Abstract
Background/Aim:
Telomere length (TL) predicts the onset of cellular senescence and correlates with longevity and age-related disease risk. While telomeres erode throughout life, adults display fixed ranking and tracking of TL, supporting the importance of the early environment in determining inter-individual variability across the life course. Given their guanine-rich structure, telomeres are highly susceptible to oxidative stress (OS). We examined maternal metal exposure, which can induce OS, in relation to newborn TL. We also considered the modifying role of maternal antioxidant intake.
Methods:
Analyses included 100 mother-newborn pairs enrolled in the Boston and New York City-based PRogramming of Intergenerational Stress Mechanisms (PRISM) pregnancy cohort. We measured As, Ba, Cd, Ni, and Pb in maternal late-pregnancy urine by ICP-MS and quantified relative leukocyte TL (rLTL) in cord blood using qPCR. We used Weighted Quantile Sum (WQS) regression to estimate the metal mixture - rLTL association and conducted repeated holdout validation to improve the stability of estimates across data partitions. We examined models stratified by high (> median) versus low (≤ median) maternal antioxidant intake, estimated from Block98 Food Frequency Questionnaires. We considered urinary creatinine, week of urine collection, maternal age, and race/ethnicity as covariates.
Results:
In adjusted models, urinary metals were inversely associated with newborn rLTL (βWQS=−0.50, 95% CI: −0.78, −0.21). The top metals contributing to the negative association included Ba (weight: 35.4%), Cd (24.5%) and Pb (26.9%). In models stratified by antioxidant intake, the significant inverse association between metals and rLTL remained only among mothers with low antioxidant intake (low: βWQS=−0.92, 95% CI: −1.53, −0.30; high: βWQS=−0.03, 95% CI: −0.58, 0.52). Results were similar in unadjusted models.
Conclusions:
Relative LTL was shorter among newborns of mothers with higher exposure to metals during pregnancy. Higher maternal antioxidant intake may mitigate the negative influence of metals on newborn rLTL.
Keywords: metals, lead (Pb), cadmium (Cd), Barium (Ba), telomere, newborn
1. Introduction
Exposure to toxic metals is ubiquitous in the United States (U.S.) and globally [1]. Naturally occurring metals in the earth’s crust are released into the air and ground water during industrial processes, including coal burning and hydraulic fracturing [2]. Other anthropogenic activities, such as agriculture, also introduce and redistribute metals throughout the environment [3]. In utero exposure to metals has been associated with altered prenatal programming and adverse pregnancy outcomes [4-6], including those that extend to childhood and adult life [7, 8]. Even low dose exposure to metals has been shown to impact the maternal-fetal interface [9], with some metals known to cross the placental barrier [10]. Metals interact with and disrupt biological processes at the cellular level; for example, they bind to DNA and nuclear/cytosolic proteins where they can inhibit function or induce oxidative stress (OS) and contribute to the deterioration of biological macromolecules [11]. Telomeres are nucleoprotein complexes at chromosome ends that progressively shorten with each cell division [12]. Critically short telomeres contribute to the onset of cellular senescence, which reduces tissue renewal and repair, leading to degraded structural components and ultimately contributing to the aging phenotype and age-related disease risk [13]. Research conducted in animals and humans supports that the initial setting of telomere length (TL) and attrition during early life are primary determinants of TL across the life course. For example, research has shown that by early adulthood most individuals display a fixed ranking and tracking of TL, suggesting that length at birth and erosion during childhood largely explain inter-individual variation observed in adults [14]. Other research conducted on adult twin pairs has also concluded that heritability and the early life environment, beginning in utero, are likely the primary determinants of TL during adulthood [15]. Thus, given the importance of TL in cellular health and aging, and growing evidence of the early life period as a critical window of telomere dynamics, it is critical to understand environmental factors that may prenatally influence the initial setting of TL.
Growing research supports that environmental exposures, including metals, may lead to accelerated telomere erosion. For example, among children, adolescents, and adults, metals exposure has been associated with shorter TL in diverse occupational [16, 17] and community-based samples [18-22], with recent research suggesting these associations may extend to the prenatal period [23-25]. An important limitation of prior studies examining prenatal exposure is that, even when multiple metals have been measured, each metal has been evaluated in isolation. Metals may act on overlapping biological pathways and may otherwise interact to elicit unique biological effects. Thus, considering metals as a mixture allows for a more comprehensive understanding of component and cumulative toxicity. In the present study, we addressed this research gap by using Weighted Quantile Sum (WQS) regression to evaluate the overall association between maternal exposure to five metals and newborn TL. We hypothesized that higher metals exposure would be associated with shorter cord blood TL and that Cd would contribute the greatest weight to the overall mixture effect. Our hypothesis regarding Cd was based on consistent evidence across accumulating studies supporting an inverse association between Cd exposure and TL [18, 19, 22, 24, 25]. Additionally, we examined effect modification by maternal dietary antioxidant intake. Telomeres are highly susceptible to OS by 8-oxoG modification given their guanine-rich structure, with persistent oxidative damage linked to telomere shortening and increased chromosome instability [26]. Antioxidants neutralize reactive oxygen species (ROS) and are localized to the placenta during pregnancy to reduce OS at the maternal-fetal interface. Whether maternal antioxidant intake modifies associations between prenatal metal exposure and newborn TL is unknown.
2. Methods
2.1. Study cohort.
Participants included a subset of the 836 mother-newborn pairs enrolled in the ongoing Programming of Intergenerational Stress Mechanisms (PRISM) pregnancy cohort. Briefly, women were recruited from prenatal clinics in Boston, MA and New York City, NY beginning to 2011. Women were considered eligible if they were English or Spanish speaking and 18 years or older. Exclusion criteria included maternal intake of ≥7 alcoholic drinks per week prior to pregnancy, any alcohol intake after pregnancy recognition, or HIV+ status. Maternal urinary metals (n=445) and newborn telomere length (n=218) were measured among a subset of the cohort; participants with these measures were randomly selected from the set of participants for whom biological samples had been collected and biobanked. The final analytic sample includes n=100 mother-newborn pairs with both metals and telomere data. No covariates of interest were missing for these 100 participants. Written informed consent was obtained from women prior to study participation in their preferred language. All study procedures were approved by the Institutional Review Boards at the Brigham and Women’s Hospital and the Icahn School of Medicine at Mount Sinai; Beth Israel Deaconess Medical Center relied on the Brigham and Women’s Hospital for review and oversight of the protocol.
2.2. Urinary metals and creatinine.
During pregnancy (mean: 32 weeks, range: 15-40 weeks) women were instructed to collect a spot urine sample in their home on the morning of a scheduled study visit. Samples were kept cool in the participant’s freezer until transport to the PRISM laboratory on the day of collection. Immediately upon receiving urine samples, they were thawed, aliquoted, and stored at −20°C. Samples (200 μL) were diluted to 10 mL with a solution containing 0.05% Triton X-100, 0.5% nitric acid, and mixed internal standard before metals analysis (arsenic [As], barium [Ba], cadmium [Cd], lead [Pb], nickel [Ni]) on an inductively coupled plasma-mass spectrometer-triple quadrupole (ICP-MS) instrument (Agilent 8800-QQQ). Quality control (QC) measures included analysis of the initial calibration, initial calibration verification, and continuing calibration verification standards: National Institute of Standards and Technology (NIST) traceable mixed-element standard solution at two concentration levels, procedural blanks and repeated analysis of 2% of samples. If QC materials did not meet control requirements, the batch was reprocessed and re-run. Daily limit of detection (LOD) values were calculated as three times the standard deviation (SD) of the concentration found in the method blank. Recoveries were determined from analyses of matrix appropriate QC standards including NIST SRM 2668 (Toxic elements in frozen human urine, levels 1 and 2). Recoveries were 90-110% for all metals. Coefficients of variation (CVs) were determined in each batch for internal urine pools fortified at mid- and high-level concentrations analyzed after initial calibration and every ten study samples. Intra-day CVs ranged from 0.3 to 21% for all elements and inter-day CVs ranged from 7 to 29% for all elements. Urine creatinine was measured using a well-established colorimetric method (LOD: 0.3125 mg/dL) and qualified by proficiency testing conducted by G-EQUAS (http://www.g-equas.de/) [27, 28]. QC measures in each batch included experimental blanks, lab urine pools, NIST SRMs (1957 and 1958) and proficiency testing material where available; batch-wise CVs of QC samples were <20% of the target concentration. We replaced metal concentrations below the LOD with the LOD divided by the square root of two. The number of samples with non-detectable values were as follows: As (n=0, 0%), Ba (n=2, 1.9%), Cd (n=3, 2.8%), Ni (n=0, 0%), Pb (n=0, 0%). Our approach to non-detects likely has minimal impact given the low frequency and because exposure levels are quantiled during the mixture analysis.
2.3. Cord blood telomere length.
Immediately following delivery cord blood samples were collected in EDTA-tubes, centrifuged to obtain the buffy coat fraction, and stored at −80°C until DNA extraction. The mean±standard deviation (SD) time to assay was 1.8±0.7 years. DNA was extracted using a Promega Wizard DNA extraction system (Madison, WI, USA). DNA quantity and quality were assessed using an Implen NanoPhotometer Pearl (Westlake Village, CA) and all values were found to be within acceptable ranges (A260/280 ratio: 1.7-2.0, minimum quantity = 30 ng). Relative leukocyte telomere length (rLTL) was measured by qPCR on a 7900HT Fast Real Time PCR System (Applied Biosystems) instrument on 384 well plates following the protocol developed by Cawthon et al [29]. Additional details of the assay, including primers used, have been previously described [30]. All samples included in the current study were run in triplicate across five plates. The same serially diluted reference DNA was included in each PCR run so that the quantity of targeted templates in each sample could be determined relative to the reference DNA sample by the standard curve method. We calculated rLTL as the average of the triplicate telomere values divided by the average of the triplicate single copy gene (β-globin) values. A measure was considered acceptable if the standard deviation among triplicate measures was <0.25. The inter-plate coefficient of variation was 5.14%; to control for inter-assay variability, a pooled DNA sample derived from adults was included in each run (median pooled rLTL across plates: 1.04, range: 0.96-1.08). For each plate, we divided the rLTL of each pooled DNA sample by the average rLTL for all pooled samples across all plates to obtain a normalizing factor. We then divided samples on a given plate by the plate-specific normalizing factor to adjust for potential batch effects [31].
2.4. Antioxidant intake.
In mid-pregnancy, mothers completed a 120-item modified Block98 Food Frequency Questionnaire (FFQ) designed to assess dietary intake, including intake of prenatal vitamins, over the prior three months. The type, consumption frequency, and portion size of each item was processed to derive corresponding micro- and macronutrient intake information using the online Block Dietary Data Systems (Berkeley, CA). Micronutrient data, including antioxidant intakes, were validated using 24-hour dietary recall among a subset of 42 participants [32]. In order to construct a composite antioxidant index, we first calculated the energy-adjusted dietary intake of seven antioxidant micronutrients (β-carotene, magnesium, selenium, zinc and vitamins A, C, and E) and then summed these values with intake derived from supplements. We then converted the distribution of each micronutrient into a percentile, which we ranked across participants. Final antioxidant index scores reflect an individual’s overall average intake across the seven micronutrients relative to other participants. We dichotomized maternal antioxidant status into high versus low based on stratification at the median. Of the 100 women included in the analytic sample, 82 had available FFQ data.
2.5. Covariates.
During pregnancy, data on maternal age, race/ethnicity, level of education, annual household income, pre-pregnancy body mass index (BMI), and smoking were collected by questionnaire administered by trained research personnel during an in-person study visit.
2.6. Statistical analysis.
2.6.1. Descriptive and missing data analyses
We visually inspected the distributions of cord blood rLTL, each urinary metal, and each covariate of interest using boxplots and histograms. We calculated geometric means (GM) and interquartile ranges (IQR) for each metal and calculated Spearman correlations between metals. We used chi-square tests and t-tests to assess differences in participant characteristics between those included in the current study and those enrolled in the cohort, but excluded from the study due to missing exposure or outcome data.
2.6.2. Regression analyses
We natural-log transformed each metal to approximate a normal distribution before regression analyses. We used WQS regression to examine urinary metals, treated as a mixture, in relation to cord blood rLTL. WQS regression was developed as a multi-pollutant method for assessing the overall effects of a mixture while allowing for collinearity between mixture components [33, 34]. Briefly, exposure variables are transformed into quantiles and compiled into a unidirectional weighted index that is regressed on the outcome in a multivariable linear model. We converted metal concentrations into deciles and constrained the model to a negative direction based on our hypothesis that the mixture would be related to shorter rLTL. We applied repeated holdout validation, an approach that combines cross-validation and bootstrap resampling, to improve the stability of weights across training and test data partitions [35]. Specifically, we generated a distribution of results by repeating WQS regression 100 times on data split randomly into 40%-60% training-test sets. We report the mean effect estimate and weights, which indicate the relative importance of each component, across the 100 iterations. We expressed estimates as the difference in rLTL per inter-quartile-range (IQR) increase in the WQS index. We considered metals with a mean weight exceeding 20% (100%/5 metals) as exposures of concern, indicating a larger contribution to the outcome than expected by chance. In addition to evaluating unadjusted models, we considered two adjustment sets that included: 1) gestational week at urine collection (weeks) and urine creatinine (mg/dL), and 2) the first set of covariates plus maternal age at delivery (years), and race/ethnicity (White vs. Black/Hispanic), which have previously been associated with TL [36-38] and may relate to maternal metals exposure [39, 40]. We conceptualized gestational age as a potential mediator of the metal-TL relationship and therefore did not adjust models for this characteristic. To account for potential selection bias, we used inverse probability weights incorporating characteristics (race/ethnicity, education, annual household income, age at delivery, and pre-pregnancy body mass index) that were significantly different between the analytic sample and the sample of mothers enrolled in the cohort, but excluded from current analyses [41]. All analyses were conducted using RStudio (v 1.2.5033) or SAS v9.4.
2.6.3. Effect modification by antioxidant intake
To evaluate effect modification we examined unadjusted and adjusted models within strata of high versus low maternal antioxidant intake, as indicated by antioxidant index scores stratified at the median.
2.6.4. Sensitivity analyses
Cigarette smoke is an established source of some metals, such as Cd [42], and has been associated with shorter TL [19, 22, 43]. Whether metals contained within cigarette smoke are the causative agent underlying associations between smoking and TL is not known. In the current analysis we were unable to adjust for maternal smoking given the relatively low prevalence (n=13, 13%), which resulted in some training and test data partitions with no variability in smoking status. As an alternative, we conducted a sensitivity analysis excluding women who smoked. We also examined full cohort models additionally adjusting for maternal education (< high school vs. ≥ high school) as an indicator of socioeconomic status, which has been linked to both TL [44] and metals exposure [45]. We were unable to consider maternal education in antioxidant-stratified models due to sparse data within levels. We also examined models adjusting for maternal pre-pregnancy body mass index (BMI), which has been linked to telomere length in prior studies [46] and could potentially influence urinary metals. To examine the potential influence of DNA storage time, we examined models adjusting for time to assay. Finally, because 1) urine is not the gold standard matrix for measuring Pb, and 2) our measure of urinary As was not speciated, we examined models excluding these components from the metal mixture.
3. Results
3.1. Sample characteristics and missing data
Compared to the analytic study sample, mothers enrolled in PRISM who were excluded due to incomplete metals or telomere data were younger (29.3 years vs. 30.9 years, p-value=0.007), heavier (pre-pregnancy BMI: 27.3 kg/m2 vs. 25.4 kg/m2, p-value = 0.010), less likely to have a high school education or more (60% vs. 80%, p-value < 0.001), less likely to have an annual household income of $50,000 or more (26% vs. 50%, p-value <0.001), and more likely to be minority (83.9% vs. 58.0%, p-value < 0.001) (Supplemental Table S1). No other significant differences were observed in sample characteristics. Table 1 provides characteristics, including rLTL and urinary metals concentrations, of mothers and newborns included in the analytic sample overall and stratified by high vs. low antioxidant intake. Women with lower antioxidant intake were more likely to be of minority race/ethnicity (69.0% vs. 40.0%, p=0.008), more likely to have less than a high school education (31.0% vs. 7.5%, p=0.014) and more likely to smoke during pregnancy (19.0% vs. 5.0%, p=0.052). Notably, women with lower antioxidant intake also had significantly higher levels of Cd (GM [IQR]: 0.20 [0.29] vs. 0.13 [0.17], p=0.013) and Ni (1.63 [1.29] vs. 1.37 [1.10], p=0.014) compared to women with higher antioxidant intake, which may in part reflect differences in smoking status. There were no other differences in sample characteristics by antioxidant level.
Table 1.
Characteristics (mean±standard deviation or N, %) of the study sample overall and stratified by higher versus lower antioxidant intake.
| Characteristic | Overall (n=100) |
Low Antioxidant (n=42) |
High Antioxidant (n=40) |
p-valuea |
|---|---|---|---|---|
| Maternal age | 30.9±5.0 | 30.2±5.5 | 31.3±4.2 | 0.305 |
| Pre-pregnancy body mass index (kg/m2) | 25.4±5.5 | 26.5±5.5 | 24.7±5.4 | 0.022 |
| Race/ethnicity | ||||
| White, non-Hispanicb | 42 (42) | 13 (31.0) | 24 (60.0) | 0.008 |
| Black/Hispanic | 58 (58) | 29 (69.0) | 16 (40.0) | 0.008 |
| <High school education | 20 (20) | 13 (31.0) | 3 (7.5) | 0.007 |
| Annual household income <$50,000 | 50 (50) | 37 (61.7) | 25 (43.1) | 0.014 |
| Cigarette smoking during pregnancy | 13 (13) | 8 (19.0) | 2 (5.0) | 0.052 |
| Gestational age at birth | 39.0±1.3 | 39.0±1.4 | 38.9±1.4 | 0.572 |
| Gestational week at urine collection | 32.2±4.6 | 31.1±4.5 | 33.7±4.1 | 0.008 |
| Urinary metalsc | ||||
| Arsenic (ng/mL) | 11.45 (16.44) | 10.66 (17.03) | 12.64 (16.75) | 0.191 |
| Barium (ng/mL) | 2.39 (3.19) | 2.51 (2.97) | 2.29 (4.02) | 0.657 |
| Cadmium (ng/mL) | 0.16 (0.21) | 0.20 (0.29) | 0.13 (0.17) | 0.013 |
| Nickel (ng/mL) | 1.46 (1.31) | 1.63 (1.29) | 1.37 (1.10) | 0.014 |
| Lead (ng/mL) | 0.45 (0.44) | 0.51 (0.48) | 0.42 (0.37) | 0.089 |
| Urinary creatininec (mg/dL) | 91.65 (66.24) | 93.09 (76.13) | 90.62 (64.81) | 0.683 |
| Cord blood relative telomere length | 2.37±0.98 | 2.33±0.94 | 2.68±0.97 | 0.102 |
P-values are from Chi-square tests of independence (categorical variables) or Student’s t-tests (continuous variables) unless otherwise noted.
Includes n=5 women that self-report race/ethnicity as other than White, Black or Hispanic. Of these 5 women, 3 have low antioxidant intake and 2 have high antioxidant intake.
Values are geometric mean (interquartile range) and p-values are from Wilcoxon rank sum tests.
3.2. Metal mixture and rLTL
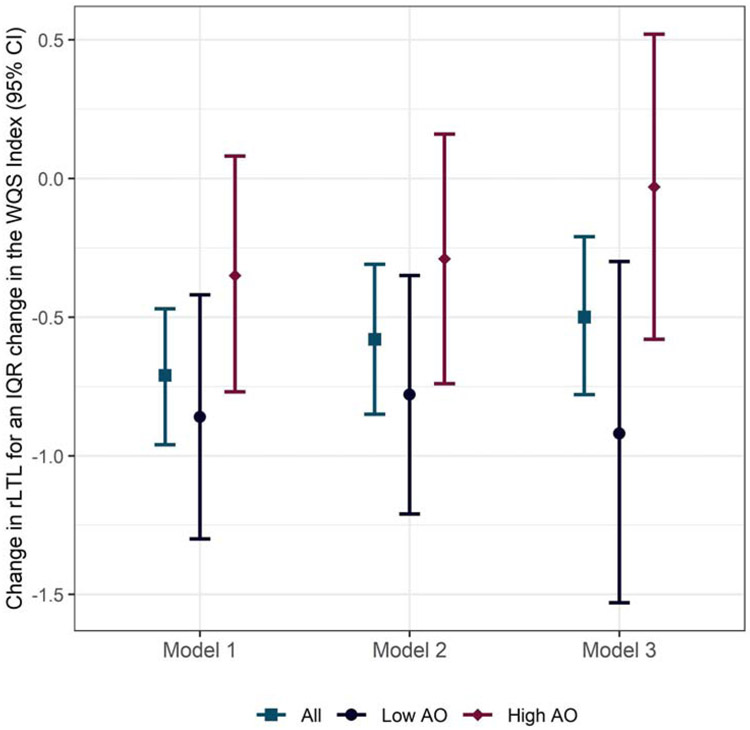
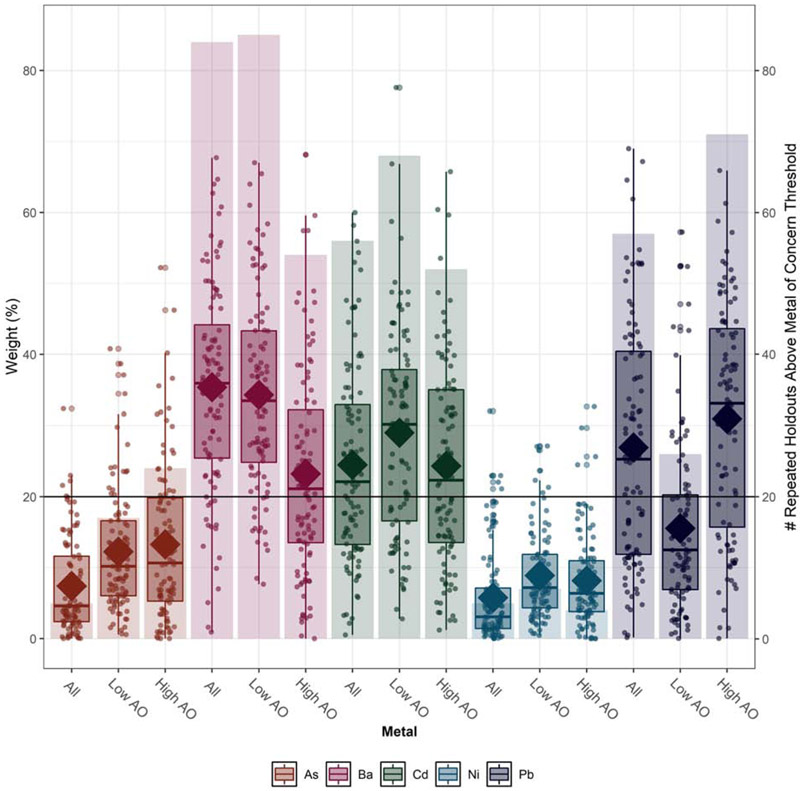
Pb and Ni were moderately to highly correlated with each other and with each of the other metals examined (Supplemental Table 2). Ba was not correlated with As or Cd, but the latter were moderately correlated with each other. Across covariate adjustment sets, higher maternal exposure to metals was associated with shorter cord blood rLTL (Figure 1). In the fully adjusted model, we observed a 0.50 unit decline (95% confidence interval [CI] = −0.78, −0.21) in rLTL for an IQR change in the WQS index. In models stratified by antioxidant intake, we observed approximately no change (βWQS=−0.03, 95% CI: −0.58, 0.52) in rLTL with increasing metals exposure among newborns of high antioxidant intake mothers, but a 0.92 unit decline (95% CI: −1.53, −0.30) among newborns of low antioxidant intake mothers (Figure 1). We constructed a weight uncertainty plot (Figure 2) that presents the mean weight for each metal (indicated by a diamond), the distribution of weights simulated using repeated holdout validation sets (indicated by boxplots), and the number of times that a given metal exceeded the threshold (20%) of concern (indicated by bars). In addition to illustrating mean weights across metals and levels of antioxidant intake, the plot characterizes the variability in weights over repeated holdouts. Across models, Ba (mean weight = 35.4%), Cd (mean weight = 24.5%), and Pb (mean weight=26.9%) had the largest contribution to the index, with weights exceeding the threshold of concern in a majority of the 100 repeated holdouts performed (Ba: n=84, Cd: n=56, Pb: n=57). Results were similar in unadjusted models and models adjusted for urinary creatinine and week of gestation at urine collection (Supplemental Table S3). In antioxidant stratified models, Pb contributed less to the overall mixture weight among women with low antioxidant intake, whereas As and Ni contributed more, although remained the smallest overall contributors. Supplemental Figures S1 and S2 present weight uncertainty plots for 1) unadjusted models and, 2) models only adjusting for urinary creatinine and week of gestation at sample collection.
Figure 1. Change in cord blood relative telomere length for an IQR increase in the maternal metals WQS Index.
Abbreviations: AO = antioxidant, IQR = inter quartile range, rLTL = relative leukocyte telomere length, WQS = weighted quantile sum.
Notes: WQS index components included: arsenic, barium, cadmium, nickel, and lead. Model 1 is unadjusted, Model 2 is adjusted for urine creatinine and week of gestation at urine collection, Model 3 is adjusted for Model 2 covariates plus maternal age and race/ethnicity.
Figure 2. WQS mixture weights for metals of concern in the sample overall and stratified by maternal antioxidant intake.
Abbreviations: AO = antioxidant, As= arsenic, Ba=barium, Cd=cadmium, Ni=nickel, Pb=lead, WQS=weighted quantile sum
Notes: Bars correspond to the right axis and indicate the number of times a metal exceeded the concern threshold of 20% in 100 repeated holdouts. Data points, boxplots, and diamonds correspond to the left axis. Data points indicate weights for each of the 100 holdouts. Box plots show 25th, 50th, and 75th percentiles, and whiskers show 10th and 90th percentiles of weights for the 100 holdouts. Closed diamonds show mean weights for the 100 holdouts. All models are adjusted for urine creatinine, week of gestation at urine collection, maternal age and race/ethnicity.
3.3. Sensitivity analyses
In fully adjusted models (creatinine, urine collection week, race/ethnicity, age) excluding smokers, the inverse association between maternal metals exposure and newborn rLTL was attenuated (βWQS=−0.29, 95% CI: −0.60, 0.03), but the rank ordering of weights remained the same (Ba: 36.8%, Pb: 24.7%, Cd: 23.0%, As: 9.9%, Ni: 5.7%). Results were not substantially changed in fully adjusted models additionally adjusting for maternal education (βWQS=−0.46, 95% CI: −0.80, −0.12; weights: Ba: 34.3%, Pb: 24.7%, Cd: 27.6%, As: 9.1%, Ni: 4.3%) or pre-pregnancy BMI (βWQS=−0.48, 95% CI: −0.77, −0.19; weights: Ba: 33.9%, Pb: 23.3%, Cd: 28.4%, As: 13.3%, Ni: 8.2%). Likewise, results from models 1) additionally adjusting for DNA storage time (Supplemental Table S4), and 2) excluding As and Pb from the metal mixture (Supplemental Table S5) were similar in magnitude and direction. In models excluding As and Pb, the rank ordering of weights for the remaining mixture components (Ba, Cd, Ni) was retained.
4. Discussion.
We found maternal urinary levels of Ba, Cd and Pb, but not As or Ni, measured during pregnancy were inversely associated with newborn rLTL. There was also evidence of effect modification by antioxidant status, such that the magnitude of inverse associations was greater among women with lower antioxidant intake.
Three prior studies have examined prenatal exposure to Cd and/or Pb in relation to newborn TL. In a study of multiple metals, maternal urinary Cd (median: 0.6 ng/mL) and As (median: 55.3 ng/mL), but not Pb (median: 1.3 ng/mL), measured during mid-pregnancy were independently associated with shorter cord blood rLTL among a sample of 409 mother-newborn pairs based in Myanmar [24]. In follow-up analyses on the same cohort, the inverse associations between Cd and rLTL was attenuated and no longer significant among women with higher intake of selenium, an essential micronutrient with antioxidant properties [47]. A separate study conducted in China (n=410) also found maternal urinary Cd (GM: 0.6 ng/mL) to be associated with shorter newborn rLTL, however, other metals were not considered [25]. Most recently, a study of 88 newborns in Argentina found cord blood Pb (GM: 14 ng/mL), but not lithium (GM: 48 ng/mL), boron (193 ng/mL), As (GM: 2.3 ng/mL), Cd (GM: <LOD), or antimony (GM: 2.5 ng/mL), to be inversely associated with cord blood rTL [23]. The inconsistent finding related to Cd between our study and that conducted in Argentina could reflect the different biological matrix examined (i.e. maternal urine versus cord blood), as well as the level of exposure (i.e. undetectable in the majority of Argentinian samples). Likewise, while the study conducted in Myanmar did not detect significant associations between increasing quartiles of Pb exposure, they did find an overall negative trend (p=0.008). Likewise, our observation of no association with As, which contrasts with the study conducted in Myanmar, may relate to the large difference in exposure level. We are not aware of prior research that has examined prenatal Ni or Ba exposure in relation to newborn rLTL.
Metal-induced OS may be one biological pathways linking metals with newborn TL. Due to their high oxidation potential, guanine-rich telomeres have heightened susceptibility to attack by free radicals [48]. ROS also produce single-strand breaks, and telomeric DNA is deficient in the repair of these breaks [48, 49]. Further, OS has been linked to reduced telomerase reverse transcriptase activity [50], which is highly expressed during gestation and is responsible for adding telomeric DNA repeats to chromosome ends. Cd is known to cross the placental barrier [10], with research conducted using murine models demonstrating wide distribution throughout the fetal system [51, 52]. The primary mode of Cd toxicity is induction of cellular apoptosis through OS [53]. During pregnancy, Cd has been shown to induce trophoblast apoptosis and OS in the placenta [54, 55], as well as promote the development of pre-eclampsia via placental oxidative DNA damage [56]. Similar to Cd, Pb can pass the placenta and upregulate ROS [57, 58], including in the intra-uterine compartment [59]. In addition to promoting OS, research suggests that Pb can directly act on DNA and chromosomal integrity [60], notably at telomere regions [61], and can alter proteins involved in telomere maintenance [62]. While less extensively studied, evidence also supports that Ba can produce OS in mammals [63, 64], may impair frontline antioxidant defense systems [65, 66], and along with Cd has been shown to compete with essential metals for protein binding sites, interfering with OS pathways [67, 68]. Animal research has found high levels of Ba to be associated with spontaneous abortion, fetal growth restriction, and neonatal death [69], and in humans, Ba during pregnancy has been associated with an increased risk for congenital heart defects [70]. While these studies collectively support that the developing fetus is sensitive to Ba toxicity, we are not aware of prior research that has examined maternal Ba exposure in relation to cord blood rLTL, although Ba exposure has notably been linked to shorter lifespan [71]. OS can also lead to chronic inflammation, which is believed to play a major role in aging and has been linked to leukocyte telomere shortening [72]. Further, metals are known to directly induce inflammation and many immune cells generate free radicals upon activation [73]. It is thus possible that mechanisms linking metals with telomere attrition involve inter-related processes involving both OS and inflammation.
We found evidence of effect modification by maternal antioxidant intake such that diets higher in antioxidant micronutrients appeared protective against metal-related TL shortening. Transport of maternal antioxidants to the intra-uterine compartment begins early during pregnancy and is suspected to play an essential role during the first trimester when the placenta has limited antioxidant capacity [74]. While we are not aware of prior research that has examined modification of metal mixture-TL associations by antioxidants, our findings are consistent with one prior study that found effect modification of the association between individual Cd exposure and newborn rLTL by selenium [47]. A role for antioxidant defenses against telomere attrition is also supported by research conducted in adults and children [75, 76]. For example, in a study of 287 children and adolescents from Spain, a significant, positive association between LTL and total dietary antioxidant capacity estimated using a FFQ was observed [77]. Research using animal models or isolated cell lines also corroborate our findings and those of prior human studies [78, 79]. Importantly, we observed several sociodemographic differences between women with high versus low antioxidant intake. While we controlled for these variables when possible and believe there is biologic plausibility to the hypothesis that antioxidant status affects telomere susceptibility, other characteristics that co-vary with antioxidant intake could underlie the observed effect heterogeneity by antioxidant status.
Exposure to Ba has historically occurred at low levels through consumption of food and drinking water; however, its industrial use (e.g., petroleum drilling, hydraulic fracturing of shale gas, steel production, agricultural chemicals) has expanded over recent decades, thereby increasing the potential for human exposure [69, 80]. In the PRISM pregnancy cohort, GM urine Ba concentrations (2.39±2.26 ng/mL) were more than double that of the general (GM=1.15 ng/mL) and female U.S. population (GM=1.03 ng/mL) [1]. Exposure to Cd and Pb, which occurs primarily through diet, tobacco smoke and air pollution [42, 81], was also higher among PRISM participants (Cd: GM=0.16 ng/mL, Pb: GM=0.45 ng/mL) compared to the U.S. overall (Cd: GM=0.13 ng/mL, Pb: GM=0.28 ng/mL) and female (Cd: GM=0.14 ng/mL, Pb: GM=0.26 ng/mL) populations [1]. While the higher exposure level among PRISM participants may reduce the generalizability of our findings to other U.S. communities with lower metals exposure, they also suggest that communities of similar sociodemographic characteristics (urban, lower income, minority) may have heightened risk for exposure and subsequent adverse health outcomes.
A key strength of this study is the use of WQS regression to create an empirically weighted index of the mixture effect. Considering the joint action of metals is key as co-exposures may combine to elicit changes not seen when considered in isolation. We additionally improved the stability of WQS estimates using repeated holdout validation, which allowed us to characterize uncertainty around the estimates, thereby adding credibility to our findings. Unfortunately, this approach precluded our ability to formally test for an antioxidant interaction due to the challenge of calculating the significance of an interaction product across bootstrapped samples. It will thus be important for future, larger studies to replicate our findings and formally investigate the interaction between metals and antioxidant status on TL. We measured metals in maternal spot urine samples collected during pregnancy, which is advantageous in that urine reflects multiple exposure sources (e.g., air, water, and food). However, while urinary Cd is considered an acceptable biomarker of longer-term exposure, concentrations of the other metals we measured reflect recent exposure only [81-84], which may in fact be consistent across time assuming relatively constant, chronic exposure conditions. While urine is an acceptable matrix for measuring As, Ba, Cd and Ni, whole blood is considered the gold standard matrix for measuring Pb. However, urinary Pb, which reflects Pb that has diffused from the plasma and been excreted through the kidneys, has more recently been shown to discriminate inter-individual variability in exposure levels [85]. Nonetheless, future research that examines blood Pb in combination with other metal exposures is needed to substantiate our findings. Our measure of As was not speciated and thus we were unable to distinguish between non-toxic, organic As largely arising from seafood consumption versus the toxic, inorganic form; it is possible that this contributed to the low As weight in the WQS index. Notably, we ran sensitivity analyses excluding Pb and As from the metal mixture and this did not substantially alter effect estimates or change the rank ordering of remaining metal components. While collecting 24-hour urine samples would have been preferable to spot urine samples, this was not feasible within the context of the PRISM cohort design and rarely done in longitudinal epidemiologic studies. Thus, we alternatively accounted for the potential influence of variation in urine dilution by adjusting models for urinary creatinine. Notably, results were similar in models with and without adjustment for creatinine, suggesting only minimal impact of urinary dilution on the observed associations. In sensitivity analyses excluding women who smoked (n=13), which is a known source of some metals, including Cd, inverse associations with newborn rLTL were attenuated, suggesting that smoke may indeed be a source of toxic metal exposure. TL may vary between different leukocyte cell types and we were unfortunately unable to account for potential variability in leukocyte cell type distributions between newborns. Little evidence suggests that metals exposure affects cell type distribution, although we acknowledge that this relationship has not been thoroughly examined in the literature. We used a validated FFQ instrument to estimate antioxidant intake and only included micronutrients that have previously been identified to correspond well with 24-hour dietary recall data in this sample [32]; however, there is potential for misclassification in our antioxidant index if mothers inaccurately recalled their dietary habits. Our study had limited statistical power due to the modest sample of mother-newborn pairs with both metals and telomere data and it will be important for future research to confirm our findings. Finally, as with all observational research, while we controlled for a core set of covariates, residual confounding by variables that were not measured is possible. A particularly important covariate that was not measured in the PRISM cohort includes parental rLTL. TL is highly heritable and it is thus possible that the associations observed here in part reflect negative effects of metals on maternal or paternal germ cell telomeres [86]. Future studies that measure both parental and newborn rLTL are needed to disentangle whether environmental exposures, including metals, have transgenerational effects, act directly on fetal rLTL, or both.
Conclusion
In conclusion, we found an inverse association between maternal exposure to metals during pregnancy and newborn rLTL after adjustment for potential confounders, with Ba, Cd, and Pb contributing the greatest weight to the mixture effect. Our finding that associations were attenuated among mothers with higher antioxidant intake sheds light on a putative underlying biological mechanism and reinforces the importance of a healthy diet during pregnancy. Environmental exposures associated with shorter TL at birth, including metals, may have implications for aging and health across the life course as longitudinal animal research and retrospective human studies suggest the initial setting of TL and attrition during early life predict lifespan [87, 88] and are a primary determinant of inter-individual variability in TL during adulthood [14, 15].
Supplementary Material
Highlights.
Urinary metals (As, Ba, Cd, Pb, Ni) are detectable during pregnancy.
Maternal metal mixtures are inversely associated with newborn telomere length.
Negative associations remain only among mothers with lower antioxidant intake.
Related underlying mechanisms may relate to oxidative stress pathways.
Acknowledgments:
The Mount Sinai HHEAR laboratory hub acknowledges Shirisha Yelamanchili who performed the measurements of creatinine in urine.
Funding: This work was supported by the National Institutes of Health [grant numbers: R01HL095606, R01ES030302, R01HD082078, R21ES021318, P30ES023515, and UG3OD023337]. During the preparation of this manuscript, WC was supported by T32HD049311.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Prevention), C.C.f.D.C.a., Fourth National Report on Human Exposure to Environmental Chemicals: Updated Tables. 2013, US Department of Health and Human Services, Centers for Disease Control and Prevention: Atlanta, GA. [Google Scholar]
- 2.Vareda JP, Valente AJM, and Duraes L, Assessment of heavy metal pollution from anthropogenic activities and remediation strategies: A review. J Environ Manage, 2019. 246: p. 101–118. [DOI] [PubMed] [Google Scholar]
- 3.He ZL, Yang XE, and Stoffella PJ, Trace elements in agroecosystems and impacts on the environment. J Trace Elem Med Biol, 2005. 19(2-3): p. 125–40. [DOI] [PubMed] [Google Scholar]
- 4.Bommarito PA, Martin E, and Fry RC, Effects of prenatal exposure to endocrine disruptors and toxic metals on the fetal epigenome. Epigenomics, 2017. 9(3): p. 333–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Freire C, et al. , Placental metal concentrations and birth outcomes: The Environment and Childhood (INMA) project. Int J Hyg Environ Health, 2019. 222(3): p. 468–478. [DOI] [PubMed] [Google Scholar]
- 6.Vilahur N, Vahter M, and Broberg K, The Epigenetic Effects of Prenatal Cadmium Exposure. Curr Environ Health Rep, 2015. 2(2): p. 195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sanders AP, Claus Henn B, and Wright RO, Perinatal and Childhood Exposure to Cadmium, Manganese, and Metal Mixtures and Effects on Cognition and Behavior: A Review of Recent Literature. Curr Environ Health Rep, 2015. 2(3): p. 284–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Young JL, Cai L, and States JC, Impact of prenatal arsenic exposure on chronic adult diseases. Syst Biol Reprod Med, 2018. 64(6): p. 469–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Geng HX and Wang L, Cadmium: Toxic effects on placental and embryonic development. Environ Toxicol Pharmacol, 2019. 67: p. 102–107. [DOI] [PubMed] [Google Scholar]
- 10.Gundacker C and Hengstschlager M, The role of the placenta in fetal exposure to heavy metals. Wien Med Wochenschr, 2012. 162(9-10): p. 201–6. [DOI] [PubMed] [Google Scholar]
- 11.Flora SJ, Mittal M, and Mehta A, Heavy metal induced oxidative stress & its possible reversal by chelation therapy. Indian J Med Res, 2008. 128(4): p. 501–23. [PubMed] [Google Scholar]
- 12.Lu W, et al. , Telomeres-structure, function, and regulation. Exp Cell Res, 2013. 319(2): p. 133–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shay JW, Telomeres and aging. Curr Opin Cell Biol, 2018. 52: p. 1–7. [DOI] [PubMed] [Google Scholar]
- 14.Benetos A, et al. , Tracking and fixed ranking of leukocyte telomere length across the adult life course. Aging Cell, 2013. 12(4): p. 615–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hjelmborg JB, et al. , The heritability of leucocyte telomere length dynamics. J Med Genet, 2015. 52(5): p. 297–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pawlas N, et al. , Telomere length, telomerase expression, and oxidative stress in lead smelters. Toxicol Ind Health, 2016. 32(12): p. 1961–1970. [DOI] [PubMed] [Google Scholar]
- 17.Wong JY, et al. , Cumulative PM(2.5) exposure and telomere length in workers exposed to welding fumes. J Toxicol Environ Health A, 2014. 77(8): p. 441–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fillman T, et al. , Association of cadmium and arsenic exposure with salivary telomere length in adolescents in Terai, Nepal. Environ Res, 2016. 149: p. 8–14. [DOI] [PubMed] [Google Scholar]
- 19.Grau-Perez M, et al. , Urinary metals and leukocyte telomere length in American Indian communities: The Strong Heart and the Strong Heart Family Study. Environ Pollut, 2019. 246: p. 311–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pawlas N, et al. , Telomere length in children environmentally exposed to low-to-moderate levels of lead. Toxicol Appl Pharmacol, 2015. 287(2): p. 111–118. [DOI] [PubMed] [Google Scholar]
- 21.Scinicariello F and Buser MC, Urinary antimony and leukocyte telomere length: An analysis of NHANES 1999-2002. Environ Res, 2016. 150: p. 513–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zota AR, et al. , Associations of cadmium and lead exposure with leukocyte telomere length: findings from National Health and Nutrition Examination Survey, 1999-2002. Am J Epidemiol, 2015. 181(2): p. 127–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Herlin M, et al. , Exploring telomere length in mother-newborn pairs in relation to exposure to multiple toxic metals and potential modifying effects by nutritional factors. BMC Med, 2019. 17(1): p. 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wai KM, et al. , Impact of prenatal heavy metal exposure on newborn leucocyte telomere length: A birth-cohort study. Environ Pollut, 2018. 243(Pt B): p. 1414–1421. [DOI] [PubMed] [Google Scholar]
- 25.Zhang L, et al. , Prenatal cadmium exposure is associated with shorter leukocyte telomere length in Chinese newborns. BMC Med, 2019. 17(1): p. 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coluzzi E, et al. , Oxidative stress induces persistent telomeric DNA damage responsible for nuclear morphology change in mammalian cells. PLoS One, 2014. 9(10): p. e110963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goen T, Schaller KH, and Drexler H, External quality assessment of human biomonitoring in the range of environmental exposure levels. Int J Hyg Environ Health, 2012. 215(2): p. 229–32. [DOI] [PubMed] [Google Scholar]
- 28.Taussky HH, A microcolorimetric determination of creatine in urine by the Jaffe reaction. J Biol Chem, 1954. 208(2): p. 853–61. [PubMed] [Google Scholar]
- 29.Cawthon RM, Telomere measurement by quantitative PCR. Nucleic Acids Res, 2002. 30(10): p. e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bosquet Enlow M, et al. , Sex differences in effects of maternal risk and protective factors in childhood and pregnancy on newborn telomere length. Psychoneuroendocrinology, 2018. 95: p. 74–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin J, et al. , Analyses and comparisons of telomerase activity and telomere length in human T and B cells: insights for epidemiology of telomere maintenance. J Immunol Methods, 2010. 352(1-2): p. 71–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brunst KJ, et al. , Validation of a Food Frequency Questionnaire for Estimating Micronutrient Intakes in an Urban US Sample of Multi-Ethnic Pregnant Women. Matern Child Health J, 2016. 20(2): p. 250–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carrico C, et al. , Characterization of Weighted Quantile Sum Regression for Highly Correlated Data in a Risk Analysis Setting. J Agric Biol Environ Stat, 2015. 20(1): p. 100–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gennings C, Sabo R, and Carney E, Identifying subsets of complex mixtures most associated with complex diseases: polychlorinated biphenyls and endometriosis as a case study. Epidemiology, 2010. 21 Suppl 4: p. S77–84. [DOI] [PubMed] [Google Scholar]
- 35.Tanner EM, Bornehag CG, and Gennings C, Repeated holdout validation for weighted quantile sum regression. MethodsX, 2019. 6: p. 2855–2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Factor-Litvak P, et al. , Leukocyte Telomere Length in Newborns: Implications for the Role of Telomeres in Human Disease. Pediatrics, 2016. 137(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ly K, et al. , Telomere length in early childhood is associated with sex and ethnicity. Sci Rep, 2019. 9(1): p. 10359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lynch SM, et al. , Race, Ethnicity, Psychosocial Factors, and Telomere Length in a Multicenter Setting. PLoS One, 2016. 11(1): p. e0146723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chang L, et al. , Study on the relationship between age and the concentrations of heavy metal elements in human bone. Ann Transl Med, 2018. 6(16): p. 320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shim YK, et al. , Prevalence and associated demographic characteristics of exposure to multiple metals and their species in human populations: The United States NHANES, 2007-2012. J Toxicol Environ Health A, 2017. 80(9): p. 502–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cole SR and Hernan MA, Constructing inverse probability weights for marginal structural models. Am J Epidemiol, 2008. 168(6): p. 656–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Registry), A.A.f.T.S.a.D., Toxicological profile for Cadmium. 2012, US Department of Health and Human Services, Public Health Service: Atlanta, GA. [Google Scholar]
- 43.Patel CJ, et al. , Systematic correlation of environmental exposure and physiological and self-reported behaviour factors with leukocyte telomere length. Int J Epidemiol, 2017. 46(1): p. 44–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Martens DS, et al. , Association of Parental Socioeconomic Status and Newborn Telomere Length. JAMA Netw Open, 2020. 3(5): p. e204057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Montazeri P, et al. , Socioeconomic position and exposure to multiple environmental chemical contaminants in six European mother-child cohorts. Int J Hyg Environ Health, 2019. 222(5): p. 864–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Martens DS, et al. , Maternal pre-pregnancy body mass index and newborn telomere length. BMC Med, 2016. 14(1): p. 148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wai KM, et al. , Protective role of selenium in the shortening of telomere length in newborns induced by in utero heavy metal exposure. Environ Res, 2020. 183: p. 109202. [DOI] [PubMed] [Google Scholar]
- 48.von Zglinicki T, Oxidative stress shortens telomeres. Trends Biochem Sci, 2002. 27(7): p. 339–44. [DOI] [PubMed] [Google Scholar]
- 49.Houben JM, et al. , Telomere length assessment: biomarker of chronic oxidative stress? Free Radic Biol Med, 2008. 44(3): p. 235–46. [DOI] [PubMed] [Google Scholar]
- 50.Gao B, et al. , Zinc finger protein 637 protects cells against oxidative stress-induced premature senescence by mTERT-mediated telomerase activity and telomere maintenance. Cell Death Dis, 2014. 5: p. e1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jacquillet G, et al. , Cadmium causes delayed effects on renal function in the offspring of cadmium-contaminated pregnant female rats. Am J Physiol Renal Physiol, 2007. 293(5): p. F1450–60. [DOI] [PubMed] [Google Scholar]
- 52.Trottier B, et al. , Maternal-fetal distribution of cadmium in the guinea pig following a low dose inhalation exposure. Toxicol Lett, 2002. 129(3): p. 189–97. [DOI] [PubMed] [Google Scholar]
- 53.Kukongviriyapan U, Apaijit K, and Kukongviriyapan V, Oxidative Stress and Cardiovascular Dysfunction Associated with Cadmium Exposure: Beneficial Effects of Curcumin and Tetrahydrocurcumin. Tohoku J Exp Med, 2016. 239(1): p. 25–38. [DOI] [PubMed] [Google Scholar]
- 54.Wang Z, et al. , Cadmium-induced teratogenicity: association with ROS-mediated endoplasmic reticulum stress in placenta. Toxicol Appl Pharmacol, 2012. 259(2): p. 236–47. [DOI] [PubMed] [Google Scholar]
- 55.Zhang W, et al. , Acute toxicity of quantum dots on late pregnancy mice: Effects of nanoscale size and surface coating. J Hazard Mater, 2016. 318: p. 61–69. [DOI] [PubMed] [Google Scholar]
- 56.Zhang X, et al. , Increased Oxidative DNA Damage in Placenta Contributes to Cadmium-Induced Preeclamptic Conditions in Rat. Biol Trace Elem Res, 2016. 170(1): p. 119–27. [DOI] [PubMed] [Google Scholar]
- 57.Patra RC, Rautray AK, and Swarup D, Oxidative stress in lead and cadmium toxicity and its amelioration. Vet Med Int, 2011. 2011: p. 457327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Verstraeten SV, Aimo L, and Oteiza PI, Aluminium and lead: molecular mechanisms of brain toxicity. Arch Toxicol, 2008. 82(11): p. 789–802. [DOI] [PubMed] [Google Scholar]
- 59.Wang J, Wu J, and Zhang Z, Oxidative stress in mouse brain exposed to lead. Ann Occup Hyg, 2006. 50(4): p. 405–9. [DOI] [PubMed] [Google Scholar]
- 60.Gastaldo J, et al. , Lead contamination results in late and slowly repairable DNA double-strand breaks and impacts upon the ATM-dependent signaling pathways. Toxicol Lett, 2007. 173(3): p. 201–14. [DOI] [PubMed] [Google Scholar]
- 61.Pottier G, et al. , Lead Exposure Induces Telomere Instability in Human Cells. PLoS One, 2013. 8(6): p. e67501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Michishita E, et al. , SIRT6 is a histone H3 lysine 9 deacetylase that modulates telomeric chromatin. Nature, 2008. 452(7186): p. 492–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Purdey M, Chronic barium intoxication disrupts sulphated proteoglycan synthesis: a hypothesis for the origins of multiple sclerosis. Med Hypotheses, 2004. 62(5): p. 746–54. [DOI] [PubMed] [Google Scholar]
- 64.Sharma B, Singh S, and Siddiqi NJ, Biomedical implications of heavy metals induced imbalances in redox systems. Biomed Res Int, 2014. 2014: p. 640754. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 65.Elwej A, et al. , Effects of barium graded doses on redox status, membrane bound ATPases and histomorphological aspect of the liver in adult rats. Toxicol Mech Methods, 2017. 27(9): p. 677–686. [DOI] [PubMed] [Google Scholar]
- 66.Khan J, et al. , Study of the effects of BaCl(2) on the level of glutathione in plasma and cytosolic fraction in whole blood. Pak J Pharm Sci, 2012. 25(4): p. 883–8. [PubMed] [Google Scholar]
- 67.Imlay JA, The mismetallation of enzymes during oxidative stress. J Biol Chem, 2014. 289(41): p. 28121–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Menon AV, Chang J, and Kim J, Mechanisms of divalent metal toxicity in affective disorders. Toxicology, 2016. 339: p. 58–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kravchenko J, et al. , A review of the health impacts of barium from natural and anthropogenic exposure. Environ Geochem Health, 2014. 36(4): p. 797–814. [DOI] [PubMed] [Google Scholar]
- 70.Zhang N, et al. , Barium exposure increases the risk of congenital heart defects occurrence in offspring. Clin Toxicol (Phila), 2018. 56(2): p. 132–139. [DOI] [PubMed] [Google Scholar]
- 71.Lv J, et al. , Effects of several environmental factors on longevity and health of the human population of Zhongxiang, Hubei, China. Biol Trace Elem Res, 2011. 143(2): p. 702–16. [DOI] [PubMed] [Google Scholar]
- 72.Zhang J, et al. , Ageing and the telomere connection: An intimate relationship with inflammation. Ageing Res Rev, 2016. 25: p. 55–69. [DOI] [PubMed] [Google Scholar]
- 73.Bondy SC, Inflammation, aging, and oxidative stress. 2016, New York, NY: Springer Science+Business Media. pages cm. [Google Scholar]
- 74.Jauniaux E, et al. , Distribution and transfer pathways of antioxidant molecules inside the first trimester human gestational sac. J Clin Endocrinol Metab, 2004. 89(3): p. 1452–8. [DOI] [PubMed] [Google Scholar]
- 75.Freitas-Simoes TM, Ros E, and Sala-Vila A, Nutrients, foods, dietary patterns and telomere length: Update of epidemiological studies and randomized trials. Metabolism, 2016. 65(4): p. 406–15. [DOI] [PubMed] [Google Scholar]
- 76.Freitas-Simoes TM, Ros E, and Sala-Vila A, Telomere length as a biomarker of accelerated aging: is it influenced by dietary intake? Curr Opin Clin Nutr Metab Care, 2018. 21(6): p. 430–436. [DOI] [PubMed] [Google Scholar]
- 77.Garcia-Calzon S, et al. , Dietary total antioxidant capacity is associated with leukocyte telomere length in a children and adolescent population. Clin Nutr, 2015. 34(4): p. 694–9. [DOI] [PubMed] [Google Scholar]
- 78.Tamura H, et al. , Long-term melatonin treatment delays ovarian aging. J Pineal Res, 2017. 62(2). [DOI] [PubMed] [Google Scholar]
- 79.Tarry-Adkins JL, et al. , Maternal diet influences DNA damage, aortic telomere length, oxidative stress, and antioxidant defense capacity in rats. FASEB J, 2008. 22(6): p. 2037–44. [DOI] [PubMed] [Google Scholar]
- 80.Vengosh A, et al. , A critical review of the risks to water resources from unconventional shale gas development and hydraulic fracturing in the United States. Environ Sci Technol, 2014. 48(15): p. 8334–48. [DOI] [PubMed] [Google Scholar]
- 81.Registry), A.A.f.T.S.a.D., Toxicologic profile for Lead (Draft for Public Comment). 2019, U.S. Department of Health and Human Services, Public Health Service: Atlanta, GA. [Google Scholar]
- 82.Registry), A.A.f.T.S.D., Toxicological profiles for Nickel. 2005, U.S. Department of Health and Human Services, Public Health Service: Atlanta, GA. [Google Scholar]
- 83.Registry), A.A.f.T.S.a.D., Toxicological profile for Barium. 2007, U.S. Department of Health and Human Services, Public Health Service: Atlanta, GA. [Google Scholar]
- 84.Registry), A.A.f.T.S.a.D., Toxicological profile for Arsenic. 2007, U.S. Department of Health and Human Services, Public Health Service: Atlanta, GA. [Google Scholar]
- 85.Sommar JN, et al. , Investigation of lead concentrations in whole blood, plasma and urine as biomarkers for biological monitoring of lead exposure. J Expo Sci Environ Epidemiol, 2014. 24(1): p. 51–7. [DOI] [PubMed] [Google Scholar]
- 86.Broer L, et al. , Meta-analysis of telomere length in 19,713 subjects reveals high heritability, stronger maternal inheritance and a paternal age effect. Eur J Hum Genet, 2013. 21(10): p. 1163–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fairlie J, et al. , Lifelong leukocyte telomere dynamics and survival in a free-living mammal. Aging Cell, 2016. 15(1): p. 140–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Heidinger BJ, et al. , Telomere length in early life predicts lifespan. Proc Natl Acad Sci U S A, 2012. 109(5): p. 1743–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.