Abstract
The endocannabinoid signaling system (ECSS) is altered by exposure to stress and both mediates and modulates the effects of stress on the brain. Considerable preclinical data support critical roles for the endocannabinoids and their target, the CB1 cannabinoid receptor, in the adaptation of the brain to repeated stress exposure. Chronic stress exposure increases vulnerability to mental illness, so the ECSS has attracted attention as a potential therapeutic target for the prevention and treatment of stress-related psychopathology. We discuss human genetic studies indicating that the ECSS contributes to risk for mental illness in those exposed to severe stress and trauma earlier in life, and we explore the potential difficulties in pharmacologic manipulation of the ECSS.
Keywords: cannabinoid receptor, 2-arachidonoylglycerol, N-arachidonoylethanolamine, fatty acid amide hydrolase, monoacylglycerol lipase
Linking Stress Exposure and Mental Health
Life is full of unexpected challenges to the steady state (“stressors”) from external and internal sources. Stressors can be physical or psychological, but all evoke similar responses that are generally beneficial in the short term. However, repeated and sustained activations of stress response systems exert “wear and tear” on the body and brain, referred to as “allostatic load” [1]. Allostatic load results from the direct effects of persistent activation of the stress response systems, together with loss of systems that normally oppose or buffer stress responses.
The health and quality of life costs of high allostatic load are enormous. Inadequate control of stress responses contributes to risk for mental health disorders [2] and increases mortality [3]. The most recent statistics provided by the National Institute of Mental Health indicate that nearly 1 in 5 individuals in the US suffer from mental illness, with women and adolescent/young adults (18–25 years of age) being most affected (https://www.nimh.nih.gov/health/statistics/index.shtml). While anxiety disorders are the most prevalent, perhaps the most startling statistic is that 17% of 15–17 year olds had a major depressive episode in the last year. Research provided by OurWorldInData.org indicate, in aggregate, that over 10% of the world’s population suffers from mental illness [4]. There are clear mechanistic links between exposure to chronic stress and mental illness, and enhancing control over stress responses (Box 1) has been proposed as an intervention to reduce the occurrence of psychopathology [5]. One link is the endocannabinoid signaling system (ECSS) that includes the cannabinoid receptors (CB1R and CB2R), endocannabinoids, and the enzymes and transport proteins that regulate endocannabinoid concentrations.
Box 1: Stress Response Systems.
Stress response systems are the processes that become engaged following stress exposure. These include the sympathetic nervous system (SNS); hypothalamic-pituitary-adrenal (HPA) axis; behavioral responses designed to avoid the threat, such as enhanced vigilance and freezing; and increased negative emotions. Stress-induced release of the neuropeptide corticotropin-releasing hormone (CRH) in the amygdala contributes to the emotional responses to stress exposure, particularly increased anxiety.
In addition, exposure to a significant stressor can result in the formation of detailed memories of the event. Traumatic memories help the individual to avoid danger in the future; however, elevated conditioning can result in significant hyperarousal to those traumatic memories and, if generalized, can result in ordinary events being interpreted as threats. Enhancement of memory is mediated by HPA axis activation through elevation of brain glucocorticoid (GC) concentrations.
The limbic system consists of multiple brain areas that are activated by stress exposure and participate in shaping the stress response. The amygdaloid complex regulates fear and anxiety and provides valence to memories through connections to the hippocampus. The hippocampus is the site of memory consolidation and participates in feedback regulation of the HPA axis. The hypothalamus integrates inputs from other limbic and sensory regions to regulate activation of the HPA axis. Subregions of the prefrontal cortex (PFC) regulate activity of other limbic regions, particularly the amygdala and hippocampus, and thereby control emotional stress responses, facilitate active coping and contribute to resilience to the effects of stress exposure in a top-down manner.
Here we review how the ECSS negatively regulates stress responses and that loss of the ECSS results in failure to successfully adapt to chronic stress. We also explore the hypothesis that differences in the ECSS contribute to inter-individual variability in the effects of stress exposure on mental health.
Enhanced understanding of the relationships among stress exposure, stress responses and the ECSS will lead to novel approaches to keep stress-induced plasticity of the brain in check and reduce the incidence of stress-related psychopathology.
The Endocannabinoid Signaling System
Inter-neuronal communication in the brain is carried out by neurotransmitters and occurs at synapses. The ECSS also operates at synapses, but via a retrograde mechanism [6, 7]. The cannabinoid receptor type 1 (CB1R, see Glossary) is located presynaptically on axon terminals, and its activation inhibits vesicular neurotransmitter release. CB1Rs are present on both glutamate- and GABA-releasing neurons, so they can modulate excitation or inhibition in a circuit depending on their cellular expression. CB1Rs are on terminals of dopaminergic, serotoninergic and noradrenergic neurons and, therefore, can also inhibit their release. The ECSS is highly expressed in the limbic system and affects synaptic transmission there and throughout the brain. A second cannabinoid receptor, CB2R, is found in the cells of the immune system, including brain microglia (Box 2).
Box 2: The ECSS and immune regulation: CB2 cannabinoid receptors.
Considerable data support a link between depression and the immune system [95]. The essential features of an inflammatory response are present in major depression, including the presence of proinflammatory cytokines in the circulation and brain and increased acute phase reactants, such as C-reactive protein (CRP). Many studies have demonstrated a significant association between depressive symptoms and concentrations of CRP and IL-6 in the circulation.
A second cannabinoid receptor, CB2R, is expressed primarily on cells of the immune system [96]. The CB2R is a G protein-coupled receptor and like the CB1R, is activated by 2-AG and AEA. CB2R activation in T cells is associated with reduced cytokine release and, in macrophages, is associated with reduced activation. CB2Rs are also expressed at very low levels in the human brain and are found in perivascular microglia [97]. CB2R presence on neurons is more controversial, although preclinical studies support the hypothesis that changes in CB2R activity can influence neuronal activity [98]. These characteristics suggest that increased activation of CB2R could suppress inflammation and thereby be a novel approach to the treatment of major depression.
Human genetic studies support a role for the CB2R in mental health. A recent GWAS study of more than 100,000 individuals identified a SNP (rs75459873) intronic to the gene for the CB2R (CNR2) as being very highly correlated with distressing psychotic experiences [99]. A recent meta-analysis of candidate gene studies found significant, consistent associations of genotype at another CNR2 SNP (rs2501431) and major depression [100]. This SNP is also associated with panic disorder in males but not females [101] and contributes to increased risk for depression and anxiety in those exposed to trauma in early life, particularly in association with the A allele in the FAAH SNP discussed in the text [102].
These findings support the hypothesis that the CB2R, through its role in the regulation of inflammation, could represent another mechanism by which the ECSS contributes to risk for the development of mental illness, particularly depression.
The endogenous CB1R ligands, endocannabinoids (eCBs), are the arachidonates 2-arachidonoylglycerol (2-AG) and N-arachidonoylethanolamine (AEA) [8]. The eCBs are “made on demand,” and their concentrations at the synapse are dependent upon the relative rates of their synthesis and degradation (Figure 1). As a consequence, activity of the ECSS can be increased by inhibitors of 2-AG and AEA catabolism (i.e. indirect agonists) in addition to mechanisms that increase eCB synthesis. Monoacylglycerol lipase (MGL) is responsible for most 2-AG catabolism in the brain [9] while fatty acid amide hydrolase (FAAH) is responsible for most brain AEA catabolism [10, 11]. There are other differences between AEA and 2-AG that add to the complexity and versatility of the ECSS (Box 3).
Figure 1: Mechanisms of regulation of the endocannabinoid signaling system.
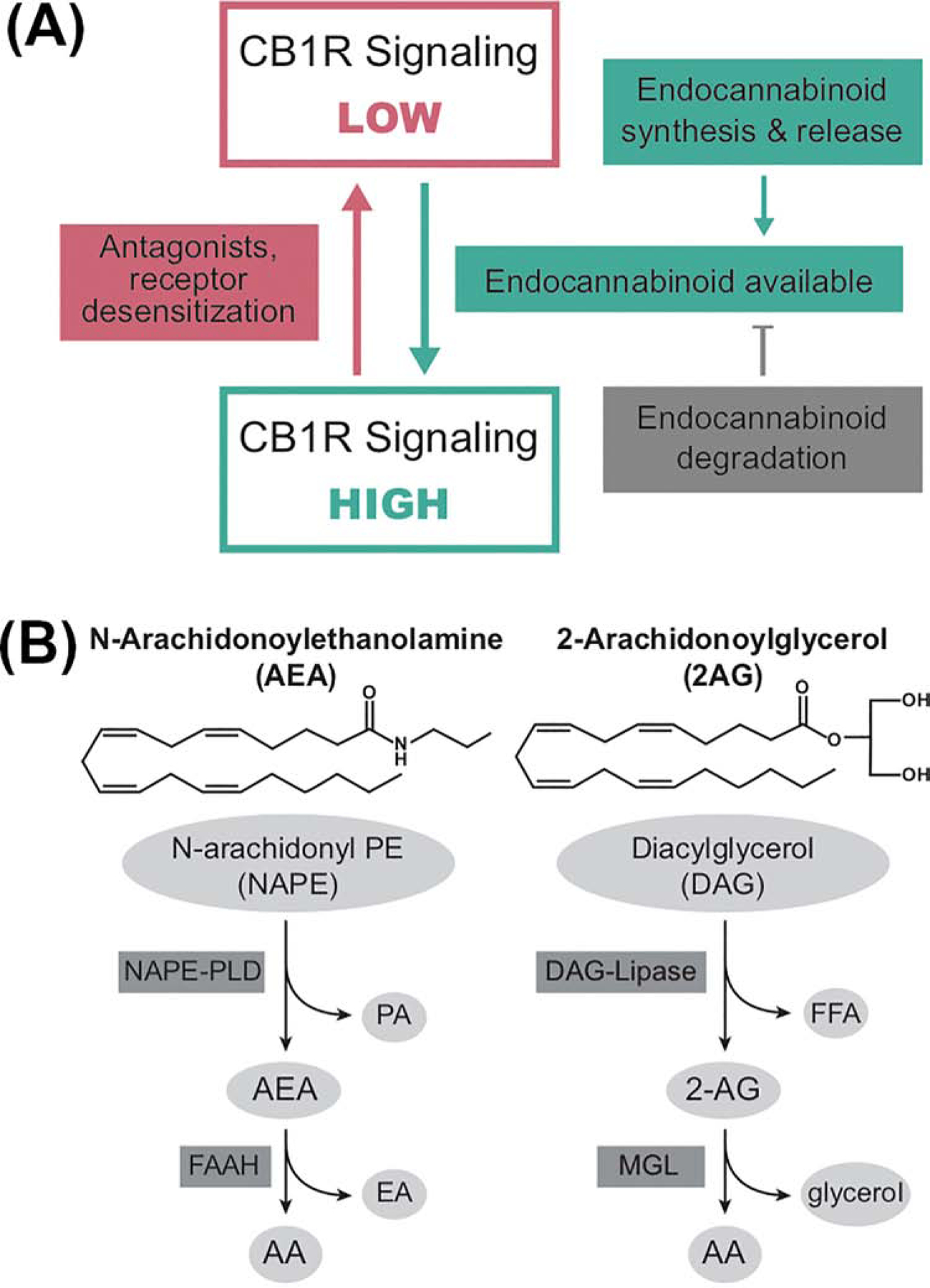
A: The endocannabinoid signaling system (ECSS) is regulated by endocannabinoid and receptor availability. The CB1 subtype of cannabinoid receptor (CB1R) can be in high and low signaling conformations. The primary mechanism regulating the ratio of receptor conformations is endocannabinoid binding to the receptor. Endocannabinoid concentrations are regulated by the relative rates of their synthesis and degradation. Thus, processes that alter the kinetics and expression of these enzymes are also regulators of CB1R activity. In addition, antagonists block the effects of endocannabinoids through occupation of the binding site, while inverse agonists block endocannabinoid binding and promote the low signaling conformation. Persistent CB1R activation, particularly by full agonists, can cause CB1R desensitization temporarily by receptor internalization or post-translational modification or more long-term via receptor down-regulation.
B: Structures and primary pathways for the synthesis and degradation of the endocannabinoids. N-Arachidonoylethanolamine (AEA) is synthesized from a low abundance lipid (N-arachidonoyl-phosphatidylethanolamine; NAPE) through the actions of a NAPE-specific phospholipase D (PLD), with the liberation of phosphatidic acid (PA). AEA is hydrolyzed to free arachidonic acid (AA) and ethanolamine (EA) by the serine hydrolase, fatty acid amide hydrolase (FAAH). 2-Arachidonoylglycerol (2-AG) is synthesized from diacylglycerol (with AA in the sn-2 position) through the actions of diacylglycerol lipase (DAG-Lipase) which results in the release of the fatty acid at the sn-1 position and 2-AG as the products. 2-AG is catabolized by hydrolysis of the ester bond by monoacylglycerol lipase (MGL) with the release of free AA and glycerol.
Box 3: AEA and 2-AG: Two ligands are better than one.
Activity of the CB1R is regulated by two endogenous agonists: AEA and 2-AG. This is an anomaly in neurobiology as most receptors have only one endogenous ligand. Characteristics of the two eCBs contribute to a versatile system that is utilized broadly throughout the brain to regulate synaptic activity. Important differences include the sites and mechanisms of synthesis and degradation of each eCB, the mechanisms that regulate their synaptic concentrations, and their efficacy at the CB1R.
While there are interesting parallels between the enzymatic mechanisms involved in the synthesis and degradation (i.e. both are made in membranes from phospholipids and are metabolized to free arachidonic acid), the enzymes involved and their regional brain expression are different [8].
Considerable functional and morphological evidence demonstrate that 2-AG encodes information regarding post-synaptic activity and conveys that information through CB1R activation to reduced presynaptic release probability. This process is phasic and underlies most forms of endocannabinoid regulation of synaptic activity.
A portion of AEA synthesis is constitutive, and sustained AEA/CB1R signaling results in tonic silencing of neurotransmission at some synapses when FAAH activity is low. FAAH, although constitutively active, can exhibit increased catalytic efficiency, likely following post-translational modification [14]. Thus, conditions that alter FAAH activity (such as activation of CRH signaling [14]) can significantly alter the signaling of AEA at its targets, including the CB receptors. Since FAAH protein is enriched in post-synaptic rather than presynaptic neurons [103], it is possible that FAAH activity regulates the amount of AEA released rather than participates in termination of AEA action at CB1R.
Available evidence suggests that 2-AG and AEA activate similar G protein-mediated signaling cascades in neurons; however, 2-AG is a full agonist while AEA is a partial agonist [104]. 2-AG also exhibits a greater ability to recruit ß-arrestin than AEA [105], which is consistent with the data discussed in the text that high 2-AG concentrations are associated with CB1R desensitization and downregulation. Thus, differences in the interactions of the eCBs with the CB1R contribute to the versatility and complexity of the ECSS and contribute to its widespread utility in the regulation of synaptic activity.
Endocannabinoid signaling links stress exposure to synaptic activity
There is an intimate connection among stress exposure, responses and the ECSS (Figure 2A). The ECSS is stress-responsive and mediates the effects of stress exposure on the brain, coupling stress-induced changes in neuropeptides and GCs to altered synaptic activity. In addition, the ECSS modulates, largely in a negative direction, the effects of stress exposure on the SNS, HPA axis, and behavioral/emotional responses.
Figure 2: Mechanisms linking stress exposure to changes in CB1R-regulation of synaptic signaling.
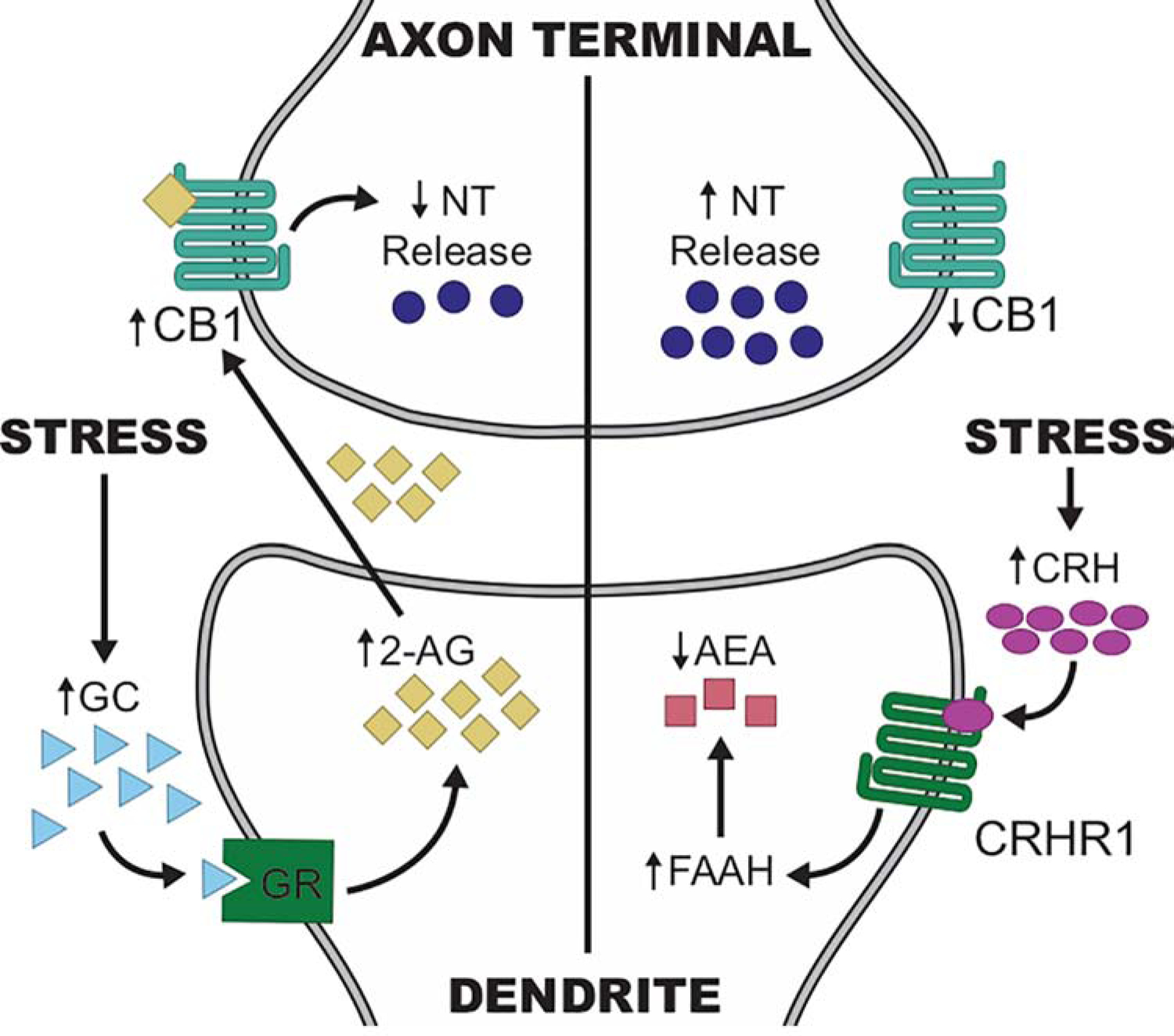
On the left hand side, stress exposure results in increased CNS glucocorticoid (GC) concentrations, which activate glucocorticoid receptors (GR) located on the plasma membrane. GR signaling triggers the synthesis of 2-arachidonoylglycerol (2-AG) which diffuses from the postsynaptic dendrite of the synthetic neuron to activate CB1 cannabinoid receptors (CB1R) on axon terminals in the immediate vicinity. The result of CB1R binding by 2-AG is reduced neurotransmitter (NT) release. On the right side, stress exposure induces release of corticotropin-releasing hormone (CRH), which binds to the G protein-coupled receptor CRHR1. CRHR1 activation results in a rapid enhancement in the activity of fatty acid amide hydrolase (FAAH), resulting in increased catabolism of N-arachidonoylethanolamine (AEA). AEA synthesis is constitutive and likely exerts tonic activation of CB1R, holding NT release low at synapses regulated by this mechanism. Activation of FAAH results in reduced AEA concentrations, leading to reduced CB1R signaling and increased NT release.
Concentrations of both of the eCBs are regulated by stress (Figure 2B). Recent studies indicate that GCs rapidly increase synaptic concentrations of 2-AG through membrane-localized glucocorticoid receptors coupled to 2-AG synthesis [12], directly linking HPA axis activation to increased 2-AG. AEA concentrations are also altered by stress exposure, although stress regulates AEA catabolism rather than synthesis. The stress-responsive neuropeptide, CRH [13], increases FAAH activity and thereby reduces AEA concentrations [14]; thus, AEA concentrations are regulated by stress-responsive neuropeptide signaling.
Plasticity Of The Endocannabinoid Signaling System In The Face Of Stress Exposure
Critical to understanding individual variability in stress responses is that ECSS activity exhibits several forms of plasticity. First, evidence is accumulating that the enzymatic processes involved in the synthesis and degradation of the eCBs are subject to modulation in response to both acute and chronic stress exposure. As a result, eCB concentrations and CB1R signaling are altered (Figure 1).
Second, availability of the pool of signaling CB1Rs and the total number of CB1Rs expressed in a cell can be regulated. For example, acute exposure to agonist results in ß-arrestin-mediated desensitization of CB1R signaling [15] while chronic exposure to exogenous agonists such as the phytocannabinoid, Δ9-tetrahydrocannabinol (THC) results in reduced CB1R density and signaling [16]. Interestingly, both genetic deletion and sustained inhibition of MGL also down-regulate the CB1R [17] although sustained inhibition of FAAH does not alter CB1R protein density [18], perhaps because AEA is not a full agonist of the receptor (Box 3). Thus, efforts to chronically activate the ECSS by direct agonists or excessive inhibition of MAGL are opposed by loss of CB1R signaling and could be counter-productive.
Habituation
Habituation of stress responses following repeated exposure to the same stressor (homotypic stress) is generally beneficial for the brain and body as it preserves metabolic resources and reduces allostatic load [19]. Sensitization of the ECSS contributes to habituation of stress responses while blockade of ECSS results in inadequate adaptation to repeated stress exposure [20]. Studies in the amygdala suggest that CB1R blockade inhibits habituation of the HPA axis response by repeated stress [21] and that reduced catabolism of 2-AG contributes to the mechanism of enhanced eCB signaling in habituation [22]. Together, these results support a role for amygdalar 2-AG-CB1R signaling in habituation to homotypic stress exposure.
Chronic stress
On the other hand, chronic stress can down-regulate the ECSS, and loss of the ECSS contributes to poor adaptation and excessive stress responses. For example, repeated homotypic stress increases FAAH activity, reducing AEA-mediated activation of CB1R, and resulting in reduced habituation and adaptation [20]. Reversal of chronic stress-induced activation of FAAH with pharmacologic inhibition maintains plasticity in the hippocampal-amygdala circuit [23] and ameliorates the effects of chronic stress to produce exaggerated startle responses and impaired extinction [24]. The effects of chronic immobilization stress in mice to enhance amygdalar microstructure and promote anxiety-like behaviors are prevented by global deletion of FAAH and reduced by pharmacologic inhibition of the enzyme [25]. Similarly, FAAH inhibition prevents the anxiogenic effects of social defeat stress in mice [26]. FAAH inhibition also ameliorates both hyperalgesia and anxiogenesis in a mouse model of combined chronic pain and chronic stress exposure [27]. Recent data indicate that enhanced expression of MGL could contribute to reduced 2-AG/CB1R signaling following chronic stress due to decreased 2-AG concentrations [28].
Chronic exposures to homotypic and variable stressors result in significant reductions in CB1R-mediated signaling in most limbic brain regions [29]. It is hypothesized that loss of CB1R signaling is the result of chronic elevation in 2-AG, which results in CB1R down-regulation as described above. Another mechanism was recently proposed based on studies in the mouse cingulate gyrus whereby chronic stress exposure down-regulates CB1R expression due to reduced histone acetylation in the promoter region of the gene [30]. An epigenetic mechanism like this could be long-lasting and might link stress exposure in early life with persistent changes in the ECSS.
Contrary to decreased CB1R expression in other brain regions, chronic stress exposure increases CB1R expression in the medial PFC (mPFC) [29]. Similarly, CB1R protein concentrations and signaling are elevated in post mortem samples of frontal cortex from humans who died by suicide [31]. This observation has clinical implications for the use of therapeutics because activators of the ECSS could worsen the effects of chronic stress in brain regions with underlying excessive CB1R signaling.
Exposure of rodents to early life stress using maternal separation [32, 33], neonatal handling [34], and housing in limited bedding [35] results in dysregulation of ECSS development, resulting in aberrant behaviors in adulthood. Exposure to high amounts of stress in early life is one of the best predictors of risk for the development of psychopathology in later life [36], and these data suggest that compromise of the ECSS could be a mechanism linking early life adversity to psychopathology in adulthood. This is further supported by data discussed below that the impact of genetic variation in the ECSS is often more significant in humans that were exposed to early life adversity.
The Endocannabinoid Signaling System Regulates Stress Responses
The ECSS modulates HPA axis and SNS responses and affects synaptic activity in brain circuits underlying the behavioral reactions to stress exposure, emotion regulation, reward processes, and fear learning and extinction (Figure 3).
Figure 3. Selected mechanisms by which the endocannabinoid signaling system (ECSS) affects responses to stress exposure.
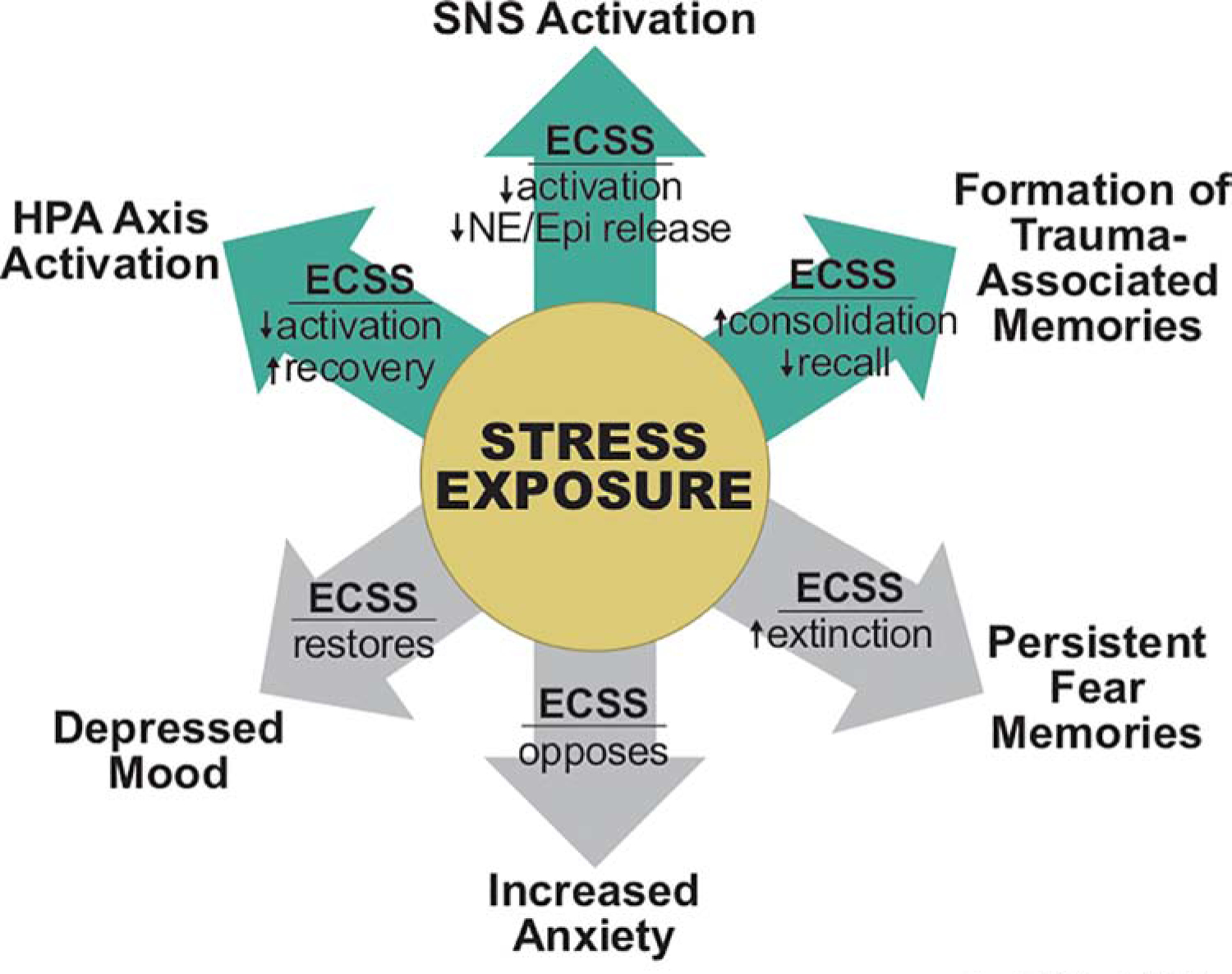
The green arrows indicate acute responses to stress exposure, which include activation of the hypothalamic-pituitary-adrenal axis (HPA axis); activation of the sympathetic nervous system (SNS), which results in the release of norepinephrine (NE) from sympathetic terminals and epinephrine (Epi) from the adrenal medulla; and enhanced memory of the traumatic event. The gray arrows indicate some of the consequences of chronic stress exposure, including depressed mood, increased anxiety and persistent fear memories. Our current understanding of the effects of an active and engaged ECSS are indicated over the arrows.
The ECSS does not appreciably affect baseline HPA axis activity but reduces its activation by acute stress exposure and mediates feedback inhibition of GCs on the HPA axis [37]. HPA axis dysregulation is a common feature of psychopathology [38]; thus, HPA axis regulation is an important mechanism by which the ECSS could moderate vulnerability for stress-induced mental illness.
CB1Rs are present on terminals of sympathetic neurons and adrenal medullary cells, and CB1R agonists inhibit release of norepinephrine (NE) and epinephrine, respectively, from these cells [39, 40]. As a result, CB1R agonists have been shown to inhibit several SNS-mediated effects, including vasoconstriction [41] and inflammation [42]. Surprisingly, this CB1R pool is also essential for the anxiogenic effects of the CB1R antagonist, rimonabant [43], and is required for stress-induced impairment of non-emotional memories [44]. The identity, source and trigger of the eCBs innervating CB1R on SNS terminals are unknown. Both 2-AG and AEA are present in the circulation, and their concentrations are increased by exposure to stress [45] (Box 4). Thus, as in the brain, the peripheral ECSS is activated by exposure to stressors and serves to buffer responses, in this case, through inhibition of NE and epinephrine release. This could be particularly relevant for the growing evidence that circulating eCB concentrations in humans are associated with measures of anxiety and depression [45]; these concentrations could reflect the contribution of a peripheral ECSS to CNS functions, such as regulation of anxiety.
Box 4: Circulating endocannabinoids: A read-out of homeostasis?
Many studies have identified associations of circulating eCB concentrations with symptoms of mental illness [45]. For example, a study of combat veterans found that suicide attempters had higher 2-AG concentrations than non-attempters [106], while another study found positive correlations between circulating AEA concentrations and mood disturbances and anger [107]. Recent studies also suggest that exercise can be used to probe ECSS status in PTSD [108] and major depression [109]. These and many other studies suggest the tantalizing hypotheses that circulating eCBs are biomarkers of ECSS function, risk for psychopathology, and therapeutic efficacy.
However, our ability to interpret the results of these studies currently is limited because the information contained in circulating eCBs is not well understood. This figure summarizes some of the possible mechanisms by which stress and other activities of daily life affect circulating eCB concentrations. Exciting new data implicate circulating eCBs in the cross-talk between the intestinal microbiome and mood [110]. Studies incorporating measures of CNS CB1R and circulating eCBs [111] support the hypothesis that the brain is a source of circulating eCBs. Exercise induces rapid changes in circulating eCBs, and skeletal muscle expresses enzymes required for eCB synthesis. Stress exposure elevates circulating eCBs, with greater stressors producing greater increases. There is evidence that both the SNS and HPA axis contribute to mobilization of eCBs into the circulation following stress exposure. In addition, recent data demonstrate that concentrations of both 2-AG [112] and AEA [113] exhibit circadian rhythms.
We hypothesize that circulating eCBs carry information to at least three CB1R pools. First, CB1R on terminals of peripheral nerves, in particular, the postganglionic neurons of the SNS; second, brain CB1R that are in close proximity to the circulation, including the hypothalamus and ventral tegmental area and, third, CB1R in metabolic organs, including adipose tissue and liver, involved in regulation of energy storage [71], In addition, circulating eCBs could engage CB2R of immune cells in the circulation.
Thus, we propose that the peripheral ECSS has features of an endocrine system; however, one in which hormone concentrations are regulated by multiple upstream processes. We hypothesize that the integrated signal carried by the circulating eCBs acts as a homeostatic “status report”. Circulating eCBs are altered by perturbations in homeostasis, including stress exposure, and the accompanying changes in multiple pools of cannabinoid receptors contribute to coordinating peripheral and central responses to the perturbation.
Anxiety-like behavioral responses to stressors
The ECSS operates in limbic brain regions involved in the processing of fear and anxiety [46, 47]. ECSS activation in rodent models generally reduces anxiety-like behaviors such as freezing or avoiding aversive environments when assessed under stressful conditions [48]. Conversely, reduced CB1R signaling in amygdalar-PFC circuits results in increased signs of anxiety and threat assessment in rodents even in the absence of stress [49]. Inhibition of FAAH and MGL are both effective strategies to reduce stress-induced anxiety in rodents [10].
The ECSS modulates the effects of trauma on anxiety and fear responses [50]. For example, treatment with CB1R agonist and FAAH inhibitors after trauma reduce the development of long-term consequences, including hyper-reactivity to the context, impairment of fear extinction and increased GR expression. On the other hand, chronic cannabinoid agonist treatment in the context of trauma reduces CB1R protein levels in the hippocampus and amygdala, and some of the beneficial effects of acute CB1R agonist treatment are lost [51].
The locus coeruleus is the source of noradrenergic innervation to the forebrain and regulates arousal, attention, vigilance and memory acquisition in response to stressors. Neuronal input into the locus coeruleus-noradrenergic system is negatively regulated by the ECSS [52], suggesting that ECSS buffers over-activation of NE-mediated responses thereby reducing hypervigilant behavior, for example [53].
Paradoxically, exogenous cannabinoid agonist can increase anxiety-like behaviors at high doses [54]. Similarly, recent studies demonstrate that anxiety can result from both over- and under-activation of FAAH in the amygdala, suggesting AEA/CB1R signaling has a narrow range of optimal activity [55]. These preclinical data are particularly interesting when taken together with the “FAAH paradox” in humans, described below.
Reward and mood
The ECSS enhances reward sensitivity, which contributes to the euphoric effects of the cannabinoid agonists in humans [56], while CB1R inhibition results in depressive-like behaviors [57]. The ECSS regulates dopamine signaling in the canonical ventral tegmental-nucleus accumbens pathway [58], and recent data suggest other ECSS-regulated circuits also participate in the effects of cannabinoids on mood and reward [59].
Considerable preclinical and clinical data demonstrate that stress, both alone and in combination with drug cues, is a trigger for reinstatement of drug seeking behavior [60]. Recent data demonstrate that stress potentiation of cue-induced drug seeking in cocaine dependent rats requires activation of the ECSS in the reward circuit [61]. These data suggest that ECSS potentiation of reward can be detrimental in some situations and therapeutic approaches to block CB1R signaling could be beneficial. However, CB1R antagonist use is fraught with difficulties (described below), so this approach might not be practical.
Aversive memories
CB1R signaling is required for glucocorticoid-induced enhancement of memory consolidation [62, 63]. For example, increases in AEA were required for the formation of strong memories of an aversive experience [64], and elevation of AEA via inhibition of FAAH rescues memory performance in the presence of high stress [65]. These studies suggest that, although CB1R signaling in other brain regions is associated with reduced impact of stress expression, AEA-CB1R signaling in the emotional memory circuit may actually strengthen the formation of aversive memories, and could therefore contribute to the risk for disorders such as posttraumatic stress disorder (PTSD). However, CB1R signaling also interferes with memory consolidation as well as memory retrieval (or recall), both of which would suggest that the ECSS could reduce the long-term storage of trauma-related memories [66].
The ECSS is essential for the appropriate extinction of conditioned fear responses [46]. Reduced CB1R signaling in the amygdala inhibits fear extinction [67] while activation of the ECSS either through direct CB1R agonists [48] or inhibition of FAAH [68] accelerates extinction. Safety learning also results in reversal of fear memory but through active learning of new responses [69]; safety learning requires hippocampal ECSS [70].
These studies suggest that the ECSS operates in the circuits involved in the formation, retrieval and extinction of memories related to fear and stress. These findings indicate that the ECSS plays an important role in risk for PTSD, for which inability to extinguish traumatic memories is a core symptom.
Human Stress Related Mental Illness and the Endocannabinoid Signaling System
Exposure to stressors can increase risk for psychological disorders, including anxiety, depression and schizophrenia. Preclinical data reviewed above indicate roles for the ECSS in the vulnerability, prevention and treatment of human stress-related psychopathologies. This section provides data obtained from human studies that support these notions.
The majority of information regarding the ECSS in human psychological function comes from pharmacological, genetic and imaging studies, together with measurement of circulating eCBs (Box 4). Our focus here is on the CB1R.
The fundamental role of the CB1R in human anxiety and depression became abundantly clear when humans were treated widely with a CB1R antagonist for the metabolic disorders. Because peripheral CB1R activation contributes to weight gain, dyslipidemia and insulin resistance [71], the CB1R antagonist, rimonabant, was developed as a treatment for obesity, metabolic disorder and type 2 diabetes. Multiple clinical trials demonstrated that rimonabant was beneficial, and the drug was used for a short while in clinical practice in Europe [72]. However, its adverse profile including anxiety, depression, and even suicide in a subset of those taking the drug triggered marked removal. Additional studies in healthy humans found that rimonabant decreases activation of brain reward circuits by pleasant stimuli and produces a negative bias in the recall of memories [73, 74]. These effects are consistent with preclinical evidence for CB1R regulation of the limbic circuits that regulate mood, fear and anxiety discussed above and provide proof-of-principle that the ECSS serves a similar role in humans.
Recent genetic data shed light on the hypothesis that individual variability in the ECSS contributes to risk and symptom severity of mental illness (See [75] and [76] for earlier and related reviews)
Endocannabinoid signaling and control of stress responses
An important role for the ECSS, particularly in the PFC, is to maintain the activity of top-down regulatory processes that dampen stress responses, providing resilience to the effects of stress exposure [77]. Support for this conclusion includes data from a study combining genetics and imaging in healthy subjects showing that single nucleotide polymorphisms (SNPs) in the gene for the CB1R (CNR1) is associated with stress-induced activation of the ventromedial PFC and cross-talk between the ventrolateral PFC and amygdala at rest [78].
Another important role for the ECSS is negative regulation of amygdalar reactivity through CB1R in the amygdala. PET imaging with radioactive CB1R antagonists can be used to estimate the level of CB1R activity [79]. This approach associated decreased CB1R activity in the amygdala with increased attention to and response to threats [80]. Another study combining genetics and imaging identified an interaction between SNPs in the genes for FAAH and CRFR1 modulates amygdalar activation and risk for anxiety disorders [81]. This finding is consistent with the role of CRHR1 in the regulation of FAAH activity identified in preclinical studies [14].
There is evidence from healthy females that CNR1 genotype moderates the effects of valence on working memory [82]. In this study of genotype at SNP rs2180619, A carriers exhibited better memory for positive inputs while G carriers had better memory for negative information and thus could be biased toward encoding negative over positive experience. This suggests the single nucleotide difference results in a large impact on memory processing.
Genetic variations in endocannabinoid signaling have greater impact in the context of stress exposure
Results of multiple studies indicate that genetic differences in the ECSS are most apparent in the context of stress exposure or trauma. For example, a very large genetic study in migraineurs found that CNR1 genotype had a significant impact on the likelihood of developing migraine headache with nausea only in those who had experienced a recent, negative life event [83]. A study of SNPs in the gene for MGL (MGLL) similarly found that genetic differences in risk for cannabis dependence were significant only in those who had experienced childhood sexual abuse [84]. Additionally, CNR1 (rs7766029) interacts significantly with financial stress to increase risk for depression and anxiety [85]. These findings are in accord with the fundamental role of the ECSS as a modulator and mediator of the effects of stress exposure on the brain. If an individual is fortunate to live a relatively stress-free life, genetic differences in the ECSS seem to have little impact on mental health.
The FAAH paradox
As presented previously, FAAH catabolizes and therefore regulates brain concentrations of AEA. A SNP in the gene for FAAH (rs324420), in which the A allele is associated with reduced FAAH activity and elevated AEA concentrations, has been extensively studied [86]. Some of the reported associations between genotype at this SNP and measures in humans are in accord with predictions of preclinical data, i.e., that low FAAH activity is associated with reduced amygdalar influence on emotions. For example, individuals homozygous for the A allele exhibit reduced stress reactivity, facilitated fear extinction, reduced amygdalar reactivity to stress and protection from negative emotional consequences of stress exposure [87, 88].
However, other studies report the opposite relationship, that the A allele at rs324420 confers increased risk for psychopathology. For example, the combination of the A allele at the FAAH SNP and repetitive stress in childhood is associated with increased vulnerability for anxiety and depression in later life [89]. Similarly, individuals with the A allele at rs324420 and a SNP in the gene for CRHR1 (CRFR1) associated with enhanced CRH signaling exhibited poor amygdalar habituation to negative stimuli, consistent with increased risk for anxiety [81]. The interaction between FAAH and CRFR1 genotypes in risk for anxiety was recently confirmed [90].
Thus, currently available studies suggest that the impact of reduced FAAH activity on anxiety and depression is changed from conferring resilience to conferring risk by the co-expression of other genetic risk factors and by exposure to early life adversity. Data such as these are essential to guide the effective use of FAAH inhibitors to treat anxiety and depressive disorders; one person’s cure could be another person’s poison.
Interventional studies
Human clinical trials of the FAAH inhibitor, JNJ42165279 are underway or recently completed for autism spectrum disorder (NCT03664232)I, phobic disorders (NCT02432703)II, depressive disorders and anxiety (NCT02498392)III, cannabis use disorder (NCT03386487)IV and facilitation of fear conditioning (NCT01665573)V A recent report of the effects of the FAAH inhibitor PF-04457845 in healthy adults found potentiation of fear extinction and reduced anxiogenic effects in response to stress exposure [91]. Based upon the evidence discussed above, it will be important to determine whether FAAH inhibition has similar beneficial effects in individuals with anxiety disorders or significant amounts of previous life adversity.
Preclinical studies have shown that several treatments for mental disorders in humans, including transcranial magnetic stimulation [92], electroconvulsive shock [93] and ketamine [94] include an ECSS component to their beneficial effects. This provides an opportunity for combining drugs, such as FAAH inhibitors, with these other modalities to perhaps improve efficacy. Finally, growing evidence supports the hypothesis that the mood-elevating effects of exercise are mediated by activation of the ECSS [45], suggesting that non-drug approaches could achieve elevation of the ECSS.
Concluding Remarks
Our understanding of the roles of the ECSS in the modulation of stress responses has grown exponentially in the last 15 years. While we still have more to learn (See Outstanding Questions Box), we are on the verge of applying this knowledge to treat and maybe even prevent psychopathology that results from exposure to trauma and excessive stress. Interventions that alter the ECSS at the time of significant stress or trauma could reduce risk for the development of stress-related psychopathology, such as PTSD. Interventions that alter the ECSS could be useful for symptom management in ongoing psychopathologies. In particular, preclinical data suggest that FAAH and MGL inhibitors could reduce anxiety and anhedonia in select individuals. The number of cannabis users continues to rise, and, while many endorse use to reduce symptoms of anxiety and depression, objective evidence for beneficial effects of cannabis are lacking (See Clinician’s Corner).
Outstanding Questions.
There are many things we do not yet know about the human endocannabinoid signaling system (ECSS) and its role as a modulator and mediator of stress exposure. Questions include:
Does the role of the ECSS change over the lifespan?
Are there sex differences in the ECSS?
What epigenetic mechanisms regulate the ECSS?
Does the role of the ECSS immediately after a stressor differ from its role once psychopathology is established?
What are the impacts of life-style choices (e.g. mindfulness practices, sleep patterns, exercise, diet, stress reduction methods) on the ECSS?
There are questions regarding the efficacy and safety of drugs that manipulate endocannabinoid signaling, including:
Will indirect agonists of the CB1R (i.e. FAAH and MGL inhibitors) be safe and effective therapeutics for symptom relief in individuals suffering with anxiety and depressive disorders?
Will only a subset of individuals receive benefit from indirect agonists for symptom management? If so, what are the characteristics of those who will benefit most?
Will use of indirect agonists as adjunctive therapies (together with transcranial magnetic stimulation, for example) provide additional or prolonged therapeutic benefits? Or reduce adverse effects?
There are questions about how individual differences in the ECSS contribute to vulnerability to stress-related disorders, including:
Does manipulation of CB1R signaling in the immediate aftermath of a traumatic incident prevent the development of PTSD or depression? If so, what else needs to be known about the individual to optimize therapy?
Can manipulation of the ECSS in adolescence mitigate against the negative effects of adversity in early childhood on mental health?
What is the impact of chronic cannabis use on the ECSS?
What information can be gained from studies of circulating endocannabinoids? Can they serve as biomarkers alone or in combination with other measures?
Box 5: Clinician’s Corner.
Many users report that cannabis mitigates symptoms of anxiety and depression [114, 115]. Laboratory studies in humans support these perceptions. For example, THC treatment of individuals with PTSD prior to threat exposure decreases amygdala reactivity and increases mPFC activation compared to placebo treatment [116]. These findings are consistent with preclinical data showing that CB1R activation in the mPFC-amygdala circuitry reduces emotional reactivity.
However, objective evidence to support beneficial effects of cannabis in the treatment of anxiety and other mental illness is limited. One recent study found beneficial effects of cannabis in depression and anxiety [117], but lack of randomization and placebo controls make it difficult to rely on this type of data or clinical judgments. A meta-analysis similarly concluded that most evidence regarding cannabis efficacy in the treatment of any human illness is of low to moderate quality and inconclusive [118–120]. However, month-long treatment with the synthetic CB1R agonist, nabilone, exhibited beneficial effects on anxiety in a placebo controlled, blinded trial of 25 individuals [121]. In another small study, nabilone treatment was effective against PTSD symptoms, particularly nightmares [122]. While these studies were small, they provide support for the potential benefit of THC and other CB1R agonists to treat anxiety disorders.
On the other hand, there is clear evidence that the use of high THC cannabis is associated with significant adverse effects that could outweigh its potential benefits [119]. Cannabis use is associated with development of dependence in about 9% of regular users [58]. Importantly, data are emerging that some individuals with underlying mental illness are at greater risk for the development of cannabis use disorder and a worsening of their psychopathology [123]. PET studies in chronic cannabis users find reduced CB1R [124, 125] and decreased FAAH [126], changes that could compromise the ECSS even further and, perhaps, worsen the disorders that one is trying to treat.
This situation leaves clinicians in a quandary as many patients report that cannabis use is beneficial. However, blinded, placebo-controlled trials of the benefits of cannabis are very difficult to do, given the hard-to-ignore effects of THC. Therefore, it could be a long time before gold standard evidence for beneficial effects of cannabis is available.
There has been increasing interest in cannabidiol (CBD), a second phytocannabinoid that does not activate CB1R and therefore does not share the potential adverse effects of THC. Strains of cannabis are available that are high in CBD and low in THC, which users find beneficial for anxiety management [127]. Preclinical studies support also demonstrate anti-anxiety effects of CBD [128], and a recent retrospective chart review supports some efficacy of CBD in the treatment of anxiety and sleep problems [129]. CBD has very few adverse effects [130] and so could be an efficacious treatment for anxiety and sleep-disorders.
Given the incalculable toll that stress exposure takes on modern urban society, we cannot afford to ignore the potential benefits that will derive from better understanding and, ultimately, individualized manipulation of the ECSS. However, successful use of therapies that manipulate the ECSS to treat mental illness requires careful attention to potential for individual differences in response.
Supplementary Material
Highlights.
Preclinical data demonstrate that the endocannabinoid signaling system is a critical stress response system that links stress to changes in synaptic activity in the CNS
Robust tone in the CNS endocannabinoid system tends to reduce the negative consequences of chronic stress
The CNS endocannabinoid system can be down-regulated by exposure to chronic stress, and its loss can contribute to increased risk for stress-induced mental illness
Prior life adversity and trauma modify the impact of polymorphisms in the genes for the CB1 cannabinoid receptor and fatty acid amide hydrolase (FAAH) on risk for psychopathology
While drugs that elevate endocannabinoid signaling show promise for the treatment of mental illness, they will be best used in the context of individual assessment of the endocannabinoid system
Acknowledgements:
The authors wish to dedicate this work to the memory of Dr. Bruce S. McEwen (1938-2020). The authors were funded during their work on this review by NIH grants R01 MH1214564 (CJH), R01 MH106574 (TADC), R56 MH116656 (TADC and CJH), R21 MH109807 (CJH), R01 DA038663 (CJH), and the Kubly Fund for Depression Research. Todd Stollenwerk is a member of the MCW-MSTP, which is partially supported by a T32 grant from NIGMS, GM080202.
Glossary
- Allele
Any of the variant forms of a gene that can be expressed; for example, single nucleotide polymorphisms (SNPs) are alleles. There are 2 copies of each allele in human genes
- 2-Arachidonoylglycerol (2-AG)
Endocannabinoid; synthesized from diacylglycerol by the enzyme diacylglycerol lipase
- Cannabidiol (CBD)
Made by the cannabis plant, is not a CB1R agonist but has anti-convulsant, anti-anxiety and sleep promoting effects that are beneficial
- Cannabinoid receptor type 1 (CB1R; gene CNR1)
A member of the G protein-coupled receptor superfamily of proteins; very highly expressed in the brain; couples to Gi/o family of heterotrimeric G proteins
- Corticotropin-releasing hormone (CRH)
Neuropeptide found in the hypothalamus and amygdala released in response to stress exposure
- Endocannabinoid (eCB)
Endogenous agonist of the cannabinoid receptors; primary endocannabinoids are N-arachidonoylethanolamine and 2-arachidonoylglycerol
- Fatty acid amide hydrolase (FAAH, gene FAAH)
Serine hydrolase that acts on fatty acyl amides and fatty acyl ethanolamines; products are the fatty acid and amine (arachidonic acid and ethanolamine, respectively, when the substrate is AEA)
- Genetic single nucleotide polymorphism (SNP)
Change in a nucleotide at a specific position in the genome that occurs in at least 0.5% of individuals SNPs can result in changes in mRNA expression or the amino acid sequence within the encoded protein, altering its expression and/or function. Thus, associations of SNP genotype with other individual characteristics are used to support mechanistic hypotheses regarding the protein of interest
- Glucocorticoids (GCs)
Steroid hormones synthesized in the adrenal cortex under the control of adrenocorticotropic hormone (ACTH); the primary glucocorticoid in rodents is corticosterone, in humans, it is cortisol
- Hypothalamic-pituitary-adrenal (HPA) axis
Endocrine (hormonal) system that promotes recovery of homeostasis following stress exposure. Parvocellular neurons in the paraventricular nucleus of the hypothalamus release corticotropin-releasing hormone (CRH) into the hypophyseal portal system which causes release of ACTH from the anterior pituitary gland into the circulation. ACTH activates melanocortin 2 receptors in the adrenal cortex to induce synthesis and release of glucocorticoids
- Monoacylglycerol lipase (MGL; gene MGLL)
Esterase that hydrolyzes the ester bond in 2-AG and other monoacylglycerols; producing free fatty acid and glycerol
- N-Arachidonoylethanolamine (AEA)
Also called anandamide, an endocannabinoid that is hypothesized to provide tonic activation of the CB1R, particularly in the amygdala
- Norepinephrine (NE)
Catecholamine that is released from post-ganglionic, sympathetic neurons and from noradrenergic neurons with cell bodies in the locus coeruleus
- Posttraumatic stress disorder (PTSD)
Anxiety disorder that occurs in response to exposure to stress or trauma. Characterized by intrusive thoughts of the trauma, such as flashbacks and nightmares; emotional numbness and avoidance of things associated with the trauma; and difficulty in concentrating, sleeping and emotional control
- Prefrontal cortex (PFC)
Part of the cerebral cortex that is vital for regulation of emotions, executive function, coping behaviors and social interactions
- Sympathetic nervous system (SNS)
Part of the autonomic nervous system, activation results in metabolic and cardiovascular changes that allow for “fight or flight” responses
- Δ9-Tetrahydrocannabinol (THC)
Made by the cannabis plant, it is a partial agonist of the CB1R and the active component in recreational and some medicinal forms of cannabis
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest:
Cecilia Hillard is a member of the board of scientific directors for Phytecs, Inc and Beryl Therapeutics.
RESOURCES
Clinical Trial Registries
REFERENCES
- 1.Romero LM et al. (2009) The Reactive Scope Model - a new model integrating homeostasis, allostasis, and stress. Horm Behav 55 (3), 375–89. [DOI] [PubMed] [Google Scholar]
- 2.Meyer HC and Lee FS (2019) Translating Developmental Neuroscience to Understand Risk for Psychiatric Disorders. Am J Psychiatry 176 (3), 179–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chiang JJ et al. (2018) Affective reactivity to daily stress and 20-year mortality risk in adults with chronic illness: Findings from the National Study of Daily Experiences. Health Psychol 37 (2), 170–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ritchie H and Roser M (2018) Mental Health. (accessed June 21 2020). [Google Scholar]
- 5.McEwen BS (2017) Neurobiological and Systemic Effects of Chronic Stress Chronic Stress (Thousand Oaks: ) 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Freund TF et al. (2003) Role of endogenous cannabinoids in synaptic signaling. Physiol Rev 83 (3), 1017–66. [DOI] [PubMed] [Google Scholar]
- 7.Augustin SM and Lovinger DM (2018) Functional Relevance of Endocannabinoid-Dependent Synaptic Plasticity in the Central Nervous System. ACS Chem Neurosci 9 (9), 2146–2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hillard CJ (2015) The Endocannabinoid Signaling System in the CNS: A Primer. Int Rev Neurobiol 125, 1–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu X et al. (2016) Coordinated regulation of endocannabinoid-mediated retrograde synaptic suppression in the cerebellum by neuronal and astrocytic monoacylglycerol lipase. Sci Rep 6, 35829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patel S et al. (2017) The endocannabinoid system as a target for novel anxiolytic drugs. Neurosci Biobehav Rev 76 (Pt A), 56–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yasmin F et al. (2020) Stress-induced modulation of endocannabinoid signaling leads to delayed strengthening of synaptic connectivity in the amygdala. Proc Natl Acad Sci U S A 117 (1), 650–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harris C et al. (2019) Cell signaling dependence of rapid glucocorticoid-induced endocannabinoid synthesis in hypothalamic neuroendocrine cells. Neurobiol Stress 10, 100158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Inda C et al. (2017) Endocrinology and the brain: corticotropin-releasing hormone signaling. Endocr Connect 6 (6), R99–R120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gray JM et al. (2015) Corticotropin-releasing hormone drives anandamide hydrolysis in the amygdala to promote anxiety. J Neurosci 35 (9), 3879–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morgan DJ et al. (2014) Mutation of putative GRK phosphorylation sites in the cannabinoid receptor 1 (CB1R) confers resistance to cannabinoid tolerance and hypersensitivity to cannabinoids in mice. J Neurosci 34 (15), 5152–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sim LJ et al. (1996) Effects of chronic treatment with delta9-tetrahydrocannabinol on cannabinoid-stimulated [35S]GTPgammaS autoradiography in rat brain. J Neurosci 16 (24), 8057–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schlosburg JE et al. (2010) Chronic monoacylglycerol lipase blockade causes functional antagonism of the endocannabinoid system. Nat Neurosci 13 (9), 1113–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Falenski KW et al. (2010) FAAH−/− mice display differential tolerance, dependence, and cannabinoid receptor adaptation after delta 9-tetrahydrocannabinol and anandamide administration. Neuropsychopharmacology 35 (8), 1775–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peters A and McEwen BS (2015) Stress habituation, body shape and cardiovascular mortality. Neurosci Biobehav Rev 56, 139–50. [DOI] [PubMed] [Google Scholar]
- 20.Patel S and Hillard CJ (2008) Adaptations in endocannabinoid signaling in response to repeated homotypic stress: A novel mechanism for stress habituation. Eur J Neurosci 27, 2821–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hill MN et al. (2010) Endogenous cannabinoid signaling is essential for stress adaptation. Proc Natl Acad Sci U S A 107 (20), 9406–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patel S et al. (2009) Repeated homotypic stress elevates 2-arachidonoylglycerol levels and enhances short-term endocannabinoid signaling at inhibitory synapses in basolateral amygdala. Neuropsychopharmacology 34 (13), 2699–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Segev A et al. (2018) Role of endocannabinoids in the hippocampus and amygdala in emotional memory and plasticity. Neuropsychopharmacology 43 (10), 2017–2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fidelman S et al. (2018) Chronic treatment with URB597 ameliorates post-stress symptoms in a rat model of PTSD. Eur Neuropsychopharmacol 28 (5), 630–642. [DOI] [PubMed] [Google Scholar]
- 25.Hill MN et al. (2013) Disruption of fatty acid amide hydrolase activity prevents the effects of chronic stress on anxiety and amygdalar microstructure. Mol Psych 18, 1125–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rossi S et al. (2010) Preservation of striatal cannabinoid CB1 receptor function correlates with the antianxiety effects of fatty acid amide hydrolase inhibition. Mol Pharmacol 78 (2), 260–8. [DOI] [PubMed] [Google Scholar]
- 27.Lomazzo E et al. (2015) Therapeutic potential of inhibitors of endocannabinoid degradation for the treatment of stress-related hyperalgesia in an animal model of chronic pain. Neuropsychopharmacology 40 (2), 488–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Longaretti A et al. (2020) Termination of acute stress response by the endocannabinoid system is regulated through lysine-specific demethylase 1-mediated transcriptional repression of 2-AG hydrolases ABHD6 and MAGL. J Neurochem. [DOI] [PubMed] [Google Scholar]
- 29.Morena M et al. (2016) Neurobiological Interactions Between Stress and the Endocannabinoid System. Neuropsychopharmacology 41 (1), 80–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lomazzo E et al. (2017) Chronic stress leads to epigenetic dysregulation in the neuropeptide-Y and cannabinoid CB1 receptor genes in the mouse cingulate cortex. Neuropharmacology 113 (Pt A), 301–313. [DOI] [PubMed] [Google Scholar]
- 31.Mato S et al. (2018) Selective up-regulation of cannabinoid CB1 receptor coupling to Go-proteins in suicide victims with mood disorders. Biochem Pharmacol 157, 258–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hill MN et al. (2019) Early life stress alters the developmental trajectory of corticolimbic endocannabinoid signaling in male rats. Neuropharmacology 146, 154–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Portero-Tresserra M et al. (2018) Maternal separation increases alcohol-drinking behaviour and reduces endocannabinoid levels in the mouse striatum and prefrontal cortex. Eur Neuropsychopharmacol 28 (4), 499–512. [DOI] [PubMed] [Google Scholar]
- 34.Vangopoulou C et al. (2018) Effects of an early life experience on rat brain cannabinoid receptors in adolescence and adulthood. IBRO Rep 5, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Atsak P et al. (2018) Glucocorticoid-endocannabinoid uncoupling mediates fear suppression deficits after early - Life stress. Psychoneuroendocrinology 91, 41–49. [DOI] [PubMed] [Google Scholar]
- 36.Galatzer-Levy IR et al. (2017) Utilization of machine learning for prediction of post-traumatic stress: a re-examination of cortisol in the prediction and pathways to non-remitting PTSD. Transl Psychiatry 7 (3), e0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hillard CJ et al. (2016) Endocannabinoid Signaling and the Hypothalamic-Pituitary-Adrenal Axis. Compr Physiol 7 (1), 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dirven BCJ et al. (2017) Epigenetic programming of the neuroendocrine stress response by adult life stress. J Mol Endocrinol 59 (1), R11–R31. [DOI] [PubMed] [Google Scholar]
- 39.Pfitzer T et al. (2005) Search for an endogenous cannabinoid-mediated effect in the sympathetic nervous system. Naunyn Schmiedebergs Arch Pharmacol 371 (1), 9–17. [DOI] [PubMed] [Google Scholar]
- 40.Niederhoffer N et al. (2001) Effects of cannabinoids on adrenaline release from adrenal medullary cells. Br J Pharmacol 134 (6), 1319–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wagner JA et al. (1997) Activation of peripheral CB1 cannabinoid receptors in haemorrhagic shock. Nature 390 (6659), 518–21. [DOI] [PubMed] [Google Scholar]
- 42.Mnich SJ et al. (2010) Anti-inflammatory properties of CB1-receptor antagonist involves beta2 adrenoceptors. J Pharmacol Exp Ther 333 (2), 445–53. [DOI] [PubMed] [Google Scholar]
- 43.Bellocchio L et al. (2013) Activation of the sympathetic nervous system mediates hypophagic and anxiety-like effects of CB(1) receptor blockade. Proc Natl Acad Sci U S A 110 (12), 4786–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Busquets-Garcia A et al. (2016) Peripheral and central CB1 cannabinoid receptors control stress-induced impairment of memory consolidation. Proc Natl Acad Sci U S A 113 (35), 9904–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hillard CJ (2018) Circulating Endocannabinoids: From Whence Do They Come and Where are They Going? Neuropsychopharmacology 43 (1), 155–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lutz B et al. (2015) The endocannabinoid system in guarding against fear, anxiety and stress. Nat Rev Neurosci 16 (12), 705–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yin AQ et al. (2019) Integrating endocannabinoid signaling in the regulation of anxiety and depression. Acta Pharmacol Sin 40 (3), 336–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lisboa SF et al. (2019) Tempering aversive/traumatic memories with cannabinoids: a review of evidence from animal and human studies. Psychopharmacology (Berl) 236 (1), 201–226. [DOI] [PubMed] [Google Scholar]
- 49.Marcus DJ et al. (2020) Endocannabinoid Signaling Collapse Mediates Stress-Induced Amygdalo-Cortical Strengthening. Neuron 105 (6), 1062–1076 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sbarski B and Akirav I (2020) Cannabinoids as therapeutics for PTSD. Pharmacol Ther, 107551. [DOI] [PubMed] [Google Scholar]
- 51.Sbarski B and Akirav I (2018) Chronic exposure to cannabinoids before an emotional trauma may have negative effects on emotional function. Eur Neuropsychopharmacol 28 (8), 955–969. [DOI] [PubMed] [Google Scholar]
- 52.Wyrofsky RR et al. (2019) Endocannabinoids, stress signaling, and the locus coeruleus-norepinephrine system. Neurobiol Stress 11, 100176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Reyes BAS et al. (2017) Cortical adrenoceptor expression, function and adaptation under conditions of cannabinoid receptor deletion. Exp Neurol 292, 179–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rey AA et al. (2012) Biphasic effects of cannabinoids in anxiety responses: CB1 and GABA(B) receptors in the balance of GABAergic and glutamatergic neurotransmission. Neuropsychopharmacology 37 (12), 2624–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Morena M et al. (2019) Upregulation of Anandamide Hydrolysis in the Basolateral Complex of Amygdala Reduces Fear Memory Expression and Indices of Stress and Anxiety. J Neurosci 39 (7), 1275–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Oomen PP et al. (2018) The acute effects of cannabis on human executive function. Behav Pharmacol 29 (7), 605–616. [DOI] [PubMed] [Google Scholar]
- 57.Hillard CJ and Liu QS (2014) Endocannabinoid signaling in the etiology and treatment of major depressive illness. Curr Pharm Des 20 (23), 3795–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Volkow ND et al. (2017) Don’t Worry, Be Happy: Endocannabinoids and Cannabis at the Intersection of Stress and Reward. Annu Rev Pharmacol Toxicol 57, 285–308. [DOI] [PubMed] [Google Scholar]
- 59.Shen CJ et al. (2019) Cannabinoid CB1 receptors in the amygdalar cholecystokinin glutamatergic afferents to nucleus accumbens modulate depressive-like behavior. Nat Med 25 (2), 337–349. [DOI] [PubMed] [Google Scholar]
- 60.Mantsch JR et al. (2016) Stress-Induced Reinstatement of Drug Seeking: 20 Years of Progress. Neuropsychopharmacology 41 (1), 335–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McReynolds JR et al. (2018) Stress Promotes Drug Seeking Through Glucocorticoid-Dependent Endocannabinoid Mobilization in the Prelimbic Cortex. Biol Psychiatry 84 (2), 85–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Campolongo P et al. (2009) Endocannabinoids in the rat basolateral amygdala enhance memory consolidation and enable glucocorticoid modulation of memory. Proc Natl Acad Sci U S A 106 (12), 4888–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sumislawski JJ et al. (2011) Reversible gating of endocannabinoid plasticity in the amygdala by chronic stress: a potential role for monoacylglycerol lipase inhibition in the prevention of stress-induced behavioral adaptation. Neuropsychopharmacology 36 (13), 2750–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Morena M et al. (2014) Endogenous cannabinoid release within prefrontal-limbic pathways affects memory consolidation of emotional training. Proc Natl Acad Sci U S A 111 (51), 18333–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Santori A et al. (2019) Anandamide modulation of circadian- and stress-dependent effects on rat short-term memory. Psychoneuroendocrinology 108, 155–162. [DOI] [PubMed] [Google Scholar]
- 66.Berardi A et al. (2016) The endocannabinoid system and Post Traumatic Stress Disorder (PTSD): From preclinical findings to innovative therapeutic approaches in clinical settings. Pharmacol Res 111, 668–678. [DOI] [PubMed] [Google Scholar]
- 67.Cavener VS et al. (2018) Inhibition of Diacylglycerol Lipase Impairs Fear Extinction in Mice. Front Neurosci 12, 479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gunduz-Cinar O et al. (2013) Convergent translational evidence of a role for anandamide in amygdala-mediated fear extinction, threat processing and stress-reactivity. Mol Psychiatry 18, 813–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kong E et al. (2014) Learning not to fear: neural correlates of learned safety. Neuropsychopharmacology 39 (3), 515–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Micale V et al. (2017) Extinction of avoidance behavior by safety learning depends on endocannabinoid signaling in the hippocampus. J Psychiatr Res 90, 46–59. [DOI] [PubMed] [Google Scholar]
- 71.Kunos G and Tam J (2011) The case for peripheral CB(1) receptor blockade in the treatment of visceral obesity and its cardiometabolic complications. Br J Pharmacol 163 (7), 1423–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Christopoulou FD and Kiortsis DN (2011) An overview of the metabolic effects of rimonabant in randomized controlled trials: potential for other cannabinoid 1 receptor blockers in obesity. J Clin Pharm Ther 36 (1), 10–8. [DOI] [PubMed] [Google Scholar]
- 73.Horder J et al. (2009) Acute administration of the cannabinoid CB1 antagonist rimonabant impairs positive affective memory in healthy volunteers. Psychopharmacology (Berl) 205 (1), 85–91. [DOI] [PubMed] [Google Scholar]
- 74.Horder J et al. (2010) Reduced neural response to reward following 7 days treatment with the cannabinoid CB(1) antagonist rimonabant in healthy volunteers. Int J Neuropsychopharmacol 13 (8), 1103–13. [DOI] [PubMed] [Google Scholar]
- 75.Hill MN and Patel S (2013) Translational evidence for the involvement of the endocannabinoid system in stress-related psychiatric illnesses. Biol Mood Anxiety Disord 3 (1), 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Karhson DS et al. (2016) Endocannabinoid signaling in social functioning: an RDoC perspective. Transl Psychiatry 6 (9), e905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Worley NB et al. (2018) Prefrontal endocannabinoids, stress controllability and resilience: A hypothesis. Prog Neuropsychopharmacol Biol Psychiatry 85, 180–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wirz L et al. (2018) An endocannabinoid receptor polymorphism modulates affective processing under stress. Soc Cogn Affect Neurosci 13 (11), 1177–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sloan ME et al. (2019) Endocannabinoid signaling in psychiatric disorders: a review of positron emission tomography studies. Acta Pharmacol Sin 40 (3), 342–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pietrzak RH et al. (2014) Cannabinoid type 1 receptor availability in the amygdala mediates threat processing in trauma survivors. Neuropsychopharmacology 39 (11), 2519–28. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 81.Demers CH et al. (2016) Interactions Between Anandamide and Corticotropin-Releasing Factor Signaling Modulate Human Amygdala Function and Risk for Anxiety Disorders: An Imaging Genetics Strategy for Modeling Molecular Interactions. Biol Psychiatry 80 (5), 356–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fairfield B et al. (2018) A variant on promoter of the cannabinoid receptor 1 gene (CNR1) moderates the effect of valence on working memory. Memory 26 (2), 260–268. [DOI] [PubMed] [Google Scholar]
- 83.Juhasz G et al. (2017) Variants in the CNR1 gene predispose to headache with nausea in the presence of life stress. Genes Brain Behav 16 (3), 384–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Carey CE et al. (2015) Monoacylglycerol lipase (MGLL) polymorphism rs604300 interacts with childhood adversity to predict cannabis dependence symptoms and amygdala habituation: Evidence from an endocannabinoid system-level analysis. J Abnorm Psychol 124 (4), 860–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gonda X et al. (2019) Effects of Different Stressors Are Modulated by Different Neurobiological Systems: The Role of GABA-A Versus CB1 Receptor Gene Variants in Anxiety and Depression. Front Cell Neurosci 13, 138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dincheva I et al. (2015) FAAH genetic variation enhances fronto-amygdala function in mouse and human. Nat Commun 6, 6395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Spagnolo PA et al. (2016) FAAH Gene Variation Moderates Stress Response and Symptom Severity in Patients with Posttraumatic Stress Disorder and Comorbid Alcohol Dependence. Alcohol Clin Exp Res 40 (11), 2426–2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mayo LM et al. (2018) Protective effects of elevated anandamide on stress and fear-related behaviors: translational evidence from humans and mice. Mol Psychiatry. [DOI] [PubMed] [Google Scholar]
- 89.Lazary J et al. (2016) Genetically reduced FAAH activity may be a risk for the development of anxiety and depression in persons with repetitive childhood trauma. Eur Neuropsychopharmacol 26 (6), 1020–8. [DOI] [PubMed] [Google Scholar]
- 90.Harris BN et al. (2019) FAAH genotype, CRFR1 genotype, and cortisol interact to predict anxiety in an aging, rural Hispanic population: A Project FRONTIER study. Neurobiol Stress 10, 100154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mayo LM et al. (2020) Elevated Anandamide, Enhanced Recall of Fear Extinction, and Attenuated Stress Responses Following Inhibition of Fatty Acid Amide Hydrolase: A Randomized, Controlled Experimental Medicine Trial. Biol Psychiatry 87 (6), 538–547. [DOI] [PubMed] [Google Scholar]
- 92.Xue SS et al. (2019) Repetitive high-frequency transcranial magnetic stimulation reverses depressive-like behaviors and protein expression at hippocampal synapses in chronic unpredictable stress-treated rats by enhancing endocannabinoid signaling. Pharmacol Biochem Behav 184, 172738. [DOI] [PubMed] [Google Scholar]
- 93.Hill MN et al. (2007) Electroconvulsive shock treatment differentially modulates cortical and subcortical endocannabinoid activity. J Neurochem 103, 47–56. [DOI] [PubMed] [Google Scholar]
- 94.Holleran KM et al. (2016) Ketamine and MAG Lipase Inhibitor-Dependent Reversal of Evolving Depressive-Like Behavior During Forced Abstinence From Alcohol Drinking. Neuropsychopharmacology 41 (8), 2062–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Raison CL and Miller AH (2017) Pathogen-Host Defense in the Evolution of Depression: Insights into Epidemiology, Genetics, Bioregional Differences and Female Preponderance. Neuropsychopharmacology 42 (1), 5–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Turcotte C et al. (2016) The CB2 receptor and its role as a regulator of inflammation. Cell Mol Life Sci 73 (23), 4449–4470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nunez E et al. (2004) Cannabinoid CB2 receptors are expressed by perivascular microglial cells in the human brain: an immunohistochemical study. Synapse 53 (4), 208–13. [DOI] [PubMed] [Google Scholar]
- 98.Li Y and Kim J (2016) Deletion of CB2 cannabinoid receptors reduces synaptic transmission and long-term potentiation in the mouse hippocampus. Hippocampus 26 (3), 275–81. [DOI] [PubMed] [Google Scholar]
- 99.Legge SE et al. (2019) Association of Genetic Liability to Psychotic Experiences With Neuropsychotic Disorders and Traits. JAMA Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kong X et al. (2019) The association of endocannabinoid receptor genes (CNR1 and CNR2) polymorphisms with depression: A meta-analysis. Medicine (Baltimore) 98 (46), e17403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Peiro AM et al. (2020) Association of cannabinoid receptor genes (CNR1 and CNR2) polymorphisms and panic disorder. Anxiety Stress Coping 33 (3), 256–265. [DOI] [PubMed] [Google Scholar]
- 102.Lazary J et al. (2019) A functional variant of CB2 receptor gene interacts with childhood trauma and FAAH gene on anxious and depressive phenotypes. J Affect Disord 257, 716–722. [DOI] [PubMed] [Google Scholar]
- 103.Gulyas AI et al. (2004) Segregation of two endocannabinoid-hydrolyzing enzymes into pre- and postsynaptic compartments in the rat hippocampus, cerebellum and amygdala. Eur J Neurosci 20 (2), 441–58. [DOI] [PubMed] [Google Scholar]
- 104.Laprairie RB et al. (2014) Type 1 Cannabinoid Receptor Ligands Display Functional Selectivity in a Cell Culture Model of Striatal Medium Spiny Projection Neurons. J Biol Chem 289, 24845–24862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ibsen MS et al. (2019) Cannabinoid CB1 and CB2 Receptor-Mediated Arrestin Translocation: Species, Subtype, and Agonist-Dependence. Front Pharmacol 10, 350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sher L et al. (2020) Endogenous cannabinoid levels and suicidality in combat veterans. Psychiatry Res 287, 112495. [DOI] [PubMed] [Google Scholar]
- 107.Belitardo de Oliveira A et al. (2019) Weight loss and improved mood after aerobic exercise training are linked to lower plasma anandamide in healthy people. Physiol Behav 201, 191–197. [DOI] [PubMed] [Google Scholar]
- 108.Crombie KM et al. (2018) Psychobiological Responses to Aerobic Exercise in Individuals With Posttraumatic Stress Disorder. J Trauma Stress 31 (1), 134–145. [DOI] [PubMed] [Google Scholar]
- 109.Meyer JD et al. (2019) Serum Endocannabinoid and Mood Changes after Exercise in Major Depressive Disorder. Med Sci Sports Exerc 51 (9), 1909–1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Butler MI et al. (2019) Man and the Microbiome: A New Theory of Everything? Annu Rev Clin Psychol 15, 371–398. [DOI] [PubMed] [Google Scholar]
- 111.Neumeister A et al. (2013) Elevated brain cannabinoid CB1 receptor availability in post-traumatic stress disorder: a positron emission tomography study. Mol Psychiatry 18 (9), 1034–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hanlon EC et al. (2015) Circadian rhythm of circulating levels of the endocannabinoid 2-arachidonoylglycerol. J Clin Endocrinol Metab 100 (1), 220–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hanlon EC (2020) Impact of circadian rhythmicity and sleep restriction on circulating endocannabinoid (eCB) N-arachidonoylethanolamine (anandamide). Psychoneuroendocrinology 111, 104471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sexton M et al. (2016) A Cross-Sectional Survey of Medical Cannabis Users: Patterns of Use and Perceived Efficacy. Cannabis Cannabinoid Res 1 (1), 131–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Betthauser K et al. (2015) Use and effects of cannabinoids in military veterans with posttraumatic stress disorder. Am J Health Syst Pharm 72 (15), 1279–84. [DOI] [PubMed] [Google Scholar]
- 116.Rabinak CA et al. (2020) Cannabinoid modulation of corticolimbic activation to threat in trauma-exposed adults: a preliminary study. Psychopharmacology (Berl). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Poli P et al. (2018) Medical Cannabis in Patients with Chronic Pain: Effect on Pain Relief, Pain Disability, and Psychological aspects. A Prospective Non randomized Single Arm Clinical Trial. Clin Ter 169 (3), e102–e107. [DOI] [PubMed] [Google Scholar]
- 118.Whiting PF et al. (2015) Cannabinoids for Medical Use: A Systematic Review and Meta-analysis. JAMA 313 (24), 2456–73. [DOI] [PubMed] [Google Scholar]
- 119.Steenkamp MM et al. (2017) Marijuana and other cannabinoids as a treatment for posttraumatic stress disorder: A literature review. Depress Anxiety 34 (3), 207–216. [DOI] [PubMed] [Google Scholar]
- 120.Orsolini L et al. (2019) Use of Medicinal Cannabis and Synthetic Cannabinoids in Post-Traumatic Stress Disorder (PTSD): A Systematic Review. Medicina (Kaunas) 55 (9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Fabre LF and McLendon D (1981) The efficacy and safety of nabilone (a synthetic cannabinoid) in the treatment of anxiety. J Clin Pharmacol 21 (8–9 Suppl), 377S–382S. [DOI] [PubMed] [Google Scholar]
- 122.Jetly R et al. (2015) The efficacy of nabilone, a synthetic cannabinoid, in the treatment of PTSD-associated nightmares: A preliminary randomized, double-blind, placebo-controlled cross-over design study. Psychoneuroendocrinology 51, 585–8. [DOI] [PubMed] [Google Scholar]
- 123.Lowe DJE et al. (2019) Cannabis and mental illness: a review. Eur Arch Psychiatry Clin Neurosci 269 (1), 107–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ceccarini J et al. (2015) [18F]MK-9470 PET measurement of cannabinoid CB1 receptor availability in chronic cannabis users. Addict Biol 20 (2), 357–67. [DOI] [PubMed] [Google Scholar]
- 125.D’Souza DC et al. (2016) Rapid Changes in Cannabinoid 1 Receptor Availability in Cannabis-Dependent Male Subjects After Abstinence From Cannabis. Biol Psychiatry Cogn Neurosci Neuroimaging 1 (1), 60–67. [DOI] [PubMed] [Google Scholar]
- 126.Boileau I et al. (2016) Fatty Acid Amide Hydrolase Binding in Brain of Cannabis Users: Imaging With the Novel Radiotracer [(11)C]CURB. Biol Psychiatry 80 (9), 691–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Andrade AK et al. (2019) Cannabinoids, interoception, and anxiety. Pharmacol Biochem Behav 180, 60–73. [DOI] [PubMed] [Google Scholar]
- 128.Papagianni EP and Stevenson CW (2019) Cannabinoid Regulation of Fear and Anxiety: an Update. Curr Psychiatry Rep 21 (6), 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Shannon S et al. (2019) Cannabidiol in Anxiety and Sleep: A Large Case Series. Perm J 23, 18–041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lattanzi S et al. (2018) Efficacy and Safety of Cannabidiol in Epilepsy: A Systematic Review and Meta-Analysis. Drugs 78 (17), 1791–1804. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


