Abstract
Aims/hypothesis:
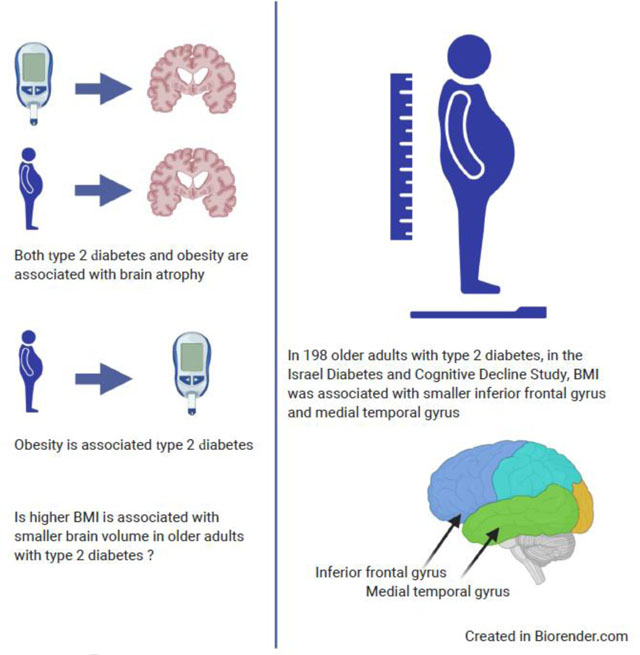
There are established relationships between adiposity (obesity) and higher dementia risk, faster cognitive decline and associated neural injury. Type 2 diabetes is strongly linked to greater adiposity and has been consistently associated with neural injury and poor cognitive outcomes. However, although obesity is a major cause of type 2 diabetes, there is limited evidence on the association of adiposity with brain atrophy among individuals with type 2 diabetes.
Methods:
We examined the association of BMI (a measure of adiposity), and of long-term trajectories of BMI (three empirically identified groups of trajectories—’normal’, ‘overweight’ and ‘obese’—using SAS macro PROC TRAJ), with regional brain volume, in a sample of older individuals (aged 64–84) with type 2 diabetes participating in the Israel Diabetes and Cognitive Decline Study (n=198).
Results:
Using linear regression, we found that greater BMI was associated with smaller volumes of the inferior frontal gyrus (IFG) (r=−0.25, p=0.001) and the middle temporal gyrus (r=−0.19; p=0.010) after adjusting for sociodemographic covariates and total intracranial volume. In addition, there were significant differences between BMI trajectory groups in IFG volume (F=4.34, p=0.014), such that a long-term trajectory of obesity was associated with a smaller volume. Additional adjustment for cardiovascular and diabetes-related potential confounders did not substantively alter the results. There were no associations of adiposity with superior frontal gyrus, middle frontal gyrus or total grey matter volumes.
Conclusions/interpretation:
In older adults with type 2 diabetes, long-term adiposity may have a detrimental impact on volume of brain regions relevant to cognitive functioning. Further studies to identify the underlying mechanisms are warranted.
Keywords: Adiposity, BMI, Brain volume, Cognitive functioning, Inferior frontal gyrus, Middle temporal gyrus, Type 2 diabetes
Graphical Abstract
Introduction
Type 2 diabetes has been associated with greater risks for cognitive decline and dementia [1]. Individuals with type 2 diabetes show greater grey matter and hippocampal atrophy [2], which may underlie their increased risk for dementia. However, the factors contributing to greater brain atrophy in type 2 diabetes are yet to be elucidated. Adiposity, commonly represented by BMI, has been associated with atrophy of the total grey matter and of the prefrontal cortex and temporal lobe [3]. Since adiposity is a major contributor to the pathogenesis of type 2 diabetes and its complications [4], it is critical to evaluate how it influences regional brain atrophy among older adults with type 2 diabetes. A few recent studies have identified a relationship between BMI and brain atrophy in type 2 diabetic individuals (including [5, 6]). Yet, a question remains regarding the risk of obesity in midlife and the reverse relationship in late age; weight loss confounds the relationship between obesity and risk for poor cognitive health [7]. Thus, we examined whether higher BMI is associated with smaller regional brain volumes in elderly individuals with type 2 diabetes, and using historical data from the Israel Diabetes and Cognitive Decline (IDCD) study, we also examined associations of trajectories of adiposity with regional brain volumes, in order to better understand whether long-term BMI relates to brain atrophy in type 2 diabetes.
Methods
Study population
Participants were recruited from the IDCD study, a longitudinal study of type 2 diabetes, associated factors and cognitive decline; a collaboration between the Icahn School of Medicine at Mount Sinai (ISMMS), New York, the Sheba Medical Center, Ramat Gan, Israel, and the Maccabi Healthcare Services (MHS), Tel Aviv, Israel, and approved by all three institutional review board committees. All participants gave written informed consent. Detailed methods of the IDCD study, and of the MHS Diabetes Registry, are described in the electronic supplementary material (ESM) Methods. The IDCD study enrolled participants with type 2 diabetes, aged ≥65 years, randomly selected from approximately 11,000 individuals with type 2 diabetes from the MHS Diabetes Registry.
Study procedures
MHS identifies potential subjects, excluding anyone with an ICD code for dementia or its subtypes, with prescribed cholinesterase inhibitors, or with a major psychiatric (e.g. schizophrenia, substance abuse) or neurological condition (e.g. stroke, Parkinson’s disease, traumatic brain injury) that could affect cognitive performance. To participate in this study, individuals must be fluent in Hebrew and have an informant (a friend or family member who can attend the assessment, to provide supplementary information, for example, reporting on their impressions of the participants’ cognitive status and day-to-day functioning. The eligibility criteria for the study are summarised in ESM Table 1. Participants are assessed at their residence (or at the Sheba Medical Center, according to their preference) by a physician, who performs a neurological and psychiatric exam and draws blood, and by a neuropsychologist, who administers the neuropsychological battery. Informed consent is signed by all participating individuals. Participants are assessed at baseline and at 18-month intervals. The longitudinal component of the study is ongoing and this brief report presents baseline data. Demographics, medical and psychiatric history, quantified functional assessment, results of the neuropsychological testing, physical testing and laboratory results are used for a physician’s diagnosis which is then discussed in a diagnostic consensus conference. Only individuals deemed as cognitively normal are included in the study at baseline. Additional detailed methods for the current study can be found in the ESM Methods and in ESM Table 1 and ESM Fig. 1.
BMI and demographic, cardiovascular and diabetes variables
BMI is a commonly used measure of adiposity [3]. BMI was calculated as kg/m2. The study physicians measure IDCD participants’ weight (in Kg) and height (in cm) at baseline, within 2 months of the MRI assessment. To calculate BMI trajectories, we used measurements prior to the baseline, taken from the Maccabi Diabetes Registry. Additional details of methods for calculation of BMI trajectories are presented in the ESM Methods. Sociodemographic covariates were age, years of education and sex. Cardiovascular risk factors (total cholesterol and systolic and diastolic blood pressure) as well as BMI, were measured as the mean of all measures in the Maccabi Diabetes Registry. Smoking was defined as never/past/current based on self-report at IDCD baseline. Type 2 diabetes-related covariates were duration of type 2 diabetes, HbA1c (the gold standard measure of glycaemic control), and glucose-lowering medication for type 2 diabetes. Additional details of methods for calculation of covariates are presented in the ESM Methods.
Estimation of brain volume
MRI scans were performed at the Diagnostic Imaging Division, Sheba Medical Center, with a 3 Tesla scanner (Signa HDxt, GE Healthcare). Details regarding MRI procedures can be found in the ESM.
Statistical analysis
Primary analyses were linear regressions performed to examine the association between BMI and brain volumes. In model 1, we controlled for sociodemographic variables (age, years of education, sex). In model 2, we added type 2 diabetes-related characteristics (HbA1c, duration of type 2 diabetes, glucose-lowering medication for type 2 diabetes) as covariates. In model 3, we added cardiovascular covariates (total cholesterol, systolic and diastolic blood pressure, and smoking status [never/past/current]) as covariates. To adjust for variability in brain volume, total intracranial brain volume was included in all analyses. Details regarding the measurement of covariates can be found in the ESM Methods.
BMI has been recorded in the MHS Diabetes Registry since 1998 for patients attending their annual visit. We thus evaluated trajectories of BMI as predictors of brain volume; details regarding the calculation of trajectories can be found in the ESM. Three trajectories were identified with linear, quadratic and cubic curves corresponding to normal, overweight and obese BMI groups, respectively. These group assignments were then analysed using ANCOVA to identify differences between trajectory groups.
Results
Participant characteristics (n=198) are presented in ESM Tables 2 and 3. Table 1 presents results of the associations of BMI with regional brain volume. Using model 1, which was adjusted for sociodemographic variables, higher BMI was significantly associated with smaller volume of the middle temporal gyrus (MTG; r= −0.19; p=0.010) and of the inferior frontal gyrus (IFG; r= −0.25, p=0.001). In model 2, which was also adjusted for type 2 diabetes-related characteristics, higher BMI remained significantly associated with smaller volume of the MTG (p=0.004) and with smaller volume of the IFG (p<0.001). In model 3, which additionally accounted for cardiovascular risk factors, the inverse association of higher BMI with smaller MTG (p=0.018) and IFG (p=0.001) volumes remained significant. BMI was not associated with the volume of the superior (p=0.618, model 3) or middle (p=0.554, model 3) frontal gyri or with total brain volume (p=0.112, model 3) in any of the models. Additional details can be found in the ESM analysis and ESM Fig. 2 and 3 (Regressions of BMI with ROIs).
Table 1.
Associations of BMI with regional brain volume
| Brain region | Model | r | β | p value |
|---|---|---|---|---|
| IFG | 1 | −0.247 | −0.227 | 0.001* |
| 2 | −0.262 | −0.235 | <0.001* | |
| 3 | −0.242 | −0.224 | 0.001* | |
| MTG | 1 | −0.185 | −0.165 | 0.010* |
| 2 | −0.205 | −0.180 | 0.004* | |
| 3 | −0.174 | −0.154 | 0.018* | |
| Superior frontal gyrus | 1 | −0.002 | −0.002 | 0.976 |
| 2 | −0.004 | −0.004 | 0.952 | |
| 3 | 0.046 | 0.043 | 0.618 | |
| Middle frontal gyrus | 1 | −0.065 | −0.061 | 0.365 |
| 2 | −0.074 | −0.068 | 0.311 | |
| 3 | −0.044 | −0.041 | 0.554 | |
| Total brain volume (grey + white matter) | 1 | −0.049 | −0.013 | 0.497 |
| 2 | −0.066 | −0.016 | 0.367 | |
| 3 | 0.117 | 0.101 | 0.112 |
Model 1 adjusted for age, years of education and sex, model 2 additionally adjusted for type 2 diabetes-related characteristics (duration of type 2 diabetes, HbA1c and glucose-lowering medication for type 2 diabetes) and model 3 additionally adjusted for other cardiovascular risk factors (total cholesterol, systolic and diastolic blood pressure, and smoking status)
p<0.05
Analysis identified three trajectories, with each characterised by (1) the BMI level at study baseline (intercept), and (2) the trend over time in the registry (ESM Fig. 4). Three types of trends of BMI over time were observed, reflecting similar categorisation to that defined by the World Health Organization: ‘normal’ (18.5–24.99 kg/m2; 43.8%), ‘overweight’ (25–29.99 kg/m2; 43.7%), and ‘obese’ (≥30 kg/m2; 12.5%). These trajectories were stable over time. As shown in Table 2, the IFG volume was significantly associated with the BMI trajectories in all models (F=4.34, p=0.014 model 1, p values <0.026 all models) and the MTG volume was significantly associated with BMI trajectories only in model 2 (p=0.036), such that the obese group had the smallest volume. None of the BMI trajectories were significantly associated with the volumes of the superior frontal gyrus, middle frontal gyrus or total brain (Table 2).
Table 2.
Associations of BMI trajectories with brain volume
| Brain region | Model | F | p value | Volume by BMI trajectory groupa, Mean (SD) |
|---|---|---|---|---|
| IFG | 1 | 4.34 | 0.014* | Normal: 0.335 (0.03) |
| 2 | 4.57 | 0.012* | Overweight: 0.333 (0.04) | |
| 3 | 3.73 | 0.026* | Obese: 0.323 (0.03) | |
| MTG | 1 | 2.96 | 0.054 | Normal: 0.426 (0.04) |
| 2 | 3.39 | 0.036* | Overweight: 0.429 (0.04) | |
| 3 | 2.23 | 0.110 | Obese: 0.418 (0.04) | |
| Superior frontal gyrus | 1 | 0.03 | 0.971 | Normal: 0.300 (0.03) |
| 2 | 0.03 | 0.968 | Overweight: 0.307 (0.04) | |
| 3 | 0.30 | 0.739 | Obese: 0.308 (0.04) | |
| Middle frontal gyrus | 1 | 0.18 | 0.836 | Normal: 0.322 (0.04) |
| 2 | 0.19 | 0.831 | Overweight: 0.326 (0.03) | |
| 3 | 0.01 | 0.990 | Obese: 0.327 (0.03) | |
| Total volume (GM+WM) | 1 | 1.25 | 0.288 | Normal: 1032.176 (112.2) |
| 2 | 1.27 | 0.283 | Overweight: 1024.724 (101.2) | |
| 3 | 1.17 | 0.312 | Obese: 1037.703 (68.1) |
Model 1 adjusted for age, years of education and sex, model 2 additionally adjusted for type 2 diabetes-related characteristics (duration of type 2 diabetes, HbA1c and glucose-lowering medication for type 2 diabetes) and model 3 additionally adjusted for other cardiovascular risk factors (total cholesterol, systolic and diastolic blood pressure, and smoking status)
p<0.05
The values for each trajectory group refers to all models
Secondary analyses that also adjusted for number of depressive symptoms and for global cognition are presented in ESM Table 4; the results remained essentially unchanged (Model 3 with depression symptoms: IFG; r=−0.22; p=0.001; MTG, r=−0.17, p=0.018; model 3 with global cognitive functioning: IFG; r=−0.23; p=0.002; MTG, r=−0.1, p=0.018). ESM Fig. 5 presents the brain regions of interests of this study. ESM Figs 6 and 7 represent the residual plots of the regressions. Additional details on the secondary analyses can be found in the ESM analysis.
Discussion
In this sample of older individuals with type 2 diabetes, higher BMI was associated with smaller volumes of IFG and MTG. These associations remained mostly unchanged when adjusted for a range of sociodemographic variables, type 2 diabetes-related characteristics and other cardiovascular risk factors. In addition, we calculated empirical trajectories of BMI, which represent long-term normal-weight, overweight and obese trajectories. Again, we found significant associations with IFG and MTG; the subgroup with a long-term obese trajectory had the smallest volumes for these brain regions. Neither BMI at the time of brain volume assessment nor trajectories of BMI were associated with the volumes of the superior or middle frontal gyri or with total brain volume.
In middle-aged and younger elderly individuals with type 2 diabetes, obesity has been associated with brain atrophy [5, 6]. Our study provides new evidence, in older adults with type 2 diabetes, of associations of smaller volumes of IFG and MTG with higher BMI, suggesting a potential role of BMI, a major cause of type 2 diabetes, in brain atrophy of older type 2 diabetic individuals. Participants in the sample tended to remain in the same BMI category over many years and those with a trajectory of obesity had lower IFG and MTG volumes. The BMI ‘paradox’ suggests that high BMI in midlife is associated with smaller brain volume and higher dementia risk, while in late life, low BMI is not associated with these outcomes [4]. Our results do not concur with this paradox and suggest that in older adults with type 2 diabetes, both late-life BMI and a long-term trajectory of obesity are associated with smaller brain volumes. These trajectories suggest that long-term obesity may be linked to brain volume loss.
Cognitive functions are impacted by both type 2 diabetes [8] and dementia. Obesity was associated with lower volume in the MTG [9], which is important for episodic memory, and the IFG, which underlies functions of decision making, attention and language [10]. This suggests that in older adults with type 2 diabetes, obesity may contribute to an increased dementia risk via injury to these regions, but longer term follow-up of the cohort will be needed to evaluate whether greater adiposity contributes to incident dementia and whether atrophy of the MTG and IFG mediate this increased risk.
Several mechanisms may underlie the BMI–brain volume associations. Adiposity is associated with hypertension and high cholesterol [11]; however, adjustment for these vascular factors in the analyses did not substantively alter the results, suggesting other mechanistic pathways. Adiposity causes chronic inflammation [11], including alterations in adipokines that affect the brain [4], and these changes may be involved in the pathogenesis of dementia [4].
This study was limited by its cross-sectional outcomes; however, longitudinal assessments are ongoing, and the trajectories of BMI support the view that our results are not due to reverse causality. Moreover, adjustment for global cognition did not substantively affect the results, suggesting that our results are not due to incipient dementia. This study included only participants with type 2 diabetes and excluded individuals without type 2 diabetes; thus, the potential causal role of type 2 diabetes in the BMI–brain relationship could not be examined in this study. BMI may not be an optimal measure of adiposity in older adults because of the redistribution of body fat with age [12], indicating the need for more direct measures of adiposity in studies of older adults. Effect sizes are relatively small but consistent across statistical models, with a broad range of adjustments for sociodemographic, diabetes-related and cardiovascular covariates. The number of obese participants in the trajectory analyses is relatively small; studies with a larger number of obese individuals are warranted. The individuals in our sample had relatively good glycaemic control, so our results may not represent older adults with type 2 diabetes with poorly controlled blood glucose. Although our hypotheses were formulated a priori, we note that results for the IFG withstand Bonferroni adjustment for multiple comparisons (p=0.01 to account for five regions of interest). Finally, to calculate duration of diabetes, we used the date the participant was included in the MHS Diabetes Registry rather than the actual time the participant was diagnosed as this information was not available, thereby underestimating the duration of diabetes in our sample. Strengths of the study include the relatively large sample of individuals who underwent MRI, strong validity of the type 2 diabetes diagnosis, assessment of multiple measures of type 2 diabetes-related characteristics and other cardiovascular risk factors, and the availability of numerous BMI assessments before the initiation of the study, permitting calculation of trajectories of BMI. Our findings provide new information regarding the relationships of long-term BMI trajectories with brain volume. Future studies are needed to determine whether interventions to reduce BMI protect against brain atrophy in older adults with type 2 diabetes.
Supplementary Material
Research in context.
What is already known about this subject?
Type 2 diabetes is associated with an increased risk for cognitive decline in older adults
Adiposity, which can be represented by BMI, is a contributor to the development of type 2 diabetes and its complications, and is associated with brain atrophy
What is the key question?
How does adiposity influence regional brain atrophy among older adults with type 2 diabetes?
What are the new findings?
In older adults with type 2 diabetes, BMI is associated with smaller volumes of the inferior frontal gyrus and middle temporal gyrus
The long-term obesity trajectory was associated with inferior frontal gyrus volume
How might this impact on clinical practice in the foreseeable future?
Potential interventions to reduce BMI in midlife may be useful for protecting against later brain atrophy in individuals with type 2 diabetes
Acknowledgements:
Some of the data were presented as an abstract at the Alzheimer’s Association International Conference in 2019.
Funding: Study funded by NIH (AG02219, AG034087, AG051545, AG053446, AG061093). We thank the LeRoy Schecter Foundation and Dr. Marina Nissim for their kind gifts which supported this study.
Abbreviations
- IDCD
Israel Diabetes and Cognitive Decline
- IFG
Inferior frontal gyrus
- MHS
Maccabi Healthcare Services
- MTG
Middle temporal gyrus
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Data availability: The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Authors’ relationships and activities: The authors declare that there are no relationships or activities that might bias, or be perceived to bias, their work.
References
- 1.Baumgart M, Snyder HM, Carrillo MC, Fazio S, Kim H, Johns H (2015) Summary of the evidence on modifiable risk factors for cognitive decline and dementia: A population-based perspective. Alzheimer’s Dement 11(6):718–726. 10.1016/j.jalz.2015.05.016 [DOI] [PubMed] [Google Scholar]
- 2.Moran C, Phan TG, Chen J, et al. (2013) Brain atrophy in type 2 diabetes. Diabetes Care 36:4036–4042. 10.2337/dc13-0143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Willette AA, Kapogiannis D (2015) Does the brain shrink as the waist expands? Ageing Res Rev 20:86–97. 10.1016/j.arr.2014.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Luchsinger JA, Gustafson DR (2009) Adiposity, type 2 diabetes and Alzheimer’s disease. J Alzheimers Dis 16(4):693–704. 10.3233/JAD-2009-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Bloemendaal L, Ijzerman RG, ten Kulve JS, et al. (2016) Alterations in white matter volume and integrity in obesity and type 2 diabetes. Metab Brain Dis 31(3):621–629. 10.1007/s11011-016-9792-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walsh EI, Shaw M, Sachdev P, Anstey KJ, Cherbuin N (2019) The impact of type 2 diabetes and body mass index on cerebral structure is modulated by brain reserve. Eur J Neurol 26(1):121–127. 10.1111/ene.13780 [DOI] [PubMed] [Google Scholar]
- 7.Pegueroles J, Jiménez A, Vilaplana E, et al. (2018) Obesity and Alzheimer’s disease, does the obesity paradox really exist? A magnetic resonance imaging study. Oncotarget 9(78):34691–34698. 10.18632/oncotarget.26162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Climie RED, Moran C, Callisaya M, et al. (2015) Abdominal obesity and brain atrophy in type 2 diabetes mellitus. PLoS One 10(11):1–11. 10.1371/journal.pone.0142589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Squire LR, Stark CEL, Clark RE (2004) The medial temporal lobe. Annu Rev Neurosci 27(1):279–306. 10.1146/annurev.neuro.27.070203.144130 [DOI] [PubMed] [Google Scholar]
- 10.Liakakis G, Nickel J, Seitz RJ (2011) Diversity of the inferior frontal gyrus-A meta-analysis of neuroimaging studies. Behav Brain Res 225(1):341–347. 10.1016/j.bbr.2011.06.022 [DOI] [PubMed] [Google Scholar]
- 11.Tchernof A, Després J-P (2013) Pathophysiology of human visceral obesity: an update. Physiol Rev 93(1):359–404. 10.1152/physrev.000332011. [DOI] [PubMed] [Google Scholar]
- 12.Letra L, Santana I, Seiça R (2014) Obesity as a risk factor for Alzheimer ‘ s disease : the role of adipocytokines. Metab Brain Dis 563–568. 10.1007/s11011-014-9501-z [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


