Abstract
Objective.
t To evaluate the accuracy of detecting 16 year old male (N=465) and female (N=162) youths who subsequently manifest opioid use disorder (OUD) at 25 years of age. We hypothesized that the combined measures of two components of etiology, heritable risk and substance use, accurately detect youths who develop OUD.
Study design.
Heritable risk was measured by the transmissible liability index (TLI). Severity of the prodrome presaging OUD was quantified by the revised Drug Use Screening Inventory (DUSI-R) containing the consumption frequency index (CFI) documenting substance use events during the past month and the overall problem density (OPD) score indicating co-occurring biopsychosocial problems. Diagnosis of OUD was formulated by a clinical committee based on results of the Structured Clinical Interview for DSM-IV in conjunction with medical and social history records.
Results.
Bivariate analysis shows that the TLI, CFI, and OPD scores at 16 years of age predict OUD at 25 years. Multivariate modeling indicates that the TLI combined with the CFI predict OUD with 86% accuracy (sensitivity = 87%; specificity = 62%). The TLI and CFI at 16 years of age mediate the association between parental substance use disorder and OUD in offspring at 25 years of age, indicating that these measures respectively evaluate risk and prodrome.
Conclusion.
These results demonstrate the feasibility of identifying youths requiring intervention to prevent OUD.
Keywords: opioids, forecasting addiction, phenotyping, risk screening, drug dependence
Over 11,000,000 Americans misused prescription opioids in 2017 (1). Prescription opioid use is especially concerning for adolescents considering that high school seniors using opioids prescribed by a physician have 33% increased risk of misuse five years later (2). The observation that each year opioid use onset delayed after age 13 lowers risk of misuse by 2% (3) underscore the importance of prevention directed at adolescents, especially considering that self-directed (ie, non-prescribed) consumption of opioids ranks second in prevalence after cannabis within the spectrum of illegal drugs (4,5). Moreover, using Schedule I opioids, particularly heroin, is frequently preceded by consuming prescription opioids (6,7).
The present longitudinal investigation examined the accuracy of forecasting opioid use disorder (OUD) manifest at 25 years of age based on measurement of two main etiological components, heritable liability (8) and substance use, at 16 years of age. Significantly, 16 years of age is the most frequent time of onset of opioid use (9) and 25 years of age is the midpoint within the period of peak OUD prevalence in the general population (10). Considering that 30% of the population receiving treatment for hazardous opioid use are younger than 24 years of age (11) and remission rate is half other addictions (12), demonstrating accurate prediction of OUD advances the opportunity to efficiently detect high risk youths so that prevention interventions can be expeditiously implemented.
METHODS
Participants were recruited by the Center for Education and Drug Abuse Research (CEDAR), a NIDA-funded longitudinal study of substance use disorder etiology (13). Men with lifetime substance use disorder consequent to using an illegal drug (N=334) or with no adult-onset psychiatric disorder (N=340), who had a 10–12 year old biological son (N=482) or daughter (N=191), were identified using random digit telephone calls, advertisement and public service announcements. In addition, approximately 25% of the men with substance use disorder were identified after discharge from addiction treatment facilities. Because prodrome severity indicated by substance use frequency cannot be meaningfully measured in 10–12 year old youths owing to low incidence of consumption onset, the evaluation was deferred until the sample attained 16 years of age. Youths were disqualified from participating in the study if they had a chronic medical disorder requiring physician monitoring, physical disability, history of neurological injury resulting in hospitalization, or an IQ below 80. Socioeconomic status of the boys (M=41.0, SD = 13.3) and girls (M=41.9, SD = 14.9) is middle class based on the Hollingshead four-factor index (14). IQ, evaluated by the WISC-III (15), is in the average range in the boys (M=107.0, SD=15.8) and girls (M=104.2, SD=16.2). African-American boys and girls respectively constituted 23% and 33% of the sample.
Attrition between baseline (age 16) and outcome (age 25) assessments was 33% in boys and 17% in girls. The most frequent reasons for attrition were relocation (including military service and incarceration) and inability to contact the participant despite deploying a comprehensive tracking protocol. Notably, the attrited and retained segments of the male sample do not differ on the TLI, CFI and OPD predictor variables. The CFI score was, however, higher among girls who attrited. Rate of substance use disorder in parents, as shown in Table 1, is not different between the attrited and retained segments of the sample. Overall, these comparisons indicate that males and females who participated in the outcome assessment are representative of the baseline sample as indicated by scores on the OUD predictors, IQ, socioeconomic status, rate of parental substance use disorder and ethnicity. In the retained segment of the sample, 6.4%, 25.1% and 22.5% qualified for opioid, alcohol or cannabis disorder.
Table 1.
Characteristics of the sample at 16 years of age who were retained or attrited at 25 years of age
| Male | Female | |||||
|---|---|---|---|---|---|---|
| Retained (N=305) |
Attrited (N=151) |
Test Statistics | Retained (N=135) |
Attrited (N=27) |
Test Statistics | |
| SUD in father (probands) | 49% | 56% | χ2=.06,p=.81 | 41% | 52% | χ2=.35,p=55 |
| SUD in mother | 20% | 26% | χ2=.07,p=79 | 20% | 39% | χ2=2.66,p=.10 |
| IQ (Mean, SD) | 108.7(15.3) | 106.3 (16.5) | t=2.61,p=.01 | 104.8(17.2) | 100.7 (14.4) | t=2.43,p=.02 |
| Family SES (Mean, SD) | 41.4(13.2) | 40.8(13.6) | t=1.50,p=.13 | 42.7 (14.7) | 41.0(16.1) | t=1.60,p=.14 |
| Ethnicity | ||||||
| • Euro-American | 77% | 78% | χ2=.98,p=32 | 67% | 59% | χ2=.08,p=78 |
| • African American | 23% | 22% | 33% | 41% | ||
| Transmissible Liability | .05(1.01) | .12(1.06) | t=−.57, p=.56 | −.23 (.97) | .04 (.87) | t=−1.04, p=.29 |
| Index (TLI) (z score) | ||||||
| Consumption Frequency | 1.86(2.34) | 2.16(2.43) | t=−1.52,p=.13 | 1.52(1.95) | 2.50 (2.34) | t=−2.79, p=.006 |
| Index (CFI)1 | ||||||
| Overall Problem Density | 18.52(12.79) | 16.28 (12.34) | t=1.58, p=.ll | 20.38(12.85) | 21.99(13.37) | t=−.48, p=.63 |
| Score (OPD) (%)2 | ||||||
Consumption events in past month
Score ranges from 0–10
Measures
Structured Clinical Interview for DSM-III-R (SCID) (16).
Diagnostic formulation of the parents and their children was conducted using the best estimate procedure (17). This procedure takes into account the respondent’s answers using an elaborated version of the SCID to conform with DSM-IV criteria in conjunction with pertinent information contained in medical and social services records. Diagnoses were formulated by a committee chaired by a psychiatrist certified in addiction psychiatry. The other members included another psychiatrist or a psychologist and Master-level clinical associates who conducted the SCID. The DSM-IV taxonomy was employed for diagnosis of the parents and their children because this study began prior to advent of the DSM-5. Notably, diagnosis based on DSM-IV criteria has excellent correspondence with DSM-5 (18).
Parental Substance Use Disorder.
Number of parents (0, 1, 2) with substance use disorder was recorded to measure magnitude of familial loading for this disorder in their children. This indicator of intergenerational risk has been shown in prior research to be heuristic for elucidating the risk for and developmental patterning to substance use disorder (19,20). Number of affected parents was recorded in this study to confirm that transmissible liability index (TLI) score mediates the association between children’s familial loading for substance use disorder and their risk for OUD. Among the boys, 48.3%, 34.6%, and 17.1% had 0, 1, or 2 affected parents. The distribution was 51.8%, 29.6%, and 18.6% in the sample of girls.
Transmissible Liability Index (TLI).
Previous reports describe the theory (21) and methods (8,22) guiding development and validation of the TLI. Briefly, transmissible liability is the component of phenotypic variance that is correlated between generations via genetic and/or environmental influences. Because liability to substance use disorder is transmissible (as it has been shown to be significantly heritable), psychological and health behavior characteristics that discriminate children of affected and unaffected parents are indicators of children’s own liability (8), making it quantifiable on a continuous scale using item response theory methods (22). Importantly, the genetic component of variance fully accounts for the correlation between the TLI score in 10–12-year-old children and their subsequent substance use disorder diagnosis (23, 24). Moreover, the TLI predicts substance use disorder better than parental diagnosis of this disorder (25). The TLI version validated for 16-year-old youth with internal reliability exceeding 0.90 (26) was self-administered. This age-specific TLI contains 65 items that for the most part assess anti-social activities (e.g., “In the past 6 months, have you stolen or attempted to steal things worth between $5 and $50”) and self-management in daily routines (e.g. “I plan and organize my work in detail”).
Drug Use Screening Inventory (revised) (DUSI-R) (27,28).
The self-administered DUSI-R measures severity of problems pertaining to: 1) substance use, 2) mental health, 3) physical health, 4) behavior self-regulation, 5) school adjustment, 6) family functioning, 7) peer relationships, 8) social skills, 9) work adjustment and 10) leisure/recreation activities. The overall problem density (OPD) score is computed by dividing the number of items in which a problem is endorsed by the total number of items encompassing the ten scales (N=149) and then multiplying the resultant quotient by 100. The overall problem density (OPD) score thus has a range of 0–100%. The mean OPD scores in the samples of boys and girls are 18.5% and 20.4%.
Convincing evidence demonstrates that liabilities to all substance use disorder categories share to large extent genetic and phenotypic variance (22,29). Accordingly, opioid use is often concurrent with consumption of other addictive substances. Measuring prodrome severity therefore requires quantifying overall involvement with addictive substances. In this study, the number of alcohol and drug use events during the past 30-days was recorded by the DUSI-R’s consumption frequency index (CFI). Consumption of twenty addictive substances was recorded in five categories to document past 30-day exposure: 0 (0 times), 1 (1–2 times), 2 (3–9 times), 3 (10–20 times) and 4 (more than 20 times). The category designations (0, 1, 2, 3, and 4) are summed across all substances to obtain the CFI. Expectedly, the three most frequently used substances were alcohol (29.6 %), tobacco (23.6 %), and cannabis (21.7 %). The mean CFI score is 1.86 in the sample of boys and 1.52 in the girls. The DUSI-R’s Lie scale, consisting of 10 items, assesses propensity to under-report problems. None of the participants were excluded from study based on the Lie scale score.
At baseline (age 16), only 6% of the sample reported lifetime opioid use, whereas 24% of the sample used opioids at least once by age 25. By age 25 (outcome), OUD was present in 6.4% of the sample (6.2% males; 6.7% females); however, information was not available regarding whether the first opioid used was a medicine prescribed by a physician, was self-directed, or involved a Schedule I substance.
Procedure
After orientation to the laboratory the parents and their children respectively signed the informed consent and assent forms approved by the University of Pittsburgh IRB. Privacy was additionally assured by a Certificate of Confidentiality issued by the National Institute on Drug Abuse to CEDAR. Next, the participants underwent a breath alcohol and urine drug screen to preclude possible biased responses consequent to substance-induced altered physiological state. The research protocol was administered individually in fixed order in a private sound-attenuated room. Upon completing the assessments, the data were reviewed by a clinical associate to ensure that all the questions were answered. Lastly, the participants were debriefed and compensated for their time and expenses.
Statistical Analyses
Analyses were conducted at the outset to confirm that the TLI and CFI/OPD are respectively valid measures of transmissible risk and OUD prodrome. Polyserial correlation evaluated the relationship between the participant’s familial loading of substance use disorder (i.e., number of affected parents) and TLI score. Point-biserial correlation estimated the association between TLI, CFI and OPD scores and OUD.
Next, multi-sample path analysis was conducted to model the relationships between number of affected parents and their children’s TLI, CFI, and OPD scores at 16 years of age, and OUD at 25 years of age. Three models were compared. Model 1 assumed that all path (standardized partial regression) coefficients, means, and variances are equal between boys and girls. Model 2 assumed that only the path coefficients are equal. Model 3 assumed that all parameters are free. Path coefficients were estimated using Mplus (30) with weighted least squares with mean and variance correction, designed for categorical and ordered data. Four indexes were computed to inform selection of the best model: 1) χ2 goodness-of-fit index, 2) root mean square error of approximation (RMSEA), 3) comparative fit index, and 4) Tucker-Lewis index. Nonsignificant χ2, RMSEA below 0.05, and comparative fit and Tucker-Lewis indexes close to 1 indicate good fit. Fit comparisons between the nested models (differing in that the parameters in the more general model are equated or absent from another model) are conducted using the difference of χ2 values between the models. This statistic has an asymptotic χ2 distribution with the degrees of freedoms equal to the difference between the degrees of freedom of the models. Mediation analyses were conducted employing the method described by Sobel (31) to ascertain whether TLI accounts for the association between number of affected parents and risk for OUD in their children. Accuracy of the TLI, CFI and OPD scores for detecting youths who subsequently develop OUD was evaluated using multiple logistic regression analysis followed by receiver operating characteristic (ROC) analysis documenting sensitivity (true positive rate), specificity (true negative rate) and overall accuracy. K-fold cross-validation, a resampling procedure used in machine learning, was employed to assess the predictive performance of the logistic model using area under the curve (AUC) for new cases to predict OUD. This predictive model thus can be generalized to new samples. It is a preferred method when there are not large enough observations in a sample. When AUC is estimated from the whole sample, it is usually overestimated because of overfitting. The k-fold cross-validation, by randomly dividing the data into k subsets (folds) to compute AUC for each fold, provides more accurate estimates, which in turn yield better predictive models. Then AUCs are averaged and a standard error for the average AUC is generated by bootstrapping. In this paper, the data were divided into five folds. Whereas this number is acceptable (32), a greater number of folds could not be specified because of the low prevalence of OUD in the sample.
RESULTS
Bivariate Correlations
As can be seen in Table 2, number of parents with substance use disorder is related to TLI score in 16-year-old boys and girls. The TLI score in turn correlates with OUD outcome in both sexes. The TLI score also correlates with CFI score in boys and girls, which, in turn, is related to OUD. In addition, the TLI and OPD scores are correlated. The OPD is also related to OUD in both sexes. As expected, the CFI and OPD scores are correlated. In sum, the TLI score covaries with prodrome severity, which, in turn, correlates with OUD diagnosis.
Table 2.
Bivariate correlations among number of substance use disorder (SUD) parents and child’s transmissible liability index (TLI), overall problem density score (OPD), consumption frequency index (CFI) and opioid use disorder (OUD)
| TLI | OPD | CFI | OUD | ||
|---|---|---|---|---|---|
| r (p-value) | r (p-value) | r (p-value) | r (p-value) | ||
| Boys | SUD Parents | .32 (<.001) | .27 (<.001) | .25 (<.001) | .10 (.06) |
| TLI | .74 (<.001) | .46 (<.001) | .32 (<.001) | ||
| OPD | .53 (<.001) | .36 (p<.001) | |||
| CFI | .34 (p<.001) | ||||
| Girls | SUD Parents | .24 (.004) | .36(<.001) | .24 (.002) | .08 (.32) |
| TLI | .70 (<.001) | .24 (.008) | .20 (.03) | ||
| OPD | .40 (<.001) | .21 (.01) | |||
| CFI | .25 (.006) |
Multivariate model
Table 3 shows that the three models have good fit; however, Models 1 (no sex differences) and 3 (all parameters are free, i.e., allowed to differ) are somewhat superior to Model 2 (only path coefficients are equal). Models 1 and 3 do not differ in their fit (the difference χ2=17.12, df=17, p=.42). Whereas both models are statistically acceptable, we adopted Model 1 because it allows including females in the analysis that would not be otherwise possible with Model 3 due to the relatively small subset who developed OUD.
Table 3.
Fit statistics of path models
| Model | χ2 | df | P | RMSEA | Comparative fit index | Tucker-Lewis Index |
|---|---|---|---|---|---|---|
| 1 | 17.58 | 19 | .55 | <.001 | .99 | .99 |
| 2 | 14.75 | 11 | .19 | .032 | .99 | .98 |
| 3 | 0.46 | 2 | .79 | <001 | .99 | .99 |
Note: Model 1 assumed that all path coefficients, means and variances are equal between boys and girls. Model 2 assumed that only the path coefficients are equal. Model 3 assumed that all parameters are free.
As indicated by the path coefficients (Figure 1), number of parents with substance use disorder predicts the TLI score (β=0.26, p < .001), which, in turn, is correlated with OPD (r = 0.53, p<.001) and CFI (r= 0.32, P < .001) scores as well as predicts OUD nine years later (β= 0.39, p<.001). As expected, OPD and CFI scores, the two facets of the OUD prodrome, are correlated (r= 0.45, p<.001); however, only the CFI predicts OUD (β= 0.17, p<.001) when TLI score is taken into account. The results of this analysis are summarized in Table IV.
Figure 1.
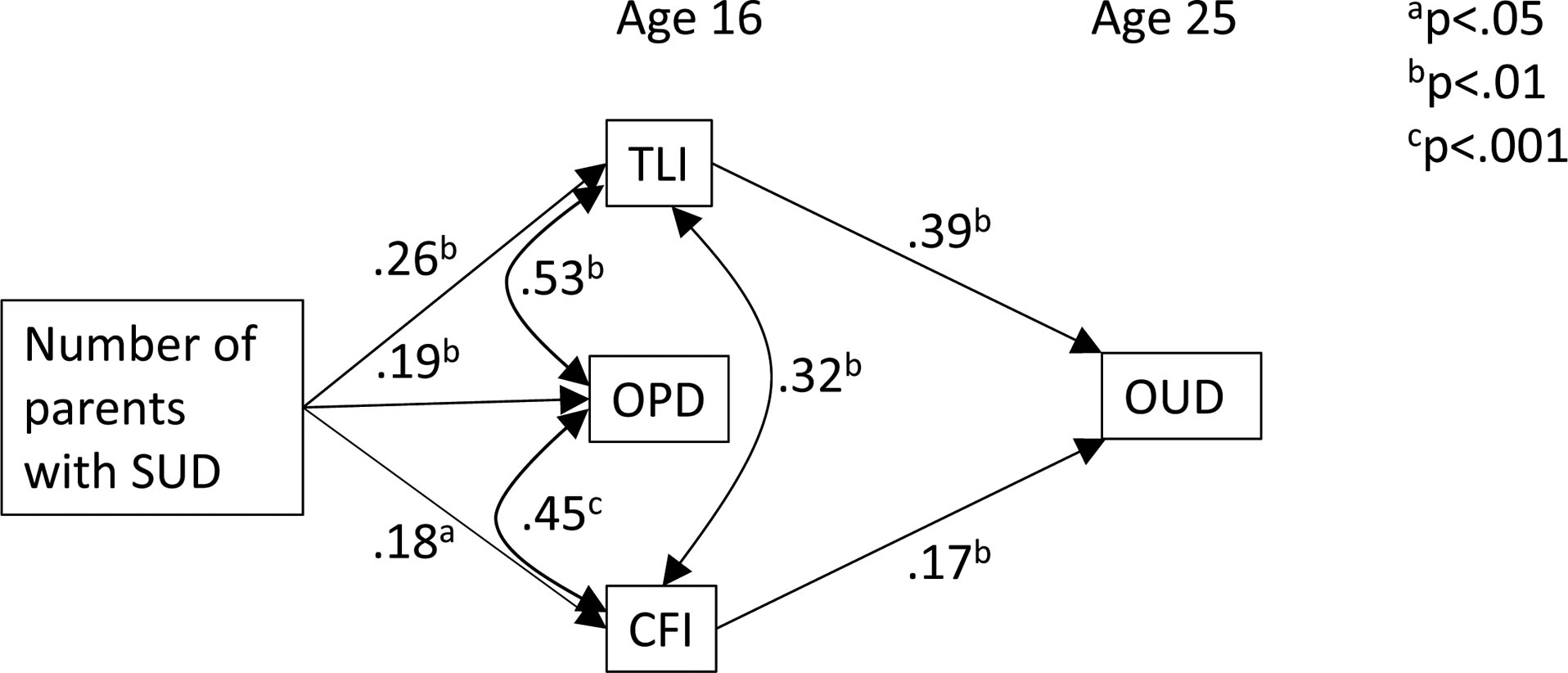
Path model depicting the relationship among parental substance use disorder (SUD), child’s transmissible liability index (TLI), consumption frequency index (CFI), and overall problem density (OPD) score at 16 years of age on current opioid use disorder (OUD) diagnosis at 25 years of age).
Table 4.
Logistic regression analysis for predicting opioid use disorder at age 25 by TLI and CFI
| B | SE | OR | p-values | 95% CI | |
|---|---|---|---|---|---|
| TLI | .39 | .06 | 1.47 | <.001 | 1.30, 1.66 |
| CFI | .17 | .05 | 1.18 | <.001 | 1.07, 1.31 |
TLI mediates the association between number of parents with substance use disorder and OUD outcome in their children (β = 0.10, z=4.62, p<.001). In effect, familial loading for substance use disorder covaries with TLI score quantifying transmissible liability. In addition, CFI mediates the relationship between number of substance use disorder parents and their children’s OUD diagnosis (β = 0.03, z=2.83, p=.005). This finding demonstrates that the CFI is a valid measure of the OUD prodrome and related to magnitude of intergenerational risk.
To evaluate the utility of the predictors for forecasting OUD while taking into account their correlations, we applied the parameters obtained in path analysis to a logistic regression model. The respective odds ratios for the TLI and CFI are respectively 1.47 (95% CI: 1.30–1.66) and 1.83 (95% CI: 1.07–1.31). As shown in Table 5 (available at www.jpeds.com), the ROC analysis based on the total sample demonstrates prediction sensitivity and specificity of 87% and 62% with overall accuracy of 86% (95% CI: 73%, 99%) using a cut-off score of 5.5%. The 5-fold cross-validation results reveal a mean overall prediction accuracy of 89% (SD=.15) with bootstrap bias-corrected 95% CI (45%, 93%) using a cut-off score of 6%. Sensitivity and specificity are respectively 75% and 89%. Notably, the AUC difference between the total sample and 5-fold mean is only 3%.
Table 5.
Results of ROC analyses based on total sample and 5-fold cross validation
| AUC | Sensitivity | Specificity | Cut-off score | |
|---|---|---|---|---|
| Total sample | 86% | 87% | 62% | 5.5% |
| Average across 5 folds | 89% | 75% | 89% | 6% |
Post-hoc analyses were additionally conducted to evaluate the accuracy of the predictor variables for detecting youths who developed alcohol and cannabis use disorder. These latter disorders were present in 25.1% and 22.5% of the sample at 25 years of age. The analyses were limited to these two outcomes owing to insufficient number of other drug disorders. With respect to alcohol use disorder, overall prediction accuracy was 68% (70% sensitivity and 55% specificity). Overall prediction accuracy for cannabis use disorder was 75% (76% sensitivity and 58% specificity). Thus, consistent with general liability to addiction, the variables forecasted three categories of substance use disorder, although most accurately for OUD.
DISCUSSION
Cost-efficient prevention of OUD is contingent on identifying the high-risk segment in the general population. The polygenic risk score, aggregating information on genetic polymorphisms identified in genome-wide association studies (33,34), is one approach. However, because heritability of OUD liability is not high (less than 0.25) (29) owing to large functional distance between gene expression and the liability phenotype, it is not surprising that the polygenic risk score is insufficiently accurate for use in clinical practice. An alternative strategy adopted herein focuses on the liability phenotype. Within this measurement framework, the 5-fold mean results indicate that transmissible (intergenerational) risk and substance use in 16-year-old youths conjointly predict OUD at 25 years of age with 89% accuracy.
Results obtained in other studies also demonstrate that it is feasible to predict OUD (35,36). The Opioid Risk Tool (37) shows very high accuracy; however, like most screening instruments, its use is circumscribed to patients taking opioids prescribed by a physician to manage pain. A more serious limitation is that symptoms of dependence, included in the set of predictor items, may not yet be present in high risk youths. The high sensitivity and specificity (>.80) reported for this risk assessment tool may thus partly be due to the fact that the person is close to or beyond diagnostic threshold for OUD, especially considering that only two symptoms are required in the DSM-5 taxonomy for diagnosis. The present study extends this line of research by showing high predictive accuracy a decade after evaluation of liability and prodrome using brief measures that can be self-administered using any device connected to the internet. Risk assessment can thus be expeditiously conducted prior to prescribing an opioid and subsequently at the time of each refill.
In addition, it is noteworthy that the TLI includes indicators of social deviancy. This is important considering that opioid users often violate the law by consuming medicinal opioids without physician prescription or using Schedule I formulations. Notably, severity of externalizing disorder in childhood covaries with magnitude of risk for developing opioid dependence in adulthood (38). Hence, the TLI may also be useful for screening youths in the juvenile justice system to measure risk for OUD and other addictions. In sum, this study extends research findings into practical application by showing that the indicators of risk for substance use disorder can be assessed in childhood, and by adolescence when substance use typically begins, the likelihood of advancing to diagnosis can be accurately determined (39,40).
Recent survey data indicate that approximately 22.3 million Americans are in recovery from substance misuse, within which 5% report that opioids were the main problem (41). The same survey found that 51%, 11%, and 10% of individuals in recovery reported that alcohol, cannabis, or cocaine was the main problem. The ancillary results obtained in this study further suggest that it is feasible to identify youths at high risk for these disorders, although predictive accuracy for alcohol and cannabis disorders may require additional measurement refinement. Nevertheless, within pediatric practice, consisting largely of health maintenance and well check-up visits, identifying high risk youths (42) is consistent with the recommendation of the American Academy of Pediatrics (43). In effect, in 15–20 minutes it is feasible to quantify risk for OUD and concomitantly current severity of substance use and associated health, psychological, and social adjustment problems.
Several caveats and limitations of this study are noted. Whereas the results lend confidence to the feasibility of routine risk screening, it should be noted that although sensitivity is high (87%), specificity is somewhat low for the total sample. A k-fold cross-validation provided somewhat different results: sensitivity was 75% and specificity was 89% with similar cut-off scores. In effect, false positive rate is 38% for the total sample, whereas it is 11% for the 5-fold cross-validation. In the light of differences we observed in sensitivity and specificity between the total and cross-validation samples, the generalizability of the predictive model for new samples should be interpreted with care. In particular, caution must be exercised before denying intervention based solely on the results of this assessment. Even though a false-positive conclusion regarding OUD prediction is less costly than non-detection of a true-positive case, further research focusing on improving measurement precision is required, particularly directed at youth in the low-risk area of the liability distribution (44). In addition, the sample is relatively small (N=627), which may have inflated parameter estimates and decreased statistical power. Also, despite the importance of sex differences in substance use disorder etiology and natural history (45–47), the size of the female sample (N=135) did not allow for sex-specific multivariate analyses, although the equally good fit of the model with the absence of sex differences and the free-parameter model may be due to the lack of power. This limitation notwithstanding, the male and female participants are indeed very similar with respect to the predictor variables, IQ, socioeconomic status and ethnicity. Nevertheless, research remains to be conducted to determine whether the accuracy of forecasting OUD using the TLI, CFI, and OPD is sex-specific. Furthermore, it should be noted that the participants were identified through proband fathers who either qualified for substance use disorder or had no disorder. The advantage of the high-risk paradigm is that it enables expeditiously accruing a sample of youths who develop substance use disorder. Non-random recruitment may, however, have produced results that are not generalizable to the population. Even though this possibility cannot be fully discounted, it is noteworthy that many studies conducted on this cohort conform to results obtained by other investigators. Lastly, OUD liability was measured by the TLI after substance use onset. Although this may have influenced the propensity to endorse certain characteristics, deferring risk assessment until mid-adolescence was necessary in order to include prodrome severity in the prediction model.
In summary, the index of transmissible liability to substance use disorder combined with past 30-day frequency of overall substance use detects 16-year old youths who qualify for OUD at 25 years of age with 86% accuracy. Considering that this assessment can be self-administered on the Web platform, currently under development, this protocol may be useful for large scale or routine screening to detect high-risk youths requiring prevention intervention.
Acknowledgments
Supported by the National Institute of Drug Abuse (P50 DA005605), Center for Disease Control (R01 CE002996), and NIDA (UG1 DA049444). The authors declare no conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.SAMHSA, 2018. Key substance use and mental health indicators in the United States: Results from the 2017 National Survey on Drug Use and Health (HHS Publication NO. SMA 18–5068, NSDUH Series H-53). Substance Abuse and Mental Health Services Administration, Center for Behavioral Health Statistics and Quality Rockville, MD. [Google Scholar]
- 2.Miech R, Johnston L, O’Malley PM, Keyes KM, Heard K. Prescription opioids in adolescence and future opioid misuse. Pediatrics 2015; 136: 1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McCabe SE, Veliz PT, Boyd CJ, Schepis TS, McCable VV, Schulenberg JE. A prospective study of nonmedical use of prescription opioids during adolescence and subsequent substance use disorder symptoms in early midlife. Drug Alc Depend 2007; 194: 377–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Palamar JJ, Kiang MV, Halkitis PN: Development and psychometric evaluation of scales that assess stigma associated with illicit drug use. Substance Use Misuse 2011; 46: 1457–1467 [DOI] [PubMed] [Google Scholar]
- 5.SAMHSA, 2012. Results from the 2011 National Survey on Drug Use and Health: Summary of National Findings. HHS Publication (NSDUH Series H-44, HHHS Publication No. SMA 12–4713). (http://www.samhsa.gov/data/NSDUH/2k11Resutls/NSDUHresults2011.htm).
- 6.Compton WM, Jones CM, Baldwin GT. Relationship between nonmedical prescription-opioid use and heroin use. N Eng J Med 2016; 374: 154–163. [DOI] [PubMed] [Google Scholar]
- 7.Cerdá M, Santaella J, Marshall BDL, Kim JH, Martins SS. Nonmedical prescription opioid use in childhood and early adolescence predicts transitions to heroin use in young adulthood: A national study. J Pediatr 2015; 167: 605–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vanyukov MM, Kirisci L, Tarter RE, Simkevitz HF, Kirillova GP, Maher BS et al. Liability to substance use disorders: 2. A measurement approach. Neurosci Biobehav Reviews 2003; 27: 517–526. [DOI] [PubMed] [Google Scholar]
- 9.Austin E, McCabe SE, Stoddard SA, Ngo QE, Boyd C. Age and cohort patterns of medical and nonmedical use of controlled medication among adolescents. J Addict Med 2015; 9:376–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Degenhardt L, Whiteford H, Hall WD. (2013). The Global Burden of Disease Projects: What have we learned about illicit drug use and dependence and their contribution to the global burden of disease? Drug Alc Rev 2013; 33: 4–12. [DOI] [PubMed] [Google Scholar]
- 11.Jones CM, McCance-Katz EF. Co-occurring substance use and mental disorders among adults with opioid use disorder. Drug Alc Depend 2019; 197: 78–83. [DOI] [PubMed] [Google Scholar]
- 12.Calabria B, Degenhardt L, Briegleb C, Vos T, Hall W, Lynskey M. et al. Systematic review of prospective studies investigating “remission” from amphetamine, cannabis, cocaine or opioid dependence. Addict Behav 2010; 35: 741–749. [DOI] [PubMed] [Google Scholar]
- 13.Tarter RE, Vanyukov MM. Introduction: Theoretical and operational framework for research into the etiology of substance use disorders. J Child Adol Sub Abuse 2001; 10: 1–12. 10.1300/J029v10n04_01. [DOI] [Google Scholar]
- 14.Hollingshead AB. Four-Factor Index of Social Status. Unpublished manuscript, Yale University; 1975. [Google Scholar]
- 15.Wechsler D Wechsler Intelligence Scale for Children manual (3rd ed.). San Antonio, TX: Psychological Corporation, 1991. [Google Scholar]
- 16.Spitzer R, Williams B, Gibbons M, First M. User’s guide for Structured Clinical Interview for DSM-III-R. New York, NY: New York State Psychiatric Institute, 1990. [Google Scholar]
- 17.Leckman JF, Sholomskas D, Thompson WD, Belanger A, Weissman MM. Best estimate of lifetime psychiatric diagnosis: a methodological study. Arch Gen Psychiatry 2981; 39: 879–883. [DOI] [PubMed] [Google Scholar]
- 18.Compton WM, Dawson DA, Goldstein RB, Grant BF. Crosswalk between DSM-IV dependence and DSM-5 substance use disorders for opioids, cannabis, cocaine and alcohol. Drug Alcohol Depend 2013; 132: 387–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kirillova GP, Vanyukov MM, Kirisci L, Reynolds M. Physical maturation, peer environment, and the ontogenesis of substance use disorders. Psychiat Res 2008; 158: 43–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kirillova G, Reynolds M, Kirisci L, Mosovsky S, Ridenour T, Tarter R et al. Familiality of addiction and its developmental mechanisms in girls. Drug Alcohol Depend 2014; 143: 213–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vanyukov MM, Tarter RE, Kirisci L, Kirillova GP, Maher BS, Clark DB. Liability to substance use disorders: 1. Common mechanisms and manifestations. Neurosci Biobehav Reviews 2003b; 27: 507–515. [DOI] [PubMed] [Google Scholar]
- 22.Vanyukov MM, Kirisci L, Moss L, Tarter RE, Reynolds MD, Maher BS et al. Measurement of the risk for substance use disorders: Phenotypic and genetic analysis of an index of common liability. Behav Gen 2009; 39: 233–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hicks BM, Iacono WG, McGue M. Index of the transmissible common liability to addiction: heritability and prospective associations with substance abuse and related outcomes. Drug Alcohol Depend 2012; 123 Suppl 1:S18–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vanyukov M, Kim K, Irons D, Kirisci L, Neale M, Ridenour T. et al. Genetic relationship between the addiction diagnosis in adults and their childhood measure of addiction liability. Behav Genet 2015; 45:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ridenour TA, Tarter RE, Kirisci L, Vanyukov MM. Could a continuous measure of individual transmissible risk be useful in clinical assessment of substance use disorder? Drug Alcohol Depend 2011; 119, 10–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kirisci L, Tarter R, Ridenour T, Reynolds M, Vanyukov M. Longitudinal modeling of transmissible risk in boys who subsequently develop cannabis use disorder. Am J Drug Alc Abuse 2013a; 39: 180–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tarter R Evaluation and treatment of adolescent substance abuse: A decision tree method. Am J Drug Alc Abuse 1990; 16: 1–46. [DOI] [PubMed] [Google Scholar]
- 28.Kirisci L, Tarter R, Mezzich A, Reynolds M. Screening current and future diagnosis of psychiatric disorder using the revised Drug Use Screening Inventory. Am J Drug Alc Abuse 2007; 34: 653–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kendler KS, Jacobson KC, Prescott CA, Neale MC. Specificity of genetic and environmental risk factors for use and abuse/dependence of cannabis, cocaine, hallucinogens, sedatives, stimulants, and opiates in male twins. Am J Psychiatry 2003;160:687–95. [DOI] [PubMed] [Google Scholar]
- 30.Muthén LK, Muthén BO. MPlus User’s Guide Fifth Edition. Los Angeles, CA: 2017. [Google Scholar]
- 31.Sobel ME. Asymptomatic confidence intervals for indirect effects in structural equation models In Leinhardt S (ed.), Sociological Methodology. Washington, DC: American Sociological Association, 290–312, 1982. [Google Scholar]
- 32.Hastie T, Tibshirani R., Friedman J (2013). The elements of Statistical Learning, Data Mining, Inference and Prediction. Springer Series in Statistics. [Google Scholar]
- 33.Gibson G On the utilization of polygenic risk scores for therapeutic targeting. PLoS Genet 2019; 15(4): e1008060 10.1371/journal.pgen.1008060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chatterjee N, Shi J, García-Closas M. Developing and evaluating polygenic risk prediction models for stratified disease prevention. Nat Rev Genet 2016; 17:392–406. doi: 10.1038/nrg.2016.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hylan TR, Von Korff M, Saunders K, Masters E, Palmer RE, Carrell D. et al. Automated prediction of risk for problem opioid use in a primary care setting. J Pain. 2015; 16:380–7. doi: 10.1016/j.jpain.2015.01.011. Epub 2015 Jan 29. [DOI] [PubMed] [Google Scholar]
- 36.Cheatle MD, Compton PA, Dhingra L, Wasser TE, O’Brien CP. Development of the Revised Opioid Risk Tool to predict opioid use disorder in patients with chronic nonmalignant pain. J Pain 2019; 20: 842–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Webster LR, Webster RM. Predicting aberrant behaviors in opioid-treated patients: Preliminary validation of the Opioid Risk Tool. Pain Med. 2005; 6:432–42. [DOI] [PubMed] [Google Scholar]
- 38.Krueger RF, Hicks BM, Patrick CJ, Carlson SR, Iacono WG, McGue M. Etiologic connections among substance dependence, antisocial behavior and personality: Modeling the externalizing spectrum. J. Abnorm. Psychol 2002; 111: 411. [PubMed] [Google Scholar]
- 39.Jing Y, Hu Z, Fan P, Wang L, Tarter R, Kirisci L et al. Analysis of substance use and its outcome by machine learning I. Evaluation of risk for substance use disorder from childhood to young adulthood. Drug Alcohol Depend 2020; 206: 107605 10.1016/j.drugalcdep.2019.107605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hu Z, Jing Y, Xue Y, Wang L, Vanyukov M, Kirisci L et al. Analysis of substance use and its outcomes by machine learning: II: Derivation and prediction of the trajectory of substance use severity. Drug Alcohol Depend 2020; 206: 107604 10.1016/j.drugalcdep.2019.107604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kelly NJ, Bergman B, Hoeppner BB, Vilsaint C, White WL. Prevalence and pathways of recovery from drug and alcohol problems in the United States population: Implications for practice, research, and policy. Drug Alcohol Depend 2017; 181: 1621–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ridenour T, White D, Bogen D, Novak S, Scherer J, Reynolds M, Zhai Z, Tarter R. Detecting initiation or risk for initiation of substance use before high school during pediatric well-child check-ups. Drug Alcohol Depend 2015; 150:54–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Committee on Substance Use. Substance use screening, brief intervention, and referral to treatment for pediatricians. Pediatrics 2019; 128: 1330–1340. [DOI] [PubMed] [Google Scholar]
- 44.Vanyukov MM, Cornelius MD, De Genna NM, Reynolds MD, Kirillova GP, Maher BS et al. Measurement of liability to addiction: Dimensional approaches. Int J Person Cent Med 2016; 6L: 250–259. [Google Scholar]
- 45.Sanchis-Segura C, Becker JB. Why we should consider sex (and study sex differences) in addiction research. Addict Biol 2016; 21:995–1006. doi: 10.1111/adb.12382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Becker JB, McClellan ML, Reed BG Sex differences, gender and addiction. J Neuroscie Res 2017; 95: 136–147.doi: 10.1002/jnr.23963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Becker JB, Hu M Sex differences in drug abuse. Front Neuroendocrin 2008; 29: 36–47. [DOI] [PMC free article] [PubMed] [Google Scholar]


