Abstract
This paper focuses on the idea that there are clear social hallmarks of aging including low lifetime socioeconomic status, adversity in childhood and adulthood, being a member of a minority group, adverse health behaviors, and adverse psychological states. The “Social Hallmarks of Aging” are analogous to the “Geroscience Hallmarks of Aging” in reflecting a set of underlying and interrelated social causes of multiple agerelated health outcomes. This paper presents empirical work incorporating the social hallmarks of aging with indicators of multiple biological hallmarks of aging as well downstream biology in explaining a range of health outcomes in order to show the relative strength of the associations of social and biological measures with important health outcomes. Social factors are strongly related to physical and cognitive functioning and multimorbidity in this older population and this remains true when the significant number of biological measures are controlled. This can be interpreted to mean that a significant amount of social variance in age-related health outcomes is not explained by these measures of biology. Indicators of the geroscience hallmarks of aging only relate modestly to the variability in human health outcomes. Attention to the social hallmarks related to human aging can usefully be incorporated into work on the biological hallmarks of aging to make greater progress in understanding human aging.
Keywords: hallmarks of aging, biological age – chronological age, social determinants of aging
Introduction
The new focus on “Geroscience” within the Biology of Aging has energized the entire aging research community to try to identify the role of a limited number of underlying biological mechanisms of aging that are at the root of a process of physiological deterioration which underlies aging- related health outcomes (Barzilai et al. 2018; Kennedy et al. 2014; Moffitt 2020; Sierra 2016). These mechanisms have been outlined by several researchers and, while they differ in details, the basic ideas are fairly similar – that a set of intertwined molecular and cellular changes are at the heart of age-related change underlying multiple age-related health conditions. For instance, Lopez-Otin et al. (2013) have listed nine “Hallmarks of Aging”: genomic instability, telomere attrition, epigenetic alterations, loss of proteostasis, deregulated nutrient sensing, mitochondrial dysfunction, cellular senescence, stem cell exhaustion, and altered intercellular communication. Franceschi et al. (2018) focus on a somewhat different set of mechanisms centered on inflammaging with 7 pillars of aging: stress, macromolecular damage, proteostasis, stem-cell regeneration, epigenetics, and metabolism. Future research will be needed to clarify the relative importance of these various mechanisms in human aging as integration of all of these measures in the study of aging of a human cohort has not been possible to date. Certainly such work Is needed before interventions to affect the aging process can be undertaken.
Determinants of age-related health outcomes in humans
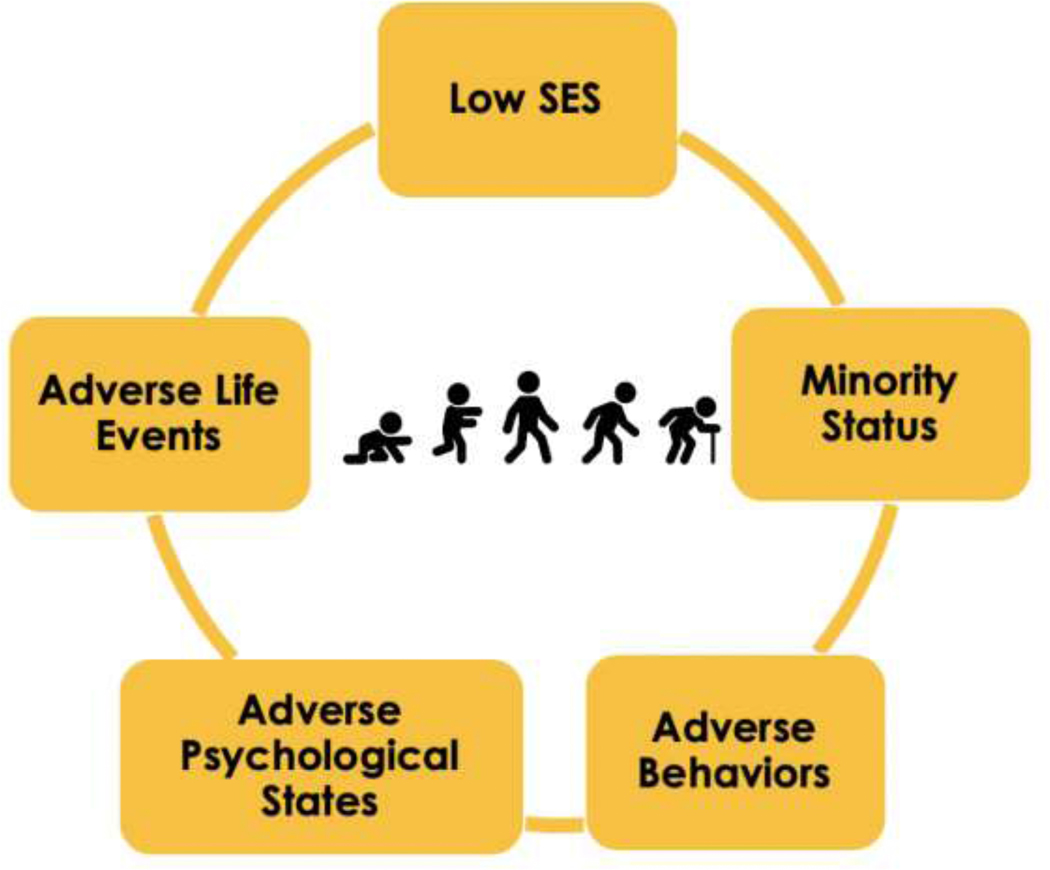
It is time to integrate this view of what causes aging-related health change with the significant body of work on human aging that makes clear the importance of what I call the “social hallmarks of aging.” Social hallmarks of aging include: low socioeconomic status, minority status, adverse life events, adverse psychological states, and adverse behaviors (Figure 1). There is an extensive body of literature presenting evidence that variability in the aging process of humans is highly related to these social factors. This is generally characterized as research on the social determinants of health (Adler and Ostrove 1999; Braveman et al. 2011). This work, however, has not presented a coherent statement that the major outcomes of aging are all related to these social processes; nor has it characterized the variability in the way social factors link to a variety of age-related health outcomes.
Figure 1:
The Social Hallmarks of Aging
Like the biological hallmarks of aging, increased levels of social adversity are associated with accelerated aging across multiple outcomes, while decreased exposure to social hallmarks retards the onset of poor health outcomes with aging (Friedman et al. 2015; Jones et al. 2019; Levine and Crimmins 2014). Like the molecular and cellular mechanisms characterized as the “Biological Hallmarks of Aging,” the social mechanisms are highly interrelated mechanisms, which are very difficult to separate either experimentally or in human lives. This view of the social hallmarks of aging reflects what recent social theory has termed the “fundamental” cause of poor health in multiple societies and across time (Link and Phelan 1995). Low social status may be the most fundamental of causes as it is connected to multiple types of resources including money, power, connections, and knowledge (Adler et al. 1994; Marmot et al. 2012). It is also connected to additional negative influences on age-linked health outcomes: experience of adverse life events, traumas, and stressors, adverse psychological states, and adverse behaviors.
These are not new ideas to the study of health outcomes in social science. The many pathways through which social status works to affect age-related health outcomes have been explored for decades. However, early studies often focused on one age-related disease or on disability, rather than “aging.” For instance, early work showed the importance of social factors in cardiovascular risk (Kraus et al. 1980; Marmot et al. 1991), on disability (Cutler et al. 2006; Manton et al. 2006), and on mortality (Cutler et al. 2006). Gradually, many studies have examined multiple age-related outcomes including physical and cognitive functioning as well as multiple diseases and mortality, or the broad set of age-related health outcomes (Elovainio et al. 2009; Seeman et al. 2001). Models of health outcomes have increasingly noted that the risk factors for morbidity and mortality from many conditions overlap and that health change with age is a process of change with multiple dimensions (Crimmmins 2015). The idea of “Social Hallmarks of Aging” is analogous to the “Geroscience Hallmarks of Aging” in reflecting a set of underlying and interrelated social causes of multiple age-related health outcomes.
Models of human health have also increasingly incorporated the idea that “aging” is a lifelong process that begins very early in life (Ben- Shlomo and Kuh 2002; Kuh et al. 2003) and that health at older ages is affected by experiences throughout life. While specific times in life, or certain experiences, may be critical and leave a long lasting health imprint, others may reflect chronic states. The relative importance of early and late life factors, or chronic or acute experiences, have not been clarified for the major health-associated conditions related to aging although there is ample evidence of effects from all of these types of influences (Barker and Thornburg 2013). The many social pathways which affect health have also been explored over decades, but these have not been clearly examined as a set of highly intertwined circumstances affecting the set of health-related changes that accompany the aging experience.
The integration of biology into social science studies of aging
The fact that social determinants “get under the skin” through multisystem biology was introduced into social science work about 3 decades ago with the recognition of the value of determining the pathways through which social factors affect health outcomes if intervention was to be successful. “Allostatic load” was one of the early formulations for including multisystem biology changes into the study of aging (Kim et al. 2018; Seeman et al. 2001). This work grew out of a network of social scientists, physicians, and biologists who incorporated the measurement of biology as possible at the time into community surveys. Allostatic load laid the foundation for the idea that multiple aspects of biology affect multiple health outcomes linked to aging (Seeman et al. 2001). As technology has changed and medical and biological knowledge have thrived, more recent work has incorporated additional aspects of biology into summarizing the physiological changes with age (Belsky et al. 2015; Levine 2013). “Biological age,” “phenotypic age” and the “pace of aging” reflect recent attempts to integrate broader and deeper biology into human aging (Belsky et al. 2017; Liu et al. 2019; Moffit et al. 2017). Incorporation of these indicators has increased ability to explain age-related outcomes in multiple studies (Hastings et al 2017; Jylhävä et al 2017).
These indicators remain at a level primarily downstream from the changes reflected in the molecular and cellular mechanisms reflected in the biological hallmarks of aging. However, recently numerous studies of human populations have incorporated measurement of some biological hallmarks. Those most frequently included are telomere length and epigenetic changes represented by DNA methylation. Telomere length is seen by many as a summary measure of aging in and of itself; it has been referred to as a “miotic clock” because of its reductions with age and associations with disease (Notterman and Mitchell 2015; Puterman et al. 2016; Zhu et al 2019). Epigenetic age acceleration, also seen as a summary measure of aging, has been measured in numerous population studies in recent years (Fiorito et al. 2019; McCrory et al. 2019). Mitochondrial function is an additional hallmark of aging which can be represented by mitochondrial copy number which has been shown to be lower at older ages and to be linked to numerous diseases and poor cognitive functioning (Ashar et al. 2015; Lee et al. 2010; Montier et al. 2009; Picard et al. 2018; Taylor and Turnbull 2005; Thyagarajan et al. 2013). The relationships among some of these variables and their relative contribution to explaining outcomes has been the topic of a number of recent papers (Belsky et al. 2017; Jylhävä et al 2017).
Empirical results on associations of social and biological hallmarks of aging with health outcomes
Studies of aging humans are a long way from including all of the biological and social hallmarks of aging in one study of lifelong human health change with aging but we should take clues from the results we have to date on where to focus future research. As indicated above social scientists have attempted to begin integrating multiple biological and social hallmarks of aging as well as more extensive physiological measures into their studies. For many indicators, the measurement may still be less than ideal; nevertheless examining indicators of the major social influences with what can be measured in biology for multiple age-related health outcomes allows us to see both how much of the variability in outcomes is explained by social factors and by measured biological factors. To promote the case that more will be learned about human aging from integrating social factors along with biological factors that underlie the aging process into multidisciplinary work, some basic empirical data are presented that link indicators of the social and biological hallmarks of aging to four aging-related health outcomes: physical and cognitive functioning, multimorbidity, and mortality. This allows us to consider how similar or different are the predictors of major health outcomes related to aging; how relatively important are social and biological factors in explaining which outcomes; and whether the biological factors we can measure are mediators of the social variables as theorized. The value of this analysis is that the associations of all of the variables can be assessed in the same data allowing the comparative approach across the measured social and biological variables. We use a large national sample of Americans over the age of 50 for this analysis.
Data and analyses.
The data used are largely cross-sectional, collected in 2016 from a large nationally representative study of Americans over the age of 56, the Health and Retirement Study (HRS) (https://hrs.isr.umich.edu/documentation). The HRS began in 1992 but it first incorporated the collection of venous blood in 2016. These blood samples are the basis of extensive biological measurement in a subsample of 4,018 people which is the basis of the analytic group used for this demonstration. The original sample of 4018 is reduced to 1159 because of missing data for some of variables. The high level of missingness occurs because of the extensive list of both social and biological variables in the models and the many different aspects of the survey that needed to be completed over numerous waves to provide the data. Details on the missing cases are shown in Supplemental Figure 1. People who are missing are more likely to be members of ethnic minority groups and have a lower level of education. They are also likely to have a higher level of physical and cognitive dysfunction and are more likely to die before the next interview.
The four aging-related health outcomes which reflect the major adverse health outcomes linked to aging include: multimorbidity (presence of 5 diseases), disability (difficulty with ADL/IADL functioning), cognitive deficiency (score on cognitive tests subtracted from highest score possible), and mortality over two years. Social hallmarks are measured by indicators of social status in childhood and adulthood, race and ethnicity (which are included with the SES variables in allocating R2), adverse experiences in childhood, adult trauma, negative psychological states, poor health behaviors, and age and gender are included in regression equations. Details on the definitions of each of these variables and their year of collection are included in Supplemental Table 1.
Biological variables included reflect a number of indicators at multiple levels, both physiological, systemic, with some at the more basic cellular and molecular levels reflecting the biological hallmarks. Physiological functioning is measured by biological age minus chronological age using a validated indicator of physiological status (Levine 2013). This version of biological age includes 10 indicators: albumin, alkaline phosphatase, CMV, puff test results, CRP, creatinine, total cholesterol, BUN, fasting glucose, and systolic blood pressure. Subtracting chronological age from biological age minus chronological age provides a measure of how much older a person appears relative to the average in his/her age group based on this combination of measures. The other three biological indicators reflect attempts to begin to measure the biological hallmarks of aging: telomere length, mitochondrial copy number, and epigenetic age acceleration. Epigenetic age acceleration is reflected in an estimate of Horvath’s initial epigenetic clock which provides an estimate of epigenetic age based on 353 DNA methylation measures (Horvath 2013). Details on the measurement of the other biological variables are provided in Crimmins et al. (2017). For the non- Hispanic white sample, a model with polygenic risk scores relevant for these health outcomes based on GWAS of 2.1 million SNPS and provided by HRS is also examined. A polygenic score for cognition is included in the cognitive model; polygenic scores for coronary artery disease, myocardial infarction, and type 2 diabetes in the multimorbidity model; and a polygenic score for longevity in the physical functioning and mortality models. Belsky (2014) has discussed the value of using polygenic risk scores in samples such as used here. In these models, if variables reflect mechanisms mediating between prior causes and outcomes, their inclusion should reduce the effect of the prior causes and they should be significant themselves. We see the biological variables, aside from the polygenic scores, as intervening mechanisms between the social variables and the health outcomes. These reflect the potential mechanisms by which social variables “get under the skin.”
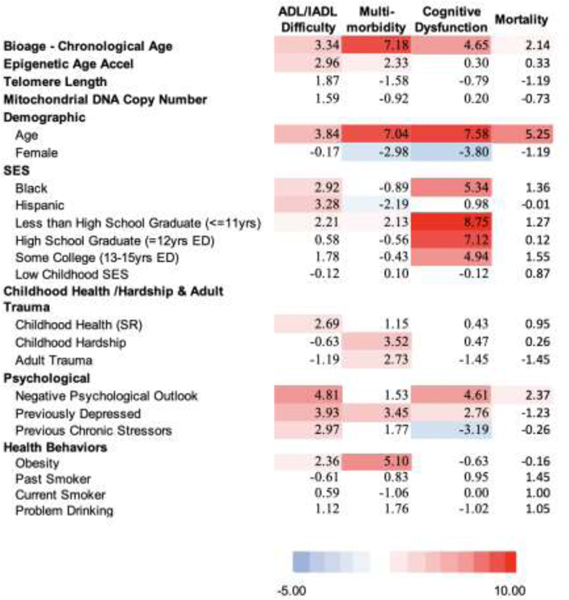
Results from four sets of equations are shown: the first includes demographic variables (age and sex) along with indicators of the social hallmarks, the second includes demographic variables and the set of biological variables, the third set incorporates all of the variables in the first two models, and the fourth set is only for non-Hispanic whites and includes the polygenic risk scores with all the other variables. Results are shown in both Table 1 and Figure 2. Table 1 shows the t values for each regression coefficient in a heat map so the strength of the relationship with the indicators can be judged. The darker the red results, the stronger the link to a poor health outcome; blue means a poor health outcome is less likely. Figure 2 attributes the variance explained (R2) to different groups of variables based on the Shapley decomposition method (Israeli 2007; Liu et al. 2019). Numbers for Figure 2 are shown in Supplemental Table 2. We are interested in how much variance is explained by the social variables, the biological variables, and how this changes when they are both included in the same models.
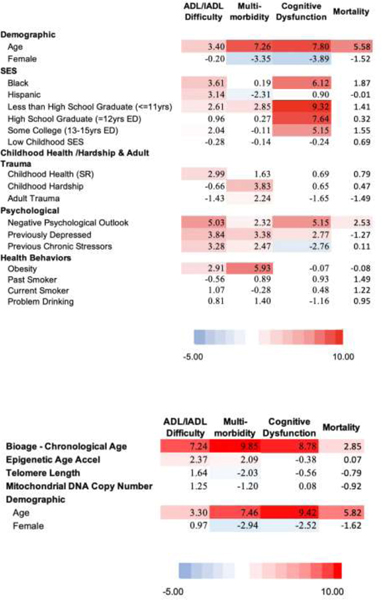
Table 1:
t values from regressions predicting 4 aging health outcomes with 3 models: Model 1 - the social hallmarks of aging, model 2 - multiple biological indicators, model 3 - includes both the social hallmarks of aging and biological indicators (N=1,159)
A. Model 1 - Social Hallmarks and Démographe Factors
B. Model 2 - Biological and Demographic Factors
C. Model 3 - Biological, Demographic, and Social Factors
D. Model 4 - Polygenic Scores, - Biological, Demographic, and Social Factors: Non-Hispanic Whites
 |
 |
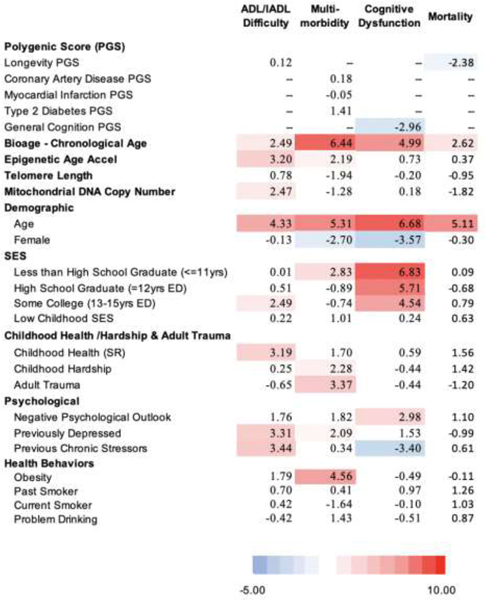 |
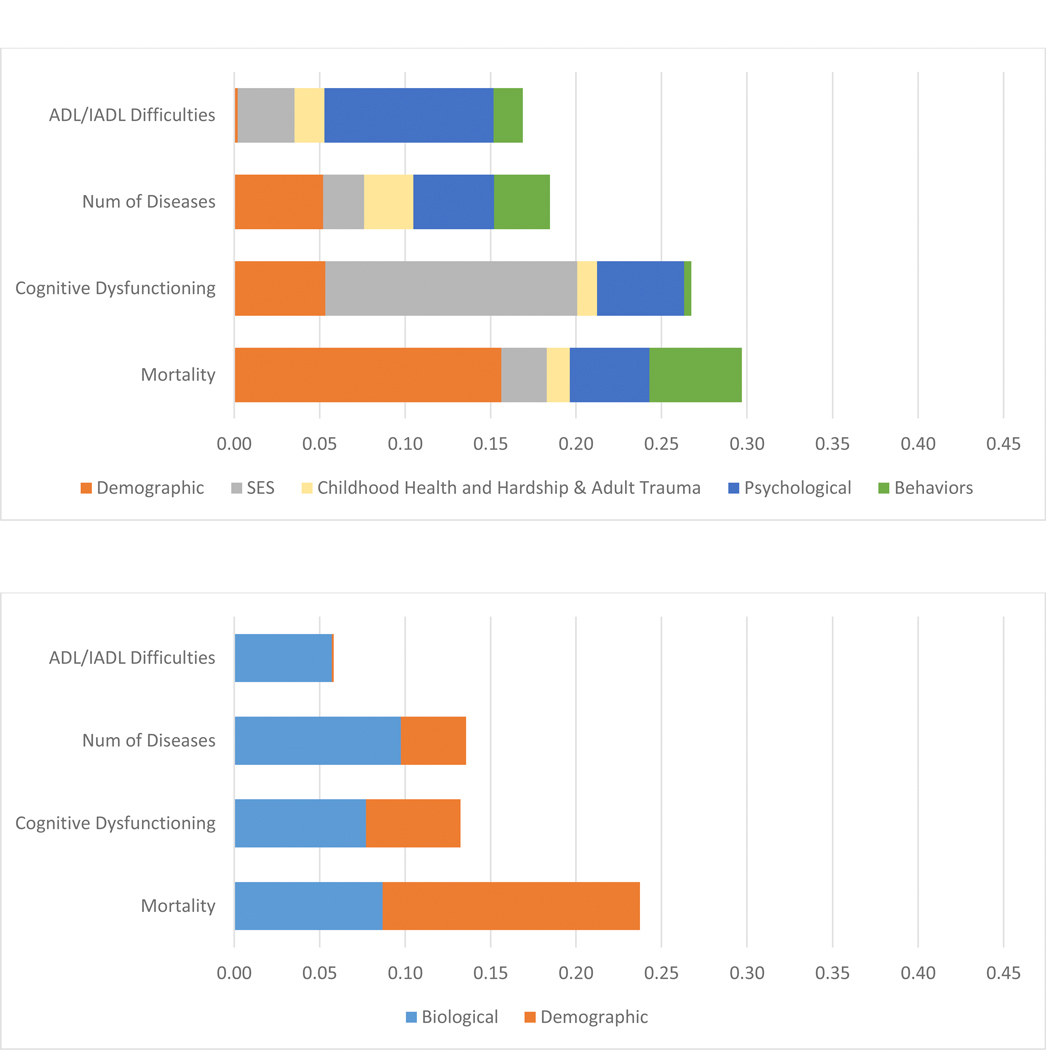
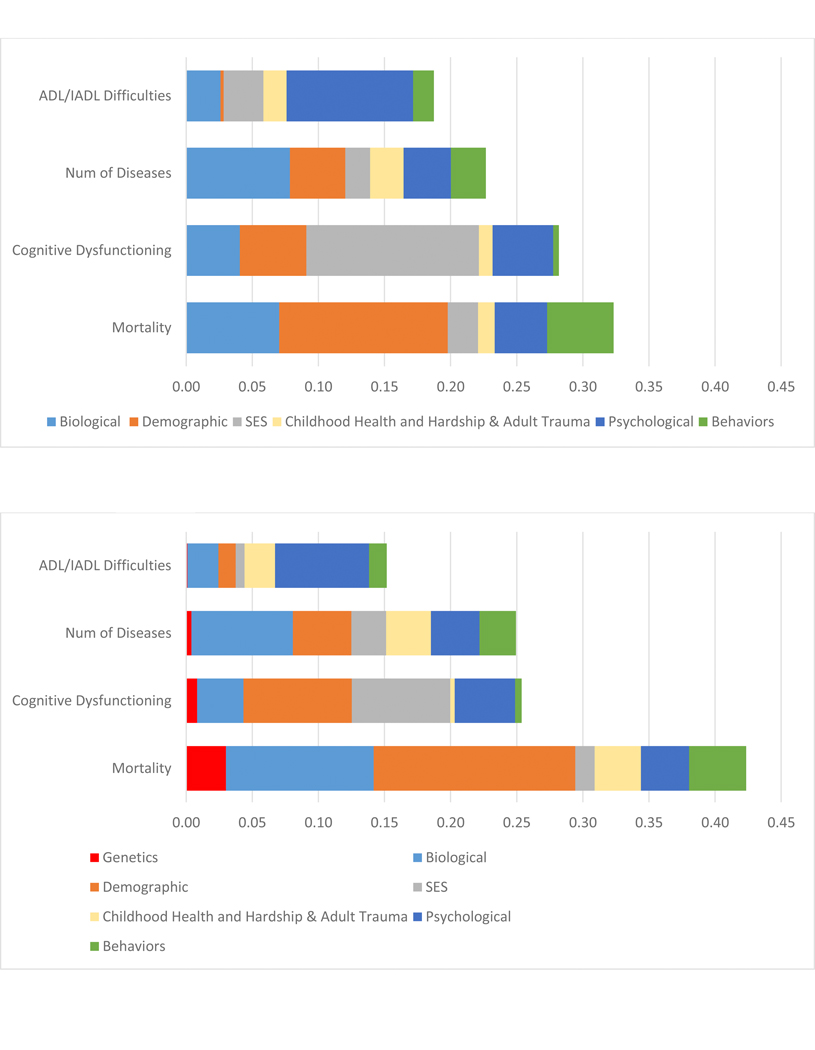
Figure 2:
R2 Decomposition resulting from regressions of the Social Hallmarks of Aging and Multiple Biological Measures on Four Age-Related Health Outcomes: Model 1 – the Social Hallmarks, Model 2 biological Measures, Model 3 both sets of measures, Model 4 adding Polygenic risk
A. Model 1 – R2 attributed to Social Hallmarks and Demographc Factors
B. Model 2 – R2 attributed to Biological and Demographic Factors
C. Model 3 - R2 attributed to Biological, Demographic, and Social Factors
D. Model 4: Non-Hispanic White Only - R2 attributed to Genetic, Biological, Demographic, and Social Factors
Models 1–3 (N=1159); Model 4 (N=832)
Decomposed R2 shown in supplemental table 1
Empirical Results.
For Model 1 (Table 1 A), the general picture is of a strong red map, meaning lower social status, more adverse circumstances, worse psychological states, and poor health behaviors all predict worse health outcomes. The exception is for mortality where only negative psychological states are related to higher mortality. This may reflect that we have data only for a two-year period when people who were sick and likely to die in the next two years may not have participated in the blood collection as indicated by the mortality among those missing and those included in the analysis. For this reason, the results on mortality should not be seen as representative of what would happen over a longer time.
The social hallmarks explain the majority of the variance explained in the outcomes, except for mortality where the majority of the variance explained reflects age and sex differences, the demographic indicators. Mortality and cognitive dysfunction have the most variance explained – between 25 and 30 percent (Figure 2 A). SES is especially important in explaining the cognitive measure.
The second model indicates a very strong link between the biological age-chronological age measure and all health outcomes. Accelerated epigenetic age is related to worse physical functioning and more diseases (Table 1B Model 2). The other biological measures do not have significant relationships with the outcomes. With age and sex controlled, the biological variables explain 9% of the variability in mortality, 8% in cognitive dysfunction, 10% of the number of diseases, and 6% of the variance in physical functioning (Figure 2 B).
As expected, the third model indicates some decrease in both the role of the social variables and the biological variables when the other set is controlled. With the social variables controlled, the biological age – chronological age has reduced explanatory power (Table 1 C). Some of the social hallmarks, particularly SES on multimorbidity, also become insignificant. This is because they were sharing explanatory power; the assumption is that the pathway goes from SES through biology. The social variables are more important than the collection of biological variables in explaining each of these outcomes. Social variables (SES, childhood health and hardship and adult trauma, psychological, and behaviors) explain 1.8 times the variance in mortality when compared to these biological variables (Figure 2 C and supplemental table 2). For multimorbidity the relative explanation of the social variables is 36% greater. The differentials in the two other measures are much greater: almost five times (4.7) as much in cognitive functioning, and six times (6.1) as much in physical functioning.
The final model which includes the polygenic risk scores, and for this reason is limited to the non Hispanic white population (Model 4 – Table 1 D). The polygenic score for longevity is statistically-significantly related to lower mortality; and the polygenic score for cognition is significantly negatively related to cognitive dysfunction. The polygenic scores explain relatively small amounts of variance: 3% for mortality and 1% for cognitive dysfunction (Figure 2 – Supplemental Table 2). The social hallmarks continue to explain most of the accounted for variance with the inclusion of the polygenic scores in this sample. Among the biological measures, biological age minus chronological age is a reasonably important explanatory variable for most outcomes, but the other hallmark of aging markers appear stronger in this sample, at least for the prediction of ADL/IADL difficulties.
Future explanations of human aging
Variability in human aging is strongly related to the social determinants of aging; and it remains so when extensive biology is introduced as mediating measures. This means that the social variability in the aging process is only partly explained by the biology introduced. Our hypothesis is that if we could fully capture the basic biological mechanisms of aging, they would explain the social variability in the process as social factors need to “get under the skin” through biology. If we fully incorporated biology, the social determinants should become insignificant.
While the field has begun to make progress in explaining some of the variation in human aging outcomes with biological data that reflects downstream biology relative to the physiological hallmarks of aging, these are not basic molecular and cellular mechanisms. Our inclusion of measures reflecting some of the hallmarks of geroscience did not provide much explanation of social variability in aging. We have far to go before we are able to include indicators of all of the hallmarks of biological aging into human population studies, but these initial measures are somewhat disappointing. The evidence presented suggests that focus on the social hallmarks of aging will help to achieve the geroscience agenda to slow human aging. Scientific advances will be made when biological approaches incorporate some of the concepts in the social hallmarks of aging in experimental design to test pathways based on what has been shown to promote earlier aging in humans. This could serve as an initial step in the process of developing measures that explain human aging. Research then needs to eventually involve human cohorts with measurement of complete life circumstances and complete biology in order to demonstrate the relative importance of hypothesized mechanisms and understand how to intervene in the aging process.
Social scientists are also a long way from fully incorporating indicators of all of the mechanisms that are related to the social hallmarks of aging. More work is needed to incorporate information on times in life as well as the specific experiences that lead to more rapid aging. Variables incorporated do not fully capture all aspects of life related to social hardship and deprivation. For instance, the above analysis includes an indicator of psychological status in the models, but did not fully incorporate indicators of the stress mechanisms so clearly outlined by Epel (2020). Social scientists can also make progress by better clarifying who is resilient to experiencing some of the social hallmarks by dividing people into those with and without “expected results” or who have unusual life patterns. Such work will set the stage for better interventions to improve health outcomes.
Supplementary Material
Highlights.
Social hallmarks are strongly related to multiple age-related health outcomes.
Biological Hallmarks are modestly related to age-related health outcomes.
An indicator of multisystem biological functioning is strongly related to health outcomes.
Integration of social and biological mechanisms is needed to understand human aging
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Adler NE, Boyce T, Chesney MA, Cohen S, Folkman S, Kahn RL, Syme SL (1994) Socioeconomic status and health. The challenge of the gradient. Am Psychol 49:15–24. 10.1037/0003-066X.49.1.15 [DOI] [PubMed] [Google Scholar]
- 2.Adler NE, Ostrove JM (1999) Socioeconomic status and health: what we know and what we don’t. Ann N Y Acad Sci 896:3–15. 10.1111/j.1749-6632.1999.tb08101.x [DOI] [PubMed] [Google Scholar]
- 3.Ashar FN, Moes A, Moore AZ, Grove ML, Chaves PH, Coresh J, Newman AB, Matteini AM, Bandeen-Roche K, Boerwinkle E, Walston JD (2015) Association of mitochondrial DNA levels with frailty and all-cause mortality. J Mol Med 93:177–186. 10.1007/s00109-014-1233-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barker DJ, Thornburg KL (2013) The obstetric origins of health for a lifetime. Clin Obstet Gynecol 56:511–519. 10.1097/GRF.0b013e31829cb9ca [DOI] [PubMed] [Google Scholar]
- 5.Barzilai N, Cuervo AM, Austad S (2018) Aging as a biological target for prevention and therapy. JAMA 320:1321–1322. 10.1001/jama.2018.9562 [DOI] [PubMed] [Google Scholar]
- 6.Belsky DW, Caspi A, Cohen HJ, Kraus WE, Ramrakha S, Poulton R, Moffitt TE (2017) Impact of early personal-history characteristics on the Pace of Aging: implications for clinical trials of therapies to slow aging and extend healthspan. Aging Cell 16:644–651. 10.1111/acel.12591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Belsky DW, Caspi A, Houts R, Cohen HJ, Corcoran DL, Danese A, Harrington H, Israel S, Levine ME, Schaefer JD, Sugden K, Williams B, Yashin AI, Poulton R, Moffitt TE (2015) Quantification of biological aging in young adults. Proc Natl Acad Sci U S A 112:E4104–4110. 10.1073/pnas.1506264112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Belsky DW, Israel S (2014) Integrating genetics and social science: genetic risk scores. Biodemography Soc Biol 60(2):137–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Belsky DW, Moffitt TE, Cohen AA, Corcoran DL, Levine ME, Prinz JA et al. (2017) Eleven telomere, epigenetic clock, and biomarker-composite quantifications of biological aging: do they measure the same thing? Am J Epidemiol 187(6):1220–1230. doi: 10.1093/aje/kwx346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ben-Shlomo Y, Kuh D (2002). A life course approach to chronic disease epidemiology: conceptual models, empirical challenges and interdisciplinary perspectives. Int J Epidemiol 31:285–293. 10.1093/ije/31.2.285 [DOI] [PubMed] [Google Scholar]
- 11.Braveman P, Egerter S, Williams DR (2011) The social determinants of health: coming of age. Annu Rev Public Health 32:381–398. 10.1146/annurev-publhealth-031210-101218 [DOI] [PubMed] [Google Scholar]
- 12.Crimmins EM (2015) Lifespan and healthspan: Past, present and promise. Gerontologist 55(6):901–911. 10.1093/geront/gnv130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crimmin EM, Faul J, Thyagarajan B, Weir DR (2017) Venous blood collection and assay protocol in the 2016 Health and Retirement Study Venous Blood Study (VBS). http://hrsonline.isr.umich.edu/modules/meta/vbs/2016/desc/HRS2016VBSDD.pdf?_ga=2.171951269.408614381.1589823285-975920407.1589823285. Accessed 20 May 2020
- 14.Cutler D, Deaeton A, Lleras-Muney A (2006) The determinants of mortality. J Econ Perspect 20(3):97–120. [Google Scholar]
- 15.Elovainio M, Ferrie JE, Singh-Manoux A, Gimeno D, De Vogli R, Shipley MJ, Vahtera J, Brunner EJ, Marmot MG, Kivimäki M (2009) Cumulative exposure to high-strain and active jobs as predictors of cognitive function: the Whitehall II study. Occup Environ Med 66:32–37. 10.1136/oem.2008.039305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Epel E (2020) The geroscience agenda: What does stress have to do with it? Ageing Res Rev. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Franceschi C, Garagnani P, Parini P, Giuliani C, Santoro A (2018) Inflammaging: a new immune-metabolic viewpoint for age-related diseases. Nat Rev Endocrinol 14: 576–590. 10.1038/s41574-018-0059-4 [DOI] [PubMed] [Google Scholar]
- 18.Friedman E, Montez JK, Sheehan CM, Guenewald TL, Seeman TE (2015) Childhood adversities and adult cardiometabolic health: does the quantity, timing, and type of adversity matter? J Aging Health 27(8):1311–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hastings W, Shalev I, Belesky D (2019) Comparability of biological aging measures in the National Health and Nutrition Examination Study, 1999–2002. Psychoneuroendocrinology 106:171–178. doi: 10.1016/j.psyneuen.2019.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Horvath S (2013) DNA methylation age of human tissues and cell types. Genome Biol 14:3156 10.1186/gb-2013-14-10-r115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Israeli OA (2007) Shapley-based decomposition of the R-Square of a linear regression. J Econ Inequal 5:199–212. 10.1007/s10888-006-9036-6 [DOI] [Google Scholar]
- 22.Fiorito G, McCrory C, Robinson O, Carmeli C, Rosales CO, Zhang Y, Colicino E, Dugué PA, Artaud F, McKay GJ, Jeong A, Mishra PP, Nøst TH, Krogh V, Panico S, Sacerdote C, Tumino R, Palli D, Matullo G, Guarrera S, Gandini M, Bochud M, Dermitzakis E, Muka T, Schwartz J, Vokonas PS, Just A, Hodge AM, Giles GG, Southey MC, Hurme MA, Young I, McKnight AJ, Kunze S, Waldenberger M, Peters A, Schwettmann L, Lund E, Baccarelli A, Milne RL, Kenny RA, Elbaz A, Brenner H, Kee F, Voortman T, Probst-Hensch N, Lehtimäki T, Elliot P, Stringhini S, Vineis P, Polidoro S; BIOS Consortium; Lifepath consortium (2019) Socioeconomic position, lifestyle habits and biomarkers of epigenetic aging: a multi-cohort analysis. Aging (Albany NY). April 14;11(7):2045–2070. doi: 10.18632/aging.101900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jones N, Gilman S, Cheng T, Drury S, Hill C, Geronimus A (2019) Life course approaches to the causes of health disparities. Am J Public Health 109(Suppl 1):S48–S55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jylhävä J, Pedersen NL, Hägg S (2017) Biological age predictors. EBioMedicine 21:29–36. doi: 10.1016/j.ebiom.2017.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kennedy BK, Berger SL, Brunet A, Campisi J, Cuervo AM, Epel ES, Franceschi C, Lithgow GJ, Morimoto RI, Pessin JE, Rando TA (2014) Geroscience: linking aging to chronic disease. Cell 159:709–713. 10.1016/j.cell.2014.10.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim P, Evans GW, Chen E, Miller G, Seeman T (2018) How socioeconomic disadvantages get under the skin and into the brain to influence health development across the lifespan In: Halfon N, Forrest CB, Lerner RM, Faustman EM (eds) Handbook of life course health development [Internet]. Cham (CH): Springer, pp 463–497. [PubMed] [Google Scholar]
- 27.Kraus JF, Borhani NO, Franti CE (1980) Socioeconomic status, ethnicity, and risk of coronary heart disease. Am J Epidemiol 111:407–414. 10.1093/oxfordjournals.aje.a112915 [DOI] [PubMed] [Google Scholar]
- 28.Kuh D, Ben-Shlomo Y, Lynch J, Hallqvist J, Power C (2003) Life course epidemiology. J Epidemiol Community Health. 57:778–783. 10.1136/jech.57.10.778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee JW, Park KD, Im JA, Kim MY, Lee DC (2010) Mitochondrial DNA copy number in peripheral blood is associated with cognitive function in apparently healthy elderly women. Clin Chim Acta 411:592–596. 10.1016/j.cca.2010.01.024 [DOI] [PubMed] [Google Scholar]
- 30.Levine ME (2013) Modeling the rate of senescence: can estimated biological age predict mortality more accurately than chronological age? J Gerontol A Biol Sci Med Sci 68:667–674. 10.1093/gerona/gls233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Levine ME, Crimmins EM (2014) Evidence of accelerated aging among African Americans and its implications for mortality. Soc Sci Med 118:27–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Link BG, Phelan J (1995) Social conditions as fundamental causes of disease. J Health Soc Behav Spec No:80–94. https://www.jstor.org/stable/2626958 [PubMed] [Google Scholar]
- 33.Liu Z, Chen X, Gill T, Ma C, Crimmins EM, Levine ME (2019) Associations of genetics, behaviors, and life course circumstances with a novel aging and healthspan measure: Evidence from the Health and Retirement Study. PLoS Med 16:e1002827. 10.1371/journal.pmed.1002827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G (2013) The hallmarks of aging. Cell 153:1194–1217. 10.1016/j.cell.2013.05.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Manton KG, Gu X, Lamb VL (2006) Change in chronic disability from 1982 to 2004/2005 as measured by long-term changes in function and health in the U.S. elderly population. Proc Natl Acad Sci U S A 103:18374–18379. 10.1073/pnas.0608483103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marmot M, Allen J, Bell R, Bloomer E, Goldblatt P (2012) Consortium for the European Review of Social Determinants of Health and the Health Divide. WHO European review of social determinants of health and the health divide. Lancet 380: 1011–1029. 10.1016/S0140-6736(12)61228-8 [DOI] [PubMed] [Google Scholar]
- 37.Marmot MG, Stansfeld S, Patel C, North F, Head J, White I, Brunner E, Feeney A, Smith GD (1991) Health inequalities among British civil servants: the Whitehall II study. Lancet 337:1387–1393. 10.1016/0140-6736(91)93068-k [DOI] [PubMed] [Google Scholar]
- 38.McCrory C, Fiorito G, Ni Cheallaigh C, Polidoro S, Karisola P, Alenius H, Layte R, Seeman T, Vineis P, Kenny RA (2019) How does socio-economic position (SEP) get biologically embedded? A comparison of allostatic load and the epigenetic clock(s). Psychoneuroendocrinology. June;1 04:64–73. doi: 10.1016/j.psyneuen.2019.02.018. Epub 2019 Feb 16 [DOI] [PubMed] [Google Scholar]
- 39.Moffit T (2020) Behavioral and Social Research to Accelerate the Geroscience Translation Agenda. Ageing Res Rev. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moffitt TE, Belsky DW, Danese A, Poulton R, Caspi A (2017) The Longitudinal Study of Aging in Human Young Adults: Knowledge gaps and research agenda. J Gerontol A Biol Sci Med Sci 72:210–215. 10.1093/gerona/glw191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Montier LLC, Deng JJ, Bai Y (2009) Number matters: control of mammalian mitochondrial DNA copy number. J Genet Genomics 36:125–131. 10.1016/S1673-8527(08)60099-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Notterman DA, Mitchell C (2015) Epigenetic and understanding the impact of social determinants of health. Pediatr Clin North Am 62:1227–1240. 10.1016/j.pcl.2015.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Picard M, Prather AA, Puterman E, Cuillerier A, Coccia M, Aschbacher K, Burelle Y, Epel ES (2018) A mitochondrial health index sensitive to mood and caregiving stress. Biol Psychiatry. 1;84(1):9–17. doi: 10.1016/j.biopsych.2018.01.012. Epub 2018 Feb 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Putterman EA, Gemmill A, Karasek D, Weir D, Adler NE, Prather AA, Epel ES (2016) Lifespan adversity and later adulthood telomere length in the nationally representative US Health and Retirement Study. Proc Natl Acad Sci U S A. October 18;113(42):E6335-E6342. doi: 10.1073/pnas.1525602113. Epub 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Seeman TE, McEwen BS, Rowe JW, Singer BH (2001) Allostatic load as a marker of cumulative biological risk: MacArthur studies of successful aging. Proc Natl Acad Sci U S A 98:4770–4775. 10.1073/pnas.081072698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sierra F (2016) The emergence of geroscience as an interdisciplinary approach to the enhancement of health span and life span. Cold Spring Harb Perspect Med 6: a025163. 10.1101/cshperspect.a025163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Taylor RW, Turnbull DM (2005) Mitochondrial DNA mutations in human disease.Nat Rev Genet 6:389–402. 10.1038/nrg1606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thyagarajan B, Wang R, Nelson H, Barcelo H, Koh WP, Yuan JM (2013) Mitochondrial DNA copy number is associated with breast cancer risk. PLoS One 8: e65968. 10.1371/journal.pone.0065968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhu Y, Liu X, Ding X, Wang F, Geng X (2019) Telomere and its role in the aging pathways: telomere shortening, cell senescence and mitochondria dysfunction. Biogerontology 20(1):1–16. doi: 10.1007/s10522-018-9769-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.