Abstract
Background & objectives:
India has been reporting the cases of coronavirus disease 2019 (COVID-19) since January 30, 2020. The Indian Council of Medical Research (ICMR) formulated and established laboratory surveillance for COVID-19. In this study, an analysis of the surveillance data was done to describe the testing performance and descriptive epidemiology of COVID-19 cases by time, place and person.
Methods:
The data were extracted from January 22 to April 30, 2020. The frequencies of testing performance were described over time and by place. We described cases by time (epidemic curve by date of specimen collection; seven-day moving average), place (area map) and person (attack rate by age, sex and contact status), and trends were represented along with public health measures and events.
Results:
Between January 22 and April 30, 2020, a total of 1,021,518 individuals were tested for severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2). Testing increased from about 250 individuals per day in the beginning of March to 50,000 specimens per day by the end of April 2020. Overall, 40,184 (3.9%) tests were reported positive. The proportion of positive cases was highest among symptomatic and asymptomatic contacts, 2-3-fold higher than among those with severe acute respiratory infection, or those with an international travel history or healthcare workers. The attack rate (per million) by age was highest among those aged 50-69 yr (63.3) and was lowest among those under 10 yr (6.1). The attack rate was higher among males (41.6) than females (24.3). The secondary attack rate was 6.0 per cent. Overall, 99.0 per cent of 736 districts reported testing and 71.1 per cent reported COVID-19 cases.
Interpretation & conclusions:
The coverage and frequency of ICMR's laboratory surveillance for SARS-CoV-2 improved over time. COVID-19 was reported from most parts of India, and the attack rate was more among men and the elderly and common among close contacts. Analysis of the data indicates that for further insight, additional surveillance tools and strategies at the national and sub-national levels are needed.
Keywords: Attack rate, contact testing, descriptive epidemiology, epidemic curve, positivity, SARS-CoV-2, testing rate
The novel severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) infection leading to coronavirus disease 2019 (COVID-19) started in Wuhan, mainland China, and spread rapidly all over the world including India1. The first case of COVID-19 was reported in India when one of the medical students returning from Wuhan University was tested positive in Kerala on January 30, 20202. India's health ministry initiated multiple methods of surveillance for COVID-19 along with various other agencies.
The Indian Council of Medical Research (ICMR) has been leading India's laboratory surveillance testing for COVID-19. In the initial phase, testing for SARS-CoV-2 was conducted through 78 selected national reference laboratories3. Constrained by the international shortage of testing reagents, the ICMR testing strategy incorporated a risk-based approach alongside clinical symptoms. This approach balanced the need for immediate deployment of nation-wide surveillance and judicious use of resources. Subsequently, sentinel surveillance for severe acute respiratory infection (SARI) was started on February 15, 20204. The ICMR led the expansion of testing capacity by using its existing laboratory network, developing standard protocols and launching an online portal for reporting5.
The infrastructure for testing included ICMR institutes and partners through the Virus Research and Diagnostics Laboratories (VRDL) Network of the Department of Health Research, Ministry of Health and Family Welfare, New Delhi. This network was established for enhancing India's capacity to diagnose and detect viruses of public health importance6. On March 17, 2020, the testing criteria were revised to expand the cases to be tested. Subsequently, on March 21, 2020, the ICMR guidelines allowed testing by private laboratories meeting the stipulated criteria. By April 12, 2020, the ICMR augmented the plan to fast-track COVID-19 testing laboratories and issued revised guidelines to use TruNAT-beta-CoV tests on April 147 and Cartridge-Based Nucleic Acid Amplification Test (CBNAAT) using Cepheid® Xpert® Xpress SARS-CoV-2 on April 19, 20208.
Thus, the ICMR's laboratory surveillance network generated valuable data with key variables of interest for characterizing the ongoing pandemic. Such an analysis may be useful to inform future course of actions. We, therefore, analyzed these surveillance data to describe the testing performance and the descriptive epidemiology of COVID-19 cases by time, place and person.
Material & Methods
Population under surveillance: The system covered the entire population of India through 426 testing centres, at the time of this analysis. Case identification was conducted by the staff of the respective local health authorities, which included the Integrated Disease Surveillance Programme (IDSP) and the State Public Health Departments of respective States/Union Territories. The testing centres complemented the IDSP and the State Public Health Departments in an effective way to augment the testing capacities for COVID-19.
Case definitions: Cases definitions used for COVID-19 surveillance are described elsewhere9. Both symptomatic suspected cases and certain asymptomatic groups, such as high-risk contacts or high-risk healthcare workers, were eligible for testing. The criteria for testing changed over time, becoming more permissive10.
Testing: The ICMR developed standard specimen collection, specimen transport and laboratory testing processes, including criteria for classifying results which are publicly available. The reported testing results are based on quantitative real-time-reverse-transcriptase polymerase chain reaction (qRT-PCR) tests.
Data collection: The testing laboratories received a specimen referral form with every sample and entered the same information into the database11. The records were extracted from the surveillance database on May 4, 2020. The dataset was truncated to include records with a date of specimen collection until April 30, 2020. Information was collected on the date of specimen collection, specimen receipt, test result, demographic characteristics, clinical information, patient category (travel and contact history, healthcare worker status) and location (District and State). All data were de-identified prior to extraction and analysis.
Data analysis
Description of tests performed by selected characteristics: The characteristics of testing were summarized by the frequency of specimen collected, number tested and the test positivity. The trends in positivity rate over time were described by calculating seven-day moving average. The number tested per million was computed by using State-specific population denominator. The number of individual tests and contacts tested per confirmed case was calculated by States. Laboratory accessibility was examined by subtracting the date of specimen collection from the specimen receipt date. An indicator for contacts tested per case was estimated by dividing the number of tests among contacts of cases with the number of positive cases.
Descriptive epidemiology of cases by time, place and person: The frequencies of characteristics of cases were described by age, gender, residence, type of exposure (contact or travel) and symptoms. The population denominators were used to calculate the attack rate by age, gender and their residence location. Secondary attack rate was estimated among contacts by dividing the number of positives among contacts with the number of contacts tested. The presence of any symptoms at the time of specimen collection was also recorded. Epidemic curve was drawn by the date of specimen collection. The time trends were annotated with that of implementation of various public health measures or some key events related to the epidemic.
Sensitivity analysis: A sensitivity analysis was done for contact tracing and secondary attack rates to account for missing data. Cases with missing exposure history were included as contacts given that travel-related cases were a minority and were further reduced due to enacted restrictions.
Results
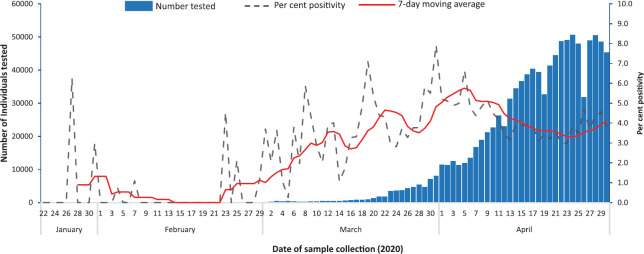
Description of tests performed by time, place and person: Between January 22 and April 30, 2020, a total of 1,021,518 individuals were tested for SARS-CoV-2. Testing increased from about 250 individuals per day in the beginning of March to 50,000 per day by the end of April 2020 (Fig. 1). This represented a 200-fold increase in testing over eight weeks. Nearly 95 per cent of throat/nasal swabs collected were received by the laboratory on the same day. Only 2.6 per cent were received on the next day and 1.8 per cent of specimens were received after 48 h.
Fig. 1.
Frequency of testing and positive tests (%) for SARS-CoV-2, India, January 22 - April 30, 2020.
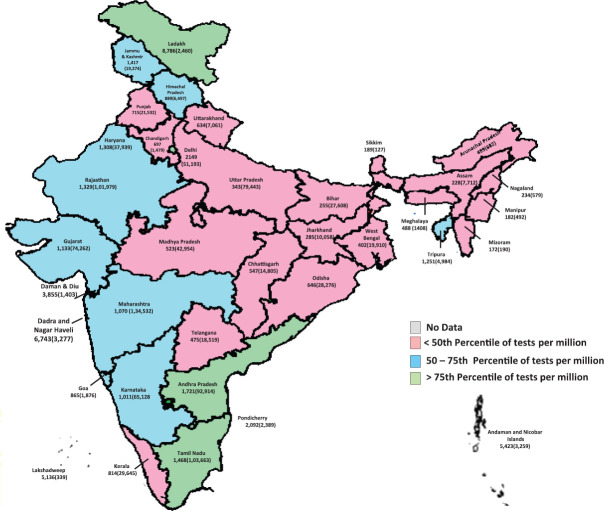
The cumulative frequency of testing was 770 individuals per million population. The testing frequency by the States varied widely (Fig. 2). In all, 729 out of 736 districts (99.0%) reported any testing. Among the States and Union Territories (UTs) with more than first quartile of Indian population (660,000), the testing frequency ranged from 182/million in Manipur to 2149/million in Delhi. The States/UTs that reported higher than all India average of tests per million were Andhra Pradesh (1721), Tamil Nadu (1468), Jammu and Kashmir (1417), Rajasthan (1329), Haryana (1308), Tripura (1251), Gujarat (1133), Maharashtra (1070), Karnataka (1011), Himachal Pradesh (889) and Kerala (814) (Table I).
Fig. 2.
SARS-CoV-2 tests per million (total individuals tested) by State/Union Territory, India, January 22 - April 30, 2020. Source: Map outline reproduced with permission from Survey of India, Department of Science & Technology.
Table I.
Frequency of districts reporting and testing (per million) for COVID-19 along with cases by State/Union Territory, India, January 22- April 30, 2020
| State/Union Territory (number of laboratories) | Reporting districts (total districts) | Affected districts (total districts) | Tests per million population (total individuals tested) | COVID-19 cases* (% tests positive) |
|---|---|---|---|---|
| Andaman and Nicobar Islands (3) | 3 (3) | 3 (3) | 5423 (3259) | 40 (1.2) |
| Andhra Pradesh (48) | 13 (13) | 12 (13) | 1721 (92,914) | 1474 (1.6) |
| Arunachal Pradesh (1) | 25 (25) | 1 (25) | 499 (682) | 1 (0.1) |
| Assam (7) | 32 (33) | 19 (33) | 228 (7712) | 94 (1.2) |
| Bihar (7) | 38 (38) | 32 (38) | 255 (27,608) | 483 (1.7) |
| Chandigarh (3) | 1 (1) | 1 (1) | 697 (1479) | 83 (5.6) |
| Chhattisgarh (4) | 28 (28) | 8 (28) | 547 (14,805) | 43 (0.3) |
| Dadra and Nagar Haveli (1) | 1 (1) | 0 (1) | 6743 (3277) | 0 (0.0) |
| Daman and Diu (0) | 2 (2) | 0 (2) | 3588 (1403) | 0 (0.0) |
| Delhi (24) | 11 (11) | 11 (11) | 2149 (51,193) | 4017 (7.8) |
| Goa (3) | 2 (2) | 2 (2) | 865 (1876) | 7 (0.4) |
| Gujarat (20) | 33 (33) | 30 (33) | 1133 (74,262) | 4694 (6.3) |
| Haryana (15) | 22 (22) | 21 (22) | 1308 (37,939) | 501 (1.3) |
| Himachal Pradesh (5) | 12 (12) | 7 (12) | 889 (6497) | 41 (0.6) |
| Jammu and Kashmir (4) | 20 (20) | 16 (20) | 1417 (19,274) | 685 (3.6) |
| Jharkhand (5) | 24 (24) | 12 (24) | 285 (10,058) | 113 (1.1) |
| Karnataka (29) | 30 (30) | 23 (30) | 1011 (65,128) | 535 (0.8) |
| Kerala (20) | 14 (14) | 14 (14) | 814 (29,645) | 491 (1.7) |
| Ladakh (1) | 1 (2) | 2 (2) | 8786 (2460) | 55 (2.2) |
| Lakshadweep | 1 (1) | 0 (1) | 5136 (339) | 0 (0.0) |
| Madhya Pradesh (13) | 55 (55) | 38 (55) | 523 (42,954) | 2605 (6.1) |
| Maharashtra (58) | 36 (36) | 36 (36) | 1070 (134,532) | 14305 (10.6) |
| Manipur (2) | 14 (16) | 2 (16) | 182 (492) | 2 (0.4) |
| Meghalaya (1) | 11 (11) | 2 (11) | 488 (1408) | 11 (0.8) |
| Mizoram (1) | 7 (8) | 1 (8) | 172 (190) | 1 (0.5) |
| Nagaland (0) | 9 (12) | 1 (12) | 234 (579) | 1 (0.2) |
| Odisha (8) | 30 (30) | 16 (30) | 646 (28,276) | 172 (0.6) |
| Puducherry (2) | 4 (4) | 2 (4) | 2092 (2389) | 8 (0.3) |
| Punjab (5) | 22 (22) | 22 (22) | 715 (21,532) | 623 (2.9) |
| Rajasthan (20) | 33 (33) | 29 (33) | 1329 (101,979) | 2402 (2.4) |
| Sikkim (1) | 4 (4) | 0 (4) | 189 (127) | 0 (0.0) |
| Tamil Nadu (50) | 38 (38) | 36 (38) | 1468 (103,663) | 2108 (2.0) |
| Telangana (20) | 33 (33) | 29 (33) | 475 (18,519) | 941 (5.1) |
| Tripura (1) | 8 (8) | 3 (8) | 1251 (4984) | 3 (0.1) |
| Uttar Pradesh (23) | 75 (75) | 66 (75) | 343 (79,443) | 2175 (2.7) |
| Uttarakhand (5) | 13 (13) | 6 (13) | 634 (7061) | 46 (0.7) |
| West Bengal (17) | 23 (23) | 20 (23) | 402 (19,910) | 1155 (5.8) |
| Data not available | - | - | 269 (16.1) | |
| India (426) | 729 (736) | 523 (736) | 770 (1,021,518) | 40,184 (3.9) |
*Distribution of cases across the states is subject to verification of residential status of the individuals tested for COVID-19.
Of the total tested, for whom data were available, 19.6 per cent (n=200,006) were asymptomatic family contacts of laboratory-confirmed cases, 6.8 per cent (n=69,315) were patients of SARI and 4.8 per cent (n=48,852) were asymptomatic healthcare workers suspected to have come in contact with laboratory-confirmed cases Table II.
Table II.
Profile of individuals tested for COVID-19 in India, January 22 - April 30, 2020
| Testing categories | Number of cases (% of total) | Number tested (% of total) | Positive (%) |
|---|---|---|---|
| Category 1: Symptomatic international travellers in the last 14 days | 523 (1.3) | 17,218 (1.7) | 3.0 |
| Category 2: Symptomatic contacts of laboratory-confirmed case | 4257 (10.6) | 41,498 (4.1) | 10.3 |
| Category 3: Symptomatic healthcare workers | 947 (2.4) | 20,429 (2.0) | 4.6 |
| Category 4: Hospitalized SARI patients | 4204 (10.5) | 69,315 (6.8) | 6.1 |
| Category 5a: Asymptomatic direct and high risk contacts of laboratory confirmed case – family members | 10,160 (25.3) | 200,006 (19.6) | 5.1 |
| Category 5b: Asymptomatic healthcare workers in contact with confirmed case without adequate protection | 1135 (2.8) | 48,852 (4.8) | 2.3 |
| Category 6: ILI identified in hot zones | 1199 (3.0) | 45,384 (4.4) | 2.6 |
| Not specified | 17,759 (44.2) | 578,816 (56.7) | 3.1 |
| Total | 40,184 | 1,021,518 | 3.9 |
SARI, severe acute respiratory infection; ILI, influenza-like illness
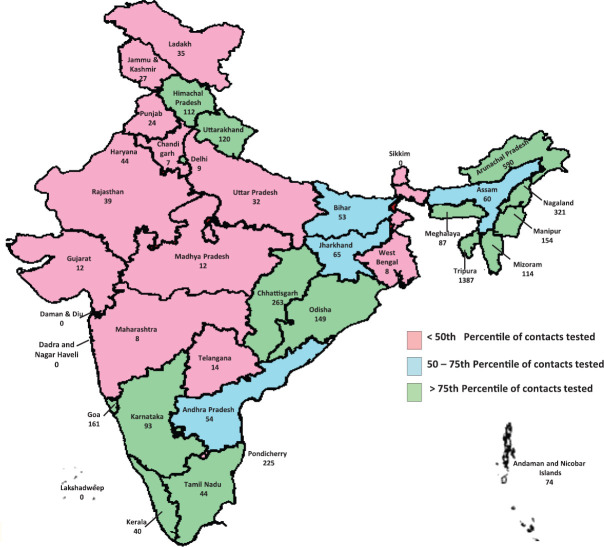
At the national level, the average number of contacts tested per laboratory-confirmed case was 6. At the State level, the average number of contacts tested per positive case ranged from 1.3 in Jharkhand to 328 in Tripura (Fig. 3). Among the top 10 States/UTs, based on the reported number of COVID-19 cases, the average number of contacts tested per positive case was more than the national average in Tamil Nadu (14.4), Uttar Pradesh (9.8), Telangana (8.1), Andhra Pradesh (7.7), Madhya Pradesh (7.6) and Rajasthan (6.3). Corrected for missing data from our sensitivity analysis, the average number of contacts tested per positive case was 20.4 at all-India level and ranged from 6.6 in Chandigarh to 1387 in Tripura (Table III).
Fig. 3.
Contacts tested per confirmed coronavirus disease 2019 case by States/Union Territory, India, January 22 - April 30, 2020. Source: Map outline reproduced with permission from Survey of India, Department of Science & Technology.
Table III.
Frequency of contacts tested per COVID-19 case by known and missing exposure category by States/Union Territory, India, January 22 - April 30, 2020
| State/Union Territory | Among symptomatic contacts or asymptomatic family members | Including uncertain contact status (unknown/not recorded or otherwise) | |||
|---|---|---|---|---|---|
| Tested | COVID-19 cases | Contacts per confirmed case | Tests | Contacts per confirmed case | |
| Andaman and Nicobar Islands | 439 | 40 | 11.0 | 2958 | 74.0 |
| Andhra Pradesh | 11,409 | 1474 | 7.7 | 79,162 | 53.7 |
| Arunachal Pradesh | 89 | 1 | 89.0 | 590 | 590.0 |
| Assam | 1428 | 94 | 15.2 | 5642 | 60.0 |
| Bihar | 2869 | 483 | 5.9 | 25,768 | 53.3 |
| Chandigarh | 320 | 83 | 3.9 | 547 | 6.6 |
| Chhattisgarh | 618 | 43 | 14.4 | 11,321 | 263.3 |
| Dadra and Nagar Haveli | 96 | 0 | 0.0 | 3148 | 0.0 |
| Daman and Diu | 0 | 0 | 0.0 | 1285 | 0.0 |
| Delhi | 8467 | 4017 | 2.1 | 35,934 | 8.9 |
| Goa | 56 | 7 | 8.0 | 1127 | 161.0 |
| Gujarat | 22,743 | 4694 | 4.8 | 54,751 | 11.7 |
| Haryana | 9702 | 501 | 19.4 | 21,995 | 43.9 |
| Himachal Pradesh | 2012 | 41 | 49.1 | 4597 | 112.1 |
| Jammu and Kashmir | 7595 | 685 | 11.1 | 18,425 | 26.9 |
| Jharkhand | 144 | 113 | 1.3 | 7363 | 65.2 |
| Karnataka | 25,361 | 535 | 47.4 | 49,861 | 93.2 |
| Kerala | 5234 | 491 | 10.7 | 19,818 | 40.4 |
| Ladakh | 574 | 55 | 10.4 | 1910 | 34.7 |
| Lakshadweep | 0 | 0 | 0.0 | 332 | 0.0 |
| Madhya Pradesh | 19,749 | 2605 | 7.6 | 30,126 | 11.6 |
| Maharashtra | 32,911 | 14,305 | 2.3 | 109,075 | 7.6 |
| Manipur | 151 | 2 | 75.5 | 307 | 153.5 |
| Meghalaya | 334 | 11 | 30.4 | 957 | 87.0 |
| Mizoram | 43 | 1 | 43.0 | 114 | 114.0 |
| Nagaland | 241 | 1 | 241.0 | 321 | 321.0 |
| Odisha | 1344 | 172 | 7.8 | 25,615 | 148.9 |
| Puducherry | 250 | 8 | 31.3 | 1799 | 224.9 |
| Punjab | 5965 | 623 | 9.6 | 14,960 | 24.0 |
| Rajasthan | 15,128 | 2402 | 6.3 | 94,279 | 39.3 |
| Sikkim | 10 | 0 | 0.0 | 36 | 0.0 |
| Tamil Nadu | 30,328 | 2108 | 14.4 | 92,981 | 44.1 |
| Telangana | 7593 | 941 | 8.1 | 13,545 | 14.4 |
| Tripura | 984 | 3 | 328.0 | 4162 | 1387.3 |
| Uttar Pradesh | 21,348 | 2175 | 9.8 | 69,772 | 32.1 |
| Uttarakhand | 1733 | 46 | 37.7 | 5541 | 120.5 |
| West Bengal | 3980 | 1155 | 3.4 | 8782 | 7.6 |
| Foreign nationals | 211 | 134 | 1.6 | 1180 | 8.8 |
| Data not available | 45 | 135 | 0.3 | 234 | 1.7 |
| India | 241,504 | 40,184 | 6.0 | 820,320 | 20.4 |
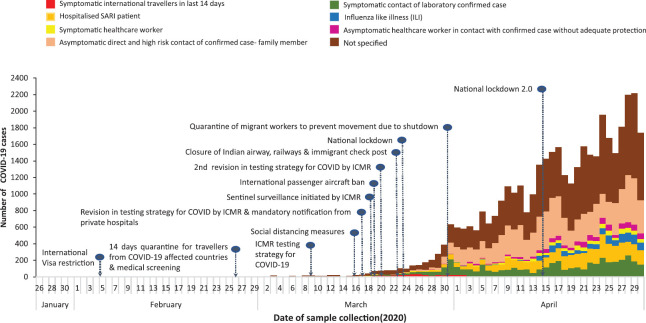
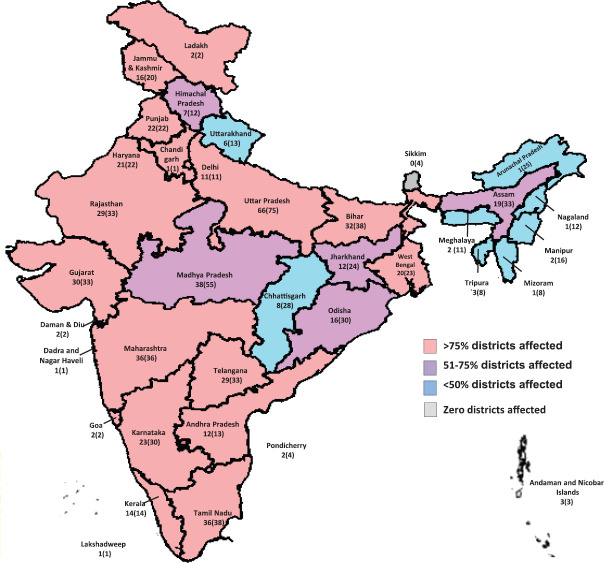
Description of COVID-19 cases by time, place and person: In all, 3.9 per cent (n=40,184) cases were positive for SARS-CoV-2. Cases reported increased over time since March 30, 2020. The proportion of detected cases reporting any international travel decreased over time (Fig. 4). The seven-day moving average for the proportion of positive tests remained between 3 and 6 per cent after March 10, 2020 (Fig. 1). COVID-19 cases have been reported from 523 of 736 (71.1%) districts in the country. States with the highest proportion of districts reporting positive cases included Delhi, Maharashtra, Kerala, Punjab, Haryana, Tamil Nadu, Andhra Pradesh and Gujarat (Fig. 5). The States/UTs with the highest test positivity were Maharashtra (10.6%), Delhi (7.8%), Gujarat (6.3%), Madhya Pradesh (6.1%) and West Bengal (5.8%) (Table I).
Fig. 4.
Coronavirus disease 2019 cases by source of exposure and date of sample collection, India, January 22 - April 30, 2020.
Fig. 5.
Proportion of districts reporting any coronavirus disease 2019 case by State/Union Territory, India, January 22 - April 30, 2020. Source: Map outline reproduced with permission from Survey of India, Department of Science & Technology.
Positivity was highest among the symptomatic contacts (10.3%) and SARI patients (6.1%). Of the 40,184 positives, 25.3 per cent were asymptomatic family contacts, 10.6 per cent were symptomatic contacts and 10.5 per cent were SARI patients Table II. Among the 12,810 cases with reported symptoms at the time of specimen collection, cough and fever were the most commonly reported symptoms (64.5 and 60%, respectively). Around one-third of cases reported sore throat and breathlessness. Gastrointestinal symptoms such as abdominal pain, nausea, vomiting and diarrhoea were reported by less than 5 per cent of cases (Table IV).
Table IV.
Symptoms reported at the time of specimen collection among COVID-19 cases, India, January 22 - April 30, 2020
| Symptom | n (%) |
|---|---|
| Cough | 8269 (64.5) |
| Fever | 7675 (60.0) |
| Breathlessness | 4083 (31.9) |
| Sore throat | 3420 (26.7) |
| Myalgia | 1599 (12.5) |
| Sputum/expectoration | 696 (5.4) |
| Rhinorrhoea | 216 (4.8) |
| Vomiting | 546 (4.3) |
| Loose stools | 396 (3.1) |
| Nausea | 316 (2.5) |
| Abdominal pain | 259 (2.0) |
| Haemoptysis | 151 (1.2) |
| Chest pain | 8 (0.1) |
| Symptomatic without details | 13 (0.03) |
Attack rate (per million population) was the highest among those aged 50-59 and 60-69 yr (64.9 and 61.8, respectively) and was lowest among those under 10 yr (6.1). While the per cent positive among tested was slightly higher among females (4.2 vs. 3.8%), the attack rate (per million population) was higher among males (41.6) (Table V).
Table V.
Profile and attack rate of COVID-19 by age and sex among tested individuals in India, January 22 - April 30, 2020
| Characteristics | Number of cases (% of the total)* | Number tested (% of the total)† | Per cent COVID-19 among tested | Attack rate (per 1,000,000) |
|---|---|---|---|---|
| Age group (yr) | ||||
| 0-9 | 1453 (3.6) | 47,830 (4.7) | 3.0 | 6.1 |
| 10-19 | 3265 (8.1) | 81,305 (8.0) | 4.0 | 12.9 |
| 20-29 | 8627 (21.5) | 272,266 (26.7) | 3.2 | 40.5 |
| 30-39 | 8422 (21.0) | 235,908 (23.1) | 3.6 | 48.5 |
| 40-49 | 6758 (16.8) | 154,585 (15.1) | 4.4 | 50.1 |
| 50-59 | 5723 (14.2) | 113,946 (11.2) | 5.0 | 64.9 |
| 60-69 | 3962 (9.9) | 73,809 (7.2) | 5.4 | 61.8 |
| 70-79 | 1512 (3.8) | 31,191 (3.1) | 4.8 | 53.2 |
| ≥80 | 462 (1.1) | 10,678 (1) | 4.3 | 40.9 |
| Sex | ||||
| Male | 25,909 (64.5) | 684,140 (67.0) | 3.8 | 41.6 |
| Female | 14,265 (35.5) | 336,949 (33.0) | 4.2 | 24.3 |
| Missing | 10 | 429 | ||
| Overall | 40,184 | 1,021,518 | 3.9 | 33.2 |
*Median age of the cases=37 yr (IQR=26, 52); †Median age of the tested=33 yr (IQR=25, 47). IQR, interquartile range
At the national level, secondary attack rate from a positive case among contacts was 6.0 per cent. Using calculations corrected for missing data from our sensitivity analysis, the secondary attack rate was 3.9 per cent. After correction, secondary attack rate was highest in Chandigarh (11.5%) and Maharashtra (10.6%) (Table VI).
Table VI.
Secondary attack rate of COVID-19 among known and uncertain contact status by State/Union Territory, India, January 22 - April 30, 2020
| State/Union Territory | Among symptomatic contacts or asymptomatic family members | Including uncertain contact status (unknown/not recorded or otherwise) | ||||
|---|---|---|---|---|---|---|
| Tested | COVID-19 cases | Secondary attack rate (%) | Tested | COVID-19 cases | Secondary attack rate (%) | |
| Andaman and Nicobar Islands | 439 | 24 | 5.5 | 2958 | 34 | 1.1 |
| Andhra Pradesh | 11,409 | 407 | 3.6 | 79,162 | 1219 | 1.5 |
| Arunachal Pradesh | 89 | 1 | 1.1 | 590 | 1 | 0.2 |
| Assam | 1428 | 45 | 3.2 | 5642 | 87 | 1.5 |
| Bihar | 2869 | 53 | 1.8 | 25,768 | 465 | 1.8 |
| Chandigarh | 320 | 60 | 18.8 | 547 | 63 | 11.5 |
| Chhattisgarh | 618 | 8 | 1.3 | 11,321 | 38 | 0.3 |
| Dadra and Nagar Haveli | 96 | 0 | 0.0 | 3148 | 0 | 0.0 |
| Daman and Diu | 0 | 0 | 0.0 | 1285 | 0 | 0.0 |
| Delhi | 8467 | 1304 | 15.4 | 35,934 | 3150 | 8.8 |
| Goa | 56 | 0 | 0.0 | 1127 | 5 | 0.4 |
| Gujarat | 22,743 | 1775 | 7.8 | 54,751 | 3163 | 5.8 |
| Haryana | 9702 | 249 | 2.6 | 21,995 | 357 | 1.6 |
| Himachal Pradesh | 2012 | 31 | 1.5 | 4597 | 36 | 0.8 |
| Jammu and Kashmir | 7595 | 496 | 6.5 | 18,425 | 655 | 3.6 |
| Jharkhand | 144 | 21 | 14.6 | 7363 | 102 | 1.4 |
| Karnataka | 25,361 | 325 | 1.3 | 49,861 | 461 | 0.9 |
| Kerala | 5234 | 158 | 3.0 | 19,818 | 337 | 1.7 |
| Ladakh | 574 | 13 | 2.3 | 1910 | 36 | 1.9 |
| Lakshadweep | 0 | 0 | 0.0 | 332 | 0 | 0.0 |
| Madhya Pradesh | 19,749 | 1416 | 7.2 | 30,126 | 2025 | 6.7 |
| Maharashtra | 32,911 | 4148 | 12.6 | 109,075 | 11,605 | 10.6 |
| Manipur | 151 | 0 | 0.0 | 307 | 1 | 0.3 |
| Meghalaya | 334 | 7 | 2.1 | 957 | 10 | 1.0 |
| Mizoram | 43 | 0 | 0.0 | 114 | 1 | 0.9 |
| Nagaland | 241 | 0 | 0.0 | 321 | 0 | 0.0 |
| Odisha | 1344 | 62 | 4.6 | 25,615 | 144 | 0.6 |
| Puducherry | 250 | 7 | 2.8 | 1799 | 8 | 0.4 |
| Punjab | 5965 | 280 | 4.7 | 14,960 | 543 | 3.6 |
| Rajasthan | 15,128 | 618 | 4.1 | 94,279 | 2256 | 2.4 |
| Sikkim | 10 | 0 | 0.0 | 36 | 0 | 0.0 |
| Tamil Nadu | 30,328 | 1152 | 3.8 | 92,981 | 1954 | 2.1 |
| Telangana | 7593 | 483 | 6.4 | 13,545 | 752 | 5.6 |
| Tripura | 984 | 1 | 0.1 | 4162 | 2 | 0.0 |
| Uttar Pradesh | 21,348 | 861 | 4.0 | 69,772 | 1922 | 2.8 |
| Uttarakhand | 1733 | 19 | 1.1 | 5541 | 39 | 0.7 |
| West Bengal | 3980 | 333 | 8.4 | 8782 | 460 | 5.2 |
| Foreign Nationals | 211 | 20 | 9.5 | 1180 | 119 | 10.1 |
| Data not available | 45 | 40 | NA | 234 | 126 | NA |
| India | 241,504 | 14,417 | 6.0 | 820,320 | 32,176 | 3.9 |
Discussion
Our analysis of ICMR's laboratory surveillance for COVID-19 documents improved coverage and frequency of testing for SARS-CoV-2 infection across the country. COVID-19 cases reported from most parts of India, were most common among close contacts, and affected men and the elderly more. A large proportion of both those tested and those positive were asymptomatic family contacts.
The national COVID-19 testing strategy formulated and implemented by the ICMR evolved with the logistics and phase of the pandemic in India. The deployment of the testing sites was scaled up over time with guidance according to the control strategy. The coverage and testing frequency was improved since launch. Almost all Indian districts reported laboratory surveillance and scaled up their capacity to test. The timeliness of specimen testing is indicated by a few delays between the specimen collection and receipt at the laboratory. Because the testing criteria, except for SARI, require exposure to a positive case, we are uncertain about the transmission among unlinked individuals in the community. The surveillance data had a large proportion of tests with missing information on exposure history. States demonstrated wide variations in contacts tested per case. It represents the robustness of contact tracing. While exposure to different contacts could vary per case, the reason for this variation needs to be further explored to improve tracing and testing strategies.
The national laboratory surveillance data provided insights on the epidemiology of COVID-19 in India. Cases were reported from all over India, and travel was no longer the primary means of exposure. While cases continued to be reported, the rate of increase slowed, as demonstrated by the relatively stable test positivity over time. The change in the trend could be attributed to multiple public health measures implemented on a wider scale. A higher attack rate of COVID-19 among men and adults has been reported widely12. It is unclear whether this difference is due to susceptibility or exposure level or represents a higher selection probability for testing. Many cases were among contacts who were asymptomatic at the time of testing. It is reported that there is a pre-symptomatic period of about two days13. With the current data, it was not possible to determine if cases remained symptom free or were pre-symptomatic. The proportion of asymptomatic at the time of testing is also affected by the criteria used for case detection. As the staff responsible for contacts tracing varies across the country, this may also affect the quality of history taking. While the contacts traced and tested improved over a period of time, there were wide variations in terms of secondary attack rates by their known contact status. Our analysis of attack rate by including those with unknown contact status group was different from that of the analysis with known contact status. While the risk to contacts will vary per case, the reason for variation across the States can be further investigated to improve the quality of isolation and quarantine measures to reduce transmission.
Strengths of SARS-CoV-2 laboratory surveillance in India include the size and scale of the network and its links to reporting units. There are several limitations as well. For certain data, such as patient category or date of symptom onset, the proportion of entries with missing data was high. Changes to the online reporting format may help to fix this. The data are also subject to the limitations of all case-based data, where the trends are influenced by changes in case detection volume and strategy, as well as various individual-level and overall system-level variations. For instance, health-seeking behaviour and access to these services are highly varying across and within several subnational settings. Our analysis trends over time and correlations with various key measures implemented at broader geographical level may have potential bias typically associated with ecological analysis. Implementing other methods of surveillance, such as population-based and sentinel-site-based, will help to understand the trends better. Finally, the sensitivity of PCR tests for SARS-CoV-2 from nasopharyngeal and oropharyngeal sampling may be limited14. Validation studies can be used to correct surveillance data for assay performance. The antibody-based tests could be performed sequentially with PCR-based testing to improve detection15.
The entire analysis was based on the date of sample collection and hence numbers reported in the analysis might vary with date of reporting considering the lag time between sample collection and reporting. Furthermore, since the data of sample collection were used in the analysis, the number of cases and tests indicated might change due to updation of records in the database. Considering this, data imported on May 4, 2020 were truncated to include records until April 30, 2020 for complete analysis.
With implementation of ICMR's laboratory-based surveillance for SARS-CoV-2, testing was available and accessible and thus contributed to improved case detection throughout the country. The network of COVID-19 testing laboratories and testing capacity continues to expand16,17. As on May 8, 2020, more than 1.4 million samples were tested18. While the cumulative number of cases continues to increase, the growth rate of reported cases has slowed. Based on our analysis the following may be recommended: ongoing national and local analysis of testing data, supplementing laboratory surveillance with additional intelligence from population-based and sentinel site strategies, moving from State to district-based indicators and further operational research to validate the utility of laboratory testing derived indicators for contact tracing and secondary attack rates.
Acknowledgment
The authors acknowledge the contributions of the following: Shailendra Singh, Anu Nagar (DHR, New Delhi); G.S. Toteja, R.S. Sharma, G.S.G. Ayyanger, Rajeev Roy, Lokesh Sharma, Pulkit Verma, Amitesh Kumar Sharma, Abhishek Yadav (ICMR, New Delhi); Ramesh T.S., Kiran Kumar H.N. (IIPH, PHFI, Bengaluru).
Footnotes
Financial support & sponsorship: None.
Conflicts of Interest: None.
References
- 1.Backer JA, Klinkenberg D, Wallinga J. Incubation period of 2019 novel coronavirus (2019-nCoV) infections among travellers from Wuhan, China, 20-28 January 2020. Eur Surveill. 2020;25:2000062. doi: 10.2807/1560-7917.ES.2020.25.5.2000062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhosale S, Kulkarni AP. Is a problem shared, a problem halved? Not always! The novel coronavirus COVID-19 outbreak. Indian J Crit Care Med. 2020;24:88–9. doi: 10.5005/jp-journals-10071-23365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Indian Council of Medical Research. Press Release: Note on COVID-19 Laboratory Preparedness in India. New Delhi: ICMR; 2020. Mar 6, [accessed on May 8, 2020]. Available from: https://wwwicmrgovin/pdf/covid/labs/ICMR_PressRelease_COVID_19pdf">https://wwwicmrgovin/pdf/covid/labs/ICMR_PressRelease_COVID_19pdf . [Google Scholar]
- 4.Gupta N, Praharaj I, Bhatnagar T, Thangaraj JWV, Giri S, Chauhan H, et al. Severe acute respiratory illness surveillance for coronavirus disease 2019, India, 2020. Indian J Med Res. 2020;151:236–40. doi: 10.4103/ijmr.IJMR_1035_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gupta N, Potdar V, Praharaj I, Giri S, Sapkal G, Yadav P, et al. Laboratory preparedness for SARS-CoV-2 testing in India: Harnessing a network of Virus Research & Diagnostic Laboratories. Indian J Med Res. 2020;151:216–25. doi: 10.4103/ijmr.IJMR_594_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Department of Health Research. Establishment of a network of laboratories for managing epidemics and Natural Calamities (VRDL) New Delhi: Ministry of Health and Fmily Welfare, Government of India; 2020. [accessed on May 2, 2020]. Available from: https://dhrgovin/schemes/establishment-network-laboratories-managing-epidemics-and-natural-calamities">https://dhrgovin/schemes/establishment-network-laboratories-managing-epidemics-and-natural-calamities . [Google Scholar]
- 7.Indian Council of Medical Research. Guidance on the use of TruenatTM beta CoV. New Delhi: ICMR, Department of Health Research; 2020. Apr 14, [accessed on May 1, 2020]. Available from: https://wwwicmrgovin/pdf/covid/update/Additional_guidance_on_TrueNat_based_COVID19_testingpdf">https://wwwicmrgovin/pdf/covid/update/Additional_guidance_on_TrueNat_based_COVID19_testingpdf . [Google Scholar]
- 8.Indian Council of Medical Research. Advisory for use of cartridge based nucleic acid amplification test (CBNAAT) using Cepheid Xpert Xpress SARS-CoV2. New Delhi: ICMR, Department of Health Research; 2020. Apr 19, [accessed on May 1, 2020]. Available from: https://wwwicmrgovin/pdf/covid/labs/Advisory_on_Cepheid_Xpert_Xpress_SARS_CoV2_testingpdf,">https://wwwicmrgovin/pdf/covid/labs/Advisory_on_Cepheid_Xpert_Xpress_SARS_CoV2_testingpdf . [Google Scholar]
- 9.National Centre for Disease Control. The updated case definitions and contact-categorisation. New Delhi: NCDC, Directorate General of Health Services, Ministry of Health and Family Welfare, Government of India; 2020. [Google Scholar]
- 10.Indian Council of Medical Research. Strategy for COVID19 testing in India (Version 4) New Delhi: ICMR; 2020. Apr 9, [accessed on April 30, 2020]. Available from: https://wwwicmrgovin/pdf/covid/strategy/Strategey_for_COVID19_Test_v4_09042020pdf">https://wwwicmrgovin/pdf/covid/strategy/Strategey_for_COVID19_Test_v4_09042020pdf . [Google Scholar]
- 11.Indian Council of Medical Research. Specimen referral form for COVID-19 (SARS-CoV2) New Delhi: ICMR; 2020. [accessed on April 30, 2020]. Available from: https://wwwicmrgovin/pdf/covid/update/SRF_v9pdf">https://wwwicmrgovin/pdf/covid/update/SRF_v9pdf . [Google Scholar]
- 12.Ge H, Wang X, Yuan X, Xiao G, Wang C, Deng T, et al. The epidemiology and clinical information about COVID-19. Eur J Clin Microbiol Infect Dis. 2020;39:1011–9. doi: 10.1007/s10096-020-03874-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wei WE, Li Z, Chiew CJ, Yong SE, Toh MP, Lee VJ. Presymptomatic transmission of SARS-CoV-2 - Singapore, January 23-March 16, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:411–5. doi: 10.15585/mmwr.mm6914e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carver C, Jones N. University of Oxford; 2020. [accessed on May 6, 2020]. Comparative accuracy of oropharyngeal and nasopharyngeal swabs for diagnosis of COVID-19 Oxford, United Kingdom: Centre for Evidence-Based Medicine, Nuffield Department of Primary Care Health Sciences. Available from: https://wwwcebmnet/covid-19/comparative-accuracy-of-oropharyngeal-and-nasopharyngeal-swabs-for-diagnosis-of-covid-19/">https://wwwcebmnet/covid-19/comparative-accuracy-of-oropharyngeal-and-nasopharyngeal-swabs-for-diagnosis-of-covid-19/ [Google Scholar]
- 15.Abbasi J. The promise and peril of antibody testing for COVID-19. JAMA. 2020;323:1881–3. doi: 10.1001/jama.2020.6170. [DOI] [PubMed] [Google Scholar]
- 16.Gupta N, Bhatnagar T, Rade K, Murhekar M, Gangakhedkar RR, Nagar A, et al. Strategic planning to augment the testing capacity for COVID-19 in India. Indian J Med Res. 2020;151:210–5. doi: 10.4103/ijmr.IJMR_1166_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Indian Council of Medical Research. Total Operational (initiated independent testing) Laboratories reporting to ICMR. New Delhi: ICMR; 2020. [accessed on May 8, 2020]. Available from: https://wwwicmrgovin/pdf/covid/labs/COVID_Testing_Labs _07052020pdf">https://wwwicmrgovin/pdf/covid/labs/COVID_Testing_Labs _07052020pdf . [Google Scholar]
- 18.Indian Council of Medical Research. SARS-CoV-2 (COVID-19) Testing: Status update. New Delhi: ICMR; 2020. May 8, [accessed on May 8, 2020]. Available from: https://wwwicmrgovin/pdf/covid/update/ICMR_testing_update_08May2020_9AM_ISTpdf,">https://wwwicmrgovin/pdf/covid/update/ICMR_testing_update_08May2020_9AM_ISTpdf . [Google Scholar]