Abstract
Streptococcus pneumoniae colonizes the human nasopharyngeal mucosa and is a leading cause of community-acquired pneumonia, acute otitis media, and bacterial meningitis. Metal ion homeostasis is vital to the survival of this pathogen across diverse biological sites and contributes significantly to colonization and invasive disease. Microarray and qRT-PCR analysis revealed an upregulation of an uncharacterized operon (SP1433–1438) in pneumococci subjected to metal-chelation by N,N,N’,Nʹ -tetrakis-(2-Pyridylmethyl)ethylenediamine (TPEN). Supplementation of zinc, cobalt, and nickel following TPEN treatment significantly abrogated induction. BLASTP comparisons and protein topology analysis predicted this locus to encode components of ATP binding cassette (ABC) transporters involved in multidrug resistance (SP1434–1435) and energy-coupling factor (ECF) transporters (SP1436–1438). Inductively coupled plasma mass spectrometry (ICP-MS) analysis identified differences in intracellular metal content in a Δ1434–8 mutant strain compared to parental T4R. Further, analysis of the secreted metabolome of WT and Δ1434–8 strains identified significant changes in pneumococcal glycolytic and amino acid metabolic pathways, indicating a shift towards mixed acid fermentation. Additionally, proteomic analysis revealed differentially expressed proteins in the Δ1434–8 mutant strain, with nearly 20% regulated by the global catabolite repressor, CcpA. Based on these findings, we propose that the transporters encoded by SP1433–1438 are involved in regulating the central metabolism of S. pneumoniae and contributing to bacterial survival during metal stress.
Graphical Abstract
Through parallel metabolomics and proteomics, we identified a previously uncharacterized genetic locus that contributes to metal ion homeostasis and central metabolism in the human pathogen Streptococcus pneumoniae.

Introduction
Streptococcus pneumoniae, or the pneumococcus, is a Gram-positive commensal of the human nasopharynx though it can also cause sinusitis and acute otitis media. Further, the pneumococcus is the leading cause of community acquired pneumonia worldwide, and infection can progress to invasive diseases including sepsis and bacterial meningitis1, 2. Throughout the course of infection, the pneumococcus must attain a variety of nutrients from the host including carbohydrates and metal ions to survive within diverse host niches. To meet these requirements, bacteria have evolved an array of ATP-binding cassette (ABC) transporters that function primarily to transport molecules such as these across cell membranes3. These systems are involved in the uptake and efflux of many substrates, including vitamin B12, iron-binding siderophores, and free metal ions4–6. These transport systems consist of importers, found only in prokaryotic systems (types I, II, and III), and exporters, found in both prokaryotic and eukaryotic systems3. Type III importers, or energy coupling factor (ECF) transporters, are some of the most recently characterized, and they differ from importer types I and II in that they lack individual substrate-binding proteins7, 8. ECF transporters have been shown to be in involved in the uptake of vitamins (thiamine and riboflavin) and metal ions (cobalt and nickel)9–11.
The uptake of metal ions is essential to microbial physiology and is known to play a large role in cellular metabolism, as many proteins have a requirement for a structural metal ion cofactor12. To exploit the necessity of metal ions for bacterial survival, the vertebrate immune system has evolved mechanisms to starve bacterial pathogens of essential metals. Granulocytic cells of the immune system produce and release proteins that sequester metal ions in a process termed “nutritional immunity”13. To counteract chelation by the immune system, bacteria utilize tightly regulated ABC transport systems to obtain metal ions. As organisms continue to use uptake and efflux systems to evade nutrient limitation by the immune response and evolve resistance to antibiotics, metal homeostasis remains an attractive target for future therapeutics. Discerning the mechanisms by which pathogens respond to and overcome metal starvation is key to understanding bacterial physiology and developing novel therapeutics to eliminate them.
Previous work from our laboratory has identified roles for zinc homeostasis in adhesion, invasion, and biofilm formation of S. pneumoniae14, 15. The pneumococcal zinc transport system, through the function of the ABC transporter permease and ATPase complex, AdcBC, zinc-binding proteins AdcA and AdcAII, and the zinc-dependent AdcR regulon have been characterized in detail14, 16–18; however, it remains unknown if other pneumococcal zinc-sensitive transport systems exist, or how zinc uptake impacts cellular metabolism. A previous study assessed the transcriptional response of the pneumococcus to zinc-deficient media; however, here we identify zinc as an effector molecule for the regulation of an additional genetic locus, not previously reported. We hypothesize that this locus encodes metal-dependent transport systems that are largely involved in the maintenance of cellular metabolism, specifically relating to oxidative stress, carbohydrate metabolism, and metal ion uptake. Additionally, this study has implemented a multi-omics approach, utilizing microarray genomics, 2D NMR metabolomics, and proteomic analysis to identify metabolic pathways of Streptococcus pneumoniae that contribute to homeostasis during metal-stress.
Materials and Methods
DNA Manipulation
S. pneumoniae strains TIGR419 and an unencapsulated T4R mutant strain with a chloramphenicol-resistant inactivated cps4A gene were grown on tryptic soy agar plates supplemented with 5% defibrinated sheep blood or in C+Y medium. Mutants of TIGR4 and T4R lacking SP1433 (Δ1433) and SP1434–1438 (Δ1434–8) were created using splicing by overlap extension (SOE) PCR method20 using an erythromycin or spectinomycin antibiotic cassette, respectively. Standard S. pneumoniae transformation procedures were used for incorporation into the genome. Mutants lacking SP1434–1438 and SP1433 were isolated by selection on blood agar plates supplemented with erythromycin (0.5 μg/mL) or spectinomycin (500 μg/mL) and confirmed by PCR and qPCR. Briefly, expected size shifts of 4675 bp and 105 bp were demonstrated using primers flanking the genes of interest, SP1434-SP1438 and SP1433. Antibiotic cassettes were confirmed to be within the genetic region of interest using combinations of primer pairs targeting the region upstream of the gene of interest and the reverse of the antibiotic cassette, and a primer targeting the downstream region of the gene of interest with a forward primer of the antibiotic cassette.
Microarray Analysis
To determine how S. pneumoniae transcription was altered during chelation, microarrays were performed as previously described with slight modifications14. Briefly, S. pneumoniae TIGR4 was cultured in C+Y medium to OD 600 nm 0.4, treated with control media or with C+Y with added TPEN at a final concentration of 20 μM for 10 minutes. RNA was then extracted using Qiagen RNeasy Mini Kit (Qiagen) and microarrays performed at the Hartwell Center for Bioinformatics and Biotechnology, St. Jude Children’s Research Hospital.
Antibiotic Sensitivity
Frozen bacterial stocks of T4R and Δ1434–8 were diluted in C+Y medium to 1×107 CFU/mL and 100 μL were spread on a blood agar plate. Discs impregnated with antibiotics at the following amounts were added to blood agar plates and were incubated overnight at 37 °C with 5% CO2 (Ciprofloxacin 5 μg, Vancomycin 30 μg, Ampicillin 10 μg, Penicillin 10 U, Ceftiofur 30 μg, Cephalothin 30 μg, and Sulfisoxazole 1 mg). Following incubation, zones of inhibition surrounding antibiotic discs were measured.
Inductively Coupled Plasma Mass Spectrometry
Bacterial cultures of T4R and Δ1434–8 were grown in C+Y medium to OD 600 nm 0.5. Six 1 mL cultures were collected for each strain and were centrifuged at 16,000xg for 5 min. Triplicate biological samples for each strain were resuspended in 1 mL C+Y medium and triplicate biological samples were resuspended in 1 mL C+Y medium with 20 μM TPEN. Samples were incubated at room temperature for 20 min. Following incubation, samples were centrifuged at 16,000xg and supernatants decanted. Pellets were heat killed at 65 °C for 2 hr. For ICP-MS analysis, pellets were resuspended in 100 μL concentrated nitric acid and were diluted 1:20 with water. An Agilent ICP-MS 7500cx was used to collect all ICP-MS data herein and the reported values are the averages of biological triplicate samples.
Quantitative Real-time PCR
For assays investigating SP1434 expression, S. pneumoniae T4R was grown in C+Y medium to OD 600 nm of 0.6 prior to the addition of the Zn2+-chelating agent TPEN (30 μM) or zinc(II), cobalt(II), iron(II), or nickel(II) ions at 200 μM. After addition of TPEN or individual metals, bacteria were incubated at 25°C for 15 min. Additionally, cultures were exposed to TPEN for 15 min with added metal spike in for an additional 15 min. Supplemented metals were TraceCERT® ICP-MS grade (Sigma-Aldrich). For assays investigating SP1433 expression, T4R, ΔadcR and Δ1433 were grown to OD 600 nm of 0.6 and treated for 15 min with TPEN (30 μM). For all samples, after incubation, 1 mL of bacterial culture was added to 2 mL of RNAprotect (Qiagen). Samples were incubated at room temperature for 5 min. Two mL of bacterial culture in RNAprotect was pelleted for 5 min at 16,000xg, pellets were resuspended in 1 mL of cold RNase free PBS and centrifuged again. Supernatants were decanted, and pellets were resuspended in 400 μL RLT buffer (Qiagen) with 2-mercaptoethanol (Sigma-Aldrich). Samples were sonicated three times (15 sec), 600 μL RLT buffer was added to each sample, and samples were transferred to 500 μL of 0.1 mM Zirconia beads. Samples were bead beat for 2 min using Mini-BeadBeater 16 (BioSpec Products). Lysates were centrifuged on tabletop centrifuge at 2,000xg for 1 min and run through Qiashredder columns (Qiagen) per manufacturer’s instructions. 100% Ethanol was added to flow through at 0.6 volume of the sample. RNA was purified using a Qiagen RNeasy Mini Kit (Qiagen) with an optional on-column DNase treatment for 30 min, RNA was quantified using a Qubit, and 5 ng of each sample served as template to synthesize cDNA using a Maxima First Strand cDNA synthesis kit (Thermo Scientific). cDNA products were diluted 1:10 and 1 μL of cDNA was used as template for qRT-PCR using Luminaris Color HiGreen High ROX qPCR Master Mix (Thermo Scientific) per manufacturer’s instructions. Fold changes were calculated using a ΔΔCT analysis and gyrA, unaltered by TPEN treatment, served as an internal control. Primer sequences can be found in Supplemental Table 4.
Secreted Metabolomics
Cultures of T4R and Δ1434–8 were grown in C+Y medium to OD 600 nm 0.2, 0.35 and 0.5. One milliliter of culture was removed and centrifuged at 16,000xg for 5 min and sampling was performed in triplicate. Culture supernatants were then filtered using 0.22 μM filters. NMR samples were made by combining the filtered supernatant (400 μL) with 200 μL of 200 mM phosphate buffer (pH 7.0) with 1.0 mM trimethylsilypropanoic acid (TMSP) in 50% D2O. The 1D and 2D NMR spectra were obtained at a temperature of 298 K on a 600 MHz Bruker Avance III cryoprobe equipped NMR spectrometer. A 1D-NOESY (noesypr1d) pulse sequence was used, and presaturation applied at 4.75 ppm during the 4 second relaxation delay and 50 millisecond mixing time to suppress the water signal. A 1 second acquisition time was used, and a total of 64 scans were collected with a 20-ppm spectral width. A modified 2D-TOCSY (dipsi2gpphzsprespe_psyche) pulse sequence was used for 2D acquisition. This sequence incorporated broadband homonuclear decoupling using PSYCHE in the t1 dimension21, 22. A zero-quantum filter was used during the 80 ms TOCSY mixing time. Water flip back pulses were used to optimize water suppression23. Each FID was collected using 2 scans, and the indirect dimension was sampled for 85 ms using 1024 complex points. Additional water suppression and solvent filtering were performed with NMRPipe using in-house scripts24. Each spectrum was calibrated using the TMSP peak as an internal standard and manually processed. Compounds in the processed spectra were identified and quantified using AMIX-Viewer v3.9.14 software (Bruker Biospin GmbH). A library of 56 pure compounds at 3.000 mM was created in-house to identify and quantify peak intensities and line widths for each compound. The output file from AMIX was a listing of concentration for each compound for each sample. Concentrations for each sample (T4R and Δ1434–8) and OD (0.2, 0.35 and 0.5) were used for the statistical analysis.
The metabolite concentrations for each sample was arranged by OD values and strain before the statistical analysis was conducted using MetaboAnalyst 25. MetaboAnalyst normalized the samples by sum with Pareto scaling. The Pareto scaling was used to emphasize the weaker metabolites and reduce the influence of the intense peaks to easily identify the biological relevance26. After normalization, the statistical methods, such as multivariate analysis, were used for data analysis. The PLS-DA data set was divided into components to identify the statistical differences between the classes. The first component (Component 1) captured the maximum variance in the data set that was the linear combination of the original predictor variables compared to the observed variables, whereas the other components (second, third, fourth, etc.) captured the remaining variance in the data set that was the linear combination and orthogonal to the first component26.
Proteomic Analysis
Four cultures of T4R and Δ1434–8 were grown in C+Y medium to OD 600 nm 0.5 medium and subjected to liquid chromatography-tandem mass spectrometry (LC-MS/MS) analysis as previously described27, 28. Briefly, proteins were isolated from bacterial pellets sonicated in NP-40 lysis buffer (0.5% NP-40, 150 mM NaCl, 20 mM CaCl2∙2H2O, 50 mM Tris, pH 7.4) supplemented with protease inhibitor cocktail (c0mplete™, Sigma-Aldrich) using a Covaris S220 focused-ultrasonicator. Protein concentration was determined using Thermo Scientific Pierce BCA Protein Assay Kit. Precipitation of 30 μg of protein was performed with methanol and chloroform (4:1), solubilized in 8 M urea, reduced (5 mM dithiothreitol (DTT) at 65 °C for 10 m) and alkylated (0.01 M iodoacetamide at 37 °C for 30 m) and digested with porcine trypsin (at 37 °C, overnight, 50:1 ratio of protein. Tryptic peptides were desalted using a C18 spin column (Thermo Fisher Scientific) and analyzed by linear trap quadropole (LTQ) Orbitrap Velos mass spectrometer equipped with an Advion nanomate electrospray ionization (ESI) source (Advion). Peptides (500 ng) were eluted from a C18 column (100 μm id × 2 cm) onto an analytical column (75 μm ID × 10 cm, C18) using a 180 m gradient with 99.9% acetonitrile, 0.1% formate at a flow rate of 400 nL/m and introduced into an LTQ-Orbitrap.
Data dependent scanning was performed by the Xcalibur v 2.1.0 software using a survey mass scan at 60,000 resolution in the Orbitrap analyzer scanning mass/charge (m/z) 400–1600 followed by collision-induced dissociation (CID) tandem mass spectrometry (MS/MS) of the 14 most intense ions in the linear ion trap analyzer29. Precursor ions were selected by the monoisotopic precursor selection (MIPS) setting with selection or rejection of ions held to a ±10 ppm window. Dynamic exclusion was set to place any selected m/z on an exclusion list for 45 s after a single MS/MS. Tandem mass spectra were searched against a Streptococcus pneumoniae serotype 4 strain ATCC BAA/TIGR4 FASTA protein database downloaded from UniProtKB to which common contaminant proteins (e.g., human keratins obtained at ftp://ftp.thegpm.org/fasta/cRAP) were appended. All MS/MS spectra were searched using Thermo Proteome Discoverer 1.3 (Thermo Fisher Scientific) considering fully tryptic peptides with up to two missed cleavage sites. Variable modifications considered during the search included methionine oxidation (15.995 Da), and cysteine carbamidomethylation (57.021 Da). Peptides were identified at 99% confidence with XCorr score cutoffs based on a reversed database search30. The protein and peptide identification results were visualized with Scaffold v 3.6.1 (Proteome Software Inc.). Protein identifications with a minimum of two peptides identified at 0.1% peptide false discovery rate (FDR) were deemed correct. Significant changes in protein expression between T4R and Δ1434–8 were identified by Fisher’s exact test at a p-value of ≤0.054 and fold change of ±1.3. Fold changes in protein expression were calculated using weighted normalized spectra with 0.5 imputation value. Various bioinformatics resources such as DAVID, KEGG, and STRING were utilized to determine the functions of the identified proteins31–33. The PRoteomics IDEntifications (PRIDE) database is a centralized, standards compliant, public data repository for proteomics data. The mass spectrometry proteomics data from this study is deposited to the ProteomeXchange Consortium via the PRIDE partner repository (PXD019773)34.
Statistics
Intercoupled plasma mass spectrometry (ICP-MS) and qRT-PCR were performed in triplicate, results were averaged together, and standard error of the mean was calculated. ICP-MS data sets were analyzed by comparing parental T4R to Δ1434–8 using the Student’s t-test, with an α value = 0.05. For qRT-PCR experiments, the samples treated with TPEN and an additional metal were compared to the TPEN 30 min control sample using a one-way ANOVA with Sidak’s multiple comparisons test with an α = 0.05. Results were deemed statistically significant when p < alpha. Statistical analyses were performed using GraphPad Prism 8.3.
Results
Identification of two uncharacterized ABC-transporters in Streptococcus pneumoniae
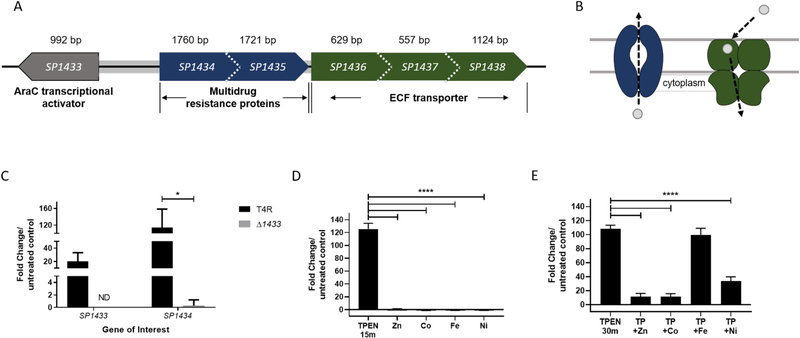
Microarray data of Streptococcus pneumoniae strain TIGR4 exposed to the chelator N,N,N’,N’-tetrakis-(2-pyridylmethyl)ethylenediamine (TPEN) identified the genetic loci SP1434–1438 as some of the most highly upregulated genes in response to metal limitation (Supplemental Table 1). Analysis using the Database of prOkaryotic OpeRons (DOOR) identified this as a single operon35. Further analysis by MicrobesOnline36 predicted this genetic locus to encode a single operon with the following association confidence levels, SP134-1435 (0.990), SP1435-1436 (0.768) SP1436-1437 (0.992) and SP1437-1438 (0.903). Co-regulation of genes SP1434–1438 was verified by detecting a significant upregulation of each gene by quantitative real time PCR (qRT-PCR) following metal-chelation with TPEN (data not shown). BLASTP comparisons of SP1434 and SP1435 proteins against non-redundant NCBI protein database predicted high homology with multidrug resistance-like ATP-binding proteins (mdlB) and both were classified as defense mechanisms by COG assignment (Fig. 1B, Supplemental Table 2). Subcellular location analyses performed with the Uniprot database predicted both proteins to have five alpha helix transmembrane domain regions and a nucleotide binding domain. Though SP1434 and SP1435 were proposed to be involved in antibiotic efflux37, when tested against a broad panel of antibiotics, a mutant strain lacking SP1434–8 was equally as sensitive as the parental T4R (Supplemental Figure 1). Further BLASTP analyses excluding S. pneumoniae homologs identified proteins IrtA and NvdA as the proteins with the highest degree of similarity with a query cover of 99% (99.8% identical) and a query cover of 100% (99.7% identical), respectively (Supplemental Table 2).
Figure 1. Identification of metal-dependent operon SP1433–1438.
Genetic locus (A) and model (B) of SP1433-SP1438. The direction of transcription is represented by the dotted arrow. C) Gene expression of SP1433 and SP1434 were assessed by qRT-PCR in T4R (black) and ΔSP1433 (gray) strain following 15 min treatment with TPEN (30 μM), *p<0.05 as determined by Student’s t-test. Non-detectable gene expression is represented by ND. Expression of SP1434 measured by qRT-PCR from RNA extracted following treatment with D) TPEN (TP) or 200 μM metal, or E) 30 min incubation with 15 min TPEN (30 μM) exposure followed by 15 min metal supplementation. All fold changes were calculated by ΔΔCT analysis with gyrA serving as internal control. ****p<0.0001 as determined by one-way ANOVA with Sidak’s multiple comparison.
The second putative transport system encoded by SP1436, SP1437, and SP1438 was classified as an energy coupling factor (ECF) transport systems by NCBI, representing the substrate-specific component (EcfS), the transmembrane transporter component (EcfT), and the ATP-binding protein (EcfA2). COG assignments of these proteins were classified as function unknown and inorganic ion and carbohydrate transport and metabolism (Supplemental Table 2). Uniprot analyses determined SP1436 to have six transmembrane domain regions, SP1437 to have a signal peptide and a transmembrane domain region, and SP1438 to have a nucleotide binding domain and an ABC transport domain (Supplemental Table 2). Homology prediction by BLASTP analyses with S. pneumoniae homologs excluded identified the closest degree of similarity to exist with a hypothetical membrane protein (query cover 100%, 100% identical), a putative thiamine permease (query cover 88%, 96% identical), and a ribose ABC transport ATPase (query cover 100%, 99.7% identical). This model of a putative ECF transport system suggests SP1436 is the substrate specific subunit, SP1437 functions as the permease, and SP1438 acts as the ATPase utilizing energy generated by ATP hydrolysis to import the unknown substrate into the cell (Fig. 1B).
Transcriptional regulation of SP1434–1438
The locus directly upstream of SP1434 encodes a previously uncharacterized AraC transcriptional regulator (SP1433). To determine if SP1433 is involved in the induction of SP1434–8 following TPEN treatment, expression of SP1433 and SP1434 were assessed by qRT-PCR in the parental T4R and a Δ1433 mutant strain. Expression of SP1433 in T4R following TPEN treatment revealed an upregulation of ≈20-fold (Fig. 1C) and expression of SP1433 was of course undetectable in a Δ1433 mutant. Though SP1434 was strongly upregulated, approximately 115-fold, in the T4R strain following TPEN exposure, treatment with TPEN failed to induce expression of SP1434 in a Δ1433 mutant strain. These data indicate involvement of SP1433 in the regulation of this operon. To identify if SP1433 contributes to expression of genes within the AdcR zinc-dependent regulon,, expression of adcA and adcAII, were analyzed in T4R and the Δ1433 mutant, though no significant differences in expression were observed between the two strains (Supplemental Figure 2A). These data suggest that SP1433 is not involved in the regulation of the canonical zinc uptake system that is within the AdcR regulon. However, to determine if SP1434 expression is regulated specifically by SP1433 or by both SP1433 and AdcR, we assessed expression of SP1434 in T4R, Δ1433, and ΔadcR strains treated with TPEN. As described above, the robust upregulation of SP1434 seen in the T4R strain was lost in the Δ1433 strain. Interestingly, expression of SP1434 was upregulated roughly 16-fold in the ΔadcR strain (Supplemental Figure 2B). Thus, these findings suggest that SP1434–1438 are likely directly regulated by SP1433 but is also further controlled by the zinc-dependent regulator, AdcR.
Metal availability alters expression of SP1434
To verify the microarray results, expression of the first gene in the locus (SP1434) was analyzed by qRT-PCR following TPEN treatment. Results from these analyses indicated robust expression in response to chelation, with SP1434 upregulated greater than 100-fold compared to an untreated control (Fig. 1D). In contrast, expression of SP1434 was unaltered in samples exposed to excess metal ion concentrations; zinc(II) (0.7-fold), cobalt(II) (−1.3), iron(II) (−1.1), and nickel(II) (−1.1) yielded similar results (Fig. 1D). To determine the effect of individual metals on SP1434 expression, samples were treated with TPEN for 15 min followed by supplementation with excess metal for 15 min. Addition of zinc or cobalt ions limited the upregulation of SP1434 by roughly 90%, and nickel(II) limited expression by 30% compared to a control treated with TPEN for 30 min. Addition of iron(II) following TPEN treatment failed to inhibit SP1434 induction, limiting upregulation by less than 10% (Fig. 1E). These data are important to note as TPEN, though most commonly used as a zinc-chelator (Kd = 2.6 × 10−16 M), is known to interact with other transition metals including copper38, nickel 39, and iron40. Collectively, these data indicate this system is responsive to multiple divalent metal ions.
SP1434–1438 alters intracellular metal availability
The considerable upregulation of this locus following metal chelation led us to investigate the intracellular metal ion concentrations within T4R and the Δ1434–8 strain using inductively coupled plasma mass spectrometry (ICP-MS). Analysis of metal accumulation during metal-sufficient conditions (C+Y medium) revealed a significant deficit in intracellular iron(Fe56) with WT containing 73 μg/L and the mutant strain containing only 38 μg/L. However, significant increases were seen in the intracellular concentrations of both manganese(II) and zinc(II) in the Δ1434–8 strain with Mn55 concentrations totaling 0.27 μg/L and 0.29 μg/L when compared to the parental T4R and Zn66 totaling 79 μg/L and 160 μg/L, respectively. (Fig. 2A, Supplemental Table 3). When samples were treated with TPEN, depicting metal ion chelation, these differences were ablated and a modestly significant difference, p=0.49, was observed in Cu63 concentrations between the two strains (Fig. 2B).
Figure 2. Intracellular metals of T4R and Δ1434–8.
Metal content of T4R (black) and Δ1434–8 (gray) were assessed by ICP-MS in A) C+Y Medium and B) C+Y 20 μM TPEN. Mean metal concentrations are displayed as concentrations in parts per billion (μg/L) standard error of the mean represented by horizontal bars. Numbers below metal ions on the x-axis represent detectable elemental isotypes.*p <0.05, **p<0.01 as determined by Student’s t-test comparing T4R to Δ1434–8 strain for individual metals analyzed.
Secreted metabolome of T4R and Δ1434–8
Metals have been shown to impact bacterial metabolism, and a recent study of Streptococcus pyogenes showed that excess zinc interfere in glucose metabolism through the inhibition of phosphofructokinase and glyceraldehyde-3-phosphate-dehydrogenase41. To determine the role of SP1434–1438 in pneumococcal metabolism, secreted metabolomics was performed on WT and Δ1434–8 using a 2D nuclear magnetic resonance (NMR) approach. Briefly, cultures of each strain were grown in C+Y media to early log (OD 600 nm 0.2), mid-log (OD 600 nm 0.35) and early stationary phase (OD 600 nm 0.5). Culture supernatants were sterile filtered, processed and quantified by NMR, using an in-house library of more than fifty metabolites. Partial least squares discriminant analysis (PLS-DA) of these samples detected significant differences in metabolite concentrations between strains of pneumococci and across the different growth phases analyzed (Fig. 3A). Whereby, the largest separation was detected across the second component from cultures collected during mid-log (OD 0.35) and early stationary (OD 0.5) growth phases, as represented by the 95% confidence interval ellipses (Fig. 3A). In addition to identifying the collective differences between strains, significant differences were observed in individual metabolites, including metabolic byproducts lactate and acetate and carbohydrates sucrose and glucose (Fig. 3B). Further, we observed a striking increase in the concentrations of cysteine, arginine, valine, and isoleucine during mid-log growth of the Δ1434–8 strain compared to the parental T4R (Fig. 3B).
Figure 3. Metabolic profile of T4R and Δ1434–8.
A) Clustering of samples within the PLS-DA plot of T4R and Δ1434–8 profiles indicate significant metabolic differences between the two strains at an OD 0.2, 0.35, and 0.5. B) PLS-DA VIP Scores plot of T4R and Δ1434–8 indicates the most significant metabolites identified between strains.
A heatmap displaying differences between the two strains throughout the growth phases and clustering of metabolites with similar behavior is shown in Supplemental Figure 3. T4R and Δ1434–8 had similar metabolite concentrations at the earliest growth phase of OD 0.2, corresponding to the similarities shown on the PLS-DA plot (Fig. 3A). Acetate, L-lysine, L-cysteine, and L-arginine concentrations decrease slowly across growth phases for Δ1434–8, whereas they are quickly catabolized in the T4R strain during mid-log growth (Supplemental Fig. 3). Broadly, the metabolites represented on the heatmap are in two classes: compounds that increase across growth phases, including L-cystine, L-threonine, and lactate, and compounds that decrease throughout growth phases, including D-glucose, L-phenylalanine, L-alanine, and L-aspartate (Supplemental Fig. 3). Some of the most significantly altered metabolites were used to construct metabolic pathways and interestingly, many alterations in the pneumococcal metabolome appear to branch from an alteration in pyruvate metabolism (Fig. 4). This metabolic map denotes a dramatic difference in pyruvate concentrations between the two strains, which then feeds into the production of amino acids L-isoleucine, L-leucine, and L-valine (Fig. 4). Pyruvate metabolism further results in the production of acetyl-CoA, which could then contribute to a downstream increased production of acetate detected in the Δ1434–8 strain (Fig. 3B, 4, Supplemental Fig. 3). It is important to note that the metabolic differences observed are not attributable to a single pathway and are instead a combined effect of changes occurring throughout several pathways. Collectively, these data indicate a stark contribution of this locus to overall cellular metabolism, with particular contributions to glucose and amino acid metabolism.
Figure 4.
Extracellular metabolite concentrations of T4R and Δ1434–8 arranged in metabolic pathways.
Proteomic analysis of T4R and Δ1434–8
To identify mechanisms contributing to differences in the metabolic profiles observed for the T4R and Δ1434–8 strains, proteomic analysis was performed using mass spectrometry-based methods. Using a Fisher’s exact t-test (p < 0.003), we identified 41-differentially expressed proteins in the Δ1434–8 strain compared to the parental T4R (Table 1). Differentially expressed proteins were analyzed through KEGG, STRING, Uniprot, and RegPrecise to determine potentially relevant metabolic and regulatory pathways33, 42–44. From these data, roughly 22% of the differentially expressed proteins, were identified within the CcpA, global catabolite repression regulon45, 46, and 2/41 are under the regulation of CodY, the known essential global nutritional regulator of S. pneumoniae47. In addition to the CcpA and CodY regulons, multiple differentially expressed proteins were identified as belonging to the ArgR, the predicted-Rex, and the CtsR regulons, indicating changes in arginine metabolism and redox stimuli between the Δ1434–8 strain and parental T4R48–51. Down-regulation of both AgaB, NanB, and BgaA in the Δ1434–8 strain suggest changes in galactose metabolism, compared to the parental T4R strain, that were not observed in the metabolomic analyses. Collectively, the differential expression of ABC-transporters or phosphotransferase systems accounted for more than 20% of the downregulated proteins in the Δ1434–8 strain. The most significantly downregulated protein (SP1690) was an uncharacterized sugar ABC transporter, further reiterating changes in carbohydrate utilization between the two strains. To gain more insight into the functions of the differentially expressed proteins, COG assignments were determined for all 41 proteins, though 9/41 (22% proteins were classified as function unknown (Fig. 5). The next abundant COG categories were classified as amino acid/carbohydrate transport and metabolism (collectively 10/41, 24%), inorganic ion transport and metabolism (5/41, 12%), energy production and conversion (4/41, 10%), and translation, ribosomal structure, and biogenesis (4/41, 10%) (Fig. 5).
Table 1. Differentially expressed proteins in ΔSP1434–8 versus T4R.
Mass spectrometry based proteomic analysis of Δ1434–8 strain compared to the parental T4R strain from cultures grown to OD 0.5 identified 41-differentially expressed proteins. Fisher’s exact t-test p<0.00274. Regulon is denoted on the left column and proteins highlighted in gray are putatively within the CcpA/CodY and Rex regulons.
| Regulon | Fold Change | Gene Name | NCBI Locus Tag | Description |
|---|---|---|---|---|
| CcpA | −10.0 | SP1557 | SP_RS07675 | uncharacterized protein, DegV (fatty-acid binding protein) |
| −5.0 | AgaB | SP_RS00340 | galactosamine PTS system transporter subunit IIB | |
| −3.3 | GatB | SP_RS03160 | Galactose PTS | |
| −2.0 | AdhB | SP_RS01400 | alcohol dehydrogenase (zinc) | |
| −2.0 | NanU | SP_RS08315 | N-acetylneuraminate ABC transporter substrate-binding protein | |
| −2.0 | NanE | SP_RS08325 | N-acetylmannosamine-6-phosphate-2-epimerase | |
| −1.4 | RaiA | SP_RS11265 | ribosomal subunit interface protein | |
| −1.3 | AdhE | SP_RS10245 | alcohol dehydrogenase (iron) | |
| 4.8 | RpiA | SP_RS04050 | ribose-5-phosphate isomerase A | |
| CodY | −3.3 | PiuA | SP_RS09285 | iron ABC-transporter |
| 1.3 | GapN | SP_RS05545 | glyceraldehyde-3-phosphate dehydrogenase | |
| ArgR | −1.7 | ArcB | SP_RS10960 | ornithine carbamoyltransferase |
| 5.3 | ArtM | SP_RS04030 | ABC-type arginine transport system | |
| CtsR | −1.4 | ClpE | SP_RS04015 | ATP-dependent Clp protease |
| −11.1 | ClpL | SP_RS01650 | ATP-dependent Clp protease ATP-binding subunit | |
| Unknown | −10.0 | PyrK | SP_RS04775 | dihydroorotate dehydrogenase electron transfer subunit, oxidoreductase |
| −10.0 | FucD | SP_RS11005 | lactaldehyde dehydrogenase fucose/iron dehydrogenase | |
| −5.0 | FolD | SP_RS04035 | tetrahydrafolate dehydrogenase, oxidoreductase activity | |
| −25.0 | SP1690 | SP_RS08350 | sugar ABC transporter substrate-binding protein | |
| −14.3 | RpmG | SP_RS10895 | 50s ribosomal protein L33 | |
| −12.5 | NanB | SP_RS08335 | neuraminidase B | |
| −12.5 | PstS2 | SP_RS10610 | phosphate ABC transporter | |
| −10.0 | SP1536 | SP_RS07565 | methyltransferase, TrmN6 | |
| −10.0 | SP1686 | SP_RS08330 | Gfo/Idh/MocA family oxidoreductase | |
| −5.0 | AgaS | SP_RS00360 | tagatose-6-phosphate-isomerase | |
| −5.0 | SpxA | SP_RS00930 | transcriptional regulator | |
| −5.0 | SP1023 | SP_RS05080 | N-acetyltransferase | |
| −5.0 | YdhJ | SP_RS06325 | homologous to: HD superfamily phosphodyrolase | |
| −5.0 | Gtf1 | SP_RS08705 | group 1 glycosyl transferase | |
| −5.0 | SP1943 | SP_RS05080 | GNAT family acetyltransferase | |
| −5.0 | SP2073 | SP_RS10560 | ABC transporter | |
| −3.3 | SP1114 | SP_RS05515 | ABC transporter ATP-binding protein | |
| −3.3 | SsuE | SP_RS07230 | NAD(P)H-dependent FMN reducatse | |
| −2.5 | SP0097 | SP_RS05515 | integral membrane domain | |
| −2.0 | BgaA | SP_RS03175 | beta-galactosidase (galactose metabolism) | |
| −1.7 | RplO | SP_RS01105 | 50s ribosomal protein L15 | |
| −1.4 | CbpA | SP_RS11185 | choline binding protein A | |
| −1.3 | SP1804 | SP_RS08955 | Alkaline shock protein, YloU | |
| 4.8 | TehB | SP_RS04845 | tellurite resistance protein (S-adenosylmethionine-dep methyltransferase) | |
| 5.4 | SP0923 | SP_RS04565 | Cof family protein, phosphatase (hydrolase) | |
| 7.4 | SP2028 | SP_RS10255 | phosphotyrosine protein phosphatase | |
Figure 5. Proteomics COG assignments.
41-differentially expressed proteins in Δ1434–8 strain compared to T4R were assigned Clusters of orthologous group assignments using EggNOG 5.0.0.
Discussion
Streptococcus pneumoniae remains one of the leading killers of children worldwide, and infections due to this organism account for more than 400,000 hospitalizations per year in the United States alone52. The pneumococcus primarily resides as a commensal of the nasopharynx, where zinc(II) concentrations are limited. Additionally, exposure to calprotectin, a protein produced by neutrophils to sequester metal ions from bacteria, further limits metal availability within the host. Pathogens must therefore utilize mechanisms to circumvent metal starvation. Though metal acquisition and regulation has been well characterized in S. pneumoniae, in this study we have identified a previously uncharacterized system that is strongly responsive to metal-limitation. It is important to note that this system has not previously appeared in studies investigating the Streptococcus pneumoniae transcriptional changes that occur in response to metal-limiting conditions53, potentially be due to differences in experimental design across studies. Furthermore, loss of this genetic locus resulted in an altered cellular metabolism. Our findings indicate that genes SP1433-1438 are encoded as a putative operon that is highly upregulated in response to chelation by the cell permeable chelator, TPEN, potentially mimicking the environment encountered during neutrophil clearance in the human nasopharynx. BLASTP and UniProt analysis revealed this genetic locus to encode components of ABC transport systems sharing homology with an antibiotic transport system and an energy coupling factor transport system. SP1434 and SP1435 were shown to be involved in pneumococcal environmental information processing and were highly responsive following deletion of the two-component regulatory system 08 (TCS08) histidine kinase54. Moreover, the pneumococcal TCS08 shares homology to the SaeRS system of Staphylococcus aureus, a system whose activity is enhanced by zinc-bound calprotectin55, 56. Taken together, these data further suggest a connection between this locus and metal ion homeostasis. Though this locus may encode for two different transport systems, the co-expression of this system collectively indicates a likely involvement in similar processes. Due to the promiscuous metal-binding affinity of TPEN, we investigated the effect of other metals on expression of SP1434. This operon was determined to be responsive to zinc, cobalt, and nickel, as supplementation following TPEN treatment led to a drastic limitation in the upregulation of SP1434 seen in TPEN treated samples alone. Additionally, we detected an upregulation of SP1434 in the parental T4R strain but no increase in expression in the strain lacking the AraC transcriptional regulator (Δ1433), identifying SP1433 as the regulator of this system. Expression of AdcR-regulon genes adcA and adcAII encoding zinc-binding lipoproteins were not affected in the Δ1433 strain, suggesting that SP1433 is likely a zinc-sensing regulator that functions to regulate SP1434–1438 independently of AdcR.
Since components of this locus share sequence similarity with metal ion transport systems, we analyzed intracellular metal concentrations of T4R and Δ1434–8 by ICP-MS. Significant differences were detected in the concentrations of manganese, zinc, and iron ions. Iron(II) is known to interact with H2O2 through Fenton chemistry to form highly reactive hydroxyl radicals57, whereas zinc and manganese are able to function as antioxidant metal ions specifically through redox-active metal antagonism and interaction with the superoxide dismutase, SodA58, 59. As metal ions are also known to be important enzymatic cofactors for proteins involved in metabolism, and H2O2 is a significant byproduct of pneumococcal metabolism, we explored how loss of this locus, and subsequent changes in intracellular metal ion concentrations, altered cellular metabolism. NMR-based metabolomics of culture supernatants identified major shifts in the metabolic profiles between strains, primarily with increased production of lactate in the T4R strain and increased production of acetate in the Δ1434–8 strain. These differences suggest a preference for mixed acid fermentation by Δ1434–8 versus homolactic acid fermentation by the parental T4R strain60. Several amino acids were also observed to exhibit differing metabolite behavior. Of particular interest were the high levels of cysteine detected in Δ1434–8 strain since this strain contains higher concentrations of zinc(II) and cysteine residues are known to interact with zinc ions with high affinity61.
Proteomic analysis validated the results obtained from the secreted metabolomics and indicated that nearly one quarter of differentially expressed proteins were regulated by the CcpA regulon, involved in carbohydrate catabolite repression. The CcpA regulon has been previously characterized as a master regulator that controls fermentation, as well as catabolism of glucose, galactose, and tagatose46. Many of the differentially expressed proteins that fall within the CcpA regulon were downregulated and are known to be involved in galactose fermentation, including GatB and the nan operon, and AgaB, a system involved in galactosamine uptake62, 63. These data could suggest preferences for galactose utilization over glucose. Additionally, CcpA regulation is thought to be important for host interactions and contributes to successful colonization, and a recent study has linked glucose metabolism to pneumococcal virulence in a chinchilla model of otitis media46, 64, 65. Previous studies have linked sialic acid and CcpA with galactose metabolism specifically through the pneumococcal nan operons63, and it is therefore interesting to note that 4/5 of the genes within this locus in the D39 background (spd_1263, spd_1264, spd_1265, and spd_1267) were found to be upregulated when grown with 0.5% sialic acid63.
In addition to the large number of genes that were identified within the CcpA regulon, results from our proteomic analysis also detected differential expression of proteins within the CodY, ArgR, and Rex regulons. CodY has been shown to be involved in the regulation of colonization and amino acid metabolism and could contribute to the differences in amino acid metabolism observed in our analyses66. Arginine metabolism has been linked to virulence in Streptococcus pneumoniae and other significant human pathogens and it is interesting to note that one of only six upregulated proteins in our analysis was an arginine transport system that is regulated by the ArgR regulon48. The Rex (redox-sensing regulator) regulon detected by RegPrecise has been characterized in both Streptococcus mutans and Staphylococcus aureus, though to our knowledge has not yet been characterized in S. pneumoniae67, 68. In both S. mutans and S. aureus Rex has been shown to sense NAD+ or NADH, and in S. aureus Rex is thought to be the central regulator of anaerobic metabolism68. Findings from our proteomics analyses indicate that the involvement of Rex in this system is at the level of a zinc(II) and iron(II)-binding alcohol dehydrogenase, both of which are also under the regulation of CcpA. An additional protein thought to be under co-regulation by Rex and CodY identified in our proteomics data is a glyceraldehyde-3-phosphate-dehydrogenase, interestingly Ong et al. have previously shown that glyceraldehyde-3-phosphate dehydrogenase was inhibited during high zinc stress in the closely related pathogen, Group A Streptococcus, further indicating a role for GapN in sensing metal ion stress41. Collectively, these data indicate major metabolic differences between our T4R strain and the Δ1434–8 strain, particularly in carbohydrate metabolism, fermentation, and amino acid metabolism.
Conclusions
In this study, we identified a previously uncharacterized regulator and locus of Streptococcus pneumoniae encoding two transport systems that are robustly expressed in metal limiting conditions. We have determined that loss of this genetic loci mutants lacking this locus (Δ1434–8) significantly alters the intracellular metal ion profile and pneumococcal metabolome. Analysis of secreted metabolites and proteome detected changes in carbohydrate metabolism, including shifts in glucose and galactose fermentation pathways. These data demonstrate that the central metabolome of Streptococcus pneumoniae is largely metal-dependent, which to our knowledge has not yet been characterized. Future studies will aim to characterize the specific substrates shuttled through the efflux and import systems initially described in this work. This work provides a strong foundation for the roles of zinc in the regulation of metabolism and from this platform we will identify key metabolic enzymes and intermediates that could be targeted for future metal-dependent therapeutics.
Supplementary Material
Acknowledgements
Thank you to Allen Shack and Moses Ayoola in the College of Basic Sciences, the staff of the Arizona State University Mass Spectrometry Core, and the staff at the University of Nebraska Spectroscopy and Biophysics core for their assistance with the work presented here. This work was supported in part an Institutional Development Award (IDeA) from the NIGMS COBRE grant number P20GM103646 (awarded to JAT) and by a National Institutes of Health grant number R15GM113152 (awarded to NCF).
References
- 1.CDC, Journal, 2015.
- 2.Doran KS, Fulde M, Gratz N, Kim BJ, Nau R, Prasadarao N, Schubert-Unkmeir A, Tuomanen EI and Valentin-Weigand PJAN, Host–pathogen interactions in bacterial meningitis, 2016, 131, 185–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ter Beek J, Guskov A and Slotboom DJ, Structural diversity of ABC transporters, Journal of General Physiology, 2014, 143, 419–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borths EL, Poolman B, Hvorup RN, Locher KP and Rees DC, In Vitro functional characterization of BtuCD-F, the Escherichia coli ABC transporter for vitamin B12 uptake, Biochemistry, 2005, 44, 16301–16309. [DOI] [PubMed] [Google Scholar]
- 5.Koster W, ABC transporter-mediated uptake of iron, siderophores, heme and vitamin B12, Research in microbiology, 2001, 152, 291–301. [DOI] [PubMed] [Google Scholar]
- 6.Claverys J-P, A new family of high-affinity ABC manganese and zinc permeases, Research in microbiology, 2001, 152, 231–243. [DOI] [PubMed] [Google Scholar]
- 7.Rodionov DA, Hebbeln P, Eudes A, ter Beek J, Rodionova IA, Erkens GB, Slotboom DJ, Gelfand MS, Osterman AL, Hanson AD and Eitinger T, A novel class of modular transporters for vitamins in prokaryotes, Journal of Bacteriology, 2009, 191, 42–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bousis S, Setyawati I, Diamanti E, Slotboom DJ and Hirsch AKH, Energy-Coupling Factor Transporters as Novel Antimicrobial Targets, Advanced Therapeutics, 2019, 2, 1800066. [Google Scholar]
- 9.Erkens GB, Majsnerowska M, ter Beek J and Slotboom DJ, Energy coupling factor-type ABC transporters for vitamin uptake in prokaryotes, Biochemistry, 2012, 51, 4390–4396. [DOI] [PubMed] [Google Scholar]
- 10.Kirsch F and Eitinger T, Transport of nickel and cobalt ions into bacterial cells by S components of ECF transporters, Biometals : an international journal on the role of metal ions in biology, biochemistry, and medicine, 2014, 27, 653–660. [DOI] [PubMed] [Google Scholar]
- 11.Josts I, Almeida Hernandez Y, Andreeva A and Tidow H, Crystal structure of a group I energy coupling factor vitamin transporter S component in complex with its cognate substrate, Cell chemical biology, 2016, 23, 827–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Waldron KJ, Rutherford JC, Ford D and Robinson NJJN, Metalloproteins and metal sensing, 2009, 460, 823. [DOI] [PubMed] [Google Scholar]
- 13.Weinberg ED, Nutritional immunity. Host’s attempt to withold iron from microbial invaders, Jama, 1975, 231, 39–41. [DOI] [PubMed] [Google Scholar]
- 14.Brown LR, Gunnell SM, Cassella AN, Keller LE, Scherkenbach LA, Mann B, Brown MW, Hill R, Fitzkee NC, Rosch JW, Tuomanen EI and Thornton JA, AdcAII of Streptococcus pneumoniae Affects Pneumococcal Invasiveness, PLOS ONE, 2016, 11, e0146785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brown LR, Caulkins RC, Schartel TE, Rosch JW, Honsa ES, Schultz-Cherry S, Meliopoulos VA, Cherry S and Thornton JA, Increased zinc availability enhances initial aggregation and biofilm formation of Streptococcus pneumoniae, Frontiers in Cellular and Infection Microbiology, 2017, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Plumptre CD, Eijkelkamp BA, Morey JR, Behr F, Counago RM, Ogunniyi AD, Kobe B, O’Mara ML, Paton JC and McDevitt CA, AdcA and AdcAII employ distinct zinc acquisition mechanisms and contribute additively to zinc homeostasis in Streptococcus pneumoniae, Molecular microbiology, 2014, 91, 834–851. [DOI] [PubMed] [Google Scholar]
- 17.Bayle L, Chimalapati S, Schoehn G, Brown J, Vernet T and Durmort C, Zinc uptake by Streptococcus pneumoniae depends on both AdcA and AdcAII and is essential for normal bacterial morphology and virulence, Molecular microbiology, 2011, 82, 904–916. [DOI] [PubMed] [Google Scholar]
- 18.Manzoor I, Shafeeq S, Afzal M and Kuipers OP, The regulation of the AdcR regulon in Streptococcus pneumoniae depends both on Zn(2+)- and Ni(2+)-Availability, Frontiers in Cellular and Infection Microbiology, 2015, 5, 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fernebro J, Andersson I, Sublett J, Morfeldt E, Novak R, Tuomanen E, Normark S and Normark BH, Capsular Expression in Streptococcus pneumoniae Negatively Affects Spontaneous and Antibiotic-Induced Lysis and Contributes to Antibiotic Tolerance, The Journal of Infectious Diseases, 2004, 189, 328–338. [DOI] [PubMed] [Google Scholar]
- 20.Horton RM, PCR-mediated recombination and mutagenesis, Molecular Biotechnology, 1995, 3, 93–99. [DOI] [PubMed] [Google Scholar]
- 21.Foroozandeh M, Adams RW, Meharry NJ, Jeannerat D, Nilsson M and Morris GA, Ultrahigh‐Resolution NMR Spectroscopy, Angewandte Chemie International Edition, 2014, 53, 6990–6992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Foroozandeh M, Adams RW, Kiraly P, Nilsson M and Morris GA, Measuring couplings in crowded NMR spectra: pure shift NMR with multiplet analysis, Chemical Communications, 2015, 51, 15410–15413. [DOI] [PubMed] [Google Scholar]
- 23.Thrippleton MJ and Keeler J, Elimination of Zero-Quantum Interference in Two-Dimensional NMR Spectra, Angewandte Chemie International Edition, 2003, 42, 3938–3941. [DOI] [PubMed] [Google Scholar]
- 24.Delaglio F, Grzesiek S, Vuister GW, Zhu G, Pfeifer J and Bax A, NMRPipe: A multidimensional spectral processing system based on UNIX pipes, Journal of Biomolecular NMR, 1995, 6, 277–293. [DOI] [PubMed] [Google Scholar]
- 25.Xia J and Wishart DS, in Current Protocols in Bioinformatics, John Wiley & Sons, Inc., 2002, DOI: 10.1002/cpbi.11. [DOI] [Google Scholar]
- 26.Worley B and Powers R, Multivariate Analysis in Metabolomics, Curr. Metabolomics, 2013, 1, 92–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shah P, Nanduri B, Swiatlo E, Ma Y and Pendarvis K, Polyamine biosynthesis and transport mechanisms are crucial for fitness and pathogenesis of Streptococcus pneumoniae, Microbiology (Reading, England), 2011, 157, 504–515. [DOI] [PubMed] [Google Scholar]
- 28.Rai AN, Thornton JA, Stokes J, Sunesara I, Swiatlo E and Nanduri B, Polyamine transporter in Streptococcus pneumoniae is essential for evading early innate immune responses in pneumococcal pneumonia, Scientific Reports, 2016, 6, 26964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Andon NL, Hollingworth S, Koller A, Greenland AJ, Yates JR 3rd and Haynes PA, Proteomic characterization of wheat amyloplasts using identification of proteins by tandem mass spectrometry, Proteomics, 2002, 2, 1156–1168. [DOI] [PubMed] [Google Scholar]
- 30.Qian WJ, Jacobs JM, Camp, DG 2nd, Monroe ME, Moore RJ, Gritsenko MA, Calvano SE, Lowry SF, Xiao W, Moldawer LL, Davis RW, Tompkins RG and Smith RD, Comparative proteome analyses of human plasma following in vivo lipopolysaccharide administration using multidimensional separations coupled with tandem mass spectrometry, Proteomics, 2005, 5, 572–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang da W, Sherman BT and Lempicki RA, Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources, Nature protocols, 2009, 4, 44–57. [DOI] [PubMed] [Google Scholar]
- 32.Yoshida M, Kashiwagi K, Kawai G, Ishihama A and Igarashi K, Polyamine enhancement of the synthesis of adenylate cyclase at the translational level and the consequential stimulation of the synthesis of the RNA polymerase ς28 subunit, Journal of Biological Chemistry, 2001, 276, 16289–16295. [DOI] [PubMed] [Google Scholar]
- 33.Szklarczyk D, Morris JH, Cook H, Kuhn M, Wyder S, Simonovic M, Santos A, Doncheva NT, Roth A, Bork P, Jensen LJ and von Mering C, The STRING database in 2017: quality-controlled protein–protein association networks, made broadly accessible, Nucleic Acids Research, 2017, 45, D362–D368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vizcaino JA, Csordas A, del-Toro N, Dianes JA, Griss J, Lavidas I, Mayer G, Perez-Riverol Y, Reisinger F, Ternent T, Xu QW, Wang R and Hermjakob H, 2016 update of the PRIDE database and its related tools, Nucleic Acids Research, 2016, 44, D447–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mao F, Dam P, Chou J, Olman V and Xu Y, DOOR: a database for prokaryotic operons, Nucleic Acids Research, 2009, 37, D459–D463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alm EJ, Huang KH, Price MN, Koche RP, Keller K, Dubchak IL and Arkin AP, The MicrobesOnline Web site for comparative genomics, Genome research, 2005, 15, 1015–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tocci N, Iannelli F, Bidossi A, Ciusa ML, Decorosi F, Viti C, Pozzi G, Ricci S and Oggioni MR, Functional Analysis of Pneumococcal Drug Efflux Pumps Associates the MATE DinF Transporter with Quinolone Susceptibility, Antimicrobial Agents and Chemotherapy, 2013, 57, 248–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hyun HJ, Sohn JH, Ha DW, Ahn YH, Koh J-Y and Yoon YH, Depletion of Intracellular Zinc and Copper with TPEN Results in Apoptosis of Cultured Human Retinal Pigment Epithelial Cells, Investigative Ophthalmology & Visual Science, 2001, 42, 460–465. [PubMed] [Google Scholar]
- 39.Nemec AA, Leikauf GD, Pitt BR, Wasserloos KJ and Barchowsky A, Nickel mobilizes intracellular zinc to induce metallothionein in human airway epithelial cells, Am J Respir Cell Mol Biol, 2009, 41, 69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arslan P, Di Virgilio F, Beltrame M, Tsien RY and Pozzan T, Cytosolic Ca2+ homeostasis in ehrlich and yoshida carcinomas. A new, membrane-permeant chelator of heavy metals reveals that these ascites tumor cell lines have normal cytosolic free Ca2+, Journal of Biological Chemistry, 1985, 260, 2719–2727. [PubMed] [Google Scholar]
- 41.Ong CL, Walker MJ and McEwan AG, Zinc disrupts central carbon metabolism and capsule biosynthesis in Streptococcus pyogenes, Sci Rep, 2015, 5, 10799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Novichkov PS, Laikova ON, Novichkova ES, Gelfand MS, Arkin AP, Dubchak I and Rodionov DA, RegPrecise: a database of curated genomic inferences of transcriptional regulatory interactions in prokaryotes, Nucleic Acids Research, 2010, 38, D111–D118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.U. Consortium, Journal, 2015, 43, D204–D212. [Google Scholar]
- 44.Kanehisa M , The KEGG database, silico simulation of biological processes, 2002, 247, 91–103. [Google Scholar]
- 45.Iyer R, Baliga NS and Camilli A, Catabolite control protein A (CcpA) contributes to virulence and regulation of sugar metabolism in Streptococcus pneumoniae, Journal of Bacteriology, 2005, 187, 8340–8349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Carvalho SM, Kloosterman TG, Kuipers OP and Neves AR, CcpA Ensures Optimal Metabolic Fitness of Streptococcus pneumoniae, PLOS ONE, 2011, 6, e26707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Caymaris S, Bootsma HJ, Martin B, Hermans PWM, Prudhomme M and Claverys J-P, The global nutritional regulator CodY is an essential protein in the human pathogen Streptococcus pneumoniae, Molecular microbiology, 2010, 78, 344–360. [DOI] [PubMed] [Google Scholar]
- 48.Kloosterman TG and Kuipers OP, Regulation of arginine acquisition and virulence gene expression in the human pathogen Streptococcus pneumoniae by transcription regulators ArgR1 and AhrC, Journal of Biological Chemistry, 2011, 286, 44594–44605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bitoun JP, Nguyen AH, Fan Y, Burne RA and Wen ZT, Transcriptional repressor Rex is involved in regulation of oxidative stress response and biofilm formation by Streptococcus mutans, FEMS microbiology letters, 2011, 320, 110–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Michel A, Agerer F, Hauck CR, Herrmann M, Ullrich J, Hacker J and Ohlsen K, Global Regulatory Impact of ClpP Protease of Staphylococcus aureus on Regulons Involved in Virulence, Oxidative Stress Response, Autolysis, and DNA Repair, Journal of Bacteriology, 2006, 188, 5783–5796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chastanet A, Prudhomme M, Claverys J-P and Msadek T, Regulation of Streptococcus pneumoniae clp Genes and Their Role in Competence Development and Stress Survival, Journal of Bacteriology, 2001, 183, 7295–7307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.CDC, Journal, 2016.
- 53.Shafeeq S, Kloosterman TG and Kuipers OPJM, Transcriptional response of Streptococcus pneumoniae to Zn2+ limitation and the repressor/activator function of AdcR, 2011, 3, 609–618. [DOI] [PubMed] [Google Scholar]
- 54.Gómez-Mejia A, Gámez G, Hirschmann S, Kluger V, Rath H, Böhm S, Voss F, Kakar N, Petruschka L, Völker U, Brückner R, Mäder U and Hammerschmidt S, Pneumococcal Metabolic Adaptation and Colonization Are Regulated by the Two-Component Regulatory System 08, mSphere, 2018, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McKessar SJ and Hakenbeck R, The Two-Component Regulatory System TCS08 Is Involved in Cellobiose Metabolism of Streptococcus pneumoniae R6, Journal of Bacteriology, 2007, 189, 1342–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cho H, Jeong DW, Liu Q, Yeo WS, Vogl T, Skaar EP, Chazin WJ and Bae T, Calprotectin Increases the Activity of the SaeRS Two Component System and Murine Mortality during Staphylococcus aureus Infections, PLoS pathogens, 2015, 11, e1005026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Koppenol WH, The centennial of the Fenton reaction, Free Radical Biology and Medicine, 1993, 15, 645–651. [DOI] [PubMed] [Google Scholar]
- 58.Powell SR, The antioxidant properties of zinc, The Journal of nutrition, 2000, 130, 1447s–1454s. [DOI] [PubMed] [Google Scholar]
- 59.Yesilkaya H, Kadioglu A, Gingles N, Alexander JE, Mitchell TJ and Andrew PW, Role of manganese-containing superoxide dismutase in oxidative stress and virulence of Streptococcus pneumoniae, Infection and immunity, 2000, 68, 2819–2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hartel T, Eylert E, Schulz C, Petruschka L, Gierok P, Grubmuller S, Lalk M, Eisenreich W and Hammerschmidt S, Characterization of central carbon metabolism of Streptococcus pneumoniae by isotopologue profiling, Journal of Biological Chemistry, 2012, 287, 4260–4274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pace NJ and Weerapana E, Zinc-binding cysteines: diverse functions and structural motifs, Biomolecules, 2014, 4, 419–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bidossi A, Mulas L, Decorosi F, Colomba L, Ricci S, Pozzi G, Deutscher J, Viti C and Oggioni MR, A Functional Genomics Approach to Establish the Complement of Carbohydrate Transporters in Streptococcus pneumoniae, PLOS ONE, 2012, 7, e33320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Afzal M, Shafeeq S, Ahmed H and Kuipers OP, Sialic Acid-Mediated Gene Expression in Streptococcus pneumoniae and Role of NanR as a Transcriptional Activator of the nan Gene Cluster, Applied and Environmental Microbiology, 2015, 81, 3121–3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Al-Bayati FAY, Kahya HFH, Damianou A, Shafeeq S, Kuipers OP, Andrew PW and Yesilkaya H, Pneumococcal galactose catabolism is controlled by multiple regulators acting on pyruvate formate lyase, Scientific Reports, 2017, 7, 43587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hu FZ, Król JE, Tsai CHS, Eutsey RA, Hiller LN, Sen B, Ahmed A, Hillman T, Buchinsky FJ, Nistico L, Dice B, Longwell M, Horsey E and Ehrlich GD, Deletion of genes involved in the ketogluconate metabolism, Entner-Doudoroff pathway, and glucose dehydrogenase increase local and invasive virulence phenotypes in Streptococcus pneumoniae, PLOS ONE, 2019, 14, e0209688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hendriksen WT, Bootsma HJ, Estevão S, Hoogenboezem T, de Jong A, de Groot R, Kuipers OP and Hermans PWM, CodY of Streptococcus pneumoniae: link between nutritional gene regulation and colonization, Journal of Bacteriology, 2008, 190, 590–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Baker JL, Derr AM, Karuppaiah K, MacGilvray ME, Kajfasz JK, Faustoferri RC, Rivera-Ramos I, Bitoun JP, Lemos JA, Wen ZT and Quivey RG, Streptococcus mutans NADH oxidase lies at the intersection of overlapping regulons controlled by oxygen and NAD+ levels, Journal of Bacteriology, 2014, 196, 2166–2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pagels M, Fuchs S, Pané-Farré J, Kohler C, Menschner L, Hecker M, McNamarra PJ, Bauer MC, Von Wachenfeldt C, Liebeke M, Lalk M, Sander G, Von Eiff C, Proctor RA and Engelmann S, Redox sensing by a Rex-family repressor is involved in the regulation of anaerobic gene expression in Staphylococcus aureus, Molecular microbiology, 2010, 76, 1142–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.