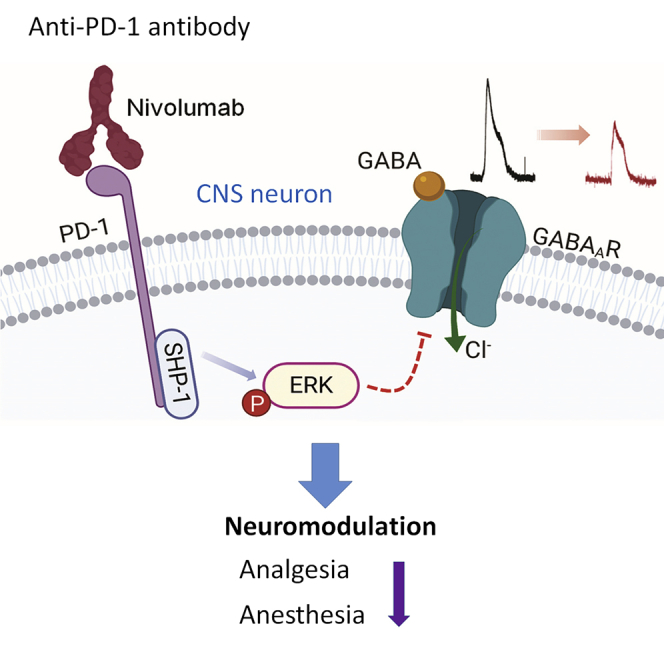
Summary
The immune checkpoint inhibitor programmed cell death protein 1 (PD-1) plays a critical role in immune regulation. Recent studies have demonstrated functional PD-1 expression in peripheral sensory neurons, which contributes to neuronal excitability, pain, and opioid analgesia. Here we report neuronal expression and function of PD-1 in the central nervous system (CNS), including the spinal cord, thalamus, and cerebral cortex. Notably, GABA-induced currents in spinal dorsal horn neurons, thalamic neurons, and cortical neurons are suppressed by the PD-1-neutralizing immunotherapeutic Nivolumab in spinal cord slices, brain slices, and dissociated cortical neurons. Reductions in GABA-mediated currents in CNS neurons were also observed in Pd1−/− mice without changes in GABA receptor expression. Mechanistically, Nivolumab binds spinal cord neurons and elicits ERK phosphorylation to suppress GABA currents. Finally, both GABA-mediated analgesia and anesthesia are impaired by Pd1 deficiency. Our findings reveal PD-1 as a CNS-neuronal inhibitor that regulates GABAergic signaling and GABA-mediated behaviors.
Subject Areas: Immunology, Molecular Biology, Neuroscience
Graphical Abstract
Highlights
-
•
Pd1 mRNA and PD-1 protein are widely expressed in spinal cord and brain neurons
-
•
GABA-induced currents in CNS neurons are suppressed by PD-1 blockade with Nivolumab
-
•
Nivolumab binds neuronal PD-1 to induce ERK activation and GABAergic inhibition
-
•
GABA-mediated pain inhibition and anesthesia is impaired after Pd1 deficiency
Immunology ; Molecular Biology; Neuroscience
Introduction
Programmed cell death protein-1 (PD-1), also known as cluster of differentiation 279 (CD279), is a member of the immunoglobulin gene superfamily. PD-1 was discovered during a screening for genes involved in apoptosis (Ishida et al., 1992). The PD-1 protein in humans is encoded by the PDCD1 gene (Shinohara et al., 1994). As PD-1 is expressed by T cells, B cells, and myeloid cells, mice lacking PD-1 (encoded by Pdcd1/Pd1) exhibit impaired immune tolerance and autoimmune features (Nishimura et al., 1999). Engagement of the PD-1 receptor with its ligand PD-L1, a T cell co-stimulatory molecule (Dong et al., 1999), leads to negative regulation of lymphocyte activation (Freeman et al., 2000). Immunotherapies using anti-PD-1 monoclonal antibodies have become the gold standard in treating various cancers (Brahmer et al., 2012; Herbst et al., 2014). Immunotherapy was also shown to improve Alzheimer disease, through activation of macrophages and clearance of cerebral amyloid-β plaques (Baruch et al., 2016), although this has been disputed (Latta-Mahieu et al., 2018).
Although neurons and immune cells are each unique cell types with regard to their morphology, gene expression, and function, primary sensory neurons in the dorsal root ganglion (DRG) express various immune regulatory proteins such as cytokines, cytokine receptors, and Toll-like receptors classically associated with host defense (Chiu et al., 2012; Donnelly et al., 2020; Liu et al., 2010; Talbot et al., 2016). We recently demonstrated that DRG nociceptive neurons of the peripheral nervous system (PNS) express functional PD-1 (Chen et al., 2017; Wang et al., 2020b). Pd1-deficient mice show hyperexcitability in sensory neurons and hypersensitivity to pain (Chen et al., 2017). PD-1 co-expression with mu opioid receptors in DRG neurons also regulates morphine analgesia (Wang et al., 2020b). It remains unknown whether neuronal PD-1 also plays an active role in the central nervous system (CNS). In this study we found that Pd1 mRNA and PD-1 protein are widely expressed by neurons in the spinal cord and different brain regions. GABAergic neurons are a major type of inhibitory neurons in the spinal cord and act as a gate control of pain (Braz et al., 2014; Melzack and Wall, 1965). GABAergic signaling in the brain plays a critical role in general anesthesia (Franks, 2008; Mihic et al., 1997). Our results demonstrate that PD-1 regulates GABA receptor signaling in spinal and brain neurons and acts as an inhibitor of CNS neuronal activity. We also find that at the behavioral level, GABA-mediated analgesia and anesthesia were compromised in mice lacking Pd1.
Results
Loss of PD-1 Function Impairs GABA-Mediated Currents in Dorsal Horn Neurons in Spinal Cord Slices
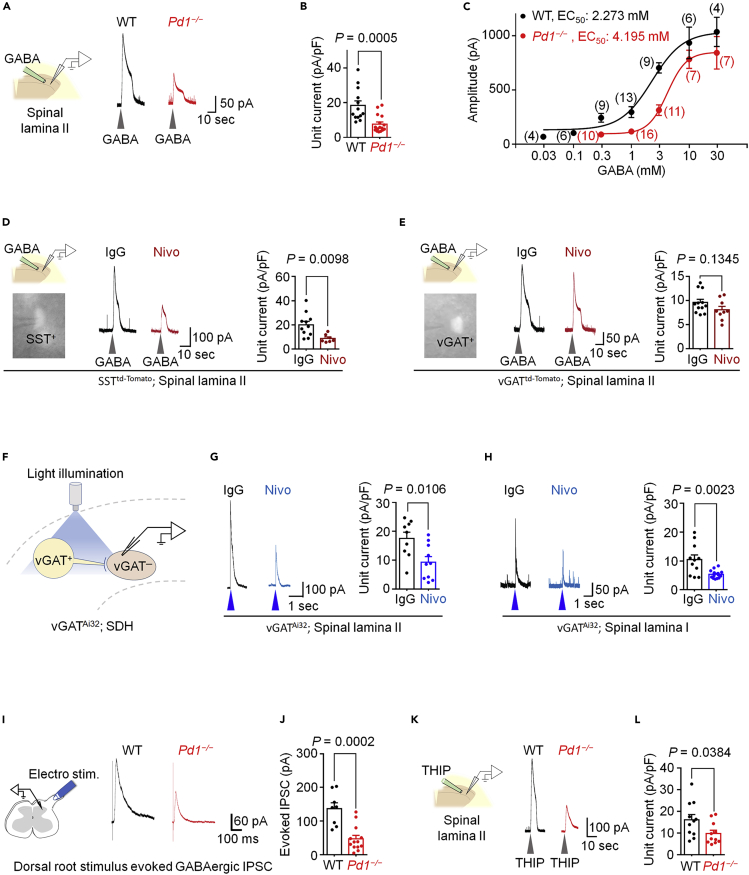
To determine the role of PD-1 in regulating the function of GABAergic neurotransmission in the spinal dorsal horn (SDH), we prepared spinal cord slices and recorded GABA-evoked currents in outer lamina II (IIo) neurons of wild-type (Pd1+/+, WT) and Pd1−/− (KO) mice (Figure 1A). Lamina IIo interneurons are predominantly excitatory and form a nociceptive circuit with primary C-afferents and lamina I projection neurons (Braz et al., 2014; Duan et al., 2018; Todd, 2010). Local application of GABA (1 mM) to spinal cord slices of WT mice induced a robust outward current (Figure 1A), but this GABA-mediated current was markedly reduced in Pd1−/− mice (Figure 1B). A dose-response curve analysis revealed a right-shift in Pd1-deficient neurons (WT: EC50 = 2.3 mM, KO: EC50 = 4.2 mM, Figure 1C).
Figure 1.
PD-1 is Required for GABAergic Signaling in SDH Neurons in Spinal Cord Slices
(A–C) GABA-induced currents in SDH lamina II neurons of WT and Pd1−/− mice. (A) Left: experimental setup for local perfusion of GABA (1 mM) in spinal cord slices. Right: representative traces of currents evoked by transient GABA perfusion.
(B) Quantification of GABA-induced unit currents from WT and Pd1−/− mice. n = 13 and 16 neurons from 4 animals.
(C) Dose-response curve plotting the average amplitude of GABA-induced currents. The curves were drawn according to the Hill equation. Values in parentheses denote the number of recorded neurons per group, each taken from 3–4 animals per group.
(D and E) GABA-induced currents in lamina II neurons of spinal cord slices from SSTtd−Tomato reporter mice (D) and vGATtd−Tomato reporter mice (E). Left top: experimental setup for GABA perfusion, as in (A). Left bottom: epifluorescence image of a spinal lamina II SST+ neuron or vGAT+ neuron with a recording pipette. Middle: traces of GABA (1 mM)-induced currents after incubation with IgG or Nivo (300 ng/mL, 2 h). Right: quantification of GABA-evoked unit currents. n = 11 (IgG, 6 mice, D); n = 7 (Nivo, 3 mice, D); n = 12 (IgG, 5 mice, E); n = 9 (Nivo, 4 mice, E).
(F–H) Blue-light-induced currents in spinal cord slices of vGATAi32 mice. (F) Schematic of experimental setup. vGAT+ neurons were stimulated with blue light (473 nm, 4 ms) to evoke endogenous GABA release, and postsynaptic currents were recorded from vGAT− neurons in lamina II (G) and lamina I (H) neurons. (G) Left: representative traces of light-induced currents from vGAT− neurons in spinal lamina II after incubation with IgG or Nivo (300 ng/mL, 2 h). Right: unit currents of light-evoked currents (n = 8 and 12 neurons from 3 animals/group). (H) Identical setup as in (G) but performed in spinal lamina I (n = 11 and 14 from 3 animals/group).
(I and J) Dorsal root stimulation evoked currents in spinal cord slices from WT and Pd1−/− mice. (I) Left: experimental setup. An electrical stimulus was applied to the spinal dorsal root to evoke GABA-evoked IPSCs. Right: representative traces of evoked IPSCs from WT and Pd1−/− mice. (J) Quantification of the amplitude of the evoked IPSCs from WT and Pd1−/− mice. n = 8 and 13 neurons recorded from 4 and 3 animals, respectively.
(K–L) Same setup as in (A and B), but using the GABAAR selective agonist THIP (1 mM; n = 11 neurons per group from 3 animals per group).
Gray arrowheads indicate the application of GABA or THIP. Blue arrowheads indicate the blue light stimulus. VH = 0 mV. Unpaired two-tailed t test. Each graph indicates the mean ± SEM.
Next we investigated whether impaired GABAergic neurotransmission in Pd1−/− mice could be recapitulated by functional blockade of PD-1 in WT mice. Nivolumab is an anti-human PD-1 monoclonal antibody, clinically used as a cancer immunotherapeutic, which we previously reported also binds mouse PD-1 on mouse sensory neurons (Chen et al., 2017). We incubated spinal cord slices with Nivolumab or human IgG4 (an isotype control antibody) for 2 h at a low concentration (300 ng/mL ≈ 2.1 nM). These spinal cord slices were prepared from reporter mice that label somatostatin-expressing (SST+) excitatory neurons (Chamessian et al., 2018; Duan et al., 2014; Haring et al., 2018) or vGAT-expressing (vGAT+) inhibitory neurons (Figures 1D and 1E). Nivolumab markedly suppressed GABA-induced currents in lamina IIo in SST+ neurons (Figure 1D), without altering GABA-induced currents in vGAT+ neurons (Figure 1E), suggesting that Nivolumab regulates GABAergic transmission primarily in excitatory neurons. We confirmed the GABAA-mediated currents by complete blockade of the current by the selective antagonist bicuculline (Figure S1A).
To specifically activate inhibitory neurons in SDH, we employed an optogenetic approach using vGAT-Cre; Ai32 mice, wherein channelrhodopsin2 is expressed in inhibitory neurons. We activated inhibitory neurons by applying blue light stimulation onto spinal cord slices (Figure 1F). Blue light induced robust GABAergic outward currents (≈18 pA/pF) in lamina IIo vGAT− neurons, in the presence of strychnine (1 μM) during recordings (Figure 1G). Remarkably, Nivolumab treatment (2.1 nM, 2 h) suppressed the current by >50% in lamina IIo neurons compared with immunoglobulin G (IgG) control (Figures 1F and 1G). As expected, this current was completely blocked by bicuculline (Figure S1B). Nivolumab also suppressed the blue-light-induced GABAergic currents in lamina I neurons (Figure 1H), supporting a broader effect of PD-1 in modulating inhibitory neurotransmission in SDH neurons. We further examined the paired-pulse ratio to assess the presynaptic modification by PD-1 in lamina IIo neurons. We observed no difference in the ratio between Nivolumab and IgG-treated neurons (Figure S1C), indicating a postsynaptic modification of inhibitory synaptic transmission by PD-1.
We next recorded evoked synaptic GABA currents in SDH by electrical stimulation of the dorsal root attached to a spinal cord slice (Figure 1I). The evoked GABA inhibitory postsynaptic currents (IPSCs) were significantly reduced in lamina IIo neurons of KO mice (p = 0.0002, versus WT, Figures 1I and 1J). α1 and α2 are two major subunits of GABAA receptors in SDH (Zeilhofer et al., 2012), and α2 subunit is the predominant one in the superficial layers of SDH (Paul et al., 2012). Western blot analysis showed comparable expression of α1 and α2 subunits in SDH of WT and KO mice (Figure S1E).
We also found that GABA-current in the SDH was mediated by inotropic GABAA receptor, as the GABAA antagonist completely blocked the current (Figures S1A). Local application of the GABAA agonist THIP (1 mM) elicited a robust outward current of ∼16.5 pA/pF in lamina IIo neurons, but this GABAA-mediated current was substantially reduced in neurons lacking Pd1 (Figures 1K–1L), showing a 5-fold change in EC50 values (WT: 0.17 mM; KO: 0.87 mM; Figure S1D). Thus, PD-1 regulates GABAergic transmission via GABAA receptors in spinal cord neurons.
Nivolumab Binds PD-1 and Activates ERK to Suppress GABA Transmission in SDH Neurons
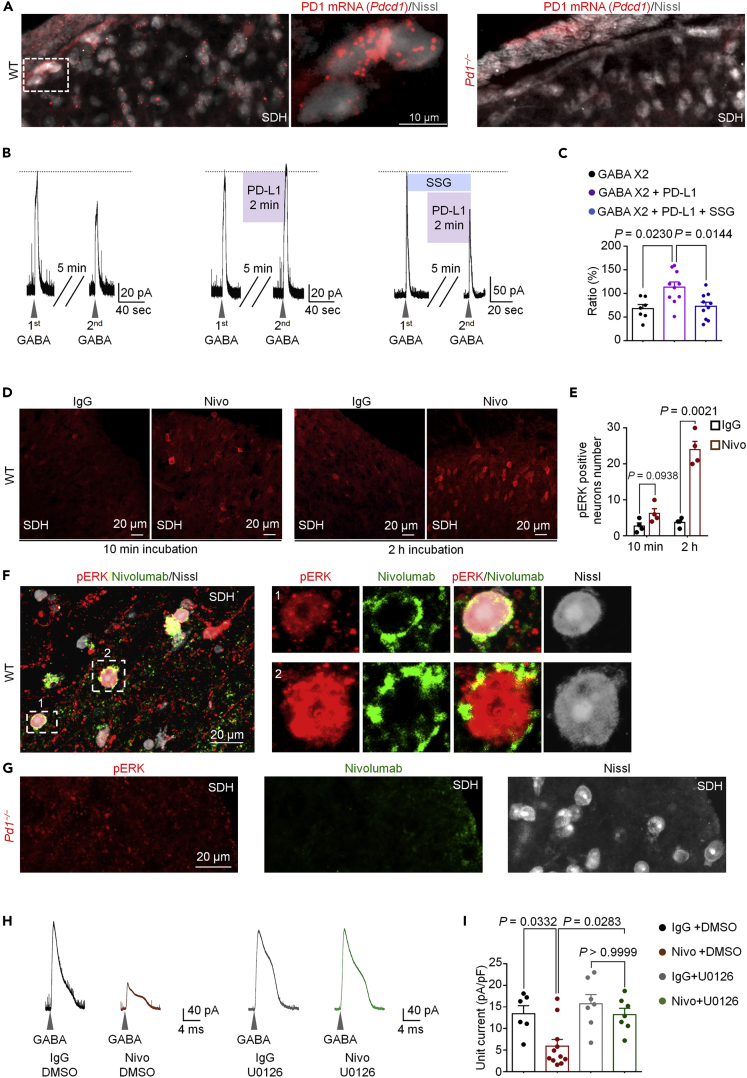
We investigated Pd1 mRNA and PD-1 protein expression in SDH neurons using in situ hybridization and immunohistochemistry. In situ hybridization with RNAscope revealed Pd1 mRNA expression in SDH neurons of WT mice but not Pd1−/− mice (Figure 2A). Immunohistochemistry showed broad expression of PD-1 in SDH neurons, including laminae I and II neurons, and this expression was abolished by blocking peptide treatment in WT mice and in Pd1−/− mice (Figures S2A). We also observed that approximately 70% of excitatory neurons and 40% of inhibitory neurons in SDH express Pd1 mRNA, supporting a primary modulation of excitatory neuron function by PD-1 (Figures S2B and S2C).
Figure 2.
Nivolumab Binds PD-1 and Activates ERK to Suppress GABA Transmission in SDH Neurons
(A) Representative images showing localization of Pd1 mRNA by fluorescent in situ hybridization in the superficial SDH in WT (left) and Pd1−/− mice (right). Middle: higher magnification image of the dashed box. SDH neurons are labeled with Nissl staining. Note that Pd1 mRNA expression is lost in Pd1−/− mice (right).
(B) Left: representative currents induced by first GABA and second application of GABA (0.3 mM) in spinal lamina II neurons. Middle, same as left panel, but the second GABA application was combined with PD-L1 (30 ng/mL). Right: same as left panel, but the second GABA application was combined with PD-L1 and the SHP-1 inhibitor SSG (11 μM).
(C) Quantification of the amplitude of second GABA-induced currents (ratio of the first one) in vehicle/ACSF group (n = 8 neurons from 3 mice), PD-L1-treated group (n = 11 from 6 mice), and PD-L1- and SSG-treated group (n = 10 from 5 mice). (D–E) Nivolumab-induced pERK expression in spinal cord slices. (D) SDH immunostained for pERK following IgG or Nivo (300 ng/mL) incubation for 10 min (left) or 2 h (middle). (E) Quantification of the number of pERK-positive neurons in the SDH after IgG or Nivo incubation. n = 4 mice per group.
(F and G) Left: double staining of pERK and Nivolumab in SDH from WT mice (F) and Pd1−/− mice (G) after Nivo treatment (1000 ng/mL, 2 hr). Higher magnification images of box-1 and box-2 are indicated in the right panel in F, with each channel displayed separately.
(H and I) Effects of U0126 on GABA-induced currents in lamina II neurons of spinal cord slices. (H) Example traces of GABA (1 mM)-induced currents after 2 h incubation with IgG and vehicle, Nivo (300 ng/mL) and vehicle, IgG and U0126 (1 μM), and Nivo (300 ng/mL), and U0126 (1 μM). (I) Unit currents of GABA currents after incubation with IgG and vehicle (n = 6 from 4 mice), Nivo and vehicle (n = 11 from 5 mice), IgG and U0126 (n = 7 from 3 mice), or Nivo and U0126 (n = 8 from 3 mice). Gray arrowheads indicate the application of GABA (1 mM). VH = 0 mV.
One-way ANOVA followed by Bonferroni post-hoc test. Data displayed indicate the mean ± SEM.
Neurokinin-1 receptor (NK1R, encoded by Tacr1) is expressed by projection neurons in the spinal lamina I (Ikeda et al., 2003; Todd, 2010), and NK1R + projection neurons exhibit marked synaptic plasticity following tissue injury and are essential for generating pathological pain (Ikeda et al., 2003; Mantyh et al., 1997; Nichols et al., 1999). Interestingly, Pd1 was expressed by 80% of Tacr1+ neurons, in further support of its role of regulating the function of these key projection neurons (Figures S2D and S2E). Pd1 transcript was absent in SDH of Pd1−/− mice (Figure S2F).
PD-L1 and PD-L2 are endogenous ligands of PD-1, and administration of PD-L1 was shown to suppress pain via PD-1 and a downstream tyrosine phosphatase SHP-1 (Chen et al., 2017). We observed that PD-1 activation by PD-L1 (30 ng/mL) enhanced GABA (0.3 mM)-induced current in SDH neurons (Figures 2B and 2C). Application of the SHP-1 inhibitor SSG (Chen et al., 2017) blocked the effect of PD-L1 in SDH neurons (Figures 2B and 2C), suggesting an involvement of SHP-1 in PD-1 modulation of GABA transmission. In situ hybridization revealed very low expression of Pdl1 mRNA (CD274) and Pdl2 (CD273) mRNA in SDH (Figure S2I). However, Pdl1 was previously detected in DRG neurons, and PD-L1 protein was also detected in DRG and spinal cord tissues by ELISA (Chen et al., 2017). It is likely that PD-1 in SDH could be activated by PD-L1 released from primary sensory neurons.
SHP-1 is known to suppress the activation (phosphorylation) of extracellular signaling-regulated kinase (pERK) in immune cells (Cerny et al., 2017). Phosphorylation of extracellular signaling-regulated kinase (pERK) in SDH neurons following intense noxious stimulation or tissue injury plays an important role in neuronal sensitization and pain hypersensitivity (Ji et al., 2009; Karim et al., 2001). Strikingly, incubation of spinal cord slices with Nivolumab for 2 h, in the absence of PD-L1 or nociceptive stimuli, was sufficient to induce pERK expression in SDH neurons (Figures 2D and 2E). Double staining revealed many SDH neurons showing both Nivolumab labeling (green) and pERK expression (red) after the Nivolumab treatment (Figure 2F). This double labeling was abolished in spinal sections of Pd1−/− mice (Figure 2G). These data suggest that (1) Nivolumab binds to SDH neurons in a PD-1-dependent manner and (2) Nivolumab binding is associated with pERK induction. We also found that the majority of pERK-labeled neurons were SST+ excitatory neurons (Figures S2G and S2H). To assess the functional contribution of ERK, we treated spinal cord slices with an ERK kinase inhibitor U0126 (1 μM), given 20 min prior to patch recording. We observed that the Nivolumab-induced inhibition of GABA currents was blocked by U0126 (Figures 2H and 2I). Collectively, these results indicate that (1) there is a constitutive activity of PD-1 in the absence of its ligands and (2) Nivolumab activates ERK to suppress GABAergic transmission in SDH neurons.
PD-1 Modulates GABA-Mediated Currents in Different Brain Regions
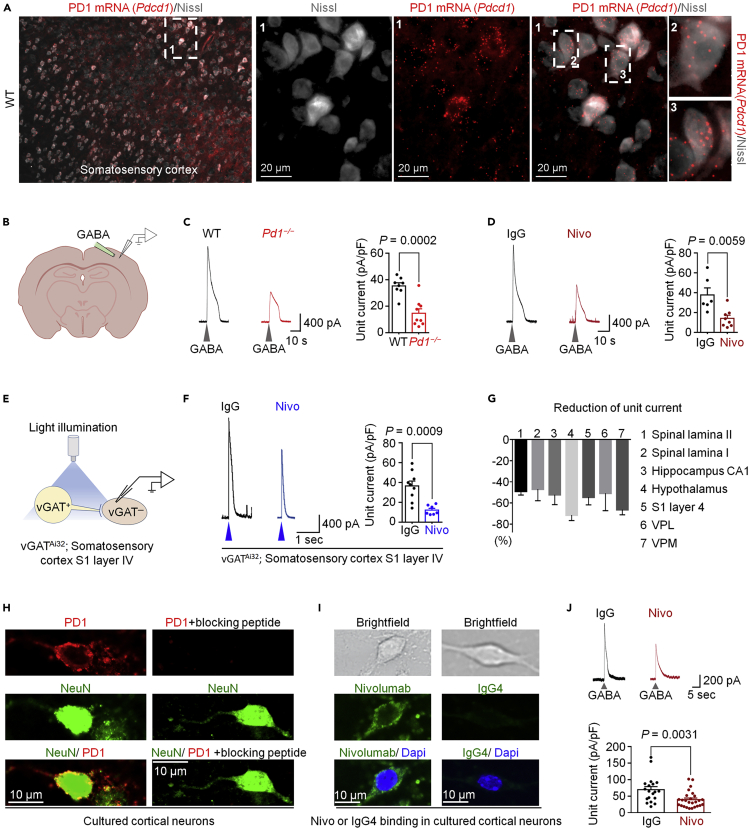
In situ hybridization and immunohistochemistry showed broad expression of Pd1 mRNA and PD-1 protein in S1 somatosensory cortex (Figures 3A and S3A). We prepared brain slices for patch clamp recordings in layer IV vGAT-negative neurons of S1 cortex in WT and Pd1−/− mice, as well as WT mice treated with Nivolumab (Figure 3B). We observed a substantial reduction of GABA-induced currents in KO mice (compared with WT mice, Figure 3C) and also in WT-S1 neurons treated with Nivolumab (compared with IgG, Figure 3D). Furthermore, in the presence of strychnine, blue-light-evoked GABAergic currents in S1-layer IV neurons was suppressed by Nivolumab (Figures 3E and 3F). Nivolumab had no effect on GABA-induced currents in S1-vGAT+ neurons (Figure S3B), suggesting that PD-1 might modulate GABA-currents selectively in excitatory cortical neurons.
Figure 3.
PD-1 Modulates GABA-Mediated Currents in Brain Slices and Dissociated Cortical Neurons
(A) Left: in situ hybridization for Pd1 mRNA expression on somatosensory cortical neurons (co-stained with Nissl in gray) in WT mice. The box is enlarged in three middle panels (labeled with 1). Boxes 2 and 3 are enlarged on the right panels.
(B–D) GABA-induced currents in S1 cortex of brain slices. (B) Experimental setup used for local perfusion of GABA in the layer IV of somatosensory cortex. (C) Left: exemplary traces of currents irritated by transient GABA (1 mM) perfusion from WT and Pd1−/− mice. Right: unit currents. n = 8 and 9 from 3 animals/group. (D) Left: exemplary traces of currents produced by GABA (1 mM) perfusion after incubation with IgG or Nivo (300 ng/mL, 2 h). Right: unit currents. n = 6 and 8 from 5 and 3 animals, respectively.
(E–G) Light-evoked GABA currents in brain slices produced from vGATAi32 mice. (E) Experimental setup showing vGAT+ neurons stimulated with blue light (473 nm, 4 ms) and postsynaptic current recorded from vGAT-negative neurons in the layer IV of somatosensory cortex. (F) Left: exemplary traces of light-induced currents from vGAT-negative neurons treated with IgG or Nivo (300 ng/mL, 2 h). Right, unit currents of light-induced currents. n = 9 and 8, from 3 animals/group. (G) Percentile reduction of light-induced unit currents by Nivolumab (300 ng/mL, 2 h) in different regions of spinal cord and brain.
(H–J) Inhibition of GABA-induced currents by Nivolumab in cultured cortical neurons. (H) Left: double staining of PD-1 and NeuN in cultured cortical neurons. Right: absence of PD-1 immunostaining upon treatment of a blocking peptide. (I) Top: bright field images of cultured cortical neurons. Middle and bottom: immunostaining for Nivo or IgG4 after Nivo or IgG4 (300 ng/mL, 2 h). (J) Upper: exemplary traces of GABA (1 mM)-induced currents in cortical neurons treated with IgG or Nivo (300 ng/mL, 2 h). Lower: unit currents of GABA currents. n = 19 and 26 from 3 animals per group. The cortical neurons were cultured for 7–8 days before experiment. Gray arrowheads indicate the application of GABA. Blue arrowheads indicate the illumination of blue light. VH = 0 mV. Unpaired two-tailed t test. All error bars indicate the mean ± SEM.
We also tested the actions of Nivolumab on blue-light-evoked GABAergic currents in neurons of the brain regions in the thalamus that are involved in pain modulation, including the ventrolateral thalamic nucleus (VPL) and ventromedial thalamic nucleus (VPM), both of which expressed PD-1 (Figure S3C). We observed a marked reduction of the light-evoked GABA currents in vGAT− neurons in the VPL and VPM by Nivolumab (Figures 3G and S3D–S3E), but no difference was observed in vGAT+ neurons in the thalamus. Compared with IgG, Nivolumab also suppressed light-induced GABA currents in vGAT− neurons in hippocampal CA1 and hypothalamic neurons (Figures 3G and S3G–S3H). The reduction of GABA currents in different brain regions ranged from 50%–70% (Figure 3G). Compared with the spinal cord, the distribution of the GABAA receptor subunits in the brain is more sophisticated, but the α1 subunit is the most abundant one in almost all the regions (Pirker et al., 2000; Sieghart et al., 1999). Western blotting showed comparable expression of α1 subunit of GABAA receptor in the somatosensory cortex, thalamus, and hypothalamus in WT and KO mice (Figures S2I–S2J). Together, these results suggest that PD-1 is a pan-neuronal modulator of GABAergic transmission in the CNS, without affecting the expression of GABAA receptor.
Spinal cord and brain slices contain non-neuronal cells such as immune cells and glial cells that may also express PD-1 to regulate neuronal activities indirectly. To this end, we assessed a direct role of PD-1 in regulating GABA transmission in dissociated cortical neurons. We observed PD-1 expression on primary cultures of cortical neurons of WT but not KO mice at 7–8 days in culture (DIV 7–8, Figure 3H). Nivolumab, but not IgG, was able to bind on cultured cortical neurons (Figure 3I). GABA application (1 mM) induced a large outward current in dissociated neurons, but this GABA current was reduced by Nivolumab treatment (300 ng/mL, 2 h, Figure 3J). Thus, inhibition of PD-1 in cortical neurons may be sufficient to alter GABA signaling.
Loss of PD-1 Results in Deficits in GABA-Mediated Analgesia and Anesthesia in Mice
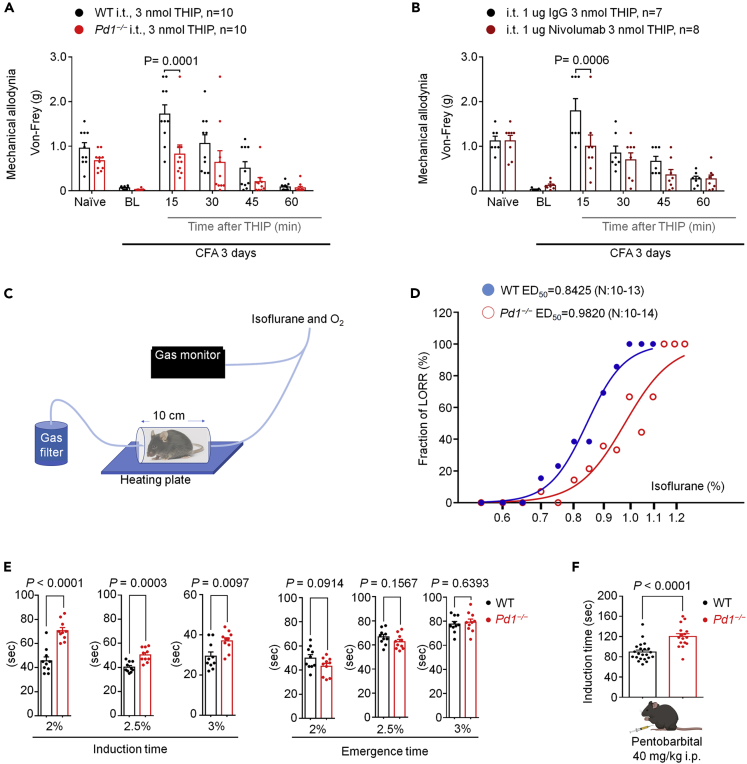
GABAergic signaling in SDH plays a critical role in pain control (Coull et al., 2005; Knabl et al., 2008; Lu et al., 2013; Zeilhofer et al., 2012). We assessed whether GABAA-mediated analgesia would require PD-1 signaling. Intrathecal administration of GABAA agonist THIP (3 nmol) produced a transient reversal of inflammatory pain induced by Complete Freund's adjuvant (CFA) in WT mice. Remarkably, this reversal of mechanical allodynia was compromised in Pd1−/− mice (Figure 4A), as well as in WT mice treated with intrathecal Nivolumab (1 μg, Figure 4B). In addition, intrathecal administration of a lower concentration of THIP (1 nmol) transiently reduced CFA-induced inflammatory pain and nerve-injury-induced neuropathic pain (mechanical allodynia), which was also impaired by Nivolumab (Figures S4A–S4B). Thus, PD-1 plays an active role in GABAA-mediated analgesia.
Figure 4.
GABAAR-Mediated Antinociception and Anesthesia is Impaired in Pd1−/− Mice
(A and B) GABAAR-mediated antinociception in WT and Pd1−/− mice. (A) von Frey testing showing the effect of intrathecal THIP (3 nmol) on CFA-induced inflammatory pain. p = 0.0001, WT versus Pd1−/−, two-way ANOVA, followed by Bonferroni's post hoc test. (B) von Frey testing showing the effect of intrathecal THIP (3 nmol) on CFA-induced inflammatory pain in mice pretreated with IgG4 or nivolumab (1 μg, i.t.). p = 0.0006, IgG4 + THIP versus nivolumab + THIP, two-way ANOVA, followed by Bonferroni's post-hoc test.
(C–F) GABAAR-mediated anesthesia in WT and Pd1−/− mice. (C) Experimental setup used to assess isoflurane anesthesia-mediated loss of righting reflex (LORR). (D) Dose-response curves of the fraction of LORR in WT or Pd1−/− mice at various concentrations of isoflurane. The curves were drawn according to the Hill equation. (E) The effects of different doses of isoflurane on induction and emergence time in WT or Pd1−/− mice (n = 10 animals in each group). Unpaired two-tailed t test. (F) The effects of sodium pentobarbital on anesthesia induction time in WT or Pd1−/− mice (n = 22 and 16 animals). Unpaired two-tailed t test.
All error bars indicate the mean ± SEM.
General anesthesia requires GABAergic signaling in multiple brain regions including the thalamus and hypothalamus (Franks, 2008; Mihic et al., 1997). We investigated isoflurane-induced general anesthesia using a gas monitor in WT and Pd1−/− mice (Figure 4C). A dose-response analysis revealed a right shift in the concentration of isoflurane (WT: EC50 = 0.8425%; KO: EC50 = 0.9820%; Figure 4D). The induction time of anesthesia (loss of turnover response) at 2%, 2.5%, and 3% isoflurane were all significantly increased in KO mice (p < 0.01, Figure 4E). In contrast, the emergence time (recovery phase) was not altered in KO mice (Figure 4E). This result could be expected, as the emergence mechanisms of general anesthesia are different from that of induction (Franks, 2008). We also evaluated the effects of Nivolumab on 1.5% isoflurane-induced general anesthesia following intracerebroventricular (ICV) injection (Figure S4C) of IgG or Nivolumab (3 μg in 5 μL) 1 h prior to assessment. We did not observe significant changes in induction and emergence time between IgG- and Nivolumab-treated mice, but observed a trend toward increase in the induction time and decrease in the emergence time (Figure S4C). The injectable anesthetic pentobarbital also binds to GABAAR, and this interaction plays an important role in the anesthetic action (Franks, 2008). Notably, the induction time of anesthesia by pentobarbital (40 mg/kg, i.p.) was prolonged in Pd1−/− mice (Figure 4F). Thus, these data provide evidence at the behavioral level that PD-1 regulates GABAergic neurotransmission by regulating the effects of multiple GABAAR-mediated types of anesthesia.
Discussion
Our study has revealed a previously unrecognized modulation of GABAergic neurotransmission by PD-1, a leading molecular target of cancer immunotherapy. We found that PD-1 blockade with Nivolumab caused a profound reduction (45%–70%) of GABA-currents across the CNS, including lamina IIo and lamina I neurons in SDH, S1 cortical neurons, thalamic neurons in the VPM and VPL, hypothalamic neurons, and hippocampal neurons (Figure 3G). Furthermore, spinal cord and cortical neurons of Pd1−/− mice showed the same degree of reduction in GABA currents (45%–60%, Figure 3G) compared with WT neurons. At behavioral levels, GABA-mediated analgesia and anesthesia were impaired in Pd1−/− mice. Furthermore, Nivolumab exhibited binding to SDH and cortical neurons and inhibited GABA currents in dissociated cortical neurons from primary cultures, providing evidence of possible direct action on neurons.
We propose that PD-1 regulates GABAA signaling via SHP-1 and ERK signaling (Figure S4D). Given that GABAA-mediated currents and analgesia were compromised in KO mice and the expression of α1 and α2 subunits of GABAA did not change in KO mice, PD-1 may interact with GABAA to regulate GABAergic signaling. The phosphatase SHP-1/2 is involved in downstream signaling of PD-1 in immune cells and DRG neurons (Chen et al., 2017; Hebeisen et al., 2013). This involvement was also demonstrated in SDH neurons (Figure 2C). It is conceivable that PD-1 regulates the activity of GABAA through SHP-1 signaling. Although PD-1 inhibition reduced GABA current, PD-1 activation by PD-L1 enhanced GABA current in SDH neurons in an SHP-dependent manner. Strikingly, Nivolumab treatment was sufficient to activate ERK in SDH neurons, in the absence of additional stimuli. Nivolumab-induced suppression of GABA currents was blocked by the ERK inhibitor U0126, providing a link between Nivolumab, ERK, and GABA transmission (Figure S4D). It is noteworthy that ERK was also implicated as a negative regulator of GABAA function via an ERK phosphorylation site (Bell-Horner et al., 2006).
Our in situ hybridization and immunohistochemistry data demonstrated a broad expression of PD-1 and Pd1 mRNA in neurons across different CNS regions. However, single-cell analysis suggested low expression levels of Pd1 mRNA in excitatory cortical neurons (Zeisel et al., 2018). This discrepancy may be due to the low sensitivity of single-cell RNA-sequencing approaches, where only the 10%–20% most highly expressed mRNAs are typically detected. Nevertheless, neuronal PD-1 expression is functional in multiple spinal cord and brain regions (Figure 3G). We cannot rule out the possibility that non-neuronal cells also contribute to the neuronal changes we saw in spinal cord and brain slices after PD-1 blockade, as inhibition of PD-1 could enhance microglia activation in SDH (Wang et al., 2020b). However, dissociated cortical neurons in culture clearly displayed binding to Nivolumab (Figure 3I), and furthermore, Nivolumab was sufficient to suppress GABA-induced currents in these dissociated neurons in cultures (Figure 3J).
Our findings suggest that immunotherapy via Nivolumab and other anti-PD1 therapeutics could impair GABA-mediated analgesia, which is consistent with reduced GABA-analgesia in Pd1−/− mice lacking Pd1. “Gate control,” an important theory for pain regulation, is mediated by GABAergic transmission in SDH, and loss of GABAergic transmission, a cardinal feature of chronic pain such as neuropathic pain, would open the gate for the pathogenesis of pain (Braz et al., 2014; Duan et al., 2018; Lu et al., 2013; Melzack and Wall, 1965; Moore et al., 2002). On the other hand, it has been shown that spinal transplantation of GABA-precursor neurons could effectively control neuropathic pain (Braz et al., 2012). Notably, our previous studies demonstrated that loss of PD-1 resulted in increased basal pain sensitivity (Chen et al., 2017; Wang et al., 2020a, 2020b), which may mimic a chronic pain condition due to impaired GABAergic transmission in SDH neurons.
An anesthesiologist in the lab initially noticed difficulty to anesthetize the Pd1−/− mice during surgery. Further quantitative analysis revealed an increase in the induction time but no change in the emergence time (Figure 4E). However, ICV administration of Nivolumab did not significantly change the induction and emergence time. It is possible that only limited brain regions are affected by ICV administration, and it requires PD-1 modulation of GABA transmission in multiple brain regions to alter anesthesia. Regarding typical nervous system side effects such as fatigue, headache, dysgeusia, vertigo and anxiety or malaise, Nivolumab is relatively safe (Kwatra et al., 2018). However, neurological issues have been reported, such as polyneuropathy and encephalopathy (Mirabile et al., 2019). We should also point out that half-life of Nivolumab in mouse is much shorter than that in human (3 days versus weeks) (Wang et al., 2020a). One systematic review found that benefits were observed with PD-1 blockade in melanomas with metastasis to the brain, and Nivolumab concentrations could reach from 35 to 150 ng/mL in CSF of these patients (van Bussel et al., 2019). As tested in this study, this concentration range may be sufficient to affect neuronal activities. Future studies are warranted to investigate the neurological effects of anti-PD-1 therapies in patients in whom significant CNS penetration of antibody has occurred after brain injury.
In summary, we have identified PD-1 as a pan-neuronal modulator of GABA receptor signaling. In particular, PD-1 is an essential signaling component for the function of GABAA across different CNS regions in the spinal cord and brain. Thus, PD-1 may act as an inhibitor for neurons as well as for immune cells. Clinical application of PD-1 inhibitors may produce beneficial effects in some disease conditions by enhancing neuronal activities, thus representing a potential neurotherapy, or may produce its beneficial actions through a combination of actions on immune cells and neurons (immunotherapy and neurotherapy).
Limitations of the Study
There are several unresolved issues in this study. First, the most direct evidence for PD-1 operating in neurons is from cultured embryonic cortical neurons, which may contain possible contamination of glial cells. Future studies are warranted to conditionally delete Pd1 selectively in neurons, glial cells, and immune cells to test the respective contribution of PD-1 in each cell type to GABAergic signaling using conditional Pd1 knockout mice. Second, the source of PD-1 ligands (e.g., PD-L1 and PD-L2) in the spinal cord remains to be identified. Because primary sensory neurons express PD-L1 (Chen et al., 2017), PD-1 could be activated by PD-L1 released from the central terminals of primary afferents in the spinal cord. We also do not rule out the possibility that PD-1 may signal in a ligand-independent manner. Additionally, we focus on spinal α1 and α2 subunits of GABAA receptors in this study, but the contribution of β and γ subunits in PD-1-mediated regulation of GABAergic transmission should also be considered in future studies. Finally, it is important to note that many basic discoveries in animals fail to translate to humans. Future clinical studies which carefully monitor anesthesia induction time on patients receiving anti-PD1 therapies are warranted to determine the clinical ramifications of these findings.
Resource Availability
Lead Contact
Ru-Rong Ji, ru-rong.ji@duke.edu.
Materials Availability
All the materials are commercially available.
Data and Code Availability
Available upon request.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
This study was supported by Duke University Anesthesiology Research Funds.
Author Contributions
C.J. and R.R.J developed the project. C.J., Z.W., C.R.D., K.W., A.S.A., X.T.M.M. and J.Z. conducted experiments; R.R.J. and C.J. wrote the manuscript. C.R.D. edited the manuscript.
Declaration of Interests
Dr. Ji is a consultant of Boston Scientific and serves on Scientific Advisory Board of Alleviate Therapeutics. These activities are not related to this study. Drs. Ji, Jiang, and Wang also filed a patent “Methods and kits for treating pain” (16/612,909) from Duke University, which is related to this study.
Published: October 23, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.101570.
Supplemental Information
References
- Baruch K., Deczkowska A., Rosenzweig N., Tsitsou-Kampeli A., Sharif A.M., Matcovitch-Natan O., Kertser A., David E., Amit I., Schwartz M. PD-1 immune checkpoint blockade reduces pathology and improves memory in mouse models of Alzheimer's disease. Nat. Med. 2016;22:135–137. doi: 10.1038/nm.4022. [DOI] [PubMed] [Google Scholar]
- Bell-Horner C.L., Dohi A., Nguyen Q., Dillon G.H., Singh M. ERK/MAPK pathway regulates GABAA receptors. J. Neurobiol. 2006;66:1467–1474. doi: 10.1002/neu.20327. [DOI] [PubMed] [Google Scholar]
- Brahmer J.R., Tykodi S.S., Chow L.Q., Hwu W.J., Topalian S.L., Hwu P., Drake C.G., Camacho L.H., Kauh J., Odunsi K. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl. J. Med. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braz J., Solorzano C., Wang X., Basbaum A.I. Transmitting pain and itch messages: a contemporary view of the spinal cord circuits that generate gate control. Neuron. 2014;82:522–536. doi: 10.1016/j.neuron.2014.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braz J.M., Sharif-Naeini R., Vogt D., Kriegstein A., Alvarez-Buylla A., Rubenstein J.L., Basbaum A.I. Forebrain GABAergic neuron precursors integrate into adult spinal cord and reduce injury-induced neuropathic pain. Neuron. 2012;74:663–675. doi: 10.1016/j.neuron.2012.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerny O., Anderson K.E., Stephens L.R., Hawkins P.T., Sebo P. cAMP signaling of adenylate cyclase toxin blocks the oxidative burst of neutrophils through epac-mediated inhibition of phospholipase C activity. J. Immunol. 2017;198:1285–1296. doi: 10.4049/jimmunol.1601309. [DOI] [PubMed] [Google Scholar]
- Chamessian A., Young M., Qadri Y., Berta T., Ji R.R., Van de Ven T. Transcriptional profiling of somatostatin interneurons in the spinal dorsal horn. Sci. Rep. 2018;8:6809. doi: 10.1038/s41598-018-25110-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G., Kim Y.H., Li H., Luo H., Liu D.L., Zhang Z.J., Lay M., Chang W., Zhang Y.Q., Ji R.R. PD-L1 inhibits acute and chronic pain by suppressing nociceptive neuron activity via PD-1. Nat. Neurosci. 2017;20:917–926. doi: 10.1038/nn.4571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu I.M., von Hehn C.A., Woolf C.J. Neurogenic inflammation and the peripheral nervous system in host defense and immunopathology. Nat. Neurosci. 2012;15:1063–1067. doi: 10.1038/nn.3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coull J.A., Beggs S., Boudreau D., Boivin D., Tsuda M., Inoue K., Gravel C., Salter M.W., De Koninck Y. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature. 2005;438:1017–1021. doi: 10.1038/nature04223. [DOI] [PubMed] [Google Scholar]
- Dong H., Zhu G., Tamada K., Chen L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat. Med. 1999;5:1365–1369. doi: 10.1038/70932. [DOI] [PubMed] [Google Scholar]
- Donnelly C.R., Chen O., Ji R.R. How do sensory neurons sense danger signals? Trends Neurosci. 2020 doi: 10.1016/j.tins.2020.07.008. S0166-2236(20)30173-30179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan B., Cheng L., Bourane S., Britz O., Padilla C., Garcia-Campmany L., Krashes M., Knowlton W., Velasquez T., Ren X. Identification of spinal circuits transmitting and gating mechanical pain. Cell. 2014;159:1417–1432. doi: 10.1016/j.cell.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan B., Cheng L., Ma Q. Spinal circuits transmitting mechanical pain and itch. Neurosci. Bull. 2018;34:186–193. doi: 10.1007/s12264-017-0136-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks N.P. General anaesthesia: from molecular targets to neuronal pathways of sleep and arousal. Nat. Rev. Neurosci. 2008;9:370–386. doi: 10.1038/nrn2372. [DOI] [PubMed] [Google Scholar]
- Freeman G.J., Long A.J., Iwai Y., Bourque K., Chernova T., Nishimura H., Fitz L.J., Malenkovich N., Okazaki T., Byrne M.C. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J. Exp. Med. 2000;192:1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haring M., Zeisel A., Hochgerner H., Rinwa P., Jakobsson J.E.T., Lonnerberg P., La Manno G., Sharma N., Borgius L., Kiehn O. Neuronal atlas of the dorsal horn defines its architecture and links sensory input to transcriptional cell types. Nat. Neurosci. 2018;21:869–880. doi: 10.1038/s41593-018-0141-1. [DOI] [PubMed] [Google Scholar]
- Hebeisen M., Baitsch L., Presotto D., Baumgaertner P., Romero P., Michielin O., Speiser D.E., Rufer N. SHP-1 phosphatase activity counteracts increased T cell receptor affinity. J. Clin. Invest. 2013;123:1044–1056. doi: 10.1172/JCI65325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbst R.S., Soria J.C., Kowanetz M., Fine G.D., Hamid O., Gordon M.S., Sosman J.A., McDermott D.F., Powderly J.D., Gettinger S.N. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515:563–567. doi: 10.1038/nature14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda H., Heinke B., Ruscheweyh R., Sandkuhler J. Synaptic plasticity in spinal lamina I projection neurons that mediate hyperalgesia. Science. 2003;299:1237–1240. doi: 10.1126/science.1080659. [DOI] [PubMed] [Google Scholar]
- Ishida Y., Agata Y., Shibahara K., Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992;11:3887–3895. doi: 10.1002/j.1460-2075.1992.tb05481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji R.R., Gereau R.W., Malcangio M., Strichartz G.R. MAP kinase and pain. Brain Res. Rev. 2009;60:135–148. doi: 10.1016/j.brainresrev.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karim F., Wang C.C., Gereau R.W. Metabotropic glutamate receptor subtypes 1 and 5 are activators of extracellular signal-regulated kinase signaling required for inflammatory pain in mice. J. Neurosci. 2001;21:3771–3779. doi: 10.1523/JNEUROSCI.21-11-03771.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knabl J., Witschi R., Hosl K., Reinold H., Zeilhofer U.B., Ahmadi S., Brockhaus J., Sergejeva M., Hess A., Brune K. Reversal of pathological pain through specific spinal GABAA receptor subtypes. Nature. 2008;451:330–334. doi: 10.1038/nature06493. [DOI] [PubMed] [Google Scholar]
- Kwatra S.G., Stander S., Kang H. PD-1 blockade-induced pruritus treated with a mu-opioid receptor antagonist. N. Engl. J. Med. 2018;379:1578–1579. doi: 10.1056/NEJMc1805637. [DOI] [PubMed] [Google Scholar]
- Latta-Mahieu M., Elmer B., Bretteville A., Wang Y., Lopez-Grancha M., Goniot P., Moindrot N., Ferrari P., Blanc V., Schussler N. Systemic immune-checkpoint blockade with anti-PD1 antibodies does not alter cerebral amyloid-beta burden in several amyloid transgenic mouse models. Glia. 2018;66:492–504. doi: 10.1002/glia.23260. [DOI] [PubMed] [Google Scholar]
- Liu T., Xu Z.Z., Park C.K., Berta T., Ji R.R. Toll-like receptor 7 mediates pruritus. Nat. Neurosci. 2010;13:1460–1462. doi: 10.1038/nn.2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y., Dong H., Gao Y., Gong Y., Ren Y., Gu N., Zhou S., Xia N., Sun Y.Y., Ji R.R. A feed-forward spinal cord glycinergic neural circuit gates mechanical allodynia. J. Clin. Invest. 2013;123:4050–4062. doi: 10.1172/JCI70026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantyh P.W., Rogers S.D., Honore P., Allen B.J., Ghilardi J.R., Li J., Daughters R.S., Lappi D.A., Wiley R.G., Simone D.A. Inhibition of hyperalgesia by ablation of lamina I spinal neurons expressing the substance P receptor. Science. 1997;278:275–279. doi: 10.1126/science.278.5336.275. [DOI] [PubMed] [Google Scholar]
- Melzack R., Wall P.D. Pain mechanisms: a new theory. Science. 1965;150:971–979. doi: 10.1126/science.150.3699.971. [DOI] [PubMed] [Google Scholar]
- Mihic S.J., Ye Q., Wick M.J., Koltchine V.V., Krasowski M.D., Finn S.E., Mascia M.P., Valenzuela C.F., Hanson K.K., Greenblatt E.P. Sites of alcohol and volatile anaesthetic action on GABA(A) and glycine receptors. Nature. 1997;389:385–389. doi: 10.1038/38738. [DOI] [PubMed] [Google Scholar]
- Mirabile A., Brioschi E., Ducceschi M., Piva S., Lazzari C., Bulotta A., Vigano M.G., Petrella G., Gianni L., Gregorc V. PD-1 inhibitors-related neurological toxicities in patients with non-small-cell lung cancer: a literature review. Cancers (Basel) 2019;11:296. doi: 10.3390/cancers11030296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore K.A., Kohno T., Karchewski L.A., Scholz J., Baba H., Woolf C.J. Partial peripheral nerve injury promotes a selective loss of GABAergic inhibition in the superficial dorsal horn of the spinal cord. J. Neurosci. 2002;22:6724–6731. doi: 10.1523/JNEUROSCI.22-15-06724.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols M.L., Allen B.J., Rogers S.D., Ghilardi J.R., Honore P., Luger N.M., Finke M.P., Li J., Lappi D.A., Simone D.A. Transmission of chronic nociception by spinal neurons expressing the substance P receptor. Science. 1999;286:1558–1561. doi: 10.1126/science.286.5444.1558. [DOI] [PubMed] [Google Scholar]
- Nishimura H., Nose M., Hiai H., Minato N., Honjo T. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity. 1999;11:141–151. doi: 10.1016/s1074-7613(00)80089-8. [DOI] [PubMed] [Google Scholar]
- Paul J., Zeilhofer H.U., Fritschy J.M. Selective distribution of GABA(A) receptor subtypes in mouse spinal dorsal horn neurons and primary afferents. J. Comp. Neurol. 2012;520:3895–3911. doi: 10.1002/cne.23129. [DOI] [PubMed] [Google Scholar]
- Pirker S., Schwarzer C., Wieselthaler A., Sieghart W., Sperk G. GABA(A) receptors: immunocytochemical distribution of 13 subunits in the adult rat brain. Neuroscience. 2000;101:815–850. doi: 10.1016/s0306-4522(00)00442-5. [DOI] [PubMed] [Google Scholar]
- Shinohara T., Taniwaki M., Ishida Y., Kawaichi M., Honjo T. Structure and chromosomal localization of the human PD-1 gene (PDCD1) Genomics. 1994;23:704–706. doi: 10.1006/geno.1994.1562. [DOI] [PubMed] [Google Scholar]
- Sieghart W., Fuchs K., Tretter V., Ebert V., Jechlinger M., Hoger H., Adamiker D. Structure and subunit composition of GABA(A) receptors. Neurochem. Int. 1999;34:379–385. doi: 10.1016/s0197-0186(99)00045-5. [DOI] [PubMed] [Google Scholar]
- Talbot S., Foster S.L., Woolf C.J. Neuroimmunity: physiology and pathology. Annu. Rev. Immunol. 2016;34:421–447. doi: 10.1146/annurev-immunol-041015-055340. [DOI] [PubMed] [Google Scholar]
- Todd A.J. Neuronal circuitry for pain processing in the dorsal horn. Nat. Rev. Neurosci. 2010;11:823–836. doi: 10.1038/nrn2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Bussel M.T.J., Beijnen J.H., Brandsma D. Intracranial antitumor responses of nivolumab and ipilimumab: a pharmacodynamic and pharmacokinetic perspective, a scoping systematic review. BMC Cancer. 2019;19:519. doi: 10.1186/s12885-019-5741-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K., Gu Y., Liao Y., Bang S., Donnelly C.R., Chen O., Tao X., Mirando A.J., Hilton M.J., Ji R.R. PD-1 blockade inhibits osteoclast formation and murine bone cancer pain. J. Clin. Invest. 2020;130:3603–3620. doi: 10.1172/JCI133334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Jiang C., He Q., Matsuda M., Han Q., Wang K., Bang S., Ding H., Ko M.C., Ji R.R. Anti-PD-1 treatment impairs opioid antinociception in rodents and nonhuman primates. Sci. Transl. Med. 2020;12:eaaw6471. doi: 10.1126/scitranslmed.aaw6471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeilhofer H.U., Benke D., Yevenes G.E. Chronic pain states: pharmacological strategies to restore diminished inhibitory spinal pain control. Annu. Rev. Pharmacol. Toxicol. 2012;52:111–133. doi: 10.1146/annurev-pharmtox-010611-134636. [DOI] [PubMed] [Google Scholar]
- Zeisel A., Hochgerner H., Lonnerberg P., Johnsson A., Memic F., van der Zwan J., Haring M., Braun E., Borm L.E., La Manno G. Molecular architecture of the mouse nervous system. Cell. 2018;174:999–1014.e22. doi: 10.1016/j.cell.2018.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Available upon request.