Abstract
Background & objectives:
Healthcare workers (HCWs) are at an elevated risk of contracting COVID-19. While intense occupational exposure associated with aerosol-generating procedures underlines the necessity of using personal protective equipment (PPE) by HCWs, high-transmission efficiency of the causative agent [severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)] could also lead to infections beyond such settings. Hydroxychloroquine (HCQ), a repurposed antimalarial drug, was empirically recommended as prophylaxis by the National COVID-19 Task Force in India to cover such added risk. Against this background, the current investigation was carried out to identify the factors associated with SARS-CoV-2 infection among HCWs in the country.
Methods:
A case-control design was adopted and participants were randomly drawn from the countrywide COVID-19 testing data portal maintained by the ICMR. The test results and contact details of HCWs, diagnosed as positive (cases) or negative (controls) for SARS-CoV-2 using real-time reverse transcription-polymerase chain reaction (qRT-PCR), were available from this database. A 20-item brief-questionnaire elicited information on place of work, procedures conducted and use of PPE.
Results:
Compared to controls, cases were slightly older (34.7 vs. 33.5 yr) and had more males (58 vs. 50%). In multivariate analyses, HCWs performing endotracheal intubation had higher odds of being SARS-CoV-2 infected [adjusted odds ratio (AOR): 4.33, 95% confidence interval (CI): 1.16-16.07]. Consumption of four or more maintenance doses of HCQ was associated with a significant decline in the odds of getting infected (AOR: 0.44; 95% CI: 0.22-0.88); a dose-response relationship existed between frequency of exposure to HCQ and such reductions (χ2 for trend=48.88; P<0.001). In addition, the use of PPE was independently associated with the reduction in odds of getting infected with SARS-CoV-2.
Interpretations & conclusions:
Until results of clinical trials for HCQ prophylaxis become available, this study provides actionable information for policymakers to protect HCWs at the forefront of COVID-19 response. The public health message of sustained intake of HCQ prophylaxis as well as appropriate PPE use need to be considered in conjunction with risk homoeostasis operating at individual levels.
Keywords: Dose-response relationship, healthcare workers, hydroxychloroquine prophylaxis, personal risk management, rapid evidence generation, SARS-CoV-2
Since its global recognition in December 2019, the novel coronavirus disease (COVID-19) pandemic has spread to over 200 countries in less than five months. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the causative agent of this disease, was noted to spread efficiently through respiratory droplets and contact routes1,2,3,4. While common presenting symptoms are fever, fatigue, dry cough, myalgia and dyspnoea, a few patients have reported having diarrhoea, nausea, vomiting and new-onset anosmia or ageusia. A considerable proportion of the SARS-CoV-2-infected individuals (around 80%) did not have any noticeable symptoms and yet were able to transmit the infection5. Such unique transmission potentials of SARS-CoV-2 and lack of definitive antiviral therapy were the reasons behind its wide-scale spread. Evidence indicates that healthcare workers (HCWs) are particularly at risk of acquiring SARS-CoV-2 infection, due to repeated occupational exposure6.
In the absence of specific treatments against COVID-19, social distancing7, use of face masks8 and frequent hand washing with alcohol rubs or soap constituted the infection prevention measures targeting general population9. However, HCWs, being exposed to a higher quantum of risk, needed additional intervention approaches for protection10. Aprons, gowns, gloves, masks, face shields and goggles addressed such needs. These protective gears serve useful purpose in settings where procedures such as nasopharyngeal swab collection, endotracheal intubation or respiratory suctioning are performed on suspected or confirmed patients of COVID-19, potentially generating aerosols from the respiratory tract11. However, caregiving in a pandemic situation would also entail the risks of transmission of SARS-CoV-2 infection to HCWs from asymptomatic individuals who are not necessarily undergoing invasive procedures2,12,13. Chemoprophylaxis for HCWs could potentially have add-on advantages to cover this additional risk.
Prophylaxis in the present context refers to the use of a short-term therapy to prevent acquisition of SARS-CoV-2 infection. Currently, there are no approved vaccines against SARS-CoV-2, which makes the alternative of using chemotherapeutic agents an attractive proposition. However, no antiviral medicines proved efficacious during the previous coronavirus outbreaks (SARS 2003; Middle East respiratory syndrome coronavirus 2012) and therefore, did not leave the therapeutic community with any viable options during the present COVID-19 pandemic14,15. Hydroxychloroquine (HCQ) came into discussion against this background16. Ability of this compound to inhibit the infection by SARS-CoV-2, as well as viral replication in cell cultures in a time- and dose-dependent manner made it a primary choice17. Furthermore, HCQ elevates the pH of endosomes and inhibits SARS-CoV-2 RNA-mediated inflammatory response18. These laboratory findings encouraged researchers to consider HCQ, originally used for malaria, as a repurposed agent for prophylaxis against SARS-CoV-219.
The National Task Force for COVID-19 in India took cognizance of this evidence and empirically recommended the use of HCQ as prophylaxis against SARS-CoV-2 infection in asymptomatic HCWs treating suspected or confirmed COVID-19 cases. Asymptomatic household contacts of confirmed COVID-19 cases were also covered by this advisory released on March 22, 202020. Around the same time, in South Korea, HCQ prophylaxis was used successfully to avert new infections after a large COVID-19 exposure event in a long-term care facility21. Scientific communications further underlined the necessity of examining the utility of such approaches in the context of high-burden, high-income countries such as Italy22. Against this backdrop, a case-control investigation was conducted to compare the risks of and protective factors against SARS-CoV-2 infection among HCWs in India.
Material & Methods
The ICMR COVID-19 Research Team developed the study proposal, which was approved by the ICMR Central Ethics Committee. Data collection for this investigation was done during May 8-23, 2020. Each participant was informed about the study purpose, and verbal consent was obtained before proceeding with telephonic interview. A data portal developed to capture the information regarding individuals undergoing testing for SARS-CoV-2 infection across India was used to identify the study participants. HCWs tested between the first week of April 2020 and the end of first week of May 2020 formed the sample pool, from which cases and controls were drawn. Symptomatic HCWs testing positive on real-time reverse transcription-polymerase chain reaction (qRT-PCR) for SARS-CoV-2 were defined as cases. Controls were symptomatic HCWs who tested negative on qRT-PCR for SARS-CoV-2 under similar considerations.
Measures: A brief 20-item interview schedule was developed to elicit the information on key issues, such as department, designation and length of employment, and use of personal protective equipment (PPE). Among exposure variables, the HCW was asked about contact with suspected or confirmed COVID-19 patients on ventilator and involvement in aerosol-generating procedures (AGPs) such as nasopharyngeal swab collection, endotracheal intubation and respiratory suction. To minimize recall bias, this enquiry was restricted to seven days before SARS-CoV-2 testing. A history of prophylactic HCQ intake with dosing details was also obtained.
Telephonic interviews: Participants were telephonically contacted by the researchers to introduce themselves, verify identities, describe the study purpose and check availability for interviews. If a participant's contact phone number in the ICMR data portal actually belonged to a treatment supporter or caregiver or relative, we reached out to the individual who was tested for COVID-19 through the primary contact. Following verbal consent, telephonic interviews, which took 5-11 min, were conducted. At the close of the interviews, participants' queries related to COVID-19 were addressed.
Sample size: It was intended to enrol cases and controls in a 1:1 ratio and match them for location (testing centre) and temporality (test date). Assuming that 50 per cent of the controls were on HCQ prophylaxis (exposure) and correlation coefficient for exposure between matched cases and controls would be 0.2, it was estimated that 484 cases would be required to detect an odds ratio of 1.50 with 80 per cent power at five per cent significance level23. These calculations were undertaken using Power Analysis Sample Size (PASS) software version 11.024.
Statistical analysis: The data captured in hard copies during the telephonic interviews were checked for quality and computerized following the necessary corrections. The association of key risk factors with SARS-CoV-2 infection was examined by comparing distributions of cases and controls across different exposures. Variables which had biologically plausible association with the outcome and were relevant for planning strategies for the prevention of SARS-CoV-2 infection in HCWs were entered into a standard logistic regression model25. STATA version 13.1 (StataCorp LP, College Station, TX, USA) was used for data analysis including trend analysis by Chi-square test.
Results
The ICMR data portal contained the results and contact details of 23,898 symptomatic HCWs who were tested for SARS-CoV-2 infection. After excluding non-Indian nationals and missing or wrong contact details from this database, 21,402 records were obtained, with 1,073 (5%) confirmed SARS-CoV-2-infected HCWs. Although it was initially decided to contact 650 cases and controls each (accounting for 25% loss over the calculated sample size of 484), only 624 and 549 individuals could be contacted in the case and control groups, respectively. Completed interview schedules of 60.58 per cent of cases (378/624) and 67.94 per cent of controls (373/549) were available for analysis. The reasons for not being able to reach out to some of the participants were: calls not picked up, wrong numbers, ineligible candidates (not HCWs), consent refusal to name a few.
Fifty eight per cent of the cases and about half of the controls were males. While the mean age of the cases was 34.73 yr [±standard deviation (SD): 9.64; median: 33.0; interquartile range (IQR): 27-40], the mean age of the controls was 33.47 yr (±SD: 9.77; median: 31.0; IQR: 26-38). Age distribution did not follow Gaussian distribution in either group. Table I presents details of the study participants.
Table I.
Profile of the healthcare workers included in the study
| Parameters | Cases (n1=378) (%) | Controls (n2=373) (%) | OR | 95% CI of OR | P |
|---|---|---|---|---|---|
| Gender | |||||
| Male | 219 (57.95) | 188 (50.40) | 1.36 | 1.02-1.81 | 0.038 |
| Female | 159 (42.06) | 185 (49.60) | Ref | ||
| Age (yr) | |||||
| 18-25 | 65 (17.2) | 86 (23.06) | 0.62 | 0.35-1.09 | 0.208 |
| 26-33 | 134 (35.45) | 135 (36.19) | 0.81 | 0.48-1.38 | |
| 34-41 | 97 (25.66) | 77 (20.64) | 1.04 | 0.59-1.8 | |
| 42-49 | 43 (11.38) | 43 (11.53) | 0.82 | 0.44-1.54 | |
| >50 | 39 (10.32) | 32 (8.58) | Ref | ||
| Occupation | |||||
| Doctor | 111 (29.37) | 123 (32.98) | 0.94 | 0.57-1.57 | 0.537 |
| Nurse/ANM | 165 (43.65) | 144 (38.61) | 1.2 | 0.74-1.96 | |
| Housekeeping staff | 16 (4.23) | 10 (2.68) | 1.68 | 0.68-4.14 | |
| Security guards | 10 (2.65) | 12 (3.22) | 0.88 | 0.34-2.25 | |
| Others | 36 (9.52) | 42 (11.26) | 0.9 | 0.48-1.67 | |
| Laboratory technician/operation theater technician | 40 (10.58) | 42 (11.26) | Ref | ||
| Duration of work in respective workplace before COVID-19 testing (yr) | |||||
| >1 | 264 (69.84) | 179 (47.99) | 2.51 | 1.86-3.39 | <0.001 |
| <1 | 114 (30.16) | 194 (52.01) | Ref |
OR, odds ratio; CI, confidence interval; Ref, reference category; ANM, auxiliary nurse midwife
Vulnerability of HCWs: Vulnerability of the study participants to SARS-CoV-2 infection was ascertained through a history of (i) placement in intensive care unit (ICU) catering to suspected or confirmed COVID-19 cases, (ii) procedures such as nasopharyngeal swab collection, intubation, respiratory suctioning and clinical specimen handling by HCWs and (iii) use of PPE. Endotracheal intubation was associated with higher odds of SARS-CoV-2 infection. Respondents who reported never using PPEs were also at a higher risk. On the other hand, when the participants were asked about individual components of PPE, usage of masks, caps, gowns and gloves was associated with reduced odds of acquiring SARS-CoV-2 infection (Table II).
Table II.
Place of work, procedure and protection details of healthcare workers
| Cases (n1=378) (%) | Controls (n2=373) (%) | OR | 95% CI of OR | P | |
|---|---|---|---|---|---|
| ICU with suspected or confirmed COVID-19 cases on ventilator | |||||
| Yes | 53 (14.02) | 40 (10.72) | 1.36 | 0.88-2.1 | 0.17 |
| No | 325 (85.98) | 333 (89.28) | Ref | ||
| Nasopharyngeal swab collection | |||||
| Yes | 18 (4.76) | 22 (5.9) | 0.8 | 0.42-1.51 | 0.488 |
| No | 360 (95.24) | 351 (94.1) | Ref | ||
| Endotracheal intubation | |||||
| Yes | 22 (5.82) | 9 (2.41) | 2.5 | 1.13-5.5 | 0.01 |
| No | 356 (94.18) | 364 (97.59) | Ref | ||
| Respiratory tract suctioning | |||||
| Yes | 15 (3.97) | 20 (5.36) | 0.73 | 0.37-1.45 | 0.365 |
| No | 363 (96.03) | 353 (94.64) | Ref | ||
| Handling clinical specimen (stool, blood, bronchoalveolar lavage) | |||||
| Yes | 42 (11.11) | 46 (12.33) | 0.89 | 0.57-1.39 | 0.603 |
| No | 336 (88.89) | 327 (87.67) | Ref | ||
| PPE usage | |||||
| Never used | 57 (15.08) | 17 (4.56) | 3.72 | 2.12-6.52 | <0.001 |
| Used in all or some cases | 321 (84.92) | 356 (95.44) | Ref | ||
| Use of PPE gears | |||||
| Masks | |||||
| Any mask use | 310 (82.01) | 346 (92.76) | 0.35 | 0.22-0.57 | <0.001 |
| No mask use | 68 (17.99) | 27 (7.24) | Ref | ||
| Cap | |||||
| Yes | 166 (43.92) | 196 (47.45) | 0.7 | 0.53-0.94 | 0.018 |
| No | 212 (56.08) | 177 (52.55) | Ref | ||
| Gown | |||||
| Yes | 152 (40.21) | 194 (52.01) | 0.62 | 0.46-0.83 | 0.001 |
| No | 226 (59.79) | 179 (47.99) | Ref | ||
| Shoe cover use | |||||
| Yes | 133 (35.19) | 127 (34.05) | 1.05 | 0.78-1.42 | 0.743 |
| No | 245 (64.81) | 246 (65.95) | Ref | ||
| Face shield or goggles | |||||
| Yes (either or both) | 163 (43.12) | 180 (48.26) | 0.81 | 0.61-1.08 | 0.158 |
| No (none) | 215 (56.88) | 193 (51.74) | Ref | ||
| Gloves | |||||
| Yes | 267 (70.63) | 322 (86.33) | 0.38 | 0.26-0.55 | <0.001 |
| No | 111 (29.37) | 51 (13.67) | Ref |
PPE, personal protective equipment; ICU, intensive care unit
Hydroxychloroquine prophylaxis: Distribution of cases and controls across exposures in univariate analysis indicated the association of risk (P=0.087) of SARS-CoV-2 infection with the lack of HCQ prophylaxis (Table III). However, the number of maintenance doses taken by HCWs following the intake of a loading dose revealed a protective dose-response relationship. Consumption of four or more maintenance doses was associated with a significant decline in the risk of SARS-CoV-2 infection among the study participants (Figure). The significant declining trend had an overall χ2 value of 48.88 (P <0.001).
Table III.
Patterns of hydroxychloroquine (HCQ) prophylaxis in healthcare workers
| Parameters | Cases (n1=378) (%) | Controls (n2=373) (%) | OR | 95% CI of OR | P |
|---|---|---|---|---|---|
| HCQ prophylaxis | |||||
| No | 206 (54.5) | 180 (48.26) | 1.28 | 0.96-1.71 | 0.087 |
| Yes | 172 (45.50) | 193 (51.74) | Ref | ||
| Number of maintenance doses of HCQ prophylaxis taken | |||||
| >6 | 12 (3.17) | 56 (15.01) | 0.19 | 0.1-0.36 | <0.001 |
| 4-5 | 42 (11.11) | 67 (17.96) | 0.55 | 0.35-0.84 | |
| 2-3 | 70 (18.52) | 37 (9.92) | 1.65 | 1.06-2.58 | |
| HCQ loading dose and irregular recall of maintenance | 48 (12.7) | 33 (8.85) | 1.27 | 0.78-2.07 | |
| None | 206 (54.5) | 180 (48.26) | Ref | ||
| Combination prophylaxis | |||||
| HCQ only | 130 (34.39) | 133 (35.66) | 0.85 | 0.62-1.17 | 0.002 |
| HCQ+azithromycin+vitamins | 25 (6.61) | 16 (4.29) | 1.36 | 0.71-2.64 | |
| HCQ+vitamins | 6 (1.59) | 25 (6.70) | 0.21 | 0.08-0.52 | |
| HCQ+non-allopathic systems of medicines or others | 11 (2.91) | 19 (5.09) | 0.51 | 0.23-1.09 | |
| No HCQ | 206 (54.5) | 180 (48.26) | Ref |
Figure.
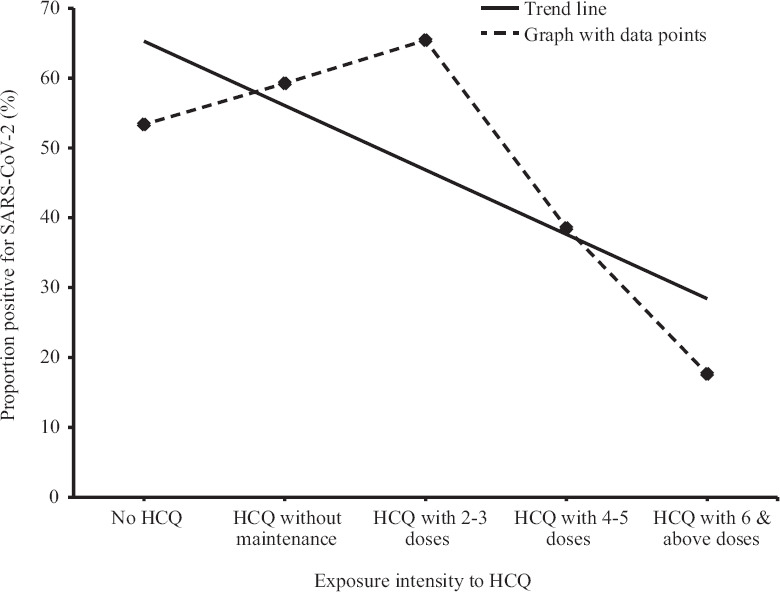
Dose-response relationship between hydroxychloroquine (HCQ) exposure and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection.
Of the 172 cases and 193 controls reporting HCQ intake, no significant difference in the occurrence of adverse drug reactions was noted. The three most common side effects of HCQ as reported by the cases and controls were nausea (5 vs. 8%), headache (6 vs. 5%) and diarrhoea (5 vs. 4%). While none of the controls on HCQ complained of palpitations, only one case (1/172, 0.6%) reported the same. Gastrointestinal symptoms such as acidity and vomiting following HCQ intake ranged from 0.6 per cent in cases to about two per cent in controls. Very few cases (0.6%) and controls (1.4%) had skin rashes after consuming HCQ.
Multivariate analysis: Factors found associated (P<0.1) with SARS-CoV-2 infection among HCWs in univariate analysis and having biological plausibility were entered into multivariate model. In case of conceivable similarity between explanatory variables, one was chosen over another to avoid collinearity. For example, PPE rather than individual items (cap, mask, gown, glove, etc.) of PPE was included in the model. Adjusted for25 gender, use of PPE, endotracheal intubation, different intensity of exposure to prophylactic HCQ and testing place with date, intake of 4-5 maintenance doses of HCQ [adjusted odds ratio (AOR): 0.44; 95% confidence interval (CI): 0.22-0.88; P=0.02] was found to independently impart the protective effect against SARS-CoV-2 infection among HCWs (Table IV). Notwithstanding this effect, the advantage of PPE usage was also independently indicated by the multivariate model. Noticeably, six or more prophylactic doses of HCQ used by HCWs had a remarkably high (>80%) protective effect against SARS-CoV-2 infection.
Table IV.
Factors independently associated with SARS-CoV-2 in healthcare workers in India
| Attributes | AOR | 95% CI of AOR | P |
|---|---|---|---|
| Male | 1.93 | 1.21-3.07 | 0.006 |
| Never used PPE | 5.33 | 2.27-12.48 | <0.001 |
| Performing endotracheal intubation | 4.33 | 1.16-16.07 | 0.029 |
| Maintenance doses of HCQ | |||
| HCQ loading dose and irregular recall of maintenance | 1.87 | 0.82-4.24 | 0.136 |
| 2-3 | 2.34 | 1.23-4.83 | 0.022 |
| 4-5 | 0.44 | 0.22-0.88 | 0.02 |
| ≥6 | 0.04 | 0.01-0.16 | <0.001 |
AOR, adjusted odds ratio; HCQ, hydroxychloroquine
Discussion
Research to inform public health responses during infectious disease emergencies is gradually gaining importance worldwide. For example, Ebola virus disease in West Africa and Nipah virus outbreak in the Indian sub-continent required quick research responses to help mitigate human sufferings in the recent past26,27. The current investigation can be considered as an example of this emerging trend. We leveraged a nationwide COVID-19 testing database to rapidly generate evidence to inform public health action.
The pivotal finding of our study was the noteworthy benefits of HCQ prophylaxis. It was identified that simply initiating HCQ prophylaxis did not reduce the odds of acquiring SARS-CoV-2 infection among HCWs. However, with the intake of four or more maintenance doses of HCQ, the protective effect started emerging, and in the adjusted multivariate model, a significant reduction (>80%) in the odds of SARS-CoV-2 infection in the HCWs was identified with the intake of six or more doses of HCQ prophylaxis. This dose-response relationship (Figure) added strength to the study outcomes. Worth noting in this context was that the National Task Force for COVID-19 in India recommended once a week maintenance dose for seven weeks (400 mg once weekly), following the loading dose (400 mg bd). Adherence to this recommended regimen is underlined by the findings of the present study. The potential antiviral and anti-inflammatory properties of HCQ28, together with the low cost of therapy, excellent oral bioavailability29, high tissue concentrations in the lungs relative to the plasma levels and acceptable safety profile lend support to this assertion17. However, HCQ prophylaxis should be taken in tandem with PPE use as indicated by the multivariate model (Table IV).
A recent registry-based analysis highlighted that HCQ did not offer therapeutic benefits to severe COVID-19 cases, and was associated with increased mortality30. This apparent disparity with the findings of the current investigation could be explained by the two different application contexts. While the observational study involving registry-analysis focussed on the treatment of hospitalized COVID-19 patients, our emphasis was on the prevention of infections among HCWs. In treatment settings, severe COVID-19 patients are likely to have a very high viral load and cytokine levels, which may not be improved by HCQ therapy31. The registry-based analysis further recorded higher frequencies of ventricular arrhythmias in patients receiving HCQ. The toxicities of HCQ are likely to be infrequent in healthy groups undergoing prophylactic therapy as observed in our study participants. Biologically, it appears plausible that HCQ prophylaxis, before onset of infection, may inhibit the virus from gaining a foothold.
While the strength of the present analysis was the involvement of a countrywide database that drew upon more than 70 COVID-19 testing laboratories spread all over India, its limitations were rooted in its observational design. However, in the absence of clinical trial results32 on safety and efficacy of HCQ chemoprophylaxis in the HCWs, this study offers evidence of public health importance. Higher prevalence of SARS-CoV-2 infection in the HCWs has been a global concern, including in countries such as Spain, Italy and the USA33,34,35, which further underscores the importance of the present findings.
The first part of the dose-response relationship curve showed an apparent increase in the odds of acquiring SARS-CoV-2 infection in HCWs who had taken 2-3 doses of HCQ prophylaxis. While this phenomenon cannot be fully explained by the data collected through the present study, lessons from other areas of public health could be of some help. The parallels36 include (i) seat-belt legislations vis-à-vis speeding and road traffic casualties, and (ii) condom use promotion with unintended effects linked to greater sexual activities. Adams37 and Wilde38 allude to models of individual risk management which have the potential to explain such apparent paradoxes. They described that the introduction of a safety device could disrupt the balance between perceived hazards and rewards of risk-taking behaviours. Within the ambit of the present discussion, we consider (i) HCQ prophylaxis as a newly identified safety device, (ii) getting infected with SARS-CoV-2 as the perceived hazard, and (iii) not adhering to conventional respiratory infection prevention measures, such as PPE use, personal hygiene and social distancing as risk-taking behaviours.
In conclusion, public health message on the role of HCQ prophylaxis for the prevention of SARS-CoV-2 infection among HCWs emerging from this study should be considered with the existing understanding of risk homoeostasis operating at individual levels.
Acknowledgment
Authors acknowledge the support in data cleaning and management provided by Servshri Ganesh Prasad Jena, Ajay Kumar and Gurmeet Singh Rana, ICMR-National Institute of Medical Statistics (ICMR-NIMS); and in data collection Ms Seema Verma, Division of Non-Communicable Diseases, ICMR, New Delhi.
Footnotes
Financial support & sponsorship: None.
Conflicts of Interest: None.
References
- 1.Liu J, Liao X, Qian S, Yuan J, Wang F, Liu Y, et al. Community transmission of severe acute respiratory syndrome coronavirus 2, Shenzhen, China, 2020. Emerg Infect Dis. 2020;26:1320–3. doi: 10.3201/eid2606.200239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chan JF, Yuan S, Kok KH, To KK, Chu H, Yang J, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: A study of a family cluster. Lancet. 2020;395:514–23. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382:1199–207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chatterjee P, Nagi N, Agarwal A, Das B, Banerjee S, Sarkar S, et al. The 2019 novel coronavirus disease (COVID-19) pandemic: A review of the current evidence. Indian J Med Res. 2020;151:147–59. doi: 10.4103/ijmr.IJMR_519_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang L, Zhang X, Zhang X, Wei Z, Zhang L, Xu J, et al. Rapid asymptomatic transmission of COVID-19 during the incubation period demonstrating strong infectivity in a cluster of youngsters aged 16-23 years outside Wuhan and characteristics of young patients with COVID-19: A prospective contact-tracing study. J Infect. 2020;80:e1–13. doi: 10.1016/j.jinf.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nguyen LH, Drew DA, Joshi AD, Guo C-G, Ma W, Mehta RS, et al. Risk of COVID-19 among frontline healthcare workers and the general community: A prospective cohort study. medRxiv. 2020 doi: 10.1016/S2468-2667(20)30164-X. doi: https://doiorg/101101/202004292008411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lewnard JA, Lo NC. Scientific and ethical basis for social-distancing interventions against COVID-19. Lancet Infect Dis. 2020 doi: 10.1016/S1473-3099(20)30190-0. doi: 101016/S1473-3099(20)30190-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prather KA, Wang CC, Schooley RT. Reducing transmission of SARS-CoV-2. Science. 2020 doi: 10.1126/science.abc6197. pii: eabc6197. [DOI] [PubMed] [Google Scholar]
- 9.World Health Organization. Coronavirus disease (COVID-19) advice for the public. Geneva: WHO; 2020. [Google Scholar]
- 10.The Lancet. COVID-19: Protecting health-care workers. Lancet. 2020;395:922. doi: 10.1016/S0140-6736(20)30644-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Verbeek JH, Rajamaki B, Ijaz S, Mischke C, Ruotsalainen JH, Mäkelä E, et al. Personal protective equipment for preventing highly infectious diseases due to exposure to contaminated body fluids in healthcare staff. Cochrane Database Syst Rev. 2016;4:CD011621. doi: 10.1002/14651858.CD011621.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bai Y, Yao L, Wei T, Tian F, Jin DY, Chen L, et al. Presumed asymptomatic carrier transmission of COVID-19. JAMA. 2020;323:1406–7. doi: 10.1001/jama.2020.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Health Organization. Novel coronavirus (2019-nCoV) - Situation Report - 2; January 22, 2020. Geneva: WHO; 2020. [Google Scholar]
- 14.Zhong NS, Zheng BJ, Li YM, Xie ZH, Chan KH, Li PH, et al. Epidemiology and cause of severe acute respiratory syndrome (SARS) in Guangdong, People's Republic of China, in February, 2003. Lancet. 2003;362:1353–8. doi: 10.1016/S0140-6736(03)14630-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ramadan N, Shaib H. Middle East respiratory syndrome coronavirus (MERS-CoV): A review. Germs. 2019;9:35–42. doi: 10.18683/germs.2019.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hashem AM, Alghamdi BS, Algaissi AA, Alshehri FS, Bukhari A, Alfaleh MA, et al. Therapeutic use of chloroquine and hydroxychloroquine in COVID-19 and other viral infections: A narrative review? Travel Med Infect Dis. 2020:101735. doi: 10.1016/j.tmaid.2020.101735. doi:10.1016/j.tmaid.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu J, Cao R, Xu M, Wang X, Zhang H, Hu H, et al. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov. 2020;6:16. doi: 10.1038/s41421-020-0156-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Al-Bari MAA. Targeting endosomal acidification by chloroquine analogs as a promising strategy for the treatment of emerging viral diseases. Pharmacol Res Perspect. 2017;5:e00293. doi: 10.1002/prp2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vincent MJ, Bergeron E, Benjannet S, Erickson BR, Rollin PE, Ksiazek TG, et al. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol J. 2005;2:69. doi: 10.1186/1743-422X-2-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National Task Force for COVID-19 in India. Recommendation for empiric use of hydroxychloroquine for prophylaxis of SARS-CoV-2 infection. 2020. [accessed on May 25, 2020]. Available from: https://wwwmohfwgovin/pdf/AdvisoryontheuseofHydroxychloroqui nasprophylaxisforSARSCoV2infectionpdf .
- 21.Lee SH, Son H, Peck KR. Can post-exposure prophylaxis for COVID-19 be considered as an outbreak response strategy in long-term care hospitals. Int J Antimicrob Agents. 2020 doi: 10.1016/j.ijantimicag.2020.105988. doi: 101016/jijantimicag2020105988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Principi N, Esposito S. Chloroquine or hydroxychloroquine for prophylaxis of COVID-19. Lancet Infect Dis. 2020 doi: 10.1016/S1473-3099(20)30296-6. pii: S1473-3099: 30296-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dupont WD. Power calculations for matched case-control studies. Biometrics. 1988;44:1157–68. [PubMed] [Google Scholar]
- 24.Hintze J. PASS 11 NCSS. LLC Kaysville, Utah, USA: NCSS; 2011. [accessed on May 25, 2020]. Available from: http://wwwncsscom">http://wwwncsscom . [Google Scholar]
- 25.Pearce N. Analysis of matched case-control studies. BMJ. 2016;352:i969. doi: 10.1136/bmj.i969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jacobsen KH, Aguirre AA, Bailey CL, Baranova AV, Crooks AT, Croitoru A, et al. Lessons from the Ebola outbreak: Action items for emerging infectious disease preparedness and response. Ecohealth. 2016;13:200–12. doi: 10.1007/s10393-016-1100-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arunkumar G, Chandni R, Mourya DT, Singh SK, Sadanandan R, Sudan P, et al. Outbreak investigation of Nipah virus disease in Kerala, India, 2018. J Infect Dis. 2019;219:1867–78. doi: 10.1093/infdis/jiy612. [DOI] [PubMed] [Google Scholar]
- 28.Zhou D, Dai SM, Tong Q. COVID-19: A recommendation to examine the effect of hydroxychloroquine in preventing infection and progression. J Antimicrob Chemother. 2020 doi: 10.1093/jac/dkaa114. doi:101093/jac/dkaa114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tett SE, Cutler DJ, Day RO, Brown KF. Bioavailability of hydroxychloroquine tablets in healthy volunteers. Br J Clin Pharmacol. 1989;27:771–9. doi: 10.1111/j.1365-2125.1989.tb03439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mehra MR, Desai SS, Ruschitzka F, Patel AN. Hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: A multinational registry analysis. Lancet. 2020 doi: 10.1016/S0140-6736(20)31180-6. S0140-6736(20)31180-6. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 31.Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ, et al. COVID-19: Consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–4. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mitjà O, Clotet B. Use of antiviral drugs to reduce COVID-19 transmission. Lancet Glob Health. 2020;8:e639–40. doi: 10.1016/S2214-109X(20)30114-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bellizzi S, Fiamma M, Arru L, Farina G, Manca A. COVID-19: The daunting experience of healthcare workers in Sardinia, Italy. Infect Control Hosp Epidemiol. 2020 doi: 10.1017/ice.2020.149. doi:101017/ice2020149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barrett ES, Horton DB, Roy J, Gennaro ML, Brooks A, Tischfield J, et al. Prevalence of SARS-CoV-2 infection in previously undiagnosed health care workers at the onset of the US COVID-19 epidemic. medRxiv. doi: 10.1186/s12879-020-05587-2. 2020042020072470; 2020 doi:101101/2020042020072470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Legido-Quigley H, Mateos-García JT, Campos VR, Gea-Sánchez M, Muntaner C, McKee M. The resilience of the Spanish health system against the COVID-19 pandemic. Lancet Public Health. 2020;5:e251–2. doi: 10.1016/S2468-2667(20)30060-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Richens J, Imrie J, Copas A. Condoms and seat belts: The parallels and the lessons. Lancet. 2000;355:400–3. doi: 10.1016/S0140-6736(99)09109-6. [DOI] [PubMed] [Google Scholar]
- 37.Adams J. Risk. 1st ed. London: Routledge, Taylor & Fransis Group; 1995. [accessed on May 28, 2020]. Available from: http://wwwjohn-adamscouk/wp-content/uploads/2017/01/RISK-BOOKpdf">http://wwwjohn-adamscouk/wp-content/uploads/2017/01/RISK-BOOKpdf . [Google Scholar]
- 38.Wilde GJS. Target risk: Dealing with the danger of death, disease and damage in everyday decisions. Toronto, ON, Canada: Castor & Columba; 1994. [Google Scholar]


