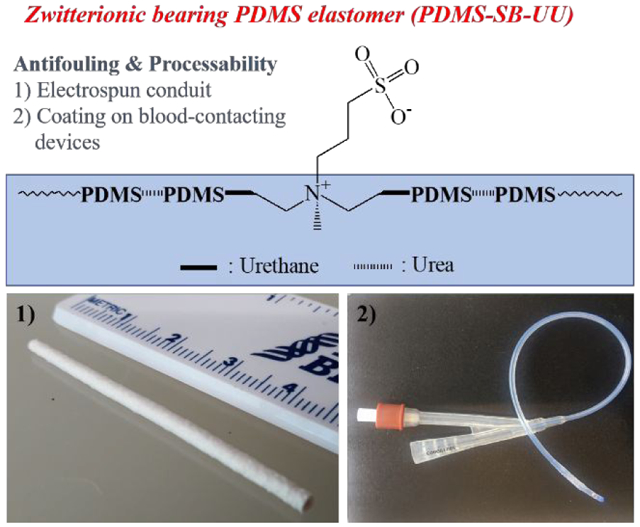
Abstract
Polydimethylsiloxane (PDMS) is commonly used in medical devices because it is non-toxic and stable against oxidative stress. Relatively high blood platelet adhesion and the need for chemical crosslinking through curing, however, limit its utility. In this research, a biostable PDMS-based polyurethane-urea bearing zwitterion sulfobetaine (PDMS-SB-UU) was synthesized for potential use in the fabrication or coating of blood-contacting devices, such as a conduits, artificial lungs, and microfluidic devices. The chemical structure and physical properties of synthesized PDMS-SB-UU were confirmed by 1H-nuclear magnetic resonance (1H-NMR), X-ray diffraction (XRD), and uniaxial stress-strain curve. In vitro stability of PDMS-SB-UU was confirmed against lipase and 30% H2O2 for 8 weeks, and PDMS-SB-UU demonstrated significantly higher resistance to fibrinogen adsorption and platelets depositions compared to control PDMS. Moreover, PDMS-SB-UU showed a lack of hemolysis and cytotoxicity with whole ovine blood and rat vascular smooth muscle cells (rSMCs), respectively. The PDMS-SB-UU was successfully processed to small-diameter (0.80 ± 0.05 mm) conduits by electrospinning and coated onto PDMS-, polyurethane-, and polypropylene-based blood-contacting biomaterials due to its unique physicochemical characteristics from its soft- and hard- segments.
Graphical Abstract
Introduction
Polydimethylsiloxanes (PDMS) are polymeric organosilicon compounds with unique properties such as low glass transition temperatures and low surface energies.1 Cured PDMS films exhibit high gas permeability, a highly hydrophobic surface, and very good stability to heat and oxidative stress. These properties are explained by a structure composed of inorganic Si-O bonds and an organic grafted methyl group. PDMS has been widely used in the fabrication of medical devices such as implantable components and microfluidic systems primarily due to its low cytotoxicity, stability, and elastomeric characteristics. The nonspecific adsorption of proteins and platelet deposition from blood onto PDMS, however, have motivated efforts to reduce its thrombogenicity.2
As with other materials used in blood-contacting medical devices, surface modification strategies have been widely studied as a means to improve the hemocompatibility of PDMS-based materials.3 These studies have shown improved blood-compatibility via covalently or non-covalently immobilized moieties that increase hydrophilicity or interact with specific proteins. However, most surface modification procedures are complex and require several steps, including oxidation of PDMS.4 The synthesis of a blood-compatible elastomeric PDMS-based copolymer may enable simpler medical device fabrication. Such a material might also find application in current PDMS-based microfluidic chips, which have been successfully developed for medical diagnosis and screening5 but also which would benefit from enhanced hemocompatibility for extended contact with whole blood.6
PDMS-based polyurethane has gained attention because it is expected to have the advantages of PDMS and polyurethane as a processable, elastomeric, and biocompatible material with low surface energy, high thermal compatibility, excellent oxidation resistance, chemical inactivity, and great molecular flexibility.1 On the other hand, zwitterionic polyurethanes have been actively studied to reduce platelet deposition. Sulfobetaine (SB), carboxybetaine (CB), and phosphorylcholine (PC) have been immobilized to the surface or backbone of polyurethanes to provide anti-fouling properties.7-13 These zwitterions reduce platelet deposition on the polyurethanes via a near zero zeta-potential and decreased non-specific protein adsorption.14 Zwitterionic polyurethane elastomers also have relatively good processability based on their thermoplastic characteristics and solubility in organic solvents.
Overall, a PDMS-based polyurethane (PU) would be an attractive candidate material for such blood-contacting applications in that it could combine appropriate mechanical properties with biostability, yet the blood-compatibility may still be insufficient. On the other hand, surface-immobilized zwitterions have shown anti-thrombotic properties. This work describes a novel zwitterionic PDMS-based polyurethane-urea (PDMS-PUU). PDMS-based zwitterionic (SB) PUU was synthesized, characterized, and assessed for blood biocompatibility and the feasibility of applying this material in the context of target medical devices.
Materials and methods
Materials
Poly(dimethylsiloxane), bis(hydroxyalkyl) terminated (PDMS-diol, Mn ~5,600), poly(dimethylsiloxane), bis(3-aminopropyl) terminated (PDMS-diamine, Mn ~2,500), N-methyldiethanolamine (MDEA, ≥99%), 4,4′-methylenebis(phenyl isocyanate) (MDI, 98%), Tin(II) 2-ethylhexanoate (Sn(Oct)2, 92.5-100.0%), 1,2-dichloroethane (DCE, anhydrous, 99.8%), methanol (MeOH, anhydrous, 99.8%), 1,3-propanesultone (PS, 98%), tetrahydrofuran (THF, anhydrous, ≥99.9%), hexamethyldisilazane (HMDS, ≥ 99%), hydrogen peroxide solution (H2O2, 30%), and lipase from Thermomyces lanuginosus (≥100,000 u/g) were purchased from Sigma-Aldrich (St. Louis, MO, USA). 1,1,1,3,3,3 Hexafluoro 2 propanol (HFIP) was purchased from Oakwood Chemical (Estill, SC, USA). Drabkin’s reagent was purchased from RICCA Chemical Company (Arlington, TX, USA). Lactate dehydrogenase (LDH) activity assay kit was purchased from Takara Bio Inc (Kusatsu, Shiga Prefecture, Japan). Sylgard® 182 Silicone Elastomer Kit was purchased from Dow Corning Corporation (Midland, MI, USA). Celltiter 96 AQueous One Solution Cell Proliferation Assay (MTS assay) was purchased from Promega (Madison, WI, USA). Dulbecco’s modified eagle medium (DMEM), heat inactivated fetal bovine serum (HI FBS), and penicillin-streptomycin (Pen Strep) were purchased from Gibco® (Gaithersburg. MD, USA). Micro BCA protein assay kit was purchased from Thermo Fisher Scientific (Waltham, MA, USA). ePTFE vascular graft (IMPRA® ePTFE Flex) was obtained from Becton Dickinson (Covington, GA, USA).
Synthesis of PDMS-based zwitterionic polyurethane-urea copolymer (PDMS-SB-UU)
A new PDMS-PUU copolymer was synthesized from Poly(dimethylsiloxane), bis(hydroxyalkyl) terminated (PDMS-diol), poly(dimethylsiloxane), bis(3-aminopropyl) terminated (PDMS-diamine), N-methyldiethanolamine (MDEA), and 4,4′-methylenebis(phenyl isocyanate) (MDI) (Scheme 1). Briefly, 1 g MDEA and 11.9 g PDMS-diol were dissolved in 150 mL DCE at 40 °C with nitrogen inlet-outlet equipment. 3.2 g MDI and a catalytic amount of Sn(Oct)2 were added to the reactor followed by the reaction at 40 °C. After 3 h of reaction time, 5.3 g PDMS-diamine in 50 mL DCE was slowly dropped in the reactor for another 12 h of reaction time at 40 °C. After the reaction, final product was precipitated and washed with excess methanol. 16 g product (PDMS-MDEA-UU, yield: 75%) was obtained after drying under vacuum at 60 °C.
Scheme 1.
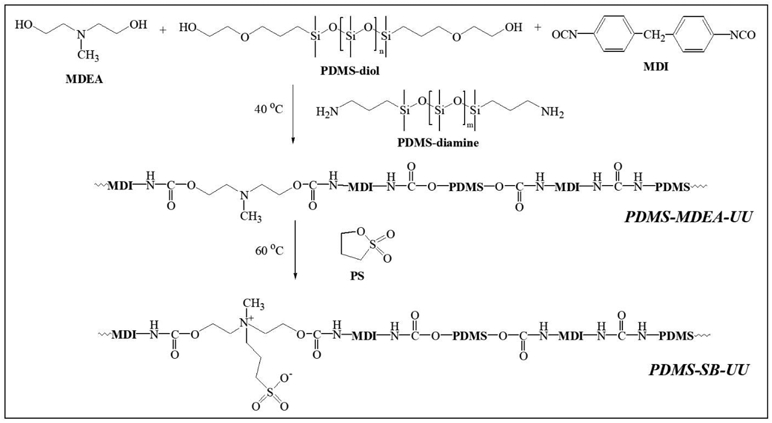
Synthesis of PDMS-based polyurethane-urea (PDMS-MDEA-UU) and zwitterionic polyurethane-urea (PDMS-SB-UU).
The PDMS-MDEA-UU was converted to zwitterionic PDMS-PUU bearing SB by reaction with 1,3-propanesultone (PS). Briefly, 5 g PDMS-MDEA-UU and 0.8 mL PS were dissolved in a mixture of 100 mL dichloroethane and 50 mL THF and then reacted at 50 °C overnight. After the reaction, the reaction solution was condensed under vacuum at 50 °C and then precipitated in excess MeOH. 3.9 g fine powder (PDMS-SB-UU, yield: 67%) was obtained after the precipitate was filtered and dried under vacuum.
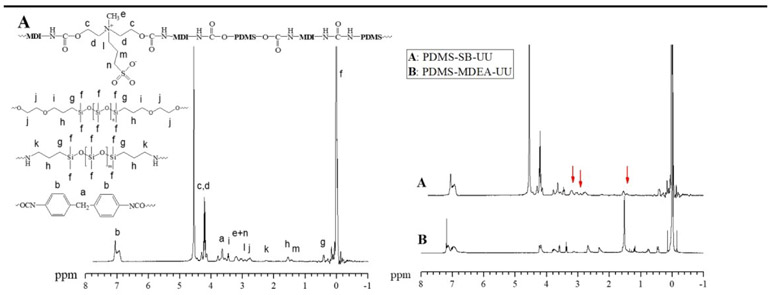
The chemical structure of synthesized copolymers PDMS-MDEA-UU and PDMS-SB-UU were confirmed by 1H-nuclear magnetic resonance (1H NMR, Bruker, Karlsruhe, Germany, Avance III, 400MHz) (Fig. 1). 1H NMR (CDCl3) of PDMS-MDEA-UU: δ −0.20~0.15 (6Hdimethylsiloxane, CH3-Si(R)-CH3), 0.38~0.50 (4HPDMS-diamine, CH2-CH2-CH2-Si(CH3)2-O), 0.69~0.81 (4HPDMS-dol, CH2-CH2-CH2-Si(CH3)2-O), 1.32~1.63 (8HPDMS-diol and PDMS-diamine, CH2-CH2-CH2-Si(CH3)2-O), 2.18~3.56 (4HPDMS-diamine, H2N-CH2-CH2-CH2-Si(CH3)2-O), 2.56~2.72 (8HPDMS-diol, OH-CH2-CH2-O-CH2-CH2), 3.07~3.17 (3HMDEA, OC-O-CH2-CH2-N+(CH3)-CH2-CH2-O-CO), 3.32~3.40 (4HPDMS-diol, OH-CH2-CH2-O-CH2-CH2), 3.60~3.84 (2HMDI, OCN-C6H4-CH2-C6H4-NCO), 4.09~4.25 (8HMDEA, OC-O-CH2-CH2-N+(CH3)-CH2-CH2-O-CO), and 6.82~7.26 (8HMDI, OCN-C6H4-CH2-C6H4-NCO). 1H-NMR (HFIP-D2) of PDMS-SB-UU: δ −0.09~0.12 (6Hdimethylsiloxane, CH3-Si(R)-CH3), 0.37~0.47 (8HPDMS-diol and PDMS-diamine, CH2-CH2-CH2-Si(CH3)2-O), 1.37~1.48 (2HSB, OC-O-CH2-CH2-(CH3)N+(CH2-CH2-CH2-SO3−)-CH2-CH2-O-CO), 1.48~1.61 (8HPDMS-diol and PDMS-diamine, CH2-CH2-CH2-Si(CH3)2-O), 2.18~2.28 (4HPDMS-diamine, H2N-CH2-CH2-CH2-Si(CH3)2-O), 2.67~2.87 (8HPDMS-diol, OH-CH2-CH2-O-CH2-CH2), 2.87~2.98 (2HSB, OC-O-CH2-CH2-(CH3)N+(CH2-CH2-CH2-SO3−)-CH2-CH2-O-CO), 3.11~3.31 (2HSB, OC-O-CH2-CH2-(CH3)N+(CH2-CH2-CH2-SO3−)-CH2-CH2-O-CO), 3.12~3.28 (3HSB, OC-O-CH2-CH2-(CH3)N+(CH2-CH2-CH2-SO3−)-CH2-CH2-O-CO), 3.40~3.51 (4HPDMS-diol, OH-CH2-CH2-O-CH2-CH2), 3.60~3.75 (2HMDI, OCN-C6H4-CH2-C6H4-NCO), 4.09~4.35 (8HSB, OC-O-CH2-CH2-(CH3)N+(CH2-CH2-CH2-SO3−)-CH2-CH2-O-CO), and 6.82~7.15 (8HMDI, OCN-C6H4-CH2-C6H4-NCO).
Fig. 1.
1H-NMR Of (A) PDMS-SB-UU in HFIP-d2 and (B) PDMS-MDEA-UU in CDCl3
Characterization of synthesized PDMS-based zwitterionic polyurethane-urea copolymer
The PDMS-MDEA-UU and PDMS-SB-UU were fabricated as films via simple solvent-casting. Briefly, 0.5 g copolymer was dissolved in 10 mL HFIP and poured into a Teflon dish (diameter: 6 cm). The copolymer solution was dried at room temperature overnight to obtain a film (thickness: 0.12 ± 0.02 mm). PDMS-control films were fabricated from a Sylgard® 182 Silicone Elastomer Kit. Briefly, Sylgard® 182 and Sylgard 182® curing agent were mixed at 10: 1 and poured into a Teflon dish (diameter: 6 cm). The PDMS was moved to a fume hood for 30 min and then moved to an oven at 60 °C to obtain a film (thickness: 0.12 ± 0.03 mm). Sample films were cut in to dumbbell-shaped strips (2×18 mm) and their mechanical strength assessed using an MTS Tytron 250 MicroForce Testing Workstation with a crosshead speed of 25 mm/min. Based on the evaluation, the initial modulus, tensile strength, and breaking strain were calculated (Table 1). The prepared solvent-cast films were also characterized by an X-ray diffraction (XRD, Bruker, Karlsruhe, Germany, D8 Discover) system to confirm their typical semi-crystalline characteristics.
Table 1.
Mechanical characteristics of PDMS-control, PDMS-SB-UU, and PDMS-SB-UU (n=3).
| Polymer | Feed ratio of MDEA or SB (wt%) |
Initial modulus (MPa) |
Tensile strength (MPa) |
Breaking Strain (%) |
|---|---|---|---|---|
| PDMS-Control | - | 0.8 ± 0.1 | 2.0 ± 1.0 | 134 ± 36 |
| PDMS-MDEA-UU | 5 | 3.9 ± 0.3 | 8.7 ± 0.4 | 334 ± 25 |
| PDMS-SB-UU | 5 | 3.6 ± 0.3 | 6.1 ± 0.6 | 227 ± 21 |
In vitro stability studies against enzyme using lipase and against oxidative stress using H2O2 solution
PDMS-MDEA-UU and PDMS-SB-UU films were prepared by solventcasting, and PDMS-control films were fabricated as described above. These films were punched in to circular samples (diameter: 8 mm) and then washed with 50% EtOH and Dulbecco’s Phosphate Buffered Saline (DPBS) several times before use. Dried samples were weighed [W0) and then immersed in 10 mL of 100 U lipase or 30% H2O2 for storage at 37 °C. The 100 U lipase or 30% H2O2 was refreshed every other week. At time points of 1, 2, 4, 6, and 8 weeks, three samples of each copolymer or control film were removed and washed several times with 1% Triton® X-100 surfactant solution, 50% ethanol, and distilled water in sequential order. The weight of washed samples (W1) was recorded after drying under vacuum at 60 °C for 2 d. The degradation of samples was evaluated by a change of mass (%) as ((W1-W0)−1) × 100.
In vitro gas permeability test of solvent-cast PDMS-SB-UU film
The gas permeability of PDMS-control and PDMS-SB-UU films for CO2 and O2 were evaluated using previously described methods.15,16 Briefly, circular film samples (diameter: 10 mm, thickness: 0.10 ± 0.03 mm) were assessed at room temperature within a custom acrylic test fixture. The test fixture positions the film between sealed inlet and outlet gas manifolds and supports the film with a porous metal mesh to prevent film deflection under application of transmural gas pressure. The inlet manifold was connected to a gas (O2 or CO2) source via a pressure regulator while the outlet manifold was connected to a capillary bubble flow meter (Supelco Inc, Bellefonte, PA, USA). A digital manometer (Series 490A Hydronic Differential Pressure Manometer, Dwyer Instruments Inc, Michigan City, IN, USA) was connected to the gas inlet and outlet to continuously measure transmural pressure across the film. After purging the entire system with the test gas, the pressure regulator was adjusted to induce a transmural pressure of 350 mmHg. Volumetric gas flow rate through the film was measured in triplicate via the bubble flow meter and used to calculate permeability based on previously described methods.15 Four samples across two different fabrication batches were used for evaluation of each film type.
In vitro anti-fouling studies against fibrinogen and platelet
Anti-fouling activity of PDMS-control, PDMS-MDEA-UU, and PDMS-SB-UU films was evaluated against fibrinogen and platelets. For the studies, films were punched in to circular samples (diameter: 8 mm, thickness: 0.12 ± 0.02 mm) and washed with 50% EtOH and then DPBS several times.
For the fibrinogen adsorption test, circular film samples were immersed in 5 mL of 0.45 g/10 mL fibrinogen solution in no additive (Z) tubes (BD Vacutainer®, Becton, Dickinson and Company, Franklin, NJ, USA) separately. The tubes were gently rocked for 2 h, the fibrinogen solution was drained, and the samples were rinsed with DPBS to remove non-adherent fibrinogen. After washing, the samples were immersed in 1 mL of 1% sodium dodecyl sulfate (SDS) solution separately using fresh polystyrene round-bottom tubes. The samples in 1% SDS were sonicated for 30 min at 50-60 Hz using an ultrasonic cleaner (Laboratory Supplies CO., INC, Hicksville, NY, USA) followed by vortexing for 5 min using a vortexer (Barnstead International, Dubuque, IA, USA). The sonication and vortexing were performed three times for detaching attached fibrinogen on the surface. After debris of the solution was spun down using a centrifuge (Sorvall® Legend RT, Marshall Scientific, Hampton, NH, USA) at 2,000 g for 15 min, 100 μL of supernatant from each tube was transferred to a 96 well plate. Diluted fibrinogen standard solution and Micro BCA working reagent were prepared following the Micro BCA protein assay kit instruction. 100 μL of BCA reagent was added to the supernatant in each well of the plate and incubated at 37 °C for 1 h. The absorbance of the plate was read at 562 nm using a microplate reader (SpectraMax, Molecular Devices, San Jose, CA, USA).
Platelet deposition on the PDMD-SB-UU was quantified from contact with whole ovine blood collected in sodium citrate tube by jugular venipuncture. National Institute of Health (NIH) guidelines for the care and use of laboratory animals were observed, and all animal procedures were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Pittsburgh. Circular film samples of PDMS-control, PDMS-MDEA-UU, PDMS-SB-UU were prepared as described above. The samples were immersed in 5 mL of fresh ovine blood in no additive (Z) tubes separately and then the tubes were gently rocked for 3 h at 37 °C. After rinsing of non-adherent platelets with DPBS, the number of deposited platelets on the samples was quantified by LDH assay or observed by scanning electron microscope (SEM, JSM 6335F, JEOL, Tokyo, Japan). For the LDH activity assay, the washed samples were immersed in 1 mL of 2% Triton™ X 100 in DPBS and then stirred for 20 min to lyse the deposited platelets on the sample. The lysis solution was centrifuged at 250 g for 10 min and then its supernatant was reacted with the LDH reagent. The absorbance of the reacted solution was recorded at 490 and 650 nm to quantify the amount of platelet deposition. To observe the morphology of the deposition of platelets on the surface of samples, after the washing, attached platelets were fixed by immersing in 2.5% glutaraldehyde solution for 2 h. The fixed platelets were dehydrated using 30, 50, 75, 95, and 100% EtOH and then treated with hexamethyldisilazane in sequence. SEM images were taken after sputter coating with gold/palladium.
In vitro hemolysis assay
Hemocompatibility of PDMS-MDEA-UU, PDMS-SB-UU, and ePTFE was evaluated following the Standard Practice for Assessment of Hemolytic Properties of Materials from the American Society for Testing and Materials (ASTM F756-17). Briefly, the hemoglobin concentration of fresh ovine blood collected in sodium citrate tube by jugular venipuncture was evaluated by a Radiometer OSM3 Hemoximeter (Kestrel Labs, Inc., Boulder, CO, USA) and then adjusted to 8 g/dL with Ca and Mg free phosphate-buffered saline (PBS) at pH 7.4. PDMS-MDEA-UU, PDMS-SB-UU, and ePTFE samples washed with 50% EtOH and DPBS were immersed in 5 mL of the whole blood in no additive (Z) tubes at 37 °C for 3 h, separately. After contacting the samples, the blood was removed and centrifuged at 750 g for 15 min. 1 mL of supernatant was taken to react with 1.0 mL Drabkin’s reagent for 15 min. The absorbance of the reacted solution was recorded at 540 nm. The adjusted ovine blood was used as a negative control. The % hemolysis (hemolytic index) was calculated as: % hemolysis= (supernatant hemoglobin concentration ×100%) / (total hemoglobin concentration in tube). According to the ASTM F756-17, a material is considered nonhemolytic if the % hemolysis is less than 2%, slightly hemolytic if between 2% and 5%, and hemolytic if greater than 5%.
In vitro cytotoxicity test
Cytotoxicity of PDMS-MDEA-UU and PDMS-SB-UU was evaluated by the extract test.17 Rat vascular smooth muscle cells (rSMC) were cultured in Dulbecco’s modified Eagle medium (DMEM) with 10% heat-inactivated fetal bovine serum (HI FBS) and 1% penicillin/streptomycin at 37 °C and 5% CO2 prior to use. For the test, rSMCs were seeded at 2.5 × 104 per 100 μL per well in 96-well plates and then kept in an incubator overnight to allow attachment of rSMCs. After unattached cells were washed out with DPBS, 100 μL of each elution medium of PDMS-control, PDMS-MDEA-UU, and PDMS-SB-UU were added to each well. The elution medium of samples was prepared by immersing 100 mg of samples in 5 mL of the cell culture medium at 37 °C for 1, 3, 7, 15, or 30 d and then kept at −80 °C prior to use.18 Negative control was polymer-free cell culture medium and positive control was 1 M acrylamide in cell culture medium filtered using a 0.2 μm membrane. After 24 h from adding the elution medium, 20 μL MTS solution was added to each well followed by incubating at 37 °C and 5% CO2 in an incubator. The absorbance of the plates was recorded at 490 nm using a microplate reader.
In vitro rSMC attachment study
Circular samples of PDMS control, PDMS-MDEA-UU and PDMS-SB-UU films were sterilized for 15 minutes in 50% ethanol three times and subsequently washed in PBS for 15 minutes three times. Samples were then exposed to UV light for 20 minutes. All of the samples were immersed in Dulbecco's Modified Eagle Medium (DMEM) with 10% of FBS and 1% of penicillin/streptomycin overnight before use. rSMC were cultured in Dulbecco's Modified Eagle Medium (DMEM) with 10% of FBS and 1% of penicillin/streptomycin. The rSMC were seeded on sterilized sample films using 50 μL of cell suspension at a concentration of 3.75 × 105 per 100 μL. Seeded sample films were then incubated at 37 °C with 5% CO2 for 4 h. After the incubation time, the seeded films were transferred to 48-well tissue culture plates (one film per well) with 200 μl of fresh medium. To test cell viability, films were transferred to new 48-well tissue culture plates and incubated in 150 μL MTS solution for 1 h. From each treated sample, 100 μL of supernatant was taken and added in a 96 well plate. The plate was read at 490 nm with a microplate reader at 24 h, 3 d and 7 d after cell seeding.
Fabrication of small-diameter conduits using PDMS-SB-UU
Small conduits (inner diameter: 0.80 ± 0.05 mm) were fabricated using PDMS-MDEA-UU and PDMS-SB-UU via electrospinning to confirm polymer processability. Briefly, 12 wt% polymer in HFIP was applied for 30 min to a rod collector (diameter 1 mm) rotating at 250 revolutions/minute with an applied voltage on the nozzle (8 kV) and collector (−8 kV), a polymer solution feed rate of 1.5 mL/h, and nozzle-to-collector distance of 18 cm. The fabricated conduit was dried at room temperature after being washed with 50% EtOH. SEM images of cross-section and lumen surface of the PDMS-SB-UU conduit were taken to observe its morphology.
Suture retention strength of electrospun PDMS-MDEA-UU, PDMS-SB-UU, and ePTFE grafts was evaluated following a protocol adapted from the methods described in ANSI/AAMI/ISO7198:1998/2001/(R) 2004 “Cardiovascular implants tubular vascular prostheses”. Briefly, Grafts were cut to 1 cm and sutured with Ti-Cron™ coated braided polyester surgical suture at a minimum distance of 2 mm from the samples free end. The suture retention strength was measured using an MTS Tytron 250 MicroForce Testing Workstation with a crosshead speed of 25 mm/min.
Coating test of PDMS-SB-UU to cured commercially available PDMS matrix
To evaluate the potential for coating PDMS-SB-UU onto PDMS-based microfluidic devices, 2% (wt/vol) PDMS-SB-UU in HFIP or DCM/HFIP (50/50) mixture were applied to a commercially available PDMS (Sylgard® 182 Silicone Elastomer Kit) matrix after thermal curing. Briefly, PDMS-control film (thickness: 431 ± 34 μm) was fabricated as described in the previous section. The PDMS-control film was punched in to a circular sample (diameter: 8 mm). The PDMS-control samples were coated with 2% (wt/vol) PDMS-SB-UU in HFIP by a simple dip-coating method. The sample was dipped in the 2% PDMS-SB-UU and allowed to dry at ambient condition. The procedure was repeated three times. To increase the penetrating amount of PDMS-SB-UU through the PDMS-control matrix, dichloromethane was used as a mixture with HFIP (DCM/HFIP= 50/50, vol/vol) to prepare 2% PDMS-SB-UU in the mixed solvent. DCM causes swelling of PDMS elastomer19 and this swollen status may increase the penetration efficiency of PDMS-SB-UU through the matrix. A PDMS-control sample was immersed in a mixed solvent for 1 h and then removed for drying. After the swollen coating, the PDMS-control sample was dip-coated as described above as well. The coating was confirmed by scanning electron microscope and energy-dispersive X-ray (SEM and EDX, Scios DualBeam, Thermo Scientific, Waltham, MA, USA) spectroscopy. For the preparation of the cross-section of the samples, coated samples were immersed in liquid nitrogen and cut by a surgical-grade blade (PERSONNA®, Verona, Virginia, USA). The cut samples were mounted and sputter-coated with gold/palladium at 2 nm. Imaging and element analysis were operated with an accelerating voltage of 5 kV, a beam current of 0.1 nA and a 10 mm working distance.
PDMS-SB-UU coating tests on blood-contacting medical devices
To evaluate potential application with clinically used medical devices, PDMS-SB-UU was coated on medical grade polyurethane indwelling catheters (ASK-04200-UPM, ARROW® international Inc.), silicone Foley catheters and a commercial polypropylene hollow fiber membrane mat (CelgardTM, Membrana, GmbH, Wuppertal, Germany) using 0.2wt% of PDMS-SB-UU in trifluoroethanol solution by dip-coating.
Statistical analyses
Data are presented as mean ± standard deviation (SD). The n-value refers to the number of replicates for each test. One-way ANOVA along with Tukey’s test formultiple comparisons was performed. P < 0.05 was considered statistically significant.
Results
Synthesis and characterization of PDMS-based zwitterionic polyurethane-urea copolymer (PDMS-SB-UU)
PDMS-PUU was successfully synthesized using PDMS-diol, MDEA, MDI, and PDMS diamine and converted to zwitterionic PDMS-PUU using PS. The PS was reacted with the tertiary amine of MDEA and formed SB. PDMS-diol and PDMS-diamine were used as a soft-segment and chain extender of the PDMS-PUUs, respectively. The conversion of MDEA to SB was confirmed from the comparison of 1H-NMR spectrum of PDMS-DMEA-UU and PDMS-SB-UU (Fig. 1). The typical methylene proton peaks of SB l, m, and n were observed at 2.87~2.98, 1.37~1.48, and 3.11~3.31 ppm, respectively. From the XRD spectrum of PDMS-control, PDMS-MDEA-UU, and PDMS-SB-UU (Supplement Fig. 1), the typical peak of PDMS at 12° was observed for all sample, moreover, the spectrum of PDMS-MDEA-UU and PDMS-SB-UU showed the typical peak of urethane at 20.5°.
Mechanical properties of PDMS-control, PDMS-MDEA-UU, and PDMS-SB-UU were characterized by uniaxial tests (Table 1). The PDMS-PUUs showed significantly higher initial modulus (MPa, PDMS-MDEA-UU: 3.9 ± 0.3 and PDMS-SB-UU: 3.6 ± 0.3), tensile strength (MPa, PDMS-MDEA-UU: 8.7 ± 0.4 and PDMS-SB-UU: 6.1 ± 0.6), and breaking strain (%, PDMS-MDEA-UU: 330 ± 25 and PDMS-SB-UU: 230 ± 21) compared to PDMS-control values of 0.8 ± 0.1 MPa, 2.0 ± 1.0 MPa, and 130 ± 36%, respectively.
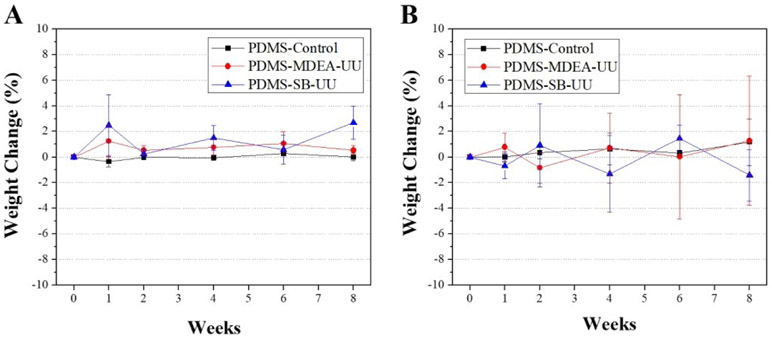
The stability of PDMS-MDEA-UU and PDMS-SB-UU against lipase or H2O2 was compared to commercially available PDMS for 8 weeks (Fig. 2). The stability was evaluated by the change of mass of the samples at 1, 2, 4, 6, and 8 weeks. There was no significant difference in change of mass relative to the control over the period.
Fig. 2.
In vitro long-term stability studies in an enzyme solution using 100 U/mL lipase (A) and oxidative treatment using 30% H2O2 solution (B). Weight change versus exposure time was determined over 8 weeks (n=3)
CO2 and O2 permeability through the solvent-cast PDMS-SB-UU films were compared with those through PDMS-control films (Supplement Fig. 2). PDMS-SB-UU exhibited no significant difference in permeability of both CO2 (3900 ± 1000 barrer) and O2 (990 ± 90 barrer) relative to PDMS-control. The CO2 and O2 permeability of PDMS-control films were 3260 ± 310 barrer and 995 ± 39 barrer, respectively, and are relatively similar to previously reported data for PDMS CO2 and O2 permeability15.
In vitro anti-fouling properties of PDMS-SB-UU
The antifouling effect of covalently immobilized zwitterionic SB group on the PDMS-PUU was evaluated. From the fibrinogen adsorption test (Supplement Fig. 3), PDMS-SB-UU showed a significantly lower fibrinogen deposition compared to PDMS-control. The amount of attached fibrinogen to the surface of PDMS-SB-UU (3.8 ± 0.2 ng) was less than half of that of PDMS-control (8.4 ± 0.1 ng). Fibrinogen deposition on PDMS-MDEA-UU (5.8 ± 0.7 ng) was significantly lower than that of PDMS-control but larger than that of PDMS-SB-UU.
Platelet deposition on the PDMS-SB-UU was also significantly reduced compared to both PDMS-control and PDMS-MDEA-UU. From SEM images of the whole ovine blood test (Fig. 3, A-C), it was clear that the surface of PDMS-control was covered by activated platelets, whereas there were few platelets and minimal evidence of activation on the surface of PDMS-SB-UU. The amount of deposition on the samples’ surface was quantified, and the result showed that the PDMS-SB-UU had almost 6.5 times less platelets deposition than PDMS-control (Fig. 3, D). The PDMS-MDEA-UU showed only 1.7 times lower platelet deposition as compared to that for the PDMS-control.
Fig. 3.
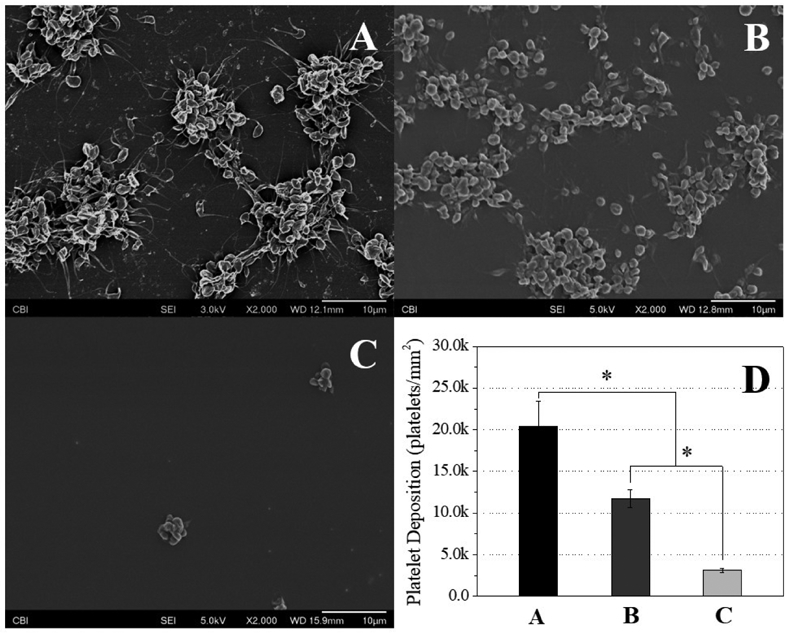
Platelet deposition studies to (A) PDMS-control, (B) PDMS-MDEA-UU, (C) PDMS-SB-UU films observed by SEM after contact with ovine blood (citrated) for 3 h at 37 °C (n=3), and (D) deposited platelet number quantified by LDH assay (n=3)
In vitro biocompatibility of PDMS-SB-UU
A hemolysis assay was performed to evaluate the hemocompatibility of PDMS-PUUs (PDMS-MDEA-UU and PDMS-SB-UU) compared to ePTFE (Supplement Fig. 4). The hemolytic index (% hemolysis) was 0.09 ± 0.02 for the negative control, 0.3 ± 0.2 for PDMS-MDEA-UU, 0.2 ± 0.2 for PDMS-SB-UU, and 0.5 ± 0.3 for ePTFE. All tested samples demonstrated a nonhemolytic effect since their hemolytic index was less than 2%. Moreover, the hemolytic index of PDMS-MDEA-UU, PDMS-SB-UU, and ePTFE showed no significant difference relative to the negative control (polymeric sample-free).
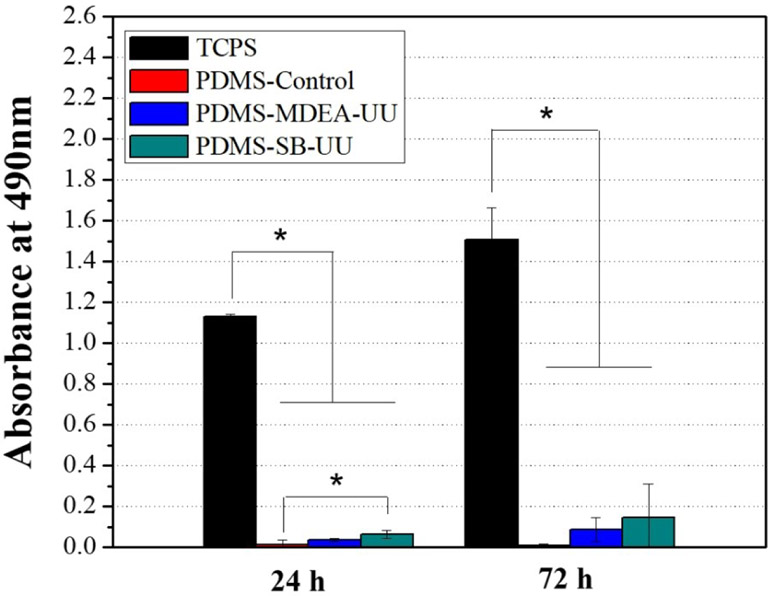
Cell cytotoxicity study by elution medium of PDMS-control, PDMS-MDEA-UU, and PDMS-SB-UU showed no significant toxic effect for all samples compared to the negative control (polymer-free cell medium) against rSMC for 30 d (Supplement Fig. 5). The cell attachment of rSMC on the surface of PDMS-control, PDMS-MDEA-UU, and PDMS-SB-UU films were compared to tissue culture polystyrene (TCPS) (Fig. 4). The PDMS-control and PDMS-UUs showed a significantly lower attachment at 72 h, but PDMS-UUs showed an increase in the number of cells over the period even at a low ratio.
Fig. 4.
Proliferation of rat aorta smooth muscle cells (rSMCs) on tissue culture polystyrene (TCPS), PDMS-control, PDMS-MDEA-UU, and PDMS-SB-UU. MTS assay was performed for the evaluation. Data are expressed as mean ± SD (n = 3)
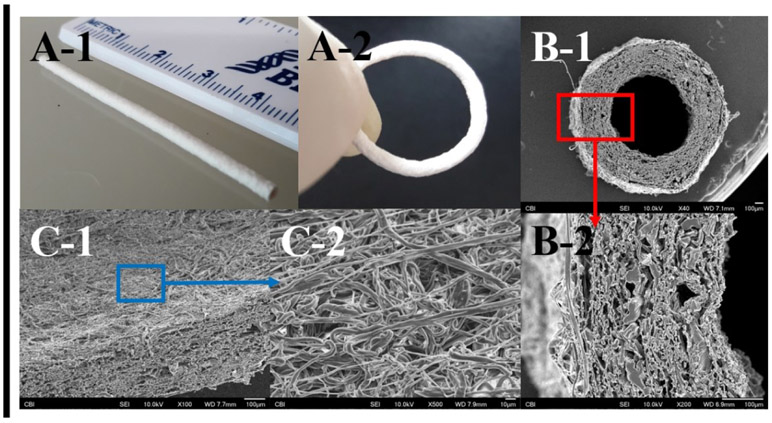
Fabrication of small diameter PDMS-SB-UU artificial conduit by electrospinning
PDMS-DMEA-UU and PDMS-SB-UU processed via electrospinning into small-diameter conduits (inner diameter: 0.80 ± 0.05 mm) are shown in Fig. 5. The length, wall thickness, and diameter of deposited fibers of the PDMS-SB-UU conduit were 5 ± 0.3 cm, 375 ± 30 μm, and 2.2 ± 0.6 μm, respectively. Also, suture retention strength of electrospun PDMS-MDEA-UU and PDMS-SB-UU was evaluated and compared with ePTFE (Supplement Fig. 6). Retention strength for PDMS-MDEA-UU (1.5 ± 0.4 N) and PDMS-SB-UU (0.69 ± 0.06 N) was significantly lower than that of ePTFE (5 ± 0.3 N).
Fig. 5.
Macroscopic images (A) and SEM images (B, C) of electrospun small diameter PDMS-SB-UU conduit.
PDMS-based material coating with PDMS-SB-UU
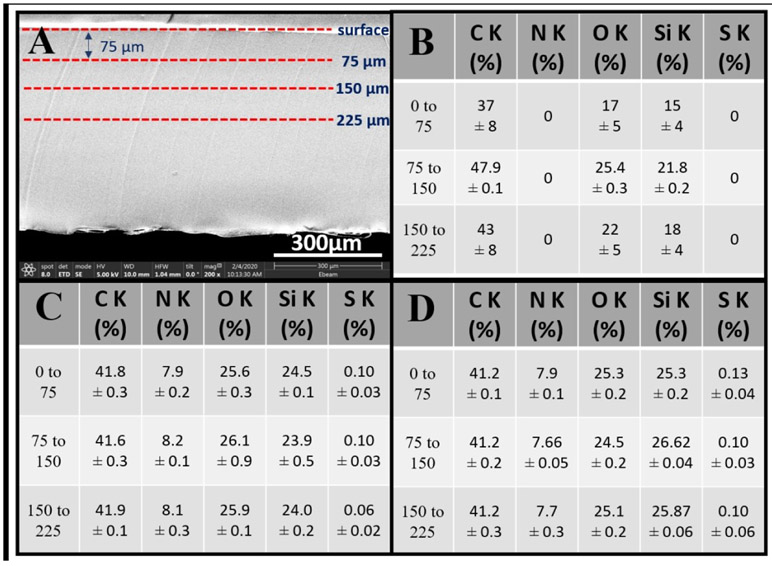
To evaluate the potential for using PDMS-SB-UU in the fabrication of a microfluidic device, the new zwitterionic PDMS-based polyurethane elastomer PDMS-SB-UU was coated onto commercially available PDMS (Sylgard® 182 Silicone Elastomer Kit). From the result (Fig. 6), PDMS-SB-UU showed a successful coating on PDMS-control deep into the middle point of the cross-section. Results for PDMS-SB-UU solution in DCM/HFIP (element % of S at surface to 75 μm, 75 to 150 μm, and 150 to 225 μm was 0.13 ± 0.04, 0.10 ± 0.03, and 0.10 ± 0.06, respectively) were more penetrating relative to those HFIP alone (element % of S at surface to 75 μm, 75 to 150 μm, and 150 to 225 μm was 0.10 ± 0.03, 0.10 ± 0.03, and 0.06 ± 0.02, respectively).
Fig. 6.
PDMS-control coating with PDMS-SB-UU (A) cross-section image for analysis, (B) atomic % of non-coated, (C) atomic % of dip-coating using 2 % (wt/vol) in HFIP, and (D) atomic % of dip-coating using 2% (wt/vol) in HFIP/DCM (1/1). Data are expressed as mean ± SD
PDMS-SB-UU coating on blood-contacting devices
To explore the potential for PDMS-SB-UU coatings on blood contacting devices, PDMS-SB-UU was applied on the surface of medical grade catheters and a commercial polypropylene hollow fiber membrane commonly used in artificial lungs. After the coating, the PDMS-SB-UU was observed to be conformally coated onto the catheter surfaces and the coated samples were smooth and exhibited no observable difference in morphology relative to the uncoated control surface. The outer surfaces of uncoated polypropylene hollow fiber control showed numerous micro- to nano-sized pores. The PDMS-SB-UU coated surface was smooth and the pores appeared to be covered by the thin coating layer (<1μm).
Discussion
PDMS-SB-UU was designed as a polymer for use in blood-contacting medical devices based on its expected biostability, fouling resistance, and semi-crystalline properties stemming from the PDMS based zwitterionic PUU structure consisting of soft- and hard-segment bearing SB. Its use could enable a straightforward method of improved thrombogenicity for blood-contacting devices such as vascular conduits, artificial lungs, and microfluidic devices.
Dacron (polyethylene terephthalate) and ePTFE are commercially available polymeric materials widely applied for the fabrication of large diameter (≥ 6 mm i.d.) artificial vascular grafts. Biostable polymeric grafts have the advantages of being ready to implant and relatively low cost compared to tissue-engineered biodegradable artificial grafts, which have not entered the clinic beyond limited exploratory studies.20 Although Dacron and ePTFE materials have been demonstrated to perform adequately in replacing large diameter blood vessels, this success decreases with the diameter of vessel replacement to the point where small diameter synthetic vascular grafts (< 4 mm i.d.) are not commonly utilized. A major reason for this failure is acute occlusion triggered by early platelet deposition, or later term hyperplasia at the anastomotic sites. Efforts to improve synthetic vascular graft biocompatibility to reduce these failure mechanisms have spanned decades, but no adequate solutions have been found.21,22 Related to the blood biocompatibility challenges of vascular grafts is the morbidity that stems from the placement of other polymeric devices acutely into the bloodstream, such as various types of catheters for sensing, delivery, collection and manipulation. Such devices need to resist thrombotic deposition on regions of action (e.g. a sensor) and generally to avoid serving as a source of thromboembolism. Blood-contacting catheters are commonly made from polysiloxanes and polyurethanes, and although these materials perform adequately in most instances, improved blood biocompatibility is desirable.6
Surface-immobilized zwitterions such as sulfobetaine (SB), phosphorylcholine (PC), or carboxybetaine (CB) have shown an antifouling effect putatively due to electrostatic hydrogen bonds between the zwitterions and water molecules to form a hydration layer.23 However, the effect is dependent on the density and length of the zwitterion. Surface modification schemes to attach zwitterions may also not be feasible for medical devices that consist of multiple materials with different physicochemical characteristics. PDMS is broadly utilized in the medical device industry due to its excellent stability against oxidative stress, good biocompatibility in many applications, and elastomeric characteristics. However, it has also shown problematic platelet deposition in some applications and has limited processing options based on the need for chemical crosslinking and thus a lack of thermo-plastic behaviour. Accordingly, a novel zwitterionic polyurethane-urea based PDMS was synthesized and evaluated with the aim of improving the anti-fouling properties and processability of PDMS.
For synthesis of the new PDMS-based zwitterionic polyurethane-urea, an aromatic diisocyanate, MDI, was employed to increase stability in an aqueous environment. Polyurethane products of aromatic diisocyantes have stronger hydrophobic intramolecular interactions than aliphatic diisocyanates.18 MDEA was immobilized to react with PS to form an SB since its short side chain has a lower steric hindrance relative to longer side chains. Therefore, PDMS-diol, MDEA, and MDI were used for the synthesis of the prepolymer, PDMS-based polyurethane-urea (PDMS-MDEA-UU). The prepolymer was processed to the PDMS-based polyurethane-urea by adding a chain extender PDMS-diamine which was chosen to increase PDMS content of the final product considering biostability. Finally, PDMS-MDEA-UU was converted to zwitterionic PDMS-PUU (PDMS-SB-UU) by reaction with PS.
The chemical structures of PDMS-MDEA-UU and PDMS-SB-UU were confirmed by the 1H-NMR spectra using hexafluoro-2-propanol (HFIP-d2, relative polarity: 0.969) and chloroform (relative polarity: 0.259), respectively (Fig. 1). Since PDMS is a part of the polyurethane-urea, the PDMS-PUUs are soluble in organic solvents, although cured PDMS by heat or ultraviolet (UV) radiation does not dissolve in organic solvents. PDMS-SB-UU showed a low solubility in chloroform and this may reveal that the PDMS-MDEA-UU became more polar after conversion to PDMS-SB-UU due to the sulfur trioxide group. Moreover, the XRD spectra of PDMS-MDEA-UU and PDMS-SB-UU showed the typical intensity of both PDMS and urethane. 1H-NMR and XRD spectrum results confirmed the successful synthesis of desired PDMS-PUUs.
PDMS-control, PDMS-MDEA-UU, and PDMS-SB-UU film samples showed soft and elastomeric properties during mechanical characterization. The results for all samples showed lower initial modulus than tensile strength and strain recovery until reaching the breaking strain (Table 1). Moreover, PDMS-MDEA-UU and PDMS-SB-UU showed higher tensile strength and breaking strain than those of the PDMS-control. These improved mechanical properties may be due to the chemical structure of PDMS-UUs consisting of the PDMS soft-segment and strong urethane urea hard-segment. PDMS-SB-UU demonstrated similar CO2 and O2 permeability relative to the PDMS-control due to the soft-segment of PDMS-SB-UU consisting of PDMS, although it has a hard segment which forms a well-arranged macromolecular domain by hydrogen bonding. Given that PDMS has been applied in a variety of blood oxygenator devices as a gas permeable membrane,2,24,25 these gas transport characteristics indicate the applicability of the new polymer as a membrane materials with improved thromboresistance.
Polymeric biomaterials can be subject to degradation in situ by oxidative stress and enzymatic activity. Oxidative stress occurs when reactive oxygen species (ROS) are transiently or chronically enhanced.26 ROS are oxygen-containing chemically-reactive radical or molecular species such as peroxides, superoxides, hydroxyl radicals, and singlet oxygen. For instance, macrophages play a major role in the formation of peroxynitrite, which contributes to polymer degradation.27 On the other hand, lipase is a major enzyme in tissues that can broadly attack the bonds common in lipids (and synthetic polymers). To confirm the biostability of newly synthesized PDMS-PUUs, the solvent-cast film of PDMS-MDEA-UU and PDMS-SB-UU were exposed to 30% H2O2 or 100 U/mL lipase at 37 °C for 8 weeks. PDMS-MDEA-UU and PDMS-SB-UU showed no measurable change in mass for 8 weeks. Moreover, there was no significant difference in mass compared to commercially available PDMS-control which was cured by heat treatment. This stability of PDMS-MDEA-UU and PDMS-SB-UU can be explained by its semi-crystalline chemical structure consisting of the PDMS soft-segment and arranged hard-segment. PDMS has superior resistance to oxidation and hydrolysis due to the unique siloxane bonded structure and hydrophobicity. A crystalline arrangement of the hard segment of PUU decreases enzymatic degradation.20
The anti-fouling properties of PDMS-MDEA-UU and PDMS-SB-UU were evaluated at a basic level using fibrinogen adsorption and platelet deposition from whole ovine blood to assess the acute resistance to these phenomena which occur over a longer time frame in the more complex in vivo setting of blood contacting medical devices. Fibrinogen was chosen as a model protein since fibrinogen is one of the coagulation factors and plays a major role in blood clotting and the support of platelet adhesion.28 Also, it has been reported that fibrinogen can support vascular smooth muscle cell adhesion and migration.29 The amount of deposition of fibrinogen was significantly less for PDMS-PUUs compared to PDMS-control (8 ± 1 ng/mm2) with PDMS-SB-UU showing the least deposition (3.79 ± 0.02 ng/mm2). This was attributed to PDMS-UUs having more hydrophilic surfaces due to the ionic groups and urethane-urea bonding. Moreover, SB has a near zero value of zeta potential, although MDEA may contribute attractive electrostatic interaction with the negative net surface charge proteins at neutral pH.
An absence of hemolysis induced by a biomaterial is a fundamental requirement for its consideration for use in a blood contacting medical device. PDMS-PUUs were evaluated for hemolytic potential related to the commonly utilized polymer for cardiovascular devices, ePTFE. Both PDMS-PUUs and ePTFE showed no elevated hemolytic activity against whole ovine blood.
To evaluate cytocompatibility of PDMS-MDEA-UU and PDMS-SB-UU, both an elution medium test and cell attachment test were conducted using rSMC. The vascular smooth muscle cell is a principal component of the normal blood vessel wall and is involved in many ‘housekeeping’ functions of the body, but the over-proliferation of vascular smooth muscle cells contributes to the incidence of restenosis of artificial vascular conduits.30 In this regard, the proposed material (PDMS-SB-UU) demonstrated potentially attractive behaviour in terms of no cytotoxicity and significantly lower rSMC attachment.
The feasibility of PDMS-SB-UU testing and use in blood-contacting devices was explored by the fabrication of small diameter PDMS-SB-UU conduits by electrospinning and coating PDMS with PDMS-SB-UU. Biostable artificial conduits and artificial lungs are good examples of blood-contacting medical devices where anti-fouling, lack of cytotoxicity, and low SMC attachment characteristics are required. PDMS-SB-UU showed good processability and the potential for use in fabrication of small diameter biostable artificial conduits. Also, PDMS-SB-UU was successfully applied to commercially available PDMS, polyurethane, and polypropylene which are the major materials use in fabricating medical-grade catheters (Supplement Fig. 7), and hollow fiber membranes for artificial lungs (Supplement Fig. 8), although future evaluation efforts are necessary to specifically confirm the functionality of such components. The goal of this initial report was to demonstrate the synthesis and processing potential of this new polymer, PDMS-SB-UU, that could be used for either the entire matrix or surface coating of several candidate blood-contacting medical devices.
Conclusions
A PDMS-based zwitterionic polyurethane-urea elastomer (PDMS-SB-UU) was successfully synthesized and demonstrated stability against 30% hydrogen peroxide and 100 U/mL lipase for 8 weeks. PDMS-SB-UU showed significantly lower adsorption of fibrinogen and platelet deposition compared to control PDMS and PDMS-MDEA-UU. PDMS-SB-UU showed no hemolytic or cytotoxic effects in whole ovine blood and with rSMCs, respectively. PDMS-SB-UU was able to be processed in to a small-diameter conduit by electrospinning and was also successfully coated on to the polymeric blood-contacting surfaces of commercial biomedical devices. Overall, the newly synthesized zwitterionic PDMS-SB-UU exhibits characteristics that indicate its potential for use in the fabrication of a variety of widely used blood-contacting medical devices.
Supplementary Material
Acknowledgments
This research was supported by the National Institutes of Health Contracts (1R03EB023620-01 & R01HL135482) and the NSF Engineering Research Center for Revolutionizing Metallic Biomaterials (ERC-RMB) (Award #0812348, National Science Foundation). The authors would like to thank the Center for Biological Imaging (CBI) of the University of Pittsburgh for their kindness assistant for the SEM images, and also thank Joseph Hanks, Teri Horgan, and others in the MIRM animal facility at University of Pittsburgh who provided the fresh ovine blood for this study. Also, the authors would like to acknowledge Tal Kurz for his assistance with gas permeability studies.
Footnotes
Electronic Supplementary Information (ESI) available: [details of any supplementary information available should be included here]. See DOI: 10.1039/x0xx00000x
Conflicts of interest
There are no conflicts to declare.
Publisher's Disclaimer: Accepted Manuscripts are published online shortly after acceptance, before technical editing, formatting and proof reading. Using this free service, authors can make their results available to the community, in citable form, before we publish the edited article. We will replace this Accepted Manuscript with the edited and formatted Advance Article as soon as it is available.
References
- 1.Ji X, Wang H, Ma X, Hou C and Ma G, RSC Adv., 2017, 7, 34086–34095. [Google Scholar]
- 2.Leung JM, Berry LR, Atkinson HM, Cornelius RM, Sandejas D, Rochow N, Selvaganapathy PR, Fusch C, Chan AK and Brash JL, J. Mater. Chem. B, 2015, 3, 6032–6036. [DOI] [PubMed] [Google Scholar]
- 3.Zhang H and Chiao M, J. Med. Biol. Eng, 2015, 35,143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gökaltun A, Kang Y. B. (Abraham), Yarmush ML, Usta OB and Asatekin A, Sci. Rep, 2019, 9, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sia SK and Whitesides GM, Electrophoresis, 2003, 24, 3563–3576. [DOI] [PubMed] [Google Scholar]
- 6.Zhang Z, Borenstein J, Guiney L, Miller R, Sukavaneshvar S and Loose C, Lab Chip, 2013, 13, 1963–1968. [DOI] [PubMed] [Google Scholar]
- 7.Malkin AD, Ye S-H, Lee EJ, Yang X, Zhu Y, Gamble LJ, Federspiel WJ and Wagner WR, J. Biomed. Mater. Res. Part B Appl. Biomater, 2018, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ye SH, Arazawa DT, Zhu Y, Shankarraman V, Malkin AD, Kimmel JD, Gamble LJ, Ishihara K, Federspiel WJ and Wagner WR, Langmuir, 2015, 31, 2463–2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fang J, Ye SH, Shankarraman V, Huang Y, Mo X and Wagner WR, Acta Biomater., 2014, 10, 4639–4649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ye SH, Chen Y, Mao Z, Gu X, Shankarraman V, Hong Y, Shanov V and Wagner WR, Langmuir. [DOI] [PubMed] [Google Scholar]
- 11.Gu X, Mao Z, Ye SH, Koo Y, Yun Y, Tiasha TR, Shanov V and Wagner WR, Colloids Surfaces B Biointerfaces, 2016, 144, 170–179. [DOI] [PubMed] [Google Scholar]
- 12.Ye SH, Chen Y, Mao Z, Gu X, Shankarraman V, Hong Y, Shanov V and Wagner WR, Langmuir, 2019, 35, 1421–1429. [DOI] [PubMed] [Google Scholar]
- 13.Ye SH, Hong Y, Sakaguchi H, Shankarraman V, Luketich SK, DAmore A and Wagner WR, ACS Appl. Mater. Interfaces, 2014, 6, 22796–22806. [DOI] [PubMed] [Google Scholar]
- 14.Schlenoff JB, Langmuir, 2014, 30, 9625–9636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Merkel TC, Bondar VI, Nagai K, Freeman BD and Pinnau I, J. Polym. Sci. Part B Polym. Phys, 2000, 38, 415–434. [Google Scholar]
- 16.Eash HJ, Jones HM, Hattler BG and Federspiel WJ, ASAIO J., 2004, 50, 491–497. [DOI] [PubMed] [Google Scholar]
- 17.Wang MO, Etheridge JM, Thompson JA, Vorwald CE, Dean D and Fisher JP, Biomacromolecules, 2013, 14, 1321–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim S, Chen Y, Ho EA and Liu S, Acta Biomater., 2017, 47, 100–112. [DOI] [PubMed] [Google Scholar]
- 19.Rumens CV, Ziai MA, Belsey KE, Batchelor JC and Holder SJ, J. Mater. Chem. C, 2015, 3, 10091–10098. [Google Scholar]
- 20.Kim S and Liu S, ACS Biomater. Sci. Eng, 2018, 4, 1479–1490. [DOI] [PubMed] [Google Scholar]
- 21.Adipurnama I, Yang M-C, Ciach T and Butruk-Raszeja B, Biomater. Sci, 2017, 5, 22–37. [DOI] [PubMed] [Google Scholar]
- 22.Pashneh-Tala S, MacNeil S and Claeyssens F, Tissue Eng. - Part B Rev, 2016, 22, 68–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Singha P, Locklin J and Handa H, Acta Biomater., 2017, 50, 20–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu WI, Rochow N, Chan E, Fusch G, Manan A, Nagpal D, Selvaganapathy PR and Fusch C, Lab Chip, 2013, 13, 2641–2650. [DOI] [PubMed] [Google Scholar]
- 25.Dabaghi M, Saraei N, Fusch G, Rochow N, Brash JL, Fusch C and Ravi Selvaganapathy P, Biomicrofluidics. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Collin F, Int. J. Mol. Sci, 2019, 20, 1–17. [Google Scholar]
- 27.Sutherland K, Mahoney JR, Coury AJ and Eaton JW, J. Clin. Invest, 1993, 92, 2360–2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weisel JW and Litvinov RI, Subcell. Biochem, 2017, 82, 405–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Naito M, Funaki C, Hayashi T, Yamada K, Asai K, Yoshimine N and Kuzuya F, Atherosclerosis, 1992, 96, 227–234. [DOI] [PubMed] [Google Scholar]
- 30.Gu X, Mao Z, Ye SH, Koo Y, Yun Y, Tiasha TR, Shanov V and Wagner WR, Colloids Surfaces B Biointerfaces, 2016, 144, 170–179. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.