Abstract
Background and Purpose:
Neurological and neurodegenerative diseases can affect the spinal cord (SC) of pediatric patients. MRI allows for in-vivo quantification of SC atrophy via cross-sectional area (CSA). The study of CSA values in the general population is important to disentangle disease-related changes from inter-subject variability. This study aimed at providing normative values for cervical CSA in children, extending our previous work performed with adults.
Methods:
Seventy-eight children (age 7–17 years) were selected from a Developmental Dyslexia study. All subjects underwent a 3T brain MRI session and any incidental findings were reported on the scans. A sagittal 1 mm3 3D T1-weighted brain acquisition extended to the upper cervical cord was used to measure CSA at C2-C3, as well as spinal canal area and skull volume (V-scale). These three metrics were linearly fitted as a function of age to extract trends and percentage annual changes. Sex differences of CSA were assessed using least squares regression analyses, adjusting for age. We tested normalization strategies proven to be effective in reducing the inter-subject variability of adults’ CSA.
Results:
CSA changed as a function of age at a faster rate when compared with skull volume (CSA: 1.82% increase, V-scale: 0.60% reduction). Sex had a statistically significant effect on CSA. Normalization methods based on canal area and skull volume reduced the CSA inter-subject variability up to 16.84%.
Conclusions:
We present CSA normative values in a large cohort of children, reporting on sources of inter-subject variability and how to reduce them applying normalization methods previously developed.
Keywords: Spinal cord, pediatric, magnetic resonance imaging, inter-subject variability, normalization strategies
INTRODUCTION
Multiple sclerosis (MS), Neuromyelitis optica (NMO), and amyotrophic lateral sclerosis (ALS) are neurodegenerative disorders of the central nervous system (CNS) that target the spinal cord (SC), causing severe disability in most patients. The SC can be affected by local inflammation, demyelination (MS, NMO), necrosis (NMO), or focal neurodegeneration (ALS).1–4 A common consequence of these different neuropathological mechanisms is SC atrophy, involving both gray (GM) and white matter (WM).5 In MS, it has been shown that SC atrophy is present from the very first stage of the disease and strongly correlates with motor disability.6
Recent advancements in MRI technology have allowed for the acquisition of high-resolution spinal cord MR images at cervical and thoracic levels. In particular, T2*-weighted and phase sensitive inversion recovery (PSIR) sequences have been developed and used to quantitatively assess GM/WM damage in neurodegenerative diseases of the CNS.7,8 In the absence of cutting-edge SC dedicated acquisitions, spinal cord atrophy at the upper cervical levels (defined in terms of cross-sectional area -CSA-) can be assessed using routinely acquired T1-weighted brain scans that include the upper cervical cord.9
To address the variability due to subject demographic and anatomical characteristics, it is crucial to have normative values of cord areas in the general population. This allows for disentanglement of differences in SC areas due to inter-subject variability from disease-related changes.
Thanks to the reliability of our 2D PSIR-based acquisition/measurement method, we could recently assess SC cervical CSA and GM areas at the C2-C3 intervertebral disc level in 129 adult healthy subjects (mean age: 41.0 ± 15.9, range: 19–79).10 We explored the influence of age and sex on SC areas, finding a stronger influence of sex than age-related decline in cord areas. We also developed and tested a series of easily implementable normalization strategies to reduce inter-subject variability in cervical cord areas.
Our previous study and others have been focused on healthy, adult subjects, whereas only very few MRI studies, with limited number of subjects, have been performed on healthy subjects younger than 18 years of age.11,12 Since many neurological and neurodegenerative diseases can affect the spinal cord of young people, we decided to extend our previous work, focusing the present study on the analysis of cervical spinal cord in young subjects.
The aim of this study was twofold:
Establish normative values/age trends for subjects younger than 18 years of age;
Verify in a younger cohort the effectiveness of the normalization strategies previously developed on adults.
METHODS
Subjects and Image Acquisition
Subjects were selected from the large, pediatric cohort at the Dyslexia Center of the University of California, San Francisco (UCSF). Inclusion criteria were: 1) age under 18 years; 2) available brain/upper cervical cord 3T MRI on a single scanner; 3) absence of incidental brain/spinal cord findings on the MRI scans. Diagnoses were made by a multidisciplinary team at the UCSF Dyslexia Center after extensive behavioral testing as previously described.13 The MRI scans were reviewed by a skilled neuroradiologist (EC with 15 years of expertise in neuroimaging).
The Committee on Human Research at UCSF approved the study protocol. Written informed consent and assent was obtained from all child participants and their parents.
Seventy-eight children (38 females, 40 males) between 7 and 17 years of age (mean age = 10.8) met eligibility criteria and were included in the study. All participants underwent MRI scanning on a Siemens 3T Prisma scanner equipped with a 64-channel head-neck coil and a 32-channel spine coil. A standard high resolution T1-weighted image of the brain (sagittal 3D-MPRAGE, 1 mm3 resolution, parallel imaging acceleration factor (iPAT) = 2, acquisition time ~5:30 min) with a large FOV extended to include the upper cervical cord was acquired for each subject, as part of the ongoing research MRI protocol. Importantly, the T1-weighted acquisition was implemented with 3D gradient nonlinearity correction applied, an acquisition correction that has been shown to be necessary to allow reliable CSA assessment at the cervical SC levels from brain scans.9
Image Analyses
CSA measurements and spinal normalizing metrics were obtained using the software Jim (version 7.0, Xinapse Systems Ltd, West Bergholt, United Kingdom; http://www.xinapse.com). To compute CSA and canal measurements analogously to previous work, the sagittal MPRAGE was resampled on the axial plane creating a packet of five contiguous slices 1 mm thick centered at the C2-C3 intervertebral disc.9,10 CSA was measured semi-automatically on these five slices and the average value computed, using the Jim “cord finder” toolkit with pre-established settings (nominal cord diameter 8 mm, number of shape coefficients 24, order of longitudinal variation 12), after manual marker placement at the cord center of each of the five slices.
To be used for the inter-subject normalizing methods, a trained operator (CA) manually measured the maximum anterior-posterior diameter and lateral diameter of the vertebral canal on the central slice of the 5-slice-packet. The product of the two diameters was computed as previously described to get the “axial_canal_product” metric.10 V-scale was obtained using SienaX (part of FSL).14
Correlations among CSA, V-scale and axial_canal_product and age/sex influence on the metrics
All statistical analyses were performed using JMP Pro 14.1.0 (SAS Institute, Cary, NC; www.jmp.com).
Pearson product-moment coefficients were computed, to assess the correlations among CSA, V-scale and axial_canal_product.
CSA, V-scale and axial_canal_product were plotted against age and a linear fit was performed to extract age trends and percentage annual changes. Even though polynomial fits may be a better choice, the number of subjects and the limited age range explored in the present study are not sufficient to go beyond linear approximations. For CSA, however, a linear fit to data in the explored age range is a reasonable choice since, according to our previous study on adults, CSA seems to increase until approximately 45 years of age and then begins to decrease.
Sex differences between CSA measures were assessed using least squares regression analyses and adjusted for age.
Test of Normalization strategies
The three best normalization strategies developed and tested in our previous work on adults were applied to the CSA values extracted for the 78 children.10
In a general form:
| Eq.1 |
where CSAimeas is the measured SC area in a given subject i, CSAipred is the resulting normalized area, a,b,c, … are the regression coefficients derived from the fits, X mean, Y mean, Z mean, … are the mean values of the skull/cord/demographic metrics of the 78 subjects group and Xi meas, Yi meas, Zi meas, … are their measured values in the subject i. The specific coefficient/metric combinations for the three different models are reported in Table 1.
Table 1.
Multi-linear regression analysis of C2-C3 CSA.
| CSA | V-scale | axial_canal_product | sex | %COV | %COVmeas | |
|---|---|---|---|---|---|---|
| Model1 | −24.15 | - | - | 8.69% | ||
| Model2 | - | 0.0807 | 3.1936 | 8.49% | ||
| Model3 | −16.76 | 0.0715 | - | 8.20% | ||
Columns 2/4 report the normalization coefficients previously derived on adults and used on the 78 children in multi-linear regression analysis with C2-C3 cross-sectional area (CSA) as outcome variables and sex, V-scale and axial_canal_product as independent variables (see Eq.1 in the text). The %COV (ratio of the group standard deviation and the respective means expressed as percentage) for the calculated values with each model for the group of 78 children are reported in column 5. %COVmeas refers to the non-normalized measured data. In the 5th column, in parenthesis, the % reduction obtained going from measured to normalized data ( 100 (%COVmeas - %COV) / %COVmeas ) is reported for each model.
Mean values of the independent variables on the total cohort of 78 subjects:
V-scalemean = 1.436
axial_canal_productmean = 252.22 mm2
The effect of the normalization by a particular selected model was evaluated by comparing the coefficient of variation (%COV, ratio of the group standard deviation and the respective means expressed as percentage) between the measured data and the values generated by the respective correction formulas.10,15,16
RESULTS
Correlations among CSA, V-scale and axial_canal_product and age/sex influence on the metrics
Pearson’s r coefficient between CSA and V-scale was −0.47 (p-value < 0.0001), between CSA and axial_canal_product was 0.43 (p-value < 0.0001), while between V-scale and axial_canal_product was −0.32 (p-value = 0.0048).
Figure 1 reports CSA, V-scale and axial_canal_product plotted against age, superimposed with the linear fit equation performed to extract percentage annual changes.
Figure 1.
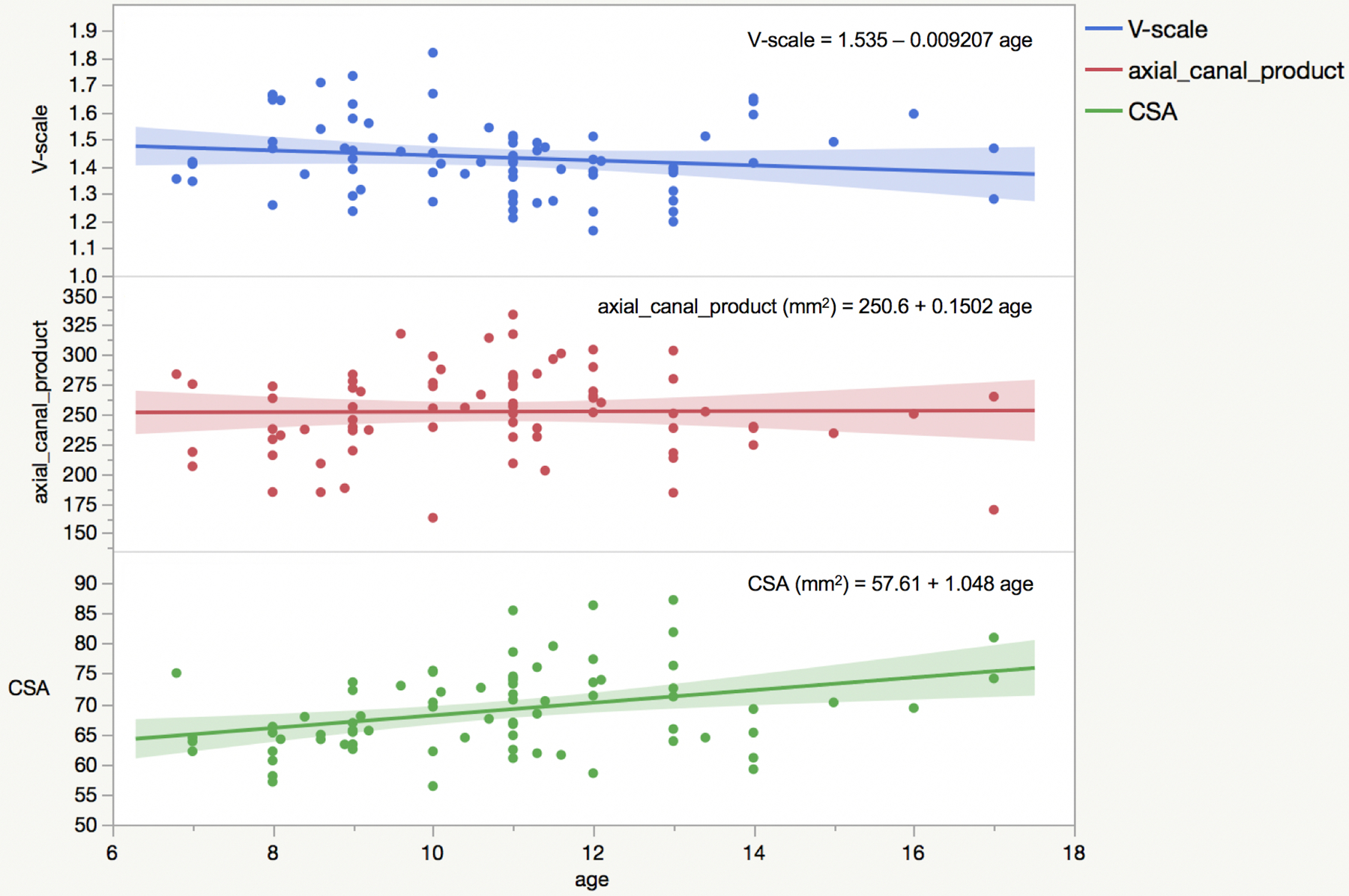
V-scale, axial_canal_product and cross-sectional area (CSA) variation with age with linear fits to the data and relative equations superimposed.
CSA and axial_canal_product increased with age, with annual % changes that were, respectively, 1.82% and 0.06%. V-scale decreased at a 0.60% annual rate (meaning the skull volume increased).
When sex was used in a least squares regression analysis, adjusting for age, males had an average CSA of 70.98 mm2, while females of 66.73 mm2 (p-value=0.0029).
Linear fits of the whole dataset in function of age provided the equations reported in Figure 1.
For CSA, the equations obtained with linear fits of the separate data for each sex were:
| Eq.2 |
| Eq.3 |
According to these equations, males had a CSA 4.16% bigger than females at 7 years of age, and 9.64% at 17 years.
The extrapolation of CSA to 20 years with the linear trend reported in Figure 1 gives 78.6 mm2. This is in line with an average area of about 80 mm2 as detected in the previous study for CSA in the 20–25 years range.10 Although different acquisition protocols were used in the two studies, we previously showed that, using Jim, they provide comparable CSA estimates.8
Test of Normalization strategies
The coefficient used in Eq.1 for the three normalization strategies (coefficient previously developed in the adult cohort of 129 subjects) and their effect (as %COV, ratio of the group standard deviation and the respective means expressed as percentage) are reported in Table 1.10
Consistent with what was found in the previous study, the most effective normalization strategy was the one using both the skull volume (V-scale) and canal dimension (Model3).10 The effect of the normalization with Model3 on CSA is reported in Figure 2 (CSA vs age stratified by sex).
Figure 2.
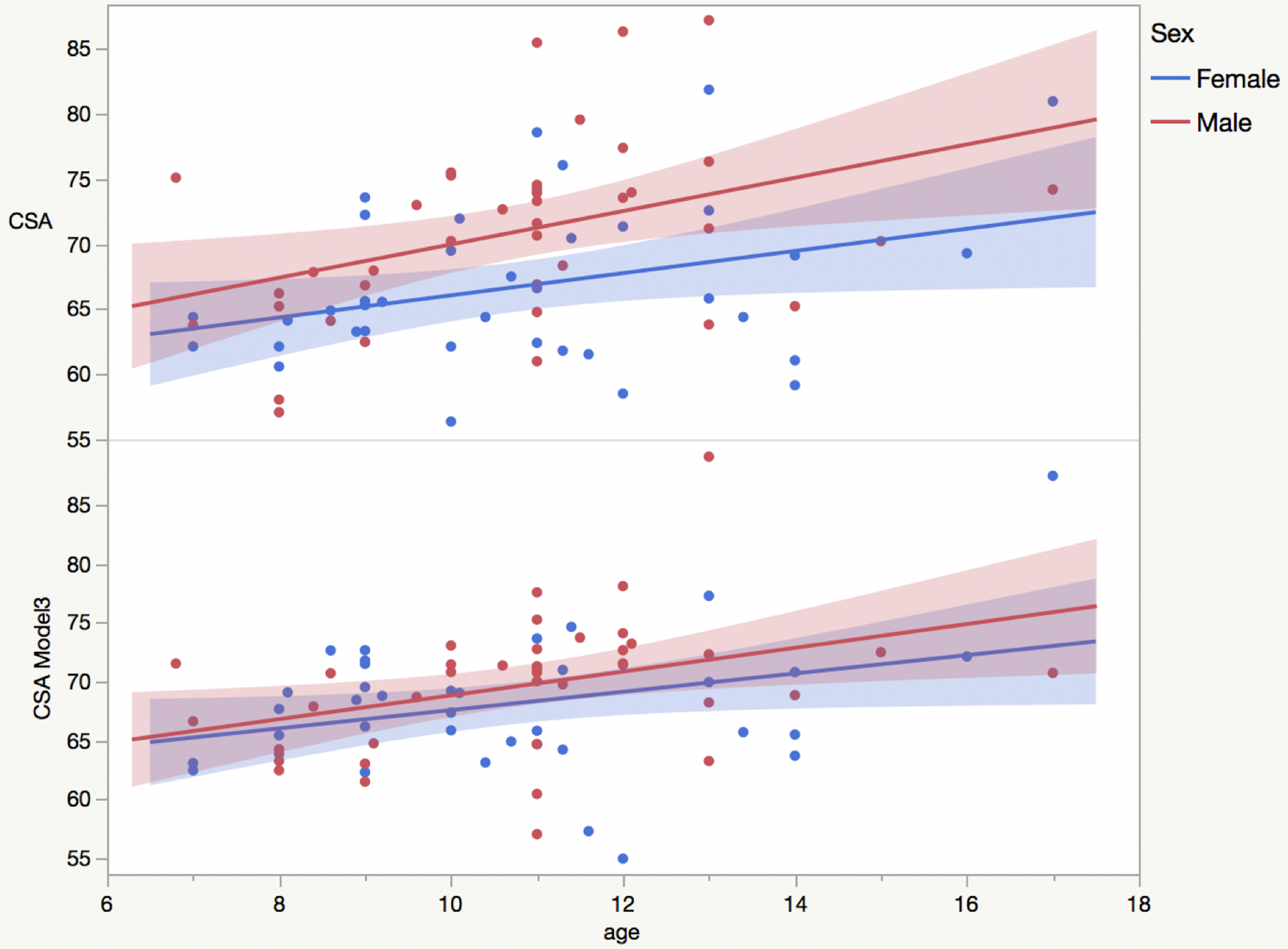
Raw cross-sectional area (CSA) and CSA normalized using Model3 as a function of age (stratified by sex: women (blue), men (red)) with linear fits to the data superimposed.
DISCUSSION
We recently studied the variability of SC areas with age and sex in 129 healthy, adult subjects, testing and developing normalization strategies to reduce the variability of these measures due to anatomical/biological differences.10 In the present study we extended the work to 78 children between 7 and 17 years of age.
The first interesting finding was observing that correlations among CSA, V-scale and canal dimensions (assessed as Pearson’s coefficients) in children were almost identical to what has been previously observed in adults. Strong correlations of CSA and skull volumes were reported in previous studies on healthy controls and MS patients.17,18,19 Our findings confirm that skull volume and V-scale are good candidates to be used as normalization factors for CSA.
While the fact that we detected correlations of similar magnitude in adults and children may suggest that CSA, skull volumes and canal area have a certain intrinsic proportionality throughout the lifespan in absence of degenerative processes, CSA during childhood seems to change at a rate faster than skull volume (1.82% for CSA vs 0.60% for V-scale), according to our data. Canal area, instead, seems to reach a maximum before 20 years. Indeed, its annual % increase was found to be very small in the age range included in this study (0.06% annual increase). This finding is in line with a pediatric computed tomography (CT) study in which the spinal canal diameter was found not to increase substantially after 4 years of age.20
The %COV, the respective mean for CSA and the sex differences in children are also similar to adults.
CSA was found to have a statistically significant larger value in males than females. This is consistent with what has been previously found in adults, including one of the few MRI studies where, instead of CSA, the authors measured the anterior-posterior and transverse diameters of the cord in healthy Chinese adolescents.10,11
Concerning the normalization strategies, the same equation derived in adults worked with a similar effect size on children in reducing their CSA inter-subject variability. Model3, using both V-scale and spinal canal dimensions, was the most effective model in children, as previously found in the adult cohort.
This study has some limitations. First, the subjects were selected from a Developmental Dyslexia (DD) study cohort. Nevertheless, DD is not expected to affect neither the spinal cord/canal area, nor the skull volume, and the selected subjects did not show any brain MRI abnormalities on conventional MRI. The second limitation is related to the use of standard brain T1-weighted images, which allowed us to only assess CSA, without being able to differentiate between GM and WM SC tissues.
Despite these limitations, this large cohort MRI study on a pediatric population will provide a helpful normative reference for future studies attempting to evaluate disease-related spinal cord area/volumetric changes in children and adolescents.
Acknowledgements and Disclosure:
The authors want to thank the participants to the study and their families.
This research was supported by the Charles and Helen Schwab Foundation.
MLGT was supported by the National Institute of Health (NINDS R01 NS050915; NIDCD K24 DC015544; NIA P01 AG019724; NIA P50 AG023501).
Footnotes
The authors have no competing interest related to the study.
REFERENCES
- 1.Bakshi R, Dandamudi VS, Neema M, et al. Measurement of brain and spinal cord atrophy by magnetic resonance imaging as a tool to monitor multiple sclerosis. J Neuroimaging 2005;15:30s–45s. [DOI] [PubMed] [Google Scholar]
- 2.Furby J, Hayton T, Anderson V, et al. Magnetic resonance imaging measures of brain and spinal cord atrophy correlate with clinical impairment in secondary progressive multiple sclerosis. Mult Scler 2008;14:1068–75. [DOI] [PubMed] [Google Scholar]
- 3.Olney NT, Bischof A, Rosen H, et al. Measurement of spinal cord atrophy using phase sensitive inversion recovery (PSIR) imaging in motor neuron disease. PLoS One 2018;13:e0208255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.El Mendili MM, Cohen-Adad J, Pelegrini-Issac M, et al. Multi-parametric spinal cord MRI as potential progression marker in amyotrophic lateral sclerosis. PLoS One 2014;9:e95516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schlaeger R, Papinutto N, Panara V, et al. Spinal cord gray matter atrophy correlates with multiple sclerosis disability. Ann Neurol 2014;76:568–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schlaeger R, Papinutto N, Zhu A, et al. Atrophy of spinal cord gray matter is detectable at an early stage of multiple sclerosis. Neurology 2017;88(16 Supplement):S2.003. [Google Scholar]
- 7.Yiannakas MC, Kearney H, Samson RS, et al. Feasibility of grey matter and white matter segmentation of the upper cervical cord in vivo: a pilot study with application to magnetisation transfer measurements. Neuroimage 2012;63:1054–9. [DOI] [PubMed] [Google Scholar]
- 8.Papinutto N, Henry RG. Evaluation of intra- and interscanner reliability of MRI protocols for spinal cord gray matter and total cross-sectional area measurements. J Magn Reson Imaging 2019;49:1078–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Papinutto N, Bakshi R, Bischof A, et al. Gradient nonlinearity effects on upper cervical spinal cord area measurement from 3D T1-weighted brain MRI acquisitions. Magn Reson Med 2018;79:1595–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Papinutto N, Asteggiano C, Bischof A, et al. Intersubject Variability and Normalization Strategies for Spinal Cord Total Cross-Sectional and Gray Matter Areas. J Neuroimaging 2020;30:110–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lao LF, Chen ZG, Qiu GX, et al. Whole-spine magnetic resonance imaging study in healthy Chinese adolescents. Orthop Surg 2013;5:164–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Isik M, Yavuz M. Analysis of MRI morphometric parameters of the pediatric cervical spine and spinal cord. J Turk Spinal Surg 2019;30:157–61 [Google Scholar]
- 13.Caverzasi E, Mandelli ML, Hoeft F, et al. Abnormal age-related cortical folding and neurite morphology in children with developmental dyslexia. Neuroimage Clin 2018;18:814–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith SM, Zhang Y, Jenkinson M, et al. Accurate, robust, and automated longitudinal and cross-sectional brain change analysis. Neuroimage 2002;17:479–89. [DOI] [PubMed] [Google Scholar]
- 15.Papinutto N, Schlaeger R, Panara V, et al. Age, gender and normalization covariates for spinal cord gray matter and total cross-sectional areas at cervical and thoracic levels: a 2D phase sensitive inversion recovery imaging study. PLoS One 2015;10:e0118576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yiannakas MC, Liechti MD, Budtarad N, et al. Gray vs. white matter segmentation of the conus medullaris: reliability and variability in healthy volunteers. J Neuroimaging 2019;29:410–7. [DOI] [PubMed] [Google Scholar]
- 17.Rashid W, Davies GR, Chard DT, et al. Upper cervical cord area in early relapsing-remitting multiple sclerosis: cross-sectional study of factors influencing cord size. J Magn Reson Imaging 2006;23:473–6. [DOI] [PubMed] [Google Scholar]
- 18.Mann RS, Constantinescu CS, Tench CR. Upper cervical spinal cord cross-sectional area in relapsing remitting multiple sclerosis: application of a new technique for measuring cross-sectional area on magnetic resonance images. J Magn Reson Imaging 2007;26:61–5. [DOI] [PubMed] [Google Scholar]
- 19.Engl C, Schmidt P, Arsic M, et al. Brain size and white matter content of cerebrospinal tracts determine the upper cervical cord area: evidence from structural brain MRI. Neuroradiology 2013;55:963–70. [DOI] [PubMed] [Google Scholar]
- 20.Johnson KT, Al-Holou WN, Anderson RC, et al. Morphometric analysis of the developing pediatric cervical spine. J Neurosurg Pediatr 2016;18:377–89. [DOI] [PubMed] [Google Scholar]


