Abstract
Background/Purpose:
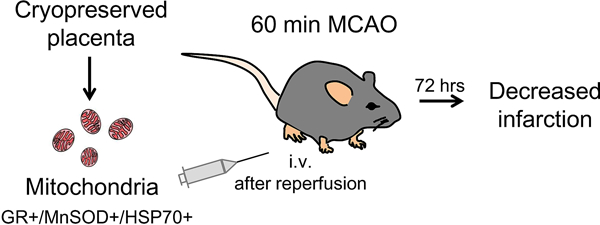
There is an urgent need to develop adjunct therapies that can be added onto reperfusion for acute ischemic stroke. Recently, mitochondrial transplantation has emerged as a promising therapeutic approach for boosting brain tissue protection. In this proof-of-concept study, we investigate the feasibility of using placenta as a source for mitochondrial transplantation in a mouse model of transient focal cerebral ischemia-reperfusion.
Methods:
Mitochondria-enriched fractions were isolated from cryopreserved mouse placenta. Mitochondrial purity and JC1 membrane potentials were assessed by flow cytometry. ATP and mitochondrial proteins were measured by luminescence intensity and western blot, respectively. Therapeutic efficacy of mitochondrial fractions was assessed in a mouse model of transient focal cerebral ischemia-reperfusion.
Results:
Flow cytometry analysis demonstrated that about 87% of placental mitochondria were viable and maintained JC1 membrane potentials after isolation. Placental mitochondrial fractions contained ATP equivalent to mitochondrial fractions isolated from skeletal muscle and brown fat tissue. Normalized mitochondrial anti-oxidant enzymes (glutathione reductase, MnSOD) and HSP70 were highly preserved in placental mitochondrial fractions. Treatment with placental mitochondrial fractions immediately after reperfusion significantly decreased infarction after focal cerebral ischemia in mice.
Conclusions:
Cryopreserved placenta can be a feasible source for viable mitochondrial isolation. Transplantation with placental mitochondria may amplify beneficial effects of reperfusion in stroke.
Keywords: Mitochondrial allograft, placenta, mitochondrial proteins, brain protection, stroke
Graphical Abstract
Flow cytometry analysis demonstrated that about 87% of placental mitochondria were viable and maintained JC1 membrane potentials after isolation. Placental mitochondrial fractions contained ATP equivalent to mitochondrial fractions isolated from skeletal muscle and brown fat tissue. Normalized mitochondrial anti-oxidant enzymes (glutathione reductase: GR, Mn-SOD) and HSP70 were highly preserved in placental mitochondrial fractions. Treatment with placental mitochondrial fractions significantly decreased infarction after transient focal cerebral ischemia in mice. This proof-of-concept study demonstrates that the cryopreserved placenta can be a source for viable mitochondria! isolation and transplantation in a mouse model of focal cerebral ischemia - reperfusion.
Introduction
Recombinant tissue plasminogen activator or endovascular thrombectomy provide effective ways to achieve reperfusion for acute ischemic stroke1. While restoring cerebral blood flow is essential for decreasing infarction, reperfusion alone may not be sufficient to fully salvage penumbral tissue in some patients where cells are dying through active cell death pathways2. Therefore, it is still important to continue the search for neuroprotective approaches that can be added onto ischemia-reperfusion1.
Mitochondria are energetic center for regulating cell viability and homeostasis3. Emerging data suggest that mitochondria may be released into extracellular space and transferred from cell to cell4. This extracellular mitochondrial mechanisms have been shown to be protective in stroke and neurodegenerative diseases5. The question is whether mitochondrial transplantation may represent a potential therapeutic approach for ischemia-reperfusion. The placenta is considered as an important resource for cell therapies even after cryopreservation6–8. In this proof-of-concept study, we asked (i) whether functional mitochondria can be isolated from cryopreserved placenta and (ii) whether transplantation of placental-derived mitochondria may be feasible in a mouse model of transient focal cerebral ischemia-reperfusion.
Methods
Data available on request from the authors:
The data that support the findings of this study are available from the corresponding author upon reasonable request.
In vivo focal ischemia model:
All experiments were performed following an institutionally approved protocol in accordance with National Institutes of Health guidelines and with the United States Public Health Service’s Policy on Human Care and Use of Laboratory Animals and following Animals in Research: Reporting In vivo Experiments (ARRIVE) guidelines. Male C57BL6 mice (11–12 weeks, Charles River Laboratories) were deeply anaesthetized with isoflurane (5% to 1.5%) in 30%/70% oxygen/nitrous oxide. After midline skin incision, 7–0 nylon monofilament coated with silicon resin was introduced through a small incision into the common carotid artery. Adequate cerebral ischemia was assessed by Laser Doppler flowmetry and by examining forelimb flexion after the mice recovered from anesthesia. Sixty minutes after occlusion, the mice were re-anesthetized, and reperfusion was established by withdrawal of the filament.
Mitochondrial isolation:
Mitochondrial enriched fractions were obtained from various tissues including snap-frozen placenta, freshly isolated skeletal muscle, freshly isolated brain, and freshly isolated brown adipose tissues and suspended in mitochondria isolation buffer - 10 mM HEPES (pH7.5) buffer containing 25 mM sucrose, 1 mM ATP, 0.1 mM ADP, 5 mM sodium succinate, and 2 mM K2HPO4. Briefly, each tissue was homogenized in glass tissue grinders in mitochondria isolation buffer at 4°C. Following removal of cellular debris with repeated centrifugation at 1,000 g at 4°C for 5 minutes, obtained supernatants were filtered by 1.2 μm filter and pellets (mitochondria-enriched fractions) were obtained by the centrifugation at 4,000 g at 4°C for 10 minutes.
ATP assay:
ATP in mitochondria-enriched fraction was determined by CellTiter-Glo luminescence (Promega). CellTiter-Glo luminescence test solution (100μL) was added to samples (10 µg protein/10 µL) and incubated for 30 min at room temperature. Luminescent signal was determined by luminescence microplate reader.
Flow cytometry analysis:
After incubating the supernatants with Mitotracker Deep Red (100 nM, Thermo Fisher Scientific, M22426) or JC1 (0.5 µM, Invitrogen, T-3168) for 30 min at 37°C, samples were analyzed for 1 minute in the “HIGH” flow rate mode by BD Fortessa. Buffer noise was excluded based on back ground signal from buffer alone. Mitochondrial membrane potential was determined by the ratio of aggregated JC1 to monomer JC1 in total Mitotracker Deep Red positive events.
Western blot analysis:
After electrophoresis and protein transferring to nitrocellulose membranes, the membranes were blocked in Tris-buffered saline containing 0.1% Tween 20 and 3% BSA for 90 min at room temperature. Membranes were then incubated overnight at 4°C with primary antibodies, anti-glutathione Reductase (1:1000, ab137513, Abcam), anti-MnSOD (1:500, ADI-SOD-111-F, Enzo Life Sciences), anti-HSP70 (1:500, ADI-SPA-810-D, Enzo Life Sciences), anti-COX IV (1:500, ab33985, Abcam). After incubation with peroxidase-conjugated secondary antibodies, visualization was enhanced by chemiluminescence (GE Healthcare, anti-mouse IgG).
Statistical analysis:
Results are expressed as mean±SD. When only two groups were compared, unpaired t-test was used. P<0.05 was considered to be statistically significant.
Results
Isolation of mitochondria-enriched fraction from cryopreserved placenta
Placentae obtained from E17 pregnant female mice were quickly frozen by liquid nitrogen and stored at −80C° for more than one week. From the snap-frozen placentae, mitochondria were isolated and resuspended in 10 mM HEPES (pH7.5) buffer containing 25 mM sucrose, 1 mM ATP, 0.1 mM ADP, 5 mM sodium succinate, and 2 mM K2HPO4 through the process described in Fig. 1A. After isolation, flow cytometry analysis demonstrated that placental mitochondria had sizes below 0.9 μm and approximately 87% of the subset was positive for a mitochondrial membrane potential-dependent dye, Mitotracker deep red (Fig. 1B). JC1 assays confirmed that the Mitotracker Deep Red-positive population demonstrated preserved mitochondrial membrane potential (Fig. 1C). Taken together, these data suggest that these isolated fractions from frozen placentae may be enriched with viable mitochondria.
Figure 1. Mitochondrial isolation from cryopreserved placenta :
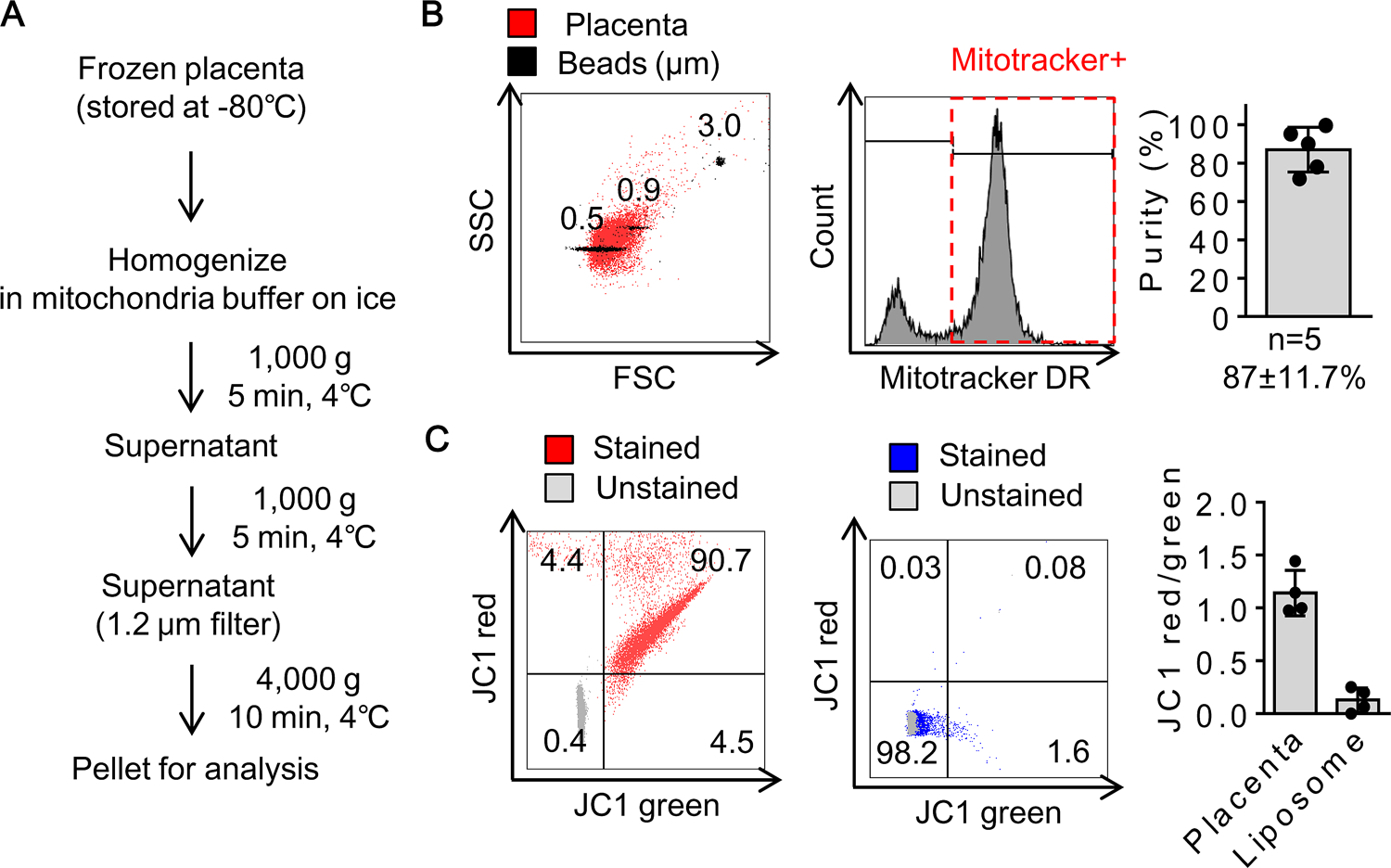
A. Diagram for mitochondrial isolation. B. Flow cytometry verified that approximately 87% of particles in fractions were Mitotracker Deep Red (DR) positive subsets (n=5). C. JC1 ratio was calculated by the number of JC1 red positive mitochondrial populations over JC1 green positive mitochondrial populations (n=4). Empty liposomes were used as negative control (n=4). Results were expressed as mean ± SD.
Measurements of ATP and mitochondrial proteins in placental mitochondria
Isolated mitochondria were then assessed by ATP measurement and western blot analysis. The placenta-derived mitochondrial fraction showed ATP levels that were equivalent to fractions obtained from skeletal muscle and brown fat tissue (Fig. 2A). Western blot demonstrated that key mitochondrial proteins including glutathione reductase, MnSOD, and HSP70 were highly expressed in the placenta-derived fractions (Fig. 2B and 2C). Interestingly, levels of mitochondrial glutathione reductase and HSP70 appeared to be higher in placenta and brain compared to muscle and brown adipose tissue.
Figure 2. ATP and mitochondrial proteins :
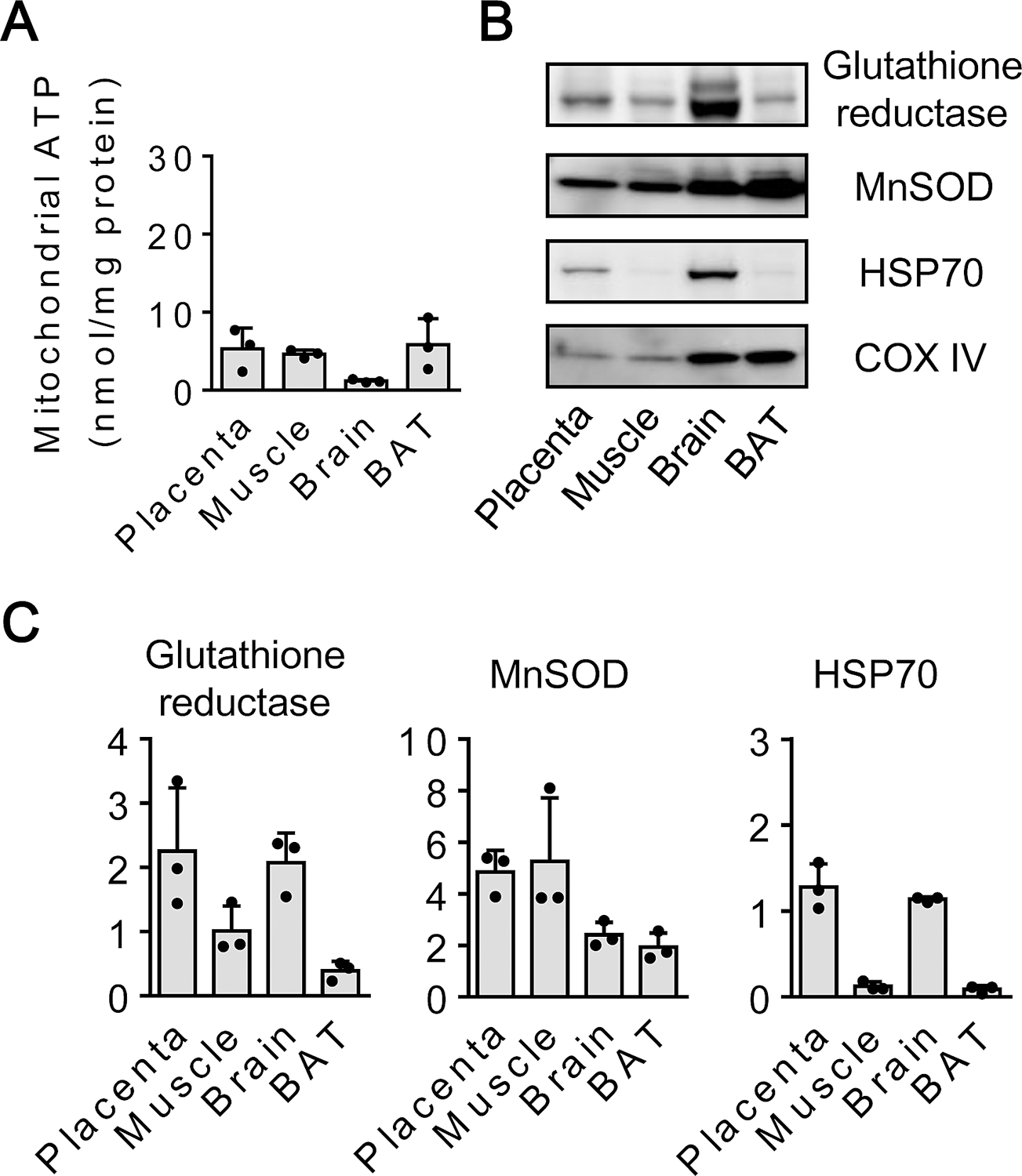
A. ATP (nmol per mg protein) in mitochondria isolated from cryopreserved placenta, fresh skeletal muscle, brain, brown adipose tissue (BAT) (n=3). B, C. Western blot analysis for mitochondrial protein expressions (n=3). Results were expressed as mean ± SD.
Protective effect of placental mitochondria in mouse focal cerebral ischemia
C57Bl6 male mice were subjected to 60 minutes of transient focal cerebral ischemia. Placental-derived mitochondrial fractions (100 μg protein/100 µL saline/mouse) were infused intravenously immediately after reperfusion with full blinding and randomization (Fig. 3A). Fluorescent imaging confirmed that transplanted mitochondria were found in brain, lung, liver, kidney and heart at 2 hours after treatment. The ability of these transplanted mitochondria to show high levels of Mitotracker Deep Red signals suggested that they retained membrane potential, and were therefore likely to be active and functional (Fig. 3B, C). After 72 hrs, mice were sacrificed and brains removed for analysis. Treatment with placental mitochondrial fractions significantly reduced infarction volumes compared to saline-treated controls (Fig. 3D).
Figure 3. Cerebroprotection induced by placental mitochondria after transient focal cerebral ischemia in mice :
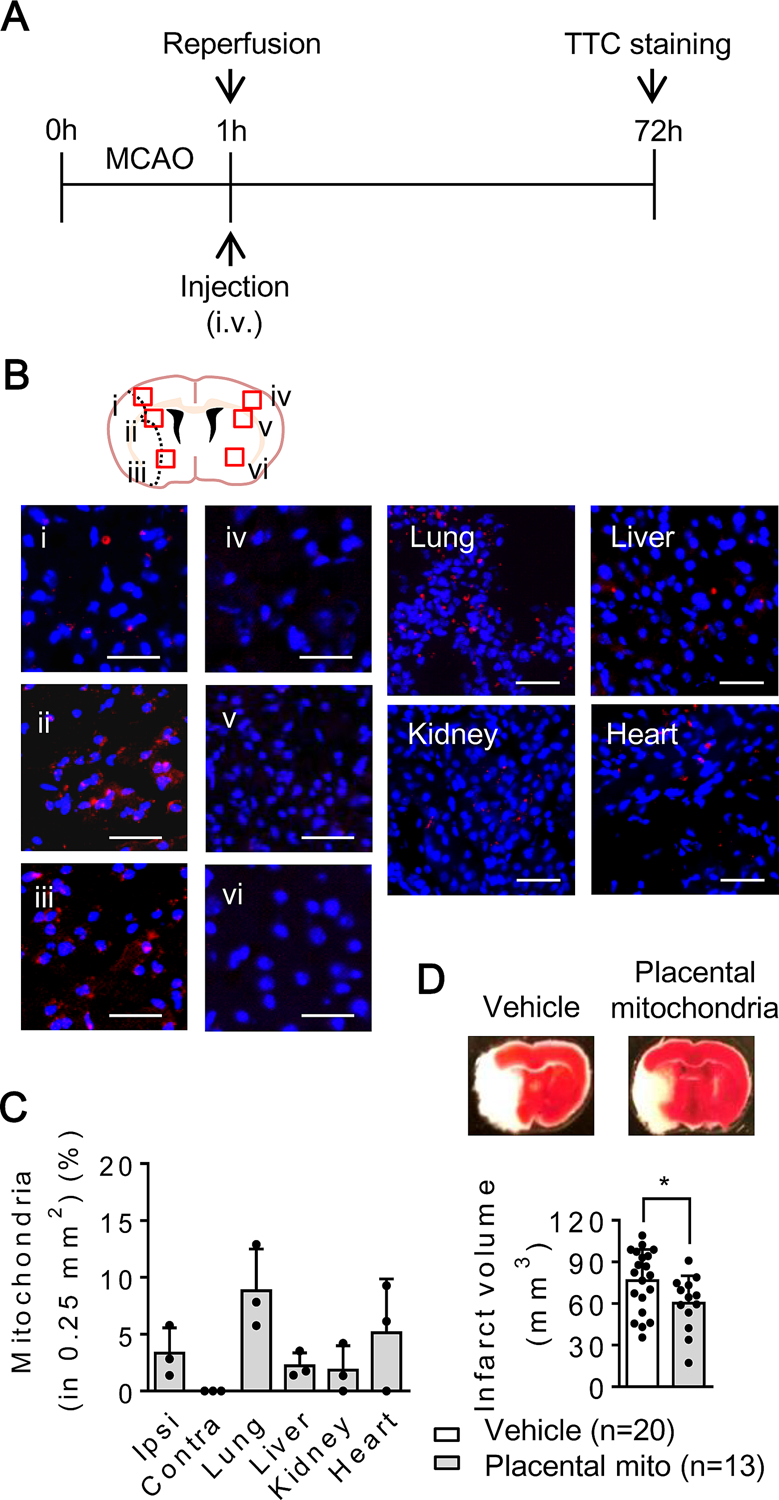
A. Male C57BL6 mice were subjected to 60 min transient focal cerebral ischemia. Treatment with mitochondria (100 µg/100 µL) or saline (100 µL) was performed right after reperfusion with fully blinding. Brain infarction was determined at 72 hours after focal cerebral ischemia. B, C. Mitochondria were labeled by Mitotracker Deep Red (100 nM) before intravenous infusion. Infused mitochondria were found in ipsilateral hemisphere, lung, liver, kidney, and heart at 2 hours after transient focal cerebral ischemia (n=3). Scale: 100 µm. D. Placental mitochondrial fraction significantly decreased brain infarction (vehicle: saline n=20, Placental mitochondrial fraction: n=13). *p<0.05, unpaired 2-tailed t-test. Results were expressed as mean ± SD.
Discussion
In this study, we found that (i) mitochondria with viable membrane potentials could be isolated from cryopreserved placenta, (ii) ATP was preserved and mitochondrial proteins were highly expressed in the placenta-derived mitochondria, and (iii) treatment with placental mitochondria was feasible in a mouse model of transient focal cerebral ischemia-reperfusion.
Mitochondria can be actively released into extracellular space and potentially transferred as a help-me signal between neurons, astrocytes, microglia, and endothelial cells in the neurovascular unit after CNS injury and disease9. Inter-cellular transfer of healthy and functional mitochondria may increase ATP, mtDNA contents10, protective mitochondrial peptides in the recipient cells and attenuate inflammation11 and cell death pathways12. Therefore, transplantation of functional extracellular mitochondria may be a promising therapeutic approach in the context of ischemic tissue damage, and ongoing investigations have assessed the use of muscle as a source for mitochondria13. However, further studies are needed to determine whether mitochondria derived from other sources may also offer therapeutic benefit. Given that placental tissues have already been used for cell-based therapies and cryopreservation may not negatively affect this particular compartment6–8, the cryopreserved placenta may offer a candidate resource for viable organelle isolation in a non-invasive way. In this proof-of-concept study, we demonstrated that viable mitochondria can indeed be derived from cryopreserved placenta, and then used to decrease infarction after transient focal cerebral ischemia in an animal model. Nevertheless, there are a few caveats that warrant further investigation. First, the underlying mechanisms of targeting, migration, and incorporation remain to be fully defined. The mitochondrial matrix is highly negatively charged around −240 mV14, suggesting that transplanted mitochondria may be attracted to cells in acidotic ischemic tissue. Further studies are warranted to rigorously map migration and incorporation mechanisms. Second, besides ischemic brain, infused mitochondria were also found in various peripheral organs including lung, liver, kidney, and heart. How the intravenous infusion of mitochondria affects other organs outside the CNS after stroke should be further investigated. Third, only a single dose, timing and delivery route were evaluated in this proof-of-concept study. All drug delivery parameters should be rigorously assessed to maximize the benefit of mitochondrial transplantation. Finally, we only tested our placental mitochondrial transplants in transient ischemia-reperfusion. This is primarily a proof-of-concept study to demonstrate feasibility. Whether this approach can work in permanent ischemia or whether there are optimal conditions for combination with reperfusion via tissue plasminogen activator or mechanical thrombectomy should be carefully explored for future translation.
Summary/Conclusions
Emerging findings in cell, animal and human studies suggest that mitochondria can be released and potentially transferred from cell to cell in the CNS. In this proof-of-concept study, we showed that placenta-derived mitochondrial transplantation may reduce infarction after cerebral ischemia-reperfusion. Further studies are warranted to investigate the therapeutic use of cryopreserved placental mitochondria in stroke.
Supplementary Material
Acknowledgement
We gratefully acknowledge the National Institutes of Health for their financial support of this work
Sources of Funding
The study was supported by NIH/NINDS (R01NS094756).
Non-standard Abbreviations and Acronyms
- ATP
Adenosine triphosphate
- CNS
Central nervous system
- MnSOD
manganese superoxide dismutase
- HSP70
heat shock protein 70
Footnotes
Competing Interests: The authors declare they have no competing financial interest.
Disclosures: There is no disclosures to report.
References
- 1.Vosler PS, Graham SH, Wechsler LR, Chen J. Mitochondrial targets for stroke: Focusing basic science research toward development of clinically translatable therapeutics. Stroke. 2009;40:3149–3155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lo EH. A new penumbra: Transitioning from injury into repair after stroke. Nat Med 2008;14:497–500 [DOI] [PubMed] [Google Scholar]
- 3.Tait SW, Green DR. Mitochondria and cell signalling. J Cell Sci 2012;125:807–815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hayakawa K, Esposito E, Wang X, Terasaki Y, Liu Y, Xing C, Ji X, Lo EH. Transfer of mitochondria from astrocytes to neurons after stroke. Nature. 2016;535:551–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nakamura Y, Park JH, Hayakawa K. Therapeutic use of extracellular mitochondria in cns injury and disease. Exp Neurol 2019;324:113114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elias M, Hoover J, Nguyen H, Reyes S, Lawton C, Borlongan CV. Stroke therapy: The potential of amniotic fluid-derived stem cells. Future Neurol 2015;10:321–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parolini O, Alviano F, Bergwerf I, Boraschi D, De Bari C, De Waele P, Dominici M, Evangelista M, Falk W, Hennerbichler S, Hess DC, Lanzoni G, Liu B, Marongiu F, McGuckin C, Mohr S, Nolli ML, Ofir R, Ponsaerts P, Romagnoli L, Solomon A, Soncini M, Strom S, Surbek D, Venkatachalam S, Wolbank S, Zeisberger S, Zeitlin A, Zisch A, Borlongan CV. Toward cell therapy using placenta-derived cells: Disease mechanisms, cell biology, preclinical studies, and regulatory aspects at the round table. Stem Cells Dev 2010;19:143–154 [DOI] [PubMed] [Google Scholar]
- 8.Nikulina MKV, Bukreieva T, Zahanich I, Kyryk V, Lobintseva G, Shablii V Cryopreservation of placenta tissue allows isolating viable mesenchymal and hematopoietic stem cells. Cytotherapy 2019;21:S78–S79 [Google Scholar]
- 9.Hayakawa K, Bruzzese M, Chou SH, Ning M, Ji X, Lo EH. Extracellular mitochondria for therapy and diagnosis in acute central nervous system injury. JAMA Neurol 2018;75:119–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hayakawa K, Chan SJ, Mandeville ET, Park JH, Bruzzese M, Montaner J, Arai K, Rosell A, Lo EH. Protective effects of endothelial progenitor cell-derived extracellular mitochondria in brain endothelium. Stem Cells. 2018;36:1404–1410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eun Jung J, Sun G, Bautista Garrido J, Obertas L, Mobley AS, Ting SM, Zhao X, Aronowski J. The mitochondria-derived peptide humanin improves recovery from intracerebral hemorrhage: Implication of mitochondria transfer and microglia phenotype change. J Neurosci 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang X, Gerdes HH. Transfer of mitochondria via tunneling nanotubes rescues apoptotic pc12 cells. Cell Death Differ 2015;22:1181–1191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang Z, Ma Z, Yan C, Pu K, Wu M, Bai J, Li Y, Wang Q. Muscle-derived autologous mitochondrial transplantation: A novel strategy for treating cerebral ischemic injury. Behav Brain Res 2019;356:322–331 [DOI] [PubMed] [Google Scholar]
- 14.Michelakis ED. Mitochondrial medicine: A new era in medicine opens new windows and brings new challenges. Circulation. 2008;117:2431–2434 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


