Abstract
Reduction of lipoprotein uptake by macrophages and stimulation of cholesterol efflux are two essential steps required for atherosclerotic plaque regression. We used the optimized mannose-functionalized dendrimeric nanoparticle (mDNP)-based platform for macrophage-specific delivery of therapeutics to simultaneously deliver SR-A siRNA (to reduce LDL uptake) and LXR ligand (LXR-L, to stimulate cholesterol efflux) – a novel “Two-pronged” approach to facilitate plaque regression. mDNP-mediated delivery of SR-A siRNA led to a significant reduction in SR-A expression with a corresponding decrease in uptake of oxLDL. Delivery of LXR-L increased expression of ABCA1/G1 and cholesterol efflux. Combined delivery of siRNA and LXR-L led to a significantly greater decrease in macrophage cholesterol content compared to either treatment alone. Administration of this in vitro optimized formulation of mDNP complexed with SR-A-siRNA and LXR-L (Two-pronged complex) to atherosclerotic LDLR−/− mice fed western diet (TD88137) led to significant regression of atherosclerotic plaques with a corresponding decrease in aortic cholesterol content.
Keywords: Nanoparticle, Macrophage, Atherosclerosis, Cholesterol influx, Cholesterol efflux, Plaque regression
1. Introduction
Uptake of modified low-density lipoprotein (mLDL) initially may be beneficial due to effective removal of this potentially toxic form of circulating LDL, but unregulated/continuous uptake of mLDL by macrophages results in the formation of lipid-laden macrophage foam cells. Accumulation of these foam cells within the artery wall leads to the development of atherosclerotic plaques, and this process is shown to start early in life[1, 2]. Recognizable or overt clinical symptoms normally appear later in life (~35–40 years of age) and current management is largely focused on reducing plasma LDL cholesterol (LDL-C) based on the observed correlation between cardiovascular disease risk and LDL-C[3, 4]. While targeted reduction in plasma LDL-C is indeed correlated to cardiovascular disease risk reduction[5, 6] likely due to attenuation of further plaque progression, the burden of existing plaques still remains and currently, no therapy is available to regress the existing plaque.
The intracellular processes involved in the maintenance of macrophage cholesterol homeostasis include influx and efflux pathways[7]. Unregulated uptake of mLDL (influx pathway) occurs via scavenger receptors, such as CD36 and SR-A, and cholesterol esters (CE) and, not unesterified or free cholesterol (FC), represents the storage form of cholesterol. It is noteworthy that while intracellular FC beyond a physiological threshold level is cytotoxic, significantly higher levels of CE can be stored without apparent toxicity and conversion of FC to CE is a potentially cyto-protective event. The efflux pathways include extracellular acceptor (e.g., HDL) dependent removal of FC (not CE) via membrane-associated cholesterol transporters, namely, ABCA1 and ABCG1. Intracellular hydrolysis of stored CE is, therefore, the rate-limiting step for FC efflux[8], and consistently, transgenic overexpression of CE hydrolase not only increases FC efflux from macrophage foam cells but also attenuates atherosclerosis[9]. Similarly, increasing the expression of cholesterol transporters, either by the introduction of transgenes or by activation of transcriptional regulator liver-x-receptor (LXR), significantly increases FC efflux[10, 11]. Based on the current understanding of macrophage cholesterol homeostasis and foam cell formation, it is apparent that reduction in influx and increase in efflux pathways are the two logical steps for therapeutic intervention.
Reduction in LDL-C is one mechanism to reduce influx, indirectly by reducing the availability of mLDL. However, preventing further influx can only reduce progression but cannot modulate reduction in existing plaques. Enhancing efflux pathways is the only logical step to reduce the lipid burden of existing plaques. Ligand-mediated activation of LXR increases FC efflux[12] and reduces atherosclerosis[13] but it also increases LXR-dependent lipogenesis in liver[14, 15] precluding clinical use. Targeted delivery of LXR ligand (LXR-L) to macrophages without significant uptake by liver can potentially circumvent this undesirable effect of increased hepatic lipogenesis. We developed mannose-functionalized dendrimeric nanoparticles (mDNP) as a platform to specifically deliver LXR-L to macrophages (via mannose receptors expressed on macrophage surface) and demonstrated a significant reduction in diet-induced atherosclerosis[16]. Based on the efficacy of this approach, we hypothesized that in addition to increasing macrophage cholesterol efflux by LXR-L, simultaneous reduction in mLDL-associated cholesterol uptake by macrophages will further enhance the efficacy of this platform in reducing macrophage cholesterol content. mLDL uptake can be effectively reduced by decreasing the expression of scavenger receptors, SR-A or CD36 and herein, we chose to reduce SR-A expression via gene knockdown using SR-A specific siRNA. By combining these two strategies into one platform, a novel “Two-pronged” approach was designed to simultaneously deliver siRNA to knock down the expression of SR-A to reduce cholesterol influx and deliver LXR-L to increase expression of cholesterol transporters ABCA1/ABCG1 to enhance cholesterol efflux (illustrated in Scheme 1). The “Two-pronged complex” was generated by combining the anti-atherogenic platform of mDNP-LXR-L validated in our earlier studies[16] with SR-A siRNA. The data presented herein demonstrate significant regression of atherosclerotic lesions by treatment with this “Two-pronged complex”.
Scheme 1.

The “Two-pronged complex” is constructed by combining the anti-atherogenic platform of mDNP-LXR-L with SR-A siRNA. This novel “Two-pronged” approach is aimed to simultaneously deliver siRNA to knock down the expression of SR-A to reduce cholesterol influx and deliver LXR-L to increase the expression of cholesterol transporters ABCA1/ABCG1 to enhance cholesterol efflux, thus leading to atherosclerotic plaque regression.
2. Materials and methods
2.1. Materials
SR-A siRNA (sc-40188) and FITC-siRNA (sc-36869) were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Ethylenediamine (EDA) core polyamidoamine (PAMAM) dendrimer generation 5.0 (technical grade) was purchased from Dendritech (Midland, MI). LXR ligand GW3965, 4-nitrophenyl chloroformate (NPC), triethylamine (TEA), HO-PEG-NH2 (Mn=3500 g/mol), tetrahydrofuran (THF), 1-ethyl-3-(−3-dimethylaminopropyl) carbodiimide hydrochloride (EDC), and Fluorescein isothiocyanate (FITC) were purchased from Sigma-Aldrich (St. Louis, MO). Mannose-PEG-NHS (Mn=3500 g/moL) was custom synthesized by JenKem Technology (Plano, TX, USA). SnakeSkin dialysis tubing with 3500 and 7000 molecular weight cut-off (MWCO) were purchased from Thermo Scientific (Rockford, IL). DMEM medium, fetal bovine serum (FBS) and Dulbecco’s Phosphate-buffered saline (DPBS) were obtained from Gibco BRL (Carlsbad, CA). Trypsin-EDTA (0.25 %), streptomycin and penicillin were obtained from Invitrogen Co., USA. Vectashield mounting medium was purchased from Vector Laboratories (Burlingame, CA). RNeasy® Mini Kit was purchased from QIAGEN. High Capacity cDNA Reverse Transcription Kit and TaqMan Universal PCR Master Mix, no AmpErase UNG were obtained from Applied Biosystems. All other chemicals were purchased from Sigma-Aldrich (St. Louis, MO).
2.2. Construction and characterization of the Two-pronged complex
Mannose functionalized dendrimer-based nanoparticle (mDNP) conjugated with LXR ligand (mDNP-LXR-L) was synthesized and characterized as previously described[16] with minor modifications since GW3965 was used as the LXR ligand (LXR-L). Briefly, LXR-L and HO-PEG-NH2 were first conjugated via the 1-ethyl-3-(−3-dimethylaminopropyl) carbodiimide hydrochloride-NHS reaction. The resulting HO-PEG-LXR-L was coupled to the amino groups on the surface of PAMAM dendrimer G5.0 to obtain DNP-LXR-L in the presence of NPC and excess triethylamine. PAMAM-G5.0 with a maximum diameter of 5.4 nm and 128 terminal amine groups, providing the suitable handle to attach the desired therapeutics, was used. The mannose-PEG-NHS was added to DNP-LXR-L in sodium bicarbonate solution. The resulting mixture was stirred overnight at room temperature followed by extensive dialysis and lyophilization to obtain mDNP-LXR-L. SR-A siRNA was complexed with mDNP-LXR-L as described earlier[17] to generate the “Two- pronged complex”. Briefly, SR-A siRNA duplex solution was added directly to the mDNP-LXR-L, mixed gently and then incubated at room temperature for 30 minutes. SR-A siRNA was also complexed to mDNP without LXR-L for delivery of SR-A siRNA alone.
2.3. Gel retardation assay
Two-pronged complex consisting of mDNP-LXR-L/SR-A siRNA (2, 4, 6, 10, 20, 40 and 50, w/w) were prepared as described above and analyzed on 0.8 % agarose gel containing ethidium bromide. The 1 kb DNA ladder (New England Biolabs, Ipswich, MA) was included as a control. Electrophoresis was performed in Tris-acetate-EDTA buffer at 100 V for 40 min. The DNA bands were visualized with a UV-light system.
2.4. Transmission electron microscopy (TEM)
Two-pronged complex (20:1, w/w) diluted 1:100 in distilled water was loaded onto a 300-mesh carbon coated copper grid, air dried at room temperature, and imaged under a transmission electron microscope (TEM) (JEM-3010, JEOL, Tokyo, Japan).
2.5. Dynamic laser scattering (DLS)
The hydrodynamic diameter of freshly prepared Two-pronged complex (20:1, w/w) diluted 1:20 v/v in distilled water was measured at room temperature using a Malvern Zetasizer Nano ZS90 (Malvern Instruments, Worcestershire, UK).
2.6. In vitro characterization
2.6.1. Cell culture
Thioglycollate-elicited mouse peritoneal macrophages (MPMs) were harvested and plated in appropriate cell culture dishes. Non-adherent cells were removed after 4 h and medium replaced with fresh growth medium (10 % FBS containing DMEM medium).
2.6.2. Cellular uptake of the Two-pronged complex by macrophages
Freshly isolate MPMs (1×106 cells/well) were plated in 2-well chamber slides. After an overnight incubation, adherent MPMs were exposed to either FITC-siRNA/mDNP-LXR-L or FITC-siRNA/DNP. After 24 h, cells were washed with DPBS, fixed with 4 % formaldehyde at room temperature for 20 min, permeabilized with 0.1 % Triton X-100 for 5 min, and the cell nuclei were counterstained with DAPI for 5 min. Cellular uptake was assessed by fluorescent imaging, a 405 nm laser line was selected for DAPI, and a 488 nm laser line was selected for FITC. Cells from a parallel experiment were collected for quantitative analysis of nanoparticle uptake using flow cytometry.
2.6.3. Intracellular trafficking
FITC-labeled SR-A siRNA was used to construct Two-pronged complex (mDNP-LXR-L/FITC-siRNA, 5 μg/0.25 μg). MPMs (1×106 cells/well) were seeded in 2-well chamber slides and adherent cells were incubated with mDNP-LXR-L/FITC-SR-A siRNA in 500μL of DMEM without FBS at 37 °C. After 6 h, the medium was replaced with fresh growth medium containing 10 % FBS. At the end of an additional 6 h incubation, the cells were rinsed with PBS, fixed with 10 % buffered formalin, permeabilized with 0.15 % Triton X-100 and counterstained with DAPI. The cells were also stained with endosome antibody Rab 5 from ThermoFisher (PA5–52369, 1:100) and then imaged under a Zeiss LSM 700 confocal laser scanning microscope.
2.6.4. Demonstration of SR-A knock down and effects of lipoprotein uptake
Freshly isolated MPMs (1×106 cells/well) were plated in 24-well culture plates and incubated overnight. Two-pronged complex (20:1, w/w) containing either non-specific (scramble) siRNA (0.25 μg/mL) or therapeutic SR-A siRNA (0.25 μg/mL) were added to the adherent MPMs. After 24 h, the medium was replaced with fresh growth medium containing 10 % FBS. Total cellular RNA (using RNeasy mini kit) and protein (in ice cold RIPA buffer) were extracted after additional 24 h. SR-A mRNA expression was quantified by qRT-PCR using optimized TaqMan™ Gene Expression Assays for MSR1 (Mm00446214_m1). Cellular SR-A protein levels were determined by Western Blot Analysis. Proteins were separated with electrophoresis, transferred onto the Immobilon-P PVDF membrane (Millipore), probed with SR-A antibody (sc-20444, Santa Cruz) and corresponding secondary antibodies labeled with IRDye™ (LI-COR Biosciences, Lincoln, NE) and detected using the Odyssey® CLx imaging system.
To evaluate whether SR-A knock down mediated by the Two-pronged complex reduced lipoprotein uptake, DiI-labeled oxLDL (DiI-oxLDL, 25 μg/mL) was added to cells pretreated for 24 h with Two-pronged complex (20:1, w/w). After an additional 24 h incubation, cells were washed with PBS to remove DiI-oxLDL in the medium, fixed in 4% buffered formalin for 20 min and counterstained with DAPI. Images were acquired by confocal microscopy (LSM 710; Carl Zeiss Q10, Germany).
2.6.5. Cellular ABCA1/G1 expression
Freshly isolated MPMs (1×106 cells/well) were plated in 24-well culture plates and incubated overnight. Adherent cells were treated with SR-A siRNA (0.25 μg/mL) or mDNP-LXR-L (5 μg/mL) or Two-pronged complex (20:1, w/w). After 24 h incubation, the medium in each well was replaced with fresh growth medium containing 10 % FBS and total cellular RNA was isolated after an additional 24h. ABCA1/G1 mRNA levels were quantified using RT-qPCR as described above with optimized TaqMan™ Gene Expression Assays for ABCA1 (Mm00442646_m 1) and ABCG1 (Mm00437390_m1).
2.6.6. Free cholesterol (FC) efflux assays
Freshly isolated MPMs (1×106 cells/well) were plated in 24-well culture plates and incubated overnight. The intracellular FC and CE pools were labeled with [3H]-cholesterol by incubating the cells for 48 h with serum-free medium containing 1 μCi/mL [3H]-cholesterol (Perkin Elmer) and 25 μg/mL acetylated LDL (AcLDL, Kalen Biomedical, Inc). The cells were then washed and incubated with serum-free medium containing either SR-A siRNA (0.25 μg/mL) or mDNP-LXR-L (5 μg/mL) or Two-pronged complex (20:1, w/w) for additional 24 h. FC efflux was initiated by replacing the medium with growth medium containing 10 % FBS and % FC efflux was evaluated as described earlier[18].
2.6.7. Lipid/Cholesterol accumulation in macrophage
Freshly isolated MPMs pretreated with either SR-A siRNA (0.25 μg/mL) or mDNP-LXR-L (5 μg/mL) or Two-pronged complex (20:1, w/w) were incubated with AcLDL (25 μg/ml) for 24 h. Following two washes in PBS, cells were fixed in 10% buffered formalin to stain with 0.2 % Oil Red O in 60 % 2-propanol for 10 minutes. Following three washes with PBS, cells were imaged using Olympus microscope (IX71, Japan). Total lipids were extracted from a parallel set and total cholesterol mass was determined as described earlier[19]. Total cellular protein was estimated following lipid extraction using a Bio-Rad dye binding assay.
2.6.8. Hepatic lipid extraction:
Liver tissue was homogenized in PBS and total lipids were extracted by the method of Bligh and Dyer[20] and normalized to total protein determined using Pierce™ BCA method.
2.7. In vivo studies
2.7.1. Animals and animal care
LDL receptor knockout mice (LDLR−/−) were purchased from The Jackson Laboratory and maintained helicobacter free in a barrier facility. All animal procedures were approved by the Institutional Animal Care and Use Committee of Virginia Commonwealth University.
2.7.2. Regression of atherosclerosis studies
To evaluate whether the Two-pronged complex could reduce the existing plaques, regression study was designed as follows. At 10 weeks of age, mice of both sexes were fed a high fat, high cholesterol Western type diet (WD, TD88137, Harlan Teklad) which contained 21 % fat, 0.15 % cholesterol, and 19.5 % casein by weight with no sodium cholate for 16 weeks and then switched to chow diet for 4 weeks. One group of mice (Baseline Group, n=20, 10 males and 10 females) were sacrificed at the end of this 4 weeks period to obtain the baseline parameters. The other mice were divided into two groups, Untreated and Treated (n=20/group, 10 males and 10 females) and were maintained on Chow diet. While mice in the Untreated group did not receive any treatment, mice in the Treated group received weekly intravenous injections of the Two-pronged complex (containing 0.1 mg/kg SR-A siRNA and 2 mg/kg mDNP-LXR-L). After 8 weeks of treatment, the mice were sacrificed to collect blood, aorta, heart and other tissues for analyses.
2.7.3. Analytical procedures
Total plasma cholesterol, triglycerides, liver function markers (AST and ALT) and kidney function marker (BUN) were determined using the Cobas c311 automated chemistry analyzer with reagents, calibrators, and controls from Roche Diagnostics. Major organs (heart, liver, spleen, lung and kidney) were fixed, sectioned and stained with H&E to monitor histological changes, if any. For gene expression analyses, total RNA from each organ was extracted using RNeasy mini kits (Qiagen) and the mRNA levels were determined using optimized TaqMan™ Gene Expression Assays for ABCA1 (Mm00442646_m1), ABCG1 (Mm00437390_m1) and MSR1 (SR-A, Mm00446214_m1). Changes in hepatic gene expression was determined using total liver RNA and the following TaqMan™ Gene Expression Assays for FAS (Mm00662319_m1), SREBP1 (Mm00550338_m1), PCSK9 (Mm01263610_m1), ApoC3 (Mm00445670_m1), ANGPTL3 (Mm00803820_m1) and ApoA1 (Mm00437568_g1).
2.7.4. Assessment of atherosclerosis
For en face analyses, aorta was dissected from the heart to the iliac bifurcation, cleaned of any surrounding tissue, opened longitudinally, pinned on black wax and fixed for 24 h in 10 % buffered formalin. The fixed aortas were imaged on a black background using a Canon Digital Camera fitted with a 60 mm, f/2.8 Macro Lens. Total area and the area occupied by the lesions in the aortic arch as well as total aorta was determined using Axiovision Image Analysis software (Carl Zeiss) as described earlier[9]. FC and CE content of the aortic arch was determined by the Lipidomics Core Laboratory at Wayne State University on a fee for service basis.
2.8. Statistical analysis
All statistical analyses were performed using GraphPad Prism Software that permits analysis of data sets with unequal variances. One-way ANOVA with Bonferroni post-hoc correction was used to compare two groups and Two-way ANOVA with Tukey’s multiple comparison tests was used to enable comparisons between multiple parameters Statistical significance for all comparisons was assigned at p< 0.05.
3. Results
3.1. Preparation and characterization of Two-pronged complex
We have earlier reported enhanced as well as specific macrophage targeting by mannose functionalization of DNP and demonstrated the use of mDNP to selectively deliver LXR-L to macrophages[16]. Taking advantage of the positive charge on the surface, negatively charged SR-A siRNA was complexed to mDNP-LXR-L (Figure 1A). As shown in Figure 1B, at the ratio of 10:1, SR-A siRNA was almost completely complexed to mDNP-LXR-L. “Two-pronged complex” where the ratio of mDNP-LXR-L to SR-A siRNA was 20:1 was, therefore, used for all the following studies. The resulting particles were of fairly uniform size as seen by TEM (Figure 1C) and mean hydrodynamic diameter of ~150 nm as well as narrow polydispersity index as characterized by DLS (Figure 1D).
Figure 1. Characterization of Two-pronged or mDNP-LXR-L-siRNA complexes.

(A) Schematic showing complexing of mDNP-LXR-L with SR-A siRNA to form the Two-pronged complex. (B) Gel retardation assay of mDNP/siRNA complexes at indicated weight ratios (2:1 to 50:1) showing that siRNA is fully complexed to mDNP-LXR-L at or above the ratio of 10:1. (C) TEM images and (D) DLS profile of the Two-pronged Complex.
3.2. Cellular uptake and intracellular trafficking of the Two-pronged complex by macrophages
Consistent with our earlier studies[16], significantly higher delivery of SR-A siRNA to MPMs was noted when delivered by mDNP- containing “Two-pronged complex” compared to DNP-containing complex (Figure 2A and 2B). Intracellular release of siRNA is required for biological activity. Therefore, the escape of siRNA from the endosomal compartment was monitored 6 h after treatment. While at 0 h, significant co-localization of endosomal marker Rab 5 and FITC labeled siRNA is noted, increased separation of FITC and Rab 5 is apparent after 6 h incubation demonstrating the release of FITC labeled siRNA from the endocytic compartment (Figure 2C).
Figure 2. Uptake and intracellular trafficking of Two-pronged complex by macrophages.

FITC labeled siRNA was complexed to either non-functionalized DNP or mannose functionalized mDNP-LXR-L and uptake by MPMs was monitored. (A) Representative images of MPMs. (B) Quantification of cell-associated FITC (mean fluorescent intensity, MFI) by flow cytometry (Mean ± SD, n=3, *P<0.05). (C) To monitor lysosomal escape of siRNA delivered via Two-pronged complex, following incubation with FITC-labeled Two-pronged complex, macrophages were fixed and stained for endosome marker Rab 5 (Red). The co-localization of FITC-labeled siRNA (Green) and endosomal marker (red) is apparent in Merged images (Yellow) at 0 h. Area enclosed in the white box is digitally enlarged and white arrows indicate escape of FITC label siRNA (Green) from the endosomal compartment after 6h incubation.
3.3. Demonstration of SR-A knock down and effects on lipoprotein uptake
Compared to scrambled/non-specific siRNA (sc-siRNA), the delivery of SR-A siRNA using the “Two-pronged complex” significantly reduced the expression of SR-A in MPMs at mRNA (Figure 3A) as well as protein level (Figure 3B and 3C). SR-A facilitates the uptake of modified LDL by macrophages and the effect of SR-A knockdown by siRNA on uptake of oxLDL was examined in MPMs treated with sc-siRNA or SR-A siRNA. No difference in uptake of DiI-labeled ox-LDL was apparent between untreated controls (No/T) and MPMs treated with complexes containing sc-siRNA (Figure 3D). Consistent with the observed reduction in SR-A expression, a dramatic reduction in uptake of DiI-labeled oxLDL was noted in MPMs treated with complexes containing SR-A siRNA demonstrating the efficacy of siRNA mediated knockdown of SR-A in reducing the uptake of modified LDL.
Figure 3. Intracellular functionality of the siRNA delivered via Two-pronged complex.

MPMs were treated with Two-pronged complex made using either non-specific scrambled siRNA (sc-siRNA) or SR-A specific siRNA (SR-A siRNA; untreated cells (No/T) were used as controls. (A) Relative mRNA expression of SR-A determined by qRT-PCR (Mean ± SD, n=3, *P<0.05). SR-A protein expression in total protein extracts from MPMs with indicated treatment and β-actin served as the housekeeping loading control. (B) Densitometric quantitation of the Western blots, data are presented in arbitrary units ((Mean ± SD, n=3, *P<0.05). (C) Representative Western blot. (D). To evaluate the effects of Two-pronged complex-mediated SR-A knockdown on mLDL uptake, MPMs with indicated treatment were exposed to DiI-labeled oxLDL and uptake monitored by imaging. Representative images are shown.
3.4. Cellular ABCA1/G1 expression and free cholesterol (FC) efflux assays
To evaluate the effects of LXR-L delivery using the “Two-pronged complex”, expression of LXR target genes, ABCA1 and ABCG1 were monitored. Compared to untreated controls (No/T), significant (p<0.0001) and a comparable increase in mRNA expression of both ABCA1 and ABCG1 was seen (Figure 4A) whether LXR-L ligand was delivered as mDNP-LXR-L or as the “Two-pronged complex” (LXR-L vs Two-pronged complex, p=0.9654). Consistent with increased expression of cholesterol transporters ABCA1 and ABCG1, time-dependent increase in cholesterol efflux from MPMs was observed (Figure 4B) which was significantly higher than untreated (No/T) controls (No/T vs LXR-L or Two-pronged p<0.0001). This increase in cholesterol efflux was comparable (p=0.8543) whether LXR-L was delivered using mDNP-LXR-L or as a Two-pronged complex demonstrating equivalent efficacy. Given that SR-A is involved in the uptake of modified LDL and not cholesterol efflux, siRNA-mediated SR-A knockdown did not affect cholesterol efflux as expected.
Figure 4. LXR-L delivered via Two-pronged complexes effectively increases target gene expression.
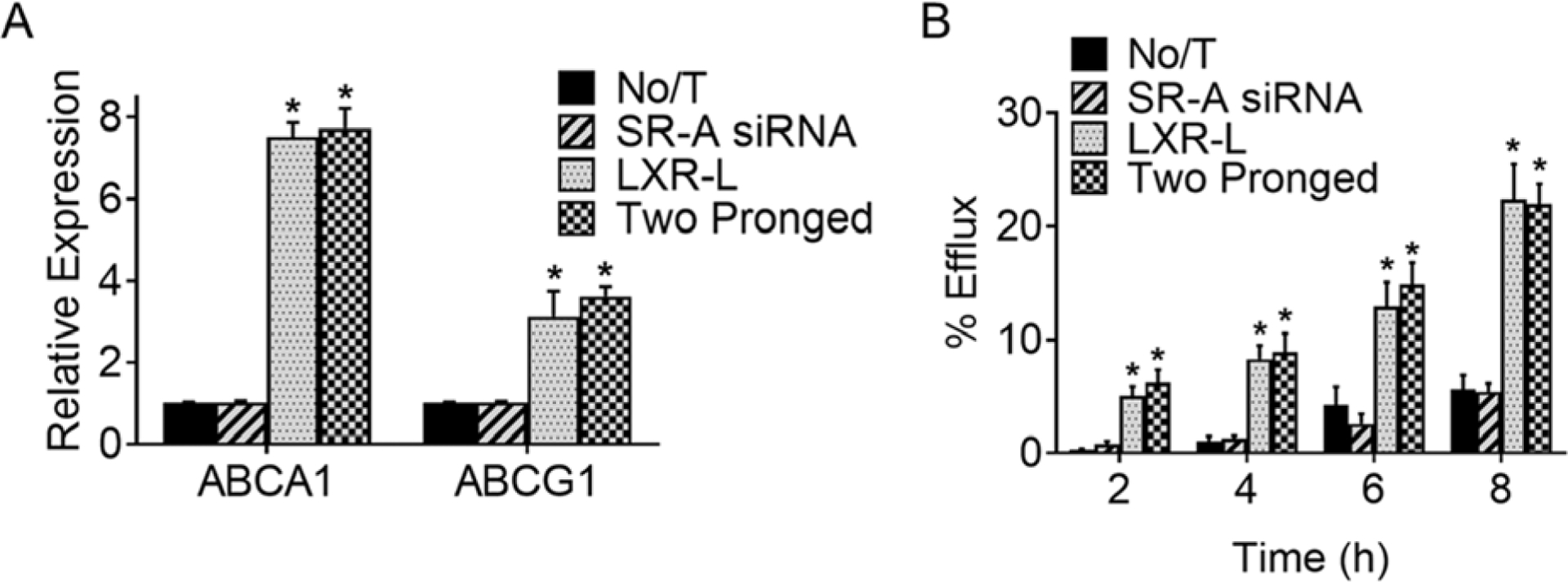
MPMs were either left untreated (No/T) or treated with mDNP complexed with SR-A siRNA (0.25 μg/ml) or mDNP LXR-L (5 μg/ml) or Two-pronged complex (20:1 w/w). (A) Total cellular RNA was extracted and used to determine the relative expression of ABCA1 and ABCG1 by qRT-PCR (Mean ± SD, n=3, *p<0.05, compared to No/T and SR-A siRNA groups. (B) After cholesterol loading with AcLDL (25 μg/ml) and labeling of intracellular cholesterol pools with [3H]-cholesterol, MPMs were treated as indicated. After 24 h, cholesterol efflux was initiated and % cholesterol efflux was determined at indicated times. Data are presented as Mean ± SD, n=3, *p<0.05 compared to No/T and SR-A siRNA groups at the same time point.
3.5. Lipid/Cholesterol accumulation in macrophage
Having validated the biological effects of SR-A knockdown and activation of LXR-L target gene expression by simultaneous delivery of SR-R siRNA and LXR-L using the “Two-pronged complex”, the combined effect on lipid accumulation in MPMs was evaluated. Reduction in mLDL uptake by siRNA-dependent knockdown of SR-A led to reduced cellular lipid burden compared to no treatment (No/T) controls as seen with reduced Oil Red O staining (Figure 5A) and significantly reduced cellular cholesterol (Figure 5B) content (No/T vs SR-A siRNA p=0.0455). Consistent with enhanced cholesterol efflux by activation of LXR-target genes ABCA1 and ABCG1, reduced Oil Red O stained lipids, as well as significantly lower cellular cholesterol content was seen following treatment with LXR-L (No/T vs LXR-L p=0.0304). Treatment with “Two-pronged complex” not only decreased cellular lipids and cholesterol content, but this reduction in cellular cholesterol content was significantly lower than that seen with SR-A siRNA or LXR-L alone (SR-A siRNA vs Two-pronged complex p=0.0017 and LXR-L vs Two-pronged Complex p=0.0023). These data suggest that simultaneous inhibition of cholesterol uptake by knocking down SR-A and increasing cholesterol efflux by LXR activation lead to a significantly greater reduction in cellular cholesterol levels.
Figure 5. Treatment with Two-pronged complex reduces cellular cholesterol accumulation.

MPMs were either left untreated (No/T) or treated with mDNP complexed with SR-A siRNA (0.25 μg/ml) or mDNP-LXR-L (5 μg/ml) or Two-pronged complex (20:1 w/w). After 24 h, lipid accumulation was induced by incubation with AcLDL (25 μg/ml) for additional 24 h. One set of cells were fixed, stained with Oil Red O and imaged (A). Total lipids were extracted from the parallel set and total cholesterol levels were determined and normalized to total cellular protein (B). Data are shown as Mean ± SD, n=3, *P<0.05 and NS-not significant.
3.6. Targeted genes expression in vivo
To assess the in vivo effects of the “Two-pronged complex”, in the first set of experiments, expression of SR-A, ABCA1 and ABCG1 in various organs was evaluated 3 or 7 days after a single intravenous injection of “Two-pronged complex”. No apparent toxicity was noted after administration of “Two-pronged complex” (data not shown). Consistent with previously demonstrated macrophage-specific delivery using mDNP-based platform[16] and in vitro efficacy shown above, expression of SR-A was significantly reduced in aortic arch containing atherosclerotic plaque and this reduction was maintained for 7 days post-administration (Figure 6A). During the same time frame, increased expression of ABCA1 (Figure 6B) and ABCG1 (Figure 6C) was also noted in the aortic arch with plaque. No significant changes in the expression of SR-A, ABCA1 or ABCG1 were noted in any of the other tissues examined confirming not only the previously reported targeted delivery using mDNP platform but also expected change in target genes.
Figure 6. Targeted gene expression following treatment with Two-pronged complex:
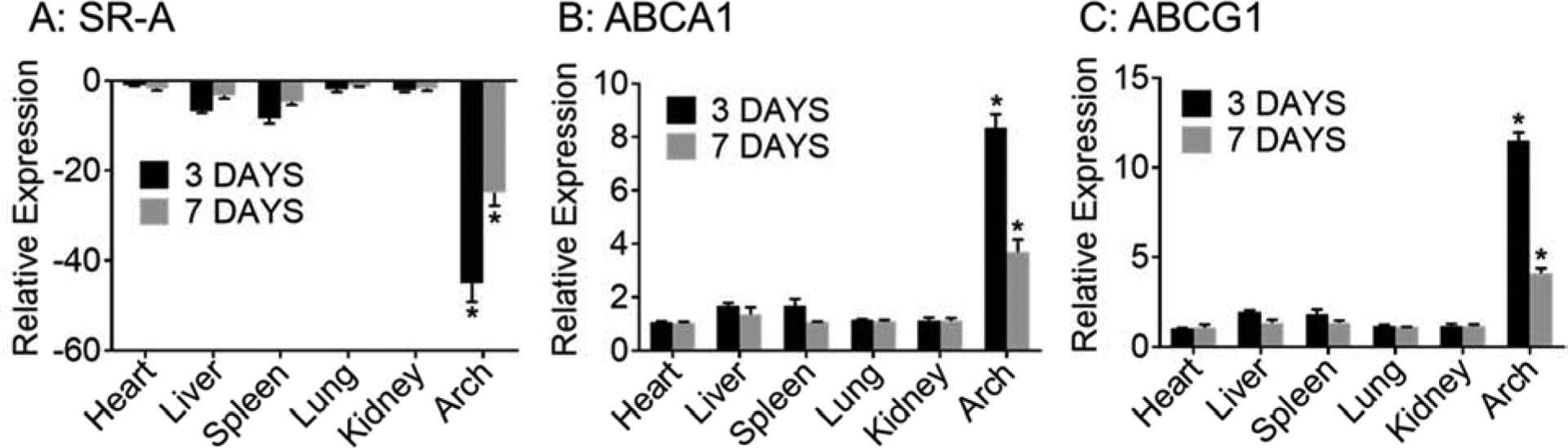
LDLR−/−mice with established atherosclerotic plaque (induced by WD feeding for 16 weeks) were given a single intravenous injection of the Two-pronged complex containing 0.1 mg/kg SR-A siRNA and 2mg/kg mDNP-LXR-L. Expression of SR-A (A), ABCA1 (B) and ABCG1 (C) was determined using total RNA from indicated tissues after 3 or 7 days. Data are presented as Mean ± SD, n=3, *p<0.05.
3.7. Assessment of atherosclerosis regression:
3.7.1. Effects of treatment with “Two-pronged complex” on tissue morphology, plasma lipids, hepatic gene expression and lipid accumulation:
We have earlier demonstrated reduction in WD-induced atherosclerosis by administration of mDNP-LXR-L[16] and in the second set of experiments, the ability of the “Two-pronged complex” to regress existing plaques was evaluated. Gross morphology of tissues, namely, heart, liver, spleen lung and kidney was not affected by 8 weekly injections of the “Two-pronged complex” (Figure 7A) indicating apparent lack of toxicity. Furthermore, plasma markers of liver (AST and ALT) and kidney function (BUN) also remain unaffected following treatment (Figure 7B). Plasma creatinine levels were below the detectable limits indicating no change in kidney function (Data not shown). Although no significant change in plasma total cholesterol was noted, plasma triglycerides were significantly reduced with treatment (Figure 7B). Activation of LXR is known to increase hepatic lipogenesis and hepatic lipid accumulation. To confirm the lack of hepatic effects using “Two-pronged complex” based on mDNP with known targeting to macrophages[16], expression of lipogenic genes (FAS and SREBP1) was monitored. No significant change in the expression of these genes was observed (Figure 7C). Consistently, total hepatic lipid levels also remained unchanged (Figure 7D) following treatment with the “Two-pronged complex”. Furthermore, treatment with the “Two-pronged complex” did not alter the expression of other genes relevant to hepatic lipid metabolism and cardiovascular function (Figure 7E).
Figure 7: Tissue morphology, plasma lipids, hepatic gene expression and lipid accumulation following treatment with “Two-pronged complex”:
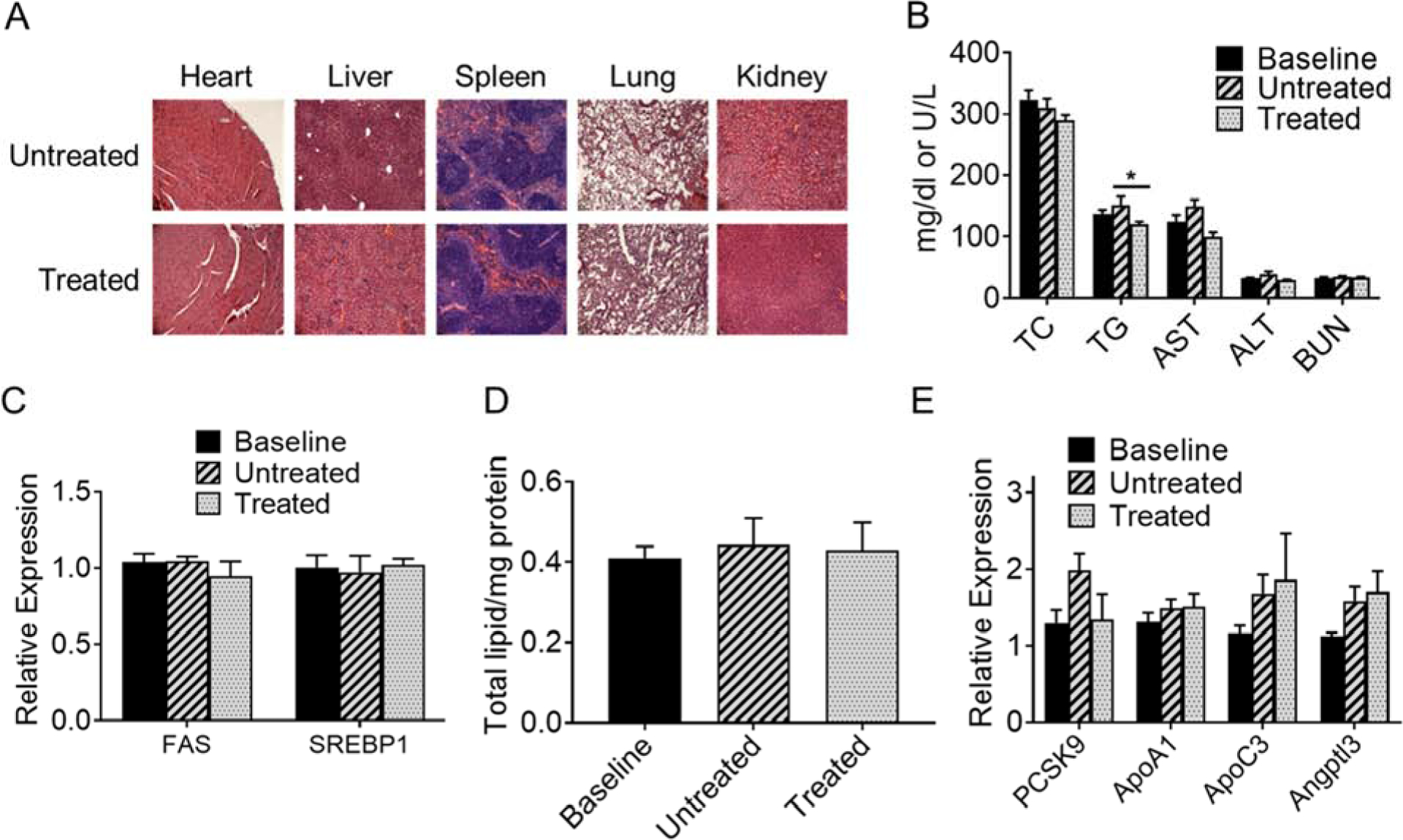
To assess the effects on regression of atherosclerosis, LDLR−/− mice were fed WD for 16 weeks, switched to chow diet for 4 weeks.While one group of mice were euthanized at this time (baseline), the other two groups were either left untreated (Untreated) or received weekly injections of the Two-Pronged complex (Treated) as described under “Methods”. (A) Indicated tissues were harvested at the time of necropsy, paraffin-embedded, sectioned, stained with H&E and imaged showing no apparent toxicity following treatment with the Two-pronged complex. (B) Plasma was analyzed for total cholesterol (TC), triglycerides (TG), liver function tests (AST and ALT) as well as kidney function test (BUN). Data are presented as Mean ± SD, n=20, *p<0.05, 10 males and 10 females. Panels C and E: Total liver RNA was prepared and used to monitor expression of indicated genes. Panel D: Total lipids were extracted from another piece of liver and normalized to total proteins. Panel E: Hepatic gene expression of indicated genes. Data are presented as Mean ± SD, n=10.
3.7.2. Regression of atherosclerosis
En face analyses were performed and representative aorta images are shown in Figure 8A. Area occupied by atherosclerotic lesions in entire aorta (Figure 8B) as well as aortic arch (Figure 8C) was determined and group comparison analyses is shown in Figure 8D. No significant difference was noted between males and females in any of the groups (P>0.05) suggesting lack of any sex-dependent effects. Despite switching to chow diet, a significant increase in total as well as aortic lesion area was observed in Untreated group compared to Baseline group. In contrast, treatment with the “Two-pronged complex” significantly reduced total (>20% decrease) and aortic arch (>19% decrease) lesion area compared to Baseline group demonstrating regression of existing atherosclerotic plaques. Comparison of Untreated vs Treated group showed that treatment with the “Two-Pronged Complex” led to >40% reduction in total and >33% reduction in aortic arch plaque area.
Figure 8. Treatment with the Two-pronged complex led to significant regression in atherosclerosis.

LDLR−/− mice were fed WD for 16 weeks, switched to chow diet for 4 weeks. One set of mice were euthanized and served as the Baseline group. The remaining mice were either left untreated (Untreated) or received weekly injections of the Two-pronged complex (Treated) as described under “Methods”. (A) Representative en face images of indicated groups; (B) Quantification of total lesion area. (C) Quantification of lesion area in the aortic arch. Data are presented as Mean ± SD, n=20, 10 males and 10 females. (D) 2-way ANOVA analyses.
To quantify the biochemical changes due to reduction in cholesterol uptake (by SR-A siRNA) and increase in cholesterol efflux (by LXR-L mediated increase in ABCA1/G1 expression), cholesterol content of aortic arches was determined. As shown in Figure 9A and 9B, compared to Baseline group, significant reduction in FC as well as in CE was observed following treated with “Two-pronged complex”. Unlike the lesion areas, FC and CE content of Baseline and Untreated group were not significantly different. It is noteworthy that while en face image analyses determine the two-dimensional lesion area, FC and CE content also include the volume of the plaque and represent a biochemical measure. Fatty acid (FA) composition of the total CE in the aortic arch was also analyzed and significant differences were noted in all CE species esterified to FA with different chain lengths and degree of unsaturation/number of double bonds (Figure 9C). For the sake of clarity, data are presented as FA with indicated double bonds for the range of carbon chain length (C16 – C24). The relative distribution of different FA in CE was not significantly different between groups (Figure 9D).
Figure 9. Treatment with the Two-pronged complex led to significant reduction in cholesterol content of the aortic arch lesions and significant reduction in CE containing fatty acids of all different chain lengths and degree of unsaturation.

Following en face analyses, the aortic arch region was cut and extracted total lipids were analyzed for total FC and CE content. (A) FC and CE content are shown as μg/arch in the indicated groups, Data are presented as Mean ± SD, n=10, 5 males and 5 females. (B) 2-way ANOVA analyses. The total CE in the aortic arch lipid extracts were also analyzed for FA content. (C) CE esterified to FA with indicated carbon chain length and number of double bonds. Data are presented as Mean ± SD, n=10, 5 males and 5 females, *p<0.05. (D) Relative distribution of FA within indicated groups.
4. Disscussion
Atherosclerotic lesions start developing in the teenage years albeit with no apparent clinical symptoms and treatment is only mandated in individuals displaying overt clinical consequences. Although several lipid-lowering therapeutic options are available that attenuate further progression of existing atherosclerotic plaques, the burden of existing disease persists. Herein, we utilized a mDNP based platform to simultaneously deliver agents to inhibit macrophage uptake of modified lipoproteins by reducing the expression of SR-A and enhance removal of stored CE by LXR-L mediated increase in expression of cholesterol transporters ABCA1 and ABCG1. The data presented clearly establish the ability of this “Two-pronged” approach to significantly reduce, not only the atherosclerotic plaque area, but also the total cholesterol content demonstrating the expected removal of cholesterol from the plaques by simultaneously limiting influx and enhancing efflux. Thus, this novel “Two-pronged approach” developed and described here holds the promise of targeting the multifactorial process of macrophage cholesterol accumulation by simultaneously increasing macrophage cholesterol efflux and inhibiting SR-A mediated influx of mLDL. Furthermore, mDNP-based functionalized nanoparticle based delivery system enables targeted delivery of LXR-L to its therapeutic target, namely macrophages, reducing the adverse hepatic effects (e.g., increased lipogenesis,[21, 22] of systemic administration enhancing the therapeutic index.[16, 23, 24].
Compared to other types of nanoparticles,[25] the most prominent and distinguishing strengths of dendrimers are their well-defined structure, such as three-dimensional, hyperbranched, monodisperse, globular nanoparticles. In addition, their size, shape, surface chemistry, flexibility/rigidity, architecture, and elemental composition can be manipulated precisely during their modulation for desired biomedical applications.[26, 27]. These features have made dendrimer as preferred nanocarriers for traditional small drugs, proteins, DNA/RNA, and in some instances, as intrinsically active nanoscale drugs.[27]. Dendrimer-based drugs, as well as diagnostic and imaging agents, are emerging as promising candidates for many nanomedicine applications. More importantly, our previous work has shown that mannose-functionalized dendrimer-based nanoplatform can be utilized to target atherosclerosis and provided strong support for the expanded utility of dendrimers.
Lipid-lowering therapies largely result in attenuating further plaque progression. Despite moderate and non-significant decrease in arterial stenosis, the associated clinical benefits are disproportionately high[28]. It is now well recognized that these clinical benefits are attributable to plaque stabilization due to reduced cholesterol influx and subsequent decrease in plaque inflammation. Strengthening of the fibrous cap further adds to the stability[29]. Since the development of intravascular ultrasound (IVUS) that provides more information on the vessel wall including the size and location of atheromatous plaques[30], several clinical trials have examined the effects of lipid-lowering by statins on changes in plaque volume. The findings have been fairly consistent with intensive lipid-lowering resulting in attenuation of plaque progression with some plaque regression; greater response being seen in patients with prior acute coronary events[31]. The regression study design used in the present study included switching from high fat high cholesterol containing WD to regular chow diet limiting fat and cholesterol intake but plasma TC was not significantly different after treatment although a significant decrease in plasma TG was noted. Although currently undefined, this observed reduction in plasma TG levels could potentially be related to modulation of macrophage phenotype as a result of reduced cholesterol accumulation that is known to not only affect macrophage lipid metabolism[32, 33] but also modulates hepatic as well as adipose tissue functions[34]. Therefore, in contrast to the numerous clinical trials with extensive lipid-lowering therapeutics, the significant regression observed following treatment with the “Two-pronged complex” (Figure 8) with no change in plasma TC levels is likely due to decreased influx and enhanced removal of FC from the existing plaques. Biochemical analyses of the atherosclerotic plaques of the aortic arch showing a significant decrease in aortic arch FC and CE further supports this conclusion (Figure 9).
A balance between cholesterol influx and efflux pathways within the macrophage is central to CE accumulation and foam cell formation. Favorable modulation of either of these pathways is expected to reduce macrophage CE content. Consistently, decrease in influx by limiting the availability of modified LDL either by reducing de novo hepatic cholesterol biosynthesis by statins or reducing intestinal cholesterol absorption by inhibiting NPC1L1 by Ezetimibe reduce foam cell formation and progression of atherosclerotic plaques. Myrecitin or Naoxintong mediated reduction in foam cell formation and atherosclerosis by reducing macrophage uptake of oxLDL via modulation of scavenger receptors SR-A or CD-36 has also been reported[35, 36]. Similarly, strategies to enhance cholesterol efflux, such as increase in intracellular CE hydrolysis[19, 37], increase in expression of macrophage cholesterol transporters [38] and availability of extracellular cholesterol acceptors increase FC efflux with subsequent reduction in macrophage foam cell formation and attenuation of atherosclerosis[39–41]. Conversely, combined deficiency of cholesterol transporters ABCA1 and ABCG1 increase atherosclerosis in mice[42, 43]. In the present study, we simultaneously modulated influx and efflux pathways using the novel “Two-pronged complex”. Significantly greater reduction in macrophage cholesterol content compared to inhibiting influx or enhancing efflux alone (Figure 5) clearly demonstrate the advantage of this approach. Dual or multiple pathway inhibition is a well-established anti-cancer approach and COMPASS trial also validated the efficacy of dual pathway inhibition as an anticoagulant strategy in reducing adverse cardiovascular events [44, 45].
We developed and established mDNP delivery platform to successfully deliver therapeutics to atherosclerotic plaque-associated macrophages with minimal uptake by liver and demonstrated the anti-atherosclerotic effects of enhancing macrophage cholesterol efflux by increasing the expression of macrophage cholesterol transporters via LXR activation without increasing LXR-mediated hepatic lipogenesis[16]. Building on this established platform, we delivered the “Two-pronged complex” to macrophages and demonstrated reduction in SR-A expression and oxLDL uptake (Figure 3) as well as increase in LXR-target genes, ABCA1 and ABCG1 and subsequent increase in FC efflux (Figure 4). Consistent with these in vitro observations and our earlier demonstration of targeted delivery of mDNPs to atherosclerotic plaques[16], changes in expression of these genes (SR-A, ABCA1 and ABCG1) were maintained for at least 7 days (Figure 6). Since reduction in foam cell formation and atherosclerosis by modulation of scavenger receptor dependent mLDL uptake has been reported[35] and we have demonstrated attenuation of atherosclerosis progression by mDNP-mediated delivery of LXR-L[16], the focus of this study was to specifically evaluate regression of atherosclerosis using only the “Two-pronged complex” and the in vivo study was planned accordingly. Technical limitations of quantitative mouse plaque imaging currently preclude the evaluation of regression of atherosclerotic plaques in vivo before and after treatment in the same animal. Therefore, comparisons are shown between Baseline Group and Untreated or Treated Group. This strategy is consistent with that used by Pennig et al[46]. Barrett et al also used a similar protocol of evaluating regression to a baseline group although plaque regression was examined following transplantation of atherosclerotic aortic arch into normolipidemic mice[47]. Reduction in total or aortic arch plaque area by ~20% and ~60% decrease in aortic arch FC or CE is comparable to the reduction in plaque area evaluated by Feig et al using the transplantation model[48]. It is noteworthy that meta analyses of 17 prospective studies published between 2001 and 2018 (Total 6333 patients) where changes in percent atheroma volume were measured by IVUS indicated that a 1% reduction in mean plaque volume was associated with a 20% reduction in the odds of major adverse cardiovascular events or MACE[49]. The observed reduction in atherosclerotic plaques of >20% following treatment with “Two-pronged complex” underscores the significance of this therapeutic strategy for reducing the burden of atherosclerotic disease.
5. Conclusions
In summary, the data presented herein not only validate the efficacy of the novel “Two-pronged complex” to modulate the respective functions of the cargo (siRNA to SR-A and LXR-L) in vitro but also demonstrate regression of atherosclerotic plaques in vivo. We[16] and others[24] have reported reduction in WD-induced atherosclerosis by nanoparticle-based delivery of LXR-L, and this study further demonstrates the application of mannose functionalized dendrimeric nanoparticle or mDNP based delivery platform to simultaneously deliver two therapeutics to facilitate plaque regression.
Acknowledgements
This work was supported in part by NIH MPI grant to SG and HY (HL140684) and VA MERIT Award to SG (IO1 BX002297). HH was supported by a Post-doctoral fellowship from the American Heart Association (17POST33660608). The Lipidomics Core Facility at Wayne State University is supported in part by National Center for Research Resources, National Institutes of Health Grant S10RR027926.
Abbreviations:
- ABCA1
ATP-binding cassette A1
- ABCG1
ATP-binding cassette G1
- CD36
cluster of differentiation 36
- CE
Cholesterol ester
- COMPASS
Cardiovascular Outcomes for People Using Anticoagulation Strategies
- DNP
Dendrimeric nanoparticles
- DLS
Dynamic light scattering
- FC
Free cholesterol
- IVUS
Intravascular ultrasound
- LDL
Low-density lipoprotein
- LDL-C
LDL cholesterol
- LXR
Liver X receptor
- LXR-L
Liver X receptor ligand
- mDNP
Mannose functionalized DNP
- mLDL
modified low-density lipoprotein
- MPMs
Mouse peritoneal macrophages
- NPC1L1
Niemann-Pick C1 Like 1
- OxLDL
Oxidized low-density lipoprotein
- PBS
Phosphate buffered saline
- siRNA
Small interfering RNA
- SR-A
Scavenger receptor class A
- TEM
Transmission electron microscopy
- WD
Western type diet
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of competing interest
The authors declare no conflict of interest.
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- [1].P.D.o.A.i.Y.R. Group, Natural history of aortic and coronary atherosclerotic lesions in youth: findings from the PDAY Study, Arterioscler. Thromb 13 (1993), 1291–1298. [DOI] [PubMed] [Google Scholar]
- [2].Strong JP, Natural history and risk factors for early human atherogenesis. Pathobiological Determinants of Atherosclerosis in Youth (PDAY) Research Group, Clin Chem 41 (1995), 134–138. [PubMed] [Google Scholar]
- [3].Collaboration PS, Blood cholesterol and vascular mortality by age, sex, and blood pressure: a meta-analysis of individual data from 61 prospective studies with 55 000 vascular deaths, Lancet 370 (2007), 1829–1839. [DOI] [PubMed] [Google Scholar]
- [4].ASSESSMENT R, Major lipids, apolipoproteins, and risk of vascular disease, JAMA 302 (2009), 19932000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Baigent C, Blackwell L, Emberson J, Holland L, Reith C, Bhala N, Peto R, Barnes E, Keech A, Simes J, Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials, Lancet 376 (2010), 1670–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, Kuder JF, Wang H, Liu T, Wasserman SM, Evolocumab and clinical outcomes in patients with cardiovascular disease, N Engl J Med 376 (2017), 1713–1722. [DOI] [PubMed] [Google Scholar]
- [7].Ghosh S, Bie J, Wang J, Yuan Q, Ghosh SS, Cholesterol removal from plaques and elimination from the body: change in paradigm to reduce risk for heart disease, Clin. Lipidol 9 (2014), 429–440. [Google Scholar]
- [8].Rothblat G, De La Llera-Moya M, Favari E, Yancey P, Kellner-Weibel G, Cellular cholesterol flux studies: methodological considerations, Atherosclerosis 163 (2002), 1–8. [DOI] [PubMed] [Google Scholar]
- [9].Zhao B, Song J, Chow WN, Clair RWS, Rudel LL, Ghosh S, Macrophage-specific transgenic expression of cholesteryl ester hydrolase significantly reduces atherosclerosis and lesion necrosis in Ldlr−/−mice, J Clin Invest 117 (2007), 2983–2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Vaisman BL, Lambert G, Amar M, Joyce C, Ito T, Shamburek RD, Cain WJ, Fruchart-Najib J, Neufeld ED, Remaley AT, ABCA1 overexpression leads to hyperalphalipoproteinemia and increased biliary cholesterol excretion in transgenic mice, J Clin Invest 108 (2001), 303–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Venkateswaran A, Laffitte BA, Joseph SB, Mak PA, Wilpitz DC, Edwards PA, Tontonoz P, Control of cellular cholesterol efflux by the nuclear oxysterol receptor LXRα, Proc Natl Acad Sci U S A 97 (2000), 12097–12102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Collins JL, Fivush AM, Watson MA, Galardi CM, Lewis MC, Moore LB, Parks DJ, Wilson JG, Tippin TK, Binz JG, Identification of a nonsteroidal liver X receptor agonist through parallel array synthesis of tertiary amines, J Med Chem 45 (2002), 1963–1966. [DOI] [PubMed] [Google Scholar]
- [13].Terasaka N, Hiroshima A, Koieyama T, Ubukata N, Morikawa Y, Nakai D, Inaba T, T - 0901317, a synthetic liver X receptor ligand, inhibits development of atherosclerosis in LDL receptor - deficient mice, FEBS Lett 536 (2003), 6–11. [DOI] [PubMed] [Google Scholar]
- [14].Schultz JR, Tu H, Luk A, Repa JJ, Medina JC, Li L, Schwendner S, Wang S, Thoolen M, Mangelsdorf DJ, Role of LXRs in control of lipogenesis, Genes Dev 14 (2000), 2831–2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Grefhorst A, Elzinga BM, Voshol PJ, Plösch T, Kok T, Bloks VW, van der Sluijs FH, Havekes LM, Romijn JA, Verkade HJ, Stimulation of lipogenesis by pharmacological activation of the liver X receptor leads to production of large, triglyceride-rich very low density lipoprotein particles, J Biol Chem 277 (2002), 34182–34190. [DOI] [PubMed] [Google Scholar]
- [16].He H, Yuan Q, Bie J, Wallace RL, Yannie PJ, Wang J, Lancina III MG, Zolotarskaya OY, Korzun W, Yang H, Ghosh S, Development of mannose functionalized dendrimeric nanoparticles for targeted delivery to macrophages: use of this platform to modulate atherosclerosis, Transl Res 193 (2018), 13–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].He H, Lancina III MG, Wang J, Korzun WJ, Yang H, Ghosh S, Bolstering cholesteryl ester hydrolysis in liver: A hepatocyte-targeting gene delivery strategy for potential alleviation of atherosclerosis, Biomaterials 130 (2017), 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Zhao B, Song J, St. Clair RW, Ghosh S, Stable overexpression of human macrophage cholesteryl ester hydrolase results in enhanced free cholesterol efflux from human THP1 macrophages, Am J Physiol Cell Physiol 292 (2007), C405–C412. [DOI] [PubMed] [Google Scholar]
- [19].Bie J, Zhao B, Marqueen KE, Wang J, Szomju B, Ghosh S, Macrophage-specific transgenic expression of cholesteryl ester hydrolase attenuates hepatic lipid accumulation and also improves glucose tolerance in ob/ob mice, Am J Physiol Endocrinol Metab 302 (2012), E1283–E1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Bligh EG, Dyer WJ, A rapid method of total lipid extraction and purification, Can J Biochem Physiol 37 (1959), 911–917. [DOI] [PubMed] [Google Scholar]
- [21].Fessler MB, The challenges and promise of targeting the Liver X Receptors for treatment of inflammatory disease, Pharmacol Ther 181 (2018), 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Lund EG, Menke JG, Sparrow CP, Liver X receptor agonists as potential therapeutic agents for dyslipidemia and atherosclerosis, Arterioscler Thromb Vasc Biol 23 (2003), 1169–1177. [DOI] [PubMed] [Google Scholar]
- [23].Guo Y, Yuan W, Yu B, Kuai R, Hu W, Morin EE, Garcia-Barrio MT, Zhang J, Moon JJ, Schwendeman A, Synthetic high-density lipoprotein-mediated targeted delivery of liver X receptors agonist promotes atherosclerosis regression, EBioMedicine 28 (2018), 225–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Zhang XQ, Even-Or O, Xu X, van Rosmalen M, Lim L, Gadde S, Farokhzad OC, Fisher EA, Nanoparticles containing a liver X receptor agonist inhibit inflammation and atherosclerosis, Adv Healthc Mater 4 (2015), 228–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Flores AM, Ye J, Jarr K-U, Hosseini-Nassab N, Smith BR, Leeper NJ, Nanoparticle therapy for vascular diseases, Arterioscler Thromb Vasc Biol 39 (2019), 635–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Kannan R, Nance E, Kannan S, Tomalia DA, Emerging concepts in dendrimer - based nanomedicine: from design principles to clinical applications, J Intern Med 276 (2014), 579–617. [DOI] [PubMed] [Google Scholar]
- [27].Svenson S, Tomalia DA, Dendrimers in biomedical applications—reflections on the field, Adv Drug Deliv Rev 64 (2012), 102–115. [DOI] [PubMed] [Google Scholar]
- [28].Brown BG, Zhao X-Q, Sacco D, Albers J, Lipid lowering and plaque regression. New insights into prevention of plaque disruption and clinical events in coronary disease, Circulation 87 (1993), 1781–1791. [DOI] [PubMed] [Google Scholar]
- [29].Farmer JA, Gotto AM Jr, Dyslipidemia and the vulnerable plaque, Prog Cardiovasc Dis 44 (2002), 415–428. [DOI] [PubMed] [Google Scholar]
- [30].Mintz GS, Nissen SE, Anderson WD, Bailey SR, Erbel R, Fitzgerald PJ, Pinto FJ, Rosenfield K, Siegel RJ, Tuzcu EM, American College of Cardiology clinical expert consensus document on standards for acquisition, measurement and reporting of intravascular ultrasound studies (ivus): A report of the american college of cardiology task force on clinical expert consensus documents developed in collaboration with the european society of cardiology endorsed by the society of cardiac angiography and interventions, J Am Coll Cardiol 37 (2001), 1478–1492. [DOI] [PubMed] [Google Scholar]
- [31].Daida H, Dohi T, Fukushima Y, Ohmura H, Miyauchi K, The Goal of Achieving Atherosclerotic Plaque Regression with Lipid-Lowering Therapy: Insights from IVUS Trials, J Atheroscler Thromb 26 (2019), 48603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Posokhova E, Khoshchenko O, Chasovskikh M, Pivovarova E, Dushkin M, Lipid synthesis in macrophages during inflammation in vivo: effect of agonists of peroxisome proliferator activated receptors α and γ and of retinoid X receptors, Biochemistry (moscow) 73 (2008), 296–304. [DOI] [PubMed] [Google Scholar]
- [33].Odegaard JI, Chawla A, Alternative macrophage activation and metabolism, Annu Rev Pathol 6 (2011), 275–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Bie J, Zhao B, Song J, Ghosh S, Improved Insulin Sensitivity in High Fat-and High Cholesterol-fed Ldlr−/− Mice with Macrophage-specific Transgenic Expression of Cholesteryl Ester Hydrolase ROLE OF MACROPHAGE INFLAMMATION AND INFILTRATION INTO ADIPOSE TISSUE, J Biol Chem 285 (2010), 13630–13637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Meng Z, Wang M, Xing J, Liu Y, Li H, Myricetin ameliorates atherosclerosis in the low-density-lipoprotein receptor knockout mice by suppression of cholesterol accumulation in macrophage foam cells, Nutr Metab (Lond) 16 (2019), 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Wang Z, Ge J, Sun A, Shi H, Zhao H, Dong Z, Zhao B, Weng X, Liu R, Li X, Naoxintong Retards Atherosclerosis by Inhibiting Foam Cell Formation through Activating PPARα Pathway, Curr Mol Med 18 (2018), 698–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Bie J, Zhao B, Ghosh S, Atherosclerotic lesion progression is attenuated by reconstitution with bone marrow from macrophage-specific cholesteryl ester hydrolase transgenic mice, Am J Physiol Regul Integr Comp Physiol 301 (2011), R967–R974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Teupser D, Kretzschmar D, Tennert C, Burkhardt R, Wilfert W, Fengler D.r., Naumann R, Sippel AE, Thiery J, Effect of macrophage overexpression of murine liver X receptor-α (LXR-α) on atherosclerosis in LDL-receptor deficient mice, Arterioscler Thromb Vasc Biol 28 (2008), 2009–2015. [DOI] [PubMed] [Google Scholar]
- [39].Uehara Y, Chiesa G, Saku K, High-density lipoprotein-targeted therapy and apolipoprotein AI mimetic peptides, Circ J 79 (2015), 2523–2528. [DOI] [PubMed] [Google Scholar]
- [40].Giannarelli C, Cimmino G, Ibanez B, Chiesa G, Garcia-Prieto J, Santos-Gallego CG, Alique-Aguilar M, Fuster V, Sirtori C, Badimon JJ, Acute ApoA-I Milano administration induces plaque regression and stabilisation in the long term, Thromb Haemost 108 (2012), 1246–1248. [DOI] [PubMed] [Google Scholar]
- [41].Gille A, D’Andrea D, Tortorici MA, Hartel G, Wright SD, CSL112 (apolipoprotein AI [human]) enhances cholesterol efflux similarly in healthy individuals and stable atherosclerotic disease patients, Arterioscler Thromb Vasc Biol 38 (2018), 953–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Out R, Hoekstra M, Habets K, Meurs I, de Waard V, Hildebrand RB, Wang Y, Chimini G, Kuiper J, Van Berkel TJ, Combined deletion of macrophage ABCA1 and ABCG1 leads to massive lipid accumulation in tissue macrophages and distinct atherosclerosis at relatively low plasma cholesterol levels, Arterioscler Thromb Vasc Biol 28 (2008), 258–264. [DOI] [PubMed] [Google Scholar]
- [43].Yvan-Charvet L, Ranalletta M, Wang N, Han S, Terasaka N, Li R, Welch C, Tall AR, Combined deficiency of ABCA1 and ABCG1 promotes foam cell accumulation and accelerates atherosclerosis in mice, J Clin Invest 117 (2007), 3900–3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Eikelboom JW, Connolly SJ, Bosch J, Dagenais GR, Hart RG, Shestakovska O, Diaz R, Alings M, Lonn EM, Anand SS, Rivaroxaban with or without aspirin in stable cardiovascular disease, N Engl J Med 377 (2017), 1319–1330. [DOI] [PubMed] [Google Scholar]
- [45].Coppens M, Weitz JI, Eikelboom JW, Synergy of dual pathway inhibition in chronic cardiovascular disease: lessons from the COMPASS trial, Circ Res 124 (2019), 416–425. [DOI] [PubMed] [Google Scholar]
- [46].Pennig J, Scherrer P, Gissler MC, Anto-Michel N, Hoppe N, L Füner C Härdtner, P. Stachon, D. Wolf, I. Hilgendorf, Glucose lowering by SGLT2-inhibitor empagliflozin accelerates atherosclerosis regression in hyperglycemic STZ-diabetic mice, Sci Rep 9 (2019), 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Barrett TJ, Distel E, Murphy AJ, Hu J, Garshick MS, Ogando Y, Liu J, Vaisar T, Heinecke JW, Berger JS, Apolipoprotein AI) Promotes Atherosclerosis Regression in Diabetic Mice by Suppressing Myelopoiesis and Plaque Inflammation, Circulation 140 (2019), 1170–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Feig JE, Pineda-Torra I, Sanson M, Bradley MN, Vengrenyuk Y, Bogunovic D, Gautier EL, Rubinstein D, Hong C, Liu J, LXR promotes the maximal egress of monocyte-derived cells from mouse aortic plaques during atherosclerosis regression, J Clin Invest 120 (2010), 4415–4424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Bhindi R, Guan M, Zhao Y, Humphries KH, Mancini GJ, Coronary atheroma regression and adverse cardiac events: A systematic review and meta-regression analysis, Atherosclerosis 284 (2019), 194–201. [DOI] [PubMed] [Google Scholar]


