Abstract
Background and Purpose:
Previous data suggest patient demographics and clinical presentation are primary predictors of motor recovery post-stroke, with minimal contributions of physical interventions. Other studies indicate consistent associations between the amount and intensity of stepping practice with locomotor outcomes. The goal of this study was to determine the relative contributions of these combined variables to locomotor outcomes post-stroke across a range of patient demographics and baseline function.
Methods:
Data were pooled from 3 separate trials evaluating the efficacy of high-intensity training (HIT), low-intensity training, and conventional interventions. Demographics, clinical characteristics and training activities from 144 participants > 1-month post-stroke were included in stepwise regression analyses to determine their relative contributions to locomotor outcomes. Subsequent latent profile analyses evaluated differences in classes of participants based on their responses to interventions.
Results:
Stepwise regressions indicate primary contributions of stepping activity on locomotor outcomes, with additional influences of age, duration post-stroke, and baseline function. Latent profile analyses revealed 2 main classes of outcomes, with the largest gains in those who received HIT and achieved the greatest amounts of stepping practice. Regression and latent profile analyses of only HIT participants indicated age, baseline function and training activities were primary determinants of locomotor gains. Participants with the smallest gains were older (~60 years), presented with slower gait speeds (<0.40 m/s) and performed 600-1000 less steps/session.
Conclusion:
Regression and cluster analyses reveal primary contributions of training interventions on mobility outcomes in patients > 1-month post-stroke. Age, duration post-stroke and baseline impairments were secondary predictors.
Clinical Trial Registration-URL:
https://clinicaltrials.gov/. Unique Identifiers: NCT02507466 and NCT01789853
Keywords: gait, rehabilitation, cluster analyses
Introduction
Restoration of locomotor function is a priority for individuals with stroke1, 2, particularly given the health care and personal costs associated with impaired mobility3, 4. Traditional rehabilitation paradigms focus on mitigating the impairments underlying walking deficits post-stroke, or normalizing gait patterns to improve mobility5, 6. However, the efficacy of these strategies is limited7. Previous studies suggest that the degree of lower extremity impairments and specific demographics (i.e., age, duration post-stroke) are primarily predictors of gains in lower extremity motor recovery8-12, with uncertain contributions of physical therapy interventions.
Conversely, other studies indicate positive associations between locomotor outcomes and specific exercise strategies13-17. Previous studies suggest therapy interventions that focus entirely on providing large amounts of stepping practice at high cardiovascular intensities often result in statistically and clinically significant gains in locomotor function as compared to conventional and/or low intensity strategies13-15, 17. Subsequent analyses reveal significant moderate associations between stepping amounts or cardiovascular intensities attained and locomotor gains achieved (i.e., dose-response relationships). More recent data suggest high-intensity training (HIT) performed in variable contexts (treadmill, stairs, obstacles, uneven surfaces) may elicit further gains in postural stability and balance confidence13, with a small decrease in stepping practice as compared to forward walking alone that does not mitigate gains in locomotor outcomes. The combined data emphasize the influence of exercise dose (i.e., type, amount and intensity) on locomotor responses, which contrast directly with previous findings indicating greater contributions of demographics and initial clinical presentation.
To address these competing hypotheses, details of training interventions from individuals with walking deficits post-stroke across a range of patient demographics and severity of impairments may allow delineation of their relative contributions to mobility outcomes. However, only a few studies have consistently monitored stepping activities during HIT in participants with subacute or chronic stroke13, 14, 17. The substantial variability in both patient demographics and interventions across studies may limit the utility of traditional regression analyses, and various cluster analyses may provide greater insight into responsiveness to interventions by identifying potential subgroups categorized by the magnitude of changes across locomotor outcomes. A range of these types of analyses are available, including the use of latent profile analyses18, which is a mixture model-based technique that hypothesizes there is an underlying hidden (i.e., latent) categorical variable that separates populations into mutually exclusive and exhaustive groups or classes of participants with similar outcomes. This and similar strategies can be used to evaluate differences between classes to evaluate the factors that contribute to responsiveness to training.
The present study was designed to evaluate contributors to locomotor outcomes post-stroke and characteristics of participants who may or may not respond to specific interventions. Data were utilized from participants with subacute or chronic stroke who enrolled in specific intervention trials evaluating the efficacy of different training strategies on locomotor outcomes. Our primary hypotheses were that training interventions and, more directly, the amount of stepping practice was the primary determinant of responsiveness to training with secondary contributions of baseline function and demographic characteristics.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Participants
Data utilized in this analysis included participants with subacute (1–6 months) or chronic (>6 months) hemiparesis following unilateral stroke previously enrolled in one of three separate trials, including a recent randomized clinical trial in patients with chronic stroke13, a smaller randomized trial in patients with subacute stroke14, and a pilot study including participants with either subacute and chronic stroke that served as the pilot study17, 19. Four different training strategies were utilized, with the primary experimental group performing HIT focused on stepping activities performed in variable contexts (high-variable)17. Comparisons groups included HIT with limited stepping variability (forward walking; high-forward), lower intensity variable walking interventions (low-variable), and conventional physical interventions (conventional). All studies used similar inclusion criteria as follows: 18–85 years old; lower-extremity Fugl-Meyer < 34; over-ground self-selected velocity (SSV) < 1.0 m/s; and medical clearance to participate. Exclusion criteria included: receiving additional physical therapy interventions outside of study interventions, presence of lower-limb contractures that significantly limited locomotor function, cardiovascular, respiratory or metabolic instability, inability to ambulate >150 feet prior to stroke, previous history of additional neurological injury, and inability to adhere to study requirements. Differences in inclusion between studies were related to duration post-stroke and ability to walk with14, 17 vs without13 physical assistance as needed, and all ambulatory and non-ambulatory participants were included. All procedures were approved by the local institutional review boards and all participants provided written consent to participate. The initial database of 144 participants with primary locomotor outcomes were included (Table 1; n=22 from the pilot study, n=32 from the randomized subacute stroke trial, and n=90 from the chronic stroke trial).
Table 1.
Demographics, training characteristics, baseline and changes in primary (ΔSSV, ΔFV, Δ6MWT and ΔTM speed) and secondary outcomes (sit-to-stand, ΔSTS; Functional Gait Assessment: ΔFGA) outcomes in all participants and each class identified through latent profile analyses [conventional, CON; high-forward, HF; high-variable, HV; low-variable, (LV)]. Classes did not include the 3 outliers.
| All (n=144) |
Class 1 (m=104) |
Class 2 (n=37) |
p-values | |
|---|---|---|---|---|
| Changes in outcomes | ||||
| ΔSSV (m/s) | 0.15±0.17 | 0.19±0.18 | 0.04±0.05 | <0.01 |
| ΔFV (m/s) | 0.24±0.26 | 0.28±0.22 | 0.05±0.05 | <0.01 |
| Δ6MWT (m) | 71±73 | 88±73 | 15±14 | <0.01 |
| ΔTM speed (m/s) | 0.30±0.25 | 0.33±0.26 | 0.18±0.16 | <0.01 |
| ΔSTS (reps/s) | 0.05±0.12 | 0.04±0.12 | 0.07±0.13 | 0.26 |
| ΔFGA (a.u.) | 2.3±3.6 | 2.4±3.8 | 2.0±3.3 | 0.55 |
| Demographics | ||||
| age (yrs) | 58±11 | 56±11 | 62±8.9 | <0.01 |
| BMI (kg/m2) | 29±6.7 | 29±6.7 | 29±7.1 | 0.89 |
| gender (F/M) | 48/96 | 37/67 | 11/26 | 0.52 |
| duration (1-6/>6 mo) | 99/45 | 30/74 | 12/25 | 0.68 |
| side of paresis (R/L) | 55/89 | 41/63 | 13/24 | 0.65 |
| lesion site: cortical | 68 | 49 | 16 | |
| subcortical/lacunar | 49 | 37 | 12 | |
| brainstem/cerebellum | 9 | 6 | 3 | 0.39 |
| multiple/diffuse | 8 | 7 | 1 | |
| not reported | 10 | 5 | 5 | |
| Baseline assessments | ||||
| Fugl-Meyer (a.u.) | 22±5.1 | 22±4.8 | 19±5.9 | <0.01 |
| AFO (N/Y) | 46/98 | 34/70 | 9/28 | 0.34 |
| SSV (m/s) | 0.45±0.28 | 0.51±0.26 | 0.26±0.24 | <0.01 |
| FV (m/s) | 0.60±0.39 | 0.68±0.36 | 0.31±0.33 | <0.01 |
| 6MWT (m) | 156±106 | 178±101 | 85±90 | <0.01 |
| TM speed (m/s) | 0.65±0.44 | 0.74±0.41 | 0.37±0.38 | <0.01 |
| STS (reps/s) | 0.28±0.15 | 0.31±0.13 | 0.18±0.14 | <0.01 |
| FGA (a.u.) | 12±5.8 | 13±5.1 | 8.1±6.0 | <0.01 |
| Training activities | ||||
| CON/HF/HV/LV | 17/30/65/32 | 10/25/51/18 | 7/4/12/14 | 0.01 |
| number of sessions | 30±7.2 | 30±7.6 | 30±6.3 | 0.83 |
| steps/session | 2460±1057 | 2677±947 | 1708±847 | <0.01 |
| total steps (x103) | 74±36 | 78±36 | 48±30 | <0.01 |
Interventions
All participants received up to 30–40 1-hr sessions of different interventions over 2–3 months. Participants wore validated accelerometers (StepWatch, Modus, Wash DC) on their paretic ankle to evaluate stepping activity during therapy sessions. Patients received ≤40 min of training per session. The primary goals of all stepping training interventions (high-variable, high-forward, low-variable) were to: 1) maximize the amount of stepping practice; 2) achieve targeted cardiovascular intensities; and, 3) increase difficulty of walking tasks as tolerated. Targeted heart rate (HR) ranges were determined using age-predicted maximum [208-(0.7*age)]20, with high-variable or high-forward targeting 70–80% HR reserve, and low-variable using 30–40% HR reserve.
For high-variable training (n=65), sessions were divided into ~10-min bouts of four different stepping tasks17. Speed-dependent treadmill training was performed with an overhead harness system in case of loss of balance, with goals to increase speeds to reach targeted HRs. Criteria for successful stepping included positive step lengths, minimal limb collapse, and sagittal/frontal plane stability, with body-weight support or swing assistance provided only as needed. Minimal consideration was directed towards gait kinematics17 unless there was a risk for orthopedic injury, with bracing or support provided as needed. Skill-dependent training was performed by applying perturbations to challenge postural stability, propulsion, and limb swing, and included walking in multiple directions, over inclines and obstacles, with leg weight/weighted vests and limited handrail use as tolerated. Over-ground training focused on achieving fastest possible speeds or performing variable tasks as described with use of a gait belt or overhead harness systems. Stair climbing was performed over static or rotating stairs (StairMaster, Vancouver, WA) using reciprocal gait patterns and progression to faster speeds and reduced handrail use as tolerated. For high-forward training (n=30), targeted intensity was also 70–80% HR reserve, although training was limited only to 20 min of forward treadmill training and 20 min of forward over-ground walking. Task difficulty was increased by increasing walking speeds within targeted intensities13. Participants in the low-variable group (n=32) performed stepping training similar to high-variable but with targeted intensities set to 30–40% HR reserve13, 16.
Participants who received conventional interventions (n=17) continued with concurrent physical therapy as possible, with details of therapeutic activities extracted from medical records14. Conventional sessions were supplemented by research staff to achieve up to 40 sessions over 10 weeks, and consisted of conventional therapy activities14, 21, including specific amounts (repetitions) of strengthening, balance, and transfer tasks, with stepping practice provided on both the treadmill and over-ground/stairs without limitations on cueing and feedback. Training intensity was targeted at 30–40% of HR reserve, consistent with HRs achieved during rehabilitation post-stroke14, 22. The treating therapist progressed patients with devices and bracing as appropriate.
Data collection
Participants completed evaluations at baseline and post-training. Primary outcomes included: (1) over-ground SSV and (2) fastest-possible velocity (FV) using a pressure-sensitive walkway (GaitMat, Chalfont, PA or GaitRite, Haverton, PA); (3) 6-minute walk test (6MWT) with instructions to walk at a normal comfortable pace; and (4) peak treadmill (TM) speed during assessments performed on a motorized TM with speeds starting at 0.1 m/s for 1–2 min and increased by 0.1 m/s every 1–2 min. The fastest TM speed that participants could walk for 1 min was considered peak TM speed. For balance measures, the Functional Gait Assessment (FGA) and Berg Balance Scales were used, and preliminary data (n=11) suggest a strong correlation between scores (r=0.95, p<0.01) with regression equations used to convert Berg to FGA (FGA=0.47*(Berg) + 3.12; unpublished results13). Balance scores are hereafter referred to as Functional Gait Assessment. Additional tests included the 5-times sit-to-stand test (transformed to repetitions/sec) and lower-limb Fugl-Meyer assessment. Training variables included average steps/sessions recorded by the ankle-worn accelerometers, number of sessions attended, and total number of steps throughout training (steps/sessions * number of sessions).
Statistical analysis
Correlation and stepwise regression and subsequent latent profile analyses evaluated the potential determinants of locomotor improvements and characteristics of responders. Correlations focused on associations between training variables (steps/session, number of sessions, and total steps) and primary walking outcomes (ΔSSV, ΔFV, Δ6MWT and ΔTM speed) with subsequent regressions performed separately for each primary dependent variable. Independent predictors included: age, gender, BMI, and duration post-stroke (1–6 months, >6 months), lesion location, lower-limb Fugl-Meyer, baseline Functional Gait Assessment and sit-to-stand, and the specific dependent variable measured at baseline (i.e., baseline 6MWT for Δ6MWT). Primary training variables utilized in regression analyses focused on number of sessions and mean steps/session (vs total steps) as these former variables may be more readily interpreted by clinicians. Given the findings, stepwise regressions were repeated for only HIT groups (high-variable/high-forward)13. Stepwise regressions were performed with α=0.05 using SPSS (v26) with primary associations identified as the independent predictor with the greater association with the dependent variable (i.e., first variable listed in regression equation). Collinearity diagnostics were monitored with variance inflation factors <5.0 considered acceptable.
Latent profile analyses were used to identify classes (i.e., subgroups or clusters) of participants with similar changes in primary locomotor outcomes (ΔSSV, ΔFV, Δ6MWT and ΔTM speed). Z-scores were used to stabilize the scales and improve the convergence of the algorithm for estimating the parameters of the Gaussian mixture model. Prior to analysis, a robust variant of the Mahalanobis distance based on the minimum covariance determinant23 was calculated to detect and delete multivariate outliers, which is an estimate of the relative distance of each specific data point to the normalized mean (z-score) and a procedure recommended prior to using cluster analytic techniques24. Four different parametrizations for the covariance structure of the Gaussian mixture model were considered including the following models: 1) equal variances across classes and covariances fixed to 0; 2) varying variances across classes and covariances fixed to 0; 3) equal variances and covariances across classes; and 4) varying variances and covariances across classes. The covariance structure of the mixture model as well as the optimal number of latent classes were determined by considering a range of indices: the Bayesian Information Criterion25, the sample-size adjusted Bayesian Information Criterior26, entropy and bootstrap likelihood ratio test27. Smaller values of Bayesian Information Criterion (or adjusted for sample-size) indicate a better model fit. The value of entropy between 0.8–1.0 reflects a sound separation of identified classes in relation to the data. The bootstrap likelihood ratio test is used to compare the fit of models that specify different number of classes but utilize the same covariance structure. Latent profile analysis was repeated for only HIT groups given similar locomotor improvements.
Post-hoc analyses assessed differences between latent class membership (independent grouping variable) for training measures, baseline impairments and demographic status (dependent variable). Chi-square tests and one-way ANOVA with post-hoc Tukey’s test were used to examine differences among clusters in categorical and continuous dependent variables. All tests were two-sided with α=0.05. Latent profile analysis was conducted using R package “tidyLPA” (version 3.6.1).28
Results
Table 1 (1st column) details changes in outcomes, demographics, baseline characteristics, and training interventions of all participants included, demonstrating substantial variability across cohorts. Preliminary correlation analyses revealed the strongest relation between total steps during training (steps/session * number sessions) and primary outcomes (range of r-values:0.48–0.60, all p<0.01), with lower but significant associations between steps/sessions (r-values:0.35–0.50) and number of sessions (r-values: 0.22–0.27; all p<0.01). Given the utility of steps/sessions and number of sessions for clinicians prescribing or providing therapy interventions, these latter data were utilized in regression analyses with associations between steps/sessions with ΔSSV and ΔFV presented in Fig 1A-B. Stepwise regressions for the primary outcomes of ΔSSV, ΔFV, Δ6MWT and ΔTM speed indicate the strongest associations with steps/session (i.e., first independent predictor listed), with secondary contributions of duration post-stroke, age, number of sessions, and baseline assessments (Equations 1-4).
Figure 1.

Dose-response relationships between stepping activity (steps/sessions) and changes in SSV (A) and FV (B) across 144 participants with subacute and chronic stroke.
| Equation 1: |
| Equation 2: |
| Equation 3: |
| Equation 4: |
Subsequent latent profile analysis was utilized to differentiate classes (i.e., clusters or groups) of participants based on their responses to training. Prior to this analysis the Mahalanobis distance was calculated to detect 3 outliers with changes that were substantially greater than the population mean (e.g., mean ΔFV=1.24±0.29 m/s). Latent profile analyses on the remaining 141 participants suggested superior fit of a 2-class model. Figure 2A indicates z-scores of changes in locomotor outcomes within each class. Class 1 represents 72% of participants (n=104) with above-average gains (z-scores>0), whereas Class 2 represents 28% (n=37) with below-average changes (z-scores<0).
Figure 2.
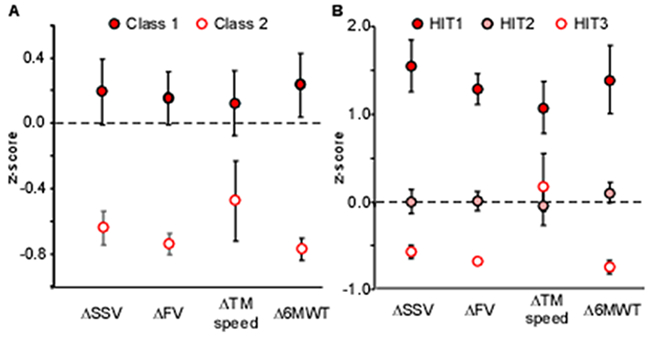
A) Mean z-scores for primary locomotor changes for 141 participants classified into Class 1 and 2. B) Mean z-scores of primary locomotor changes for 92 HIT participants classified into HIT1, HIT2, and HIT3 (errors bars indicate confidence intervals).
Table 1 delineates the clinical and demographic characteristics and changes in outcomes in the two classes. Between-class analyses indicated locomotor gains were significantly different as anticipated, with 0.20–0.35 m/s differences in gait speed changes (ΔSSV, ΔFV, ΔTM speed) and 70 m differences in Δ6MWT. Age was the only demographic variable that was statistically different between classes, although all measures of baseline function and Fugl-Meyer were significantly lower in Class 2. For training characteristics, a greater proportion of non-HIT training (low-variable/conventional) was observed in Class 2, and differences in total steps and steps/session (Class 1 > Class 2).
Given the significant contributions of training interventions to outcomes, regression and latent profile analyses were repeated with only HIT (high-forward/high-forward) groups. Table 2 (1st column) delineates the demographics, clinical characteristics, training activities and locomotor outcomes in HIT groups combined. Correlations again indicate total steps were better correlated with primary outcomes (r-valus:0.40–0.58), with significant associations with other training variables (steps/session, 0.24–0.45; number sessions, 0.28–0.35; all p<0.01). Retaining both steps/session and number of sessions as independent predictors in the stepwise regression revealed primary associations between age and locomotor outcomes (Equation 5-8), with secondary contributions of training variables or other demographics or clinical characteristics.
Table 2.
Demographics and baseline/change in outcomes for participants who performed HIT and each class identified through latent profile analyses; * indicates significantly different from other groups; † indicates differences between two groups. Classes did not include the 3 outliers.
| All (n=95) |
HIT1 (m=26) |
HIT2 (n=44) |
HIT3 (n=22) |
p-values | |
|---|---|---|---|---|---|
| Changes in outcomes | |||||
| ΔSSV (m/s) | 0.21±0.17 | 0.42±0.15* | 0.15±0.09* | 0.05±0.04* | <0.01 |
| ΔFV (m/s) | 0.32±0.27 | 0.58±0.13* | 0.24±0.10* | 0.07±0.03* | <0.01 |
| Δ6MWT (m) | 92±76 | 172±83* | 78±32* | 17±16* | <0.01 |
| ΔTM speed (m/s) | 0.39±0.24 | 0.57±0.22* | 0.29±0.19 | 0.34±0.22 | <0.01 |
| ΔSTS (reps/s) | 0.05±0.13 | 0.07±0.15 | 0.05±0.11 | 0.02±0.15 | 0.44 |
| ΔFGA (a.u.) | 2.3±3.5 | 3.2±3.2 | 2.2±3.8 | 1.6±3.3 | 0.29 |
| Demographics | |||||
| age (yrs) | 58±11 | 51±10* | 60±9.3 | 62±8.0 | <0.01 |
| BMI (kg/m2) | 29±6.8 | 28±6.6 | 31±6.8 | 29±7.1 | 0.25 |
| gender (F/M) | 29/66 | 11/15 | 14/30 | 4/18 | 0.20 |
| duration (<6/>6 mo) | 27/68 | 10/16 | 9/35 | 6/16 | 0.26 |
| side of paresis (R/L) | 38/57 | 11/15 | 19/25 | 7/15 | 0.65 |
| lesion site: cortical | 46 | 11 | 19 | 13 | |
| subcortical/lacunar | 32 | 11 | 16 | 5 | |
| brainstem/cerebellum | 6 | 1 | 4 | 1 | 0.53 |
| multiple/diffuse | 5 | 2 | 3 | 0 | |
| not reported | 6 | 1 | 2 | 3 | |
| Baseline assessments | |||||
| Fugl-Meyer | 22±5.2 | 22±5.1 | 23±4.8 | 19±5.4* | <0.01 |
| AFO (N/Y) | 36/59 | 10/16 | 17/27 | 6/16 | 0.63 |
| SSV (m/s) | 0.45±0.29 | 0.45±0.29 | 0.50±0.27† | 0.30±0.28† | 0.02 |
| FV (m/s) | 0.60±0.39 | 0.60±0.39 | 0.68±0.37† | 0.38±0.38† | 0.01 |
| 6MWT (m) | 156±105 | 166±113 | 172±100† | 99±91† | 0.02 |
| TM speed (m/s) | 0.65±0.42 | 0.72±0.41 | 0.73±0.41 | 0.35±0.34* | <0.01 |
| STS (reps/s) | 0.29±0.15 | 0.32±0.13 | 0.30±0.16 | 0.24±0.14 | 0.16 |
| FGA (a.u.) | 12±5.6 | 13±5.8 | 13±5.1 | 9.3±5.8* | 0.02 |
| Training activities | |||||
| HF/HV | 30/65 | 7/19 | 16/28 | 6/26 | 0.63 |
| number of sessions | 31±7.3 | 34±5.2† | 29±8.1† | 30±6.1 | <0.01 |
| steps/session | 2826±865 | 3144±683 | 2844±848 | 2234±740* | <0.01 |
| total steps (x103) | 87±32 | 106±25* | 76±36 | 67±25 | <0.01 |
| Equation 5: |
| Equation 6: |
| Equation 7: |
| Equation 8: |
Latent profile analysis of only the HIT groups (n=92) suggested good fit of a 3-class model. Class-specific mean z-scores are presented in Figure 2B, with classes ranked by their relative responsiveness (HIT1>HIT2>HIT3; Table 2). Substantial differences in locomotor gains were observed between HIT1 and HIT2, with smaller but significant differences between HIT2 and HIT3 except TM speed. Post-hoc ANOVA revealed younger participants in the highest responders (HIT1>HIT2/HIT3). Differences in baseline function and impairments were consistently lower in the lowest responding group (HIT3). Additional differences in training sessions were observed between HIT1 vs HIT2, with lower steps/session and total steps in HIT3.
Discussion
The present study details the relative contributions of physical interventions, demographics, and clinical presentation on locomotor outcomes in individuals post-stroke using both regression and latent profile analyses. Variables thought to contribute primarily to recovery (age, duration post-stroke and baseline deficits)9, 10 were important contributors to locomotor gains. However, training-related variables, namely total steps and steps/sessions during HIT, were strong predictors of gains in primary locomotor outcomes as previously shown29, 30. While total steps throughout the duration of training demonstrated greater association to locomotor outcomes, steps/sessions and number of sessions may be more tangible training parameters that clinicians can readily interpret and apply in clinical practice. Subsequent latent profile analyses resulted in two classes of participants based on responses to training, and revealed between-group differences in training characteristics, age, and baseline clinical presentation.
Regression analyses performed in only those participants who performed HIT revealed greater primary contributions of age and training activities, with additional influences of baseline function. In this latter analysis, HIT1 demonstrating the largest improvements was younger than HIT2 but with similar baseline function, while HIT3 with the smallest gains were older and presented with the lowest baseline function. Despite attempts to provide similar interventions in all HIT participants, this latter group also received the least amount of stepping practice. Reduced stepping activity in HIT3 may be due both to initial baseline function or other factors not captured here (e.g., exercise tolerance and willingness to participate). While gains in HIT3 approached thresholds for small minimally important clinical differences (0.05 m/s for speed, 20 m for 6MWT)31, the changes as compared to those observed in HIT1/HIT2 emphasize the limitations of performing HIT in selected individuals.
The present findings contrast with studies emphasizing the primary contributions of demographics and baseline function to resultant neurologic or functional recovery and little association with therapy activities9. Differences between these competing hypotheses may be due to two factors. First, few studies measure the amounts and intensities of interventions performed, and their assessment may be important to delineate the contributions of physical therapy strategies29, 30. In addition, the primary measures to evaluate recovery post-stroke vary between studies. Specifically, many researchers focus on the assessment of the Fugl-Meyer or other paretic limb impairments9 as a measure of recovery. While valuable, these measures do not directly assess activity limitations. Conversely, clinical locomotor outcomes are used prominently in studies evaluating the efficacy of different physical interventions given their relation with health, participation and mortality4, 32. Both types of measures are important for determining recovery post-stroke, although estimate different constructs that require differentiation by the neuroscience and rehabilitation community.
Limitations of the current study include the relatively small sample size, although the pooled data represent the largest cohort of participants post-stroke with accurate stepping data during physical therapy interventions. While a potential strength of the current analyses is the use of similar inclusion criteria and outcomes across studies, the present sample excluded patients early post-stroke (< 1 month) during which larger gains are typically observed and the benefits of rehabilitation may be greater12. Similar types of analyses are possible on those populations with availability of data detailing demographics, baseline function, and therapy activities.
Another primary limitation is the inability to provide precise lesion locations or estimates of the integrity of descending pathways using imaging or electrophysiological measures (e.g., transcranial stimulation). The importance of the magnitude and location of lesions is well-recognized33-35, and detailed anatomical or electrophysiological measures of corticospinal integrity may serve as biomarkers for predictive algorithm of motor recovery post-stroke. Interestingly, however, recent studies of the lower limb recovery suggest evaluation of corticospinal integrity through selected imaging analyses or with transcranial stimulation may be of limited utility of to predict or estimate motor function9, 36. Continuing work related to this area of research should provide valuable insight into brain-behavior relations for lower limb recovery and locomotor function post-stroke.
In summary, correlation, regression and latent profile analyses suggest that participation in HIT providing substantial amounts of stepping practice at higher cardiovascular intensities were primary determinants of changes in locomotor function in ambulatory individuals > 1-month post-stroke. Secondary analysis of only those participants who performed HIT reinforce the important contributions of age and baseline function. Given these findings, the possibility of more widespread clinical implementation of HIT in patients > 1-month post-stroke may be warranted with appropriate safety considerations37 and with explicit understanding that specific patient populations may demonstrate smaller gains with such training. Further research is necessary to evaluate the comparative efficacy of this strategy to other treatment protocols < 1-month stroke.
Acknowledgements/Sources of Funding:
Funding was provided by NIDILRR-90RT5027, NIH-NINDS-NS079751, DOD-W81XWH-18–1-0796, and ISCBIRF-4785314.
Abbreviations
- 6MWT
6-min walk test
- FV
fastest velocity
- HIT
high-intensity training
- HR
heart rate
- SSV
self-selected walking velocity
- TM
treadmill
- FGA
Functional Gait Assessment
Footnotes
Disclosures/Conflict of Interest: Dr. Hornby is co-owner and Dr. Henderson is partially employed by the Institute for Knowledge Translation, which provides continuing education for rehabilitation professionals.
References
- 1.Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS et al. Executive summary: Heart disease and stroke statistics−-2012 update: A report from the american heart association. Circulation. 2012;125:188–197 [DOI] [PubMed] [Google Scholar]
- 2.Roger VL, O’Donnell CJ. Population health, outcomes research, and prevention: Example of the american heart association 2020 goals. Circulation. Cardiovascular quality and outcomes. 2012;5:6–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rajsic S, Gothe H, Borba HH, Sroczynski G, Vujicic J, Toell T, Siebert U. Economic burden of stroke: A systematic review on post-stroke care. Eur J Health Econ. 2019;20:107–134 [DOI] [PubMed] [Google Scholar]
- 4.Studenski S, Perera S, Patel K, Rosano C, Faulkner K, Inzitari M, Brach J, Chandler J, Cawthon P, Connor EB, et al. Gait speed and survival in older adults. Jama. 2011;305:50–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O’Sullivan SB, Schmitz TJ, and Fulk G Physical rehabilitation. Philadelphia, PA: F.A. Davis; 2013. [Google Scholar]
- 6.Umphred DA, Lazaro RT, Roller M and Burton G Neurological rehabilitation. Maryland Heights, MO: Mosby; 2012. [Google Scholar]
- 7.Hornby TG, Reisman DS, Ward IG, Scheets PL, Miller A, Haddad D, Fox EJ, Fritz NE, Hawkins K, Henderson CE, et al. Clinical practice guideline to improve locomotor function following chronic stroke, incomplete spinal cord injury, and brain injury. Journal of neurologic physical therapy : JNPT. 2020;44:49–100 [DOI] [PubMed] [Google Scholar]
- 8.Smith MC, Barber PA, Stinear CM. The twist algorithm predicts time to walking independently after stroke. Neurorehabilitation and neural repair. 2017;31:955–964 [DOI] [PubMed] [Google Scholar]
- 9.Smith MC, Byblow WD, Barber PA, Stinear CM. Proportional recovery from lower limb motor impairment after stroke. Stroke; a journal of cerebral circulation. 2017;48:1400–1403 [DOI] [PubMed] [Google Scholar]
- 10.Stinear CM, Byblow WD, Ackerley SJ, Barber PA, Smith MC. Predicting recovery potential for individual stroke patients increases rehabilitation efficiency. Stroke; a journal of cerebral circulation. 2017;48:1011–1019 [DOI] [PubMed] [Google Scholar]
- 11.Dobkin BH, Nadeau SE, Behrman AL, Wu SS, Rose DK, Bowden M, Studenski S, Lu X, Duncan PW. Prediction of responders for outcome measures of locomotor experience applied post stroke trial. Journal of rehabilitation research and development. 2014;51:39–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duncan PW, Lai SM, Keighley J. Defining post-stroke recovery: Implications for design and interpretation of drug trials. Neuropharmacology. 2000;39:835–841 [DOI] [PubMed] [Google Scholar]
- 13.Hornby TG, Henderson CE, Plawecki A, Lucas E, Lotter J, Holthus M, Brazg G, Fahey M, Woodward J, Ardestani M et al. Contributions of stepping intensity and variability to mobility in individuals poststroke. Stroke; a journal of cerebral circulation. 2019;50:2492–2499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hornby TG, Holleran CL, Hennessy PW, Leddy AL, Connolly M, Camardo J, Woodward J, Mahtani G, Lovell L, Roth EJ. Variable intensive early walking poststroke (views): A randomized controlled trial. Neurorehabilitation and neural repair. 2016;30:440–450 [DOI] [PubMed] [Google Scholar]
- 15.Moore JL, Roth EJ, Killian C, Hornby TG. Locomotor training improves daily stepping activity and gait efficiency in individuals poststroke who have reached a “plateau” in recovery. Stroke; a journal of cerebral circulation. 2010;41:129–135 [DOI] [PubMed] [Google Scholar]
- 16.Holleran CL, Rodriguez KS, Echauz A, Leech KA, Hornby TG. Potential contributions of training intensity on locomotor performance in individuals with chronic stroke. Journal of neurologic physical therapy : JNPT. 2015;39:95–102 [DOI] [PubMed] [Google Scholar]
- 17.Holleran CL, Straube DD, Kinnaird CR, Leddy AL, Hornby TG. Feasibility and potential efficacy of high-intensity stepping training in variable contexts in subacute and chronic stroke. Neurorehabilitation and neural repair. 2014;28:643–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McLachlan G PD. Finite mixture models. . New York, NY: NY: John Wiley & Sons;; 2000. [Google Scholar]
- 19.Straube DD, Holleran CL, Kinnaird CR, Leddy AL, Hennessy PW, Hornby TG. Effects of dynamic stepping training on nonlocomotor tasks in individuals poststroke. Physical therapy. 2014;94:921–933 [DOI] [PubMed] [Google Scholar]
- 20.Tanaka H, Monahan KD, Seals DR. Age-predicted maximal heart rate revisited. Journal of the American College of Cardiology. 2001;37:153–156 [DOI] [PubMed] [Google Scholar]
- 21.Lang CE, Macdonald JR, Reisman DS, Boyd L, Jacobson Kimberley T, Schindler-Ivens SM, Hornby TG, Ross SA, Scheets PL. Observation of amounts of movement practice provided during stroke rehabilitation. Archives of physical medicine and rehabilitation. 2009;90:1692–1698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.MacKay-Lyons MJ, Makrides L. Cardiovascular stress during a contemporary stroke rehabilitation program: Is the intensity adequate to induce a training effect? Archives of physical medicine and rehabilitation. 2002;83:1378–1383 [DOI] [PubMed] [Google Scholar]
- 23.Leys C KO, Dominicy Y, Ley C. .Detecting multivariate outliers: Use a robust variant of the mahalanobis distance. Journal of Experimental Social Psychology. 2018;74:150–156 [Google Scholar]
- 24.Hair JF AR, Tatham RL, Black WC. Multivariate data analysis. . . Upper Saddle River, NJ: Prentice-Hall;; 1998. [Google Scholar]
- 25.G. S. Estimating the dimension of a model. The Annals of Statistics. 1978::461–464 [Google Scholar]
- 26.SL. S. Application of model-selection criteria to some problems in multivariate analysis. Psychometrika. 1987;52:333–343. [Google Scholar]
- 27.Nylund KL AT, Muthén BO. Deciding on the number of classes in latent class analysis and growth mixture modeling: A monte carlo simulation study. . . Structural Equation Modeling: A Multidisciplinary Journal. 2007;4:535–569. [Google Scholar]
- 28.Rosenberg JM BP, Anderson DJ, Van Lissa CJ, Schmidt JA. An r package to easily carry out latent profile analysis (lpa) using open-source or commercial software. . Journal of Open Source Software. . 2018;3 978 [Google Scholar]
- 29.Hornby TG, Moore JL, Lovell L, Roth EJ. Influence of skill and exercise training parameters on locomotor recovery during stroke rehabilitation. Current opinion in neurology. 2016;29:677–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hornby TG, Straube DS, Kinnaird CR, Holleran CL, Echauz AJ, Rodriguez KS, et al. Importance of specificity, amount, and intensity of locomotor training to improve ambulatory function in patients poststroke. Topics in stroke rehabilitation. 2011;18:293–307 [DOI] [PubMed] [Google Scholar]
- 31.Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults. Journal of the American Geriatrics Society. 2006;54:743–749 [DOI] [PubMed] [Google Scholar]
- 32.Middleton A, Fritz SL, Lusardi M. Walking speed: The functional vital sign. Journal of aging and physical activity. 2015;23:314–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boyd LA, Hayward KS, Ward NS, Stinear CM, Rosso C, Fisher RJ, Carter AR, Leff AP, Copland DA, Carey LM, et al. Biomarkers of stroke recovery: Consensus-based core recommendations from the stroke recovery and rehabilitation roundtable. Neurorehabilitation and neural repair. 2017;31:864–876 [DOI] [PubMed] [Google Scholar]
- 34.Stinear CM, Smith MC, Byblow WD. Prediction tools for stroke rehabilitation. Stroke; a journal of cerebral circulation. 2019;50:3314–3322 [DOI] [PubMed] [Google Scholar]
- 35.Milot MH, Cramer SC. Biomarkers of recovery after stroke. Current opinion in neurology. 2008;21:654–659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sivaramakrishnan A, Madhavan S. Absence of a transcranial magnetic stimulation-induced lower limb corticomotor response does not affect walking speed in chronic stroke survivors. Stroke; a journal of cerebral circulation. 2018;49:2004–2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pescatello LS. American college of sports medicine guidelines for exercise testing and prescription. 2014:456 [Google Scholar]


