Abstract
Ticks deposit salivary proteins into the skin during a bite to mediate acquisition of a blood meal. Acquired resistance to tick bites has been demonstrated to prevent Borrelia burgdorferi sensu lato (s.l.) transmission. However, the mechanism of resistance, as well as the protective antigens, have remained elusive. To address these unknowns, we utilized a guinea pig model of tick resistance and a mouse model of permissiveness. Guinea pigs developed immunity after multiple Ixodes scapularis tick infestations, characterized by rapid tick detachment and impaired feeding. In comparison, mice tolerated at least 6 infestations with no significant impact on feeding. We analyzed the bite sites by RNA-sequencing and histology, identifying several inflammatory pathways in tick immune animals, such as FcεRI signaling and complement activation, and activation of coagulation pathways that could impair local blood flow. Together, these results identify important pathways altered during tick rejection and potential tick proteins that could serve as vaccine candidates.
Keywords: Ixodes scapularis, Lyme Disease, vaccine, tick immunity, tick saliva, RNA-sequencing
1. Introduction
Borrelia burgdorferi sensu lato (s.l.) is the causative agent of Lyme disease, and remains the most common tick-borne disease, with more than 30,000 cases reported annually in the US (Hinckley et al., 2014; Rosenberg et al., 2018). Borrelia burgdorferi s.l. is transmitted to vertebrates through the saliva of the blacklegged tick, Ixodes scapularis. Disease can manifest with several systemic symptoms, such as carditis, arthritis and neurologic symptoms. There are currently no available human vaccines against B. burgdorferi s.l., leaving an urgent need for prevention and post-exposure therapy. Recently, strategies to develop vaccines against tick salivary proteins have emerged as an exciting strategy to disrupt tick feeding, which can also impair pathogen transmission (Maruyama et al., 2017; Mulenga et al., 1999; Narasimhan et al., 2020, 2007; Trimnell et al., 2005).
Tick feeding is a dynamic process that involves attaching to the host by penetrating the epidermis and inserting the barbed mouthparts, followed by secretion of cement to help adhere the mouthparts to the host’s skin. Once attached, the tick secretes proteins through the saliva into the host tissue (Simo et al., 2017). The secretion of salivary proteins occurs throughout the multi-day long feeding period and the composition changes throughout the feeding process to accommodate the changing environment at the tick bite site (Kim et al., 2016; Ribeiro et al., 2006). Several studies have identified salivary proteins involved in feeding, including those with functions in inhibiting coagulation, inducing vasodilation, suppressing the immune response and inhibiting complement activation at the bite site (Dai et al., 2010; Das et al., 2001; Francischetti et al., 2005; Ramamoorthi et al., 2005; Ribeiro et al., 1985; Schuijt et al., 2011). The functions of these proteins are essential for the feeding process. For example, RNAi knockdown of the anticoagulant salivary protein 14 (SALP14) results in significantly impaired tick feeding on mice (Narasimhan et al., 2004). In addition to facilitating the acquisition of a blood meal, salivary proteins can augment pathogen transmission. SALP15 binds to the outer surface protein C (OspC) on B. burgdorferi s.l. and protects the spirochetes from Ag-specific antibodies (Ramamoorthi et al., 2005). Additionally, needle co-inoculation of Salp15 and B. burgdorferi s.l. significantly enhanced transmission and infection in mice as compared to the spirochetes alone (Ramamoorthi et al., 2005).
Several studies report that multiple species, such as guinea pigs, rabbits and cattle, develop resistance to ticks after multiple tick exposures (Wikel, 1996). Repeat tick exposure induces an adaptive memory response in these animals, primarily targeted against the salivary proteins injected into the host at the bite site (Brown et al., 1984; Das et al., 2001; Ramachandra and Wikel, 1995). Importantly, acquired tick resistance in animals can prevent the transmission of B. burgdorferi s.l. (Narasimhan et al., 2007). Furthermore, a long-term memory response and tick resistance can be induced by immunizing guinea pigs with tick saliva or salivary gland extract (SGE) (Brown et al., 1984; Narasimhan et al., 2020). By contrast, white-footed mice, Peromyscus leucopus, are the natural host for I. scapularis and do not develop robust resistance to I. scapularis nymphs (Anderson et al., 2017). To identify potential mechanisms that determine resistance, a histological comparison of guinea pigs (resistant host) and P. leucopus (permissive host) after repeated exposures to ticks was performed (Anderson et al., 2017), with the finding that dermal inflammation alters the skin architecture in resistant guinea pigs and impairs tick attachment during feeding. In comparison, the immune response towards the bite in mice did not elicit the same changes to the dermal structure.
In this study, we sought to identify molecular mechanisms involved in mediating tick resistance. We utilized RNA-sequencing to identify key pathways differentially activated at the bite site of tick immune guinea pigs, as well as in tick permissive mice. Our results help elucidate the drivers of acquired tick resistance and thus may guide vaccine development.
2. Materials and methods
2.1. Animal experiments
4–5-weeks old female BALB/cAnNCrl and CRL:CD-1(ICR) mice (Charles River, MA) were utilized for tick challenge experiments. Outbred 400 g female Hartley guinea pigs (Crl:HA, Charles River, MA) were used for tick challenge experiments. Female New Zealand white rabbits (Crl:KBL NZW, Charles River, MA) were used for tick saliva collection experiments, as described previously (Narasimhan et al., 2020). Ixodes scapularis nymphs and adults were obtained from Oklahoma State University (Stillwater, OK). Ticks were maintained at 85% relative humidity with a 14 h light and 10 h dark period at 23 °C. Animal care and housing were performed according to the Guide for the Care and Use of laboratory Animals of National Institutes of Health, USA. All protocols were approved by the Yale University Institutional Animal Care and Use Committee, (approval number 2020–07941), and performed in a Biosafety Level 2 animal facility.
2.2. Tick challenge experiments
Mice were anesthetized by intraperitoneal injection of a 10 mg/kg ketamine/xylazine mixture and 10–15 I. scapularis nymphs were applied to the neck area of the mice. In our experience, shaving mice prior to tick attachment has no impact on tick feeding or the development of tick immunity. Ticks were allowed to attach on the mice before being housed in individual cages with wire platforms above water to capture the engorged ticks. For repeat tick exposures in mice, ticks were allowed to feed to repletion and rested for 2 weeks before the next challenge. For repeat tick challenges in mice, 3 mice per group were used.
Guinea pigs were anesthetized by intramuscular injection of 40 mg/kg ketamine/xylazine mixture. The backs of the animals were carefully shaved and 15–25 I. scapularis nymphs were applied to the skin. The initial attachment of ticks in guinea pigs is more variable than in mice, therefore, a higher number of ticks were applied to guinea pigs to obtain a sufficient number of ticks to assess rejection. Experiments in our lab indicate that tick immunity is not dependent on the number of ticks applied to the guinea pig. Conversely, we have not observed induction of tick immunity when a higher number of ticks are applied to mice. Ticks were allowed to attach to the animals and then returned to their cages. Guinea pigs were housed individually in cages with a wire platform above water to capture the engorged ticks. For repeat tick exposure experiments, the animals were rested for 2 weeks after the previous challenge and then challenged again. Three animals per group were utilized for tick challenges in guinea pigs.
Animals were monitored daily for tick attachment, tick recovery and erythema at the bite site. Recovered ticks were rinsed in water, dried and body weight was recorded as a measure of tick engorgement.
2.3. Passive serum transfer
Sera were collected from the following groups of guinea pigs: naïve guinea pigs; after three exposures to I. scapularis nymphs; saliva immunization; or Ova immunization. Guinea pig sera (200 μl) was pooled from 3 guinea pigs/group and delivered to BALB/c mice by intraperitoneal injection 1 day prior to tick challenge.
2.4. Immunization
Tick saliva was collected from adult I. scapularis as described previously (Narasimhan et al., 2020). Briefly, adult ticks were applied to the ears of white rabbits and allowed to feed to repletion. Pilocarpine was applied to the dorsal scutum to induce salivation. Saliva was harvested from the tick mouthparts using a micropipette, pooled and stored at −80°C. Guinea pigs and mice were immunized subcutaneously with 5 μl of tick saliva (1 μg total protein) diluted in 200 μl sterile PBS on day 0. Control animals received PBS. In the indicated experiments, aluminum hydroxide gel 2% (InvivoGen, San Diego, CA, USA) was used with saliva immunizations mixed 1:1 in a total volume of 200 μl. Animals were then boosted with the same dose on days 14 and 28. Retro-orbital bleeds were performed before each immunization. Sera was stored at −80°C and used at a later time to measure Ag-specific antibody concentrations. Animals were challenged with I. scapularis nymphs two weeks after the final boost.
2.5. Enzyme Linked Immunosorbent Assay (ELISA)
To determine Ag-specific antibodies in the immunized and tick immune animals, ELISA was performed as described previously (Narasimhan et al., 2020). Briefly, 96-well plates were coated overnight at 4 °C with 200 ng of saliva or recombinant tick salivary lectin pathway inhibitor (TSLPI) diluted in carbonate-bicarbonate buffer, pH 9.6 (Narasimhan et al., 2020). Plates were washed and blocked in PBS with 3% BSA and 0.02% Tween 20. Guinea pig and mouse sera were serially diluted and incubated in the wells for 2 hours and 37 °C. The sera dilutions utilized for ELISA are indicated on the figures. Wells were washed, and reactivity was detected using goat anti-guinea pig IgG-HRP (ThermoFisher, Waltham, MA, USA catalog # A18769; 1:10,000 dilution) or goat anti-mouse IgG-HRP (ThermoFisher, Waltham, MA, USA; catalog # 31430; 1:2000 dilution) and TMB substrate (ThermoFisher, Waltham, MA, USA).
2.6. Generation of recombinant protein
RNA was isolated from the salivary glands of fully-fed I. scapularis ticks (RNeasy Plus kit, Qiagen) and cDNA was synthesized (iScript cDNA synthesis kit, BioRad). As published previously, tslpi was cloned into the pEZT-D-lux plasmid and confirmed by sequencing (Narasimhan et al., 2020). The pEZT-D-lux-TSLPI plasmid was transfected into Expi293F cells (ThermoFisher, Waltham, MA, USA) using the ExpiFectamine 293 Transfection kit (ThermoFisher, Waltham, MA, USA) according to the manufacture’s protocol. TSLPI was purified from the supernatant using Ni-NTA Agarose (Qiagen, Germantown, MD, USA) and quantified using the BCA protein assay kit (ThermoFisher, Waltham, MA, USA).
2.6. RNA isolation
Guinea pigs were euthanized using 1 ml/kg Euthasol (390 mg pentobarbital sodium, 50 mg phenytoin sodium/ml) (RXV CIII) delivered by cardiac injection. Mice were euthanized by carbon dioxide asphyxiation followed by terminal exsanguination by cardiac puncture. 6 mm biopsy punches at the tick bite sites (2 sites/animal) were obtained and stored in RNAlater Stabilization Solution (ThermoFisher, Waltham, MA, USA). RNA was isolated from the skin biopsies using RNeasy Plus kit (Qiagen, Germantown, MD, USA).
2.7. Histopathology
Guinea pigs and mice were euthanized and the skin at the bite site, as well as normal unbitten skin, were harvested, immersion-fixed flat in 10% neutral buffered formalin (VWR International, Radnor, PA, USA), and processed to paraffin by routine methods. Prior to embedding, the skin samples were “bread-loafed” as described previously (Narasimhan et al., 2019), sectioned at 5 microns and stained by hematoxylin and eosin (H&E) using routine methods. Stained sections were evaluated for pathological changes in epidermis, dermis, edema, necrosis and inflammation. Analysis was performed by an experienced veterinarian trained in veterinary pathology (CBJ) who was blinded to experimental manipulation. Samples were assigned scores from 0 to 5, with values of 0 (normal), 1 (minimal), 2 (mild), 3 (moderate), 4 (marked) and 5 (severe). This is a scoring system to assess the degree of inflammation based on the gross abundance of inflammatory cells at the bite site. This scoring system has been utilized in previous studies as well (Narasimhan et al., 2019). Due to a lack of antibodies to identify immune cells in guinea pigs, cells were identified based on morphology by a trained pathologist. The nature of the inflammatory infiltrate was characterized by cell type, neutrophil (heterophil for guinea pigs), eosinophils, and mononuclear (macrophage, lymphocyte, and plasma cell). Slides were examined and photographed using an AxioImager.A1 microscope with an AxioCam MRc5 camera using Axiovision software (Zeiss Microscopy, White Plains, NY, USA). Images were optimized using Adobe Photoshop.
2.8. Sequencing and pathway analysis
RNA submitted for library preparation using TruSeq (Illumina, San Diego, CA, USA) and sequenced using Illumina HiSeq 2500 by paired-end sequencing by Yale Centre for Genome Analaysis (YCGA). Raw paired-end read data were trimmed and quality selected for reads with quality values above 30 and read length of 45+ using Partek v8.0.19. Samples were processed and normalized using Partek v8.0.19 and aligned to the Mus musculus reference genome using STAR v.2.3.1. Gene expression and differential expression analysis were performed using Partek. Differentially expressed genes (fold change ≥ 2; P value <0.05) were selected and utilized for pathway analysis using Ingenuity Pathway Analysis (IPA) (Qiagen, Germantown, MD, USA). For the data from guinea pigs, the reads were trimmed for quality and filtered for length using trimmomatic. Only those bases with quality values of Q30+ and read lengths of 45+ were used for further analysis. The trimmed reads were aligned to guinea pig genome (Cavea porcellus, Cavpor 3.0 from Ensembl), with associated annotation file, and indexed with hisat2. The alignment files were processed with stringTie, and ballgown to generate per gene counts. These count matrices were processed further with R and DESeq to obtain significantly differentially expressed genes. The guinea pig genome is not annotated in IPA; therefore, we converted the guinea pig’s Ensembl gene symbol to the Entrez ID gene ID for mice using the online tool Biomart (https://m.ensembl.org/biomart/martview/6bb2814849d206e495bc70ca75f8fe1c) in order to be used in IPA. Human gene symbols were also utilized to confirm these results. Larger sample sizes and additional validation of pathway activation will be required in future studies to confirm these findings.
2.9. Statistical analysis
Statistical analysis was performed using Prism 7.0 software (GraphPad Software, CA). Data are represented as mean ± standard error of the mean (SEM). Statistical significance tests were determined using two-way ANOVA, one-way ANOVA and two-tailed student t-test (unpaired and nonparametric). P value < 0.05 was considered statistically significant. The number of animals used (n) for each experiment is provided in the figure legends. Ingenuity Pathway Analysis (Qiagen) was used to identify differentially activated pathways. Differentially expressed genes were identified as −2 ≥ fold change ≤ 2 and P value < 0.05. Gene expression was determined relative to unbitten, normal skin from the respective species. Venn diagrams were generated using the online tool http://bioinformatics.psb.ugent.be/webtools/Venn/.
3. Results
3.1. Repeat tick exposure in guinea pigs induces protective immunity
To develop tick immune guinea pigs, we repeatedly applied I. scapularis nymphs for a total of 3 tick exposures, with a two-week rest period between infestations. Ticks were rapidly rejected by the guinea pigs upon a 2nd or 3rd exposure to tick feeding. After the 2nd tick exposure, guinea pigs are no longer permissive to tick feeding and will be referred throughout this study as “tick immune” (Fig. 1A). At 48 hours post-tick attachment, 82.5% of ticks were attached and appeared to be feeding successfully on guinea pigs exposed to ticks for the first time. In comparison, feeding was significantly reduced on guinea pigs exposed to ticks for the 3rd time, with only 15.1% of ticks still attached (P value < 0.001). Additionally, erythema was rapidly induced within 24 hours of attachment in guinea pigs previously exposed to ticks; whereas erythema was not observed in naïve guinea pigs (Fig. 1B). The rapid detachment and erythema at the bite site significantly impaired tick feeding. Ticks applied to naïve guinea pigs fed to repletion, as determined by an average engorgement weight of 3.1 mg. In comparison, tick recovery and feeding were significantly impaired on guinea pigs exposed to ticks for the 3rd time, with an average engorgement weight of 0.24 mg (P value < 0.0001) (Fig. 1C).
Figure 1. Repeat tick exposure in guinea pigs and mice.
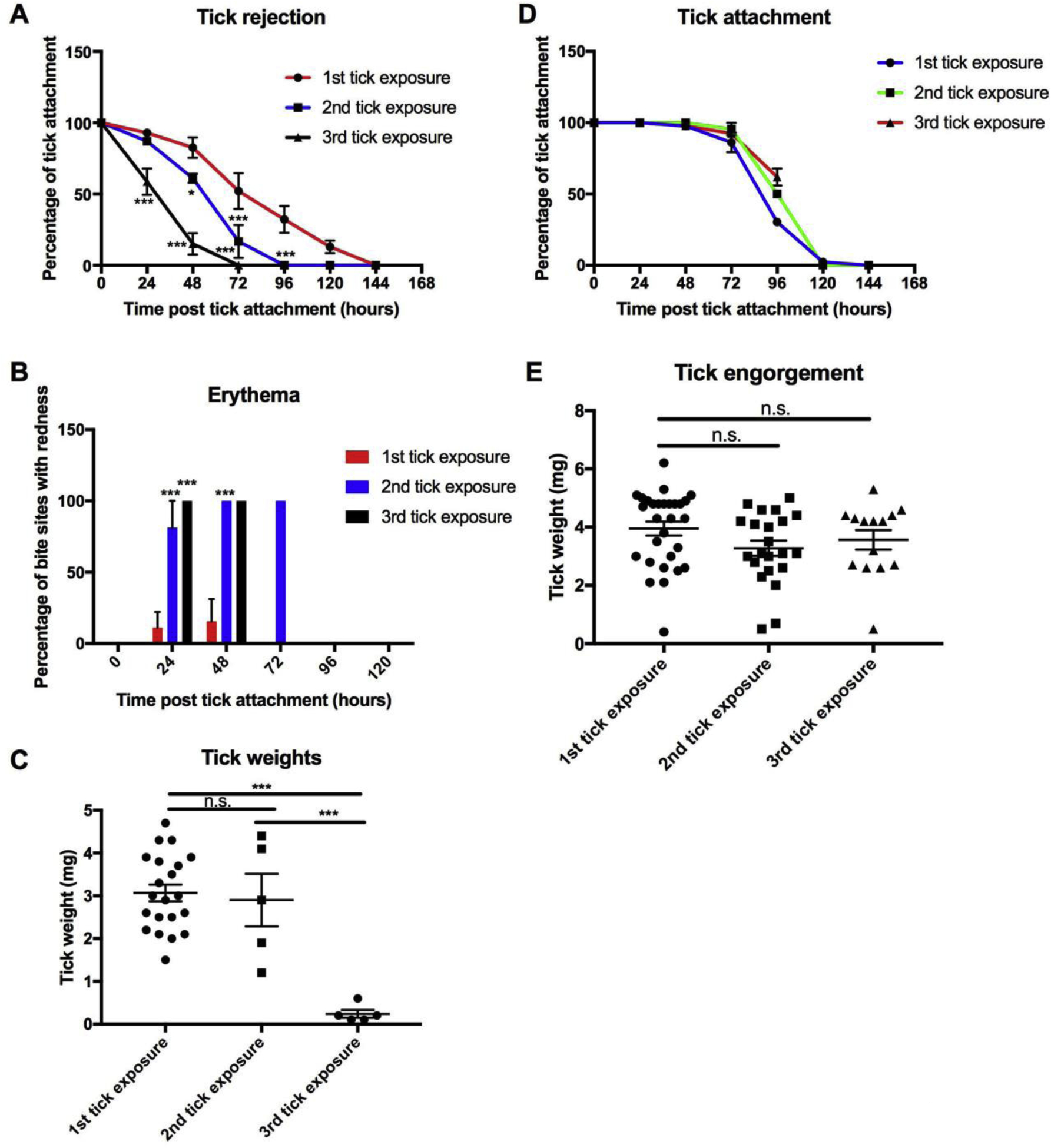
A Tick attachment was monitored in guinea pigs (n=3) after one, two and three exposures. B Induction of erythema was monitored in guinea pigs after repeated tick exposures. Guinea pigs previously exposed to ticks develop erythema at the bite within 24 hours after tick challenge. C Tick engorgement weights were measured to assess tick feeding. Repeat tick exposure resulted in decreased engorgement weights, as well as a decrease in tick recovery. D Tick attachment was monitored in mice (n=3) after one, two and three exposures. E Tick engorgement weights were obtained after feeding on mice after the indicated number of tick exposures. Data are represented as mean ± SEM. Statistical significance was determined in: A and B by two-way ANOVA; C and E by one-way ANOVA (n.s. = no significance; * P value < 0.05; ** P value ≤ 0.01; *** P value ≤ 0.001).
To compare the effects of repeat tick exposure in mice, ticks were applied to BALB/c mice using the same schedule as for the guinea pigs. However, unlike guinea pigs, repeated tick exposure had no impact on tick feeding success for subsequent tick infestations (Fig. 1D). The attachment of ticks applied to mice were similar with no significant difference between the 1st, 2nd and 3rd tick exposures (Fig. 1D). Additionally, there was no evidence of itching, tick removal or erythema at the bite site. Tick recovery and engorgement weights were not significantly different (P value > 0.05) after the 1st, 2nd and 3rd tick exposures (4.0 mg, 3.3 mg and 3.6 mg, respectively) (Fig. 1E). To confirm these results, the same experiments were performed in outbred CD-1 mice (Supplemental Fig. S1). We performed 6 tick infestations with no significant impact on feeding in CD-1 mice, suggesting that the lack of immunity is not strain specific.
As published previously in guinea pigs, immunization with saliva can also elicit tick resistance, although not as robust as from natural tick feeding (Narasimhan et al., 2020). To determine if a similar impact occurs in mice, mice were immunized with saliva in the presence and absence of aluminum hydroxide and challenged with ticks (Supplemental Fig. S2). In contrast to guinea pigs, saliva immunization had no impact on feeding in mice.
3.2. Activation of the humoral immune response following repeat tick feeding in mice and guinea pigs
To determine if resistance and permissiveness to repeat tick exposures was due to differences in the memory response, we analyzed the generation of tick specific antibody responses in guinea pigs and mice repeatedly exposed to ticks. Serum was collected from the guinea pigs and mice prior to each tick feeding. Guinea pigs and mice immunized with 5 μl saliva (1 μg of protein) in the absence of an adjuvant were used as controls. Saliva specific antibodies were significantly induced in guinea pigs immunized with saliva (P value < 0.001) (Fig. 2A). However, substantial saliva specific antibodies were not detected in guinea pigs following multiple tick exposures, despite eliciting robust tick rejection (Figs. 2A and 2B). To further confirm the difference in the humoral response between saliva immunized (no adjuvant) and tick immune guinea pigs, we analyzed the antibody response against recombinant Tick Salivary Lectin Pathway Inhibitor (TSLPI). We selected TSLPI as a marker for the humoral response against tick salivary proteins based on previous studies that identified TSLPI as an immunodominant protein in guinea pigs immunized with tick saliva (Narasimhan et al., 2020). Tick immune guinea pigs, however, did not induce a significant antibody response towards TSLPI (Fig. 2C). Conversely, mice exposed to multiple tick exposures developed saliva- and TSLPI-specific antibody responses, despite not inducing tick rejections (Figs. 2D, 2E and 2F). To further assess the lack of protective role of salivary protein-specific antibodies we performed a passive serum transfer from tick immune guinea pigs to mice. No significant difference was observed in attachment, recovery (86.7% versus 88.9%; P value = 0.8) or engorgement weights (4.24 mg versus 3.8 mg; P value = 0.1) between mice that received naïve or tick immune serum, respectively (Supplemental Fig. S3). Additionally, passive serum transfer from guinea pigs immunized with tick saliva (no adjuvant) or control guinea pigs did not confer protection to mice, despite eliciting a robust antibody response towards tick salivary proteins (Supplemental Fig. S3). Of note, we were unable to examine additional isotypes of the Ag-specific antibodies in guinea pigs due to a lack of commercially available reagents.
Figure 2. Induction of tick-specific antibody responses in guinea pigs and mice.
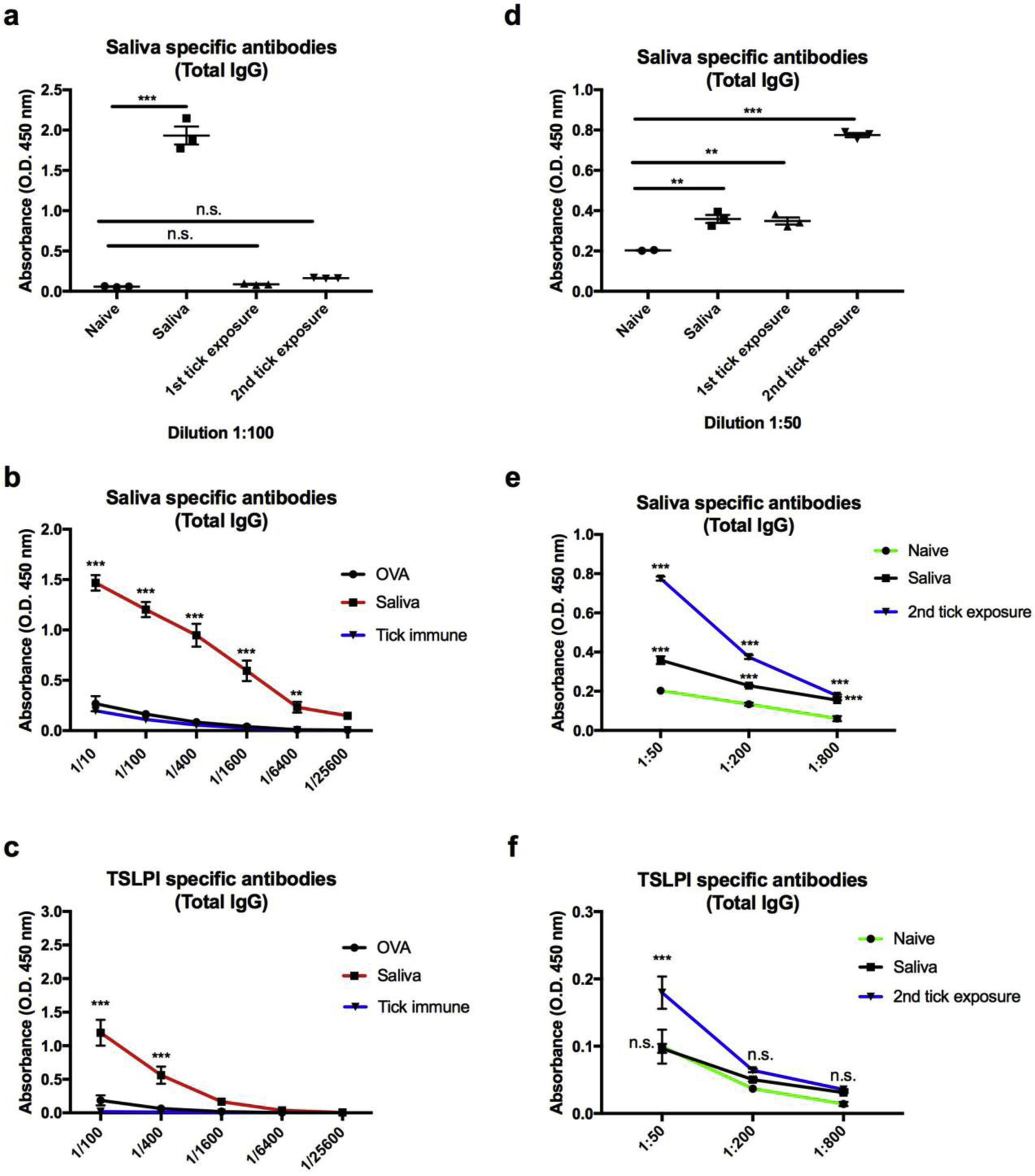
A-C Serum was obtained from guinea pigs (n=3) immunized with saliva immediately prior to tick challenge or two weeks after the the indicated tick exposure. A Sera from guinea pigs exposed to multiple tick infestation or immunization with saliva was measured for reactivity against saliva specific proteins (1:50 dilution). B Sera obtained from naïve guinea pigs, saliva immunized guinea pigs or tick immune guinea pigs (sera obtained prior to 3rd tick infestation) was measured for saliva specific antibodies by ELISA at the indicated dilutions. C Sera from the indicated guinea pigs was also measured for reactivity towards recombinant TSLPI by ELISA at the indicated dilutions. D-F Serum was obtained from BALB/c mice prior to immunization (n=2), after immunization with saliva (n=3) or two weeks after the indicated tick exposure (n=3) and ELISAs were performed against saliva or recombinant TSLPI. Data are represented as mean ± SEM. Statistical significance was determined in: A and D using one-way ANOVA; B, C, E and F by two-way ANOVA (n.s. = no significance; * P value < 0.05; ** P value ≤ 0.01; *** P value ≤ 0.001).
3.3. Differential pathway activation upon tick challenge in guinea pigs and mice immunized with tick saliva or repeat tick infestation
To further investigate the mechanisms driving tick immunity, we analyzed the host response at the bite site in challenged animals by RNA-seq. We chose day 4 for this assessment, based upon histological analysis characterizing the kinetics of immune infiltration at the bite site of tick immune guinea pigs with the finding that immune cell infiltration was most intense at day 4 post-challenge (Anderson et al., 2017). We collected skin samples for RNA isolation on day 4 post-tick challenge from guinea pigs and mice previously exposed to ticks, as well as saliva immunized animals. RNA isolated from the skin of naïve guinea pigs or mice (no tick challenge) was used as the respective controls. We examined the gene expression changes at the bite site of animals exposed to ticks for the 1st, 2nd and 3rd time, as well as in saliva immunized animals, relative to normal skin.
RNA-seq analysis identified several differentially expressed genes relative to normal skin (−2 ≥ fold change ≥ 2; P value < 0.05) (Supplemental Table S1). Guinea pigs that were fed on by ticks for the first time had 58 genes up-regulated and 27 genes down-regulated. In comparison, 258 genes were significantly up-regulated and 482 genes down-regulated in guinea pigs fed upon by ticks for the 2nd time. Similarly, 50 genes were up-regulated and 384 genes were down-regulated in guinea pigs fed upon by ticks for the 3rd time. By contrast, saliva immunization resulted in only 65 genes up-regulated and 8 genes down-regulated upon tick challenge. Of the 434 genes differentially expressed after the 3rd tick exposure, 340 (78.3%) overlapped with the genes differentially expressed after the 2nd tick exposure (Fig. 3A). An additional 400 genes were differentially expressed after the 2nd tick exposure, most of which are involved in immune response pathways (Figs. 3B and Supplemental Table S1). Overall, a majority of the differentially expressed genes in tick immune animals were downregulated, suggesting that the host is inhibiting pathways critical for successful feeding or that the tick’s ability to influence host pathways favorable to the tick are no longer intact.
Figure 3. Differential pathway activation in guinea pigs after repeat tick exposure.
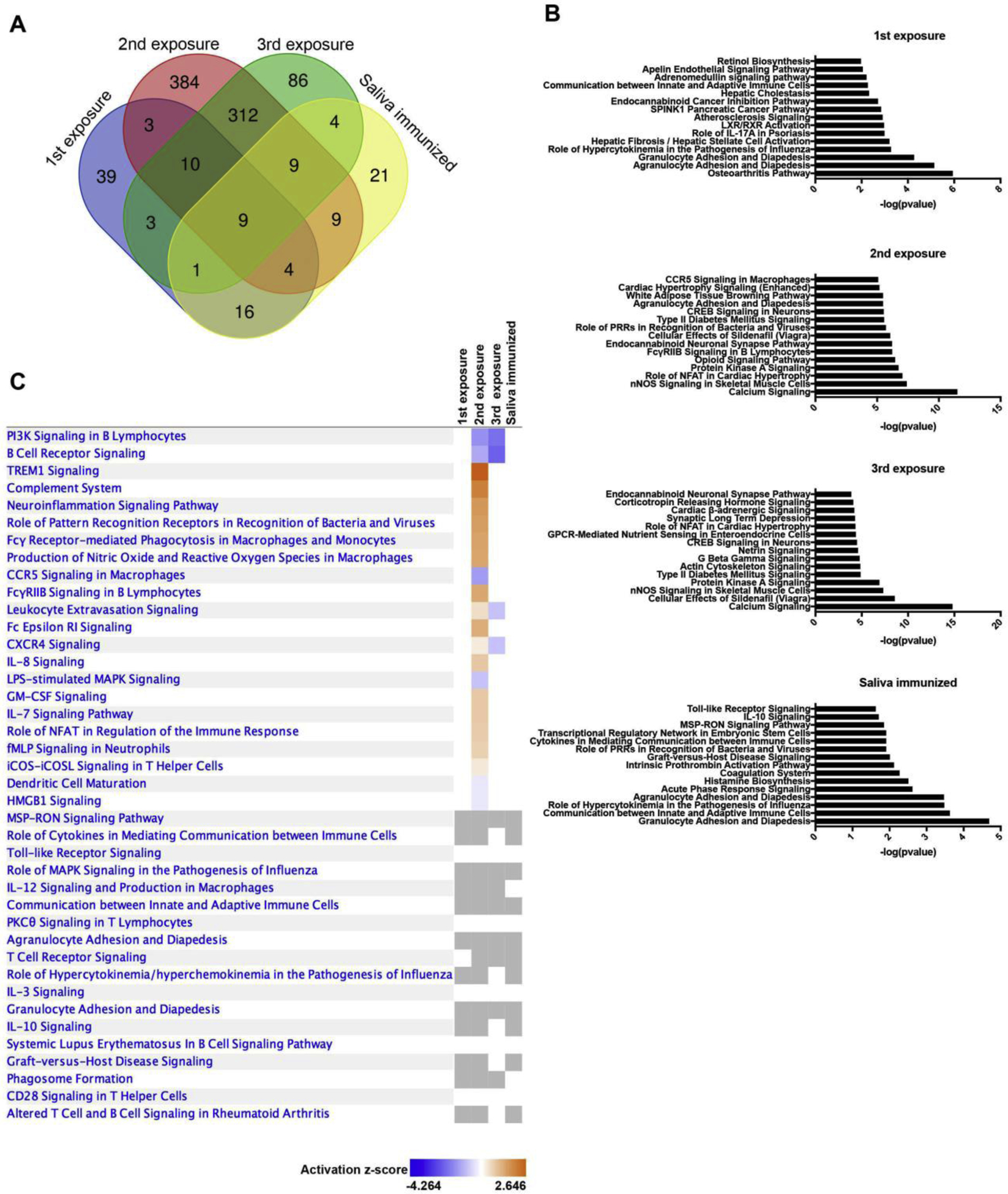
A RNA-sequencing was performed at the bite site of guinea pigs 4 days after: the first tick exposure (n=3); 2nd tick exposures (n=2); third tick exposures (n=2), saliva immunized (n=3); and unbitten normal skin from a naïve mouse (n=3). Differentially expressed genes were defined as at least a 2-fold change and P value <0.05. These data are presented in a Venn diagram to indicate overlap between the different treatment groups. B Pathways analysis was performed using the differentially expressed genes. The top 15 significantly altered pathways are indicated (P value < 0.05). C A heat map of differentially activated pathways (−2 ≥ z-score ≥ 2) are represented.
To determine the biological relevance of these changes, we analyzed these data sets using Ingenuity Pathway Analysis (P value < 0.05; −2 ≥ z-score ≥ 2) (Supplemental Table S2). Immunization with saliva resulted in distinct differences in pathway activation as compared to guinea pigs during the 1st tick challenge (Fig. 3B); however, there were similarities in the lack of pathways activated between the two groups (Fig. 3C). Guinea pigs challenged with I. scapularis for the 2nd and 3rd time resulted in the most significant changes (Figs. 3B and 3C). Most of the changes were associated with metabolic functions and calcium signaling (Fig. 3B). Guinea pigs exposed for the 2nd and 3rd time exhibited downregulation of several pathways involved in tissue repair and wound healing, such as actin cytoskeleton signaling, integrin signaling and VEGF signaling. Additionally, activation of coagulation was observed in guinea pigs immunized with saliva, as well as after the 2nd tick exposure (Figs. 3B and Supplemental Table S2). Activation of the coagulation system was characterized by upregulation of coagulation factor V (F5), coagulation factor XII (F12), plasminogen activator, urokinase receptor (PLAUR) and serpin family E member I (SERPINE1). Ticks acquire a blood meal over the course of several days and combat the wound healing and coagulation pathways through proteins secreted in the saliva, such as the anticoagulant protein SALP14.
Activation of innate and adaptive immune responses were also detected in guinea pigs with repeat tick exposures (Fig. 3C). Consistent with previous literature describing the importance of histamine release from basophils and mast cells, we observed activation of FcεRI signaling at the bite site (Fig. 3C) (Brown and Askenase, 1985, 1981; Kemp and Bourne, 1980; Wada et al., 2010). Repeat tick exposure also resulted in upregulation of pattern recognition receptor signaling, IL-8 signaling and TREM1 signaling (Figs. 3C and Supplemental Table S2).
RNA-seq analysis in the skin at the bite site in repeatedly tick-infested and saliva immunized BALB/c mice revealed several distinct differences as compared to changes measured in the guinea pigs (Figs. 4A and Supplemental Table S3). Mice exposed to I. scapularis for the 1st time resulted in 519 up-regulated genes and 246 down-regulated. Mice exposed to ticks for the 2nd time resulted in 187 genes up-regulated and 258 genes down-regulated. The 3rd tick exposure induced expression of 130 genes and down-regulation of 105 genes. Lastly, tick challenge following saliva immunization resulted in 239 up-regulated genes and 246 down-regulated genes.
Figure 4. Differential pathway activation in mice after repeat tick exposure.
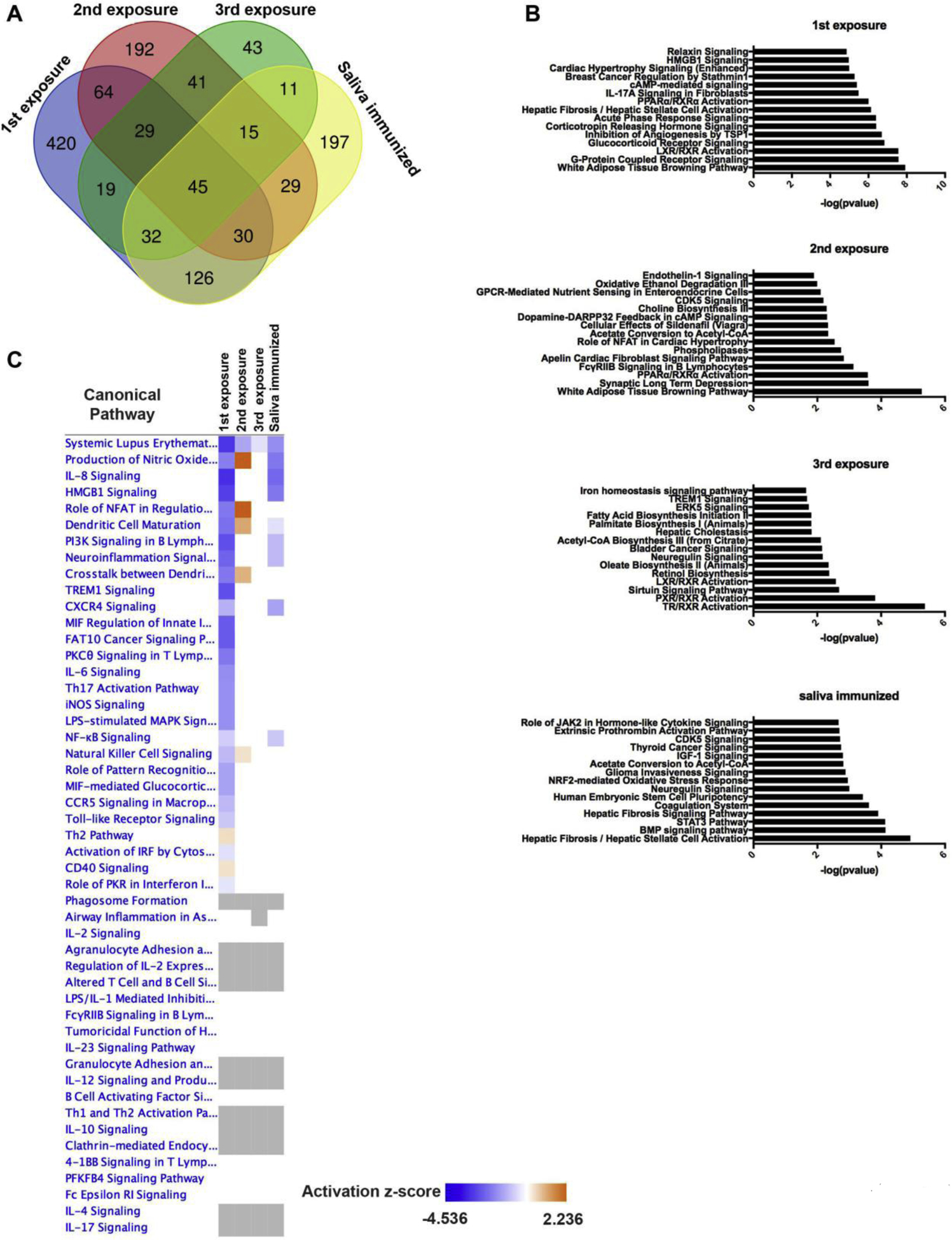
A RNA-sequencing was performed at the bite site of mice 4 days after: the first tick exposure (n=3); 2nd tick exposures (n=3); third tick exposures (n=3), saliva immunized (n=1); and unbitten normal skin from a naïve mouse (n=2). Differentially expressed genes were defined as at least a 2-fold change and P value <0.05. These data are presented in a Venn diagram to indicate overlap between the different treatment groups. B Pathways analysis was performed using the differentially expressed genes. The top 15 significantly altered pathways are indicated (P value < 0.05). C A heat map of differentially activated pathways (−2 ≥ z-score ≥ 2) are represented.
As performed with the guinea pig dataset, we analyzed the mice datasets using IPA. In contrast to guinea pigs, there were very few pathways differentially activated (P value < 0.05; −2 ≥ z-score ≥ 2) (Supplemental Table S4) with the most significant changes occurring after the 1st tick bite (Figs. 4B and 4C). After this feed, several immune pathways were down-regulated such as IL-8 signaling, Th17 activation, Toll-like receptor signaling, TREM1 signaling and IL-6 signaling. However, after the 2nd and 3rd tick feedings, these pathways were no longer down-regulated and had similar levels of activation as normal skin (Figs. 4C and Supplemental Table S4). Mice challenged with ticks following saliva immunization resulted in downregulation in several pathways as wells, such as CXCR4 signaling, PI3K signaling in B lymphocytes, HMGB1 signaling, IL-8 signaling and systemic lupus erythematosus in B cell signaling. Overall, these data suggest that mice are not able to activate the same innate and adaptive immune response towards tick antigens as observed in guinea pigs. These results are consistent with previous literature describing the immunosuppressive function of salivary proteins (Anguita et al., 2002; Hovius et al., 2008; Schuijt et al., 2011, 2008; Simo et al., 2017).
3.4. Immune infiltration between mice and guinea pigs repeatedly exposed to ticks
We next compared the immune cell infiltration at the bite site of mice and guinea pigs repeatedly exposed to ticks, as well as in saliva immunized animals. Skin was harvested at the bite site of animals 4 days after attachment. Guinea pigs challenged with ticks for the first time resulted in minimal to mild inflammation, characterized by a predominance of eosinophils and neutrophils over mononuclear cells (Fig. 5). Guinea pigs challenged for a second and third time resulted in increased severity of inflammation. Tick feeding resulted in marked recruitment of eosinophils, and heterophils (equivalent to neutrophils in other mammalian species) over mononuclear cells. In addition to the increased inflammatory infiltrate, there was prominent acanthosis with ortho- and parakeratotic epidermal hyperkeratosis observed at the bite site. Interestingly, similar results were observed in saliva immunized animals. Here, the bite site also resulted in marked recruitment of eosinophils, with fewer mononuclear cells.
Figure 5. Guinea pig histopathology of normal non-bite and bite site skin after repeat tick exposures or saliva immunization.
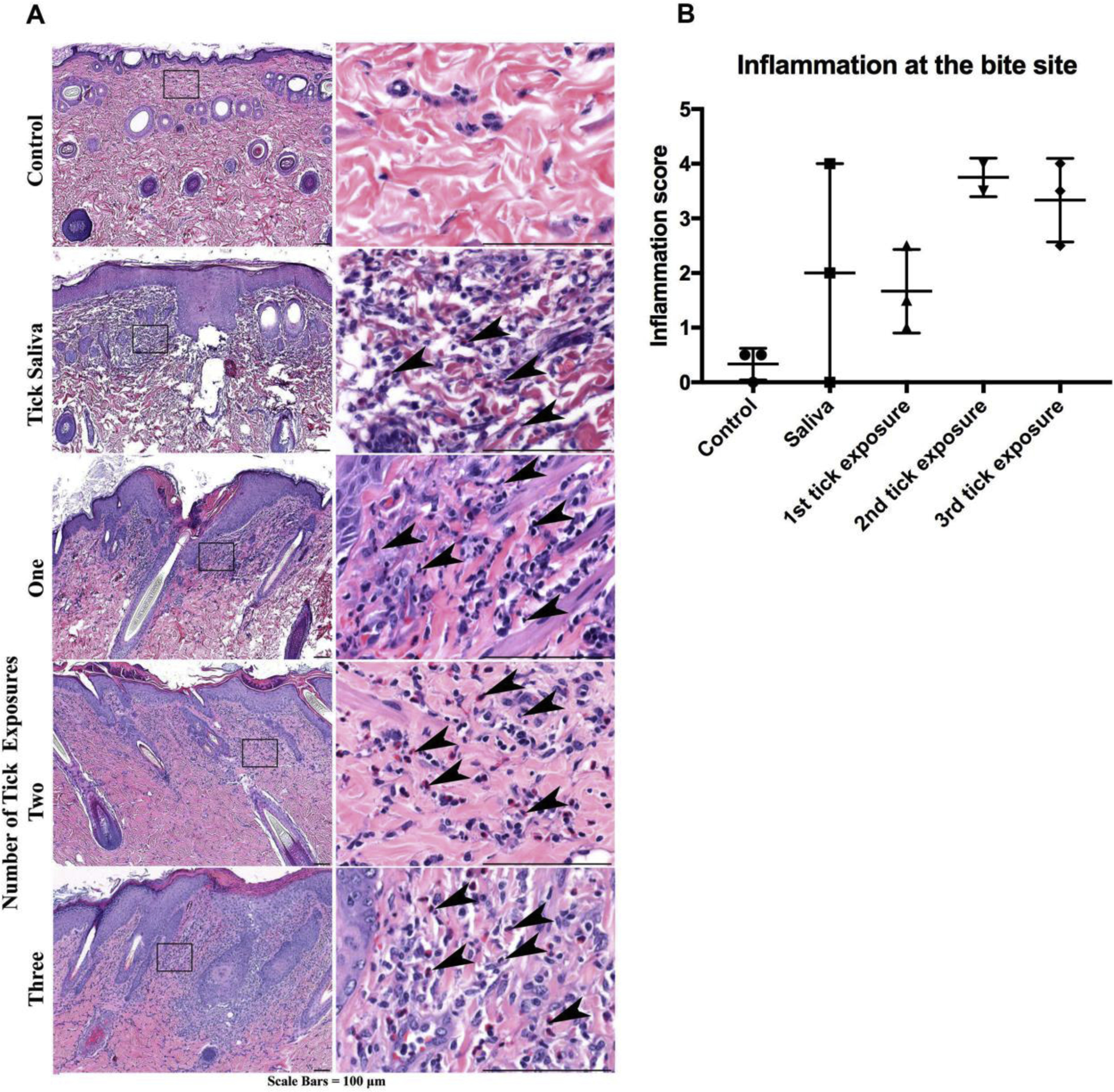
A Compared to non-bite sites, skin at the bite sites harvested 4 days after tick attachment show increased inflammation with heterophils/eosinophils (arrowheads) equal to or in excess over mononuclear cells (representative images). B Inflammation severity scores. Data are represented as mean ± SEM.
Mice exposed to ticks for the first time also had minimal to mild inflammation, characterized primarily by neutrophils and eosinophils over mononuclear cells (Fig. 6). Although multiple tick exposures do not provide protection, the bite sites in these mice elicited a robust (moderate to severe) inflammatory response. The bite site in previously exposed mice was characterized by recruitment of predominantly mononuclear cells, with fewer neutrophils and eosinophils (Fig. 6). Similarly, tick challenge in saliva immunized mice resulted in infiltration where mononuclear cells predominate over eosinophils and neutrophils. Overall, repeat tick exposure results in recruitment of immune cells to the bite site in guinea pigs and mice. However, the bite site in guinea pigs was predominately composed of eosinophils and heterophils, whereas the site in mice was predominately comprised of mononuclear cells and few eosinophils. While acanthosis with ortho- and parakeratotic epidermal hyperkeratosis was also observed at the bite sites in mice, it was markedly reduced when compared to what was observed in the guinea pigs at the same time point, reflecting a difference in response between the species.
Figure 6. Mouse histopathology of normal non-bite and bite site skin after repeat tick exposures or saliva immunization.
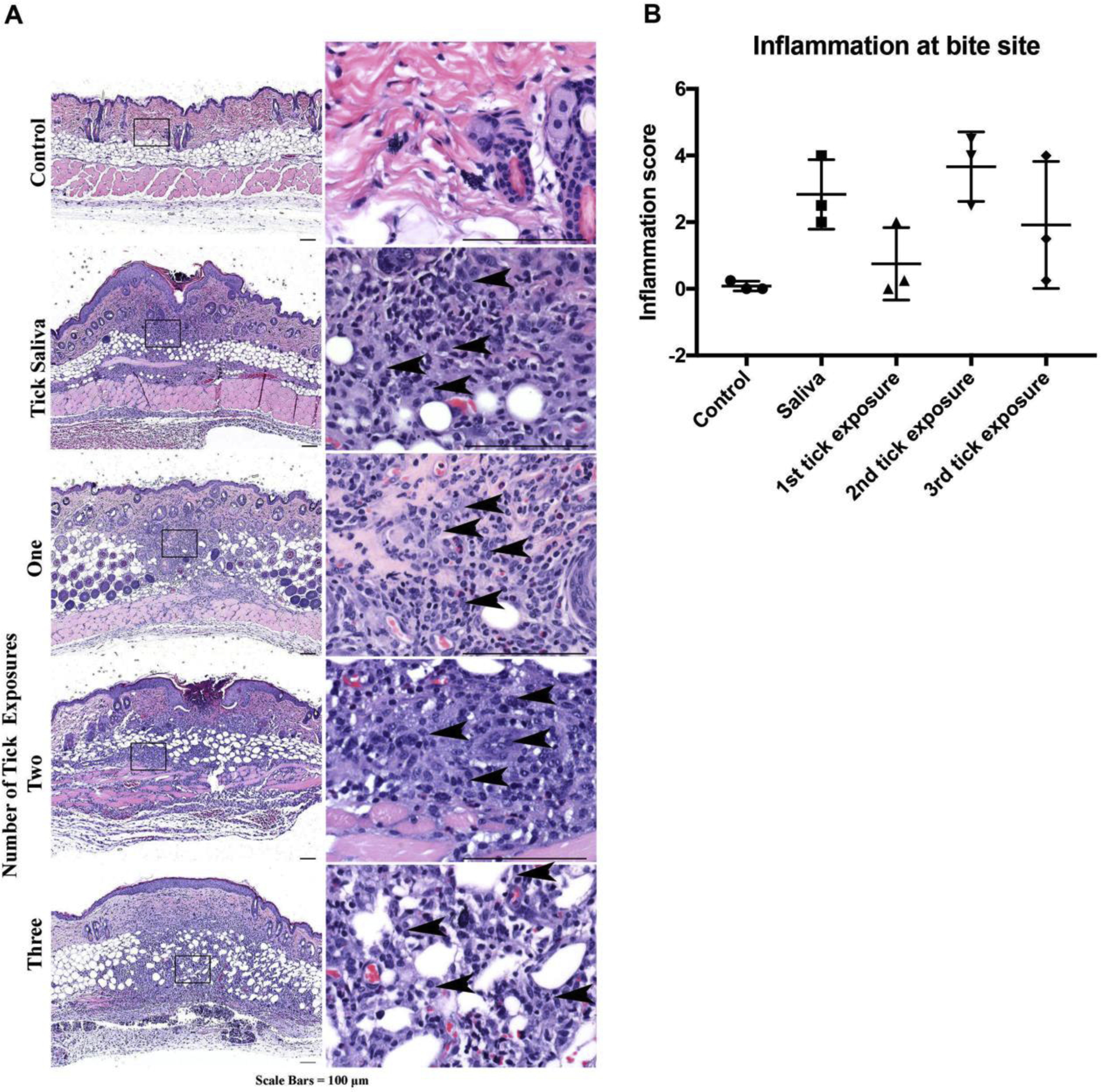
A Compared to non-bite, skin at the bite sites (harvested 4 days after tick attachment) show increased inflammation composed predominantly of mononuclear cells (arrowheads) over neutrophils/eosinophils (representative images). B Inflammation severity scores. Data are represented as mean ± SEM.
4. Discussion
The mechanisms mediating tick rejection are not completely understood (Allen, 1973; Wikel, 1996; Wikel and Allen, 1976). In a guinea pig model of tick resistance, depletion of complement abolished tick rejection in tick immune guinea pigs upon challenge, suggesting complement-mediated protection (Wikel, 1979; Wikel and Allen, 1978). However, passive transfer of lymphoid cells alone has also been shown to be effective in transferring partial immunity from an immune guinea pig to a naïve guinea pig (Wikel and Allen, 1976). Studies from our lab, as well as others, have indicated that serum transfer can only confer partial protection, suggesting that antibodies are not the only protective factor (Brown and Askenase, 1981; Narasimhan et al., 2007). Additionally, depletion of basophils in tick immune guinea pigs prior to tick challenge abolished tick rejection (Brown et al., 1982). To further demonstrate the role of basophils, delivery of histamine receptor antagonists (H1R and H2R) prior tick challenge also eliminated tick rejection in tick immune guinea pigs. Accumulation of basophils has also been shown to be critical for development of tick resistance in mice repeatedly infested with Haemaphysalis longicornis (Wada et al., 2010). Collectively, these studies highlight the essential function of complement, as well as basophil degranulation and histamine release through either an antibody mediated type I hypersensitivity reaction or a T lymphocyte mediated cutaneous basophil hypersensitivity response at the bite site (Dvorak, 1976; Karasuyama et al., 2018; Rajan, 2003; Wikel, 1996). Structural changes to the dermal layer have also been proposed to mediate tick rejection by impairing the ability of the tick to maintain attachment to the skin (Anderson et al., 2017). Identification of protective tick antigens and a complete understanding of the immune profile of the cells involved at the tick bite site are critical to guide the generation of a tick vaccine.
In this study we sought to identify molecular processes involved in tick rejection. We utilized a guinea pig model that develops resistance to tick bites after multiple tick exposures, as well as a mouse model that does not develop tick resistance after multiple exposures to nymphs. Of note, guinea pigs were shaved prior to tick infestation in order to increase tick attachment. However, shaving mice did not impact tick rejection or the development of tick immunity. Our results confirm the importance of several immune pathways previously described for tick rejection in guinea pigs, such as complement activation and FcεRI signaling (Dai et al., 2010; Kemp and Bourne, 1980; Paesen et al., 1999; Wikel, 1979; Wikel and Allen, 1978). Importantly, activation of these pathways was not detected in mice. Although complement activation and FcεRI signaling contribute towards tick rejection, these pathways are unlikely to be the only such factors. For example, we identified eosinophils as the predominate infiltrating immune cell in guinea pigs following saliva immunization or repeat tick infestation. In comparison, mononuclear cells are the predominant cell at the bite site of mice immunized with saliva or following repeat tick infestation.
The immunomodulatory effects of tick saliva and salivary proteins have been well documented, such as activation of Th2 cytokines by T cells following incubation with purified saliva (Langhansova et al., 2015; Simo et al., 2017). Additionally, several tick salivary proteins have been shown to be critical for successful tick feeding (Simo et al., 2017). However, immunization with tick saliva only provides partial tick rejection as compared to tick immunity conferred by natural tick feeding in guinea pigs. These results suggest that tick immunity in guinea pigs can be a result of multiple factors that impact development of tick resistance, such as the duration of antigen delivery and innate immune activation pathways triggered by the physical bite process (Glatz et al., 2017). Future studies are required to examine the impact of these processes for their role in eliciting tick immunity, which could provide important information for the design and delivery of a tick vaccine.
In addition to immune pathways, we observed local skin changes at the bite site that likely impact blood flow and the ability of the tick to obtain a blood meal, including local hyperkeratosis (Anderson et al., 2017). Ticks encode several salivary proteins in order to maintain the flow of blood throughout the feeding process by inhibiting coagulation (Narasimhan et al., 2004). Coagulation can be triggered upon an acute injury to skin or collagen exposure (Reinke and Sorg, 2012). Here, we identified activation of the coagulation system in animals immunized with saliva or upon the 2nd tick infestation, including activation of coagulation factor V (FV). Both of these groups demonstrated significant tick rejection, suggesting activation of these pathways could be important for impairing tick feeding. Ixodes scapularis encode several proteins involved in preventing activation of the coagulation pathway (Narasimhan et al., 2004; Ribeiro et al., 1985; Schuijt et al., 2013). Previous studies have demonstrated that knockdown of the anticoagulant protein SALP14 results in decreased tick feeding (Narasimhan et al., 2004). Immunization with recombinant SALP14 elicits erythema at the bite site and partial tick rejection in guinea pigs (Narasimhan et al., 2020). Furthermore, in a naïve animal, I. scapularis prevent FV activation by secreting the salivary protein Tick Inhibitor of factor Xa towards factor V (TIX-5) into the bite site, which prevents activation of FV by interacting with FXa (Schuijt et al., 2013). Based on our results and previous findings, we hypothesize guinea pigs reject tick feeding by inhibiting the activity of critical salivary proteins involved in preventing coagulation. Future studies will examine vaccine strategies aimed at targeting these proteins.
Guinea pigs immunized with tick saliva have protective immunity with SALP14-specific antibodies (Narasimhan et al., 2020). Yet, guinea pigs exposed to multiple tick exposures develop low antibody titers against SALP14 (Narasimhan et al., 2020). One possibility is the protection elicited by immunization with tick saliva occurs through a separate mechanism than protection by repeat tick exposures. While the immune cells at the bite site are similar, pathway analysis suggests that the inflammatory response is different between the two groups upon tick challenge. In tick immune animals, we measured activation of several inflammatory pathways, such as NFAT signaling (Fig. 3), most of which were absent in saliva immunized animals. Another possibility is that the tick antigens targeted are different between saliva immunized and tick immune animals. Analysis by ELISA revealed several tick antigens recognized in saliva immunized serum (Fig. 2) (Narasimhan et al., 2020), yet we were unable to detect significant antibodies against salivary proteins in tick immune animals. Although SALP14 specific antibodies were not detected in the serum of tick immune animals, we cannot rule out the impact of a T cell response against SALP14.
Many of the genes differentially expressed in guinea pig skin following the 2nd and 3rd infestation are shared; however, the 2nd infestation resulted in additional activation of several immune pathways absent from samples collected following the 3rd infestation. A likely explanation for this is timing of sample collection. Samples were collected 4 days after tick attachment. Tick rejection was complete on day 4 in animals exposed to tick for the 2nd time. In comparison, rejection was more rapid with only 15.1% attached on day 2 and rejection complete on day 3 in animals exposed for the 3rd infestation. Therefore, the gene expression profile obtained from the 2nd infestation likely represent active rejection; whereas samples obtained from the 3rd infestation likely represent post-rejection.
Ixodes scapularis ticks feed on a variety of animals, such as mice, birds, rabbits, canines and reptiles. The function of salivary proteins in modulating the host to support tick feeding has been predominately studied in mice. These salivary proteins function to inhibit the host’s innate and adaptive defense mechanisms, while also maintaining the flow of blood to the bite site (Francischetti et al., 2005; Narasimhan et al., 2007; Ribeiro et al., 1985; Simo et al., 2017; Singer and Clark, 1999). The function of these proteins relies on direct interactions between tick and mouse proteins, such as SALP15 binding to dendritic cells and T cells to suppress the inflammatory response (Anguita et al., 2002; Hovius et al., 2008). In contrast to mice, guinea pigs are not the natural host for I. scapularis. Whether tick salivary proteins are able to elicit similar immunomodulatory effects in guinea pigs as observed in mice has not been determined. Along these lines, a previous study demonstrated that the composition of I. scapularis saliva deposited into guinea pig skin is distinct from the saliva deposited into the skin of mice during feeding (Narasimhan et al., 2019). These results raise the possibility that the antigens targeted by the adaptive immune system of guinea pigs may not be present when ticks feed on mice, which can partially explain the inability to transfer immunity from guinea pigs to mice.
The observations in our study indicate that despite significant recruitment of inflammatory cells towards the bite site after immunization or repeat tick infestation, mice were not able to inhibit tick feeding. Our data indicate several cellular and humoral immune pathways are altered during the first exposure to ticks; however, these pathways are unchanged in subsequent challenges. The lack of acquired tick immunity in mice could be a result of co-evolution between ticks and mice that allow ticks to circumvent induction of tick immunity. Immunity to I. scapularis has not been documented in mice, with the exception of humanized mice engineered to express HLA DR3 (human HLA class II) in class II negative mice (Shattuck et al., 2014). Repeat tick infestation in HLA DR3 transgenic mice resulted in a bias towards Th2 cytokine production, partial tick immunity and partial inhibition of B. burgdorferi s.l. transmission. The exact mechanisms mediating immunity in this model are not completely understood, but suggest that protective antigens are not properly presented on mouse MHC II, whereas they can be presented by human HLA (Shattuck et al., 2014).
Overall, our findings have similarities with previous studies that examined the bite site of tick susceptible and resistant animals following multiple tick infestations. Similar to our study, RNA-sequencing analysis at the bite site of cattle challenged with multiple Rhipicephalus microplus infestations resulted in up-regulation of genes involved in skin remodeling and basophil activation in resistant animals (More et al., 2019). In line with our identification of eosinophil recruitment to the bite site of tick immune guinea pigs, repeat tick infestation in cattle resulted in up-regulation of CCL13, a chemokine important for recruitment of eosinophils (More et al., 2019).
5. Conclusions
In this study, we examined the molecular interaction at the tick bite site after multiple infestations from I. scapularis in guinea pigs and mice. Our data demonstrate that I. scapularis are able to feed repeatedly on mice, possibly by inhibiting activation of inflammatory pathways or shifting the immune response towards tolerance. In contrast, guinea pigs develop rapid tick immunity and rejection. Examination of the bite site of tick immune animals revealed upregulation of several inflammatory pathways absent in mice. Future studies will examine immunization strategies in mice to activate the immune response towards tick proteins. Additionally, we plan to optimize immunization strategies and vaccine cocktails to target salivary proteins involved in coagulation, such as SALP14 and TIX-5 (Schuijt et al., 2013). Inhibiting tick feeding and inducing tick rejection can be an effective strategy to prevent transmission of tick-borne pathogens, particularly against pathogens such as B. burgdorferi s.l. that require 24–48 hours for transmission.
Supplementary Material
Acknowledgements
We wish to thank Michael Schadt at the Comparative Research Pathology Service in the Department of Comparative Medicine (Yale) for the histology preparation. This work was supported in part by the NIH grant AI138949PO1 to EF, the John Monsky and Jennifer Weis Monsky Lyme Disease Research Fund and the Steven and Alexandra Cohen Foundation to EF. CK is funded by a NIH Immuno-Hematopathology Research Training Grant (T32HL007974).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare no competing interests.
Data availability
Data and reagents are available upon request.
References
- Allen JR, 1973. Tick resistance: basophils in skin reactions of resistant guinea pigs. Int J Parasitol 3, 195–200. [DOI] [PubMed] [Google Scholar]
- Anderson JM, Moore IN, Nagata BM, Ribeiro JMC, Valenzuela JG, Sonenshine DE, 2017. Ticks, Ixodes scapularis, Feed Repeatedly on White-Footed Mice despite Strong Inflammatory Response: An Expanding Paradigm for Understanding Tick-Host Interactions. Front Immunol 8, 1784 10.3389/fimmu.2017.01784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anguita J, Ramamoorthi N, Hovius JW, Das S, Thomas V, Persinski R, Conze D, Askenase PW, Rincon M, Kantor FS, Fikrig E, 2002. Salp15, an Ixodes scapularis salivary protein, inhibits CD4(+) T cell activation. Immunity 16, 849–859. 10.1016/s1074-7613(02)00325-4 [DOI] [PubMed] [Google Scholar]
- Brown SJ, Askenase PW, 1985. Rejection of ticks from guinea pigs by anti-hapten-antibody-mediated degranulation of basophils at cutaneous basophil hypersensitivity sites: role of mediators other than histamine. J Immunol 134, 1160–1165. [PubMed] [Google Scholar]
- Brown SJ, Askenase PW, 1981. Cutaneous basophil responses and immune resistance of guinea pigs to ticks: passive transfer with peritoneal exudate cells or serum. J Immunol 127, 2163–2167. [PubMed] [Google Scholar]
- Brown SJ, Galli SJ, Gleich GJ, Askenase PW, 1982. Ablation of immunity to Amblyomma americanum by anti-basophil serum: cooperation between basophils and eosinophils in expression of immunity to ectoparasites (ticks) in guinea pigs. J Immunol 129, 790–796. [PubMed] [Google Scholar]
- Brown SJ, Shapiro SZ, Askenase PW, 1984. Characterization of tick antigens inducing host immune resistance. I. Immunization of guinea pigs with Amblyomma americanum-derived salivary gland extracts and identification of an important salivary gland protein antigen with guinea pig anti-tick antibod. J Immunol 133, 3319–3325. [PubMed] [Google Scholar]
- Dai J, Narasimhan S, Zhang L, Liu L, Wang P, Fikrig E, 2010. Tick histamine release factor is critical for Ixodes scapularis engorgement and transmission of the lyme disease agent. PLoS Pathog 6, e1001205 10.1371/journal.ppat.1001205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das S, Banerjee G, DePonte K, Marcantonio N, Kantor FS, Fikrig E, 2001. Salp25D, an Ixodes scapularis antioxidant, is 1 of 14 immunodominant antigens in engorged tick salivary glands. J Infect Dis 184, 1056–1064. 10.1086/323351 [DOI] [PubMed] [Google Scholar]
- Dvorak HF, 1976. Cutaneous basophil hypersensitivity. J Allergy Clin Immunol 58, 229–240. [DOI] [PubMed] [Google Scholar]
- Francischetti IM, Mather TN, Ribeiro JM, 2005. Tick saliva is a potent inhibitor of endothelial cell proliferation and angiogenesis. Thromb Haemost 94, 167–174. 10.1160/TH04-09-0566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glatz M, Means T, Haas J, Steere AC, Mullegger RR, 2017. Characterization of the early local immune response to Ixodes ricinus tick bites in human skin. Exp Dermatol 26, 263–269. 10.1111/exd.13207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinckley AF, Connally NP, Meek JI, Johnson BJ, Kemperman MM, Feldman KA, White JL, Mead PS, 2014. Lyme disease testing by large commercial laboratories in the United States. Clin Infect Dis 59, 676–681. 10.1093/cid/ciu397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovius JW, de Jong MA, den Dunnen J, Litjens M, Fikrig E, van der Poll T, Gringhuis SI, Geijtenbeek TB, 2008. Salp15 binding to DC-SIGN inhibits cytokine expression by impairing both nucleosome remodeling and mRNA stabilization. PLoS Pathog 4, e31 10.1371/journal.ppat.0040031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karasuyama H, Tabakawa Y, Ohta T, Wada T, Yoshikawa S, 2018. Crucial Role for Basophils in Acquired Protective Immunity to Tick Infestation. Front Physiol 9, 1769 10.3389/fphys.2018.01769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp DH, Bourne A, 1980. Boophilus microplus: the effect of histamine on the attachment of cattle-tick larvae--studies in vivo and in vitro. Parasitology 80, 487–496. 10.1017/s0031182000000950 [DOI] [PubMed] [Google Scholar]
- Kim TK, Tirloni L, Pinto AF, Moresco J, Yates JR 3rd, da Silva Vaz I Jr., Mulenga A, 2016. Ixodes scapularis Tick Saliva Proteins Sequentially Secreted Every 24 h during Blood Feeding. PLoS Negl Trop Dis 10, e0004323 10.1371/journal.pntd.0004323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langhansova H, Bopp T, Schmitt E, Kopecky J, 2015. Tick saliva increases production of three chemokines including monocyte chemoattractant protein-1, a histamine-releasing cytokine. Parasite Immunol 37, 92–96. 10.1111/pim.12168 [DOI] [PubMed] [Google Scholar]
- Maruyama SR, Garcia GR, Teixeira FR, Brandao LG, Anderson JM, Ribeiro JMC, Valenzuela JG, Horackova J, Verissimo CJ, Katiki LM, Banin TM, Zangirolamo AF, Gardinassi LG, Ferreira BR, de Miranda-Santos IKF, 2017. Mining a differential sialotranscriptome of Rhipicephalus microplus guides antigen discovery to formulate a vaccine that reduces tick infestations. Parasit Vectors 10, 206 10.1186/s13071-017-2136-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- More DD, Cardoso FF, Mudadu MA, Malago-Jr W, Gulias-Gomes CC, Sollero BP, Ibelli AMG, Coutinho LL, Regitano LCA, 2019. Network analysis uncovers putative genes affecting resistance to tick infestation in Braford cattle skin. BMC Genomics 20, 998 10.1186/s12864-019-6360-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulenga A, Sugimoto C, Sako Y, Ohashi K, Musoke A, Shubash M, Onuma M, 1999. Molecular characterization of a Haemaphysalis longicornis tick salivary gland-associated 29-kilodalton protein and its effect as a vaccine against tick infestation in rabbits. Infect Immun 67, 1652–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narasimhan S, Booth CJ, DePonte K, Wu MJ, Liang X, Mohanty S, Kantor F, Fikrig E, 2019. Host-specific expression of Ixodes scapularis salivary genes. Ticks Tick Borne Dis 10, 386–397. 10.1016/j.ttbdis.2018.12.001 [DOI] [PubMed] [Google Scholar]
- Narasimhan S, Deponte K, Marcantonio N, Liang X, Royce TE, Nelson KF, Booth CJ, Koski B, Anderson JF, Kantor F, Fikrig E, 2007. Immunity against Ixodes scapularis salivary proteins expressed within 24 hours of attachment thwarts tick feeding and impairs Borrelia transmission. PLoS One 2, e451 10.1371/journal.pone.0000451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narasimhan S, Kurokawa C, Diktas H, Strank NO, Cerny J, Murfin K, Cao Y, Lynn G, Trentleman J, Wu MJ, DePonte K, Kantor F, Anguita J, Hovius J, Fikrig E, 2020. Ixodes scapularis saliva components that elicit responses associated with acquired tick-resistance. Ticks Tick Borne Dis 101369. 10.1016/j.ttbdis.2019.101369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narasimhan S, Montgomery RR, DePonte K, Tschudi C, Marcantonio N, Anderson JF, Sauer JR, Cappello M, Kantor FS, Fikrig E, 2004. Disruption of Ixodes scapularis anticoagulation by using RNA interference. Proc Natl Acad Sci U S A 101, 1141–1146. 10.1073/pnas.0307669100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paesen GC, Adams PL, Harlos K, Nuttall PA, Stuart DI, 1999. Tick histamine-binding proteins: isolation, cloning, and three-dimensional structure. Mol Cell 3, 661–671. 10.1016/s1097-2765(00)80359-7 [DOI] [PubMed] [Google Scholar]
- Rajan TV, 2003. The Gell-Coombs classification of hypersensitivity reactions: a reinterpretation. Trends Immunol 24, 376–379. [DOI] [PubMed] [Google Scholar]
- Ramachandra RN, Wikel SK, 1995. Effects of Dermacentor andersoni (Acari: Ixodidae) salivary gland extracts on Bos indicus and B. taurus lymphocytes and macrophages: in vitro cytokine elaboration and lymphocyte blastogenesis. J Med Entomol 32, 338–345. [DOI] [PubMed] [Google Scholar]
- Ramamoorthi N, Narasimhan S, Pal U, Bao F, Yang XF, Fish D, Anguita J, Norgard MV, Kantor FS, Anderson JF, Koski RA, Fikrig E, 2005. The Lyme disease agent exploits a tick protein to infect the mammalian host. Nature 436, 573–577. 10.1038/nature03812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinke JM, Sorg H, 2012. Wound repair and regeneration. Eur Surg Res 49, 35–43. 10.1159/000339613 [DOI] [PubMed] [Google Scholar]
- Ribeiro JM, Alarcon-Chaidez F, Francischetti IM, Mans BJ, Mather TN, Valenzuela JG, Wikel SK, 2006. An annotated catalog of salivary gland transcripts from Ixodes scapularis ticks. Insect Biochem Mol Biol 36, 111–129. 10.1016/j.ibmb.2005.11.005 [DOI] [PubMed] [Google Scholar]
- Ribeiro JM, Makoul GT, Levine J, Robinson DR, Spielman A, 1985. Antihemostatic, antiinflammatory, and immunosuppressive properties of the saliva of a tick, Ixodes dammini. J Exp Med 161, 332–344. 10.1084/jem.161.2.332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg R, Lindsey NP, Fischer M, Gregory CJ, Hinckley AF, Mead PS, Paz-Bailey G, Waterman SH, Drexler NA, Kersh GJ, Hooks H, Partridge SK, Visser SN, Beard CB, Petersen LR, 2018. Vital Signs: Trends in Reported Vectorborne Disease Cases - United States and Territories, 2004–2016. MMWR Morb Mortal Wkly Rep 67, 496–501. 10.15585/mmwr.mm6717e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuijt TJ, Bakhtiari K, Daffre S, Deponte K, Wielders SJ, Marquart JA, Hovius JW, van der Poll T, Fikrig E, Bunce MW, Camire RM, Nicolaes GA, Meijers JC, van ‘t Veer C, 2013. Factor Xa activation of factor V is of paramount importance in initiating the coagulation system: lessons from a tick salivary protein. Circulation 128, 254–266. 10.1161/CIRCULATIONAHA.113.003191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuijt TJ, Coumou J, Narasimhan S, Dai J, Deponte K, Wouters D, Brouwer M, Oei A, Roelofs JJ, van Dam AP, van der Poll T, Van’t Veer C, Hovius JW, Fikrig E, 2011. A tick mannose-binding lectin inhibitor interferes with the vertebrate complement cascade to enhance transmission of the lyme disease agent. Cell Host Microbe 10, 136–146. 10.1016/j.chom.2011.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuijt TJ, Hovius JW, van Burgel ND, Ramamoorthi N, Fikrig E, van Dam AP, 2008. The tick salivary protein Salp15 inhibits the killing of serum-sensitive Borrelia burgdorferi sensu lato isolates. Infect Immun 76, 2888–2894. 10.1128/IAI.00232-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shattuck WM, Dyer MC, Desrosiers J, Fast LD, Terry FE, Martin WD, Moise L, De Groot AS, Mather TN, 2014. Partial pathogen protection by tick-bite sensitization and epitope recognition in peptide-immunized HLA DR3 transgenic mice. Hum Vaccin Immunother 10, 3048–3059. 10.4161/21645515.2014.985498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simo L, Kazimirova M, Richardson J, Bonnet SI, 2017. The Essential Role of Tick Salivary Glands and Saliva in Tick Feeding and Pathogen Transmission. Front Cell Infect Microbiol 7, 281 10.3389/fcimb.2017.00281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer AJ, Clark RA, 1999. Cutaneous wound healing. N Engl J Med 341, 738–746. 10.1056/NEJM199909023411006 [DOI] [PubMed] [Google Scholar]
- Trimnell AR, Davies GM, Lissina O, Hails RS, Nuttall PA, 2005. A cross-reactive tick cement antigen is a candidate broad-spectrum tick vaccine. Vaccine 23, 4329–4341. 10.1016/j.vaccine.2005.03.041 [DOI] [PubMed] [Google Scholar]
- Wada T, Ishiwata K, Koseki H, Ishikura T, Ugajin T, Ohnuma N, Obata K, Ishikawa R, Yoshikawa S, Mukai K, Kawano Y, Minegishi Y, Yokozeki H, Watanabe N, Karasuyama H, 2010. Selective ablation of basophils in mice reveals their nonredundant role in acquired immunity against ticks. J Clin Invest 120, 2867–2875. 10.1172/JCI42680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikel SK, 1996. Host immunity to ticks. Annu Rev Entomol 41, 1–22. 10.1146/annurev.en.41.010196.000245 [DOI] [PubMed] [Google Scholar]
- Wikel SK, 1979. Acquired resistance to ticks: expression of resistance by C4-deficient guinea pigs. Am J Trop Med Hyg 28, 586–590. [PubMed] [Google Scholar]
- Wikel SK, Allen JR, 1978. Acquired resistance to ticks. III. Cobra venom factor and the resistance response. Immunology 32, 457–465. [PMC free article] [PubMed] [Google Scholar]
- Wikel SK, Allen JR, 1976. Acquired resistance to ticks. I. Passive transfer of resistance. Immunology 30, 311–316. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


