Abstract
Background and Objectives:
Family-based behavioral treatment (FBT) is the recommended treatment for children with common obesity. However, there is a large variability in short- and long-term treatment response and mechanisms for unsuccessful treatment outcomes are not fully understood. In this study, we tested if brain response to visual food cues among children with obesity before treatment predicted weight or behavioral outcomes during a 6-mo. behavioral weight management program and/or long-term relative weight maintenance over a 1-year follow-up period.
Subjects and Methods:
Thirty-seven children with obesity (age 9–11y, 62% male) who entered active FBT (attended 2 or more sessions) and had outcome data. Brain activation was assessed at pre-treatment by functional magnetic resonance imaging across an a priori set of appetite-processing brain regions that included the ventral and dorsal striatum, medial orbitofrontal cortex, amygdala, substantia nigra/ventral tegmental area and insula in response to viewing food images before and after a standardized meal.
Results:
Children with more robust reductions in brain activation to high-calorie food cue images following a meal had greater declines in BMI z-score during FBT (r= 0.42; 95% CI: 0.09, 0.66; P=0.02) and greater improvements in Healthy Eating Index scores (r= −0.41; 95% CI: −0.67, −0.06; P=0.02). In whole-brain analyses, greater activation in the ventromedial prefrontal cortex, specifically by high-calorie food cues, was predictive of better treatment outcomes (whole-brain cluster corrected P=0.02). There were no significant predictors of relative weight maintenance and initial behavioral or hormonal measures did not predict FBT outcomes.
Conclusions:
Children’s brain responses to a meal prior to obesity treatment were related to treatment-based weight outcomes, suggesting that neurophysiologic factors and appetitive drive, more so than initial hormone status or behavioral characteristics, limit intervention success.
Keywords: Functional magnetic resonance imaging (fMRI), childhood obesity, obesity treatment
INTRODUCTION
Childhood obesity prevalence has more than tripled during the last four decades in the US (1). Intensive (≥26 contact hours over at least 6 months) (2) family-based behavioral treatment (FBT) is recommended for children aged 6 or more years old with common obesity (3), with demonstrated immediate and long-term improvements in weight status (4, 5). Nonetheless, variability in treatment response persists and only a minority of initially treatment-responsive children sustain success following treatment cessation (5–7). Both child and parental factors have been examined as potential treatment predictors. Male sex, less insulin resistance, stronger initial weight reduction, and parents’ response to obesity treatment have some evidence of being beneficial (2, 8–15). One study found that children who experience food as highly reinforcing exhibit poorer FBT response (16). However, there is little consistency across studies regarding factors reliably predicting FBT outcomes. For example, findings conflict as to whether younger child age and higher initial weight status hinder or facilitate long-term outcomes (17, 18).
A growing literature (19) derived from functional magnetic resonance imaging (fMRI) studies demonstrates that adults (20–22) and children (23–25) with obesity exhibit alterations in appetitive processing within brain regions regulating attentional, reward, salience, and motivational aspects of food consumption. Among children with obesity, relative to healthy weight controls, phenotypic responses include enhanced activation to food cues when fasted (or pre-meal) within reward regions (23, 24) and reduced ability of food intake to suppress reward-related activation by food cues (23). Such neurobiological responses have been linked prospectively to greater ad libitum food intake (26) and weight gain in young women (27). In adults, fMRI studies suggest that heightened responsiveness to food cues does inhibit treatment success by diet or bariatric surgery (28, 29). However, data about whether food cue responsiveness influences treatment outcomes among children are lacking.
Among children with obesity before treatment, we used fMRI to assess brain activation by visual food images before and after a standardized meal and targeted a set of key appetite-processing brain regions: the ventral and dorsal striatum (VS, DS), medial orbitofrontal cortex (mOFC), amygdala, substantia nigra/ventral tegmental area, and insula. The degree of response to high-calorie food cues within these regions is a marker of subjective satiety and, critically, also predicts food choice and caloric intake (26, 30). We have previously shown that 9–11 year old children with obesity exhibit an attenuated satiety response averaged across these appetite-processing regions compared to children of healthy weight (25). We therefore tested if activation by food images among children with obesity--measured pre-treatment--predicted weight or behavioral outcomes during a 6-mo. FBT program and/or long-term relative weight maintenance over 1 year of non-contact follow-up.
MATERIALS AND METHODS
Participants
Children with obesity, age 9–11 years, with at least one overweight parent (BMI ≥27 kg/m2) were recruited through advertisements and direct mailings. Children were screened for eligibility over the phone (N=369). Exclusion criteria included contraindications to MRI; serious medical conditions (e.g., diabetes, cognitive disorders); current use of medications known to alter appetite, body weight, or brain response (e.g., stimulants); and inability to consume study foods (e.g., allergies, vegetarianism). Fifty-eight parents provided informed consent and children provided informed assent. Children were assessed at baseline (N=55 of which 53 started FBT), following FBT treatment (N=41) and at 6-months (N=35) and 1-year (N=34) follow up visits (75%, 64% and 62% retention rates, respectively). The current study considered participants who entered FBT (defined as attending 2 or more FBT sessions) with both baseline and post-FBT assessments (N=41), of which N=4 were excluded from analyses (N=3 due to unusable MRI, N=1 due to child baseline BMI <90th percentile), resulting in the analysis sample of N=37. Of these participants, 32 completed the 6-month and 30 the 1-year follow up visit (See Consort Diagram, Supplemental Figure 2). The study was approved by the Seattle Children’s Institutional Review Board.
Study Procedures
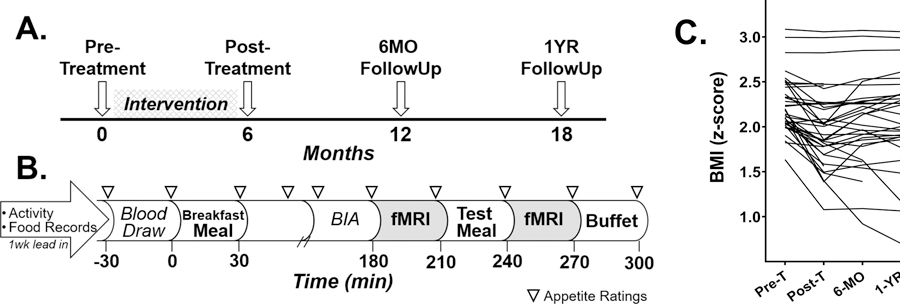
On average, pre-treatment study visits occurred ~2 weeks prior to FBT and post-treatment study visits occurred ~6 days following the last FBT session. Follow up visits were 6 and 12 months after completion of FBT (Figure 1A).
Figure 1.
Study paradigm detailing the longitundial design and pre-treatment study visit day and individual trajectores of children’s BIM z-scores throughout study. Study participants underwent a pre-treatment study visit, attended a family-based behavioral treatment (FBT) weight loss intervention for 6 months, then returned for 3 follow-up study vists (A). Study procedures for the pre-treatment visit included a 1-week lead-in period during which participants wore activity monitors and completed 3 food records (B). Participants arrived fasted, had a blood draw, were provided a standardized breakfast meal representing 10% of their estimated daily caloric need, underwent bioelectrical impedance analysis (BIA) to assess body composition, completed questionnaires, and underwent the first fMRI 3 hours (180 minutes) after the breakfast meal. Following the first fMRI, participants were provided a standarized test meal of macaroni and cheese represeting 33% of their estimated daily caloric need and underwent a 2nd fMRI 30 minutes later which was followed by an ad libitum buffet meal. Serial appetite ratings were completed approximately every 30 minutes throughtout the pre-treatment visit (represented by triangles). Individual BMI z-scores from pre-treatment to 1 year after completion of a 6-month FBT intervention (C). Pre-T, pre-treatment; Post-T, post-treatment.
Lead-in procedures took place during the week prior to the pre-treatment study visit and at the end of FBT. Children (with parental assistance) completed three food records (2 weekday, 1 weekend) and wore accelerometers (GT3X Actigraph LLC, Pensacola, FL) to assess activity. Food records were reviewed by research staff, then data were processed to determine nutrient parameters (Nutrition Data Systems for Research, version 5.0–2015) and calculate the Healthy Eating Index-2010 (HEI) (31). Accelerometers were worn around the waist, above the right hip, for 7 consecutive days and only removed while sleeping or showering/swimming. Data were captured in 30-second epochs and wear time validated by the Troiano algorithm (ActiLife v6). The Evenson cut point was applied to valid data to determine average daily minutes of moderate and vigorous intensity physical activity (MVPA) (32).
Figure 1B details pre-treatment study visit procedures as previously described (25). Briefly, participants had a fasting blood draw, consumed a standardized breakfast (milk, toast with butter and jam) representing 10% of their estimated daily caloric requirements (by the Mifflin-St Jeor equation with a standard activity factor of 1.3) (33) and underwent body composition measurements (Bioelectrical Impedance Analysis (BIA; Quantum II. RJL Systems, Detroit, MI)) (34, 35). Hydration was standardized for BIA to 12 oz. (355ml) of water intake prior to assessment. Three hours after breakfast, participants underwent the first fMRI, then consumed a standardized test meal (macaroni and cheese) titrated to meet 33% of estimated daily caloric needs (N=2 consumed <90% of provided meal), followed by the second fMRI and a 30-minute ad libitum buffet meal (~5,000 kcal). Buffet items were familiar to children and varied in nutritional and hedonic properties (e.g., pizza, fruit, cookies). Macronutrients and kilocalories consumed at the buffet were calculated (ProNutra, VioCare, Inc., Princeton, NJ). Participants were unaware that their food consumption was monitored until a subsequent debriefing. Subjective appetite ratings of hunger and fullness were assessed by visual analog scale every 30–60 minutes during the study visit (36). Change in appetite ratings by a meal (post – pre meal values) and area under the curve were calculated.
Post-treatment, assessments including lead-in procedures, body composition, waist circumference, and fasting blood draws were repeated. Height and weight were measured at Week 1 and end of FBT as well as at the 6-month and 1-year follow up visits using standardized procedures (http://www.cdc.gov/nchs/nhanes.htm). Child BMI z-score (with LMS values by sex and age) and BMI % over 95th percentile were calculated (https://www.cdc.gov/growthcharts/percentile_data_files.htm). To assess changes during FBT, change variables were calculated as post-treatment – pre-treatment. After FBT, change variables were calculated from each follow up visit to post-treatment (i.e., 6-months – post-treatment; 1-year – post-treatment).
Family-based therapy (FBT) weight loss intervention.
The FBT intervention was adapted from evidence-based programs (4–7). Briefly, children, accompanied by a least one parent or caregiver, attended 24 weekly in-person sessions. Sessions included a 30-minute meeting between a professional interventionist and the parent/child pair to individualize treatment and separate child and parent 45-minute group sessions. The intervention focused on food and physical activity education, parenting around food and physical activity, and behavioral skills use (e.g., self-monitoring (food and activity), environmental control (e.g., increasing access to healthy foods and opportunities to be active), and contingency management (e.g., rewards for decreasing intake of unhealthy foods)) (5). An eating plan was developed for each family based on an adaptation of Epstein’s Stoplight Diet (37). Foods and beverages are categorized as Green, Yellow, or Red based on fat and sugar cut-points by food group (e.g., dairy foods have higher sugar cut-points than meats due to higher naturally occurring sugars). Children and parents set goals for reducing weekly consumption of Red foods and increasing fruits and vegetables (all categorized as Green foods), combined with daily caloric range goals for children to lose approximately 0.5 pound per week (5). Children were encouraged to increase physical activity to 90 minutes/day and parents up to 60 minutes/day and reduce sedentary behavior.
Plasma Leptin and HOMA-IR
Plasma glucose and insulin were measured by glucose oxidase and commercially available immunoassay (Millipore Corp., St. Charles, MI), respectively. The homeostasis model assessment of insulin resistance (HOMA-IR) (38) was calculated. Plasma leptin was measured by ELISA (R&D Systems, Minneapolis, MN). Intra-assay coefficients of variation (CV) were <3% and inter-assay CVs were ≤4%.
fMRI Paradigm, Acquisition, Processing and Analyses
Details of the fMRI paradigm, acquisitions, processing and analyses are published elsewhere (25). Briefly, child-friendly images were presented in distinct sets of non-food blocks (N=7) alternated with high-calorie (N=3) and low-calorie (N=3) food blocks (13 blocks total per set with 10 images each presented for 2.4 seconds). Attention to the fMRI task was similar during both sessions (determined by a memory test after each session where subjects had to differentiate between images they saw in the scanner and distractor images (Pre-meal 84±9 vs. Post-meal 82±10 % correct. T=1.66 P=0.11)). Acquisitions and processing are equivalent to details in our previous study (25), processing code available upon request.
First-level data were modeled (FMRI Expert Analysis Tool) and condition effects were estimated from the average response across blocks for our contrasts of interest (high-calorie foods vs. objects and low-calorie foods, low-calorie foods vs. objects). fMRI data were excluded for excessive motion (N=4 post-meal). Creation of the functional-anatomic ROI masks (Supplemental Figure 1) has been previously described (25). Mean activation (parameter estimate) within each region for contrasts of interest was extracted using functional-anatomic ROI masks. Regional mean parameter estimates were averaged to obtain the primary outcome of average activation, for each subject, across 6 a priori brain regions (medial orbitofrontal cortex, substantia nigra/ventral tegmental area, bilateral amygdala, dorsal striatum, ventral striatum, and insula) which play a role in appetitive processing and have been established as markers of satiety and predictors of food choice (26, 30).
An exploratory whole-brain, mixed effects analysis was performed in FMRIB’s Local Analysis of Mixed Effects to determine if brain activation by contrasts of interest was associated with BMI z-score change. Z-statistic images were whole-brain corrected for multiple comparisons using a cluster threshold correction with an individual voxel threshold at Z=2.3 and a corrected cluster significance threshold of P<0.05. For descriptive purposes, scatter plots were generated by extracting mean parameter estimates of activation from significant clusters to show the association of pre-meal brain activation and BMI z-score change.
Statistics
Data are reported as mean±SD and are unadjusted unless otherwise noted. Per-protocol and unless otherwise noted, analyses included subjects who completed both baseline and post-FBT assessments and attended 2 or more FBT sessions. Linear mixed models including fixed effects (e.g., time) with the restricted maximum likelihood estimation tested differences between pre- and post-treatment for descriptive variables. Non-normally distributed variables were transformed for regression analyses where possible, otherwise local-linear kernel (Epanechnikov) nonparametric regression models were applied. Simple and multiple linear regression models tested associations and Pearson’s correlation coefficients were calculated for descriptive purposes. To assess associations between pre-treatment measures and relative weight maintenance during the 1-year follow up, repeated measures regression models were used and included the change in BMI z-score from post-treatment to each follow-up visit (6-months and 1-year) as the outcome variable and a time variable for the repeated measure. For purposes of sensitivity analyses, we imputed post-treatment BMI z-score values from last measured weight and height for children who completed baseline assessments and entered FBT (N=43), but did not return for post-treatment assessment to create “last available” change in BMI z-score. Whole-brain cluster analyses were performed in FSL otherwise, all statistics were completed in STATA (v. 15.1) using extracted values (parameter estimates) for fMRI measures; graphing was completed in GraphPad Prism (v. 6.05).
RESULTS
Participant characteristics and response to FBT
The final sample consisted of 37 children with obesity (Table 1; 62% male) with a mean age of 10.5 yr (SD=0.9) (Figure 1A). Parents reported children’s race/ethnicity as 54% White, Non-Hispanic and 46% as Non-White or Hispanic. Families attended an average of 19 (SD=5) FBT sessions out of 24 (range 2–24 sessions attended). Thirty-two of 37 children included in the study sample returned for the 6-month follow-up assessment (86%) and 30 for the 1-year follow-up assessment (81%).
Table 1.
Participant characteristics for key behavioral and hormonal measures.
| Pre-Treatment | Post-Treatment | P-value | Change | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | N | Mean | SD | N | Mean | SD | N | ||
| Height, cm | 148 | 8.2 | 37 | 151 | 8.3 | 37 | <0.0001 | 3.38 | 0.96 | 37 |
| Weight, kg | 65.2 | 18.4 | 37 | 64.3 | 20.1 | 37 | 0.29 | −0.89 | 5.14 | 37 |
| BMI, kg/m2 | 29.5 | 7.0 | 37 | 28.1 | 7.6 | 37 | <0.0001 | −1.47 | 2.18 | 37 |
| BMI z-score | 2.24 | 0.31 | 37 | 2.01 | 0.44 | 37 | <0.0001 | −0.23 | 0.23 | 37 |
| BMI % over the 95th | 28.5 | 28.7 | 37 | 19.5 | 30.9 | 37 | <0.0001 | −9.01 | 9.26 | 37 |
| Fat mass, kg | 31.6 | 14.2 | 37 | 30.6 | 16.2 | 35 | 0.06 | −1.29 | 4.07 | 35 |
| Fat mass, % | 48.1 | 6.8 | 37 | 45.3 | 8.5 | 35 | <0.0001 | −2.81 | 3.50 | 35 |
| Lean mass, kg | 32.4 | 5.2 | 37 | 34.0 | 5.6 | 35 | <0.0001 | 1.55 | 1.69 | 35 |
| Lean mass, % | 51.9 | 6.8 | 37 | 54.7 | 8.5 | 35 | <0.0001 | 2.81 | 3.50 | 35 |
| Waist circumference, cm | 96.1 | 14.1 | 37 | 95.8 | 16.5 | 37 | 0.77 | −0.28 | 5.75 | 37 |
| Activity, avg min/d | 35.8 | 21.4 | 37 | 38.1 | 23.8 | 35 | 0.55 | 2.37 | 23.8 | 35 |
| Healthy Eating Index | 54.1 | 12.0 | 37 | 60.8 | 12.4 | 34 | 0.008 | 6.07 | 14.4 | 34 |
| Leptin, ng/mL | 34.1 | 22.0 | 35 | 27.3 | 19.6 | 35 | 0.007 | −7.43 | 15.5 | 33 |
| HOMA-IR | 3.95 | 3.75 | 33 | 4.56 | 3.62 | 34 | 0.28 | 0.69 | 3.41 | 30 |
Data reported as mean (SD). Pre-treatment vs. Post-treatment comparisons are unadjusted and were done by linear mixed model. Height, weight and BMI parameters measured at Week 1 and Week 24 of FBT. All other measures were assessed at pre-treatment and post-treatment study visits. BMI parameters were calculated with LMS values by sex and age and BMI % over the 95th was calculated as the percent the current BMI was over the estimated 95% BMI percentile (https://www.cdc.gov/growthcharts/percentile_data_files.htm). Body composition parameters were by bioelectrical impedance analysis. Activity is moderate and vigorous levels (average min/d from 7 days of consecutive wear). Healthy Eating Index calculated based on 2010 criteria.
On average, children reduced absolute and relative weight status during FBT, while gaining height (Table 1). Leptin concentrations decreased, whereas HOMA-IR and waist circumference measures demonstrated little change. Behaviorally, daily MVPA minutes did not change significantly, however HEI scores, demonstrated improvement. Children’s weight status outcomes varied considerably during FBT (Figure 1C). Following FBT completion, most children’s BMI z-score increased (Figure 1C) and this pattern was similar for other indicators of child weight status (Supplemental Table 1).
Pre-treatment factors associated with response to FBT
Pre-treatment child BMI z-score was not a significant predictor of BMI z-score change during treatment although a trend was present for children of higher body fat percentage to have greater reduction in BMI z-score during treatment (Table 2). Pre-treatment behaviors of total daily physical activity, diet quality by HEI score, subjective appetite ratings, and measured food intake at an ad libitum buffet following fMRI scans were unrelated to change in child BMI z-score from pre- to post-treatment (Table 2). Pre-treatment plasma leptin concentrations (log transformed) and HOMA-IR also did not predict change in BMI z-score during FBT. Using a nonparametric regression model, families who attended more treatment sessions experienced greater child weight management success (Observed estimate −0.23, P<0.001).
Table 2.
Associations of pre-treatment demographic, behavioral, and hormonal characteristics with change in BMI z-score by FBT.
| Ba | 95% CIb | βc | P-value | |
|---|---|---|---|---|
| Sex (F vs. M) | −0.057 | −0.220, 0.106 | −0.12 | 0.48 |
| Ethnicity (White/Non-Hispanic vs. Non-white/Hispanic) | 0.088 | −0.068, 0.245 | 0.19 | 0.26 |
| Annual Household Income (Below vs. Above median) | −0.032 | −0.195, 0.132 | −0.067 | 0.69 |
| BMI z-score | 0.195 | −0.052, 0.443 | 0.26 | 0.12 |
| Fat mass % | 0.011 | 0.000, 0.022 | 0.32 | 0.05 |
| Activity | 0.002 | −0.002, 0.005 | 0.15 | 0.37 |
| Healthy Eating Index | 0.003 | −0.003, 0.010 | 0.17 | 0.30 |
| Change in Hunger score by meal | 0.001 | −0.002, 0.004 | 0.14 | 0.41 |
| Change in Fullness score by meal | −0.001 | −0.004, 0.001 | −0.20 | 0.24 |
| Hunger scores during study (AUC) | −4.52e-06 | −1.63e-05, 7.23e-06 | −0.13 | 0.44 |
| Fullness scores during study (AUC) | −3.67e-06 | −1.98e-05, 1.25e05 | −0.08 | 0.65 |
| Calories consumed at ad libitum buffet (% of daily need) | −0.001 | −0.005, 0.003 | −0.11 | 0.52 |
| Log(Leptin) | 0.038 | −0.088, 0.164 | 0.10 | 0.55 |
| HOMA-IR | 0.009 | −0.014, 0.031 | 0.14 | 0.43 |
Unstandardized regression coefficient.
95% CI of the unstandardized regression coefficient.
Standardized regression coefficient. Negative regression coefficients indicate greater reduction of BMI z-score by FBT associated with increased value of independent variable. All independent predictors measured pre-treatment (BMI z-score at Week 1 of FBT). Annual household income was calculated as below or above local regional median (https://www.deptofnumbers.com/income/washington/seattle/) for the reporting period. Activity is moderate and vigorous levels (average min/d from 7 days of consecutive wear). Healthy Eating Index calculated based on 2010 criteria. Hunger and fullness ratings assessed by visual analog scale. Change in appetite ratings were calculated from before to after the standardized meal. Hunger and fullness scores during study are area under the curve (AUC) for hunger and fullness ratings calculated by the trapizoidal method and included all serial ratings throughout the pre-treatment study visit day. N=37 (except for N=36 change in appetite scores, N=35 log(leptin), 33 HOMA).
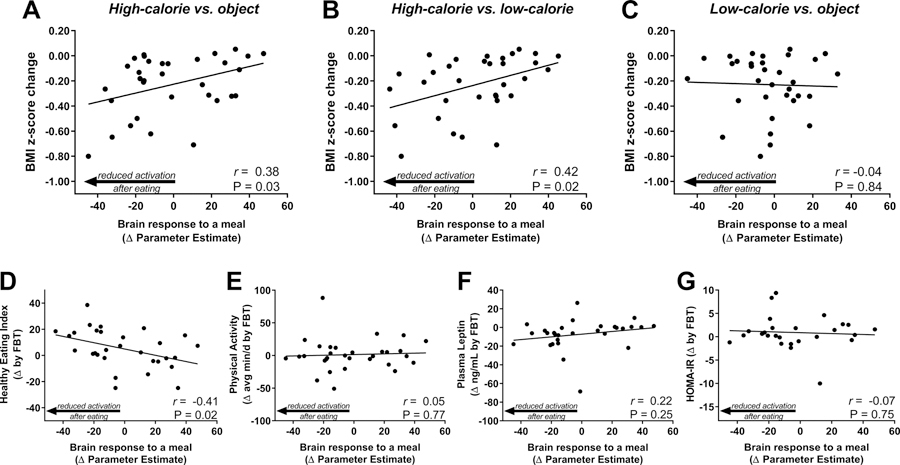
We next tested whether brain activation by visual food cues assessed pre-treatment was related to changes in child weight status during the FBT intervention. Pre-meal brain activation within a priori regions of interest was not associated with change in BMI z-score (post-treatment – pre-treatment) for any contrast (High-Calorie vs. Object (β= −3.20×10−3, P=0.20), High-Calorie vs. Low-Calorie (β= −2.51×10−3, P=0.24), or Low-Calorie vs. Objects (β= 0.30×10−3, P=0.92). However, in response to consuming a standardized meal, the pre- to post-meal change in mean brain activation by high-calorie visual food cues (vs. Objects β= 3.53×10−3, P=0.03, Figure 2A; vs. Low-calorie β= 4.04×10−3, P=0.02, Figure 2B), but not low-calorie food cues (vs. Objects β= −0.48×10−3, P=0.84, Figure 2C), was related to child BMI z-score change during treatment. Specifically, children with obesity showing more robust reductions in brain activation to high-calorie food cues following a meal had greater declines in BMI z-score during FBT. Conversely, participants who sustained brain activation by high-calorie food cues despite meal consumption had less or no success with weight loss during treatment. Adjusting for pre-treatment BMI z-score did not alter these associations (data not shown). Additionally, using imputed data for children with valid baseline BMI z-score and fMRI studies (from both pre- and post-meal sessions), but who did not have a post-treatment BMI z-score measure, we determined that pre- to post-meal change in mean brain activation by high-calorie visual food cues (vs. objects) at baseline remained significantly related to change in BMI z-score (β=3.44×10−3, P=0.03, N=43) but was unrelated to treatment attendance (β= −0.08, P=0.16).
Figure 2.
Association of pre-treatment brain response to a meal with change in BMI z-score, behavioral, and hormonal outcomes by FBT. Reduction in brain activation by high-calorie (A, B), but not low-calorie (C), food cues in response to a standardized meal (post-pre meal activation) predicted greater reduction in BMI z-score during FBT. Change in brain activation by high-calorie food (vs. object) images in response to a standardized meal (post-pre meal) was associated with FBT-induced changes Health Eating Index scores from 3 24-hr food records (D), but was unrelated to FBT-induced changes in physical activity by actigraphy (E), plasma leptin (F), and HOMA-IR (G). Physical activity includes moderate and vigorous, healthy eating index was determined based on 2010 criteria. P-value by linear regression, Pearson’s correlation coefficients calculated for descriptive purposes.
Pre-treatment factors associated with relative weight maintenance
Pre-treatment anthropometric, behavioral, and hormonal measures were unrelated to relative weight maintenance (change in child BMI z-score from each follow-up visit to post-treatment) during the 1-year follow up period. Specifically, pre-treatment BMI z-score, waist circumference, and fat mass were also not significantly associated with relative weight maintenance during the 1-year follow up (β= 0.06 z-score, P=0.38; β= 0.60×10−3 cm, P=0.71; β= −0.53×10−3 % P=0.88, respectively). Similarly, pre-treatment measures of physical activity, diet quality by HEI score, and ad libitum caloric intake did not predict relative weight maintenance (β= −0.43×10−3 average minutes, P=0.70; β= 2.06×10−3 HEI score, P=0.30; β= −0.43×10−3 % estimated daily caloric need, P=0.72, respectively). Pre-treatment measures of plasma leptin (log transformed) and HOMA-IR scores were also unrelated to relative weight maintenance (β= 0.02 log(leptin) (ng/mL), P=0.55; β= 0.27×10−3 HOMA-IR, P=0.94, respectively). Finally, unlike the initial treatment response during FBT, pre-treatment brain response to a meal by visual food cues in regions associated with appetite regulation was not related to child BMI z-score changes during follow-up (high-calorie vs. objects β= −0.67×10−3, P=0.54; low-calorie vs. objects β= 0.37×10−3, P=0.82; high-calorie vs. low-calorie β= −0.83×10−3, P=0.44). Adjusting for pre-treatment BMI z-score, change in BMI z-score during FBT, or BMI z-score at the conclusion of FBT did not alter outcomes (data not shown).
Components of treatment response associated with pre-treatment brain activation
We next examined if activation predicted other treatment outcomes that are potential mediators of successful weight status change. Children with obesity who, pre-treatment, showed more robust reductions in brain activation to high-calorie food cues following a meal also had greater improvements in HEI scores during FBT (Figure 2D). This did not appear to be explained by global adherence to recommend behavior change as brain activation by food cues showed no association with changes in physical activity during treatment (Figure 2E). Changes in leptin and HOMA-IR during FBT were unrelated to the pre-treatment brain activation (Figure 2F, G).
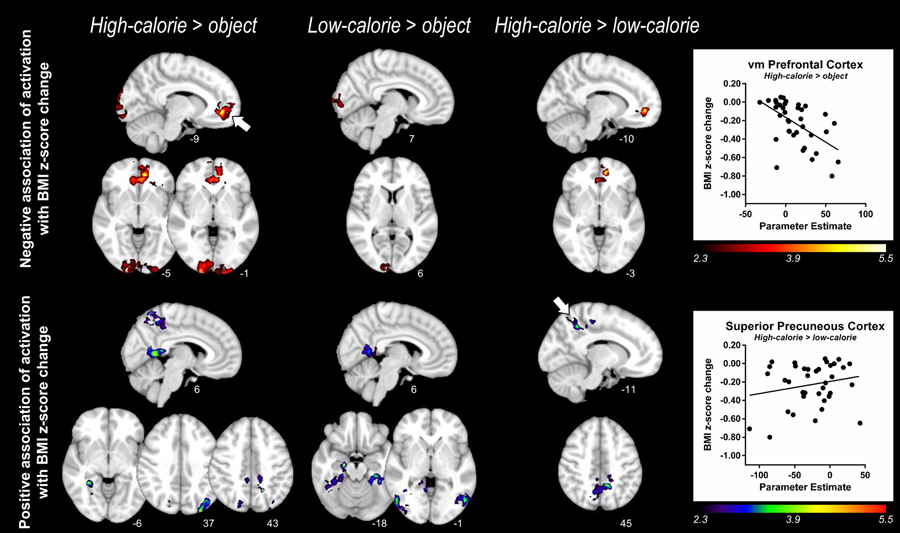
Exploratory whole-brain cluster analyses
Using a voxelwise approach, we identified clusters of activation associated with BMI z-score change. Negative correlations reflect regions in which greater activation pre-treatment correlated with greater BMI z-score reduction during FBT (Table 3, Figure 3). Greater pre-meal activation by high-calorie food images (vs. objects or low-calorie food) in the ventromedial prefrontal cortex (vmPFC) correlated with greater reductions in BMI z-score during FBT. Clusters in the occipital pole emerged in which greater pre-meal activation by either high- or low-calorie food cues (vs. objects) correlated with BMI z-score reduction.
Table 3.
Brain regions in which pre-meal brain activation by food cues associated with change in BMI z-score by FBT.
| Primary Gray Matter Anatomic Area of Z max A | B | MNI coordinatesC | Brodmann AreaD | Cluster Size (# voxels) |
Z maxE | P | Other anatomic areas in clusterF | ||
|---|---|---|---|---|---|---|---|---|---|
| x | y | z | |||||||
| Negative Association: Greater pre-meal brain activation is associated with greater reduction in BMI z-score | |||||||||
| High-calorie foods > objects | |||||||||
| Occipital Pole | R | 20 | −104 | −1 | 18 | 15598 | 4.44 | <0.0001 | occipital pole (B), occipital fusiform gyrus, intracalcarine cortex, lingual gyrus |
| vm Prefrontal Cortex (Paracingulate Gyrus) | L | −9 | 45 | −5 | 32 | 10361 | 5.25 | <0.0001 | vmPFC (paracingulate gyrus) (B), frontal medial cortex-mOFC (B), frontal orbital cortex, cingulate gyrus (B), frontal pole |
| Low-calorie foods > objects | |||||||||
| Occipital Pole | R | 7 | −102 | 6 | 17 | 2620 | 3.35 | 0.01 | ------- |
| High-calorie foods > low-calorie foods | |||||||||
| Vm Prefrontal Cortex (Paracingulate Gyrus) | L | −10 | 49 | −3 | 32 | 2518 | 5.42 | 0.02 | frontal medial cortex-mOFC (B), cingulate cortex (B), frontal pole |
| Positive Association: Greater pre-meal brain activation is associated with little or no change in BMI z-score | |||||||||
| High-calorie foods > objects | |||||||||
| Superior Precuneous Cortex | L | −17 | −56 | 43 | n/a | 9444 | 4.32 | <0.0001 | precuneous cortex (B), superior parietal lobule, cingulate gyrus, postcentral gyrus (B), precentral Gyrus, lateral occipital cortex (R) |
| Inferior Precuneous Cortex | R | 6 | −53 | 6 | n/a | 4154 | 4.14 | 0.0008 | cingulate gyrus (B), lingual gyrus, intracalcarine cortex |
| Lateral Occipital Cortex | L | −30 | 88 | 37 | 19 | 3071 | 4.12 | 0.007 | ------- |
| Lingual Gyrus | R | 31 | −45 | −6 | 19 | 2742 | 3.99 | 0.01 | temporal occipital fusiform cortex, parahippocampal gyrus, temporal fusiform cortex |
| Low-calorie foods > objects | |||||||||
| Hippocampus | R | 19 | −15 | −18 | 54 | 10615 | 4.20 | <0.0001 | parahippocampal gyrus, lingual gyrus, temporal occipital fusiform cortex, temporal fusiform cortex, amygdala, lateral occipital cortex, inferior temporal gyrus |
| Lateral Occipital Cortex | L | −52 | −69 | −1 | 19 | 3958 | 3.79 | 0.0006 | middle temporal gyrus |
| Lingual Gyrus | L | −13 | −51 | −10 | 19 | 3726 | 3.84 | 0.001 | temporal occipital fusiform cortex, temporal fusiform cortex, parahippocampal gyrus |
| Inferior Precuneous Cortex | R | 6 | −55 | 7 | 18 | 3106 | 3.30 | 0.004 | lingual gyrus, cingulate gyrus, intracalcarine cortex |
| High-calorie foods > low-calorie foods | |||||||||
| Superior Precuneous Cortex | L | −11 | −50 | 45 | 31 | 9169 | 3.88 | <0.0001 | precuneous cortex (B), precentral gyrus, postcentral gyrus, lateral occipital cortex |
A negative association indicates greater pre-meal brain activation in identified clusters (by contrast indicated) is associated with greater reduction in BMI z-score by FBT. A positive association indicates greater pre-meal brain activation in identified clusters (by contrast indicated) is associated with little or no change in BMI z-score by FBT. Cerebellum was not included in cluster analyses.
Harvard-Oxford Atlas identified regions of local maximum (*if local maximum appeared in white matter closest gray matter structure within cluster is listed),
Hemisphere of local maximum (L: Left, R: Right),
Montreal Neurological Institute (MNI) coordinates of peak location,
Brodmann area of local maximum,
maximum Z-score,
Other areas identified within the cluster (areas listed are ipsilateral to primary area unless otherwise indicated; L: Left hemisphere, R: Right hemisphere, B: Bilateral; regions in bold were included in functional-anatomic a priori regions of interest analyses (See Supplemental Figure 1). Z-statistic images were corrected for multiple comparisons with a cluster-threshold correction (individual voxel threshold Z=2.3, corrected cluster significant threshold P=0.05).
Figure 3.
Whole-brain cluster analyses of pre-meal brain activation in association with change in BMI z-score by FBT. The top panel shows significant clusters of activation prior to the standardized meal in which increased activation to high-calorie foods (vs. objects (left); vs. low-calorie foods (right)) and low-calorie foods (vs. objects (center)) is associated with greater BMI z-score reductions by FBT. Scatter plot in top panel represents mean parameter estimates of activation derived from the significant cluster in the ventromedial prefrontal cortex (vm PFC) region shown in top left panel (indicated by arrow, High-calorie > object) versus BMI z-score change by FBT. The bottom panel shows significant clusters of activation (pre-meal) in which increased activation to high-calorie (vs. objects (left); vs. low-calorie foods (right)) and low-calorie foods (vs. objects (center)) is associated with little or no change in BMI z-score by FBT. Scatter plot in bottom panel represents mean parameter estimates of activation derived from the significant cluster in the superior precuneous cortex region shown in bottom right panel (indicated by arrow, high-calorie > low-calorie) versus BMI z-score change by FBT. Z statistic maps were whole-brain corrected for multiple comparisons using a cluster threshold correction with the individual voxel threshold at Z = 2.3 and a corrected cluster significant threshold of P < 0.05. Color scales provided Z values of functional activation and numbers below each brain image are Montreal Neurological Institute coordinates. N=37 children with obesity. See Table 3 for further details of the cluster statistics.
Positive associations were present signifying clusters in which greater pre-meal activation by high-calorie food cues correlated with less BMI z-score reduction (Table 3, Figure 3). Greater activation by high-calorie food cues (vs. low-calorie) in the superior portion of the precuneus was related to less BMI z-score reduction. Clusters centered in the inferior portions of the bilateral precuneus (overlapping with the posterior cingulate cortex) were present for both high- and low-calorie (vs. objects) contrasts and therefore appear to reflect nonspecific reduced activation to food cue viewing. Other regions demonstrating positive associations included the left lateral occipital cortex and right lingual gyrus (Table 3). There were no significant clusters in which the change in activation by a meal pre-treatment predicted change in child BMI z-score during FBT for any of the 3 contrasts.
DISCUSSION
The current study reveals that children’s neurobiology entering FBT impacts their treatment success. Specifically, the signature brain finding associated with worse outcomes during FBT was a failure to reduce activation by high-calorie food cues from before to after eating, whereas children showing activation reductions had more success during treatment. The examined brain regions are critical to the control of motivation, incentive salience, and reward. Based on mechanistic studies manipulating satiety (30, 39), the appropriate response to food intake within these regions is a suppression of activation by high-calorie food cues. Heightened post-meal activation in regions including the ventral striatum, amygdala, mOFC, and insula are characteristic of hyperphagic conditions of childhood such as Prader-Willi syndrome (40, 41) and hypothalamic obesity (42). Children with common forms of obesity also exhibit blunted response to food intake in these regions relative to healthy weight peers (23, 25). However, there is variability in brain activation response among children with obesity. Children with obesity whose brain response was more similar to healthy weight peers (i.e., reduced activation following a meal) were more successful in improving their weight status in FBT. Intriguingly, an association between pre-treatment neural response pattern and change in dietary quality during FBT was also evident such that the children who exhibited reduced brain activation after a meal before entering treatment had greater improvements in diet quality during treatment. Thus, child’s neurobiological responses to food intake can be a physiological barrier or facilitator to modifying eating habits sufficiently to achieve treatment success.
The brain regions selected are individually and collectively identified as key modulators of appetitive drive (43, 44). They encompass corticolimbic pathways in which dopaminergic signaling predominate, link neurons in the ventral tegmental area to ventral striatum and medial OFC, and together drive motivated behaviors. In conjunction, the dorsal striatum houses additional pathways linked to reward perception (45) as well as response inhibition (46), whereas the amygdala encodes rewarding cues (47) and insular cortex represents the primary taste cortex (48). Reactivity to high-calorie food cues in many of these regions increases with fasting (39). Responsivity to high-calorie food cues within these regions has also been related to eating a greater proportion of calories from fat (30) and to consumption of a greater number of total calories (26) at an ad libitum buffet meal. Thus, the persistence of reactivity within appetite processing regions after eating has been postulated to reflect abnormal appetite drive in children with obesity (23, 25). A failure to reduce activation within these regions post-meal was associated with less improvement in children’s dietary quality as measured by Healthy Eating Index scores. The lack of improvement in dietary quality may have been a contributing factor to insufficient treatment outcomes among these children, as limiting highly energetic, low-nutrient foods is central to the FBT approach. Our fMRI findings are in line with a prior study of 25 adults undergoing behavioral weight loss in which greater activation in a nucleus accumbens ROI predicted less weight change (29). In another study of 18 adults before and 12-month post sleeve gastrectomy, nucleus accumbens and hypothalamic activation predicted less 12-month weight loss (28). In addition, observational studies have identified heightened responsiveness to food cues in the nucleus accumbens (27) as a risk factor for greater weight gain. Thus, the current study supports the behavioral and clinical relevance of responsivity to food cues as a marker of motivational aspects of food intake in young children.
A parallel literature emphasizes the importance of cognitive control and self-regulatory capacity to successful weight loss (49, 50). Some children with obesity demonstrate engagement of dorsolateral prefrontal cortical regions (40), potentially consistent with inhibitory control. Interestingly, our whole-brain analyses identified the vmPFC as a region in which more pre-meal activation, specifically by high-calorie food cues, was predictive of better treatment outcomes. This region is implicated in subjective reward value (51) and the evaluation of the health value of a food (52) and a decline in activation in this region has been associated with weight loss by caloric restriction in adults (53). One possible interpretation is that children who went on to be successful were already better at cognitively integrating a health-related valuation of food cues (54). In the current study, pre-meal activation in regions associated with visual imagery and episodic memory (55), such as the precuneous, and object recognition and encoding visual memory (occipital cortex and lingual gyrus) (56, 57) were associated with less BMI z-score reduction, suggesting that visual perception and memory processing of food cues may be additional components of appetite regulation that have clinical significance in children with obesity. While we did not find pre-treatment executive control regions to be significantly related to treatment outcomes, engagement of executive control during treatment may be the more important contributor to successful weight control (53, 58). Alternatively, cognitive control might be less important than appetitive drive in weight loss among young children, given that more maturation of executive control occurs later during adolescence.
While among functional neuroimaging studies the current study has a large sample size, low dropout rates, and employed evidence-based treatment consistent with recommended intensity and content, limitations persist. We were only able to detect temporal associations and cannot state that the fMRI measures were causally related to child weight status change during treatment. Moreover, the focus was on pre-treatment characteristics that predicted outcomes to treatment, but changes in neurobiological or other factors during treatment may be equally important, particularly to long-term outcomes.
Childhood obesity is a risk factor for negative health consequences (59). Understanding the diminution of treatment effectiveness in these children is clinically relevant because even modest reductions of BMI z-scores by 0.125 or more can result in significant improvements of several parameters of the metabolic syndrome (60). Evaluation of and adaptations to FBT that specifically address satiety responsiveness might help some children and families better implement behavior change and be more successful in treatment.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the National Institutes of Health award R01DK098466 (CLR), P30DK035816 (University of Washington Nutrition and Obesity Research Center); and the University of Washington Institute of Translational Health Sciences (UL1TR002319). We would like to thank Sue Kearns and Holly Callahan for their contributions to study planning and execution, Mark Abbey-Lambertz for support during treatment visits and execution of study assessments, and Gabrielle D’Ambrosio, Habiba Mohamed and Cordelia Franklin for their excellent support for performing the study visits.
Footnotes
Competing Interests Statement: The authors have declared that no conflict of interest exists.
Supplementary information is available at IJO’s website.
REFERENCES
- 1.Fryar CD, Carroll MD, OGden CL. Prevalence of overweight and obesity among children and adolescents: United States, 1962–1965 through 2011–2012. Health E-Stats. 2014.
- 2.U. S. Preventive Services Task Force, Grossman DC, Bibbins-Domingo K, Curry SJ, Barry MJ, Davidson KW, et al. Screening for Obesity in Children and Adolescents: US Preventive Services Task Force Recommendation Statement. JAMA. 2017;317(23):2417–26. [DOI] [PubMed] [Google Scholar]
- 3.Styne DM, Arslanian SA, Connor EL, Farooqi IS, Murad MH, Silverstein JH, et al. Pediatric Obesity-Assessment, Treatment, and Prevention: An Endocrine Society Clinical Practice Guideline. The Journal of clinical endocrinology and metabolism. 2017;102(3):709–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Epstein LH, Paluch RA, Roemmich JN, Beecher MD. Family-based obesity treatment, then and now: twenty-five years of pediatric obesity treatment. Health Psychol. 2007;26(4):381–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saelens BE, Lozano P, Scholz K. A randomized clinical trial comparing delivery of behavioral pediatric obesity treatment using standard and enhanced motivational approaches. J Pediatr Psychol. 2013;38(9):954–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mead E, Brown T, Rees K, Azevedo LB, Whittaker V, Jones D, et al. Diet, physical activity and behavioural interventions for the treatment of overweight or obese children from the age of 6 to 11 years. The Cochrane database of systematic reviews. 2017;6:CD012651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilfley DE, Stein RI, Saelens BE, Mockus DS, Matt GE, Hayden-Wade HA, et al. Efficacy of maintenance treatment approaches for childhood overweight: a randomized controlled trial. JAMA. 2007;298(14):1661–73. [DOI] [PubMed] [Google Scholar]
- 8.Danielsson P, Kowalski J, Ekblom O, Marcus C. Response of severely obese children and adolescents to behavioral treatment. Arch Pediatr Adolesc Med. 2012;166(12):1103–8. [DOI] [PubMed] [Google Scholar]
- 9.Goldschmidt AB, Best JR, Stein RI, Saelens BE, Epstein LH, Wilfley DE. Predictors of child weight loss and maintenance among family-based treatment completers. J Consult Clin Psychol. 2014;82(6):1140–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reinehr T, Kleber M, Lass N, Toschke AM. Body mass index patterns over 5 y in obese children motivated to participate in a 1-y lifestyle intervention: age as a predictor of long-term success. Am J Clin Nutr. 2010;91(5):1165–71. [DOI] [PubMed] [Google Scholar]
- 11.Sabin MA, Ford A, Hunt L, Jamal R, Crowne EC, Shield JP. Which factors are associated with a successful outcome in a weight management programme for obese children? J Eval Clin Pract. 2007;13(3):364–8. [DOI] [PubMed] [Google Scholar]
- 12.Shalitin S, Phillip M, Krepel-Volsky S. Predictors of successful weight reduction and maintenance in obese children and adolescents. Acta Paediatr. 2016;105(1):e42–6. [DOI] [PubMed] [Google Scholar]
- 13.Goldschmidt AB, Stein RI, Saelens BE, Theim KR, Epstein LH, Wilfley DE. Importance of early weight change in a pediatric weight management trial. Pediatrics. 2011;128(1):e33–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Madsen KA, Garber AK, Mietus-Snyder ML, Orrell-Valente JK, Tran CT, Wlasiuk L, et al. A clinic-based lifestyle intervention for pediatric obesity: efficacy and behavioral and biochemical predictors of response. J Pediatr Endocrinol Metab. 2009;22(9):805–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wiegand S, Keller KM, Lob-Corzilius T, Pott W, Reinehr T, Robl M, et al. Predicting weight loss and maintenance in overweight/obese pediatric patients. Horm Res Paediatr. 2014;82(6):380–7. [DOI] [PubMed] [Google Scholar]
- 16.Best JR, Theim KR, Gredysa DM, Stein RI, Welch RR, Saelens BE, et al. Behavioral economic predictors of overweight children’s weight loss. J Consult Clin Psychol. 2012;80(6):1086–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moens E, Braet C, Van Winckel M. An 8-year follow-up of treated obese children: children’s, process and parental predictors of successful outcome. Behav Res Ther. 2010;48(7):626–33. [DOI] [PubMed] [Google Scholar]
- 18.Kumar S, King EC, Christison AL, Kelly AS, Ariza AJ, Borzutzky C, et al. Health Outcomes of Youth in Clinical Pediatric Weight Management Programs in POWER. J Pediatr. 2019;208:57–65 e4. [DOI] [PubMed] [Google Scholar]
- 19.Burger KS, Berner LA. A functional neuroimaging review of obesity, appetitive hormones and ingestive behavior. Physiol Behav. 2014;136:121–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martin LE, Holsen LM, Chambers RJ, Bruce AS, Brooks WM, Zarcone JR, et al. Neural Mechanisms Associated With Food Motivation in Obese and Healthy Weight Adults. Obesity. 2010;18(2):254–60. [DOI] [PubMed] [Google Scholar]
- 21.Rothemund Y, Preuschhof C, Bohner G, Bauknecht HC, Klingebiel R, Flor H, et al. Differential activation of the dorsal striatum by high-calorie visual food stimuli in obese individuals. Neuroimage. 2007;37(2):410–21. [DOI] [PubMed] [Google Scholar]
- 22.Stoeckel LE, Weller RE, Cook EW, Twieg DB, Knowlton RC, Cox JE. Widespread reward-system activation in obese women in response to pictures of high-calorie foods. Neuroimage. 2008;41(2):636–47. [DOI] [PubMed] [Google Scholar]
- 23.Bruce AS, Holsen LM, Chambers RJ, Martin LE, Brooks WM, Zarcone JR, et al. Obese children show hyperactivation to food pictures in brain networks linked to motivation, reward and cognitive control. Int J Obes. 2010;34(10):1494–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davids S, Lauffer H, Thoms K, Jagdhuhn M, Hirschfeld H, Domin M, et al. Increased dorsolateral prefrontal cortex activation in obese children during observation of food stimuli. Int J Obes. 2010;34(1):94–104. [DOI] [PubMed] [Google Scholar]
- 25.Roth CL, Melhorn SJ, Elfers CT, Scholz K, De Leon MRB, Rowland M, et al. Central Nervous System and Peripheral Hormone Responses to a Meal in Children. J Clin Endocrinol Metab. 2019;104(5):1471–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Melhorn SJ, Askren MK, Chung WK, Kratz M, Bosch TA, Tyagi V, et al. FTO genotype impacts food intake and corticolimbic activation. Am J Clin Nutr. 2018;107(2):145–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Demos KE, Heatherton TF, Kelley WM. Individual differences in nucleus accumbens activity to food and sexual images predict weight gain and sexual behavior. J Neurosci. 2012;32(16):5549–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holsen LM, Davidson P, Cerit H, Hye T, Moondra P, Haimovici F, et al. Neural predictors of 12-month weight loss outcomes following bariatric surgery. Int J Obes. 2018;42(4):785–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murdaugh DL, Cox JE, Cook EW 3rd, Weller RE. fMRI reactivity to high-calorie food pictures predicts short- and long-term outcome in a weight-loss program. Neuroimage. 2012;59(3):2709–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mehta S, Melhorn SJ, Smeraglio A, Tyagi V, Grabowski T, Schwartz MW, et al. Regional brain response to visual food cues is a marker of satiety that predicts food choice. Am J Clin Nutr. 2012;96(5):989–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guenther PM, Casavale KO, Reedy J, Kirkpatrick SI, Hiza HA, Kuczynski KJ, et al. Update of the Healthy Eating Index: HEI-2010. J Acad Nutr Diet. 2013;113(4):569–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Evenson KR, Catellier DJ, Gill K, Ondrak KS, McMurray RG. Calibration of two objective measures of physical activity for children. J Sports Sci. 2008;26(14):1557–65. [DOI] [PubMed] [Google Scholar]
- 33.Mifflin MD, St Jeor ST, Hill LA, Scott BJ, Daugherty SA, Koh YO. A new predictive equation for resting energy expenditure in healthy individuals. Am J Clin Nutr. 1990;51(2):241–7. [DOI] [PubMed] [Google Scholar]
- 34.Cleary J, Daniells S, Okely AD, Batterham M, Nicholls J. Predictive validity of four bioelectrical impedance equations in determining percent fat mass in overweight and obese children. J Am Diet Assoc. 2008;108(1):136–9. [DOI] [PubMed] [Google Scholar]
- 35.Schaefer F, Georgi M, Zieger A, Scharer K. Usefulness of bioelectric impedance and skinfold measurements in predicting fat-free mass derived from total body potassium in children. Pediatr Res. 1994;35(5):617–24. [PubMed] [Google Scholar]
- 36.Shields BJ, Palermo TM, Powers JD, Grewe SD, Smith GA. Predictors of a child’s ability to use a visual analogue scale. Child Care Health Dev. 2003;29(4):281–90. [DOI] [PubMed] [Google Scholar]
- 37.Epstein LH, Squires S. The stoplight diet for children. Boston, MA: Little, Brown and Co; 1988. [Google Scholar]
- 38.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–9. [DOI] [PubMed] [Google Scholar]
- 39.Goldstone AP, de Hernandez CGP, Beaver JD, Muhammed K, Croese C, Bell G, et al. Fasting biases brain reward systems towards high-calorie foods. Eur J Neurosci. 2009;30(8):1625–35. [DOI] [PubMed] [Google Scholar]
- 40.Holsen LM, Savage CR, Martin LE, Bruce AS, Lepping RJ, Ko E, et al. Importance of reward and prefrontal circuitry in hunger and satiety: Prader-Willi syndrome vs simple obesity. International journal of obesity. 2012;36(4):638–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Holsen LM, Zarcone JR, Brooks WM, Butler MG, Thompson TI, Ahluwalia JS, et al. Neural mechanisms underlying hyperphagia in Prader-Willi syndrome. Obesity. 2006;14(6):1028–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roth CL, Aylward E, Liang O, Kleinhans NM, Pauley G, Schur EA. Functional Neuroimaging in Craniopharyngioma: A Useful Tool to Better Understand Hypothalamic Obesity? Obes Facts. 2012;5(2):243–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kelley AE, Baldo BA, Pratt WE, Will MJ. Corticostriatal-hypothalamic circuitry and food motivation: integration of energy, action and reward. Physiol Behav. 2005;86(5):773–95. [DOI] [PubMed] [Google Scholar]
- 44.Palmiter RD. Dopamine signaling in the dorsal striatum is essential for motivated behaviors: lessons from dopamine-deficient mice. Ann N Y Acad Sci. 2008;1129:35–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Small DM, Jones-Gotman M, Dagher A. Feeding-induced dopamine release in dorsal striatum correlates with meal pleasantness ratings in healthy human volunteers. Neuroimage. 2003;19(4):1709–15. [DOI] [PubMed] [Google Scholar]
- 46.Ghahremani DG, Lee B, Robertson CL, Tabibnia G, Morgan AT, De Shetler N, et al. Striatal Dopamine D2/D3 Receptors Mediate Response Inhibition and Related Activity in Frontostriatal Neural Circuitry in Humans. J Neurosci. 2012;32(21):7316–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gottfried JA, O’Doherty J, Dolan RJ. Encoding predictive reward value in human amygdala and orbitofrontal cortex. Science. 2003;301(5636):1104–7. [DOI] [PubMed] [Google Scholar]
- 48.Rolls ET. Taste, olfactory and food texture reward processing in the brain and obesity. Int J Obes. 2011;35(4):550–61. [DOI] [PubMed] [Google Scholar]
- 49.Weygandt M, Mai K, Dommes E, Leupelt V, Hackmack K, Kahnt T, et al. The role of neural impulse control mechanisms for dietary success in obesity. Neuroimage. 2013;83:669–78. [DOI] [PubMed] [Google Scholar]
- 50.Goldman RL, Canterberry M, Borckardt JJ, Madan A, Byrne TK, George MS, et al. Executive control circuitry differentiates degree of success in weight loss following gastric-bypass surgery. Obesity. 2013;21(11):2189–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bartra O, McGuire JT, Kable JW. The valuation system: a coordinate-based meta-analysis of BOLD fMRI experiments examining neural correlates of subjective value. Neuroimage. 2013;76:412–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hare TA, Camerer CF, Rangel A. Self-control in decision-making involves modulation of the vmPFC valuation system. Science. 2009;324(5927):646–8. [DOI] [PubMed] [Google Scholar]
- 53.Neseliler S, Hu W, Larcher K, Zacchia M, Dadar M, Scala SG, et al. Neurocognitive and Hormonal Correlates of Voluntary Weight Loss in Humans. Cell Metab. 2019;29(1):39–49 e4. [DOI] [PubMed] [Google Scholar]
- 54.Hare TA, Malmaud J, Rangel A. Focusing attention on the health aspects of foods changes value signals in vmPFC and improves dietary choice. J Neurosci. 2011;31(30):11077–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain : a journal of neurology. 2006;129(Pt 3):564–83. [DOI] [PubMed] [Google Scholar]
- 56.Golby A, Silverberg G, Race E, Gabrieli S, O’Shea J, Knierim K, et al. Memory encoding in Alzheimer’s disease: an fMRI study of explicit and implicit memory. Brain : a journal of neurology. 2005;128(Pt 4):773–87. [DOI] [PubMed] [Google Scholar]
- 57.Grill-Spector K, Kourtzi Z, Kanwisher N. The lateral occipital complex and its role in object recognition. Vision Res. 2001;41(10–11):1409–22. [DOI] [PubMed] [Google Scholar]
- 58.Gettens KM, Gorin AA. Executive function in weight loss and weight loss maintenance: a conceptual review and novel neuropsychological model of weight control. J Behav Med. 2017;40(5):687–701. [DOI] [PubMed] [Google Scholar]
- 59.Juonala M, Magnussen CG, Berenson GS, Venn A, Burns TL, Sabin MA, et al. Childhood adiposity, adult adiposity, and cardiovascular risk factors. N Engl J Med. 2011;365(20):1876–85. [DOI] [PubMed] [Google Scholar]
- 60.Reinehr T, Lass N, Toschke C, Rothermel J, Lanzinger S, Holl RW. Which Amount of BMI-SDS Reduction Is Necessary to Improve Cardiovascular Risk Factors in Overweight Children? J Clin Endocrinol Metab. 2016;101(8):3171–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.