Abstract
Sensory neurons are activated by physical and chemical stimuli, eliciting sensations such as temperature, touch, pain, and itch. From an evolutionary perspective, sensing danger is essential for organismal survival. Upon infection and injury, immune cells respond to pathogen/damage-associated molecular patterns (PAMPs/DAMPs) through pattern recognition receptors (PRRs) such as Toll-like receptors (TLRs), and produce inflammatory mediators that activate sensory neurons through neuro–immune interactions. Sensory neurons also express TLRs and other PRRs that directly sense danger signals after injury or during infection, leading to pain, itch, or analgesia. In addition to slow-acting canonical TLR signaling, TLRs function uniquely in sensory neurons through non-canonical coupling to ion channels, enabling rapid modulation of neuronal activity. We discuss how sensory neurons utilize TLRs and other PRR pathways to detect danger signals in their environment.
Detecting Danger: Shared Roles for Sensory Neurons and Immune Cells
During evolution, among the most fundamental functions is the ability of organisms to detect ‘danger signals’ indicating that organismal survival is threatened, such as signals produced by neoplastic host cells and invading pathogens. To accomplish this task, vertebrates have coevolved two crucial systems to aid in host protection: the immune system and sensory neurons of the peripheral nervous system (PNS). Unlike other sensory organs for vision, hearing, smell, taste, and olfaction, in which their sensory neurons (cell bodies and their innervations) are restricted to specific body part for a particular sensory function, PNS neurons in the dorsal root ganglia (DRG, see Glossary) and trigeminal ganglia (TG) innervate the entire somatic and orofacial body surface [1]. Thus, like immune cells, afferents from peripheral sensory neurons are pervasive throughout the body, providing a mechanism to rapidly alert organisms to changes in their internal or external environment as well as a complementary system to sense and respond to danger signals produced by internal or external threats.
To sense danger signals, both the immune system and PNS sensory neurons rely on a limited number of signaling pathways that they utilize in overlapping and distinct ways to accomplish their unique roles in host protection. In this review we discuss how immune cells and sensory neurons recognize danger signals via activation of these pathways, with a particular emphasis on pattern recognition receptors (PRRs) such as TLRs.
How Do Immune Cells Sense Danger and Elicit Pain?
Immune Cells Sense Environmental Danger via PRRs
Immune cells initiate host defense in response to cues derived from invading microorganisms, and they do so through specialized host-intrinsic PRRs that recognize conserved microbe-associated molecular patterns (MAMPs) and pathogen-associated molecular patterns (PAMPs, Figure 1; the distinction between MAMPs and PAMPs is discussed in Box 1). The TLRs were the first PRRs to be identified and are perhaps the best-characterized to date, initiating innate immune responses upon their activation by MAMPs/PAMPs [2–6]. Since their initial discovery in Drosophila melanogaster, TLRs have been found across a wide range of invertebrate and vertebrate species where they participate in a diverse range of functions, underscoring the ubiquity of these proteins across eukaryotes [5–7]. Based on their subcellular localizations, TLRs can be subdivided into (i) surface-localized TLRs (TLRs 1, 2, 4, 5, and 6), and (ii) intracellularly localized TLRs (TLRs 3, 7/8, and 9) that are present in subcellular compartments such as endosomes, or the endoplasmic reticulum in the case of TLR9. Different sets of PAMPs activate specific TLRs, for instance: bacterial lipoproteins for TLRs 1 and 2, double-stranded RNA (dsRNA) for TLR3, lipopolysaccharide (LPS) for TLR4, flagellin for TLR5, single-stranded RNA (ssRNA) for TLR7 and 8, and unmethylated CpG motifs for TLR9. Activation of TLRs by PAMPs leads in turn to the activation of intracellular signaling pathways including MyD88 (myeloid differentiation primary response 88), NF-κB (nuclear factor κ light-chain enhancer of activated B cells), and MAPK (mitogen-activated protein kinase) pathways. Activation of these intracellular signaling pathways results in the synthesis and secretion of various inflammatory mediators (IFMs) including the proinflammatory cytokines and chemokines IL-1β, TNF-α, CCL-2, and CXCL1, as well as proinflammatory enzymes such as cyclooxygenase 2 (COX-2) that promotes the synthesis of prostaglandin E2 (PGE2) (Figure 1) [8,9]. Notably, the activation of both endosomal and surface-localized TLRs relies on a host of accessory proteins and cofactors that facilitate ligand binding and/or receptor signaling, including CD14 (several endosomal and surface TLRs), CD36 (TLR2/6 and TLR4/6 heterodimers) [10], and MD2 (TLR4) [11].
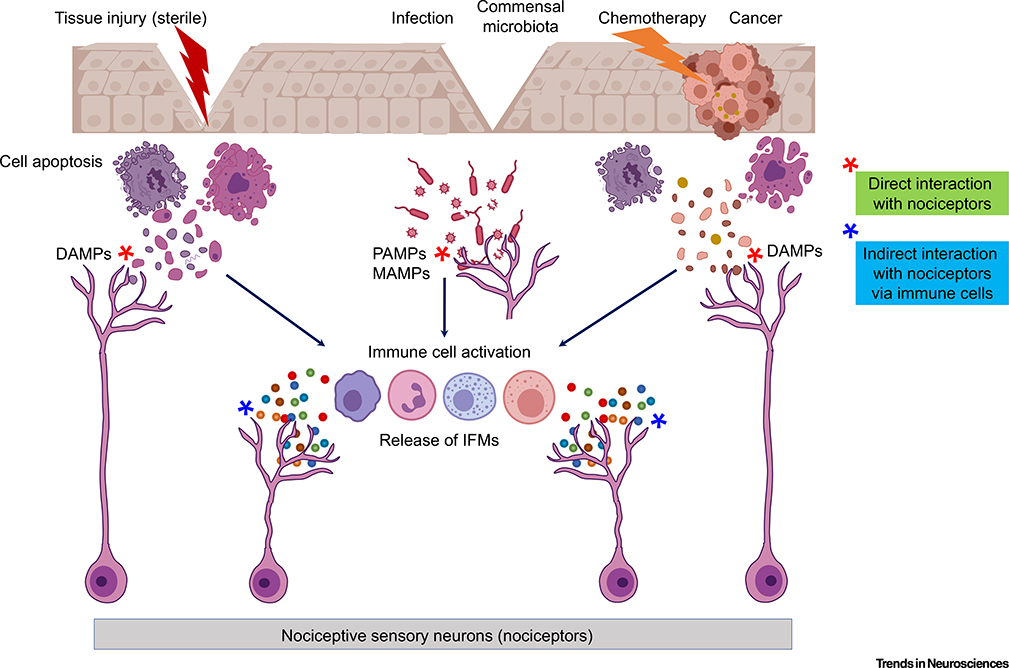
Figure 1. Danger Sensing by Immune Cells and Nociceptors and Neuro–Immune Interactions.
Sterile tissue injury occurring without barrier disruption, neoplastic cancer cells, and chemotherapeutic drugs employed to treat cancers lead to the release of damage-associated molecular patterns (DAMPs) by host cells. Epithelial barrier leakage can enable entry of microbe-associated molecular patterns (MAMPs) produced by the resident skin commensal microbiota. In addition, epithelial barrier disruption (e.g., by tissue damage) can enable entry of bacterial and viral pathogens leading to infections. Immune cells recognize pathogenic microorganisms by sensing conserved microbe/pathogen-associated molecular patterns (PAMPs). Notably, these danger signals (e.g., DAMPs and PAMPs) activate pattern recognition receptors (PRRs) on immune cells, leading to the production and release of cytokines/chemokines and inflammatory mediators (IFMs) that interact with nociceptor terminals. In addition to well-established indirect interactions with nociceptors via immune cells (neuro–immune interactions; indicated with blue stars), emerging evidence also suggests direct interaction of nociceptor terminals with PAMPs/MAMPs and DAMPs (red stars). Activation of nociceptors by indirect and direct mechanisms results in pain, as well as in analgesia and itch (pruritus).
Box 1. What Are Danger Signals?
A fundamental property of the immune system is the ability to identify and respond selectively to signals that warrant an active immune response. This idea, originally formulated by Frank Burnet in 1959 and developed in the context of initiating immunity against invading pathogens, became known as the self versus non-self theory [115]. Thirty years later, Charles Janeway refined this model with his pattern recognition theory which proposed that immune cells utilize genetically encoded PRRs to selectively recognize PAMPs that are unique to microorganisms [116]. Recognizing that this model fails to explain immunity in the context of sterile inflammation (i.e., in the absence of infection), Polly Matzinger later followed with the danger theory, which proposed that damaged host cells can also release host-intrinsic danger signals that can themselves initiate immune responses and were later termed DAMPs [117]. It is now widely appreciated that PAMPs and DAMPs are recognized by many different PRRs, and together these receptors accomplish the difficult task of detecting a wide variety of potentially hazardous stimuli.
It is also important to note that there is no general agreement on the term ‘PAMP’. Virtually all PAMPs are conserved microbial structures that are invariably present in all microorganisms (e.g., LPS and flagellin), and thus are not restricted to pathogens. Given our growing appreciation of the commensal microbiota, which can activate the same host PRRs using the same ligands, it has been suggested that these molecules be renamed microbe-associated molecular patterns (MAMPs) [118]. However, it has also been argued that a functional distinction exists between the terms MAMPs and PAMPs because only the term PAMP describes a signal produced by a pathogenic microorganism, as opposed to MAMPs produced by the commensal microbiota. Moreover, this emerging body of research has led to further evolution in our understanding of PRR biology, raising the idea that the consequences of PAMP/MAMP activation of PRRs may rely on additional contextual signals [2].
Likewise, the term ‘DAMP’ has been disputed among researchers in interpreting its abbreviation [12], with some arguing that the original term ‘damage-associated molecular pattern’ should be replaced by ‘danger-associated molecular pattern’. The expansion to the latter term has been argued to be more inclusive, including processes that significantly deviate from physiological or homeostatic conditions (e.g., factors associated with hypoxia, reactive oxygen species, etc.) and molecules that are induced in response to damage (e.g., cytokines, neuropeptides, etc.). In this review we chose to use the well-established terms ‘pathogen-associated molecular patterns’ and ‘damage-associated molecular patterns’, recognizing and paying homage to other terms and appreciating the caveats outlined here.
Beyond recognition of exogenous microorganism-derived PAMPs and MAMPs, TLRs also recognize endogenous (e.g., self-derived) damage-associated molecular patterns (DAMPs, Box 1) that are released by damaged, dying, or dead cells in a variety of sterile inflammatory conditions [12,13] (Figure 1). Notably, injury-induced DAMPs exert their functions through many of the same PRRs that are activated by PAMPs. For example, following tissue damage, HMGB1 (high-mobility group Box 1) is rapidly produced and acts as an endogenous ligand of TLR4 [14]. PAMP and DAMP-mediated PRR activation can activate nociceptor neurons through two pathways: (i) an indirect pathway in which PRR activation in immune cells leads to the induction of IFMs that themselves activate nociceptors, or (ii) a direct pathway in which danger signals directly activate PRRs expressed by nociceptors (Figure 1).
MicroRNAs (miRNAs) are small non-coding ssRNAs that are canonically recognized to regulate gene expression post-transcriptionally, but they can also be secreted extracellularly and act as DAMPs. miRNAs are dysregulated in human diseases such as cancers, and circulating miRNAs serve as disease biomarkers [15]. For example, let-7b is released from cancer cells and engulfed by immune cells in the tumor microenvironment, and let-7b binding to TLR7 in the intracellular compartments of immune cells results in NF-κB activation and secretion of IL-6 and TNF-α that can promote cancer cell growth and metastasis [16] (Figure 2). In addition to immune cells, glial cells such as microglia and astrocytes also express TLRs such as TLR2, TLR4, and TLR7 that contribute to neuroinflammation, neuropathic pain, and opioid tolerance [17–20].
Figure 2. Schematic Indicating Common Pattern Recognition Receptor (PRR) Pathways Used by Immune Cells to Recognize Danger Signals.
Toll-like receptors (TLRs) 1, 2, 4, 5, and 6 are present on the cell surface and recognize pathogen-associated molecular patterns (PAMPs) that are present in bacteria, such as lipopolysaccharide (LPS), lipopeptides, and flagellin. Intracellular TLRs, including TLRs 3, 7/8, 9, 11, 12, and 13 (TLR12/13 are not present in humans), recognize PAMPs such as single- or double-stranded RNA (ss/dsRNA) produced by viruses, unmethylated CpG motifs produced by bacteria, and damage-associated molecular patterns (DAMPs) such as microRNAs that can be released by host cells following tissue injury. Activation of TLRs leads to activation of the signaling adaptor MyD88, that activates IKK/NF-κB-dependent transcriptional changes leading to the upregulation of cytokines and inflammatory mediators (IFMs). In addition to TLRs, surface PRRs such as C-type lectin receptors (CLRs), N-formyl peptide receptors (FPRs), the receptor for advanced glycation end products (RAGE), as well as additional intracellular PRRs such as the cytosolic dsRNA sensor RIG-I and the dsDNA-sensing cGAS/STING pathway, serve to detect PAMPs and DAMPs, leading to transcriptional induction of IFMs, cytokines, and chemokines. Finally, several PRRs such as RIG-I and AIM-2, as well as exogenous stimuli (e.g., ROS, low pH, ATP), lead to activation of the NLRP3 inflammasome, and this drives the maturation and release of the proinflammatory cytokines IL-1β and IL-18. Extracellular IL-1α can also be cleaved by matrix metalloproteases (MMP-9 and MMP-2) under neuropathic pain [119]. The net result of this process is to initiate immunity, induce local inflammation, and amplify the immune response. Abbreviations: cGAMP, cyclic GAMP-AMP; cGAS, cGAMP synthase; ER, endoplasmic reticulum; mtDNA, mitochondrial DNA.
Importantly, MAMPs, PAMPs, and DAMPs are also recognized by a variety of host-intrinsic PRRs beyond the TLRs, many of which were discovered as key members of the danger-sensing machinery only recently [21–25]. These include the NOD-like receptors (NLRs), the intracellular RNA-sensing retinoic acid-inducible gene I (RIG-I)-like receptors, the carbohydrate-binding C-type lectin receptors (CLRs), and cytosolic DNA sensors (CDSs) such as cGAS and STING1 (recently renamed stimulator of interferon response cGAMP interactor 1) (Figure 2), and how these PRRs regulate innate immunity has been the subject of several excellent reviews [26–29]. Notably, activation of multiple PRR pathways also activates the NLRP3 inflammasome, leading to cleavage of pro-IL-1α and its family member pro-IL-18 to generate their mature and active forms (Figure 2), and the NLRP3 inflammasome is a key contributor to inflammation, cell death, and chronic pain [24,30–32].
Immune Cells Induce Pain via Interaction with Nociceptors
Inflammation can be defined as the body’s immune response to potentially harmful stimuli, and is characterized by five cardinal signs: loss of function, redness, swelling, heat, and pain [33,34]. Pain is sensed by peripheral nociceptor cells, a unique population of somatosensory neurons that are activated in response to noxious or potentially damaging stimuli. In many respects, immune cells and primary sensory neurons share a common function: detecting danger signals or otherwise potentially hazardous stimuli [35]. With their cell bodies located in the DRG and TG, nociceptors extend afferent projections to peripheral tissues such as skin, muscles, joints, and visceral organs. Nociceptors extend free nerve endings to the skin, although some of these nerve endings may also be encapsulated by specialized terminal Schwann cells [36]. At the nociceptor afferent terminals, many different molecular sensors are present, including (but not limited to) G protein-coupled receptors (GPCRs), transient receptor potential channels (e.g., TRPA1, TRPV1), and sodium channels (e.g., NaV1.7, NaV1.8, and NaV1.9) [8,9]. Peripheral nerve terminals of nociceptors can detect a variety of inflammatory mediators such as PGE2 and proinflammatory cytokines such as IL-1β and TNF-α, as well as proinflammatory chemokines including CCL2. Nociceptors are direct sensors of these cytokines and chemokines because activation of cytokine/chemokine receptors rapidly alters nociceptor activity and excitability through functional coupling with TRP channels and sodium channels via intracellular signaling pathways [37–39] (Figure 3A).
Figure 3. Nociceptive Sensory Neurons Sense Danger Signals via Indirect and Direct Mechanisms.
(A) Schematic indicating the inflammatory mediator (IFM) and cytokine receptors expressed and utilized by nociceptive sensory neurons to sense danger signals communicated by immune cells following damage/pathogen-associated molecular pattern (DAMP/PAMP) activation of pattern recognition receptors (PRRs). NGF, TNF, IL-1β, CCL2, and CXCL1 can induce the opening of TRPA1 and TRPV1 ion channels as well as of the voltage-gated sodium channels Nav1.8 and Nav1.9. This process requires the activation of downstream signaling pathways such as MAPK (ERK, p38, and JNK), PI3K, PKA, and PKC pathways. Collectively, nociceptors sensing these immune-derived danger indicators lead to nociceptor activation, ultimately resulting in pain. (B) Peripheral nerve terminals of nociceptive sensory neurons can directly detect pathogens and their products (PAMPs), as well as endogenous DAMPs and danger signals. Staphylococcus aureus can directly activate nociceptors through the production of hemolysins and N-formyl peptides. Hemolysins can penetrate the neuronal membrane via toxin pore assembly, leading to ionic influx, neuronal depolarization, and increased excitability. N-formyl peptides act via FPR1 receptors in nociceptive neurons, increasing neuronal excitability. Gram-negative bacteria can activate nociceptors via N-formyl peptides (through FPR1) or by lipopolysaccharide (LPS), and this increases neuronal excitability through TLR4 coupling to the non-selective ion channel TRPV1. TLR2 is activated by bacteria-derived zymosan and is likely to be coupled with TRPA1 and TRPV1 [49]. TLR3 is activated by double-stranded RNAs (dsRNAs) and functionally interacts with TRPV1 [50]. Several Mycobacterium species have also been found to regulate nociceptor activity. M. ulcerans, for example, can silence nociceptor activity via mycolactone-mediated activation of AT2R and subsequent activation of the TRAAK potassium channel, leading to hyperpolarization. Conversely, sulfolipid-1 (SL-1) secreted by M. tuberculosis can activate nociceptive nerve terminals in the lungs to evoke coughing. Endogenous microRNAs such as let-7b and miR-21 can activate TLR7 and TLR8. In addition to endosomes, TLR7 is uniquely localized to the cell membrane of nociceptive neurons, where it is functionally coupled with TRPA1 to induce inward currents and neuron excitability. TRPA1 can also be directly activated by miR-711 produced by lymphoma [97]. Dashed arrows represent speculative modulation. Abbreviations: AT2R, angiotensin II receptor; FPR1, formyl peptide receptor 1; MAPK, mitogen-activated protein kinase; PGE2, prostaglandin E2; PTX, pertussis toxin; TLR, Toll-like receptor.
How Do Nociceptors Directly Sense Danger Signals?
Nociceptors Express PRRs
Peripheral nociceptors in the DRG represent a heterogeneous population that can be defined by their degree of myelination, somal diameter, electrophysiological properties, and/or their expression of molecular markers such as neuropeptides and surface receptors. Over the past decade our understanding of this heterogeneity has greatly benefited from advances in sequencing technologies. In a study using transcriptional profiling analysis at the whole-population and single-cell RNA-Seq (scRNA-Seq) levels [40], the authors identified six distinct groups of mouse DRG neurons. Around the same time, another study [41] reported scRNA-Seq analysis of 622 DRG neurons and identified 11 distinct types of mouse DRG neurons, and this was later expanded to 17 subtypes following a larger study of 2309 DRG neurons by the same group [42]. Later work using high-coverage scRNA-Seq with functional characterization [43] identified 10 major types and 14 subtypes of mouse DRG neurons. Recently, another study employed whole-transcriptome deep sequencing (dRNA-Seq) of eight genetically defined mouse DRG neuron subtypes using a genetic labeling strategy to identify the molecular determinants underpinning the physiological properties of each neuron subtype [44]. Importantly, each of these studies generated searchable datasets to explore gene expression in defined sensory neuron subpopulations, collectively expanding our understanding of sensory neuron gene expression.
Using these studies and many others, nociceptor expression of a variety of PRRs has been demonstrated by a variety of methods, including scRNA-Seq, dRNA-Seq, single-cell PCR (sc-PCR), in situ hybridization, immunohistochemistry, and electrophysiology [41,42,44–48]. Nociceptors in the DRG and TG express several types of TLRs, including TLR2, TLR3, TLR4, TLR7, and TLR8 [46,49–54], as well as the downstream signaling adaptor MyD88 [55] (Figures 3B and 4A). Tlr4 mRNA has the highest expression among all TLR transcripts and is primarily expressed by peptidergic nociceptor cells, as revealed by scRNA-Seq and dRNA-Seq [41,42,44]. dRNA-Seq also reveals low-level expression of Tlr3 transcripts in both peptidergic and non-peptidergic nociceptors [44]. RNA-Seq fails to detect other transcripts of Tlr family members in nociceptors [41,42,44].
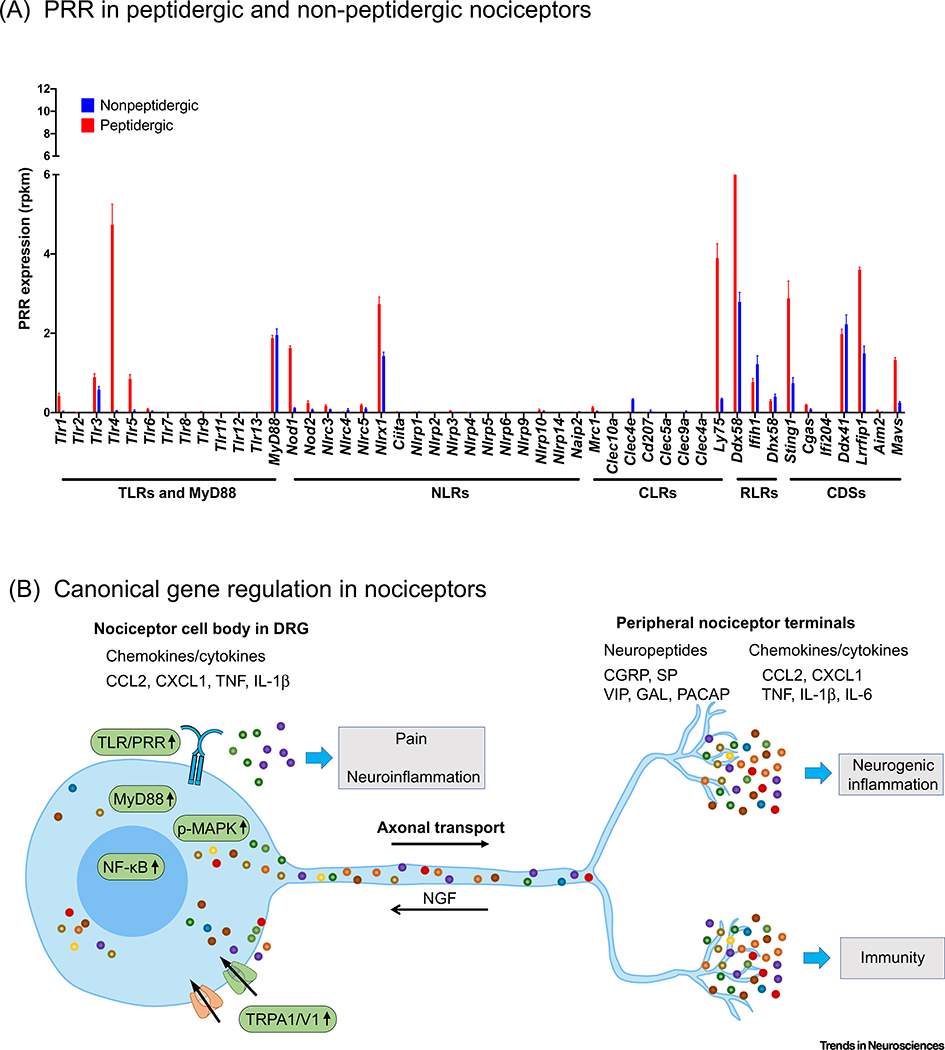
Figure 4. Expression of Pattern Recognition Receptors (PRRs) and Canonical Gene Regulation in Peripheral Nociceptors.
(A) Transcriptomic profiling of PRR expression in peptidergic and nonpeptidergic dorsal root ganglion (DRG) nociceptor cells, the two broad classes of peripheral nociceptor. PRRs from the major categories were explored: Toll-like receptors (TLRs), NOD-like receptors (NLRs), C-type lectin receptors (CLRs), RIG-I-like receptors (RLRs), and cytosolic DNA sensors (CDSs). Sequencing data were analyzed from the database generated by Zheng et al. [44], who conducted deep RNA-Seq profiling of defined DRG subtypes using a genetic labeling strategy in mice; data indicate mRNA levels in reads per kilobase, per million mapped reads (rpkm; bars represent the mean ± SEM; n = 3 individual mice sequenced per group). Peptidergic nociceptors exhibit high expression of Tlr4, Nod1, Nlrx1, Ly75, Ddx58, Sting1, and Lrrfip1. Nonpeptidergic nociceptors exhibit lower expression of all PRRs compared to peptidergic nociceptors, but have noteworthy expression of Nlrx1, Ddx58, Ifih1, Ddx41, and Lrrfip1. (B) Canonical gene regulation in nociceptors following PRR activation after injury and infection. Nerve injury and chemotherapy agents upregulate TLRs such as TLR4, TLR7, and TLR8, leading to activation of the MyD88/NF-κB or MAPK signaling pathways and increased synthesis of nociceptor mediators including cytokines and chemokines (TNF, IL-1α, IL-6, CCL2, and CXCL1) and neuropeptides (SP, CGRP, VIP, GAL, and PACAP). Axonal transport occurs in both directions, and retrograde axonal transport of nerve growth factor (NGF) was shown to activate p38 MAPK and induce the expression of TRPA1/V1 [80,105]. Injury- and infection-induced upregulation of TRPA1/V1 further promotes the release of these nociceptor mediators in terminals, and these not only sensitize peripheral nociceptors to promote pain but also contribute to neurogenic inflammation and immunity. Some of these mediators can also be released from the cell body of nociceptors to contribute to neuroinflammation and immune cell infiltration into sensory ganglia [55].
Beyond the TLRs, fewer studies to date have investigated the function in sensory neurons of other PRR families such as the NLRs, RLRs, CLRs, and CDSs. By comparing normalized read counts in genetically labeled peptidergic and nonpeptidergic nociceptors in the DRG using the database generated by [44], it is clear that many additional PRRs are highly expressed by nociceptive sensory neurons (Figure 4A), and may be of functional significance. In interpreting these data, one can look at Tlr4 as a reference point because the role of TLR4 in peptidergic nociceptors and pain is well established [56,57]. In addition to the TLRs, several NLR genes (Nlrx1, Nod1), CLRs (Ly75), RLRs (Ddx58, the gene encoding RIG-I), and CDSs (Sting1) exhibit particularly high expression in nociceptive neurons, which is largely mirrored by scRNA-Seq databases [41,42]. However, it is also important to note that scRNA-Seq is fundamentally different from whole-transcriptome RNA-Seq in that only the top 10–20% most abundant mRNAs are typically captured by scRNA-Seq, leaving the majority of genes, and especially those which exhibit lower expression, undetected [58].
In examining these datasets, it becomes clear that many PRRs are present at the mRNA level, suggesting a possible functional role. However, it is important to acknowledge that this does not necessarily indicate functional significance or even the presence of protein. Conversely, the inability to detect genes by RNA-Seq does not necessarily imply the absence of gene expression, protein, or physiological function. Illustrating this point, neither Ciita nor Nlrc5 is abundantly detected in nociceptors by either sequencing method, but one or both must be functionally active because these proteins are crucial for MHC-I transactivation across cell types [59]. Further, several TLRs that were found to be absent from sensory neurons in sequencing studies, such as Tlr7 and Tlr8, have been demonstrated to be present at the protein level and/or are functionally upregulated in injury states [52,53]. Moreover, behavioral and electrophysiological studies using Tlr7−/− and Tlr8−/− mice have demonstrated these receptors play important roles in nociception in vivo [46,52,60]. Across many fields in biology, the widespread use of RNA-Seq has been noted to produce somewhat conflicting results, and many such discrepancies can be attributed to issues related to RNA isolation or processing methodologies, mRNA stability, and mRNA localization and translation efficiency, among others [61]. Notably, it is recognized that there is only a weak positive correlation (R2 = 0.41) between the cellular concentrations of proteins and the abundance of their corresponding mRNAs [62]. Although the overall protein expression level should be a better predictor of the function of the studied gene than the level of mRNA, the anatomical (e.g., axon vs soma) and subcellular locations (e.g., cell surface vs endosome) of these proteins in sensory neurons are more important for their specific function [63]. Thus, although these studies generate a vast array of data and suggest prospective targets for future studies aimed at identifying new PRRs involved in nociception, a deeper examination of each target using a combination of biochemical, immunohistochemical, and functional approaches remains paramount and will likely yield new regulators of sensory function.
Non-Canonical Interaction between TLRs and TRP Channels in Nociceptors
The TRP cation channels TRPA1 and TRPV1 are activated by two common natural compounds, mustard oil (allyl isothiocyanate) and capsaicin, respectively. Localized to the cell membrane and serving as key molecular sensors of temperature and pain, TRPA1 and TRPV1 play a crucial role in the pathogenesis of pathological pain conditions such as inflammatory pain and cancer pain [64–67]. For this reason, targeting TRP channel function has long been proposed as a therapeutic approach to enable pain control. In addition to their important role in the genesis of pain, activation of TRP channels also contributes to neurogenic inflammation. Over the past decade a host of studies have revealed functional coupling between TLRs and TRP channels in sensory neurons, uncovering a highly unusual and non-canonical TLR function of great interest to sensory neurobiology and immunology.
Several lines of evidence support the notion that TLR4 is directly coupled with TRPV1 in DRG and TG nociceptors. For example, acute application of LPS to dissociated TG neurons evoked rapid (seconds to minutes) inward currents and potentiated capsaicin-induced responses (inward currents, Ca2+ influx, and CGRP release) in trigeminal neurons [68]. Furthermore, capsaicin-induced acute pain is also markedly reduced in Tlr4 knockout mice, and distinct subdomains of TLR4 and TRPV1 have been identified that may possibly mediate their physical association [69]. In rodents and humans, systemic treatment with the chemotherapy agent paclitaxel is associated with TLR4 activation in nociceptors [54,56].
There is also evidence for functional coupling between TLR3 and TRPV1. RNA-Seq profiling of mouse DRG nociceptors reveals Tlr3 expression in TRPV1+ DRG neurons, and immunohistochemistry reveals colocalization of TLR3 and TRPV1 in DRG nociceptors [50]. Notably, extracellular application of the TLR3 agonist poly(I:C), as well as double-stranded RNAs (dsRNAs) extracted from brain, can induce rapid Tlr3-dependent inward currents in nociceptors within 1 minute [50]. In support of functional coupling between TLR3 and TRPV1, capsaicin-evoked excitatory postsynaptic currents (EPSCs) in spinal cord neurons are abolished in Tlr3 knockout mice, whereas poly(I:C)-evoked EPSCs are abrogated in Trpv1 knockout mice. Thus, although these data provide evidence for functional and bidirectional interaction between TLR3 and TRPV1, the precise mechanism by which these two receptors are functionally coupled to one another remains unknown.
Immunohistochemistry and electrophysiology revealed that TLR7 is also present on the cell surface of mouse nociceptor neurons [46,60]. The miRNA let-7b was identified as an endogenous ligand of TLR7 in mouse DRG neurons, and addition of synthetic let-7b RNA causes rapid activation and excitation of nociceptors through the activation of TLR7 and subsequent coupling of TLR7 and TRPA1 [60]. Thus, this action, occurring on the order of seconds after ligand application, requires both TLR7 and TRPA1 but not the canonical MyD88 signaling. Notably, coimmunoprecipitation demonstrated that let-7b enhances the interaction between TLR7 and TRPA1. In vivo, administration of let-7b via intraplantar (e.g., hindpaw) injection elicits rapid and dose-dependent spontaneous pain (licking and flinching the affected paw) in the first several minutes after the injection, an effect that is abolished in mice lacking Tlr7 or Trpa1 [60]. Thus, a functional and direct link exists between TLR7 and TRPA1, enabling rapid modulation of TRPA1 to regulate inflammatory pain.
Nociceptors Rapidly Respond to Infection for Nociception and Antinociception
Infections caused by bacterial and viral pathogens are commonly associated with altered pain sensitivity [70,71]. It was generally believed that bacterial infections cause pain indirectly through the intermediating effects of immune cells and the inflammatory substances they secrete (Figures 1 and 3A). However, emerging evidence suggests that pathogens can also directly activate nociceptors and elicit pain using novel mechanisms (Figure 3B) [33,71]. For example, Staphylococcus aureus produces a pore-forming toxin called α-hemolysin that enables it to destroy host cells. Intriguingly, this toxin also causes pain in mice by directly forming pores in nociceptors, permitting cation influx and action potential firing [71]. In dental pulp, LPS from bacteria modulates nociceptor activity through TLR4-mediated nociceptor sensitization of TRPV1 [68]. In addition, bacteria have been demonstrated to secrete metabolic byproducts such as N-formyl peptides that directly activate nociceptors via formyl peptide receptor 1 (FPR1). In a recent study it was also demonstrated that the pulmonary tuberculosis-causing bacterium, Mycobacterium tuberculosis, releases sulfolipid-1 that acts on peripheral nociceptors to induce cough through a possible interaction with TRPV1 [72]. Conversely, in some instances bacterial infections can also suppress pain. For example, Mycobacterium ulcerans causes extensive skin lesions and Buruli ulcers, but does not evoke pain. Instead, M. ulcerans can secrete the mycobacterial polyketide mycolactone to elicit analgesia through activation of type 2 angiotensin II receptors (AT2Rs) and subsequent activation of K2P potassium channels KCNK4 in nociceptors (Figure 3B) [73]. Thus, by suppressing nociception, bacteria may evade host detection to allow the infection to continue unmitigated. Similarly, exploring how the commensal microbiome interacts with sensory neurons through PRRs or other receptors under physiological and pathological conditions is an important area for future investigation [74].
Nociceptors Respond to Injury and Infection via Transcriptional and Translational Regulation
Conventional TLR signaling through transcriptional activation requires MyD88, an adaptor for all TLRs with the exception of TLR3. Canonical TLR signaling via transcriptional activation is also evident in sensory neurons because selective deletion of Myd88 in sensory neurons results in a reduction in neuropathic pain and inflammatory pain, especially in the chronic phases (Figure 4B) [55,75]. Notably, MyD88 is upregulated in DRG nociceptors after nerve injury and chemotherapy, and the homodimerization inhibitory peptide for MyD88 signaling reduced neuropathic pain in rats after intrathecal administration [54,76]. After nerve injury, TLR7 is upregulated in nociceptive neurons and enhances neuropathic pain by activating the NF-κB and ERK signaling pathways in primary sensory neurons [53]. TLR8 is localized in the endosomes and lysosomes of nociceptors, and nerve injury causes TLR8 upregulation in non-peptidergic nociceptors. This upregulation results in ERK activation in nociceptors, leading to increased production of inflammatory mediators (TNF, IL-1β, IL-6, and CCL2) and nociceptor hyperexcitability, thus driving neuropathic pain [52]. Furthermore, miR-21, an endogenous ligand of TLR8, is also upregulated in DRG neurons after nerve injury, and miR-21 drives neuropathic pain via TLR8 [52]. Importantly, activation of MAPKs (ERK, p38, and JNK) regulates nociceptor function via transcriptional, translational, and post-translational mechanisms [77,78], and translational control in nociceptors plays an active role in pain persistence [79–81].
Nociceptors also sense the presence of viruses, and viral infections are frequently associated with acute pain. Several types of viruses, such as herpes simplex virus (HSV) and varicella zoster virus (VZV), exhibit neurotropism and preferentially infect sensory neurons. Upon infecting sensory neurons, these viruses enter a latent phase that is characterized by a period of dormancy, which is subsequently followed by viral reactivation that can be exceptionally painful. In this manner, sensory neurons offer a particularly advantageous target for neurotropic viruses because they present a post-mitotic refuge that extends from both the PNS and the CNS, with peripheral projections that extend into the upper layers of the epidermis. This may also explain the robust expression of several viral sensor genes in nociceptive neurons (Figure 4A), including Ddx58, Sting1, and Ddx41. Interestingly, intraplantar administration of rats with HSV-1 has been shown to induce antinociception, increasing mechanical sensory thresholds in the ipsilateral paw [82]. Moreover, HSV-1/HSV-2 infection of rodent DRG neurons has also been shown to decrease sensory neuron excitability and sodium currents [83,84]. During reactivation of HSV, patients frequently experience paresthesia (numbness, tingling) followed by acute pain following ulceration. Thus, HSV may inhibit or potentiate pain depending on the stage of the infection. How viruses regulate sensory function via transcriptional and translational regulation; reciprocally, how sensory neurons contribute to host defense against viruses is an area of research that is still in its infancy and stands out as an important avenue for future studies.
Mechanosensitive Sensory Neurons Express TLR5 for Tactile Allodynia
Peripheral mechanoreceptive sensory neurons (mechanoreceptors) are crucial for transducing a wide array of tactile information. In mice, at least five subtypes of low-threshold mechanoreceptors (LTMRs) have been identified that innervate hairy skin to communicate tactile information, including myelinated Aδ LTMRs, Aβ rapidly adapting (RA)-LTMRs, Aβ slowly adapting (SA)-LTMRs, Aβ field-LTMRs, and unmyelinated C-LTMRs [85]. Notably, dRNA-Seq analyses of DRG neurons have demonstrated that Tlr5 is mainly expressed by LTMRs [41] (Figure 5A). dRNA-Seq of DRG neurons further revealed that Aα RA-LTMRs exhibit the highest expression of Tlr5, and Aα SA-LTMRs and Aδ LTMRs also show significant Tlr5 expression [44] (Figure 5A). In situ hybridization also revealed Tlr5 expression in medium-sized DRG neurons, consistent with expression in Aα LTMRs [48] (Figure 5B). Interestingly, activation of TLR5 by its prototypical ligand flagellin induced calcium responses in mouse DRG neurons [86]. Furthermore, in both mouse and human DRG neurons, application of flagellin results in increased membrane permeability to QX-314, a membrane-impermeable lidocaine derivative, and subsequent silencing of TLR5-expressing A-fibers, without affecting the function of C-fibers [48]. Moreover, a combination of flagellin and QX-314 blocked Aα fiber-evoked compound potentials in sciatic nerve [48] and Aα fiber-evoked synaptic transmission in spinal cord neurons [86]. Tactile hypersensitivity or mechanical allodynia is a common feature of acute and chronic pain [87]. Notably, the combination treatment of flagellin and QX-314 blocked mechanical allodynia in mice after nerve injury and chemotherapy [48]. These data demonstrate that mechanoreceptive sensory neurons utilize TLR5 as a danger-sensing mechanism to detect bacteria, a mechanism that has the potential to be used to develop novel therapeutics to treat chronic pain (mechanical allodynia) [48]. In the future, it will also be important to identify other mechanisms used by mechanosensitive neurons to detect danger signals. Although large Aα mechanoreceptors mediate mechanical allodynia under injured and disease conditions (e.g., nerve injury), activation of these large fibers in steady-state conditions can also produce analgesia according to the ‘gate control’ theory through a mechanism in which Aα input suppresses nociceptive C-fiber input (closing the gate) via a spinal cord inhibitory circuit [88–90]. In this context, flagellin could be a MAMP produced by commensal microbiota that acts on mechanoreceptors to ‘close the gate’, thereby suppressing pain sensitivity. Thus, although speculative, it is possible that bacteria-mediated TLR5 activation may produce both pain and analgesia, depending on the status of the ‘gate’.
Figure 5. Dorsal Root Ganglion (DRG) Mechanoreceptors Express Toll-Like Receptor TLR5 and Other Pattern Recognition Receptors (PRRs).
(A) In situ hybridization (ISH) for Tlr5 in DRG sections, in concert with NF200 immunostaining to label large-diameter mechanosensory neurons (reproduced, with permission, from [48]). Hybridization with a sense control demonstrates the specificity of Tlr5 ISH. Notably, the vast majority of TLR5+ neurons also express NF200 and have medium to large soma, as indicated by yellow arrows. Blue and white arrows indicate singly stained neurons. Scale, 100 μm. (B) Traces of sodium currents recorded from A-fiber and C-fiber neurons from wild-type (WT) and Tlr5−/− mice in the absence (black) or presence (red) of flagellin (0.3 nM) and QX-314 (5 mM). These data demonstrate that coapplication of flagellin and QX-314 silences A-fiber neurons in a TLR5-dependent manner, without affecting C-fiber neurons (reproduced, with permission, from [48]). (C) Transcriptomic profiling of PRR expression in several low-threshold mechanoreceptor (LTMR) populations using the deep RNA-Seq database generated by Zheng et al. [44]; normalized mRNA levels are indicated in reads per kilobase, per million mapped reads (rpkm; bars represent the mean ± SEM from n = 3–5 individual mice per group). The DRG populations indicated include Aβ RA-LTMRs (pink, rapidly adapting), Aβ SA-LTMRs (teal, slowly adapting), and Aβ field-LTMRs (dark purple).
PRRs in Pruriceptors Regulate Itch
Itch and pain are unique sensations mediated by pruriceptors and nociceptors, respectively. Based on physical, molecular, and functional characteristics, mouse and human pruriceptors and nociceptors substantially overlap, representing a subset of nociceptors [41,91]. TRPV1-expressing nociceptors are crucially required for pruritus [92], and several subpopulations of nociceptors including those expressing MrgprA3 and somatostatin are specifically linked to itch [93,94]. Functionally unique populations of pruriceptors are represented within the DRG and can be differentiated by their responses to different pruritogens (e.g., itch-evoking compounds). For example, in rodents, acute chemical itch induced by histamine occurs via activation of the H1 and H4 receptors, whereas chloroquine induces histamine-independent itch via activation of the MrgprA3 receptor [95]. Pruritogens activate GPCRs to elicit itch via TRPA1 and TRPV1. For example, TRPV1 is required for histamine-induced itch, whereas TRPA1 is required for chloroquine-induced itch [92,96]. Thus, as in pain, TRPA1 and TRPV1 are key molecular integrators for itch. Intriguingly, in mice, rapid activation of TRPA1 by injection of miR-711 induces potent pruritus without producing neurogenic inflammation [97]. Chronic itch after skin injury also requires TRPA1 [97,98]. Several types of nociceptor-expressed TLRs, such as TLR2, TLR3, TLR4, and TLR7, have been implicated in itch modulation via functional coupling to TRPA1 or/and TRPV1 [46,49,50,99]. Both histamine-dependent and independent itch was abolished after Tlr3 deficiency, in part owing to impaired TPRV1 function in spinal dorsal horn central terminals [50]. Histamine-induced pruritus was also attenuated in Tlr4 knockout mice owing to TRPV1 dysfunction [99]. Another study demonstrated that Tlr4 deletion in mice also impairs chronic itch [100]. In addition, TLR2 regulates histamine-dependent and histamine-independent acute itch via activation of TRPA1 and TRPV1. Notably, different TLR2 agonists evoke distinct pain and itch responses by differentially targeting TLR1/2 and TLR2/6 heterodimers [49].
Different neurocircuits have been implicated in regulating chemical itch and mechanical itch [89,101]. Recently, TLR5-expressing Aα fibers were also reported to modulate mechanical itch [86]. These Aα fibers project to deep lamina II of the spinal cord and make synaptic contacts with Ucn3-positive interneurons [86], and silencing these fibers by coapplication of QX-314 and flagellin abolished Aβ-evoked EPSCs in Ucn3+ interneurons in spinal cord slices [86]. Flagellin is sufficient induce mechanical itch as well as spontaneous scratching via activation of TLR5 [86], suggesting a mechanism by which bacterial infection causes pruritus. Future studies aimed at investigating whether bacterial and/or fungal pathogens contribute to pathological itch conditions through direct activation of PRRs in pruriceptors are likely to yield new insights.
PRRs in Sensory Neurons Regulate Neuroinflammation and Local Immunity
Sensory neurons not only express receptors that can respond to inflammatory mediators but also produce cytokines, chemokines, and neuropeptides to regulate local immunity (Figure 4B) [35]. Activation of MyD88 by TLR agonists in turn activates the NF-κB and MAP kinase pathways, leading to the production of inflammatory mediators and neuropeptides by DRG neurons [52,53]. Deletion of Myd88 selectively in peripheral nociceptors resulted in downregulation of CCL2 expression in sensory neurons, thus impairing macrophage infiltration into DRG following nerve injury and chemotherapy [55,75]. Although present at low abundance (<0.5% of total cells), the mouse DRG hosts many different types of immune cells including macrophages, monocytes, neutrophils, CD4+ helper T cells, CD8+ cytotoxic T cells, and other antigen-presenting cells [55,102]. In rodents, paclitaxel treatment causes significant increases in innate and adaptive immune subsets in mouse DRG. In addition, paclitaxel treatment also reduces the abundance of anti-inflammatory T cells in DRG [55], potentially perturbing the steady-state neuroimmune microenvironment. Strikingly, all these changes in DRG-resident innate and adaptive immune cells require MyD88 in nociceptive neurons [55]. Further studies will be necessary to identify the specific mediators produced by nociceptors that control the proliferation and migration of different immune cell types to and from sensory ganglia under normal and pathological conditions. Likewise, additional studies to explore the role of the various innate and adaptive immune cell types present within the DRG under homeostatic and injury conditions are warranted.
Neuropeptides such as CGRP, SP, and VIP have also emerged as potent regulators of inflammation and immunity. Notably, activation of PRRs (e.g., TLR4 by LPS) and TRPA1/V1 results in increased release of the neuropeptide CGRP and neurogenic inflammation [68,103]. Peripheral inflammation further upregulates the expression of ion channels (TRPA1 and TRPV1) and neuropeptides (SP and CGRP) in DRG neurons via MAPK [80,104,105]. Notably, barrier surfaces such as the skin, gut, and lung are densely innervated by nociceptors, and secretion of neuropeptides (CGRP, SP, VIP) has been demonstrated to play a role in protective immunity, pain, or neurogenic inflammation via activation of tissue-resident or newly recruited immune subsets [71,106–109]. Notably, after nerve injury, the expression of several neuropeptides including VIP, galanin (GAL), and pituitary adenylate cyclase-activating polypeptide (PACAP) is upregulated in injured nociceptors (Figure 4B) [110–113]. By contrast, axonal injury upregulates neuropeptide Y (NPY) in A-fiber neurons, which contributes to tactile allodynia [114]. It will be of great interest to identify additional mediators released by C-fiber and A-fiber neurons that contribute to immunity, homeostasis, and pathology in different organs following PRR activation by MAMPs, PAMPs, and DAMPs.
Concluding Remarks and Future Perspectives
Immune cells and sensory neurons share a common function that is fundamentally required for organismal survival – the detection of danger signals [35]. To do so, both immune cells and sensory neurons utilize a relatively limited number of genetically encoded PRRs such as TLRs that enable the detection of danger signals (e.g., PAMPs and DAMPs) derived from infection, injury, or neoplastic disease processes. At its most fundamental level, the recognition of danger signals by TLRs in immune cells causes changes in gene expression that initiate and amplify the innate immune response, promoting inflammation and activating host defense. Similarly, the steady-state function of sensory neurons is to allow organisms to detect a variety of physical stimuli (e.g., temperature and tactile information), thereby enabling host defense in response to a variety of external sensory cues ranging from noxious temperatures to potentially hazardous insects. Viewing sensory neurons in this light, the adoption and unique utilization of TLRs by sensory neurons to detect danger signals that are frequently indicative of pathology is perhaps unsurprising, and further expands the repertoire of functions of sensory neurons. Philosophically, this interpretation of sensory neuron function could be an argument to view these cells as surveillance cells alongside classical innate immune cells. Given that sensory neurons are fundamentally unique from immune cells in the sense that they are electrically excitable and rely on ion channels to rapidly respond to various stimuli, eliciting immediate responses to danger signals by utilizing cellular machinery (TLRs) that typically involves a relatively slow transcription-dependent mechanism (MyD88) poses a unique problem. To this end, sensory neurons couple DAMP- and PAMP-sensing TLRs with ion channels that directly gate neuronal excitability, thus providing a direct path for the danger-sensing machinery to rapidly alter sensory neuron excitability and evoke sensations such as pain, itch, or analgesia. The net result of this coupling depends on the precise cell types, signals, and receptors involved, and mediators produced by injured host cells and infectious agents yield an array of behavioral outcomes including pain, itch, and analgesia. However, many unresolved issues remain (see Outstanding Questions), including the crucial question of how TLRs enable rapid modulation of ion channel activity and thereby potentiate or suppress neuron excitability. The identification of these mechanisms, as well as additional PRR signaling pathways that sensory neurons may use to potentiate or suppress neuron excitability, will crucially impact on our understanding of sensory neuron function and may also reveal promising new therapeutic strategies to treat infectious diseases, chronic pain, and chronic itch.
Outstanding Questions.
How are TLRs functionally coupled with ion channels to enable rapid modulation of sensory neuron excitability? Although it is well established that several TLRs are functionally coupled with ion channels, leading to pain, itch, or analgesia, a fundamental question that remains concerns how TLRs are coupled to ion channels (directly or indirectly) in a manner that allows them to alter neuronal excitability on the order of seconds to minutes. Future studies employing biophysical, biochemical, and proteomic techniques will help address this question.
Do TLRs or other PRRs regulate mechanoreception under steady-state conditions or pathological conditions? Mechanical allodynia is a hallmark of chronic pain. The case study of TLR5 coactivation with its natural ligand flagellin and QX-314 demonstrates the potential promise of this avenue of research in treating mechanical allodynia associated with chronic pain. In future studies, it will also be important to understand whether and how TLRs or other PRRs regulate physiological mechanoreception.
How do other PRRs expressed in sensory neurons contribute to sensory function? RNA-Seq profiling of peripheral sensory neurons has revealed that they express many PRRs whose functions are well established in immune cells but remain unexplored in sensory neurons. How these PRRs contribute to sensory neuron function and to the ability of sensory neurons to detect exogenous or endogenous danger signals is an important open question.
How do nociceptors and other sensory neurons contribute to host defense against bacterial and viral infections? What is the role of nociceptors in engaging with the commensal microbiota? Nociceptors extend peripheral afferents that penetrate deep into barrier surfaces such as the gut lining and the epidermis. Taken in conjunction with the diverse array of PRRs they express, nociceptors would thus appear well poised to participate in initiating host defense to invading pathogens or in sensing mediators produced by the commensal microbiota.
Highlights.
The detection of potentially hazardous stimuli is a fundamental property of both immune cells and peripheral sensory neurons. To detect danger signals, both cell types utilize specialized PRRs that recognize PAMPs from invading pathogens and DAMPs from damaged host cells.
Sensory neurons express DAMP/PAMP-sensing TLRs that signal non-canonically through coupling to ion channels to effect rapid modulation of sensory neuron excitability. TLRs can also alter neuronal excitability via a slower MyD88-mediated canonical pathway.
DAMP and PAMP-mediated modulation of peripheral sensory neuron excitability can evoke pain, itch, or analgesia.
Databases emerging from RNA-Seq profiling of peripheral sensory neurons indicate that they express many additional PRRs beyond TLRs.
Acknowledgments
The laboratory of R-R.J. is funded by Duke Anesthesiology research funds. C.R.D. received support from the John J. Bonica Trainee Fellowship and the National Institutes of Health (T32 GM008600). Illustrations were created using BioRender.
Glossary
- Allodynia
pain evoked by a stimulus that ordinarily does not provoke pain. In the context of chronic or neuropathic pain, cold allodynia (caused by application of a non-noxious cool stimulus) and mechanical allodynia (caused by a non-noxious tactile stimulus) is frequently observed in both rodents and humans.
- Analgesia
absence of pain in response to a stimulus that would ordinarily evoke pain.
- Damage-associated molecular patterns (DAMPs)
molecular signals released from dead, dying, damaged, or aberrant host cells that typically promote an inflammatory response.
- Dorsal root ganglion (DRG)
bilateral clusters of somatosensory neurons that are present at each spinal level. DRG neurons extend both peripheral afferent nerve fibers to target tissues through which they receive sensory input, and also extend central projections to the dorsal horn of the spinal cord where they relay this input to higher-order neurons. An analogous system is present in the trigeminal ganglion (TG), that afferently innervates craniofacial tissues and relays sensory input to nuclei located in the brainstem.
- Mechanoreceptor
specialized sensory neurons that normally detect mechanical stimuli. In the peripheral nervous system (PNS), different subtypes of mechanoreceptors are present that are specialized for the detection of different mechanical stimuli (e.g., vibration, light touch, firm touch).
- Neurogenic inflammation
inflammation evoked by the release of neuropeptides and inflammatory mediators that are produced directly by peripheral nociceptor afferents.
- Nociceptor
sensory neurons that normally respond to noxious (painful) and potentially injurious stimuli, including both physical and chemical stimuli. Nociceptors in the PNS can be referred to as peripheral nociceptors, of which there are several subtypes. Activation of nociceptors leads to pain, whereas inhibition of activated nociceptors (antinociception) can inhibit pain.
- Nociceptor sensitization
heightened response of a nociceptor to its normal input, and/or responsiveness to a normally subthreshold input. Sensitization of peripheral nociceptors is termed peripheral sensitization, whereas sensitization of nociceptive neurons in the central nervous system is termed central sensitization.
- Pathogen-associated molecular patterns (PAMPs)
molecular signals produced by exogenous microorganisms but not by host cells. In the context of infection, PAMPs typically promote an inflammatory response.
- Pattern recognition receptors (PRRs)
germline-encoded host-intrinsic receptors that detect PAMPs and DAMPs released from sites of damage, including infectious agents, neoplastic host cells, or injured tissues.
References
- 1.Chiu IM et al. (2012) Neurogenic inflammation and the peripheral nervous system in host defense and immunopathology. Nat. Neurosci 15, 1063–1067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fitzgerald KA and Kagan JC (2020) Toll-like receptors and the control of immunity. Cell 180, 1044–1066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barton GM and Medzhitov R (2003) Toll-like receptor signaling pathways. Science 300, 1524–1525 [DOI] [PubMed] [Google Scholar]
- 4.Poltorak A et al. (1998) Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 282, 2085–2088 [DOI] [PubMed] [Google Scholar]
- 5.Lemaitre B et al. (1996) The dorsoventral regulatory gene cassette spätzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell 86, 973–983 [DOI] [PubMed] [Google Scholar]
- 6.Medzhitov R et al. (1997) A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature 388, 394–397 [DOI] [PubMed] [Google Scholar]
- 7.Tassia MG et al. (2017) Toll-like receptor pathway evolution in deuterostomes. Proc. Natl. Acad. Sci. U. S. A 114, 7055–7060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gold MS and Gebhart GF (2010) Nociceptor sensitization in pain pathogenesis. Nat. Med 16, 1248–1257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ji RR et al. (2014) Emerging targets in neuroinflammation-driven chronic pain. Nat. Rev. Drug Discov 13, 533–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stewart CR et al. (2010) CD36 ligands promote sterile inflammation through assembly of a Toll-like receptor 4 and 6 heterodimer. Nat. Immunol 11, 155–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shimazu R et al. (1999) MD-2, a molecule that confers lipopolysaccharide responsiveness on Toll-like receptor 4. J. Exp. Med 189, 1777–1782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gong T et al. (2020) DAMP-sensing receptors in sterile inflammation and inflammatory diseases. Nat. Rev. Immunol 20, 95–112 [DOI] [PubMed] [Google Scholar]
- 13.Wang H et al. (1999) HMG-1 as a late mediator of endotoxin lethality in mice. Science 285, 248–251 [DOI] [PubMed] [Google Scholar]
- 14.Yang H et al. (2010) A critical cysteine is required for HMGB1 binding to Toll-like receptor 4 and activation of macrophage cytokine release. Proc. Natl. Acad. Sci. U. S. A 107, 11942–11947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen X et al. (2008) Characterization of microRNAs in serum: a novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res 18, 997–1006 [DOI] [PubMed] [Google Scholar]
- 16.Fabbri M (2012) TLRs as miRNA receptors. Cancer Res 72, 6333–6337 [DOI] [PubMed] [Google Scholar]
- 17.Liu T et al. (2012) Emerging role of Toll-like receptors in the control of pain and itch. Neurosci. Bull 28, 131–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Watkins LR et al. (2009) The ‘toll’ of opioid-induced glial activation: improving the clinical efficacy of opioids by targeting glia. Trends Pharmacol. Sci 30, 581–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tanga FY et al. (2005) The CNS role of Toll-like receptor 4 in innate neuroimmunity and painful neuropathy. Proc. Natl. Acad. Sci. U. S. A 102, 5856–5861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim D et al. (2007) A critical role of toll-like receptor 2 in nerve injury-induced spinal cord glial cell activation and pain hypersensitivity. J. Biol. Chem 282, 14975–14983 [DOI] [PubMed] [Google Scholar]
- 21.Yoneyama M et al. (2004) The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat. Immunol 5, 730–737 [DOI] [PubMed] [Google Scholar]
- 22.Sun L et al. (2013) Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science 339, 786–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ishikawa H and Barber GN (2008) STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature 455, 674–678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Agostini L et al. (2004) NALP3 forms an IL-1beta-processing inflammasome with increased activity in Muckle-Wells autoinflammatory disorder. Immunity 20, 319–325 [DOI] [PubMed] [Google Scholar]
- 25.Seth RB et al. (2005) Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-kappaB and IRF 3. Cell 122, 669–682 [DOI] [PubMed] [Google Scholar]
- 26.Brubaker SW et al. (2015) Innate immune pattern recognition: a cell biological perspective. Annu. Rev. Immunol 33, 257–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chow KT et al. (2018) RIG-I and other RNA sensors in antiviral immunity. Annu. Rev. Immunol 36, 667–694 [DOI] [PubMed] [Google Scholar]
- 28.Ting JP et al. (2010) How the noninflammasome NLRs function in the innate immune system. Science 327, 286–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Motwani M et al. (2019) DNA sensing by the cGAS-STING pathway in health and disease. Nat. Rev. Genet 20, 657–674 [DOI] [PubMed] [Google Scholar]
- 30.Swanson KV et al. (2019) The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat. Rev. Immunol 19, 477–489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grace PM et al. (2016) Morphine paradoxically prolongs neuropathic pain in rats by amplifying spinal NLRP3 inflammasome activation. Proc. Natl. Acad. Sci. U. S. A 113, E3441–E3450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cowie AM et al. (2019) NOD-like receptor protein 3 inflammasome drives postoperative mechanical pain in a sex-dependent manner. Pain 160, 1794–1816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ji RR et al. (2016) Pain regulation by non-neuronal cells and inflammation. Science 354, 572–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Medzhitov R (2010) Inflammation 2010: new adventures of an old flame. Cell 140, 771–776 [DOI] [PubMed] [Google Scholar]
- 35.Talbot S et al. (2016) Neuroimmunity: physiology and pathology. Annu. Rev. Immunol 34, 421–447 [DOI] [PubMed] [Google Scholar]
- 36.Abdo H et al. (2019) Specialized cutaneous Schwann cells initiate pain sensation. Science 365, 695–699 [DOI] [PubMed] [Google Scholar]
- 37.Binshtok AM et al. (2008) Nociceptors are interleukin-1beta sensors. J. Neurosci 28, 14062–14073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jung H et al. (2008) Monocyte chemoattractant protein-1 functions as a neuromodulator in dorsal root ganglia neurons. J. Neurochem 104, 254–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jin X and Gereau RW (2006) Acute p38-mediated modulation of tetrodotoxin-resistant sodium channels in mouse sensory neurons by tumor necrosis factor-alpha. J. Neurosci 26, 246–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chiu IM et al. (2014) Transcriptional profiling at whole population and single cell levels reveals somatosensory neuron molecular diversity. Elife 3, e04660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Usoskin D et al. (2015) Unbiased classification of sensory neuron types by large-scale single-cell RNA sequencing. Nat. Neurosci 18, 145–153 [DOI] [PubMed] [Google Scholar]
- 42.Zeisel A et al. (2018) Molecular architecture of the mouse nervous system. Cell 174, 999–1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li CL et al. (2016) Somatosensory neuron types identified by high-coverage single-cell RNA-sequencing and functional heterogeneity. Cell Res 26, 967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zheng Y et al. (2019) Deep sequencing of somatosensory neurons reveals molecular determinants of intrinsic physiological properties. Neuron 103, 598–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goethals S et al. (2010) Toll-like receptor expression in the peripheral nerve. Glia 58, 1701–1709 [DOI] [PubMed] [Google Scholar]
- 46.Liu T et al. (2010) Toll-like receptor 7 mediates pruritus. Nat. Neurosci 13, 1460–1462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wadachi R and Hargreaves KM (2006) Trigeminal nociceptors express TLR-4 and CD14: a mechanism for pain due to infection. J. Dent. Res 85, 49–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xu ZZ et al. (2015) Inhibition of mechanical allodynia in neuropathic pain by TLR5-mediated A-fiber blockade. Nat. Med 21, 1326–1331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang TT et al. (2020) Activation of different heterodimers of TLR2 distinctly mediates pain and itch. Neuroscience 429, 245–255 [DOI] [PubMed] [Google Scholar]
- 50.Liu T et al. (2012) TLR3 deficiency impairs spinal cord synaptic transmission, central sensitization, and pruritus in mice. J. Clin. Invest 122, 2195–2207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Qi J et al. (2011) Painful pathways induced by TLR stimulation of dorsal root ganglion neurons. J. Immunol 186, 6417–6426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang ZJ et al. (2018) TLR8 and its endogenous ligand miR-21 contribute to neuropathic pain in murine DRG. J. Exp. Med 215, 3019–3037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.He L et al. (2020) Toll-like receptor 7 contributes to neuropathic pain by activating NF-kappaB in primary sensory neurons. Brain Behav. Immun 87, 840–851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li Y et al. (2014) Toll-like receptor 4 signaling contributes to paclitaxel-induced peripheral neuropathy. J. Pain 15, 712–725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu XJ et al. (2014) Nociceptive neurons regulate innate and adaptive immunity and neuropathic pain through MyD88 adapter. Cell Res 24, 1374–1377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li Y et al. (2015) The cancer chemotherapeutic paclitaxel increases human and rodent sensory neuron responses to TRPV1 by activation of TLR4. J. Neurosci 35, 13487–13500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bruno K et al. (2018) Targeting toll-like receptor-4 (TLR4) - an emerging therapeutic target for persistent pain states. Pain 159, 1908–1915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Islam S et al. (2014) Quantitative single-cell RNA-seq with unique molecular identifiers. Nat. Methods 11, 163–166 [DOI] [PubMed] [Google Scholar]
- 59.Kobayashi KS and van den Elsen PJ (2012) NLRC5: a key regulator of MHC class I-dependent immune responses. Nat. Rev. Immunol 12, 813–820 [DOI] [PubMed] [Google Scholar]
- 60.Park CK et al. (2014) Extracellular microRNAs activate nociceptor neurons to elicit pain via TLR7 and TRPA1. Neuron 82, 47–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu Y et al. (2016) On the dependency of cellular protein levels on mRNA abundance. Cell 165, 535–550 [DOI] [PubMed] [Google Scholar]
- 62.Vogel C and Marcotte EM (2012) Insights into the regulation of protein abundance from proteomic and transcriptomic analyses. Nat. Rev. Genet 13, 227–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Riccio A et al. (1997) An NGF-TrkA-mediated retrograde signal to transcription factor CREB in sympathetic neurons. Science 277, 1097–1100 [DOI] [PubMed] [Google Scholar]
- 64.Caterina MJ et al. (2000) Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science 288, 306–313 [DOI] [PubMed] [Google Scholar]
- 65.Bautista DM et al. (2006) TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell 124, 1269–1282 [DOI] [PubMed] [Google Scholar]
- 66.Patapoutian A et al. (2009) Transient receptor potential channels: targeting pain at the source. Nat. Rev. Drug Discov 8, 55–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Moore C et al. (2018) Regulation of pain and itch by TRP channels. Neurosci. Bull 34, 120–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Diogenes A et al. (2011) LPS sensitizes TRPV1 via activation of TLR4 in trigeminal sensory neurons. J. Dent. Res 90, 759–764 [DOI] [PubMed] [Google Scholar]
- 69.Min H et al. (2018) Association of TRPV1 and TLR4 through the TIR domain potentiates TRPV1 activity by blocking activation-induced desensitization. Mol. Pain 14, 1744806918812636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chiu IM (2018) Infection, pain, and itch. Neurosci. Bull 34, 109–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chiu IM et al. (2013) Bacteria activate sensory neurons that modulate pain and inflammation. Nature 501, 52–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ruhl CR et al. (2020) Mycobacterium tuberculosis sulfolipid-1 activates nociceptive neurons and induces cough. Cell 181, 293–305.e11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Marion E et al. (2014) Mycobacterial toxin induces analgesia in Buruli ulcer by targeting the angiotensin pathways. Cell 157, 1565–1576 [DOI] [PubMed] [Google Scholar]
- 74.Shen S et al. (2017) Gut microbiota is critical for the induction of chemotherapy-induced pain. Nat. Neurosci 20, 1213–1216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liu XJ et al. (2016) TLR signaling adaptor protein MyD88 in primary sensory neurons contributes to persistent inflammatory and neuropathic pain and neuroinflammation. Sci. Rep 6, 28188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liu F et al. (2017) Suppression of MyD88-dependent signaling alleviates neuropathic pain induced by peripheral nerve injury in the rat. J. Neuroinflammation 14, 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kato G et al. (2009) Organization of intralaminar and translaminar neuronal connectivity in the superficial spinal dorsal horn. J. Neurosci 29, 5088–5099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Obata K and Noguchi K (2004) MAPK activation in nociceptive neurons and pain hypersensitivity. Life Sci 74, 2643–2653 [DOI] [PubMed] [Google Scholar]
- 79.Megat S et al. (2019) Nociceptor translational profiling reveals the Ragulator-Rag GTPase complex as a critical generator of neuropathic pain. J. Neurosci 39, 393–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ji RR et al. (2002) p38 MAPK activation by NGF in primary sensory neurons after inflammation increases TRPV1 levels and maintains heat hyperalgesia. Neuron 36, 57–68 [DOI] [PubMed] [Google Scholar]
- 81.Khoutorsky A and Price TJ (2018) Translational control mechanisms in persistent pain. Trends Neurosci 41, 100–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Andoh T et al. (1995) Paresthesia induced by cutaneous infection with herpes simplex virus in rats. Neurosci. Lett 190, 101–104 [DOI] [PubMed] [Google Scholar]
- 83.Fukuda J et al. (1983) Specific reduction in Na currents after infection with herpes simplex virus in cultured mammalian nerve cells. Brain Res 268, 367–371 [DOI] [PubMed] [Google Scholar]
- 84.Oakes SG et al. (1981) Electrophysiological changes of HSV-1-infected dorsal root ganglia neurons in culture. J. Neuropathol. Exp. Neurol 40, 380–389 [DOI] [PubMed] [Google Scholar]
- 85.Abraira VE and Ginty DD (2013) The sensory neurons of touch. Neuron 79, 618–639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pan H et al. (2019) Identification of a spinal circuit for mechanical and persistent spontaneous itch. Neuron 103, 1135–1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Costigan M et al. (2009) Neuropathic pain: a maladaptive response of the nervous system to damage. Annu. Rev. Neurosci 32, 1–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Braz J et al. (2014) Transmitting pain and itch messages: a contemporary view of the spinal cord circuits that generate gate control. Neuron 82, 522–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Duan B et al. (2018) Spinal circuits transmitting mechanical pain and itch. Neurosci. Bull 34, 186–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lu Y et al. (2013) A feed-forward spinal cord glycinergic neural circuit gates mechanical allodynia. J. Clin. Invest 123, 4050–4062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.LaMotte RH et al. (2013) Sensory neurons and circuits mediating itch. Nat. Rev. Neurosci 15, 19–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Imamachi N et al. (2009) TRPV1-expressing primary afferents generate behavioral responses to pruritogens via multiple mechanisms. Proc. Natl. Acad. Sci. U. S. A 106, 11330–11335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Han L et al. (2013) A subpopulation of nociceptors specifically linked to itch. Nat. Neurosci 16, 174–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Huang J et al. (2018) Circuit dissection of the role of somatostatin in itch and pain. Nat. Neurosci 21, 707–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Liu Q et al. (2009) Sensory neuron-specific GPCR Mrgprs are itch receptors mediating chloroquine-induced pruritus. Cell 139, 1353–1365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wilson SR et al. (2011) TRPA1 is required for histamine-independent, Mas-related G protein-coupled receptor-mediated itch. Nat. Neurosci 14, 595–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Han Q et al. (2018) miRNA-711 binds and activates TRPA1 extracellularly to evoke acute and chronic pruritus. Neuron 99, 449–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wilson SR et al. (2013) The ion channel TRPA1 is required for chronic itch. J. Neurosci 33, 9283–9294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Min H et al. (2014) TLR4 enhances histamine-mediated pruritus by potentiating TRPV1 activity. Mol. Brain 7, 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Liu T et al. (2016) Toll-like receptor 4 contributes to chronic itch, alloknesis, and spinal astrocyte activation in male mice. Pain 157, 806–817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bourane S et al. (2015) Gate control of mechanical itch by a subpopulation of spinal cord interneurons. Science 350, 550–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hu P et al. (2007) Immune cell involvement in dorsal root ganglia and spinal cord after chronic constriction or transection of the rat sciatic nerve. Brain Behav. Immun 21, 599–616 [DOI] [PubMed] [Google Scholar]
- 103.Meseguer V et al. (2014) TRPA1 channels mediate acute neurogenic inflammation and pain produced by bacterial endotoxins. Nat. Commun 5, 3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Woolf CJ and Costigan M (1999) Transcriptional and post-translational plasticity and the generation of inflammatory pain. Proc. Natl. Acad. Sci. U. S. A 96, 7723–7730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Obata K et al. (2005) TRPA1 induced in sensory neurons contributes to cold hyperalgesia after inflammation and nerve injury. J. Clin. Invest 115, 2393–2401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cohen JA et al. (2019) Cutaneous TRPV1+ neurons trigger protective innate type 17 anticipatory immunity. Cell 178, 919–932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lai NY et al. (2019) Gut-innervating nociceptor neurons regulate Peyer’s patch microfold cells and SFB levels to mediate Salmonella host defense. Cell 180, 33–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Green DP et al. (2019) A mast-cell-specific receptor mediates neurogenic inflammation and pain. Neuron 101, 412–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Talbot S et al. (2015) Silencing nociceptor neurons reduces allergic airway inflammation. Neuron 87, 341–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hokfelt T et al. (1994) Messenger plasticity in primary sensory neurons following axotomy and its functional implications. Trends Neurosci 17, 22–30 [DOI] [PubMed] [Google Scholar]
- 111.Zhang Q et al. (1995) Expression of pituitary adenylate cyclase-activating polypeptide in dorsal root ganglia following axotomy: time course and coexistence. Brain Res 705, 149–158 [DOI] [PubMed] [Google Scholar]
- 112.Xiao HS et al. (2002) Identification of gene expression profile of dorsal root ganglion in the rat peripheral axotomy model of neuropathic pain. Proc. Natl. Acad. Sci. U. S. A 99, 8360–8365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ji RR et al. (1994) Galanin antisense oligonucleotides reduce galanin levels in dorsal root ganglia and induce autotomy in rats after axotomy. Proc. Natl. Acad. Sci. U. S. A 91, 12540–12543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ossipov MH et al. (2002) Selective mediation of nerve injury-induced tactile hypersensitivity by neuropeptide Y. J. Neurosci 22, 9858–9867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Burnet F (1959) The Clonal Selection Theory of Acquired Immunity, Vanderbilt University Press [Google Scholar]
- 116.Janeway CA Jr, (1989) Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb. Symp. Quant. Biol 54, 1–13 [DOI] [PubMed] [Google Scholar]
- 117.Matzinger P (1994) Tolerance, danger, and the extended family. Annu. Rev. Immunol 12, 991–1045 [DOI] [PubMed] [Google Scholar]
- 118.Boller T and Felix G (2009) A renaissance of elicitors: perception of microbe-associated molecular patterns and danger signals by pattern-recognition receptors. Annu. Rev. Plant Biol 60, 379–406 [DOI] [PubMed] [Google Scholar]
- 119.Kawasaki Y et al. (2008) Distinct roles of matrix metalloproteases in the early- and late-phase development of neuropathic pain. Nat. Med 14, 331–336 [DOI] [PMC free article] [PubMed] [Google Scholar]