Abstract
Chronic pain remains challenging to both diagnose and treat. These challenges, in part, arise from limited systems-level understanding of the basic mechanisms that process nociceptive information and ultimately instantiate a subjectively available experience of pain. Here I provide a framework – the Distributed Nociceptive System – for understanding nociceptive mechanisms at a systems level by integrating the concepts of neural population coding with distributed processing. Within this framework, widespread engagement of populations of neurons produces representations of nociceptive information that are highly resilient to disruption. The Distributed Nociceptive System provides a foundation for understanding complex spatial aspects of chronic pain and provides an impetus for non-pharmacological cognitive and physical therapies that can effectively target the highly distributed system that gives rise to an experience of pain.
Keywords: Nociception, pain, recruitment, bilateral, population coding, biomarkers
The Need for a Systems-Level Framework for Understanding Pain Mechanisms
Chronic pain remains a tremendous problem world-wide. Patients suffer tremendously from dismissal of their symptoms and uncertainty about their diagnoses [1], and once properly diagnosed, frequently receive pharmacologic treatments with limited efficacy [2]. Many of the challenges of treating pain arise from our limited understanding of central nervous system mechanisms that process nociceptive information into a subjectively available experience of pain. Current reductionistic approaches focusing on single neurons, single molecules, or single brain regions have only provided incomplete explanations for the complex symptoms of pain and have been largely ineffective in the development of new treatments. Conversely, many therapies for chronic pain that potentially target multiple central nervous system mechanisms and that are often efficacious, such as cognitive behavioral therapy (CBT) and physical therapy, are underutilized and under-reimbursed due to limited understanding of how they work.
A systems-level framework for understanding pain is urgently needed. Here, I integrate two neglected concepts - population coding [3] and distributed processing [4] - to provide a framework for understanding nociceptive mechanisms across the spinal cord and the brain. I refer to this framework as the Distributed Nociceptive System. The central tenet of this framework is that the extraction and utilization of nociceptive information is a process that can be accomplished separately and largely independently by multiple sites within the central nervous system. As such, processing of nociceptive information can occur in a highly distributed fashion, yielding a system that is very resistant to disruption (Fig. 1).
FIGURE 1. Distributed versus Serial Processing of Information.
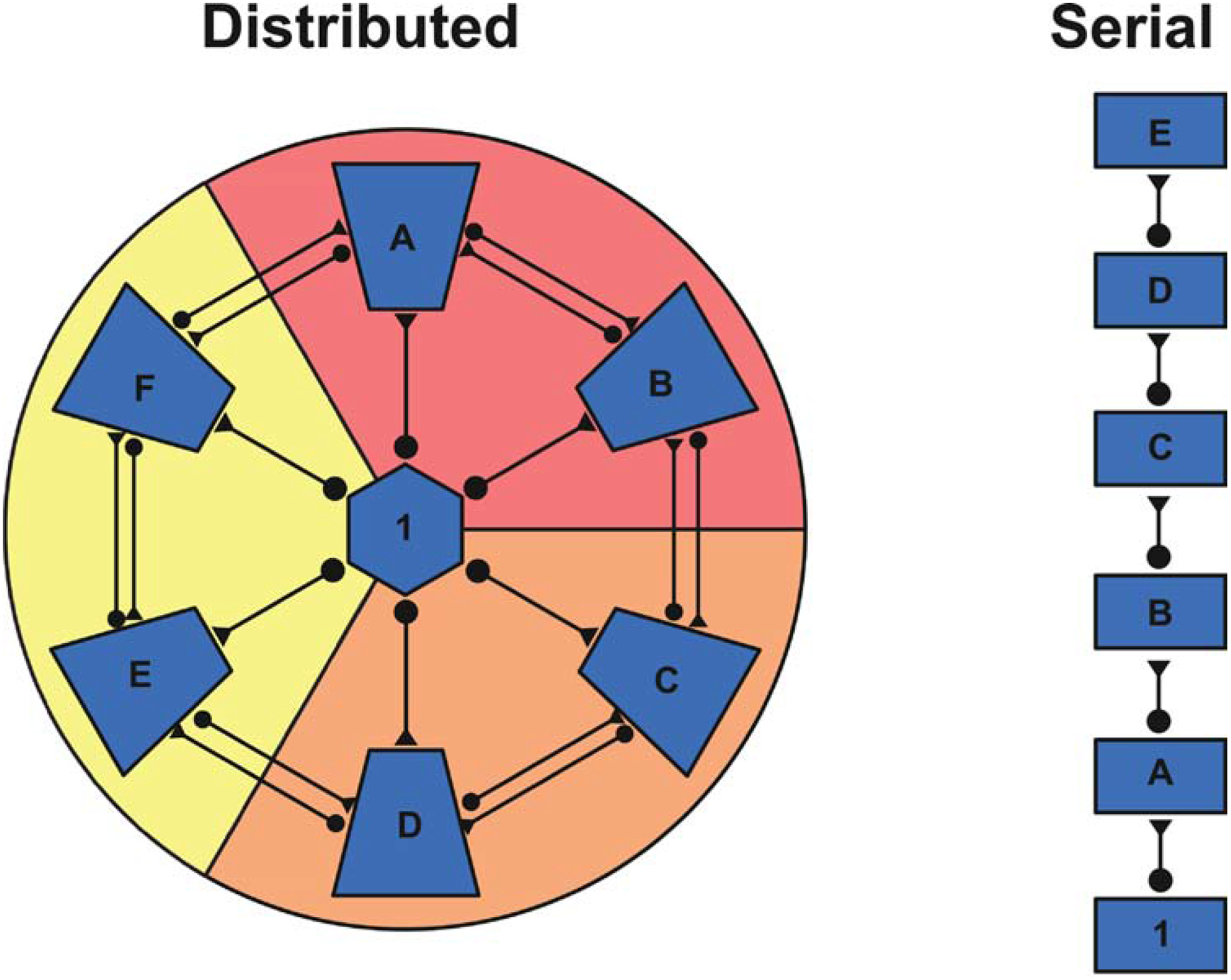
Distributed processing of information can produce a system that is highly resilient to injury whereas serial organizations are significantly more susceptible to disruption. For example, in the distributed system, information flowing from region 1 reaches areas A-F via parallel pathways. In contrast, information flowing from region 1 sequentially needs to pass through all areas prior to reaching area E. Thus, damage to region B would result in minimal disruption of information flow in the distributed organization, while information processing in the serial system would be significantly impacted. Furthermore, a distributed system lends itself to the processing of relatively degenerate information. For example, the modules denoted in red, orange, and yellow could instantiate a representation of information independently of one another. In contrast, serial systems may be crucial when extracting complex features from incoming information.
Distributed Processing of Nociceptive Information within the Spinal Cord
The earliest stage of nociceptive processing within the central nervous system occurs within the spinal cord. Investigations of spinal cord transected animals as well as humans indicate that the spinal cord by itself has tremendous capacity to encode multiple dimensions of sensory features and to formulate complex motor responses to noxious stimuli. The isolated spinal cord can encode noxious stimulus intensity [5, 6], stimulus location [7], and can formulate dynamic withdrawal responses to widespread, spatially complex nociceptive stimuli [7].
Distribution of Nociceptive Processing across Multiple Laminae
The complex processing of nociceptive input is distributed across multiple anatomical regions of the spinal cord. When viewed as a cross section, the spinal cord grey matter has multiple anatomically and functionally distinct laminae encompassing the dorsal and ventral horns [8]. Traditionally, the dorsal horns have been viewed as being engaged in sensory processing, whereas the ventral horns as engaged in motor processing. In addition, most of the ascending nociceptive input has been ascribed to neurons within laminae I and V ipsilateral to the stimulated body regions. In sharp contrast with these traditional views, afferent nociceptive processing is distributed across multiple spinal laminae of both the dorsal and ventral horns. Nociceptive neurons with ascending projections have been identified in the superficial dorsal horn (lamina I) [9], deep dorsal horn (laminae V, VI) [9], ventral horn (lamina VII) [10] of primates, and central gray (lamina X) of other species [11].
Within these laminae, there are two types of nociceptive neurons – wide dynamic range (WDR) neurons [12] (Fig. 2) and nociceptive specific (NS) neurons [13]. Both types of neurons respond vigorously to stimuli that would be sufficient to elicit tissue damage. However, NS neurons exhibit minimal responses to innocuous stimuli (with some exceptions for innocuous cool stimuli), while WDR neurons respond robustly to innocuous tactile stimuli. Multiple lines of evidence indicate that WDR neurons are capable of encoding noxious stimulus intensity [6, 14]. However, when thought about as single neurons, the responsiveness of WDR neurons to innocuous tactile stimuli has long remained difficult to reconcile with their roles in the encoding of noxious stimulus intensity, and represented a topic of intense and bitter controversy. The resolution of this controversy, one could argue, relies not on considering single WDR neurons in isolation, but thinking about how populations of these neurons work together to encode the distinction between noxious and innocuous stimuli, as well as noxious stimulus intensity [9]. The complex receptive field organization of these WDR neurons provides a critical substrate for a population coding mechanism (Fig. 2). Given this organization, neuron recruitment within the population would be predicted to represent a key dimension of nociceptive coding.
FIGURE 2. Wide Dynamic Range (WDR) Receptive Field Organization: An Architecture for Neural Recruitment and Population Coding.
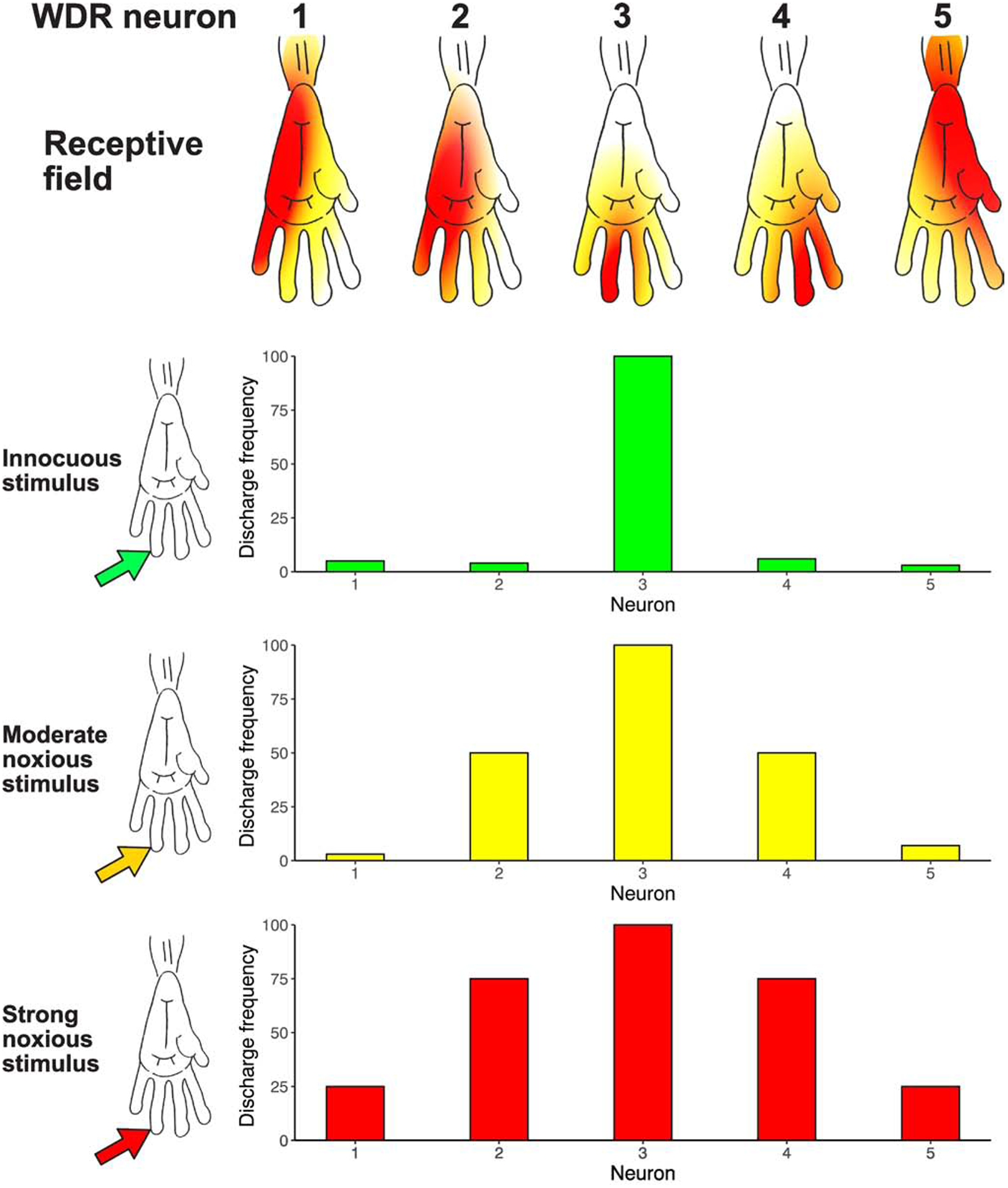
WDR neurons have a complex center/surround receptive field organization [9], and respond to both noxious stimuli as well as light touch in spatially distinct portions of their receptive fields. In this conceptual figure of neurons in the monkey spinal cord, the central receptive field zone (red) of each neuron is responsive to both noxious and innocuous stimuli, while the peripheral surround zone (orange to yellow) is responsive to only noxious stimuli. Moreover, the peripheral surround zone has gradients of sensitivity, such that increasingly intense noxious stimuli are necessary to activate progressively peripheral regions of the surround excitatory receptive field. Given that WDR neurons have relatively large surround receptive fields - frequently encompassing an entire limb or body quadrant - there is likely a substantial overlap of these large surround fields on any given region of the body surface. Accordingly, an innocuous noxious stimulus (green arrow) would recruit a relatively small portion of the population of spinal WDR neurons due to the relatively small receptive field sizes of the central receptive field zone (WDR Neuron #3, red receptive field zone). In contrast, progressively intense noxious stimuli (yellow arrow, red arrow) would be predicted to activate progressively larger portions of the WDR population. Neurons whose central receptive field zones were located within the stimulated area would be activated (WDR Neuron #3), but importantly, neurons whose excitatory surround receptive fields were stimulated would be recruited as well (WDR Neurons #2 and #4 with a moderate noxious stimulus [orange receptive field zone], and then WDR Neurons #1 and 5 with intense noxious stimulus [yellow receptive field zone]). Thus, while single WDR neurons cannot provide sufficient information to distinguish a noxious from an innocuous stimulus, populations of these neurons acting in concert can provide sufficient information to support this distinction [9]. More importantly, noxious stimulus intensity can be encoded by progressive recruitment of increasing numbers of WDR neurons. Portions of this figure are adapted from [82], with permission.
To further determine if neuron recruitment can contribute to sensory aspects of pain, responses in humans undergoing percutaneous electrical stimulation of the anterolateral quadrant of the spinal cord in preparation for a cordotomy were examined [15, 16]. This region of the spinal cord white matter contains axons from spinal neurons that project supraspinally. Increasing stimulus intensities caused a shift of sensations from non-painful to painful as the higher voltages recruited greater numbers of axons. Moreover, numbers of neurons may be traded off against frequency of discharge, such that progressively higher increases in stimulus frequency could elicit shifts from non-painful to painful sensations at relatively low voltages. As such, both the numbers of neurons active, as well as their discharge frequencies, are key components of nociceptive coding and, importantly, may contribute to sensory perceptions.
Spinal Neuron Recruitment and the Coding of Pain
The prediction of differential neural recruitment was borne out in early, autoradiographic, functional imaging studies in the rat spinal cord [16, 17] (Fig. 3). Progressive increases in noxious stimulus intensity applied to the distal hindpaw produced progressive increases in spinal cord activation (Fig. 3A). Low stimulus intensities (45°C) activated the segment L4 in the somatotopic epicenter. As noxious stimulus intensities increased (49°C), activation extended from L2 to L5. In contrast to noxious stimulation, innocuous brushing produced minimal recruitment of activation – restricted to L4. (Fig. 3B).
FIGURE 3. Spinal Cord Recruitment of Activation in Models of Acute and Chronic Pain.
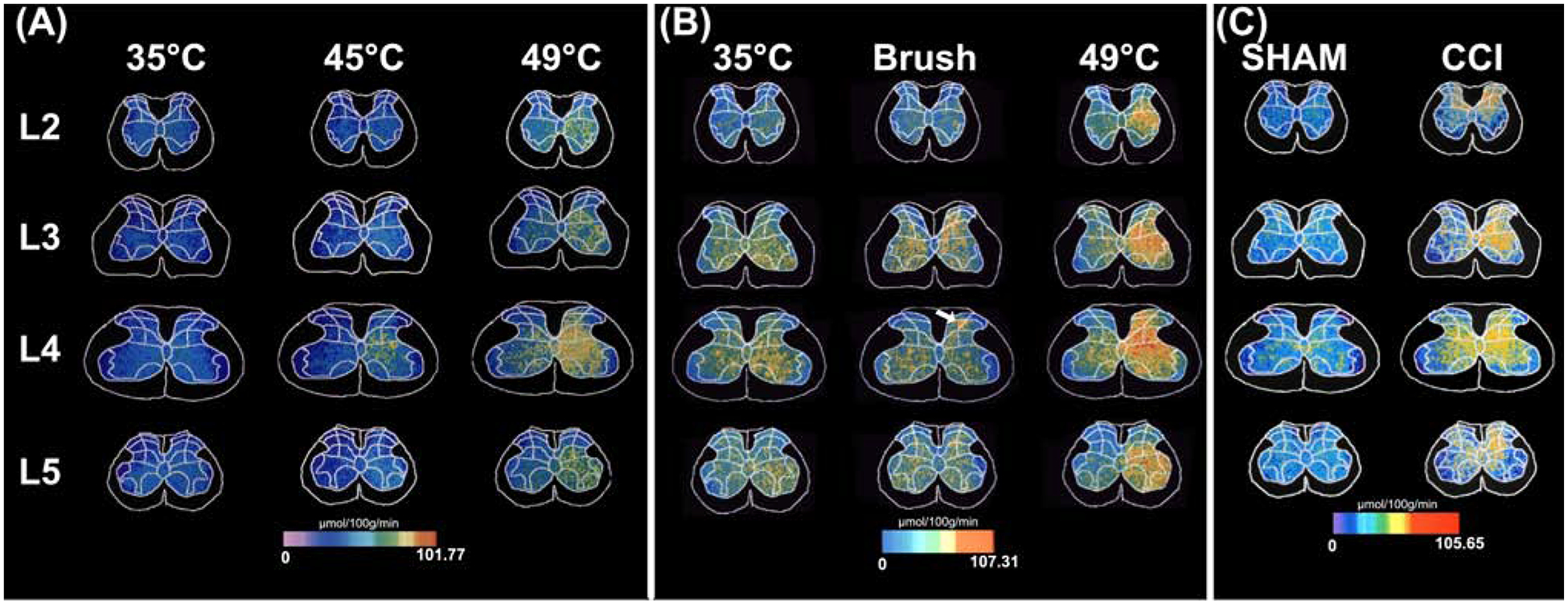
Activation of the rat spinal cord during noxious input was quantified using the 14C-2-deoxyglucose autoradiographic method. This tracer gets taken up by cells within the spinal cord in proportion to glucose utilization, and as such, can provide a marker for neural activity. A. Graded thermal stimuli were applied to the distal hind paw of the rat [17]. As stimulus temperatures increased from neutral (35°C), to mildly noxious (45°C) and to intensely noxious (49°C), graded increases in activity were noted ipsilaterally (right side of the images) within L4, the somatotopic focus of distal hind paw. These increases were located in the superficial dorsal horn, deep dorsal horn, as well as the ventral horn. During intensely noxious stimulation, activation extended far outside of L4, such that significant increases ranged ipsilaterally from L2 through L5, and contralaterally within the deep dorsal horn and ventral horn of L4. This finding confirms predictions of neuron recruitment during graded noxious stimulation inferred from receptive field properties of WDR neurons [9]. B. Vigorous, yet innocuous, brushing of the distal hind paw produced highly focal activation within the intermediate dorsal horn (arrow). This activation was spatially restricted in comparison with the substantial rostral-caudal recruitment of activation evoked by intensely noxious (49°C) stimulation [83]. This finding confirms predictions that noxious stimuli recruit far more neural activity than innocuous stimuli, and further supports the theory that populations of WDR neurons can encode the distinction between noxious and innocuous stimuli [9]. C. Following chronic constriction injury (CCI) of the sciatic nerve - a rat model of neuropathic pain - spinal activation increased dramatically relative to sham operated controls [18]. CCI-related activation extended rostro-caudally from L2 through L5. Moreover, substantial contralateral activation was detected. This extensive ipsilateral and contralateral spread of activity may give rise to the extensive radiation of pain that frequently occurs during complex regional pain syndrome in humans [22]. Modified from [17, 18, 83], with permission.
In addition to the unilateral rostro-caudal recruitment of activation with increasing noxious stimulus intensities, activation also increased contralaterally in the deep dorsal horn and ventral horn [17]. (Fig. 3). These regions have neurons with bilateral receptive fields that ascend to the brain [10]. Imaging studies in rats with a model of nerve injury pain reveal a tremendous recruitment of spinal activation, with extensive rostro-caudal spread, but importantly, significant bilateral involvement [18]. (Fig. 3C). At an anatomic level, recruitment of activation may be supported by both extensive rostro-caudal arborization of primary nociceptive afferents as well as by wide-ranging propriospinal interconnections that extend both rostro-caudally as well as contralaterally (Fig. 4).
Figure 4. Schematic Illustration of Anatomic Substrates Supporting Spinal Distribution of Nociceptive Input.
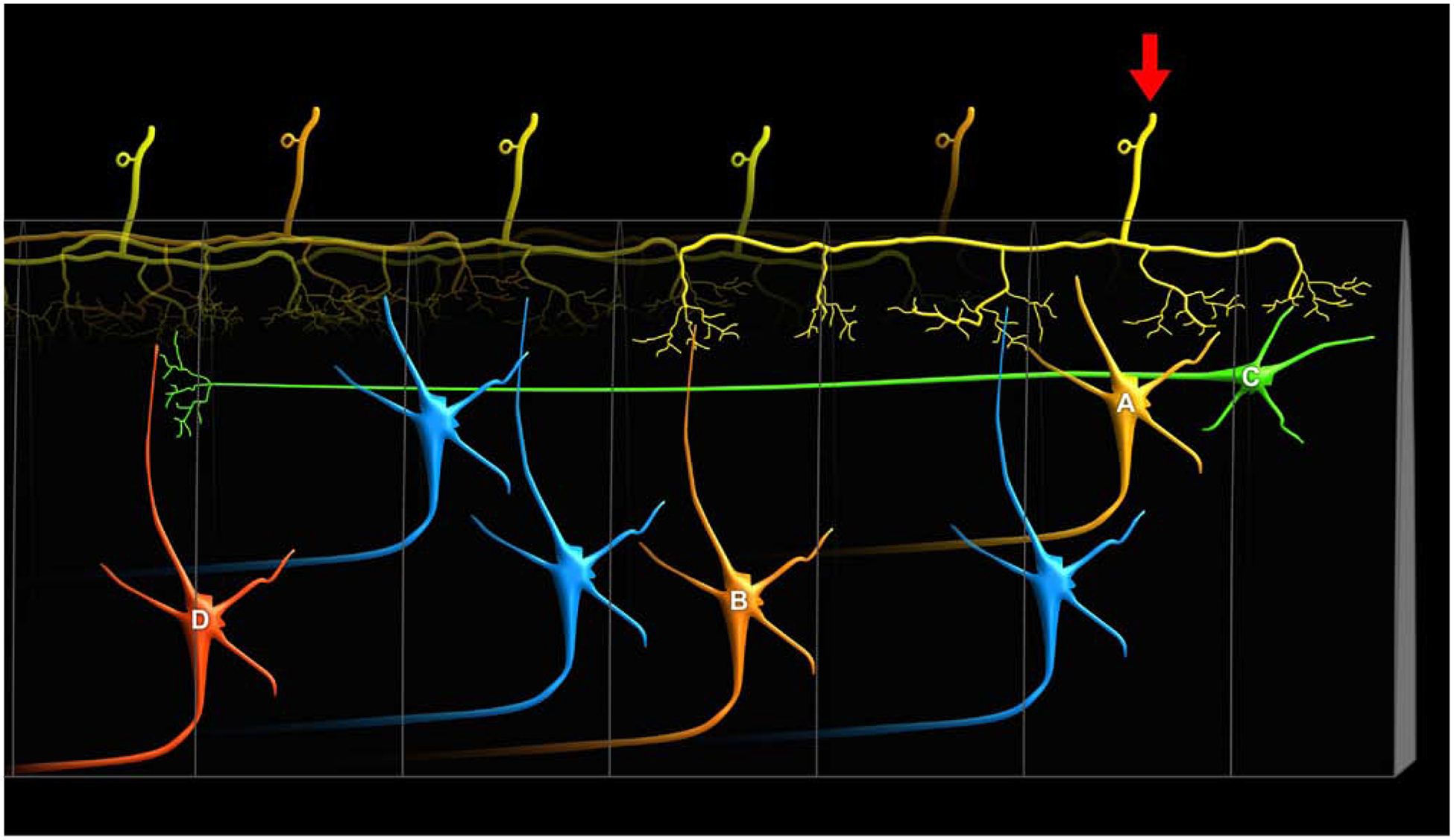
There are several anatomical pathways that can support the substantial rostro-caudal distribution of nociceptive activity within the spinal cord. First, when both A-delta and C-fiber afferents enter the spinal cord, they branch considerably in the rostro-caudal direction and traverse relatively long distances within Lissauer’s tract prior to entering the dorsal horn [84–86]. These distances are sufficiently large to extend 3 to 7 spinal cord segments (yellow primary afferent denoted by arrow). Accordingly, they can activate ascending neurons within the segment in which they enter (yellow neuron A), as well as neurons several segments rostral or caudal to the segment of entry (orange neuron B). Second, there are substantial propriospinal interconnections that may be sufficient to transmit nociceptive information even further along the rostral-caudal axis of the spinal cord [87, 88], as well as across the midline to the contralateral dorsal horn [89]. These neurons have cell bodies that reside in laminae I, V and VII, and can project more than eight spinal segments (green neuron C). Moreover, they can be activated by noxious stimuli [88]. While typically thought about in the context of motor control, specifically coordinating forelimb with hindlimb activity, they may also provide a substrate for wide ranging facilitation or inhibition of neurons across many spinal segments (red neuron D) [87]. For example, stimuli applied to sites as remote as the forelimb and face can inhibit the responses of noxious stimuli applied to the hindlimb of spinal cord transected monkeys [90]. This integration of sensory input over vast portions of the spinal cord cannot be explained simply by the branching of primary afferents, suggesting that proprio-spinal interconnections are critically involved. Thus, spinal distribution of nociceptive input and potential neuron recruitment may be driven by both widely branching primary afferents as well as by propriospinal interconnections. In addition, this widespread distribution of nociceptive information is key for multi-segmental spatial summation of pain [25].
Dynamic expansion of receptive fields of nociceptive neurons may represent a key factor for neuron recruitment. Relatively brief (20s) barrages of C-fiber input can evoke nearly 400% increases in receptive field sizes of nociceptive neurons in the rat dorsal horn, a portion of which project supraspinally [19]. In humans, demonstration of neuron recruitment has remained elusive. However, clever behavioral designs can provide important insights. Comprehensive mapping of the reflex receptive fields of muscles involved in nociceptive withdrawal represents a process to interrogate the spinal nociceptive processing mechanisms that drive the withdrawal reflex. Using this process, injections of capsaicin – an intensely noxious stimulus – have been shown to dramatically expand reflex receptive fields in both intact and spinal cord transected individuals [20]. Moreover, chronic pain patients exhibit a generalized expansion of reflex receptive fields in comparison with pain-free volunteers [21]. Accordingly, with such enlarged receptive fields, the spatial distribution of nociceptive processing in the spinal cord would be predicted to increase substantially.
Aspects of Pain Explained by the Neuron Recruitment Model
Spatial aspects of pain have long remained difficult to explain and can pose tremendous clinical problems. In particular, spread of pain away from the site of injury or tissue damage can complicate diagnoses by obscuring the location of the key problem. Spread of chronic pain and sensory disturbances occurs frequently. For example, spread of pain during complex regional pain to multiple limbs occurs in a high proportion of patients [22]. Patients with pain that radiates exhibit contralateral radiation most frequently, but significant proportions exhibit ipsilateral radiation [22]. The spread of pain in humans spatially mirrors the pronounced ipsilateral and bilateral spread of activation in the rat spinal cord following chronic constriction injury of the sciatic nerve, a model of chronic pain (Fig. 3C) [18]. As such, recruitment of spinal activation outside of the epicenter of activation would provide a putative initial substrate for the generation of a perceptual awareness of spread of pain by supraspinal mechanisms.
Spatial summation of pain is another phenomenon that can potentially be explained by the population recruitment model. Spatial summation of pain would be defined as two simultaneous noxious stimuli evoking more pain than one noxious stimulus alone. In humans, spatial summation of pain has been demonstrated to occur locally within the same dermatome [23] and across adjacent dermatomes [24]. However, consistent with the widespread recruitment of activation in the rat spinal cord during noxious stimulation (Fig. 3A), spatial summation of pain has also been demonstrated to occur even when stimuli are separated by 6 dermatomes in human participants [25]. Such widespread interactions between noxious stimuli could be supported by both multi-segmental distribution of afferent input as well as propriospinal interconnections (Fig. 4). In chronic pain, spatial interactions among noxious stimuli are substantially altered, with spatial inhibition converting to spatial summation in some instances. For example, in the conditioned pain modulation paradigm – a quantitative sensory testing paradigm where a “test” noxious stimulus is normally inhibited by a remotely applied “conditioning” noxious stimulus – patients with temporomandibular disorder have been shown to exhibit summation instead of inhibition [26]. Such summation would be consistent with the widespread and bilateral recruitment of spinal activation seen in animal models of chronic pain [18].
Regulation of Population Recruitment by Lateral Inhibition
Mechanisms regulating population recruitment and the excitatory receptive field sizes of nociceptive neurons supporting population recruitment remain remarkably poorly understood. In the rat, excitatory receptive field sizes shrink considerably during the course of development [27]. During this period, glycinergic inhibitory control emerges [28]. In adult monkeys, the application of strychnine, a glycine antagonist, causes the receptive fields of WDR neurons in the trigeminal dorsal horn to expand considerably [29]. Thus, inhibitory regulation of receptive field sizes may play a key role in the regulation of population recruitment.
In humans, spatial summation of pain is both non-linear across distance and is generally sub-additive. Specifically, noxious stimuli that are delivered in close proximity (<10 cm) exhibit surprisingly less spatial summation than those that are further apart [23, 30]. Such diminished activation could be due to increasing engagement of inhibition vs. excitation when stimuli are in close proximity. This hypothesis was tested using an innovative laser stimulation paradigm where distinct patterns of noxious heat could be “drawn” on the skin [30]. Comparisons of pain evoked by two points separated by 8 cm with pain evoked by a continuous line of stimuli revealed that the two-point stimuli evoked significantly more pain than the line stimuli, despite the fact that considerably more skin was stimulated by the line stimuli. This suggests that when stimuli are in close proximity, a relatively greater degree of inhibition is engaged than excitation. As such, lateral inhibition would be predicted to effectively shrink excitatory receptive fields and minimize the total population recruitment.
In the visual system, the dynamic engagement of inhibition is critical for regulation of neural responses supporting attention [31]. Such attention-mediated regulation of inhibition could also be critical for regulation of recruitment within the nociceptive system. In a rare example in an awake behaving monkey, a nociceptive neuron exhibited exclusively unilateral responses until an attentional cue was given that instructed the monkey to attend to the contralateral side of its face [32]. The neuron’s receptive field then expanded to cross the midline and occupy a large portion of the contralateral face. As noted above, the expansion of excitatory receptive fields by reduction of inhibition would be likely to enhance neural recruitment at the population level.
Descending facilitation can regulate the responses of spinal neurons, and as such, may provide a mechanism for dynamic regulation of receptive field properties and population coding. For example, many of the spinal neurons with bilateral receptive fields in the monkey rely on top-down facilitatory signals to sustain their extensive receptive fields. Spinal cord transection considerably shrinks receptive fields down to a more typical body quadrant [10]. Moreover, these descending responses may be dynamically engaged by attention. During thermal discrimination paradigms involving noxious heat, WDR neurons within the monkey spinal cord exhibit a brief discharge at the presentation of the visual cue initiating the trial, consistent with a top-down signal [33]. Attention has been demonstrated to alter spinal activity in human participants, indicating the involvement of a dynamic regulation of nociceptive processing in response to a cognitive set [34]. Regions of the ACC have direct projections to laminae V-VII [35] and could be well positioned to provide attentional information to spinal neurons.
Using psychophysical techniques, spatial summation of pain has been shown to be dynamically regulated by attention [36]. When participants were instructed to provide one overall rating of two noxious stimuli (typically used in studies of spatial summation), substantial spatial summation of pain was detected. However, when participants were instructed to divide their attention and provide separate ratings of each of the simultaneous stimuli, spatial summation of pain was abolished. Moreover, inhibition at the distal stimulus site was noted. This distal inhibition was present irrespective of whether the proximal stimulus was noxious or was an innocuous control delivered by an active probe. If the proximal probe was completely inactivated in clear sight of the participant and there was minimal attentional allocation required, the distal inhibition was abolished.
Taken together, these findings strongly suggest that attention can regulate spatial aspects of receptive field properties. This attentional regulation may be of substantial clinical importance for the treatment of chronic pain. Single point attentional training in patients with CRPS has been shown to reduce pain ratings and minimize pain-related disability [37]. As such, attentional paradigms could represent a productive approach for treating pain conditions, particularly those with extensive spatial spread.
Distributed Processing of Pain within the Brain
Distributed Transmission of Nociceptive Input from the Spinal Cord to the Brain
The idea that transmission of nociceptive input from the spinal cord to the brain is distributed over multiple pathways is far from new [38]. Examination of patients who had undergone surgical transection of the anterolateral quadrant of the spinal cord in order to abolish chronic pain revealed that pain often returns after several weeks and can be readily evoked by various stimuli in the areas that were affected by the cordotomy.
One of the simplest explanations for the transient efficacy of cordotomies is that other pathways somehow become engaged over time. One potential candidate for such pathways would be spinothalamic projections that arise from deep dorsal horn/ventral horn neurons that have bilateral and/or whole body receptive fields [10]. Similarly, other spinothalamic neurons have been identified that have ascending projections that travel ipsilaterally instead of contralaterally to the cell body [39]. Thus, the neural architecture of ascending pathways is sufficient to distribute the transmission of nociceptive information across bilateral routes.
Multiple Thalamic Nuclei Receive Spinothalamic Input Sufficient to Convey Nociceptive Information
Spinothalamic input to the thalamus is distributed over multiple nuclei that have distinct connections with cerebral cortical regions involved in pain [40] (Fig. 5, Key Figure). In primates, nociceptive neurons have been identified in the ventroposterior lateral nucleus [41] – one of the most widely recognized targets of the spinothalamic tract, but many other nuclei also receive direct spinothalamic input and have nociceptive neurons. These nuclei include the ventroposterior inferior nucleus [42], medial dorsal nucleus [43], and intralaminar nuclei [41, 43], as well as nuclei in the posterior complex [41, 43].
FIGURE 5. (KEY FIGURE). Anatomic Substrates for Distribution of Nociceptive Input throughout the Cerebral Cortex.
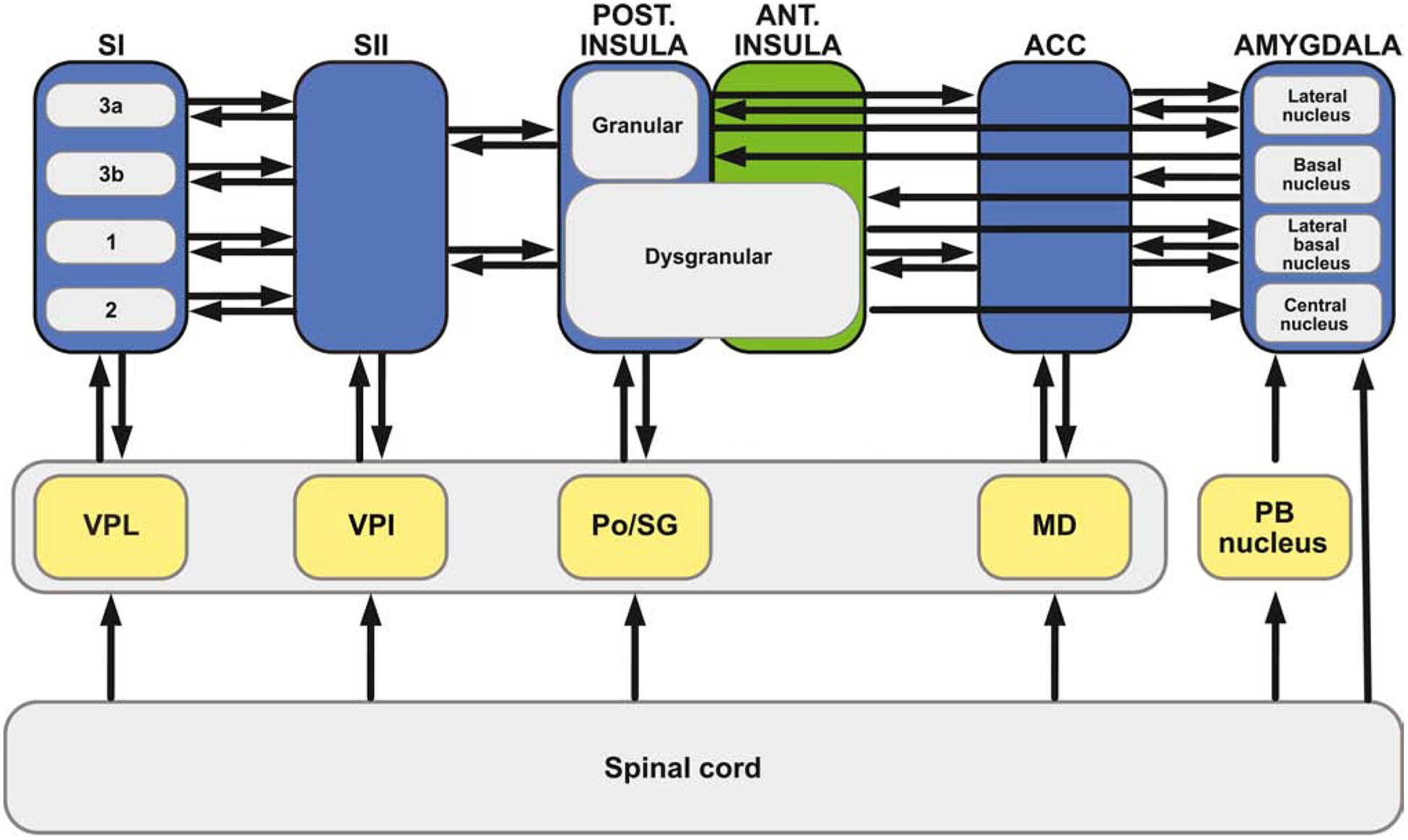
Anatomic studies in primates suggest that nociceptive input may arrive in the cerebral cortex in a highly distributed fashion via parallel routes from the thalamus (middle tier gray shading). Much of this transmission can occur in a di-synaptic fashion, such that a spinal neuron projects directly to a thalamic neuron or other subcortical neuron (yellow), which in turn, projects directly to a cortical neuron (blue) [40]. Cortical regions receiving this parallel, di-synaptic input include the primary somatosensory cortex (SI), secondary somatosensory cortex (SII), the posterior insular cortex, and the anterior cingulate cortex. Of these regions, SI gets input from ventroposterior lateral nucleus (VPL) [91], with subregions 3a,3b,1,and 2 each receiving input [92]; SII gets input from ventroposterior inferior nucleus (VPI) [93]; the granular and dysgranular portions of insula get input from the posterior-suprageniculate (Po-SG) complex [93, 94]; and the cingulate cortex gets input from the mediodorsal nucleus (MD) [95, 96] as well as the parafasicular nucleus [96]; In many instances, this thalamic connectivity is somewhat reciprocal: SI projects to VPL [97], SII projects to VPI [98], the ACC projects to MD [99]. In addition to thalamic routes, subcortical regions such as the globus pallidus (not shown) and central nucleus of the amygdala also receive direct spinal inputs [49], as well as di-synaptic input [40] via non-spinothalamic routes, such as the spinoparabrachial (PB) amygdaloid pathway [100]. This massively parallel architecture provides a clear substrate for direct and widespread distribution of nociceptive information across multiple cerebro-cortical regions.
Extensive intra-cortical connectivity augments distribution of afferent nociceptive input within the cerebral cortex. Multiple regions within SI - areas 3a, 3b, 1, and 2- are reciprocally connected with SII [101, 102]. SII is reciprocally connected with the granular and dysgranular portions of the insula [102–104]. The granular and dysgranular portions of the insula both project to multiple subregions of the anterior and anterior midcingulate cortex including areas 32, perigenual area 24, as well as 24a, 24b, and 24c (not shown) [105]. In turn, the granular and dysgranular portions of the insula receive inputs from portions of area 24 [103, 106]. The granular insula projects to the lateral nucleus of the amygdala [102], while the dysgranular insula projects to lateral and lateral basal nuclei of the amygdala [102], as well as to the central nucleus of the amygdala [107]. Both granular and dysgranular portions of the insula receive inputs from the basal and basal accessory nuclei of the amygdala [108], while anterior and anterior midcingulate areas 24a and 24b have inputs from the lateral basal and accessory basal nuclei of amygdala [109]. Area 24 also projects to the lateral basal nucleus of the amygdala [106].
Classical views of pain have emphasized the distinction between lateral and medial spinothalamic tracts, and their corresponding projections to lateral and medial nuclei of the thalamus. These views attribute sensory-discriminative processing to the lateral nuclei and affective motivational processing to the medial nuclei [44]. The receptive fields of nociceptive neurons in the lateral thalamus are generally much smaller than those in the medial thalamus, and as such, have been believed to better support discrimination of sensory features than those in the medial thalamus [44]. However, distinctions between how the medial and lateral thalamic nuclei process nociceptive input may be more subtle than typically thought. For example, the same spinothalamic nociceptive neuron can project to both lateral and medial nuclei of the thalamus [10], raising questions about how separate the ascending projections really are. Moreover, studies in awake, behaving monkeys indicate that intralaminar neurons in the medial thalamus encode noxious stimulus intensity with accuracy sufficient to support behavioral discrimination [45]. Accordingly, sensory-discriminative processing of nociceptive stimulus intensity is distributed across both medial and lateral thalamic nuclei.
Ascending Transmission of Nociceptive Information is Distributed Beyond the Spinothalamic Pathways.
In addition to the spinothalamic pathways, nociceptive input from the spinal cord ascends in a parallel fashion to many other brain regions. Anatomic studies in monkeys have identified spinal projections to the ponto-medullary reticular formation [46], parabrachial nucleus [40, 47], locus coeruleus [40], periaqueductal grey, superior colliculus, midbrain reticular formation [40, 47, 48], globus pallidus, central nucleus of the amygdala, and hypothalamus [40, 49]. Many of these regions project to the thalamus or other regions that would allow further transmission of nociceptive information.
Distributed Processing of Noxious Stimulus Intensity
Multiple cerebral cortical regions receive parallel input from the thalamus and are only one synapse away from direct spinal nociceptive input [40] (Fig. 5). Moreover, these cortical regions are highly interconnected, allowing parallel flow of information (Fig. 5). Consistent with this anatomic organization, human studies indicate that classic somatosensory regions such as SI and SII are activated in parallel by noxious stimulation [50, 51]. Moreover, intracranial EEG reveals that regions such as the posterior insula, SII, mid-cingulate cortex, and amygdala are also activated in parallel [52]. Other regions without direct spinal nociceptive input - the anterior insula, frontal operculum, precuneus, and dorsolateral prefrontal cortex - are activated next. Finally, regions such as the posterior parietal cortex and perigenual cingulate cortex are activated last [52].
Consistent with this parallel distribution of nociceptive input to the thalamus and cerebral cortex, a wide range of human brain regions exhibit graded increases in activation in response to graded increases in noxious stimulus intensity. These include bilateral portions of the thalamus, contralateral SI, bilateral SII, bilateral posterior insula, bilateral anterior insula, bilateral anterior cingulate, and bilateral portions of the putamen [53]. Moreover, these regions are activated in a fashion related to perceived pain intensity. This widespread distribution of intensity-related information is notable in that it encompasses regions ipsilateral to stimulation, as well as regions like the anterior cingulate cortex that are typically associated with affective processing rather than sensory-discriminative processing.
Recruitment at the Cerebral Cortical Level
Demonstration of population recruitment within individual cerebral cortical regions has been limited by the use of neuroimaging techniques that rely on examination of BOLD or CBF, and as such, volumes of signal change are difficult to relate back directly to the spatial extent of neural activity. However, recruitment of ipsilateral activity with increasingly intense noxious stimulation has been observed in both psychophysical studies as well as neuroimaging studies. For example, in a callosectomized patient, weakly noxious, but not innocuous, stimuli were detected by the ipsilateral hemisphere but were rated lower than when the contralateral hemisphere provided ratings [54]. Once strongly noxious stimulus levels were reached, both contralateral and ipsilateral hemispheres reported nearly equal pain intensity [54]. Similarly, in imaging studies of graded noxious stimuli in healthy volunteers, the ipsilateral SII and insular cortex required greater noxious stimulus intensities than the corresponding contralateral regions before detectable activation was elicited [53]. These patterns of activity mirror the bilateral recruitment of activation observed in the rat spinal cord during graded noxious stimulation [17]. Further, when taken together with the presence of nociceptive neurons with bilateral receptive fields within the spinal cord [10] and thalamus [45], these patterns suggest that recruitment of ipsilateral cortical activity can be driven by sub-cortical input. In chronic pain patients who have bilateral pain from a unilateral injury, bilateral cortical activation may provide the substrate for the instantiation of a conscious experience of bilateral pain.
Extensive Brain Lesions Fail to Disrupt the Capacity to Experience Pain Intensity
The ability of the brain to continue to generate a subjectively available experience of pain intensity in the face of a myriad of lesions stands in sharp contrast to the processing of light touch. For example, lesions of SI significantly disrupt the ability to perceive light touch, but the ability to perceive pain still remains [55]. Similarly, the ability to perceive pain is preserved with lesions to SII [56], anterior insula [56, 57], posterior insula [56], ACC [58, 59], and prefrontal cortex [60, 61], as well as to regions providing subcortical input to the prefrontal cortex [62].
In addition to focal lesions, pain is preserved despite widespread lesions. Combined quantitative sensory testing (QST)/imaging studies in patients with removal of one entire cerebral hemisphere reveal that pain is highly preserved when noxious stimuli are delivered ipsilateral to the remaining hemisphere [63]. One case of a patient with lesions of both the insula and ACC indicated that both pain intensity and pain unpleasantness was preserved [64], similar to that observed for lesions of the ACC [58, 59] and insula [57]. In what can only be described as a horrific example of the resiliency of the nociceptive system, a patient with post-herpetic neuralgia of the face was treated sequentially with multiple neurosurgical procedures including total resection of the sensory root of the trigeminal nerve, excision of the contralateral somatosensory cortex for the face, excision of the ipsilateral somatosensory cortex for the face, and a bilateral prefrontal lobotomy [65]. The patient still complained of a steady burning pain (although his relatives noted that it did not appear as troublesome).
Degenerate Mechanisms – A Key Element Necessary for Distributed Nociceptive Processing
The lesion data discussed above, when taken together with the neurophysiological data of the widespread processing of pain intensity, suggest that the encoding of noxious stimulus intensity occurs through a relatively degenerate mechanism that is highly conserved across multiple, functionally distinct central nervous system regions. Consistent with the concept of degeneracy [66, 67], both WDR and NS neurons are largely conserved from the spinal cord, through the thalamus [68], to multiple cerebral cortical regions including SI [69] and the ACC [70]. Similarly, stimulus-response relationships are also conserved across much of the brain [53]. As such, this degenerate mechanism of intensity coding may provide the ability to instantiate a subjectively available experience of pain through multiple different and independent brain mechanisms. However, the generation of pain by multiple distinct mechanisms may pose a significant challenge for the application of brain-based markers for the detection of pain (Text Box 1).
TEXT BOX 1. The Distributed Nociceptive System and Challenges in Development of Brain-Based Biomarkers for Pain.
Machine learning techniques have allowed the identification of signatures for pain that encompass large portions of the brain, more effectively capturing the distributed processing of nociceptive information than traditional massive univariate approaches [81]. With the advent of these technologies, there is currently an aggressive search for “objective” biomarkers for pain using neuroimaging techniques. This search is driven not only by concerns about the validity and biases in subjective reports of pain, but also by a genuine need to assess pain in individuals who are too young, or too neurologically impaired to effectively communicate their pain. However, the development of such markers needs to take into account not only the distributed nature of nociceptive information processing, but also the possibility that different individuals may instantiate a subjectively available experience of pain using highly variable subsets of brain regions known to be involved in nociceptive processing. This becomes a particular concern when markers developed in neurologically intact individuals who can communicate their pain are applied to severely neurologically impaired individuals who are rendered unable to communicate. The organization of brain networks in such individuals may be massively distorted, yet they may still be able to have an experience of pain. Moreover, individually unique patterns of activation, or patterns of activation that occur uniquely in specific groups of individuals who were under-represented during marker development, pose significant concerns for the utilization of such biomarkers. Failure to detect pain with such markers will inevitably result in the undertreatment of pain.
Pain – a Distributed Problem That Calls for a Distributed Treatment
Conventional pharmacological therapy for chronic pain is startlingly ineffective [2], and new drugs often struggle to outperform placebo [2]. The limited efficacy of pharmacotherapy for chronic pain may, in part, result from the distribution of nociceptive processing across dozens of neurotransmitters and neuromodulators [71]. Thus, targeting a single neurotransmitter system is not sufficient to adequately disrupt nociceptive processing when there are a myriad of other neurotransmitter systems that are sufficient to maintain a state of chronic pain. When taken together with the neurophysiological evidence of distributed processing addressed above, treatments that effectively target distributed systems are clearly needed.
Emerging evidence indicates that multidisciplinary treatments incorporating CBT can substantially alter pain by simultaneously targeting multiple brain regions [72–76]. The majority of these studies use BOLD-based functional connectivity analyses to delineate brain changes occurring during CBT. Given that this approach necessitates focus on the connectivity of an individual brain region (or several regions) rather than assessments of activation across the whole brain, such studies provide a promising, yet fragmentary view of the widespread effects of CBT [72]. For example, combined cognitive behavioral, physical, and occupational therapy substantially alters the connectivity of the amygdala in patients with complex regional pain syndrome. Specifically, decreased connectivity of the left amygdala with multiple regions of the prefrontal cortex, motor cortex, cingulate cortex, anterior insula was identified after this multidisciplinary rehabilitation therapy [73]. CBT also alters connectivity of the amygdala with nodes of the default mode network in a group of patients with diverse chronic pain conditions [74]. In patients with fibromyalgia, connectivity between SI and the default mode network as well as connectivity between SI and the insula was reduced after CBT [75]. These reductions in connectivity occurred simultaneously with reductions in catastrophizing. Similarly, decreased catastrophizing following CBT was associated with widespread increases in grey matter density in the dorsolateral and ventrolateral prefrontal cortices, somatosensory cortex, anterior cingulate cortex, and posterior parietal cortex in a group of chronic pain patients with pain of different etiologies [76]. Thus, when taken together, this emerging evidence of the mechanisms supporting CBT for chronic pain strongly suggests that CBT simultaneously engages multiple systems for pain modulation.
Mindfulness meditation represents another cognitive technique for pain modulation that alters multiple brain regions. In healthy individuals experiencing experimental heat stimuli, mindfulness meditation causes substantial reductions in both pain intensity and unpleasantness [77, 78]. In contrast to the majority of studies which have used BOLD fMRI to examine brain changes during CBT, two mindfulness studies have used arterial spin labeled (ASL) fMRI to examine steady-state activation/deactivation during active practice of mindfulness. During mindfulness meditation, the anterior cingulate cortex, anterior insular cortex, and orbitofrontal cortex are activated and the posterior cingulate cortex/precuneus are deactivated [77, 78]. The thalamus also displays notable deactivation [77, 78]. Individuals who had the greatest reductions in pain intensity during mindfulness meditation had the greatest activation of the anterior insula and anterior cingulate cortex, while individuals who had the greatest reductions in pain unpleasantness had the greatest activation of the orbitofrontal cortex and the greatest deactivation of the thalamus [77]. In comparison with a topically applied placebo cream, meditation produces greater reductions in both pain intensity and pain unpleasantness, and produces markedly distinct patterns of activation, with placebo producing deactivations rather than activations of the cingulate cortex [78]. Consistent with the idea that such cognitive techniques are not engaging a single focal modulatory mechanism, naloxone, an opioid antagonist, does not block pain reductions evoked by mindfulness meditation [79].
Concluding Remarks and Future Perspectives
The processing of nociceptive information is markedly different from the traditional, highly hierarchical view of processing in other sensory modalities [80]. Extraction and utilization of sensory features of nociceptive input can occur at low levels of the central nervous system, and can also be accomplished using multiple, distinct subsets of brain regions. Accordingly, the Distributed Nociceptive System is highly resilient to injury, but is similarly difficult to disrupt via treatment in cases of clinical pain. Current treatments, I would argue, still rely far too heavily on focal pharmacological modalities and fail to capitalize on the tremendous power of the brain to regulate pain. Multimodal treatments including pharmacological, cognitive, and physical components that simultaneously target multiple portions of the Distributed Nociceptive System are critically needed for the treatment of both acute and chronic pain.
Highlights.
Multiple converging lines of anatomic, neurophysiological, and behavioral evidence from studies of humans, primates, as well as rodents support the conceptual framework of the Distributed Nociceptive System.
Nociceptive stimulus intensity is encoded by a population-based mechanism involving both the numbers of neurons recruited and the frequencies at which they discharge.
Nociceptive processing is highly distributed at multiple levels of the neuraxis.
The highly distributed processing, in combination with the degenerate nature of mechanisms encoding nociceptive stimulus intensity, produces a system that is highly resilient to disruption.
Given the distributed nature of nociceptive processing, the development of improved therapies which simultaneously target multiple regions of the Distributed Nociceptive System is critically needed for the treatment of both clinical and chronic pain.
Outstanding Questions.
The brain has a remarkable ability to preserve the capacity for instantiating an experience of pain in the presence of very large lesions. What are the minimal central nervous system circuits necessary to construct a subjectively available experience of pain? Are there multiple distinct circuits that are sufficient? Given the degeneracy of nociceptive intensity coding mechanisms and the highly distributed architecture that supports it, multiple distinct circuits are a likely possibility.
Do different neurologically normal individuals instantiate an experience of pain using different components of the Distributed Nociceptive System? This question is critical for the use of brain activation-based markers for pain. If different individuals construct an experience of pain from different mechanisms, then one marker for pain will not be sufficient to adequately detect the presence of pain across all individuals.
Is a cerebral cortex even necessary to instantiate an experience of pain? Are sub-cortical, or even spinal circuits, sufficiently complex/rich to process noxious input to a degree that it transcends from nociception into a minimal form of consciousness of pain?
Acknowledgements
The author gratefully acknowledges Donald D. Price, Ph.D. for his ideas about population coding and neuron recruitment. Supported by NIH R01 NS08539, R01 NS101321, R01 AT010171, and R01 AR074795.
Glossary
- Degenerate processing
The capacity of different structures and/or mechanisms to produce similar functional outputs
- Dermatome
The body region primarily innervated by a given spinal nerve
- Distributed processing
A model of information processing in which processing is spread across multiple individual elements in at least a partially parallel organization
- Nociception
Processing of information evoked by a stimulus that actually damages the body or holds the immediate potential to damage the body
- Population coding
Representation of information accomplished by the collective action of groups of neurons
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Neville A et al. (2019) Diagnostic Uncertainty in Youth With Chronic Pain and Their Parents. Journal of Pain 20 (9), 1080–1090. [DOI] [PubMed] [Google Scholar]
- 2.Finnerup NB et al. (2018) Neuropathic pain clinical trials: factors associated with decreases in estimated drug efficacy. Pain 159 (11), 2339–2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Price DD et al. (1978) Spatial and temporal transformations of input to spinothalamic tract neurons and their relation to somatic sensations. J Neurophysiol 41 (4), 933–47. [DOI] [PubMed] [Google Scholar]
- 4.Coghill RC et al. (1994) Distributed processing of pain and vibration by the human brain. J Neurosci 14 (7), 4095–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sherrington CS (1908) The integrative action of the nervous system, Archibald Constable.
- 6.Coghill RC et al. (1993) Wide dynamic range but not nociceptive specific neurons encode multidimensional features of prolonged repetitive heat pain. J.Neurophysiol 69, 703–716. [DOI] [PubMed] [Google Scholar]
- 7.Weng HR and Schouenborg J (1996) Cutaneous inhibitory receptive fields of withdrawal reflexes in the decerebrate spinal rat. J Physiol 493 (Pt 1), 253–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rexed B (1954) A cytoarchitectonic atlas of the spinal cord in the cat. J.Comp.Neurol 100, 297–379. [DOI] [PubMed] [Google Scholar]
- 9.Price DD et al. (1978) Spatial and temporal transformations of input to spinothalamic tract neurons and their relation to somatic sensations. J.Neurophysiol 41, 933–947. [DOI] [PubMed] [Google Scholar]
- 10.Giesler GJ et al. (1981) Spinothalamic tract neurons that project to medial and/or lateral thalamic nuclei: evidence for a physiologically novel population of spinal cord neurons. J.Neurophysiol 46, 1285–1286. [DOI] [PubMed] [Google Scholar]
- 11.Nahin RL et al. (1983) Anatomical and physiological studies of the gray matter surrounding the spinal cord central canal. J.Comp.Neurol 220, 321–335. [DOI] [PubMed] [Google Scholar]
- 12.Mendell LM and Wall PD (1965) Response of single dorsal cord cells to peripheral cutaneous unmyelinated fibres. Nature 206, 97–99. [DOI] [PubMed] [Google Scholar]
- 13.Christensen BN and Perl ER (1970) Spinal neurons specifically excited by noxious or thermal stimuli: marginal zone of the dorsal horn. J.Neurophysiol 33, 292–307. [DOI] [PubMed] [Google Scholar]
- 14.Maixner W et al. (1986) Wide-dynamic-range dorsal horn neurons participate in the encoding process by which monkeys percieve the intensity of noxious heat stimuli. Brain Res. 374, 385–388. [DOI] [PubMed] [Google Scholar]
- 15.Mayer DJ et al. (1975) Neurophysiological characterization of the anterolateral spinal cord neurons contributing to pain perception in man. Pain 1, 51–58. [DOI] [PubMed] [Google Scholar]
- 16.Coghill RC et al. (1993) The roles of spatial recruitment and discharge frequency in spinal cord coding of pain: a combined electrophysiological and imaging investigation. Pain 53 (3), 295–309. [DOI] [PubMed] [Google Scholar]
- 17.Coghill RC et al. (1991) Spatial distribution of nociceptive processing in the rat spinal cord. J.Neurophysiol 65, 133–140. [DOI] [PubMed] [Google Scholar]
- 18.Mao J et al. (1992) Spatial patterns of spinal cord [14C]-2-deoxyglucose metabolic activity in a rat model of painful peripheral mononeuropathy. Pain 50, 89–100. [DOI] [PubMed] [Google Scholar]
- 19.Cook AJ et al. (1987) Dynamic receptive field plasticity in rat spinal cord dorsal horn following C-primary afferent input. Nature 325 (7000), 151–3. [DOI] [PubMed] [Google Scholar]
- 20.Biurrun Manresa JA et al. (2014) Central sensitization in spinal cord injured humans assessed by reflex receptive fields. Clin Neurophysiol 125 (2), 352–62. [DOI] [PubMed] [Google Scholar]
- 21.Neziri AY et al. (2010) Generalized expansion of nociceptive reflex receptive fields in chronic pain patients. Pain 151 (3), 798–805. [DOI] [PubMed] [Google Scholar]
- 22.van Rijn MA et al. (2011) Spreading of complex regional pain syndrome: not a random process. J Neural Transm (Vienna) 118 (9), 1301–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Price DD et al. (1989) Spatial summation of heat-induced pain: influence of stimulus area and spatial separation of stimuli on perceived pain sensation intensity and unpleasantness. J.Neurophysiol 62, 1270–1279. [DOI] [PubMed] [Google Scholar]
- 24.Douglass DK et al. (1992) Spatial summation in human thermal pain perception: comparison within and between dermatomes. Pain 50 (2), 197–202. [DOI] [PubMed] [Google Scholar]
- 25.Quevedo AS and Coghill RC (2009) Filling-in, spatial summation, and radiation of pain: evidence for a neural population code in the nociceptive system. J Neurophysiol 102 (6), 3544–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.King CD et al. (2009) Deficiency in endogenous modulation of prolonged heat pain in patients with Irritable Bowel Syndrome and Temporomandibular Disorder. Pain 143 (3), 172–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fitzgerald M (1985) The post-natal development of cutaneous afferent fibre input and receptive field organization in the rat dorsal horn. J Physiol 364, 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koch SC et al. (2012) C-fiber activity-dependent maturation of glycinergic inhibition in the spinal dorsal horn of the postnatal rat. Proc Natl Acad Sci U S A 109 (30), 12201–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yokota T et al. (1979) Effects of strychnine upon different classes of trigeminal subnucleus caudalis neurons. Brain Res. 168, 430–434. [DOI] [PubMed] [Google Scholar]
- 30.Quevedo AS et al. (2017) Lateral inhibition during nociceptive processing. Pain 158 (6), 1046–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Compte A and Wang XJ (2006) Tuning curve shift by attention modulation in cortical neurons: a computational study of its mechanisms. Cereb Cortex 16 (6), 761–78. [DOI] [PubMed] [Google Scholar]
- 32.Hayes RL et al. (1981) Neuronal activity in medullary dorsal horn of awake monkeys trained in a thermal discrimination task. II. Behavioral modulation of responses to thermal and mechanical stimuli. J Neurophysiol 46 (3), 428–43. [DOI] [PubMed] [Google Scholar]
- 33.Duncan GH et al. (1987) Task-related responses of monkey medullary dorsal horn neurons. J Neurophysiol 57 (1), 289–310. [DOI] [PubMed] [Google Scholar]
- 34.Sprenger C et al. (2012) Attention modulates spinal cord responses to pain. Curr Biol 22 (11), 1019–22. [DOI] [PubMed] [Google Scholar]
- 35.Morecraft RJ et al. (2007) Amygdala interconnections with the cingulate motor cortex in the rhesus monkey. J Comp Neurol 500 (1), 134–65. [DOI] [PubMed] [Google Scholar]
- 36.Quevedo AS and Coghill RC (2007) Attentional modulation of spatial integration of pain: evidence for dynamic spatial tuning. J Neurosci 27 (43), 11635–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moseley GL et al. (2008) Tactile discrimination, but not tactile stimulation alone, reduces chronic limb pain. Pain 137 (3), 600–8. [DOI] [PubMed] [Google Scholar]
- 38.Noordenbos W (1959) Pain, 0 edn., Elsevier. [Google Scholar]
- 39.Lu GW and Willis WD (1999) Branching and/or collateral projections of spinal dorsal horn neurons. Brain Res Brain Res Rev 29 (1), 50–82. [DOI] [PubMed] [Google Scholar]
- 40.Dum RP et al. (2009) The spinothalamic system targets motor and sensory areas in the cerebral cortex of monkeys. J Neurosci 29 (45), 14223–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Perl ER and Whitlock DG (1961) Somatic stimuli exciting spinothalamic projections to thalamic neurons in cat and monkey. Exp Neurol 3, 256–96. [DOI] [PubMed] [Google Scholar]
- 42.Pollin B and Albe-Fessard D (1979) Organization of somatic thalamus in monkeys with and without section of dorsal spinal tracts. Brain Res 173 (3), 431–49. [DOI] [PubMed] [Google Scholar]
- 43.Casey KL (1966) Unit analysis of nociceptive mechanisms in the thalamus of the awake squirrel monkey. J Neurophysiol 29 (4), 727–50. [DOI] [PubMed] [Google Scholar]
- 44.Melzack R and Casey KL (1968) Sensory, motivational, and central control determinants of pain In The Skin Senses (0 edn) (Kenshalo DR ed), pp. 423–435, Thomas. [Google Scholar]
- 45.Bushnell MC and Duncan GH (1989) Sensory snd affective aspects of pain perception: is medial thalamus restricted to emotional issues? Experimental Brain Research 78, 415–418. [DOI] [PubMed] [Google Scholar]
- 46.Kevetter GA et al. (1982) Cells of origin of the spinoreticular tract in the monkey. J Comp Neurol 207 (1), 61–74. [DOI] [PubMed] [Google Scholar]
- 47.Wiberg M et al. (1987) Somatosensory projection to the mesencephalon: an anatomical study in the monkey. J Comp Neurol 264 (1), 92–117. [DOI] [PubMed] [Google Scholar]
- 48.Yezierski RP (1988) Spinomesencephalic tract: projections from the lumbosacral spinal cord of the rat, cat, and monkey. J Comp Neurol 267 (1), 131–46. [DOI] [PubMed] [Google Scholar]
- 49.Newman HM et al. (1996) Direct spinal projections to limbic and striatal areas: anterograde transport studies from the upper cervical spinal cord and the cervical enlargement in squirrel monkey and rat. J Comp Neurol 365 (4), 640–58. [DOI] [PubMed] [Google Scholar]
- 50.Ploner M et al. (1999) Parallel activation of primary and secondary somatosensory cortices in human pain processing. J Neurophysiol 81 (6), 3100–4. [DOI] [PubMed] [Google Scholar]
- 51.Liang M et al. (2011) Parallel processing of nociceptive and non-nociceptive somatosensory information in the human primary and secondary somatosensory cortices: evidence from dynamic causal modeling of functional magnetic resonance imaging data. J Neurosci 31 (24), 8976–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bastuji H et al. (2016) Pain networks from the inside: Spatiotemporal analysis of brain responses leading from nociception to conscious perception. Hum Brain Mapp 37 (12), 4301–4315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Coghill RC et al. (1999) Pain intensity processing within the human brain: A bilateral, distributed mechanism. J.Neurophysiol 82, 1934–1943. [DOI] [PubMed] [Google Scholar]
- 54.Stein BE et al. (1989) Pain perception in a man with total corpus callosum transection. Pain 38, 51–56. [DOI] [PubMed] [Google Scholar]
- 55.Knecht S et al. (1996) Parallel and serial processing of haptic information in man: effects of parietal lesions on sensorimotor hand function. Neuropsychologia 34, 669–687. [DOI] [PubMed] [Google Scholar]
- 56.Greenspan JD et al. (1999) Pain sensitivity alterations as a function of lesion location in the parasylvian cortex. Pain 81 (3), 273–82. [DOI] [PubMed] [Google Scholar]
- 57.Starr CJ et al. (2009) Roles of the insular cortex in the modulation of pain: insights from brain lesions. J Neurosci 29 (9), 2684–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Davis KD et al. (1994) Altered pain and temperature perception following cingulotomy and capsulotomy in a patient with schizoaffective disorder. Pain 59, 189–199. [DOI] [PubMed] [Google Scholar]
- 59.Greenspan JD et al. (2008) Quantitative somatic sensory testing and functional imaging of the response to painful stimuli before and after cingulotomy for obsessive-compulsive disorder (OCD). Eur J Pain 12 (8), 990–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hardy JD et al. (1952) Pain Sensations and Reactions, 0 edn., Williams & Wilkins. [Google Scholar]
- 61.King HE et al. (1950) Cutaneous thresholds for pain before and after unilateral prefrontal lobotomy. J.Nerv.Ment.Dis 112, 93–96. [PubMed] [Google Scholar]
- 62.Talbot JD et al. (1995) Evaluation of pain perception after anterior capsulotomy A case report. Somatosensory and Motor Research 12, 115–126. [DOI] [PubMed] [Google Scholar]
- 63.Olausson H et al. (2001) Cortical activation by tactile and painful stimuli in hemispherectomized patients. Brain 124 (Pt 5), 916–27. [DOI] [PubMed] [Google Scholar]
- 64.Feinstein JS et al. (2016) Preserved emotional awareness of pain in a patient with extensive bilateral damage to the insula, anterior cingulate, and amygdala. Brain Struct Funct 221 (3), 1499–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sugar O and Bucy PC (1951) Postherpetic trigeminal neuralgia. AMA Arch Neurol Psychiatry 65 (2), 131–45. [DOI] [PubMed] [Google Scholar]
- 66.Tononi G et al. (1999) Measures of degeneracy and redundancy in biological networks. Proc Natl Acad Sci U S A 96 (6), 3257–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Edelman GM and Gally JA (2001) Degeneracy and complexity in biological systems. Proc Natl Acad Sci U S A 98 (24), 13763–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kenshalo DR et al. (1980) Responses of neurons in primate ventral posterior lateral nucleus to noxious stimuli. J.Neurophysiol 43, 1594–1614. [DOI] [PubMed] [Google Scholar]
- 69.Kenshalo DR Jr. and Isensee O (1983) Responses of primate SI cortical neurons to noxious stimuli. J.Neurophysiol 50, 1479–1496. [DOI] [PubMed] [Google Scholar]
- 70.Sikes RW and Vogt BA (1992) Nociceptive neurons in area 24 of rabbit cingulate cortex. J.Neurophysiol 68, 1720–1732. [DOI] [PubMed] [Google Scholar]
- 71.Millan MJ (2002) Descending control of pain. Prog Neurobiol 66 (6), 355–474. [DOI] [PubMed] [Google Scholar]
- 72.Cunningham NR et al. (2019) Brain mechanisms impacted by psychological therapies for pain: identifying targets for optimization of treatment effects. Pain Rep 4 (4), e767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Simons LE et al. (2014) The responsive amygdala: treatment-induced alterations in functional connectivity in pediatric complex regional pain syndrome. Pain 155 (9), 1727–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shpaner M et al. (2014) Unlearning chronic pain: A randomized controlled trial to investigate changes in intrinsic brain connectivity following Cognitive Behavioral Therapy. Neuroimage Clin 5, 365–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lazaridou A et al. (2017) Effects of Cognitive-Behavioral Therapy (CBT) on Brain Connectivity Supporting Catastrophizing in Fibromyalgia. Clin J Pain 33 (3), 215–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Seminowicz DA et al. (2013) Cognitive-behavioral therapy increases prefrontal cortex gray matter in patients with chronic pain. J Pain 14 (12), 1573–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zeidan F et al. (2011) Brain mechanisms supporting the modulation of pain by mindfulness meditation. J Neurosci 31 (14), 5540–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zeidan F et al. (2015) Mindfulness Meditation-Based Pain Relief Employs Different Neural Mechanisms Than Placebo and Sham Mindfulness Meditation-Induced Analgesia. J Neurosci 35 (46), 15307–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zeidan F et al. (2016) Mindfulness-Meditation-Based Pain Relief Is Not Mediated by Endogenous Opioids. J Neurosci 36 (11), 3391–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Felleman DJ and Van Essen DC (1991) Distributed hierarchical processing in the primate cerebral cortex. Cerebral Cortex 1, 1–47. [DOI] [PubMed] [Google Scholar]
- 81.Wager TD et al. (2013) An fMRI-based neurologic signature of physical pain. N Engl J Med 368 (15), 1388–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Price DD (1999) Psychological mechanisms of pain and analgesia, 0 edn., IASP Press. [Google Scholar]
- 83.Coghill RC et al. (1993) The roles of spatial recruitment and discharge frequency in spinal cord coding of pain: a combined electrophysiological and imaging investigation. Pain 53, 295–309. [DOI] [PubMed] [Google Scholar]
- 84.Sugiura Y et al. (1986) Central projections of identified, unmyelinated (C) afferent fibers innervating mammalian skin. Science 234 (4774), 358–61. [DOI] [PubMed] [Google Scholar]
- 85.Lidierth M (2007) Long-range projections of Adelta primary afferents in the Lissauer tract of the rat. Neurosci Lett 425 (2), 126–30. [DOI] [PubMed] [Google Scholar]
- 86.Traub RJ et al. (1986) The rostral projection of small diameter primary afferents in Lissauer’s tract. Brain Res 399 (1), 185–9. [DOI] [PubMed] [Google Scholar]
- 87.Burton H and Loewy AD (1976) Descending projections from the marginal cell layer and other regions of the monkey spinal cord. Brain Res 116 (3), 485–91. [DOI] [PubMed] [Google Scholar]
- 88.Skinner RD et al. (1980) Responses of long descending propriospinal neurons to natural and electrical types of stimuli in cat. Brain Res 196 (2), 387–403. [DOI] [PubMed] [Google Scholar]
- 89.Petko M and Antal M (2000) Propriospinal afferent and efferent connections of the lateral and medial areas of the dorsal horn (laminae I-IV) in the rat lumbar spinal cord. J Comp Neurol 422 (2), 312–25. [DOI] [PubMed] [Google Scholar]
- 90.Gerhart KD et al. (1981) Inhibitory receptive fields of primate spinothalamic tract cells. J Neurophysiol 46 (6), 1309–25. [DOI] [PubMed] [Google Scholar]
- 91.Whitsel BL et al. (1978) Thalamic projections to S-I in macaque monkey. J Comp Neurol 178 (3), 385–409. [DOI] [PubMed] [Google Scholar]
- 92.Jones EG and Friedman DP (1982) Projection pattern of functional components of thalamic ventrobasal complex on monkey somatosensory cortex. J Neurophysiol 48 (2), 521–44. [DOI] [PubMed] [Google Scholar]
- 93.Friedman DP and Murray EA (1986) Thalamic connectivity of the second somatosensory area and neighboring somatosensory fields of the lateral sulcus of the Macaque. J.Comp.Neurol 252, 348–373. [DOI] [PubMed] [Google Scholar]
- 94.Mufson EJ and Mesulam MM (1984) Thalamic connections of the insula in the rhesus monkey and comments on the paralimbic connectivity of the medial pulvinar nucleus. J.Comp.Neurol 227, 109–120. [DOI] [PubMed] [Google Scholar]
- 95.Hatanaka N et al. (2003) Thalamocortical and intracortical connections of monkey cingulate motor areas. J Comp Neurol 462 (1), 121–38. [DOI] [PubMed] [Google Scholar]
- 96.Vogt BA et al. (1987) Cingulate cortex of the rhesus monkey: I. Cytoarchitecture and thalamic afferents. J.Comp.Neurol 262, 256–270. [DOI] [PubMed] [Google Scholar]
- 97.Darian-Smith C et al. (1999) Comparing thalamocortical and corticothalamic microstructure and spatial reciprocity in the macaque ventral posterolateral nucleus (VPLc) and medial pulvinar. J Comp Neurol 410 (2), 211–34. [PubMed] [Google Scholar]
- 98.Burton H (1984) Corticothalamic connections from the second somatosensory area and neighboring regions in the lateral sulcus of macaque monkeys. Brain Res 309 (2), 368–72. [PubMed] [Google Scholar]
- 99.Yeterian EH and Pandya DN (1988) Corticothalamic connections of paralimbic regions in the rhesus monkey. J Comp Neurol 269 (1), 130–46. [DOI] [PubMed] [Google Scholar]
- 100.Pritchard TC et al. (2000) Projections of the parabrachial nucleus in the old world monkey. Exp Neurol 165 (1), 101–17. [DOI] [PubMed] [Google Scholar]
- 101.Friedman DP et al. (1980) Representation pattern in the second somatic sensory area of the monkey cerebral cortex. J.Comp.Neurol 192, 21–41. [DOI] [PubMed] [Google Scholar]
- 102.Friedman DP et al. (1986) Cortical connections of the somatosensory fields of the lateral sulcus of macaques: evidence for a corticolimbic pathway for touch. J.Comp.Neurol 252, 323–347. [DOI] [PubMed] [Google Scholar]
- 103.Mufson EJ and Mesulam M (1982) Insula of the old world monkey. II. Afferent cortical input and comments on the claustrum. J.Comp.Neurol 212, 23–37. [DOI] [PubMed] [Google Scholar]
- 104.Mesulam M-M and Mufson EJ (1982) Insula of the old world monkey. III: Efferent cortical output and comments on function. J.Comp.Neurol 212, 38–52. [DOI] [PubMed] [Google Scholar]
- 105.Morecraft RJ et al. (2012) Cytoarchitecture and cortical connections of the anterior cingulate and adjacent somatomotor fields in the rhesus monkey. Brain Res Bull 87 (4–5), 457–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Pandya DN et al. (1981) Efferent connections of the cingulate gyrus in the rhesus monkey. Experimental Brain Research 42, 319–330. [DOI] [PubMed] [Google Scholar]
- 107.Stefanacci L and Amaral DG (2002) Some observations on cortical inputs to the macaque monkey amygdala: an anterograde tracing study. J Comp Neurol 451 (4), 301–23. [DOI] [PubMed] [Google Scholar]
- 108.Amaral DG and Price JL (1984) Amygdalo-cortical projections in the monkey (Macaca fascicularis). J Comp Neurol 230 (4), 465–96. [DOI] [PubMed] [Google Scholar]
- 109.Vogt BA and Pandya DN (1987) Cingulate cortex of the rhesus monkey: II. Cortical afferents. J.Comp.Neurol 262, 271–289. [DOI] [PubMed] [Google Scholar]


