Abstract
Background:
Limited evidence exists regarding differences in outcomes between angiotensin II receptor blockers (ARBs) and angiotensin-converting enzyme inhibitors (ACEIs) among older nursing home (NH) residents after acute myocardial infarction (AMI). The purpose of our study was to estimate the post-AMI effects of ARBs versus ACEIs on mortality, rehospitalization, and functional decline outcomes in this important population.
Methods:
This retrospective cohort study used national Medicare claims linked to Minimum Data Set assessments. The study population included individuals aged ≥65 years who resided in a U.S. NH ≥30 days, were hospitalized for AMI between May 2007 and March 2010, and returned to the NH. We compared 90-day mortality, rehospitalization, and functional decline outcomes between ARB and ACEI users with inverse-probability-of-treatment-weighted binomial and multinomial logistic regression models.
Results:
Among 2,765 NH residents, 270 (9.8%) were ARB and 2,495 (90.2%) ACEI users. The mean age of ARB versus ACEI users was 82.3 versus 82.7 years. No marked differences existed between ARB versus ACEI users for mortality (OR=1.18, 95% CI 0.78–1.79), rehospitalization (OR=1.22, 95% CI 0.90–1.65), or functional decline (OR=1.23, 95% CI 0.88–1.74). In subgroup analyses, ARBs were associated with increased mortality and rehospitalization in individuals with moderate to severe cognitive impairment, and increased rehospitalization in those <85 years.
Conclusions:
Our findings concord with prior data and suggest that clinicians can prescribe either ARBs or ACEIs post-AMI for secondary prevention in NH residents, though the subgroup findings merit further scrutiny and replication. Providers should consider factors like patient preferences, class-specific adverse events, and costs to guide prescribing.
Keywords: long-term care facilities, cardiovascular disease, secondary prevention, renin-angiotensin-aldosterone system inhibitors, pharmacoepidemiology
1. INTRODUCTION
Renin-angiotensin-aldosterone system (RAAS) inhibitors, particularly angiotensin II receptor blockers (ARBs) and angiotensin-converting enzyme inhibitors (ACEIs), are an integral part of guideline-recommended therapy for secondary prevention following an acute myocardial infarction (AMI)(1,2). Data from randomized clinical trials (RCTs) and observational studies supports a mortality benefit from using either ARBs or ACEIs after AMI in older adults ≥65 years (3–10). Clinicians therefore have the option of selecting between ARBs and ACEIs. However, data from direct comparisons of the two RAAS classes are scarce (11). Such comparative effectiveness data are especially lacking in older adults who are frail, multimorbid, and cognitively or functionally impaired, despite the significant challenges these conditions present to prescribing and care management by cardiovascular and geriatric healthcare professionals.(12,13)
The absence of data is especially marked for older adults residing long-term in nursing homes (NHs), as these individuals tend to be the oldest, frailest, and most medically complex U.S. subpopulation. Frailty among NH residents manifests as a decreased ability to recover from physiologic insults, including medication exposures, and often presents with the phenotype of weight loss, sarcopenia, or lack of independence in activities of daily living (ADLs)(14–16). Although mechanistically RAAS inhibitors should produce similar effects regardless of age, frailty, or other potential physiologic changes post-AMI, confirmatory empirical data are scarce as nearly all frail individuals were excluded from RCTs, even those that enrolled older adults (17). An absence of observational studies of ARB versus ACEI use among these individuals has also contributed to the lack of relevant data. This absence of observational studies is primarily due to a scarcity of sufficiently large, high-quality data sources on older NH residents. Therefore, the potential for disparate effects between ARBs and ACEIs in this subpopulation should not be overlooked (17). If differences were to exist between ARBs and ACEIs, especially for key subgroups, information on the comparative effectiveness of the two classes in older NH residents would be necessary to systematically optimize health outcomes after AMI. This is especially true for functional outcomes since such outcomes have rarely been studied for ARBs and ACEIs.
We compared the effectiveness of ARB versus ACEI use on 90-day mortality, rehospitalization, and functional decline among older NH residents after AMI. We hypothesized that there would be no meaningful overall differences in those outcomes between users of the two drug classes. We anticipated that if this hypothesis were correct, our results would help to emphasize that the selection between ARBs and ACEIs for older NH residents post-AMI should be based on other important factors like patient preferences, known adverse effects (e.g., ACEI-induced cough and angioedema), drug costs, and drug availability given increasingly common drug shortages.
2. METHODS
2.1. Study Design and Data Source
This was a retrospective new-user cohort study using existing (18–24) national Medicare data linked to the Minimum Data Set (MDS) version 2.0 and Online Survey Certification and Reporting System (OSCAR) data. The MDS is a comprehensive, clinical assessment instrument used to document health status of NH residents, including demographic, medical, functional status, psychological, and cognitive status information. All NH facilities are required to report their residents’ characteristics through MDS assessments at least quarterly to receive Medicare or Medicaid funding. These assessments occur more frequently for patients with a major recent change in clinical status and those receiving care under the Medicare Skilled Nursing Facility (SNF) benefit. The OSCAR data provides facility-level information on NH characteristics, staffing levels, and quality indicators. Medicare claims include information on inpatient care (Part A), outpatient care (Part B), and prescription drug dispensings (Part D). Part D covers over 90% of NH residents and is the sole source of prescription drug coverage for nearly all of these individuals. A previously validated residential history file algorithm was used to track the timing and location of health service use (25). The data employed in our study are subject to a data use agreement with the Centers for Medicare and Medicaid Services and cannot be made available to other researchers.
2.2. Study Population
The study population was a previously established (18–24) national cohort of long-stay NH residents aged ≥65 years who were hospitalized for AMI (ICD-9 codes 410.XX or 411.1 in principal or secondary position on inpatient claim), had not taken an ARB or ACEI for at least 12 months before the AMI, and were readmitted to a U.S. NH directly after hospital discharge between May 1, 2007 and December 31, 2010 (eFigure 1). We excluded patients with extremely poor functional status before the AMI hospitalization (ADL score ≥24) because they had little opportunity for further functional decline (see Outcomes below) (18). Previous non-users were selected to evaluate the decision to initiate either ARBs or ACEIs after AMI, distinct from the decision to continue these agents in patients who had already been taking them before their AMI. Additional details of the cohort have been previously described (18–24).
2.3. Exposures and Causal Contrast of Interest
ARB or ACEI initiation after AMI was identified in Medicare Part D prescription drug claims (individual drugs listed in eTable 1) (18–24). The causal contrast of interest was defined as the effect of initiating ARBs versus ACEIs, regardless of subsequent treatment discontinuation or switching among treatment groups. This is the observational study analogue of the intention-to-treat analyses in randomized controlled trials because patients are analyzed according to the treatment that was initially dispensed (26–28).
2.4. Outcomes
The three outcomes were 90-day mortality, all-cause rehospitalization, and functional decline. We used data from Medicare Part A and enrollment files to identify hospital readmissions and date of death. Functional decline was defined as an increase of 3 points on the validated 28-point MDS Morris scale of independence in Activities of Daily Living (ADL) between the prehospital baseline assessment and the first available assessment after hospitalization up to 3 months after discharge (29). This measure indicates the degree of dependence on staff assistance in seven areas of ADL function (bed mobility, transfer, locomotion, dressing, eating, toilet use, personal hygiene), which are summed to create a validated score that ranges from 0 (no assistance required) to 28 (total dependence in ADL functioning)(30). Increases in this score over time have been validated as a measure of functional decline, and a 3-point increase corresponds to a major loss of independence in one ADL or incremental losses in two or more ADLs (18,29).
2.5. Follow-up
Follow-up started on day 14 (index date) after hospital discharge and continued up to 90 days. We excluded individuals who died or were hospitalized within 14 days of hospital discharge because reliable ascertainment of ARB and ACEI use is difficult in such short-stay situations. Follow-up therefore started on day 14 (index date) after hospital discharge and continued up to 90 days (18). We chose a primary follow-up period of 90 days because it is long enough to be clinically meaningful, but short enough that many of the highly vulnerable NH residents in our study population had not yet died. Death is a common competing outcome that complicates the interpretation of longer-term functional and hospitalization outcomes.
Multinomial outcome variables with three levels were created for the rehospitalization and functional decline outcomes. For the rehospitalization outcome, at the end of the 90-day follow-up, participants were classified as alive without rehospitalization (level 1 of the multinomial outcome), having had a rehospitalization (level 2), or having died without a rehospitalization (level 3). Similarly, for the functional decline outcome, at the end of the 90-day follow-up period, participants were classified as alive without functional decline, having had functional decline documented on an MDS assessment in that period, or having died without evidence of functional decline on the MDS. For the death outcome, individuals were simply categorized as alive or dead at 90 days.
2.6. Baseline Characteristics
Variables that could potentially confound the relationship between ARBs or ACEIs and outcomes were prespecified and all measured prior to the index date. A complete list of these 91 characteristics and details about their measurement are provided in eTable 2.
2.7. Statistical Analyses
We adjusted for confounding by baseline covariates using methods that rely on estimating the propensity score (i.e., the probability of receiving ARBs versus ACEIs, conditional on covariates). We estimated the propensity scores via a flexible logistic regression model that used the aforementioned 91 baseline variables (eTable 2) to predict the use of ARBs versus ACEIs. The initial model achieved good balance in measured covariates (see below) with fair discrimination (c-statistic = 0.74) and was thus used for all analyses. We used the propensity score to construct inverse probability of treatment weights (IPTW). Standardized mean differences after IPTW weighting were used to assess covariate balance across treatment groups. Weighting resulted in good covariate balance based on standardized mean differences (eTable 3).
We estimated odds ratios (ORs) with 95% confidence intervals (CIs) using IPTW multinomial logistic regression models to compare new ARB users versus ACEI users for rehospitalization and functional decline. Multinomial models enabled us to account for the competing risk of death. The robust (Huber-White) estimator of the sampling variance was employed for those analyses. For the mortality outcome, we used IPTW binomial logistic regression models. As an alternative to the ORs, we estimated 90-day risk differences with 95% CIs calculated using non-parametric bootstrapping with 10,000 replicates. Statistical significance was defined as a p-value < 0.05.
Additionally, we conducted stability analyses for 180-day and 365-day post-AMI mortality, rehospitalization, and functional decline outcomes to provide confidence in the validity of the main findings. To evaluate whether the association between RAAS inhibitor classes and outcomes varied across patient characteristics, we conducted pre-specified subgroup analyses including 6 subgroups based on age, sex, cognitive function, functional status, intensive care unit (ICU)/coronary care unit (CCU) stay, and polypharmacy (31). Sensitivity analyses using the E-value were performed to assess potential or unmeasured confounding (32). Additional study methods are further described in Appendix I (located in the online supplement).
2.8. Software
Data were analyzed using SAS, version 9.4 (SAS Institute, Inc., Cary, NC) and Stata, version 14.0 (Stata Corp., College Station, TX), software.
3. RESULTS
3.1. Study Cohort
Of the 8,437 NH residents who met all other eligibility criteria, 2,765 (32.7%) were initiated on a RAAS inhibitor after AMI (eFigure 1). Our study cohort thus included 270 (9.8%) new ARB users and 2,495 (90.2%) new ACEI users after AMI (Table 1). Prior to IPT weighting, the mean (standard deviation (SD)) age of the study cohort was 82.3 (9.1) years in the ARB group versus 82.7 (8.2) years in the ACEI group. The study population was primarily female (73.3% vs. 67.8%) and white race (83.0% vs. 82.7%). We observed that 37.8% of the ARB group versus 49.3% of the ACEI group had moderate to severe cognitive impairment prior to the AMI hospitalization and 24.4% versus 30.5% had extensive impairment in their physical functioning. Hypertension, chronic heart failure, Alzheimer’s disease, and diabetes mellitus were the most common comorbid conditions across both groups. Approximately 68% of ARB users versus 53% of ACEI users were on ≥11 medications. The median (interquartile range (IQR)) pre-AMI length of NH stay was 147 (60, 753) days for the ARB group versus 396 (90, 1,120) days for the ACEI group. After IPT weighting, covariates such as comorbidities, medications, and healthcare utilization factors were well-balanced between ARB and ACEI users (eTable 3).Most NH facility characteristics were also similar between ARB and ACEI users (eTable 4), including NH size, percent occupancy, and quality indicators. ARB users were more likely to reside in for-profit NHs than ACEI users (79.3% vs. 72.9%).
Table 1.
Characteristics of new ARB and ACEI users among older NH residents hospitalized for AMI.
| No. (%)a | ||
|---|---|---|
| Characteristics | ARBs (n=270) | ACEIs (n=2495) |
| Age, mean (SD), years | 82.3 (9.1) | 82.7 (8.2) |
| 65 to <85 | 157 (58.2) | 1,406 (56.4) |
| ≥85 | 113 (41.9) | 1,089 (43.7) |
| Male sex | 72 (26.7) | 803 (32.2) |
| Race | ||
| White | 224 (83.0) | 2064 (82.7) |
| Non-Whiteb | 46 (17.0) | 431 (17.3) |
| Body Mass Index mean (SD), kg/m2 | 26.8 (6.8) | 26.2 (6.4) |
| Chronic Conditions | ||
| Chronic Heart Failure | 154 (57.0) | 1429 (57.3) |
| Atrial Fibrillation | 71 (26.3) | 656 (26.3) |
| Alzheimer’s Disease | 81 (30.0) | 967 (38.8) |
| Angina Pectoris | 30 (11.1) | 209 (8.4) |
| Chronic Obstructive Pulmonary Disease | 78 (28.9) | 660 (26.5) |
| Diabetes Mellitus | 93 (34.4) | 759 (30.4) |
| Dyslipidemia | 72 (26.7) | 551 (22.1) |
| Hypertension | 184 (68.1) | 1454 (58.3) |
| Peripheral Vascular Disease | 33 (12.2) | 209 (8.4) |
| Tachyarrhythmias | 22 (8.1) | 127 (5.1) |
| Unstable Angina | 30 (11.1) | 223 (8.9) |
| Elixhauser comorbidity score | ||
| 0 to 2 | 66 (24.4) | 736 (29.5) |
| 3 to 4 | 157 (58.1) | 1388 (55.6) |
| ≥5 | 47 (17.4) | 371 (14.9) |
| ADL status before hospitalizationc | ||
| Independent to limited assistance required | 113 (41.9) | 987 (39.6) |
| Extensive assistance required | 91 (33.7) | 748 (30.0) |
| Extensive dependency | 66 (24.4) | 760 (30.5) |
| Cognitive status before hospitalizationd | ||
| Intact cognition | 79 (29.3) | 439 (17.6) |
| Borderline intact to mild impairment | 89 (33.0) | 826 (33.1) |
| Moderate to severe impairment | 102 (37.8) | 1230 (49.3) |
| CHESS score before hospitalizatione | ||
| No health instability (0) | 135 (50.0) | 1415 (56.7) |
| Minimal health instability (1) | 91 (33.7) | 705 (28.3) |
| Low to high health instability (2 to 4) | 44 (16.3) | 375 (15.0) |
| Number of medications before hospitalization, mean (SD) | 13.0 (4.9) | 11.2 (4.9) |
| 0 to 10 | 87 (32.2) | 1172 (47.0) |
| 11 to 14 | 81 (30.0) | 758 (30.4) |
| ≥15 | 102 (37.8) | 565 (22.6) |
| Medication use before hospitalization | ||
| Anticoagulant | 16 (5.9) | 211 (8.5) |
| Antiplatelet | 26 (9.6) | 270 (10.8) |
| Calcium channel blockers | 46 (17.0) | 365 (14.6) |
| Loop diuretic | 62 (23.0) | 675 (27.1) |
| Thiazide diuretic | 14 (5.2) | 104 (4.2) |
| Vasodilator | 32 (11.9) | 332 (13.3) |
| Alpha blocker | 15 (5.6) | 137 (5.5) |
| Nitrate | 30 (11.1) | 315 (12.6) |
| Selective serotonin reuptake inhibitor | 63 (23.3) | 733 (29.4) |
| Atypical antipsychotic | 16 (5.9) | 238 (9.5) |
| Hypnotic | 15 (5.6) | 196 (7.9) |
| NH length of stay before AMI, median (IQR), days | 147 (60, 753) | 396 (90, 1120) |
| Length of hospital stay for AMI, median (IQR), days | 7 (5, 10) | 6 (4, 9) |
| No. of days in ICU or CCU | ||
| 0 | 110 (40.7) | 927 (37.2) |
| 1–2 | 63 (23.3) | 696 (27.9) |
| ≥3 | 97 (35.9) | 872 (34.9) |
Percentages have been rounded and may not total 100.
Due to the Data Use Agreement with and the Cell Size Suppression Policy of the Centers for Medicare and Medicaid Services, we are unable to report groups containing 0–10 participants and therefore cannot further stratify the “Non-White” race category.
Measured by the Morris 28-point scale of independence in ADLs and categorized as 0 to 14 (independent to limited assistance required), 15 to 19 (extensive assistance required), and 20 or higher (extensive dependency).
Measured by the Cognitive Performance Scale and trichotomized as 0 (intact), 1 to 2 (borderline intact to mild impairment), and 3 to 5 (moderate to severe impairment; roughly equivalent to a Folstein Mini-Mental State Examination score of ≤14 of 30).
Scores ranging from 0 to 5, with higher scores indicating greater health instability.
ACEI angiotensin-converting enzyme inhibitor, ADL activities of daily living, AMI acute myocardial infarction, ARB angiotensin II receptor blocker, CCU coronary care unit, CHESS Changes in Health, End-stage Disease, Signs, and Symptoms, ICU intensive care unit, IQR interquartile range, NH nursing home, SD standard deviation.
During 90-day follow-up, 287 of 2,765 participants died (10.4%); 713 (25.8%) were rehospitalized; and 473 (17.1%) experienced a significant functional decline.
3.2. Outcomes of ARB versus ACEI Use
Before IPT weighting, outcomes (Table 2) were not markedly different between ARB and ACEI users for 90-day mortality (OR=0.83, 95% CI 0.53–1.28) and re-hospitalization (OR=1.29, 95% CI 0.98–1.71), though ARB users had a greater risk of functional decline (OR=1.45, 95%CI 1.07–1.98). After IPT weighting, outcomes remained comparable between treatment groups for 90-day mortality (IPTW adjusted OR=1.18, 95% CI 0.78–1.79) and re-hospitalization (IPTW adjusted OR=1.22, 95% CI 0.90–1.65). The IPTW estimate for functional decline attenuated toward the null (IPTW adjusted OR=1.23, 95% CI 0.88–1.74), suggesting no marked difference between ARB and ACEI users.
Table 2.
Effect of ARB use versus ACEI use following myocardial infarction on 90-day outcomes among older NH residents before and after IPTW.
| Percent with Outcome (%) | Unweighted OR (95% CLs) | IPTW OR (95% CLs) | Unweighted Absolute Risk Difference (95% CLs)a,b | IPTW Absolute Risk Difference (95% CLs)a,b | ||
|---|---|---|---|---|---|---|
| Outcome | ARB | ACEI | ||||
| Mortality | 8.9 | 10.5 | 0.83 (0.53–1.28) | 1.18 (0.78–1.79) | −1.65 (−5.49, 2.18) | 1.68 (−3.48, 6.84) |
| Rehospitalization | 30.7 | 25.3 | 1.29 (0.98–1.71) | 1.22 (0.90–1.65) | 5.61 (−0.15, 11.37) | 3.71 (−2.84, 10.26) |
| Functional Decline | 22.6 | 16.5 | 1.45 (1.07–1.98) | 1.23 (0.88–1.74) | 6.08 (0.88, 11.28) | 2.78 (−2.76, 8.33) |
Reported as a percent rather than a proportion.
Confidence intervals estimated using bootstrapping with 10,000 replicates.
ACEI angiotensin-converting enzyme inhibitor, ARB angiotensin II receptor blocker, CL confidence limit, IPTW inverse probability of treatment-weighted, OR odds ratio.
3.3. Treatment Effects in Subgroups
In the IPTW weighted cohort, we observed that ARB use was associated with an increased likelihood of 90-day mortality (Figure 1) compared to ACEI use among individuals with moderate to severe cognitive impairment (OR=1.75, 95%CI 1.07–2.88), but not among individuals with intact cognition to mild impairment (OR=0.57, 95%CI 0.25–1.33)(p value=0.03 for effect modification by cognitive impairment). We observed a similar pattern for the rehospitalization outcome (Figure 2)(p value=0.02 for effect modification by cognitive impairment). Additionally, we found that ARB use was associated with an increased likelihood of 90-day rehospitalization (Figure 2) compared to ACEI use among individuals aged <85 (OR=1.70, 95%CI 1.16–2.47), but not among those aged ≥85 (OR=0.70, 95%CI 0.40–1.19)(p value=0.03 for effect modification by age). Functional decline between ARB and ACEI users was similar across a variety of patient characteristics (Figure 3).
Figure 1. Subgroup analyses of the effect of ARB use versus ACEI use on mortality among older NH residents after myocardial infarction.
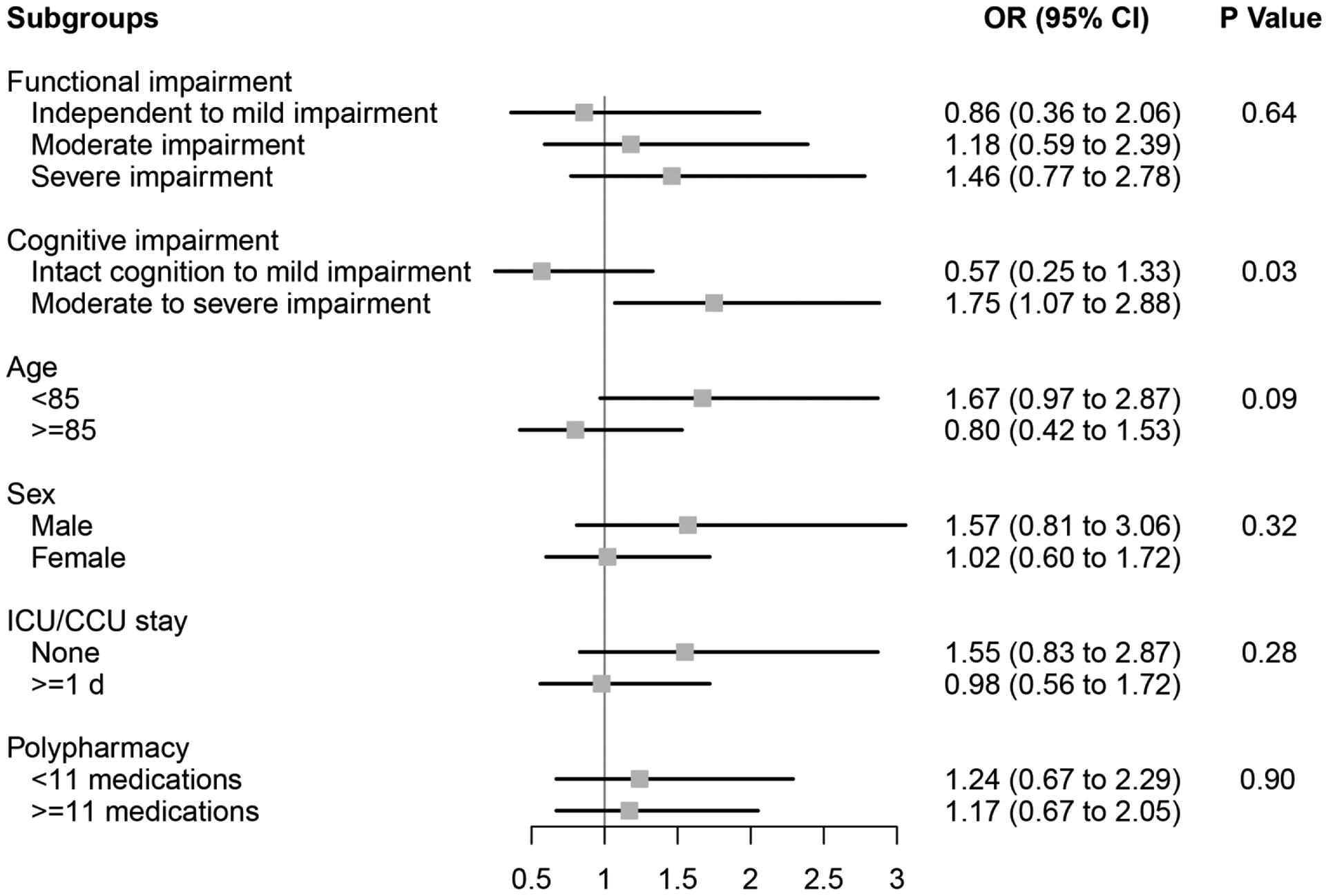
Functional impairment measured using the Minimum Data Set 28-point Activities of Daily Living (ADL) Scale; Independent to mild impairment is represented by an ADL score of 0 to 14 (independent to limited assistance required with ADLs), moderate impairment is represented by an ADL score of 15 to 19 (extensive assistance required with ADLs), and severe impairment is represented by an ADL score of ≥20 (extensive dependency in ADLs).
Cognitive impairment measured using the Minimum Data Set Cognitive Performance Score (CPS); Intact to Mild Impairment is represented by a CPS score of 0 to 2, and Moderate to Severe Impairment is a score of ≥3 (roughly equivalent to a Folstein Mini-Mental State Examination score of ≤14 of 30).
ACEI angiotensin-converting enzyme inhibitor, ARB angiotensin II receptor blocker, CCU coronary care unit, CI confidence interval, ICU intensive care unit, NH nursing home, OR odds ratio.
P Values for effect modification by subgroup characteristic.
Figure 2. Subgroup analyses of the effect of ARB use versus ACEI use on rehospitalization among older NH residents after myocardial infarction.
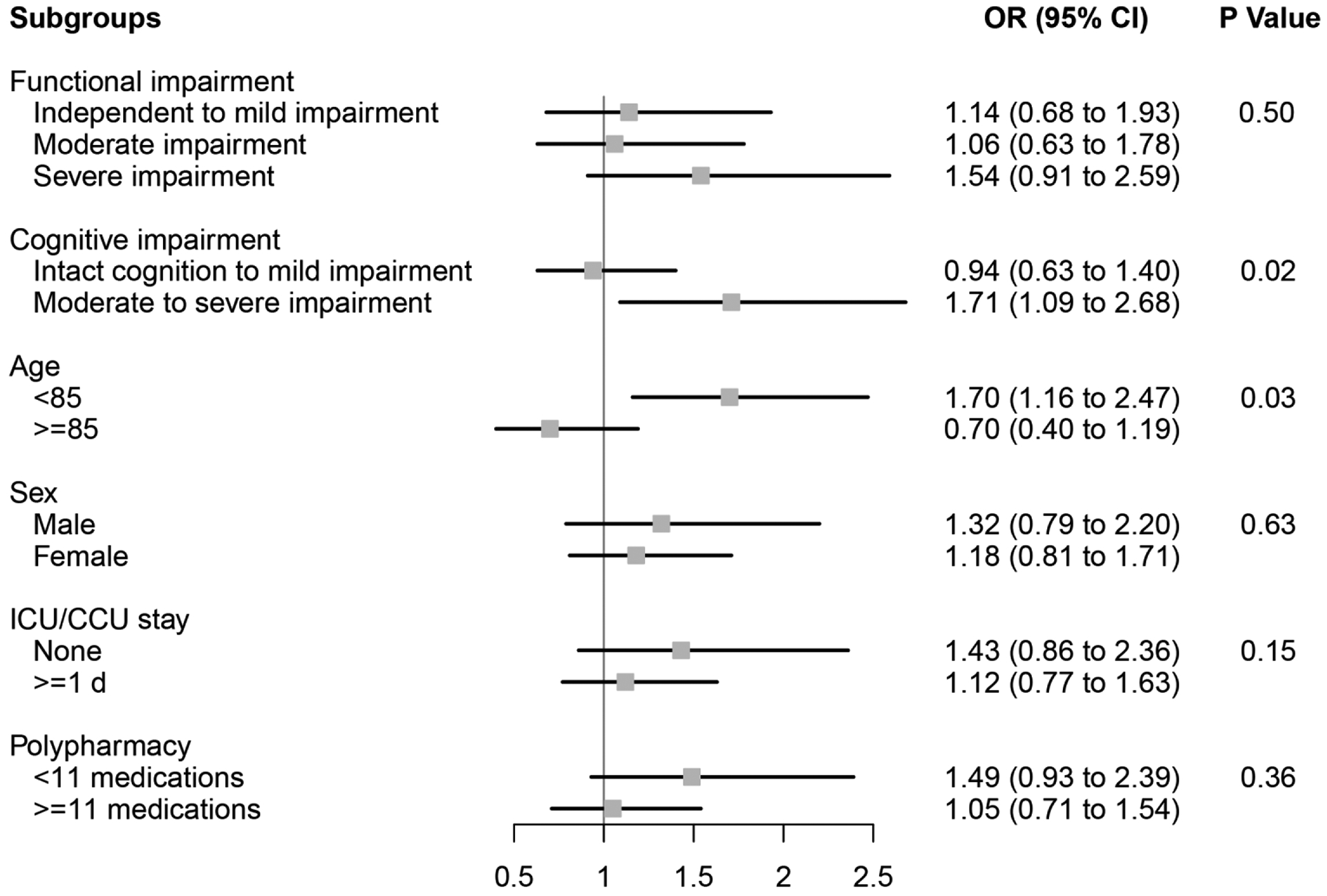
Functional impairment measured using the Minimum Data Set 28-point Activities of Daily Living (ADL) Scale; Independent to mild impairment is represented by an ADL score of 0 to 14 (independent to limited assistance required with ADLs), moderate impairment is represented by an ADL score of 15 to 19 (extensive assistance required with ADLs), and severe impairment is represented by an ADL score of ≥20 (extensive dependency in ADLs).
Cognitive impairment measured using the Minimum Data Set Cognitive Performance Score (CPS); Intact to Mild Impairment is represented by a CPS score of 0 to 2, and Moderate to Severe Impairment is a score of ≥3 (roughly equivalent to a Folstein Mini-Mental State Examination score of ≤14 of 30).
ACEI angiotensin-converting enzyme inhibitor, ARB angiotensin II receptor blocker, CCU coronary care unit, CI confidence interval, ICU intensive care unit, NH nursing home, OR odds ratio.
P Values for effect modification by subgroup characteristic.
Figure 3. Subgroup analyses of the effect of ARB use versus ACEI use on functioning among older NH residents after myocardial infarction.
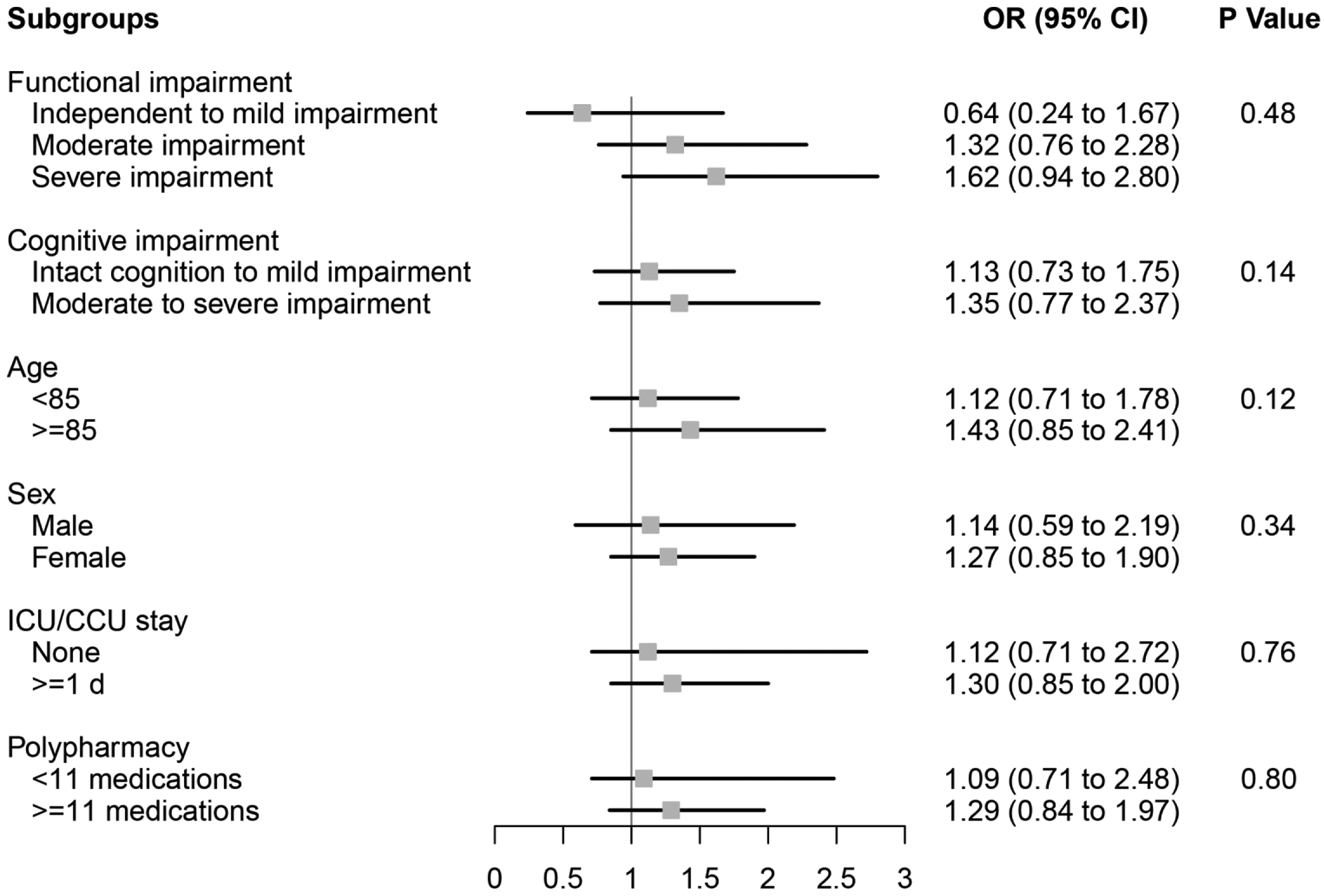
Functional impairment measured using the Minimum Data Set 28-point Activities of Daily Living (ADL) Scale; Independent to mild impairment is represented by an ADL score of 0 to 14 (independent to limited assistance required with ADLs), moderate impairment is represented by an ADL score of 15 to 19 (extensive assistance required with ADLs), and severe impairment is represented by an ADL score of ≥20 (extensive dependency in ADLs).
Cognitive impairment measured using the Minimum Data Set Cognitive Performance Score (CPS); Intact to Mild Impairment is represented by a CPS score of 0 to 2, and Moderate to Severe Impairment is a score of ≥3 (roughly equivalent to a Folstein Mini-Mental State Examination score of ≤14 of 30).
ACEI angiotensin-converting enzyme inhibitor, ARB angiotensin II receptor blocker, CCU coronary care unit, CI confidence interval, ICU intensive care unit, NH nursing home, OR odds ratio.
P Values for effect modification by subgroup characteristic.
3.4. Stability Analyses
The 180-day and 365-day mortality, rehospitalization, and functional decline results were consistent with the results of the main analysis (eTables 5 and 6).
3.5. Sensitivity Analyses
The E-values were 2.90 for the 90-day mortality estimate among individuals with moderate to severe cognitive impairment, 1.94 for the 90-day rehospitalization estimate among individuals with moderate to severe cognitive impairment, and 1.93 for the 90-day rehospitalization estimate among individuals aged <85, suggesting moderate sensitivity of the subgroup findings to unmeasured confounding.
4. DISCUSSION
In this national retrospective cohort study, we found that 90-day mortality, rehospitalization, and functional decline outcomes did not markedly differ between older ARB and ACEI users residing in NHs after AMI. However, in subgroup analyses, we observed that ARBs were associated with increased mortality and rehospitalization in individuals with moderate to severe cognitive impairment, and increased rehospitalization in those <85 years. Given the absence of a clear mechanism or explanation for the subgroup findings, they merit further scrutiny and replication. Since use of either ARBs or ACEIs resulted in comparable outcomes post-AMI in our overall NH population, providers, patients, and caregivers should consider basing their decisions about which class to use on other considerations. These considerations might include patient preferences, known adverse events specific to one of the two medication classes (e.g., ACEI-induced cough and angioedema), drug costs based on patients’ individual insurance and prescription drug coverage, and medication availability in the face of drug shortages and recalls.
Data on the comparative effectiveness of ARBs and ACEIs among older NH residents are scarce, both after AMI and in general. The two landmark trials comparing ARBs and ACEIs, the Valsartan in Acute Myocardial Infarction (VALIANT) trial and the Optimal Trial in Myocardial Infarction with Angiotensin II Antagonist Losartan (OPTIMAAL), enrolled study populations with mean ages of 65 and 67 years, respectively (versus 83 in our study)(33–35). Neither study provided explicit information on trial participants’ frailty status, cognitive status, functional status, multimorbidity, or polypharmacy (34,35). Both studies observed no difference in all-cause mortality between ARB and ACEI users. Despite arising from a much frailer and older population, our results are consistent with the estimates from VALIANT and OPTIMAAL, providing evidence to support our hypothesis that outcomes should not differ between ARBs and ACEIs.
Some observational studies have attempted to extend the findings of VALIANT and OPTIMAAL to older adults in real-world settings, though like the RCTs, their study populations were not as old or frail as ours. One such study used 1994–2004 Medicare Parts A and B claims linked to prescription drug data for New Jersey and Pennsylvania residents to examine 1-year all-cause mortality between ARB and ACEI users post-AMI (10). In an analysis restricting to new users, the investigators found no difference in 1-year mortality between ARB and ACEI users (multivariable-adjusted HR=1.09, 95%CI 0.85–1.39). The population of this study may be most comparable to our own. Yet, their study population was an average of about 3 years younger than ours, less than 6% of their population versus 100% of ours resided in a NH, and the 1-year mortality of their population was 14.3% versus 39.2% in ours, all of which suggests our study population was much frailer. Despite the greater importance of all-cause mortality, all-cause rehospitalization, and functional outcomes to many older adults, other observational studies have focused on cardiovascular or other cause-specific mortality or rehospitalization outcomes (36,37). Little attention has been paid to functioning. Also of note, few studies have examined use of RAAS inhibitors in the immediate post-acute AMI period, though use may be particularly impactful during that critical time period.
The differential effects of ARBs versus ACEIs we observed in subgroups defined by cognitive impairment and age were surprising. The most likely explanation for the finding that ARBs were associated with worse outcomes is residual or unmeasured confounding, especially given the magnitude of the estimates. An alternative, though less likely, explanation is that there may truly be some biological effects that differ between ARBs and ACEIs. The biological mechanisms for such effects are unclear. Related to the cognitive impairment subgroup findings, a number of studies have examined the effects of RAAS agents on cognition and dementia, but few have directly compared how the effects of ARBs and ACEIs differ or if cognitive status might modify effects (38–41). However, the RAAS does exist in the brain (42).
4.1. Limitations
The findings of our study must be interpreted in light of several limitations.
First, due to the observational nature of our study, we cannot rule out the possibility of residual confounding. For example, we were unable to measure baseline left ventricular ejection fraction (LVEF), baseline severity of CHF, baseline renal function, or accurately differentiate ST-segment–elevation MI from non–ST-segment–elevation MI, which may have influenced prescribing of ACEIs over ARBs as relevant guidelines differentiate size and precision of treatment effect based on the aforementioned factors (1,2,43,44). Disease-related concerns for ARB or ACEI use that may warrant close monitoring or avoiding the drug classes altogether (e.g., history of bilateral renal artery stenosis) and procedures conducted during the index hospital admission (e.g., percutaneous coronary intervention, coronary catheterization, coronary artery bypass graft) were also challenging to accurately measure. Additionally, prior studies have demonstrated substantial geographic variation in ARB versus ACEI prescribing (45). Since census region was the smallest geographic unit we could include in our propensity score estimation models due to the relatively limited number of ARB users, residual confounding by smaller geographic units may remain.
Second, the small number of ARB users relative to ACEI users limited our statistical power and served as a barrier to detecting small or moderate magnitude effects. The smaller number of ARB users is understandable given that guidelines for both ST-segment–elevation MI and non–ST-segment–elevation MI generally recommended ACEIs as first-line and ARBs for those who were ACEI intolerant (1,2,46). Nonetheless, to our knowledge, this is the largest study of older NH residents comparing ARBs and ACEIs.
Third, we did not have a validated measure of cardiovascular cause-specific mortality. It is possible that ARBs and ACEIs have differential effects on cardiovascular disease mortality, but not all-cause mortality. Additionally, due to the relatively small size of the ARB group and the absence of well-validated measures, we were not able to examine adverse event outcomes like hypotension, hyperkalemia, renal dysfunction, or cough. To enable robust assessment of ARB and ACEI exposure, we also excluded patients who died or were rehospitalized within the first 14 days of hospital discharge. This exclusion prevented us from evaluating the effect of medication on outcomes during this period.
Fourth, we did not examine the comparative effectiveness of individual drugs within or between the ARB and ACEI classes, though some evidence from less frail populations than our own has suggested effects may differ by individual drug (47). We also were unable to conduct analyses of ARB and ACEI doses due to the nature of our data.
Fifth, our study used older data from 2007 to 2010, which may affect the generalizability of our results to more recent time periods if the distributions of important treatment effect modifiers have changed over time.
Finally, data on functional outcomes assessments was limited to intermittent reporting through MDS assessments. We were unable to measure functional decline continuously like mortality and rehospitalization. If functional decline occurred between an MDS assessment and death, that outcome would have been unmeasured. However, it is unlikely that this issue would have differentially affected ARB or ACEI users. Other limitations of the datasets and study cohort have been previously discussed (18–24).
However, several factors support the robustness of our findings. We collected extensive data on 91 measured covariates and many covariates were well-balanced between treatment groups even before IPT weighting. Furthermore, in prior work, we conducted a companion validation study using national data from the Department of Veterans Affairs, which contains information on vital signs (e.g., blood pressure, pulse), laboratory test results (e.g., estimated glomerular filtration rate (eGFR), peak troponin), procedures (e.g., revascularization procedure during index AMI hospitalization) and measures of cardiac function (e.g., LVEF) that was missing from our linked Medicare and MDS data (18). Those analyses suggest that our results would not be substantially altered by the inclusion of these missing variables (e.g., LVEF, eGFR). Finally, comparing two active treatments with a shared indication (i.e., an active-comparator design) rather than an active treatment versus no treatment reduces both measured and unmeasured confounding.
4.2. Conclusion
In summary, use of an ARB or ACEI was associated with similar 90-day mortality, rehospitalization, and functional decline outcomes in older NH residents post-AMI. Our findings concord with prior data and suggest that clinicians can reasonably prescribe either an ARB or ACEI after AMI for secondary prevention. Carefully designed randomized controlled trials could be considered to further evaluate if cognitive status truly modifies the effects of ARBs versus ACEIs on rehospitalization and mortality outcomes. In the meantime, providers, patients, and their caregivers should consider other factors like patient preferences, known adverse events specific to one of the two medication classes or individual drugs within them, and drug costs to guide their selection between ARBs and ACEIs.
Supplementary Material
Key Points:
Limited evidence exists regarding differences in outcomes between ARBs and ACEIs among older nursing home residents after AMI.
No marked differences existed between ARB versus ACEI users for mortality (OR=1.18, 95% CI 0.78–1.79), rehospitalization (OR=1.22, 95% CI 0.90–1.65), or functional decline (OR=1.23, 95% CI 0.88–1.74).
Clinicians should decide between ARBs or ACEIs for older nursing home residents post-AMI based on factors other than effectiveness.
ACKNOWLEDGEMENTS
Funding: This study was supported by grants R01HL111032 and K24AG049057 from the National Institutes of Health and by award 5K12HS022998 from the Agency for Healthcare Research and Quality (Dr. Zullo). Dr. Zullo was also supported by a Department of Veterans Affairs Office of Academic Affiliations Advanced Fellowship in Health Services Research and Development, a grant from the National Institute on Aging (R21AG061632), and a grant from the National Institute of General Medical Sciences (U54GM115677).The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Meeting Presentations: Parts of this paper were presented at the Eastern States Conference for Pharmacy Residents and Preceptors Meeting, April 30, 2019, Hershey, Pennsylvania.
Conflicts of Interest: Drs. Zullo, Riester, Erqou, Wu, Rudolph, and Steinman declare that they have no potential conflicts of interest that might be relevant to the contents of this manuscript.
The institutional review boards of Brown University; the University of California San Francisco; and the San Francisco Veterans Affairs (VA) Health Care System approved the study protocol. Informed consent was not required.
REFERENCES
- 1.American College of Emergency P, Society for Cardiovascular A, Interventions, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Journal of the American College of Cardiology. 2013;61(4):e78–140. DOI: 10.1016/j.jacc.2012.11.019. [DOI] [PubMed] [Google Scholar]
- 2.Amsterdam EA, Wenger NK, Brindis RG, et al. 2014 AHA/ACC Guideline for the Management of Patients with Non-ST-Elevation Acute Coronary Syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Journal of the American College of Cardiology. 2014;64(24):e139–e228. DOI: 10.1016/j.jacc.2014.09.017. [DOI] [PubMed] [Google Scholar]
- 3.Swedberg K, Held P, Kjekshus J, et al. Effects of the early administration of enalapril on mortality in patients with acute myocardial infarction. Results of the Cooperative New Scandinavian Enalapril Survival Study II (CONSENSUS II). The New England journal of medicine. 1992;327(10):678–684. DOI: 10.1056/NEJM199209033271002. [DOI] [PubMed] [Google Scholar]
- 4.Gruppo Italiano per lo Studio Della Sopravvivenza Nell’Infarto Miocardico. Six-month effects of early treatment with lisinopril and transdermal glyceryl trinitrate singly and together withdrawn six weeks after acute myocardial infarction: the GISSI-3 trial. Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto Miocardico. Journal of the American College of Cardiology. 1996;27(2):337–344. [PubMed] [Google Scholar]
- 5.ISIS-4 (Fourth International Study of Infarct Survival) Collaborative Group. ISIS-4: a randomised factorial trial assessing early oral captopril, oral mononitrate, and intravenous magnesium sulphate in 58,050 patients with suspected acute myocardial infarction. ISIS-4 (Fourth International Study of Infarct Survival) Collaborative Group. Lancet. 1995;345(8951):669–685. [PubMed] [Google Scholar]
- 6.Ambrosioni E, Borghi C, Magnani B. The effect of the angiotensin-converting-enzyme inhibitor zofenopril on mortality and morbidity after anterior myocardial infarction. The Survival of Myocardial Infarction Long-Term Evaluation (SMILE) Study Investigators. The New England journal of medicine. 1995;332(2):80–85. DOI: 10.1056/NEJM199501123320203. [DOI] [PubMed] [Google Scholar]
- 7.Pfeffer MA, Braunwald E, Moye LA, et al. Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction. Results of the survival and ventricular enlargement trial. The SAVE Investigators. The New England journal of medicine. 1992;327(10):669–677. DOI: 10.1056/NEJM199209033271001. [DOI] [PubMed] [Google Scholar]
- 8.The Acute Infarction Ramipril Efficacy (AIRE) Study Investigators. Effect of ramipril on mortality and morbidity of survivors of acute myocardial infarction with clinical evidence of heart failure. The Acute Infarction Ramipril Efficacy (AIRE) Study Investigators. Lancet. 1993;342(8875):821–828. [PubMed] [Google Scholar]
- 9.Kober L, Torp-Pedersen C, Carlsen JE, et al. A clinical trial of the angiotensin-converting-enzyme inhibitor trandolapril in patients with left ventricular dysfunction after myocardial infarction. Trandolapril Cardiac Evaluation (TRACE) Study Group. The New England journal of medicine. 1995;333(25):1670–1676. DOI: 10.1056/NEJM199512213332503. [DOI] [PubMed] [Google Scholar]
- 10.Winkelmayer WC, Fischer MA, Schneeweiss S, et al. Angiotensin inhibition after myocardial infarction: does drug class matter? Journal of general internal medicine. 2006;21(12):1242–1247. DOI: 10.1111/j.1525-1497.2006.00590.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ricci F, Di Castelnuovo A, Savarese G, et al. ACE-inhibitors versus angiotensin receptor blockers for prevention of events in cardiovascular patients without heart failure - A network meta-analysis. International journal of cardiology. 2016;217:128–134. DOI: 10.1016/j.ijcard.2016.04.132. [DOI] [PubMed] [Google Scholar]
- 12.Krishnaswami A, Steinman MA, Goyal P, et al. Deprescribing in Older Adults With Cardiovascular Disease. Journal of the American College of Cardiology. 2019;73(20):2584–2595. DOI: 10.1016/j.jacc.2019.03.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zullo AR, Gray SL, Holmes HM, et al. Screening for Medication Appropriateness in Older Adults. Clin Geriatr Med. 2018;34(1):39–54. DOI: 10.1016/j.cger.2017.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. The journals of gerontology Series A, Biological sciences and medical sciences. 2001;56(3):M146–156. [DOI] [PubMed] [Google Scholar]
- 15.Fried LP, Ferrucci L, Darer J, et al. Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. The journals of gerontology Series A, Biological sciences and medical sciences. 2004;59(3):255–263. [DOI] [PubMed] [Google Scholar]
- 16.Rockwood K, Mitnitski A. Frailty defined by deficit accumulation and geriatric medicine defined by frailty. Clin Geriatr Med. 2011;27(1):17–26. DOI: 10.1016/j.cger.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 17.Fleg JL, Forman DE, Berra K, et al. Secondary prevention of atherosclerotic cardiovascular disease in older adults: a scientific statement from the American Heart Association. Circulation. 2013;128(22):2422–2446. DOI: 10.1161/01.cir.0000436752.99896.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steinman MA, Zullo AR, Lee Y, et al. Association of beta-Blockers With Functional Outcomes, Death, and Rehospitalization in Older Nursing Home Residents After Acute Myocardial Infarction. JAMA internal medicine. 2017;177(2):254–262. DOI: 10.1001/jamainternmed.2016.7701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zullo AR, Hersey M, Lee Y, et al. Outcomes of “Diabetes- Friendly” versus “Diabetes- Unfriendly” Beta- blockers in Older Nursing Home Residents with Diabetes after Acute Myocardial Infarction. Diabetes, obesity & metabolism. 2018;20(12):2724–2732. DOI: 10.1111/dom.13451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zullo AR, Lee Y, Daiello LA, et al. Beta-Blocker Use in U.S. Nursing Home Residents After Myocardial Infarction: A National Study. Journal of the American Geriatrics Society. 2017;65(4):754–762. DOI: 10.1111/jgs.14671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zullo AR, Mogul A, Corsi K, et al. Association Between Secondary Prevention Medication Use and Outcomes in Frail Older Adults After Acute Myocardial Infarction. Circulation Cardiovascular quality and outcomes. 2019;12(4):e004942 DOI: 10.1161/CIRCOUTCOMES.118.004942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zullo AR, Olean M, Berry SD, et al. Patient-Important Adverse Events of beta-blockers in Frail Older Adults after Acute Myocardial Infarction. The journals of gerontology Series A, Biological sciences and medical sciences. 2018. DOI: 10.1093/gerona/gly191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zullo AR, Sharmin S, Lee Y, et al. Secondary Prevention Medication Use After Myocardial Infarction in U.S. Nursing Home Residents. Journal of the American Geriatrics Society. 2017;65(11):2397–2404. DOI: 10.1111/jgs.15144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zullo AR, Ofori-Asenso R, Wood M, et al. Effects of Statins for Secondary Prevention on Functioning and Other Outcomes Among Nursing Home Residents. Journal of the American Medical Directors Association. 2020;21(4):500–507 e508. DOI: 10.1016/j.jamda.2020.01.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Intrator O, Hiris J, Berg K, et al. The residential history file: studying nursing home residents’ long-term care histories(*). Health services research. 2011;46(1 Pt 1):120–137. DOI: 10.1111/j.1475-6773.2010.01194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Danaei G, Rodriguez LA, Cantero OF, et al. Observational data for comparative effectiveness research: an emulation of randomised trials of statins and primary prevention of coronary heart disease. Statistical methods in medical research. 2013;22(1):70–96. DOI: 10.1177/0962280211403603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hernan MA, Robins JM. Using Big Data to Emulate a Target Trial When a Randomized Trial Is Not Available. American journal of epidemiology. 2016;183(8):758–764. DOI: 10.1093/aje/kwv254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huitfeldt A, Hernan MA, Kalager M, et al. Comparative Effectiveness Research Using Observational Data: Active Comparators to Emulate Target Trials with Inactive Comparators. EGEMS (Wash DC). 2016;4(1):1234 DOI: 10.13063/2327-9214.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carpenter GI, Hastie CL, Morris JN, et al. Measuring change in activities of daily living in nursing home residents with moderate to severe cognitive impairment. BMC geriatrics. 2006;6:7 DOI: 10.1186/1471-2318-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morris JN, Fries BE, Morris SA. Scaling ADLs within the MDS. The journals of gerontology Series A, Biological sciences and medical sciences. 1999;54(11):M546–553. [DOI] [PubMed] [Google Scholar]
- 31.VanderWeele TJ. On the distinction between interaction and effect modification. Epidemiology (Cambridge, Mass). 2009;20(6):863–871. DOI: 10.1097/EDE.0b013e3181ba333c. [DOI] [PubMed] [Google Scholar]
- 32.VanderWeele TJ, Ding P. Sensitivity Analysis in Observational Research: Introducing the E-Value. Annals of internal medicine. 2017;167(4):268–274. DOI: 10.7326/M16-2607. [DOI] [PubMed] [Google Scholar]
- 33.Velazquez EJ, Pfeffer MA, McMurray JV, et al. VALsartan In Acute myocardial iNfarcTion (VALIANT) trial: baseline characteristics in context. Eur J Heart Fail. 2003;5(4):537–544. DOI: 10.1016/s1388-9842(03)00112-0. [DOI] [PubMed] [Google Scholar]
- 34.Dickstein K, Kjekshus J, Group OSCotOS. Effects of losartan and captopril on mortality and morbidity in high-risk patients after acute myocardial infarction: the OPTIMAAL randomised trial. Optimal Trial in Myocardial Infarction with Angiotensin II Antagonist Losartan. Lancet. 2002;360(9335):752–760. [DOI] [PubMed] [Google Scholar]
- 35.Pfeffer MA, McMurray JJ, Velazquez EJ, et al. Valsartan, captopril, or both in myocardial infarction complicated by heart failure, left ventricular dysfunction, or both. The New England journal of medicine. 2003;349(20):1893–1906. DOI: 10.1056/NEJMoa032292. [DOI] [PubMed] [Google Scholar]
- 36.Ko D, Azizi P, Koh M, et al. Comparative effectiveness of ACE inhibitors and angiotensin receptor blockers in patients with prior myocardial infarction. Open Heart. 2019;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hara M, Sakata Y, Nakatani D, et al. Comparison of 5-year survival after acute myocardial infarction using angiotensin-converting enzyme inhibitor versus angiotensin II receptor blocker. The American journal of cardiology. 2014;114(1):1–8. DOI: 10.1016/j.amjcard.2014.03.055. [DOI] [PubMed] [Google Scholar]
- 38.Zhuang S, Wang HF, Wang X, et al. The association of renin-angiotensin system blockade use with the risks of cognitive impairment of aging and Alzheimer’s disease: A meta-analysis. J Clin Neurosci. 2016;33:32–38. DOI: 10.1016/j.jocn.2016.02.036. [DOI] [PubMed] [Google Scholar]
- 39.Zhuang S, Wang HF, Li J, et al. Renin-angiotensin system blockade use and risks of cognitive decline and dementia: A meta-analysis. Neurosci Lett. 2016;624:53–61. DOI: 10.1016/j.neulet.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 40.Stuhec M, Keuschler J, Serra-Mestres J, et al. Effects of different antihypertensive medication groups on cognitive function in older patients: A systematic review. Eur Psychiatry. 2017;46:1–15. DOI: 10.1016/j.eurpsy.2017.07.015. [DOI] [PubMed] [Google Scholar]
- 41.Barthold D, Joyce G, Wharton W, et al. The association of multiple anti-hypertensive medication classes with Alzheimer’s disease incidence across sex, race, and ethnicity. PloS one. 2018;13(11):e0206705 DOI: 10.1371/journal.pone.0206705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wright JW, Harding JW. The brain RAS and Alzheimer’s disease. Exp Neurol. 2010;223(2):326–333. DOI: 10.1016/j.expneurol.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 43.Canadian Cardiovascular S, American Academy of Family P, American College of C, et al. 2007 focused update of the ACC/AHA 2004 guidelines for the management of patients with ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Journal of the American College of Cardiology. 2008;51(2):210–247. DOI: 10.1016/j.jacc.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 44.Anderson JL, Adams CD, Antman EM, et al. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-Elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines for the Management of Patients With Unstable Angina/Non-ST-Elevation Myocardial Infarction) developed in collaboration with the American College of Emergency Physicians, the Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation and the Society for Academic Emergency Medicine. Journal of the American College of Cardiology. 2007;50(7):e1–e157. DOI: 10.1016/j.jacc.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 45.Donohue JM, Morden NE, Gellad WF, et al. Sources of regional variation in Medicare Part D drug spending. The New England journal of medicine. 2012;366(6):530–538. DOI: 10.1056/NEJMsa1104816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Amsterdam EA, Wenger NK, Brindis RG, et al. 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;130(25):e344–426. DOI: 10.1161/CIR.0000000000000134. [DOI] [PubMed] [Google Scholar]
- 47.Pilote L, Abrahamowicz M, Rodrigues E, et al. Mortality rates in elderly patients who take different angiotensin-converting enzyme inhibitors after acute myocardial infarction: a class effect? Annals of internal medicine. 2004;141(2):102–112. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


