Abstract
Objective:
To identify timing, incidence, and risk factors for ipsilateral re-amputation within 12 months of first dysvascular amputation and to determine specific subgroups of patients at each amputation level that are at increased risk.
Methods:
A retrospective cohort study evaluating 7187 patients with first unilateral transmetatarsal (TM), transtibial (TT), or transfemoral (TF) amputation secondary to diabetes and/or peripheral artery disease (PAD) were identified in the VA Surgical Quality Improvement Program database between 2004 and 2014. Re-amputation was defined as any subsequent ipsilateral soft tissue/bony revision or amputation to a higher level. Twenty-three potential pre-operative risk factors (and nine potential interactions) were identified. A backward stepwise Cox regression was used to identify risk factors. Incidence rates and hazard ratios (HR) with 95% confidence intervals (CI) were computed.
Results:
The median time to highest level of re-amputation in the first year was 33 (interquartile range, 13–73) days. Risk of requiring at least one re-amputation was 41% (TM), 25% (TT), and 9% (TF). Risk factors associated with requiring re-amputation included chronic obstructive pulmonary disease, elevated white blood cell count, abnormal ankle brachial index (ABI), history of revascularisation, and alcohol misuse. TM patients who had diabetes only (HR 1.9; 95% CI 1.4–2.5), diabetes with an abnormal ankle brachial index (ABI) score (HR 2.4; 95% CI 1.8–3.2), and kidney failure (HR 1.7; 95% CI 1.3–2.1) were at the greatest risk of re-amputation. TT amputees who were smokers were also at an increased risk (HR 1.4; 95% CI 1.2–1.6).
Conclusion:
This research identified important risk factors for failure of primary healing and need for re-amputation at the TM and TT level. If considering a TM amputation, caution should be exercised in patients with diabetes, in particular those with an abnormal ABI and/or renal failure. At the TT level, caution should be exercised in those who smoke.
Keywords: Amputation, Dysvascular, Re-amputation, Risk factors
INTRODUCTION
Wound complications after lower extremity amputation secondary to peripheral artery disease and diabetes account for up to 49% of re-admissions.1,2 More importantly, they result in additional re-amputations, a delay in return to functional mobility,3 and an increase in patient and family burden associated with continuing care of the affected limb.4
The risks of re-amputation in the dysvascular patient population are not well established. Prior studies are limited by inadequate follow up1,2,5 to fully capture the extent of wound healing complications.1 Additionally, prior analyses5–8 often did not determine laterality of either the first or subsequent amputations, making it difficult to determine whether subsequent amputations were ipsilateral or contralateral. The majority of prior investigations did not include transmetatarsal (TM) amputations in the analysis cohort.4,9–11 Their inclusion is important because it is an amputation level that is being performed increasingly to attempt limb salvage and is associated with high re-amputation rates.11–13
The identification of patients with a high risk of healing failure has been advocated to improve amputation level selection.13–15 In a recent systematic review, renal failure, limb ischaemia, and HbA1c were associated with an increased risk of healing failure after TM amputation, although ABI was not.13 Abnormal ABI did not emerge as a predictor in a re-amputation prediction model presented in a recent publication.12 However, this study excluded all patients who died in the first year after amputation. As a reduced ABI is associated with an increased risk of death,16 excluding those who died from the analysis may have minimised the risk of ABI on re-amputation.12 Other studies have shown that smoking, prior revascularisation, and diabetes are risk factors for re-amputation.17
The present study sought to overcome some of the methodological limitations of prior research to determine the re-amputation risk at each major amputation level and to identify specific subgroups of patients at higher risk.
METHODS
This retrospective cohort study used administrative, quality improvement and clinical data from two Veterans Administration (VA) data sources: the Veterans Affairs Surgical Quality Improvement Program (VASQIP) dataset and the VA Corporate Data Warehouse (CDW).
The VASQIP dataset was used to define the inception cohort as well as several pre-amputation risk factors. VASQIP data includes pre-operative variables from all 110 VA Medical Centre inpatient surgical programmes. Data are abstracted by nurse data managers and are limited to the first 36 cases performed during an eight day cycle. The eight day cycle results in study samples beginning on different days of the week, which ensures that representative samples from each VA hospital’s cases are captured.
The CDW is a national repository comprising data and tools to support clinical research and other administrative goals. Historical data in the CDW go back to 1999 and current data are added nightly. The CDW includes multiple data domains, such as laboratory, pharmacy, and inpatient diagnoses, and procedures based on the VA’s electronic health record.
Data from the VA CDW were merged with the VASQIP data and used to ensure that the amputation surgical procedure defined in the VASQIP dataset was an incident amputation using a five year look back strategy.18 The CDW was also used to acquire demographic data and additional risk factors that were not available in VASQIP. The CDW’s Vital Status File was used to identify time to death in those who died during the one year follow up period after first major amputation.19 Subsequent amputations were also identified from the CDW, as VASQIP data only follow patients for 30 days after surgery.
Study sample
Patients undergoing their first unilateral TM, transtibial (TT), or transfemoral (TF) amputation between 1 October 2004 and 31 December 2014 secondary to diabetes and/or peripheral artery disease (PAD), aged ≥ 40 years were included in the study. ICD-9 procedure codes and current procedural terminology codes used for establishing amputation level and ICD-9 diagnosis codes used to define co-morbid medical conditions have been reported previously.18 Patients were excluded if they had specific pre-operative diagnoses of coma, paraplegia, quadriplegia, disseminated cancer, tumour of the central nervous system, or were ventilator dependent. Patients with these conditions often undergo TF amputation because preservation of a more distal amputation to ensure ambulatory mobility is questionable. Patients were also excluded in the following circumstances: 1) if they had a BMI <15 or >52 kg/m2 (implausible or a very sick patient), 2) if they died on the day of surgery, because they did not contribute any time at risk, and 3) if the amputation laterality could not be ascertained (Fig. 1). This study was approved by the local facility institutional review board.
Figure 1.
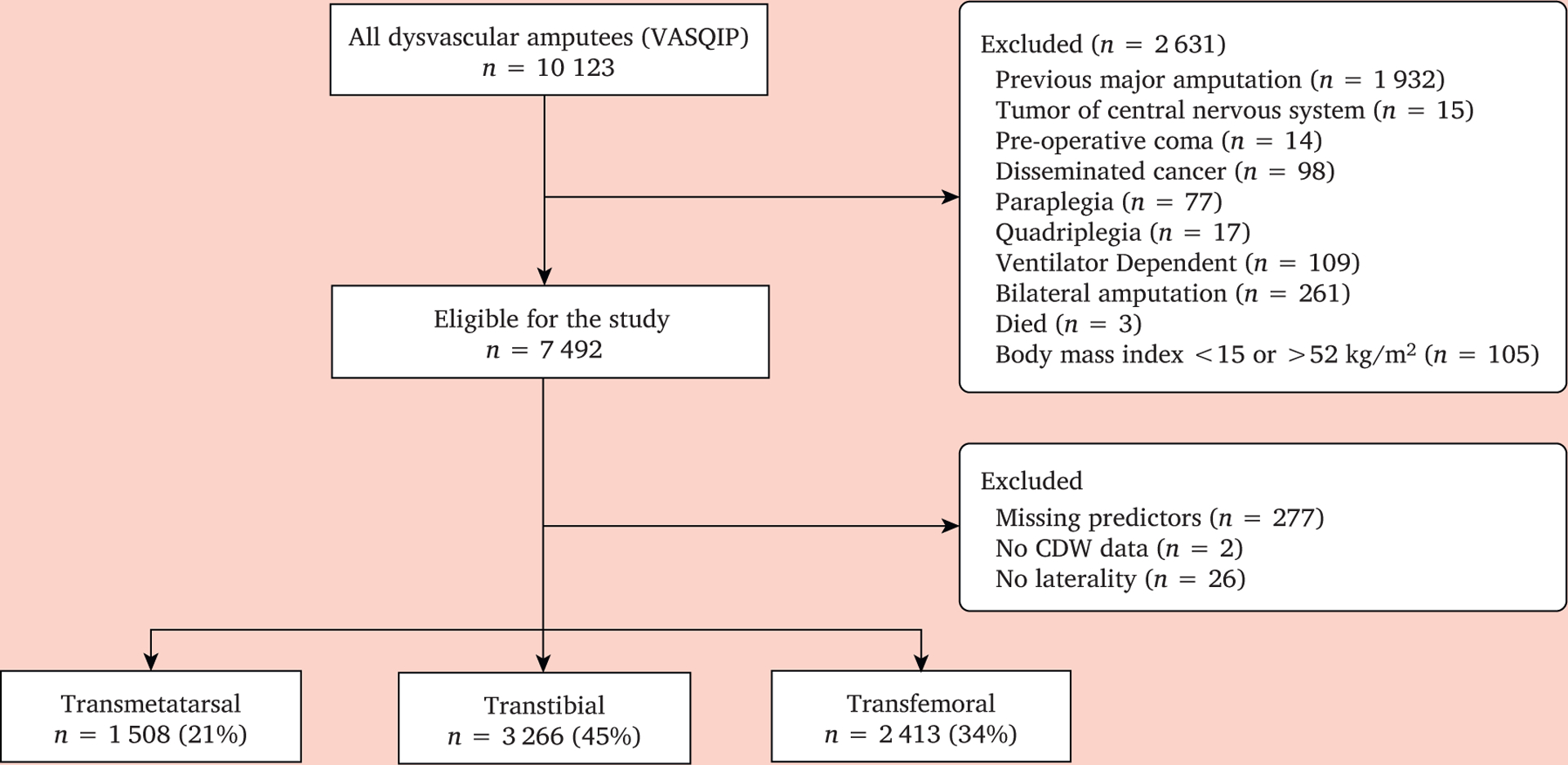
Strobe diagram depicting total numbers acquired by Veterans Affairs Surgical Quality Improvement Program (VASQIP) and total number excluded to achieve final analysis cohort of 7 187 patients.
Defining incident amputation
To ensure that patients undergoing their first major unilateral amputation were included, a five year look back algorithm was used. The presence of any diagnostic or procedure code during this interval that was related to amputation or its treatment resulted in the exclusion of those patients.18 Patients with a prior toe or ray amputation were not excluded from the cohort. For guillotine procedures at the TT and TF level, the presumption was that a closure procedure would be performed within three weeks of the guillotine procedure; therefore, searches were performed forward three weeks for the next procedure code to classify the index level.18
Classifying laterality for incident amputations and ipsilateral re-amputations
To ensure subsequent amputations were correctly classified as ipsilateral, a natural language processing (NLP) algorithm was developed to determine the laterality of each amputation procedure. Additional NLP algorithms were developed to identify and categorise the laterality of all prior revascularisation procedures, as well as ankle brachial index (ABI) scores. The validation accuracy (i.e. comparison of the NLP classifications to a random sample of 100 reviewed charts) for amputation (incident and subsequent), revascularisation, and abnormal ABI scores were 95%, 92%, and 91%, respectively.18
Risk factors
Twenty-three possible risk factors were extracted from VASQIP and CDW data sources based on prior associations in the literature and/or clinical expert consensus based on a team of investigators that included physical and internal medicine physicians, vascular surgeons, and rehabilitation psychologists. A three category variable was created to capture the nature of the dysvascular disease. Patients fell into one of the following three categories: no diabetes (PAD only), diabetes only (no PAD), and diabetes and PAD. Peripheral artery disease was assumed if the patient had an abnormal ABI (<0.9). This variable was created to determine whether there was an increased risk of re-amputation with diabetes and abnormal perfusion compared with diabetes only or no diabetes specifically in patients undergoing TM amputation. Kidney failure was defined as an estimated glomerular filtration rate (eGFR) of <15 mL/min/1.73 m2. Not all patients with kidney failure (63%) were necessarily on dialysis, which is consistent with the literature.20,21 Therefore, these were treated as separate risk factors. Revascularisation on the ipsilateral side prior to surgery included open, endovascular, or both.
Outcomes
The primary outcome of this study was the final ipsilateral re-amputation within one year of the index amputation. Re-amputation was defined as the presence of an amputation procedure code for soft tissue or bony revision at the same or higher anatomical level ipsilateral to the incident procedure. This event was chosen as the primary outcome because the goal was to capture the overall impact of failure of primary healing on the need for additional re-amputation procedures. Secondary outcomes were ipsilateral re-amputation to a higher level (e.g. TM to TT) and the number of revision procedures during the first year after incident amputation. The time to the final re-amputation was calculated by subtracting the date of the final amputation procedure from the date of the initial amputation procedure.
Statistical methods
Survival analysis was performed to compare the frequency of re-amputations along with the timing of their occurrence. Patients were followed over the course of a one year observation period. Calendar dates of failure events (final amputation) and censoring times (as a result of death) were converted to the number of days from first amputation. All patients contributed time to the survival analysis but were censored at date of death. Those who survived and did not experience a re-amputation were right censored at 365 days after first amputation. There were few missing data for the 23 candidate predictor variables and therefore a “complete case” analysis was performed (n = 7 187; >96% of those eligible). The proportion of missing data was so small it was determined unlikely to change the results; therefore, no other measures were used, such as multiple imputation. Nine potential interactions were considered based on clinical experience or prior literature: six with TM amputation (married, alcohol abuse, illicit drug use, kidney failure, diabetes without and with an abnormal ABI), two with TT amputation (guillotine amputation and smoking), and one with TF amputation (smoking). Married, alcohol abuse and illicit drug use were included because of their potential effect on compliance with weight bearing restrictions to support wound healing, while kidney failure, and diabetes (with and without an abnormal ABI) were included because of their potential direct effect on wound healing, especially in the small vessel association with diabetes. Smoking was included as a potential interaction with TT and TF amputation because of its association with large vessel disease; guillotine amputation was included with TT amputation because of its potential effect on wound healing. Associations with continuous predictors were assumed to be linear (on the logit scale). To derive a final model, a backward stepwise Cox regression was used with a cutoff of p < .050 for variable retention. Incidence rates (hazards) were computed by taking the ratio of the number of events and total time at risk. Unadjusted and adjusted hazard ratios (HR) and their 95% confidence intervals (CI) were calculated assuming a Cox proportional hazard regression model for all risk factors. Analyses were performed using Stata software, version 15.0 (College Station, TX, USA). The underlying assumption of proportional hazards necessary for the Cox regression model was assessed post-estimation both qualitatively and quantitatively. First, log–log plots of the cumulative hazard function against time were inspected to verify parallel trends (using the stphplot command). Furthermore, Schoenfeld residuals were analysed to determine whether the slope of the line appeared different from zero. The global test for proportional hazards was also used to determine whether any individual risk factors were contributing to the violation of proportional hazards.
RESULTS
Patient characteristics
The “complete case” cohort included 7 187 individuals with dysvascular amputation (1 508 [21%] TM amputees, 3 266 [45%] TT amputees, and 2 413 [34%] TF amputees; Fig. 1). The distributions of the 23 risk factors by incident amputation level are summarised in Table 1. Twenty-six per cent (n = 1 898) of the study cohort died during the study period, with a median time to death of 97 (interquartile range [IQR] 35, 200) days.
Table 1.
Risk factors for one year re-amputation risk by major amputation level among 7 187 patients with first unilateral amputation secondary to diabetes and/or peripheral artery disease
| Predictors for amputation at any level | Transmetatarsal (n = 1 508) | Transtibial (n = 3 266) | Transfemoral (n = 2 413) |
|---|---|---|---|
| Demographics | |||
| Age — years | 65.6 ± 10.0 | 66.8 ± 10.2 | 70.7 ± 10.7 |
| Male | 1480 (99.1) | 3227 (99.1) | 2385 (99.0) |
| Married | 547 (36.6) | 1357 (41.7) | 1015 (42.1) |
| Race | |||
| Caucasian | 900 (60.3) | 2014 (61.9) | 1310 (54.4) |
| Black | 461 (30.9) | 996 (30.6) | 797 (33.1) |
| Hispanic | 115 (7.7) | 218 (6.7) | 288 (12.0) |
| Other | 17(1.1) | 28 (0.9) | 14 (0.6) |
| Comorbidities | |||
| Diabetes | |||
| No | 255 (17.1) | 724 (22.2) | 844 (35.0) |
| Yes without abnormal ABI | 701 (47.0) | 1295 (39.8) | 814 (33.8) |
| Yes with abnormal ABI | 537 (36.0) | 1237 (38.0) | 751 (31.2) |
| Dialysis in two weeks pre-op | 201 (13.5) | 483 (14.8) | 229 (9.5) |
| History of chronic obstructive pulmonary disease | 198 (13.3) | 589 (18.1) | 529 (22.0) |
| Kidney failure | 153 (10.3) | 353 (10.8) | 151 (6.3) |
| Health factors | |||
| Smoker within one year pre-op | 541 (36.2) | 1299 (39.9) | 986 (40.9) |
| >2 alcohol drinks/day in past two weeks | 105 (7.0) | 261 (8.0) | 206 (8.6) |
| Illicit drugs within one year pre-op* | 174 (11.7) | 292 (9.0) | 183 (7.6) |
| Nutritional status | |||
| >10% weight loss in six months pre-op | 110 (7.4) | 302 (9.3) | 285 (11.8) |
| Body mass index — kg/m2 | 27.5 ± 6.1 | 26.9 ± 6.1 | 24.8 ± 5.7 |
| Mental health | |||
| Any mental health diagnosis† | 642 (43.0) | 1343 (41.3) | 994 (41.3) |
| Mental health outpatient visits (>1 past year) | 0.2 ± 0.4 | 0.2 ± 0.4 | 0.1 ± .3 |
| Physical function | |||
| Independent | 970 (65.0) | 1841 (56.5) | 888 (36.9) |
| Partially dependent | 453 (30.3) | 1207 (37.1) | 984 (40.9) |
| Totally dependent | 70 (4.7) | 208 (6.4) | 537 (22.3) |
| Medications | |||
| Outpatient antiplatelet medication | 268 (18.0) | 567 (17.4) | 396 (16.4) |
| Pre-operative laboratory values | |||
| White blood cell count — count per (μL | 23.1 ± 16.0 | 24.8 ± 18.2 | 22.9 ± 16.0 |
| Platelets — count per mL | 323.3 ± 133.4 | 337.2 ± 140.3 | 335.0 ± 144.3 |
| Potassium — mEq/L | 4.3 ± 0.5 | 4.2 ± 0.6 | 4.2 ± 0.5 |
| Haematocrit — % | 32.1 ± 5.1 | 31.9 ± 5.1 | 32.0 ± 5.2 |
| Vascular/limb status | |||
| Any revascularisation in past year | 534 (35.8) | 1363 (41.9) | 1099 (45.6) |
| Abnormal ankle brachial index (<0.9) | 677 (44.9) | 1656 (50.7) | 1187 (49.2) |
Data are provided as n (%) or mean ± standard deviation unless stated otherwise. ABI = ankle brachial index; TT = transtibial; y = years.
Included both dependent and non-dependent drug use (ICD9 304.x and 305.x).
Any diagnosis of depression, anxiety, post-traumatic stress disorder, bipolar, or schizophrenia.
Incident risks, rates, and median time to an ipsilateral revision by incident amputation level
The risks of re-amputation during the study period in the 1634 patients who underwent any ipsilateral re-amputation were 40% (n = 606) in the TM group, 25% (n = 809) in the TT group, and 9% (n = 219) in the TF group. The re-amputation risk estimates by amputation level over the 365 day observation period are shown in Fig. 2. The median time to the final re-amputation was 33 (IQR 13, 73) days: 28 (IQR 9, 84) in the TM group, 35 (IQR 15, 64) in the TT group, and 37.5 (IQR 17, 100) in the TF group. The overall difference in time to the final re-amputation by amputation level did not reach statistical significance (p = .080). Nearly 50% of all revisions occurred in the first month, 75% in first two months, and 90% by 5 months. The incidence rates were 65/100 person years in the TM group, 37/100 person years in the TT group, and 13/100 person years in the TF group (Table 2, the rates are higher than the risks because the denominator for the rate was “time at risk” rather than the full one year at risk).
Figure 2.
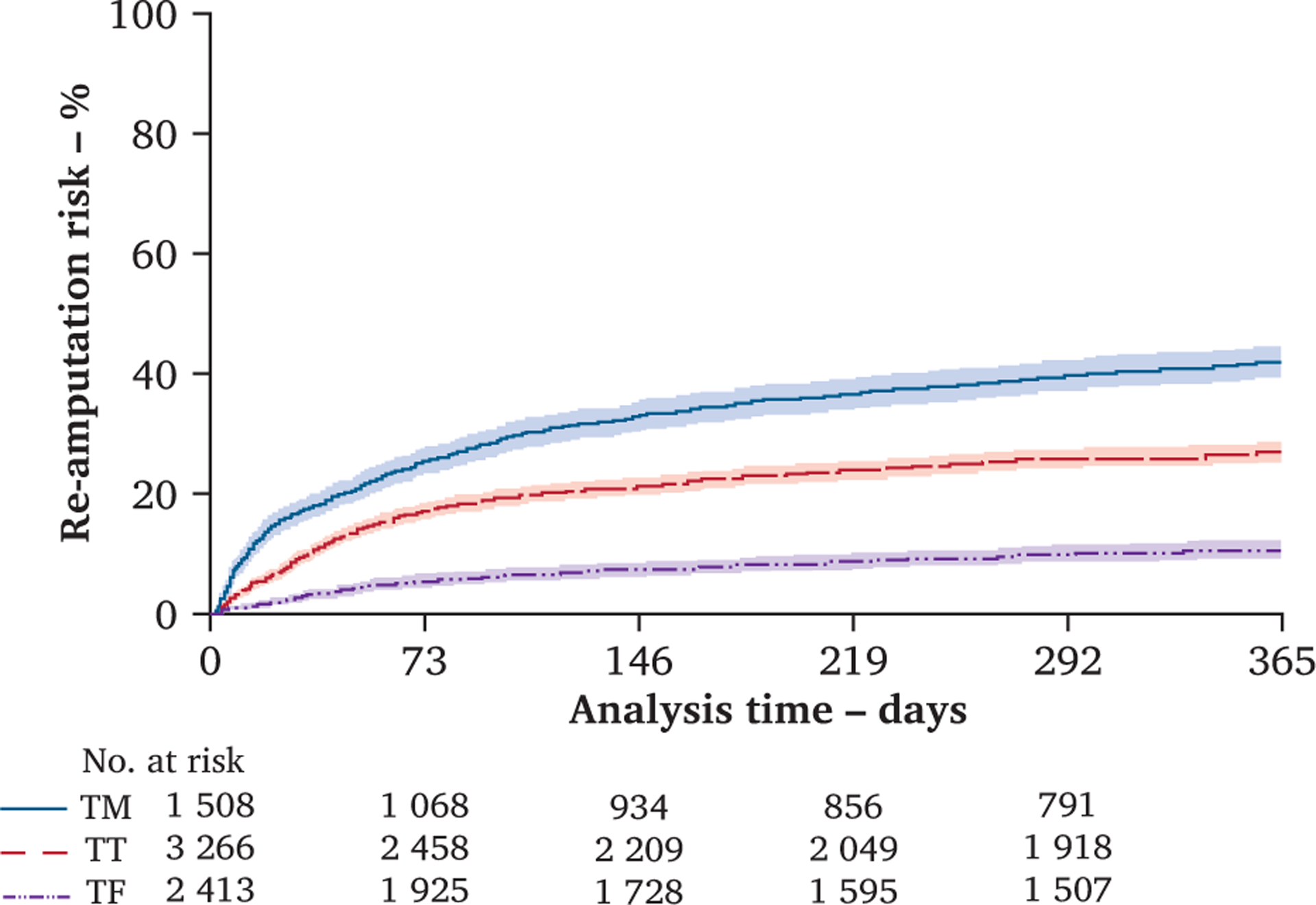
Re-amputation risks over the 365 day observational period comparing patients who undergo a first dysvascular transmetatarsal (TM), transtibial (TT), and transfemoral (TF) amputation levels demonstrating the greatest risk in those who undergo a TM amputation, followed by a TT amputation, and lowest risk in those who undergo a TF amputation (n = 7 187). Patients are censored at time of re-amputation, death or surviving without death or re-amputation 365 days after incident amputation. Shaded areas represent 95% confidence intervals. Number of patients by amputation level remaining at risk for a re-amputation at each time point included.
Table 2.
Risk factors for re-amputation within the first 365 days after major incident amputation secondary to diabetes and/or peripheral artery disease among 7187 patients (factors retained in the final Cox regression model)
| Outcome | Unadjusted HR (95% CI) | Adjusted HR (95% CI)* |
|---|---|---|
| Amputation level† | ||
| Transtibial | 0.58 (0.52–0.64) | 0.87 (0.67–1.1) |
| Transfemoral | 0.20 (0.17–0.24) | 0.34 (0.26–0.45) |
| Ever diagnosed with COPD | 1.1 (0.97–1.3) | 1.2 (1.0–1.4) |
| Diabetes‡ | 1.0 (0.98–1.1) | 0.84 (0.77–0.91) |
| Elevated white blood cell counts (>11 000) | 1.5 (1.4–1.7) | 1.7 (1.5–1.8) |
| Abnormal ABI | 1.1 (1.0–1.2) | 1.2 (1.0–1.3) |
| Alcohol misuse | 1.3 (1.1–1.5) | 1.2 (1.1–1.5) |
| History of revascularisation | 1.4 (1.2–1.6) | 1.1 (1.0–1.3) |
| Interactions | ||
| TM amputation with diabetes (no abnormal ABI) | 1.9 (1.7–2.2) | 1.7 (1.3–2.3) |
| TM amputation with diabetes (abnormal ABI) | 2.6 (2.3–3.0) | 2.4 (1.8–3.2) |
| TM amputation with kidney failure | 3.3 (2.7–4.1) | 1.7 (1.3–2.1) |
| TT amputation and current smoker | 1.5 (1.4–1.7) | 1.4 (1.2–1.6) |
CI = confidence interval; HR = hazard ratio; COPD = chronic obstructive pulmonary disease; ABI = ankle brachial index; TM = transmetatarsal; TT = transtibial.
Adjusted for all risk factors and interactions in the model.
Transmetatarsal amputation is the reference level.
Categorical variable with “no diabetes” as reference (1 = diabetes without abnormal ABI; 2 = diabetes with abnormal ABI).
Risk of revision to a higher level and multiple revisions by amputation level
The risks of revision to a higher level in the TM and TT groups were 29% (n = 432) and 15% (n = 474), respectively. Fig. 3 illustrates the proportion of patients who require re-amputation relative to those who do not at each amputation level and the final level of amputation in each re-amputation group. The difference in the proportion of patients requiring revision to a higher level in the TM and TT groups was statistically significant (p < .001). No re-amputations to a higher level were observed in the TF group. Among those who experienced a re-amputation at the same or higher level in the first year, 25% in the TM group experienced two or more of these procedures compared with 16% and 11% in the TT and TF groups, respectively (p < .001).
Figure 3.
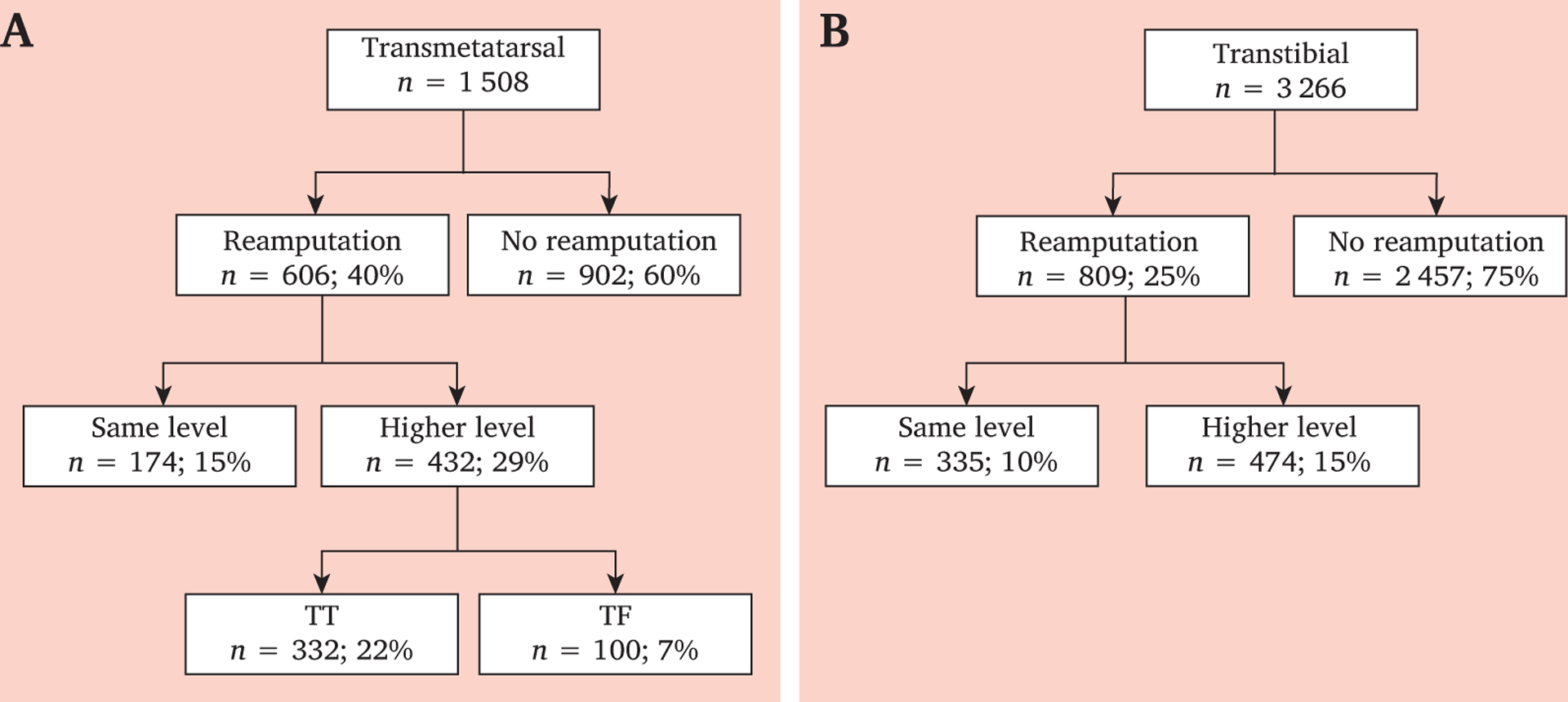
Numbers of re-amputation at the same or a higher level among first (A) transmetatarsal (TM) and (B) transtibial (TT) dysvascular amputations with percentages of individuals undergoing initial TM or TT dysvascular amputation.
Risk factors for an ipsilateral revision by incident amputation level
The final Cox regression model included seven main effects and four interaction terms (Table 2). After controlling for the other main effects in the model, and in the presence of several interactions, the risk of re-amputation among first TT amputation group compared with first TM amputation was less, but not statistically significant (HR 0.87; 95% CI 0.67–1.1; p = .33). However, the TF to TM comparison was significant (HR 0.34; 95% CI 0.26e.045; p < .001). Risk factors associated with an increased risk of revision included COPD, elevated white blood cell count, alcohol misuse, abnormal ABI in past year, and history of revascularisation in the past year.
Subgroups of patients that may be at greater risk of revision
Nine interactions were modelled to determine whether specific interactions uniquely affected re-amputation risk at a specific amputation level, and four were retained in the final model (Table 2). Those retained in the final model included patients undergoing TM amputation with the following conditions: diabetes without an abnormal ABI (HR 1.7; 95% CI 1.3–2.3; p < .001), diabetes with an abnormal ABI (HR 2.4; 95% CI 1.8–3.2; p < .001), and those in kidney failure (HR 1.7; 95% CI 1.3–2.1; p < .001). The re-amputation risks (%) by the presence or absence of diabetes with or without an abnormal ABI over the 365 day observation period can be seen in Fig. 4. Also retained were those undergoing TT amputation who reported smoking in the past year (HR 1.4; 95% CI 1.2–1.6; p < .001). The final model violated the global test for proportional hazards (p = .010). The primary contributor to this violation was the TM and kidney failure interaction (χ2 = 8.95; p = .003). When this interaction was removed, the model no= longer violated proportional hazards. When keeping this interaction in the model, the log–log plot demonstrates that the early risk of re-amputation was not proportional in the TM group; however, it was proportional for the remaining time at risk (Fig. S1) and therefore was retained in the final model. The Schoenfeld residuals suggest that PH hazards was not violated in this final model (Fig. S2).
Figure 4.
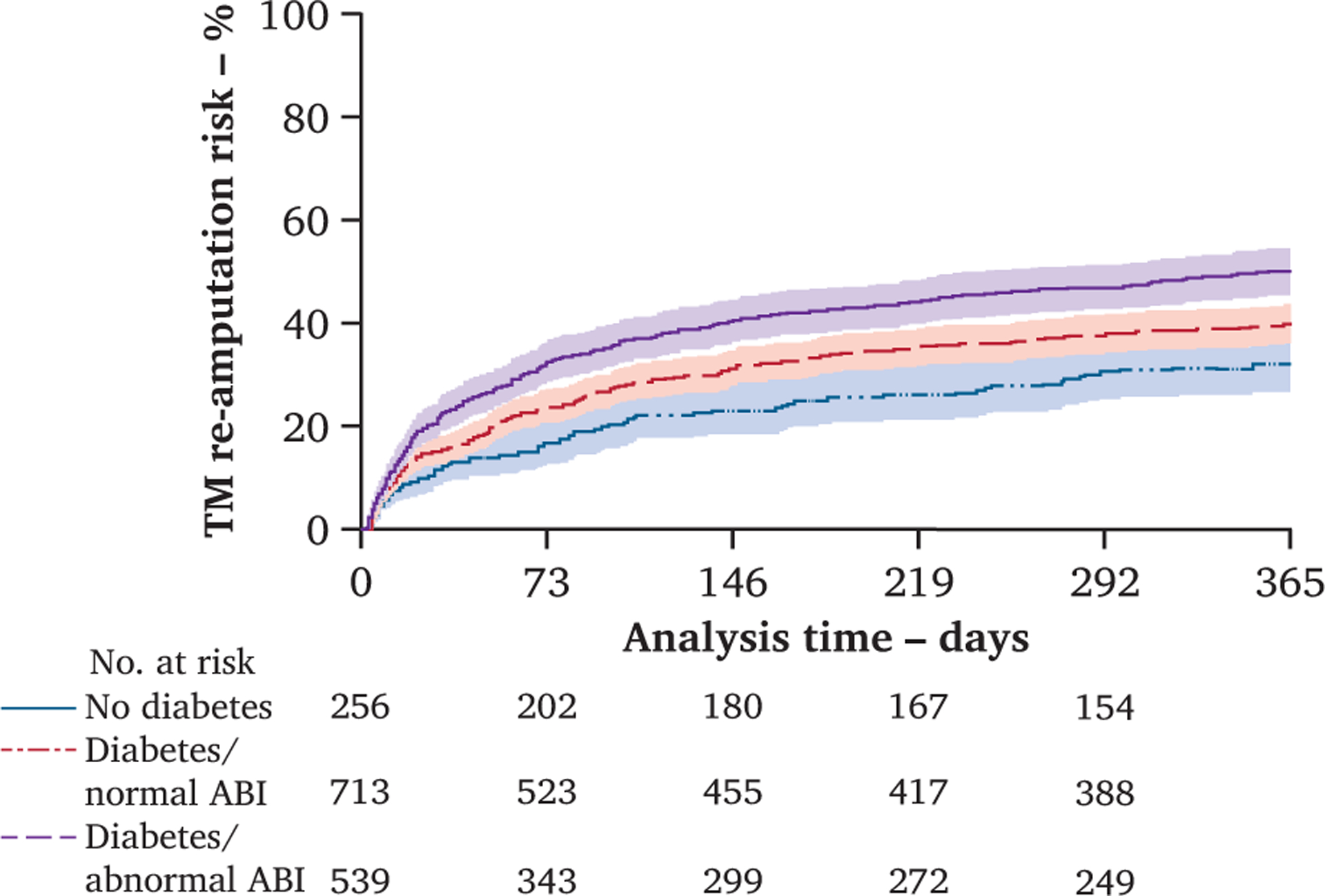
Re-amputation risk over the 365 day observational period in those who undergo a first dysvascular transmetatarsal (TM) amputation demonstrating the greatest risk in those with diabetes and an abnormal ankle-brachial index (ABI), followed by diabetes without an abnormal ABI, and lowest risk in those without diabetes (n = 7 187). Patients are censored at time of re-amputation, death or surviving without death or re-amputation 365 days after incident amputation. Shaded areas represent 95% confidence intervals. Number of patients by amputation level remaining at risk for a re-amputation at each time point included.
DISCUSSION
To the authors’ knowledge, this is the first study examining the one year risk of ipsilateral re-amputation after first major amputation in patients with PAD and/or diabetes, comparing TM, TT, and TF amputation in the same population. Risk factors of re-amputation were identified together with subgroups of patients at greater risk of failure of primary healing. The recent Global Vascular Guidelines on Chronic Limb Threatening Ischaemia have identified the difficulties associated with choosing an amputation level that will result in primary healing, as well as its importance in optimising prosthetic mobility and functional outcome.22
This study confirms the high risk of re-amputation in patients with PAD and diabetes, and the disproportionately higher risk after TM amputation.5,11,13 The crude risk of re-amputation within one year of initial TM amputation is 40%, which is similar to a prior study that evaluated the long term failure rate (53%)5 and another reporting re amputation within 30 days of the initial amputation (26%).11 Revision to a higher amputation level occurred in 29% of those undergoing TM amputation, while only one half that number required revision to a higher level after TT amputation. More importantly, of those that require re-amputation at the TM level, 71% will ultimately require a re-amputation to a higher level. This suggests that repeated attempts to salvage the TM level will be largely unsuccessful and that a higher level of amputation may be the appropriate clinical course.
This study also identified the prolonged time course associated with healing difficulties. Fifty per cent of re-amputations occurred within the first month; however, 40% did not have their final re-amputation until five months after amputation. Although not established in this investigation, the intervening time period between the initial amputation and the final re-amputation is often associated with repeated wound care visits, limitations in ambulatory mobility and dependence in care. This may lead to deconditioning, muscle atrophy, and joint contracture and ultimately limit the potential for ambulation once healed.
Risk factors for re-amputation not previously reported include COPD, an elevated white blood cell count, alcohol misuse, and an abnormal ABI. The sensitivity of ABI as a measure of impaired perfusion and its relationship to re-amputation risk is controversial. Vessel incompressibility can artificially elevate the measured ABI in patients with diabetes. Prior small case series have not demonstrated an association between an abnormal ABI and an increased risk of re-amputation,11,14,23 while others, including a systematic review, have shown an association between a reduced ABI and risk of amputation in patients with diabetes.24,25 Abnormal ABI did not emerge as a predictor of re-amputation in a recent publication.18 This may be related to the fact that patients who died in the first year were excluded from the analysis. The well established relationship between abnormal ABI and death16 suggests that by excluding those who died in the analysis, this may have minimised the potential effect of ABI on re-amputation risk in that prediction model.
Comparing risk factors for re-amputation identified in this investigation with prior studies is challenging because of variations in cohort (diabetes vs. diabetes and/or PAD, any amputation vs. first amputation), cohort source (hospital vs. regional vs. national data), varying definitions (re-amputation to a higher level vs. any additional amputation surgery), and varying risk factors included in the modelling. A recent study that included dysvascular amputation extending from toe to transfemoral identified similar associations with diabetes, smoking, and previous revascularisation.17
This study has limitations. Using survival analysis with death as a censored event it is also a competing risk. For those that died prior to a re-amputation, it is uncertain whether they may have later required a re-amputation had they survived. In addition, the outcome was defined as the final re-amputation procedure, whether it was a local revision or a re-amputation to a higher level. This definition was arrived at through expert panel input. The panel collectively determined that this event best captured the impact of an amputation level decision that resulted in a failure of primary healing. A patient who had an early re-amputation, which then healed, contributed less time at risk compared with one who had several procedures before achieving a final re-amputation. As the incidence rates closely mirrored the proportional risks at each amputation level, it is not likely there was an appreciable effect on the magnitude of the risks and the risk factors.
Future research should determine whether targeted intervention strategies could be employed to modify pre-amputation risk factors or post-amputation care for patients at high risk. Furthermore, future research should focus on the downstream consequences of re-amputation including death, functional mobility, prosthetic use, qualify of life, social integration, and healthcare costs.
Conclusion
Multiple factors contribute to risk of re-amputation at all amputation levels and there are factors that differentially contribute to risk at specific amputation levels. Patients who undergo a TM amputation and have either diabetes or renal failure are at a 1.7 times greater risk. Those with diabetes and an abnormal ABI are at more than double the risk. At the TT amputation level, the re-amputation risk is increased in patients who smoked in the prior year. Knowledge of the risk factors associated with re-amputation is important in amputation level selection and setting patient expectations as part of the surgical consent process.
Supplementary Material
WHAT THIS PAPER ADDS.
The recent Global Vascular Guidelines on Chronic Limb Threatening Ischaemia emphasise the difficulties in choosing an amputation level that will result in primary healing. Primary healing of the amputation surgical site is important in optimising prosthetic mobility and functional outcome. This investigation identified risk factors associated with failure of primary healing in the dysvascular lower extremity amputee, as well as the specific interaction factors that contribute uniquely to risk at each amputation level. This information may be used to identify patients at high risk of re-amputation, modify amputation level selection, and provide additional evidence to enhance informed consent discussions.
FUNDING
This material is based upon work supported by the US Department of Veterans Affairs, Office of Research and Development, Rehabilitation Research and Development Grants number (O1474-R) and (1 I01 RX002960-01). The opinions expressed are those of the authors and not necessarily those of the Department of Veterans Affairs or the United States Government.
Footnotes
CONFLICT OF INTEREST
None.
APPENDIX A. SUPPLEMENTARY DATA
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ejvs.2020.06.026.
REFERENCES
- 1.Hickson LJ, Rule AD, Thorsteinsdottir B, Shields RC, Porter IE, Fleming MD, et al. Predictors of early mortality and readmissions among dialysis patients undergoing lower extremity amputation. J Vasc Surg 2018;68:1505–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Curran T, Zhang JQ, Lo RC, Fokkema M, McCallum JC, Buck DB, et al. Risk factors and indications for readmission after lower extremity amputation in the American college of surgeons national surgical quality improvement Program. J Vasc Surg 2014;60:1315–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stone PA, Flaherty SK, Aburahma AF, Hass SM, Jackson JM, Hayes JD, et al. Factors affecting perioperative mortality and wound-related complications following major lower extremity amputations. Ann Vasc Surg 2006;20:209–16. [DOI] [PubMed] [Google Scholar]
- 4.Hasanadka R, McLafferty RB, Moore CJ, Hood DB, Ramsey DE, Hodgson KJ. Predictors of wound complications following major amputation for critical limb ischemia. J Vasc Surg 2011;54:1374–82. [DOI] [PubMed] [Google Scholar]
- 5.O’Brien PJ, Cox MW, Shortell CK, Scarborough JE. Risk factors for early failure of surgical amputations: an analysis of 8,878 isolated lower extremity amputation procedures. J Am Coll Surg 2013;216: 836–42. [DOI] [PubMed] [Google Scholar]
- 6.Brown BJ, Crone CG, Attinger CE. Amputation in the diabetic to maximize function. Semin Vasc Surg 2012;25:115–21. [DOI] [PubMed] [Google Scholar]
- 7.Dillingham TR, Pezzin LE. Rehabilitation setting and associated mortality and medical stability among persons with amputations. Arch Phys Med Rehabil 2008;89:1038–45. [DOI] [PubMed] [Google Scholar]
- 8.Dillingham TR, Pezzin LE, Shore AD. Re-amputation, mortality, and health care costs among persons with dysvascular lower-limb amputations. Arch Phys Med Rehabil 2005;86:480–6. [DOI] [PubMed] [Google Scholar]
- 9.Aulivola B, Hile CN, Hamdan AD, Sheahan MG, Veraldi JR, Skillman JJ, et al. Major lower extremity amputation: outcome of a modern series. Arch Surg 2004;139:395–9. [DOI] [PubMed] [Google Scholar]
- 10.Kono Y, Muder RR. Identifying the incidence of and risk factors for re-amputation among patients who underwent foot amputation. Ann Vasc Surg 2012;26:1120–6. [DOI] [PubMed] [Google Scholar]
- 11.Landry GJ, Silverman DA, Liem TK, Mitchell EL, Moneta GL. Predictors of healing and functional outcome following transmetatarsal amputations. Arch Surg 2011;146:1005–9. [DOI] [PubMed] [Google Scholar]
- 12.Czerniecki JM, Thompson ML, Littman AJ, Boyko EJ, Landry GJ, Henderson WG, et al. Predicting re-amputation risk in patients undergoing lower extremity amputation due to the complications of peripheral artery disease and/or diabetes. Br J Surg 2019;28: 11160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thorud JC, Plemmons B, Buckley CJ, Shibuya N, Jupiter DC. Mortality after nontraumatic major amputation among patients with diabetes and peripheral vascular disease: a systematic review. J Foot Ankle Surg 2016;55:591–9. [DOI] [PubMed] [Google Scholar]
- 14.Anthony T, Roberts J, Modrall JG, Huerta S, Asolati M, Neufeld J, et al. Transmetatarsal amputation: assessment of current selection criteria. Am J Surg 2006;192:e8–11. [DOI] [PubMed] [Google Scholar]
- 15.Phair J, DeCarlo C, Scher L, Koleilat I, Shariff S, Lipsitz EC, et al. Risk factors for unplanned readmission and stump complications after major lower extremity amputation. J Vasc Surg 2018;67: 848–56. [DOI] [PubMed] [Google Scholar]
- 16.Diehm C, Allenberg JR, Pittrow D, Mahn M, Tepohl G, Haberl RL, et al. Mortality and vascular morbidity in older adults with asymptomatic versus symptomatic peripheral artery disease. Circulation 2009;120:2053–61. [DOI] [PubMed] [Google Scholar]
- 17.Font-Jimenez I, Llaurado-Serra M, Roig-Garcia M, De Los Mozos-Perez B, Acebedo-Urdiales S. Retrospective study of the evolution of the incidence of non-traumatic lower-extremity amputations (2007–2013) and risk factors of re-amputation. Prim Care Diabetes 2016;10:434–41. [DOI] [PubMed] [Google Scholar]
- 18.Czernieck JM, Thompson ML, Boyko EJ, Landry G, Littman AJ, Henderson W, et al. Predicting re-amputation risk in patients undergoing dysvascular lower extremity ampuptation. Br J Surg 2019;106:1026–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sohn MW, Arnold N, Maynard C, Hynes DM. Accuracy and completeness of mortality data in the department of Veterans Affairs. Popul Health Metr 2006;4:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rosansky SJ, Eggers P, Jackson K, Glassock R, Clark WF. Early start of hemodialysis may be harmful. Arch Intern Med 2011;171: 396–403. [DOI] [PubMed] [Google Scholar]
- 21.Tattersall J, Dekker F, Heimburger O, Jager KJ, Lameire N, Lindley E, et al. When to start dialysis: updated guidance following publication of the Initiating Dialysis Early and Late (IDEAL) study. Nephrol Dial Transpl 2011;26:2082–6. [Google Scholar]
- 22.Conte MS, Bradbury AW, Kolh P, White JV, Dick F, Fitridge R, et al. Global vascular Guidelines on the management of chronic limb-threatening ischemia. Eur J Vasc Endovasc Surg 2019;58:S1e 109 e133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Toursarkissian B, Hagino RT, Khan K, Schoolfield J, Shireman PK, Harkless L. Healing of transmetatarsal amputation in the diabetic patient: is angiography predictive? Ann Vasc Surg 2005;19:769–73. [DOI] [PubMed] [Google Scholar]
- 24.Brownrigg JR, Hinchliffe RJ, Apelqvist J, Boyko EJ, Fitridge R, Mills JL, et al. Effectiveness of bedside investigations to diagnose peripheral artery disease among people with diabetes mellitus: a systematic review. Diabetes Metab Res Rev 2016;32: 119–27. [DOI] [PubMed] [Google Scholar]
- 25.Tsai CY, Chu SY, Wen YW, Hsu LA, Chen CC, Peng SH, et al. The value of Doppler waveform analysis in predicting major lower extremity amputation among dialysis patients treated for diabetic foot ulcers. Diabetes Res Clin Pract 2013;100:181–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


