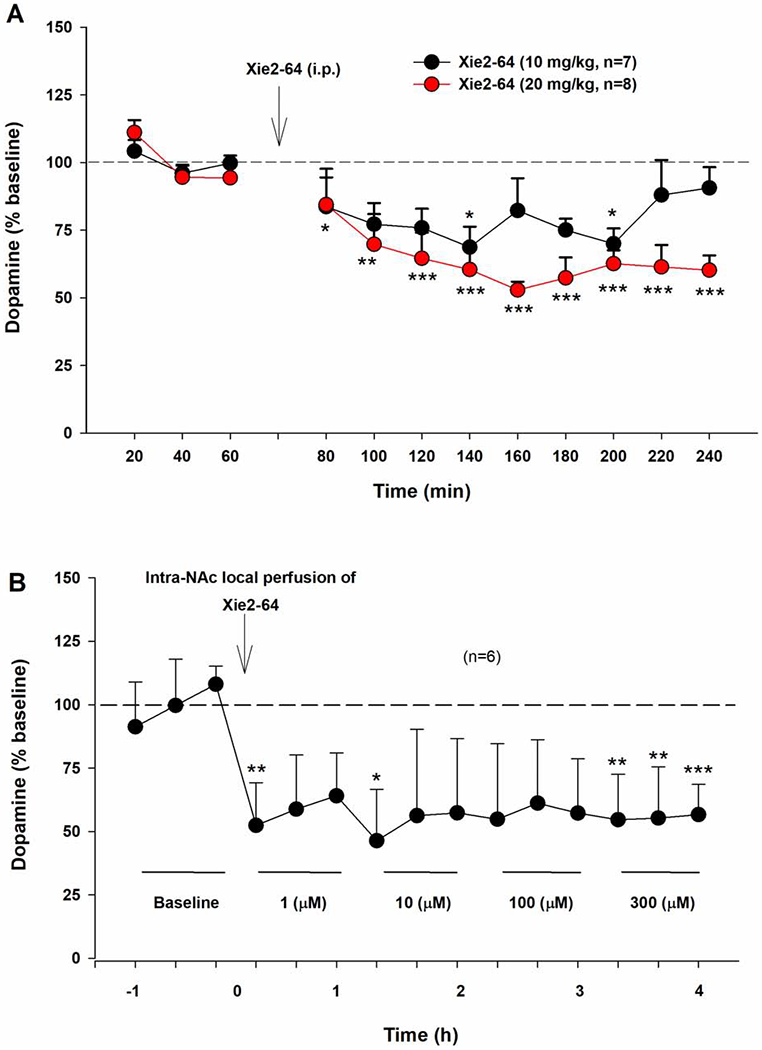
Impact of Xie2-64 on extracellular DA in the NAc as assessed by in vivo microdialysis. A, Systemic administration of Xie2-64 dose-dependently reduces extracellular DA in the nucleus accumbens (NAc) for over two hours post-administration. B, Intra-NAc local perfusion of Xie2-64 also decreased extracellular DA in the NAc. ***p < 0.001, **p < 0.01, *p < 0.05 compared to baseline.
3.6. Potential targets and off-targets of Xie2-64 in the brain
Finally, we used DAKB-GPCRs (https://www.cbligand.org/dakb-gpcrs/) and our established chemoinformatics tools (Chen et al., 2019) to perform systems pharmacological analysis on Xie2-64 for its potential targets and off-targets in the brain. We found that Xie2-64 docked into the collected proteins from our database. As targets with higher docking scores and similarity scores may have higher binding affinities, and therefore a greater chance of interacting with ligands, predicted proteins were ranked based on their docking scores and similarity scores. We found that Xie2-64 has the highest probability of binding to CB2Rs and CB1Rs, followed by DA D3Rs and several subtypes of serotonin and opioid receptors (Fig. 5).
Fig. 5:
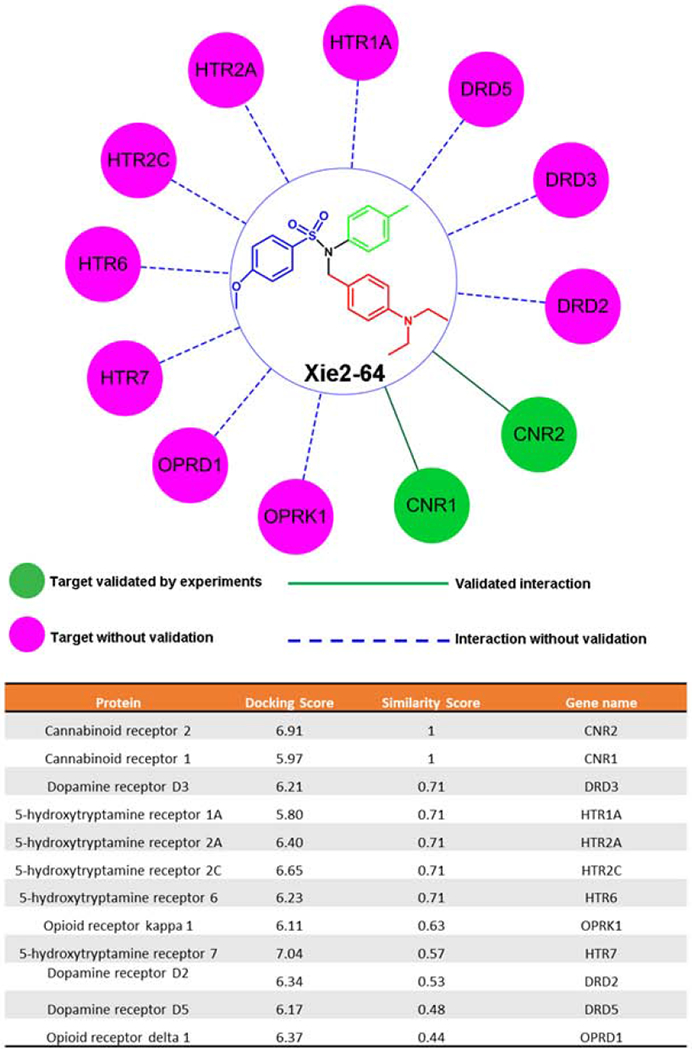
The predicted targets of Xie2-64 and the docking and similarity score of Xie2-64 by the machine learning-trained models – computational systems pharmacology-target mapping (CSP-Target Mapping), DAKB-GPCRs (https://www.cbligand.org/dakb-gpcrs/), and target identification program.
4. Discussion
Taken together, the results of the present study suggest that the novel CB2R ligand Xie2-64 may represent a viable therapeutic option for cocaine use disorder, as assessed by the findings that Xie2-64 and SR144528 (a well-characterized CB2R antagonist/inverse agonist) dose-dependently reduced cocaine self-administration and cocaine intake under multiple reinforcement schedules of reinforcement in rats and wild-type mice.
In addition, Xie2-64 dose-dependently inhibited cocaine-enhanced brain-stimulation reward maintained by optical stimulation of VTA DA neurons. The oICSS procedure is a new animal model we developed in transgenic mice to evaluate DA-dependent behaviors and brain reward function (Han et al., 2017; Jordan et al., 2019a; Newman et al., 2019). oICSS is sensitive to drug-induced changes in brain reward and mechanistically cell type-specific, and thus potentially superior to classical ICSS mediated by electrical stimulation of the medial forebrain bundle (Carlezon and Chartoff, 2007; Spiller et al., 2019). In the classical electrical ICSS paradigm, drug-induced leftward or upward shifts in the rate-frequency functions are interpreted to indicate an increase in reward, while rightward or downward shifts are interpreted to indicate a reduction in reward or aversion (Carlezon and Chartoff, 2007). Consistent with the findings in electrical ICSS models, we observed a cocaine-induced increase in oICSS responding. Similarly, oxycodone also produces a dose-dependent increase in oICSS in DAT-Cre mice (Jordan et al., 2019b). These findings suggest that cocaine and brain-stimulation produce additive or synergistic rewarding effects. In contrast to cocaine or oxycodone, Xie2-64 or SR144528 alone produced significant decreases in oICSS responding, while pretreatment with Xie2-64 attenuated cocaine-enhanced oICSS, suggesting that a DA-dependent mechanism may underlie the effects of Xie2-64 to reduce cocaine seeking. This conclusion is further supported by our finding that systemic or intra-NAc local administration of Xie2-64 also decreased NAc DA release. Systemic administration of SR144528 also inhibited cocaine-primed reinstatement of cocaine-seeking behavior in prior studies (Adamczyk et al., 2012). The reduction in cocaine self-administration or oICSS is unlikely due to sedation or non-specific locomotor impairment after Xie2-64 administration, since this compound did not alter inactive lever responding during cocaine self-administration or locomotor activity associated with VTA DA stimulation. Notably, the doses of Xie2-64 that were effective in reducing cocaine reward were significantly higher than those of SR144528 that has significantly lower selectively for CB2R over CB1R (Table 1). These differences in effective dosing may be due to off-target effects or differences in the compounds’ pharmacokinetic (PK) profiles, such as in absorption, metabolism, and blood-brain barrier penetration that will be further addressed in the future studies.
The neural mechanisms through which Xie2-64 inhibits NAc DA release are unclear. There are several possibilities. First, Xie2-64 or SR144528 may directly act on CB2R on VTA DA neurons or DA terminals in the NAc, producing an inhibitory effect on DA release in the NAc. This is supported by recent findings that within the VTA that CB2Rs are expressed mainly on DA neurons (Xi et al., 2011; Zhang et al., 2015; Zhang et al., 2014), while CB1Rs are expressed mainly on glutamate and GABA neurons (Han et al., 2017; Szabo et al., 2002). Activation of CB2R by JWH-133 inhibits VTA DA neuronal firing and DA release in the NAc (Xi et al., 2011; Zhang et al., 2014; Zhang et al., 2015). We note that Xie2-64 is not a CB2R agonist, but a human CB2R inverse agonist, as assessed by forskolin-induced cyclic AMP production assays in ex vivo CHO cells expressing human CB2R (Yang et al., 2013; Zhang et al., 2011). In theory, if a CB2R agonist (JWH133) produces a reduction in DA release, a CB2R inverse agonist (Xie2-64) should produce an increase in extracellular DA in the NAc. Contrary to this expectation, we found unexpectedly that Xie2-64, when administered systemically or locally into the NAc, produced a dose-dependent reduction in extracellular DA and in DA-dependent oICSS, suggesting that Xie2-64 might behave as a CB2R agonist in rodents in vivo. It was recently reported that CB2Rs display significant species and in vitro versus in vivo differences in gene and receptor structure and functional responses to CB2R ligands (Zhang et al., 2015). For example, AM1241 was initially reported to be an agonist at human CB2Rs (Yao et al., 2006), but later reported as an inverse agonist at rodent CB2Rs (Bingham et al., 2007). Similarly, JWH-133 was reported to inhibit DA release in the NAc in vivo (Xi et al., 2011; Zhang et al., 2014), but increases K+-evoked [3H]-DA release in midbrain slices in vitro (López-Ramirez et al., 2020). Blockade of DA D2R reverses JWH-133 effects – from increase to decrease in K+-induced [3H]-DA release, suggesting that CB2R responding to receptor ligands is endogenous DA- or D2R-dependent (López-Ramírez et al., 2020).
A second possibility is that Xie2-64 may act on CB2Rs expressed in other types of neurons that indirectly modulate VTA DA neurons or DA release in the NAc. For example, previous studies have reported CB2R expression in glutamate neurons in the cortex and hippocampus (Li and Kim, 2016; Stempel et al., 2016; Stumpf et al., 2018) and GABA neurons in the cortex and NAc (den Boon et al., 2012; Morgan et al., 2009; Zhang et al., 2020). Thus, Xie2-64-induced reduction in NAc DA release could be a net effect of CB2R actions on GABA and glutamate neurons that project to the VTA and NAc.
A third possibility is that Xie2-64 may act on other non-CB2R targets. This is supported by our finding that Xie2-64, at higher doses (20 mg/kg), produced a moderate reduction in cocaine self-administration in CB2-KO mice, although this reduction was not statistically significant. To further address this issue, we used several machine learning-trained models – computational systems pharmacology-target mapping (CSP-Target Mapping) and drug abuse related G-protein coupling receptors specific chemogenomics knowledgebase (DAKB-GPCRs) (https://www.cbligand.org/dakb-gpcrs/) to predict the potential off-targets of Xie2-64. Our results show that in addition to CB2Rs, Xie2-64 may also bind to CB1Rs, DA receptors (D2R, D3R, D5R), serotonin receptors (5HT1A, 5HT2A, 5HT2C, 5HT6, 5HT7), and opioid receptors (OPRK and OPRD) under certain circumstances. By using these tools, we have previously predicted that some cannabinoids may bind to serotonin receptors and opioid receptors, which have been confirmed in recent studies. For example, Δ9-THC may behave as an agonist at μ opioid receptor (Soderstrom et al., 2017), while HU 210, a CB1R/CB2R agonist, may also bind to 5HT2 or GPR55 receptors (Turcotte et al., 2016), and cannabidiol may act as an agonist toward 5HT1A (Soderstrom et al., 2017; Galaj et al., 2020). Clearly, further experiments are required to determine the role of non-CB2R in the effects of cannabinoid receptor ligands.
Whatever the mechanisms, the current finding that Xie2-64 is effective in attenuating cocaine reward in both rats and mice suggests that this compound represents an improvement over other CB2R agonists such as JWH-133, which was more effective in mice than in rats in reducing cocaine self-administration (Zhang et al., 2015). Notably, Xie2-64 was not a potent antagonist to the rewarding effects of cocaine. Under “low-cost” reinforcement conditions, such as easy access to high doses of cocaine under low-demand FR schedules, Xie2-64 has little effect, suggesting that the pharmacological effects of Xie2-64 depend upon cocaine dose or reinforcement schedule.
In conclusion, the present study suggests that the novel CB2R ligand, Xie2-64, can attenuate cocaine abuse-related behaviors in both rats and mice. The CB2R may, therefore, represent a valuable new therapeutic target for the treatment of cocaine use disorders.
Highlights.
CB1R ligands have been studied as addiction treatments, but failed clinical trials.
Xie2-64, a novel CB2R inverse agonist, inhibits cocaine reward and cocaine seeking
Xie2-64 reduces extracellular dopamine levels in the nucleus accumbens.
CB2R inverse agonists warrant further studies as new treatments for cocaine use disorder.
Acknowledgements and Funding Sources
This research was supported by NIDA-IRP (Z1A DA000424) and P30DA035778 (Xie). All rights are reserved by NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
None of the authors have any conflicts of interest.
References
- Adamczyk P, Miszkiel J, McCreary AC, Filip M, Papp M, Przegalinski E, 2012. The effects of cannabinoid CB1, CB2 and vanilloid TRPV1 receptor antagonists on cocaine addictive behavior in rats. Brain Res 1444, 45–54. [DOI] [PubMed] [Google Scholar]
- Atwood BK, Mackie K 2010. CB2: a cannabinoid receptor with an identity crisis. Br J Pharmacol 160, 467–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bingham B, Jones PG, Uveges AJ, Kotnis S, Lu P, Smith VA, Sun SC, Resnick L, Chlenov M, He Y, Strassle BW, Cummons TA, Piesla MJ, Harrison JE, Whiteside GT, Kennedy JD, 2007. Species-specific in vitro pharmacological effects of the cannabinoid receptor 2 (CB2) selective ligand AM1241 and its resolved enantiomers. Br J Pharmacol 151, 1061–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canseco-Alba A, Schanz N, Sanabria B, Zhao J, Lin Z, Liu QR, Onaivi ES, 2019. Behavioral effects of psychostimulants in mutant mice with cell-type specific deletion of CB2 cannabinoid receptors in dopamine neurons. Behav Brain Res 360, 286–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlezon WA Jr., Chartoff EH, 2007. Intracranial self-stimulation (ICSS) in rodents to study the neurobiology of motivation. Nat Protoc 2, 2987–2995. [DOI] [PubMed] [Google Scholar]
- Chen M, Jing Y, Wang L, Feng Z, Xie XQ, 2019. DAKB-GPCRs: An Integrated Computational Platform for Drug Abuse Related GPCRs. J Chem Inf Model 59, 1283–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delis F, Polissidis A, Poulia N, Justinova Z, Nomikos GG, Goldberg SR, Antoniou K, 2017. Attenuation of Cocaine-Induced Conditioned Place Preference and Motor Activity via Cannabinoid CB2 Receptor Agonism and CB1 Receptor Antagonism in Rats. Int J Neuropsychopharmacol 20, 269–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Boon FS, Chameau P, Schaafsma-Zhao Q, van Aken W, Bari M, Oddi S, Kruse CG, Maccarrone M, Wadman WJ, Werkman TR, 2012. Excitability of prefrontal cortical pyramidal neurons is modulated by activation of intracellular type-2 cannabinoid receptors. Proc Natl Acad Sci U S A 109, 3534–3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsch DG, 2016. A Personal Retrospective: Elevating Anandamide (AEA) by Targeting Fatty Acid Amide Hydrolase (FAAH) and the Fatty Acid Binding Proteins (FABPs). Front Pharmacol 7, 370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devane WA, Dysarz FA 3rd, Johnson MR, Melvin LS, Howlett AC, 1988. Determination and characterization of a cannabinoid receptor in rat brain. Mol Pharmacol 34, 605–613. [PubMed] [Google Scholar]
- Foster DJ, Wilson JM, Remke DH, Mahmood MS, Uddin MJ, Wess J, Patel S, Marnett LJ, Niswender CM, Jones CK, Xiang Z, Lindsley CW, Rook JM, Conn PJ, 2016. Antipsychotic-like Effects of M4 Positive Allosteric Modulators Are Mediated by CB2 Receptor-Dependent Inhibition of Dopamine Release. Neuron 91, 1244–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao JT, Jordan CJ, Bi GH, He Y, Yang HJ, Gardner EL, Xi ZX, 2018. Deletion of the type 2 metabotropic glutamate receptor increases heroin abuse vulnerability in transgenic rats. Neuropsychopharmacology 43, 2615–2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galaj E, Bi GH, Yang HJ, Xi ZX 2020, Cannabidiol attenuates the rewarding effects of cocaine in rats by CB2, 5-HT1A and TRPV1 receptor mechanisms. Neuropharm 167:107740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galaj E, Xi ZX 2019. Potential of Cannabinoid Receptor Ligands as Treatment for Substance Use Disorders. CNS Drugs. 33:1001–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil-Ordonez A, Martin-Fontecha M, Ortega-Gutierrez S, Lopez-Rodriguez ML, 2018. Monoacylglycerol lipase (MAGL) as a promising therapeutic target. Biochem Pharmacol 157, 18–32. [DOI] [PubMed] [Google Scholar]
- Gobira PH, Oliveira AC, Gomes JS, da Silveira VT, Asth L, Bastos JR, Batista EM, Issy AC, Okine BN, de Oliveira AC, Ribeiro FM, Del Bel EA, Aguiar DC, Finn DP, Moreira FA, 2019. Opposing roles of CB1 and CB2 cannabinoid receptors in the stimulant and rewarding effects of cocaine. Br J Pharmacol 176, 1541–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Cuevas G, Martin-Fardon R, Kerr TM, Stouffer DG, Parsons LH, Hammell DC, Banks SL, Stinchcomb AL, Weiss F, 2018. Unique treatment potential of cannabidiol for the prevention of relapse to drug use: preclinical proof of principle. Neuropsychopharmacology 43, 2036–2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin G, Fernando SR, Ross RA, McKay NG, Ashford ML, Shire D, Huffman JW, Yu S, Lainton JA, Pertwee RG, 1997. Evidence for the presence of CB2-like cannabinoid receptors on peripheral nerve terminals. Eur J Pharmacol 339, 53–61. [DOI] [PubMed] [Google Scholar]
- Han X, He Y, Bi GH, Zhang HY, Song R, Liu QR, Egan JM, Gardner EL, Li J, Xi ZX, 2017. CB1 Receptor Activation on VgluT2-Expressing Glutamatergic Neurons Underlies Delta(9)-Tetrahydrocannabinol (Delta(9)-THC)-Induced Aversive Effects in Mice. Sci Rep 7, 12315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Galaj E, Bi GH, Wang XF, Gardner E, Xi ZX. 2020. β-Caryophyllene, a dietary terpenoid, inhibits nicotine taking and nicotine seeking in rodents. Br J Pharmacol. 177:2058–2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howlett AC, 1998. The CB1 cannabinoid receptor in the brain. Neurobiol Dis 5, 405–416. [DOI] [PubMed] [Google Scholar]
- Hu J, Feng Z, Ma S, Zhang Y, Tong Q, Alqarni MH, Gou X, Xie XQ, 2016. Difference and Influence of Inactive and Active States of Cannabinoid Receptor Subtype CB2: From Conformation to Drug Discovery. J Chem Inf Model 56, 1152–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huffman JW, Liddle J, Yu S, Aung MM, Abood ME, Wiley JL, Martin BR, 1999. 3-(1′,1′-Dimethylbutyl)-1-deoxy-delta8-THC and related compounds: synthesis of selective ligands for the CB2 receptor. Bioorg Med Chem 7, 2905–2914. [DOI] [PubMed] [Google Scholar]
- Hughes A, Williams MR, Lipari RN, Van Horn S , 2016. State Estimates of Past Year Cocaine Use Among Adults: 2014 and 2015 The CBHSQ Report. Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration, Rockville, MD. [PubMed] [Google Scholar]
- Jordan CJ, Cao J, Newman AH, Xi ZX, 2019a. Progress in agonist therapy for substance use disorders: Lessons learned from methadone and buprenorphine. Neuropharmacology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan CJ, Humburg B, Rice M, Bi GH, You ZB, Shaik AB, Cao J, Bonifazi A, Gadiano A, Rais R, Slusher B, Newman AH, Xi ZX, 2019b. The highly selective dopamine D3R antagonist, RVK4-40, attenuates oxycodone reward and augments analgesia in rodents. Neuropharmacology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan CJ, Xi ZX, 2019. Progress in brain cannabinoid CB2 receptor research: From genes to behavior. Neurosci Biobehav Rev 98, 208–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keck TM, Yang HJ, Bi GH, Huang Y, Zhang HY, Srivastava R, Gardner EL, Newman AH, Xi ZX, 2013. Fenobam sulfate inhibits cocaine-taking and cocaine-seeking behavior in rats: implications for addiction treatment in humans. Psychopharmacology (Berl) 229, 253–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanciego JL, Barroso-Chinea P, Rico AJ, Conte-Perales L, Callen L, Roda E, Gomez-Bautista V, Lopez IP, Lluis C, Labandeira-Garcia JL, Franco R, 2011. Expression of the mRNA coding the cannabinoid receptor 2 in the pallidal complex of Macaca fascicularis. J Psychopharmacol 25, 97–104. [DOI] [PubMed] [Google Scholar]
- Le Foll B, Gorelick DA, Goldberg SR, 2009. The future of endocannabinoid-oriented clinical research after CB1 antagonists. Psychopharmacology (Berl) 205, 171–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Hua T, Vemuri K, Ho JH, Wu Y, Wu L, Popov P, Benchama O, Zvonok N, Locke K, Qu L, Han GW, Iyer MR, Cinar R, Coffey NJ, Wang J, Wu M, Katritch V, Zhao S, Kunos G, Bohn LM, Makriyannis A, Stevens RC, Liu ZJ, 2019. Crystal Structure of the Human Cannabinoid Receptor CB2. Cell 176, 459–467 e413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Kim J, 2016. Deletion of CB2 cannabinoid receptors reduces synaptic transmission and long-term potentiation in the mouse hippocampus. Hippocampus 26, 275–281. [DOI] [PubMed] [Google Scholar]
- Munro S, Thomas KL, Abu-Shaar M, 1993. Molecular characterization of a peripheral receptor for cannabinoids. Nature 365, 61–65. [DOI] [PubMed] [Google Scholar]
- Newman AH, Cao J, Keighron JD, Jordan CJ, Bi GH, Liang Y, Abramyan AM, Avelar AJ, Tschumi CW, Beckstead MJ, Shi L, Tanda G, Xi ZX, 2019. Translating the atypical dopamine uptake inhibitor hypothesis toward therapeutics for treatment of psychostimulant use disorders. Neuropsychopharmacology 44, 1435–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaldi-Carmona M, Barth F, Millan J, Derocq JM, Casellas P, Congy C, Oustric D, Sarran M, Bouaboula M, Calandra B, Portier M, Shire D, Breliere JC, Le Fur GL, 1998. SR 144528, the first potent and selective antagonist of the CB2 cannabinoid receptor. J Pharmacol Exp Ther 284, 644–650. [PubMed] [Google Scholar]
- Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, Ideker T, 2003. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res 13, 2498–2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan ME, Gowin JL, Ramchandani VA, Hurd YL, Le Foll B, 2017. The endocannabinoid system as a target for addiction treatment: Trials and tribulations. Neuropharmacology 124, 73–83. [DOI] [PubMed] [Google Scholar]
- Soderstrom K, Soliman E, Van Dross R, 2017. Cannabinoids Modulate Neuronal Activity and Cancer by CB1 and CB2 Receptor-Independent Mechanisms. Front Pharmacol 8, 720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solimini R, Rotolo MC, Pichini S, Pacifici R, 2017. Neurological Disorders in Medical Use of Cannabis: An Update. CNS Neurol Disord Drug Targets 16, 527–533. [DOI] [PubMed] [Google Scholar]
- Spiller KJ, Bi GH, He Y, Galaj E, Gardner EL, Xi ZX, 2019. Cannabinoid CB1 and CB2 receptor mechanisms underlie cannabis reward and aversion in rats. Br J Pharmacol 176, 1268–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stempel AV, Stumpf A, Zhang HY, Ozdogan T, Pannasch U, Theis AK, Otte DM, Wojtalla A, Racz I, Ponomarenko A, Xi ZX, Zimmer A, Schmitz D, 2016. Cannabinoid Type 2 Receptors Mediate a Cell Type-Specific Plasticity in the Hippocampus. Neuron 90, 795–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumpf A, Parthier D, Sammons RP, Stempel AV, Breustedt J, Rost BR, Schmitz D, 2018. Cannabinoid type 2 receptors mediate a cell type-specific self-inhibition in cortical neurons. Neuropharmacology 139, 217–225. [DOI] [PubMed] [Google Scholar]
- Szabo B, Siemes S, Wallmichrath I, 2002. Inhibition of GABAergic neurotransmission in the ventral tegmental area by cannabinoids. Eur J Neurosci 15, 2057–2061. [DOI] [PubMed] [Google Scholar]
- Turcotte C, Blanchet MR, Laviolette M, Flamand N, 2016. The CB2 receptor and its role as a regulator of inflammation. Cell Mol Life Sci 73, 4449–4470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Sickle MD, Duncan M, Kingsley PJ, Mouihate A, Urbani P, Mackie K, Stella N, Makriyannis A, Piomelli D, Davison JS, Marnett LJ, Di Marzo V, Pittman QJ, Patel KD, Sharkey KA, 2005. Identification and functional characterization of brainstem cannabinoid CB2 receptors. Science 310, 329–332. [DOI] [PubMed] [Google Scholar]
- Xi ZX, Gilbert JG, Peng XQ, Pak AC, Li X, Gardner EL, 2006. Cannabinoid CB1 receptor antagonist AM251 inhibits cocaine-primed relapse in rats: role of glutamate in the nucleus accumbens. J Neurosci 26, 8531–8536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi ZX, Peng XQ, Li X, Song R, Zhang HY, Liu QR, Yang HJ, Bi GH, Li J, Gardner EL, 2011. Brain cannabinoid CB(2) receptors modulate cocaine’s actions in mice. Nat Neurosci 14, 1160–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing C, Zhuang Y, Xu TH, Feng Z, Zhou XE, Chen M, Wang L, Meng X, Xue Y, Wang J, Liu H, McGuire TF, Zhao G, Melcher K, Zhang C, Xu HE, Xie XQ, 2020. Cryo-EM Structure of the Human Cannabinoid Receptor CB2-Gi Signaling Complex. Cell 180, 645–654 e613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang P, Wang L, Feng R, Almehizia AA, Tong Q, Myint KZ, Ouyang Q, Alqarni MH, Wang L, Xie XQ, 2013. Novel triaryl sulfonamide derivatives as selective cannabinoid receptor 2 inverse agonists and osteoclast inhibitors: discovery, optimization, and biological evaluation. J Med Chem 56, 2045–2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao BB, Mukherjee S, Fan Y, Garrison TR, Daza AV, Grayson GK, Hooker BA, Dart MJ, Sullivan JP, Meyer MD, 2006. In vitro pharmacological characterization of AM1241: a protean agonist at the cannabinoid CB2 receptor? Br J Pharmacol 149, 145–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang HY, Bi GH, Li X, Li J, Qu H, Zhang SJ, Li CY, Onaivi ES, Gardner EL, Xi ZX, Liu QR, 2015. Species differences in cannabinoid receptor 2 and receptor responses to cocaine self-administration in mice and rats. Neuropsychopharmacology 40, 1037–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang HY, Gao M, Liu QR, Bi GH, Li X, Yang HJ, Gardner EL, Wu J, Xi ZX, 2014. Cannabinoid CB2 receptors modulate midbrain dopamine neuronal activity and dopamine-related behavior in mice. Proc Natl Acad Sci U S A 111, E5007–5015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Xie Z, Wang L, Schreiter B, Lazo JS, Gertsch J, Xie XQ, 2011. Mutagenesis and computer modeling studies of a GPCR conserved residue W5.43(194) in ligand recognition and signal transduction for CB2 receptor. Int Immunopharmacol 11, 1303–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Zhou S, Yang P, Tian Y, Feng Z, Xie XQ, Liu Y, 2018. Targeted inhibition of the type 2 cannabinoid receptor is a novel approach to reduce renal fibrosis. Kidney Int 94, 756–772. [DOI] [PMC free article] [PubMed] [Google Scholar]


