Abstract
Background & objectives:
A cluster of SARS-CoV-2 infection occurred among Italian tourists visiting India. We report here the epidemiological, clinical, radiological and laboratory findings of the first cluster of SARS-CoV-2 infection among the tourists.
Methods:
Information was collected on demographic details, travel and exposure history, comorbidities, timelines of events, date of symptom onset and duration of hospitalization from the 16 Italian tourists and an Indian with laboratory-confirmed SARS-CoV-2 infection. The clinical, laboratory, radiologic and treatment data was abstracted from their medical records and all tourists were followed up till their recovery or discharge or death. Throat and deep nasal swab specimens were collected on days 3, 8, 15, 18, 23 and 25 to evaluate viral clearance.
Results:
A group of 23 Italian tourists reached New Delhi, India, on February 21, 2020 and along with three Indians visited several tourist places in Rajasthan. By March 3, 2020, 17 of the 26 (attack rate: 65.4%) had become positive for SARS-CoV-2 infection. Of these 17 patients, nine were symptomatic, while eight did not show any symptoms. Of the nine who developed symptoms, six were mild, one was severe and two were critically ill. The median duration between the day of confirmation for COVID-19 and RT-PCR negativity was 18 days (range: 12-23 days). Two patients died with a case fatality of 11.8 per cent.
Interpretation & conclusions:
This study reconfirms higher rates of transmission among close contacts and therefore, public health measures such as physical distancing, personal hygiene and infection control measures are necessary to prevent transmission.
Keywords: Contact, COVID-19, India, SARS-CoV-2, transmission
In December 2019, Wuhan, an industrial hub in the Hubei province of People's Republic of China witnessed the emergence of a new acute respiratory tract illness1,2. The illness named by the WHO as coronavirus disease 2019 (COVID-19) is caused by a novel beta-coronavirus (SARS-CoV-2)3. On March 11, 2020, the WHO officially declared COVID-19 a pandemic4. In the following weeks there was a rapid spread of the illness to the other provinces in China as well as other countries. As on April 30, 2020, more than three million laboratory-confirmed cases, including 217,769 deaths, were reported from more than 200 countries/territories/areas5.
In India, the first laboratory-confirmed case was reported from Kerala on January 30, 2020. By April 30, 2020, India had reported 33,610 cases and 1075 deaths6. Studies on familial and hospital clusters confirmed person-to-person transmission of SARS-CoV-27,8. A cluster of SARS-CoV-2 infection was reported among Italian tourists visiting India. We investigated this cluster to describe the epidemiological, clinical, radiological and laboratory findings.
Material & Methods
A detailed investigation of the cluster of SARS-CoV-2 infection among 16 Italian tourists and an Indian during March-April 2020 was conducted. The study team visited these tourists admitted in the three different hospitals (2 in Delhi and 1 in Jaipur) in India. Data were collected in a structured questionnaire through in-person interview of the infected tourists. The information was collected on demographic details, travel and exposure history, comorbidities, timelines of events, date of symptom onset and duration of hospitalization. The clinical, laboratory, radiologic and treatment data were extracted from their medical records. All the 17 patients (two Italian tourists in Jaipur and the remaining 15 in Delhi) were followed up till their recovery or discharge or death. Asymptomatic patients were followed up for occurrence of any symptoms. Throat and deep nasal swab specimens were collected on days 3, 8, 15, 18, 23 and 25 to evaluate viral clearance. The laboratory test for SARS-CoV-2 infection was based on the detection of unique sequences of virus RNA by nucleic acid amplification test by real-time reverse transcription-polymerase chain reaction (qRT-PCR). The first-line screening assay targeted the SARS-CoV-2-specific E (envelope) gene. Confirmatory assays targeted the RdRp (RNA dependent RNA polymerase) gene and ORF (open reading frame)-1b genes9.
Statistical analysis: Continuous variables were summarized using median and interquartile range (IQR) values. Categorical values were described as count with percentages. Distribution of symptomatic cases by date of onset of symptoms and clinical course of illness was described using bar charts.
Results
On February 29, 2020, an Italian tourist was hospitalized at the Sawai Man Singh (SMS) Medical College, Jaipur, with symptoms of fever, cough and difficulty in breathing. Upper respiratory samples (nasal and throat swabs) collected from the patient tested positive for SARS-CoV-2 infection on March 2, 2020. The index patient was a part of a group of 23 Italian tourists who reached New Delhi on February 21, 2020. This group along with three Indians (tour guide, driver and conductor) visited several tourist places in Rajasthan by a tourist coach.
The index patient, 69 yr old male, resident of Lombardy Province, Italy and family physician by profession developed fever, cough and difficulty in breathing on February 23, 2020. He, however, continued the tour along with his group, travelled mostly on the last seat of coach and occasionally skipped visiting a few tourist places preferring to stay in the hotel. On arriving at Jaipur on February 28, 2020, he first saw a private healthcare provider and then visited a private hospital from where he was referred to the SMS Medical College, Jaipur, for qRT-PCR for SARS-CoV-2 detection. Following testing positive for SARS-CoV-2, he was isolated in the infectious disease ward. His wife (70 yr), who did not have any symptoms also tested positive for SARS-CoV-2 and was isolated along with the index patient.
The remaining 24 members of the group (21 Italians and 3 Indians) returned to Delhi on March 2, 2020 by the same coach and were quarantined. All the 24 individuals were initially asymptomatic. Their throat and nasal swabs were collected on March 3, 2020. Fifteen persons (including 14 Italian tourists and one Indian) tested positive and were isolated.
In total, 17 of the 26 (23 tourists and three Indians) were COVID-19 positive with an attack rate of 65.4 per cent. The median age of the COVID-19-positive individuals was 69 yr [interquartile range (IQR): 65-70] and nine (52.9%) were female. Of the 17 patients, nine (52.9%) had or developed symptoms, whereas eight (47.1%) did not show any symptoms. Of the nine who developed symptoms, six (66.7%) had mild infection, one had severe infection and two (22.2%) were critically ill.
Among the affected, one had symptoms of COVID-19 on day 0 of hospitalization and six developed symptoms by day 3, while two became symptomatic by day 8. By day 12, symptoms subsided in all the mild patients (Fig. 1). The most common symptoms were fever (66.7%), cough (44.4%) and sore throat (33.3%). The median interval between the day of confirmation for COVID-19 and RT-PCR negativity was 18 days (range: 12-23 days). The median duration of hospital stay was 20 days (range: 14-37 days). Eleven of the 17 (64.7%) had any chronic comorbidities, the most common being hypertension (n=7, 63.6%), dyslipidaemia (n=3, 27.3%) and diabetes mellitus (n=2, 18.2%). Of the seven who had hypertension, two progressed to severe/critical illness, whereas one became RT-PCR negative on day 23. The median duration between day of confirmation of infection and RT-PCR negativity among symptomatic and asymptomatic (18 vs. 18, P=0.5) and those with and without comorbidity (18 vs. 18, P=1.0) was not different.
Fig. 1.
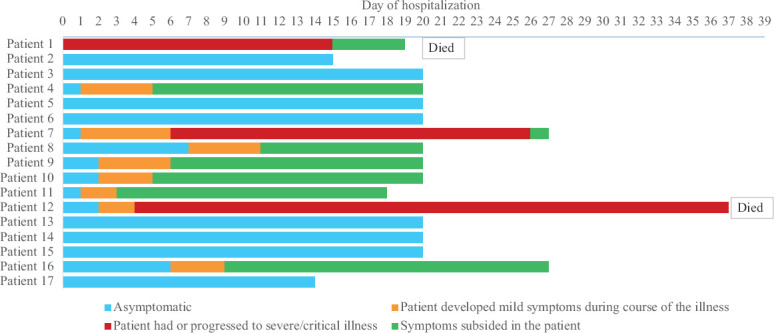
Clinical course of 16 Italian tourists and one Indian, tested positive for COVID-19.
Except for the severely and critically ill patients, the rest were stable, and their biochemical, haematological parameters and chest radiography findings were normal (Table). Apart from the supportive care, two patients with mild symptoms were started on tablet lopinavir/ritonavir. The case summaries of the three severely and critically ill patients are presented. Blood samples were collected at different time intervals from these patients by the treating hospitals to monitor the progression of the patient illness. The results of the laboratory parameters of the severely and critical ill patients are given using the median and range values.
Table I.
Laboratory investigations of asymptomatic/mildly symptomatic Italian tourists
| Investigations | Asymptomatic/mild symptomatic COVID-19 (n=14) |
|---|---|
| Hematologic investigations, median (IQR) | |
| Total leucocyte count/µl | 4710 (3890-5050) |
| Platelet count/µl | 163,000 (152,000-208,000) |
| NLR | 1.6 (1.1-2.3) |
| Biochemical investigations, median (IQR) | |
| AST (IU/l) | 21 (13-29) |
| ALT (IU/l) | 30 (25-35) |
| Serum bilirubin (g/dl) | 0.45 (0.3-0.6) |
| Serum urea (mg/dl) | 32.5 (28-36) |
| Serum creatinine (mg/dl) | 0.75 (0.7-0.9) |
| C-reactive protein levels (mg/l) | 5 (5-6.3) |
NLR, neutrophil lymphocyte ratio; IQR, interquartile range; AST, aspartate aminotransferase; ALT, alanine aminotransferase; COVID 19, coronavirus disease 2019
Patient 1: The index patient, an ex-smoker with a history of chronic bronchitis and emphysema, was admitted in the intensive care unit with complaints of fever, cough, sore throat and breathlessness for a duration of six days. On admission, the patient was afebrile, tachypnoeic, tachycardic and normotensive with a low oxygen saturation on ambient air. The results of the laboratory investigations were as follows: total leucocyte count 16,740 (9,900-16,850) cells/μl, neutrophil lymphocyte ratio (NLR) 8.5, platelet count 110,000 (86,000-382,000) cells/μl; alanine aminotransferase (ALT) 37 (30-50) IU/l, aspartate aminotransferase (AST) 31 (30-54) IU/l, blood urea 37 (24-43) mg/dl and serum creatinine 0.93 (0.7-0.95) mg/dl. Investigations revealed leucocytosis, thrombocytopenia and increased NLR. Chest X-ray showed bilateral infiltrates. Besides supportive care, he was given lopinavir/ritonavir, oseltamivir, chloroquine, corticosteroids, broad-spectrum antibiotics and supplementary oxygen. In view of worsening hypoxia, he required non-invasive ventilation (NIV). His condition stabilized following which he was weaned off the ventilator. On day 13 of hospitalisation, his COVID-19 test results turned negative. He was transferred to a private hospital where he later died due to cardiac arrest.
Patient 7: A 66 yr old male patient, a known hypertensive, initially had only symptoms of fever, cough and sore throat. On day 6 of admission, he developed shortness of breath and complained of chest congestion and had low SpO2. He was started on lopinavir/ritonavir and administered supplemental oxygen to maintain his SpO2 above 97 per cent. The results of the laboratory investigations were: total leucocyte count 12,950 (6,770-13,960) cells/μl, NLR 4.5 (1.6-15.8), platelet count 340,000 (140,000-464,000) cells/μl, C-reactive protein (CRP) 148.9 (44.8-367.2) mg/l, ALT 21 (18-68) IU/l, AST 41 (28-66) IU/l, blood urea 33.5 (25-43) mg/dl and serum creatinine 0.7 (0.5-0.9) mg/dl. He had an increased leucocyte count, NLR and CRP levels, but his kidney function tests and liver function tests were within normal limits. The chest X-ray showed increasing infiltrates. He was shifted to 60 per cent Venturi oxygen masks and was prescribed antibiotics. On day 18, he tested negative for COVID-19 by qRT-PCR. All his symptoms subsided except for his shortness of breath. He required oxygen support for seven more days and computed tomography (CT) chest imaging findings were suggestive of bilateral pneumonia. By day 26, he became stable with an SpO2 of 90-94 per cent without oxygen support and was discharged.
Patient 12: A 77 yr old Italian woman, a known hypertensive, remained asymptomatic for three days. On day 4 of hospitalization, she developed low-grade fever and mild cough. Her oxygen saturation dropped; and she was started on tablet lopinavir/ritonavir (400 mg/100 mg) twice daily along with supplemental oxygen. With increasing oxygen requirement, she was switched to a high-flow nasal cannulas for two hours and then to NIV. On day 11 of hospitalization, her condition further worsened necessitating endotracheal intubation and invasive ventilatory support. She also received a dose of tocilizumab 480 mg. The results of the laboratory investigations were: total leucocyte count 11,285 (6,490-18,530) cells/μl, NLR 15.4 (7.2-30.8), platelet count 93,500 (70,000-134,000) cells/μl, CRP 62.4 (33.6-228.5) mg/l, ALT 64 (17-341) IU/l, AST 33 (22-97) IU/l, blood urea 107 (59-183) mg/dl and serum creatinine 0.7 (0.5-1) mg/dl. Her blood reports showed leucocytosis with neutrophilic predominance, thrombocytopenia and elevated CRP levels. She became hypotensive and had tachycardia on day 12 which responded to fluid administration (guided by inferior vena cava collapsibility), vasopressors and 200 mg/day of hydrocortisone infusion. Her chest CT images showed bilateral (B/L) consolidation, B/L pleural effusion and ground glass opacities. She developed blood stream infection, critical illness myopathy and ventilator dependence during the course of her illness. On day 18, her COVID-19 test result was negative. On day 36, she became haemodynamically unstable and was supported with inotropes. On day 37, she went into cardiac arrest and died.
Discussion
The epidemiological investigation of this cluster of 17 cases was consistent with person-to-person transmission already reported in other studies4,5,10. Two possible scenarios of transmission existed in this cluster. First, the index patient could have been infected during his medical practice in Italy and later transmitted the infection to his co-tourists. According to the WHO, there were only three COVID-19 cases reported from Italy on February 21, 202011, but by February 28, 2020, Italy had 888 cases including 21 deaths due to SARS-CoV-2. Most cases had occurred in the Lombardy and Veneto regions of Northern Italy with local transmission being the main source of SARS-CoV-2 infections12. Second, the tourists could have individually picked up the infection from Italy before starting their trip to India. The first scenario appears mostly likely, considering the duration of onset of symptoms (12 days since their arrival in India except for the index case), the duration of viral clearance being more than 14 days in other tourists and no history of contact or exposure to any suspected or confirmed COVID-19 positive patients in Italy. In view of the first scenario and considering February 28, 2020 as the last day of exposure/contact of affected individuals with index case, the median incubation period was 5.5 days (range: 4-11 days) (Fig. 2).
Fig. 2.
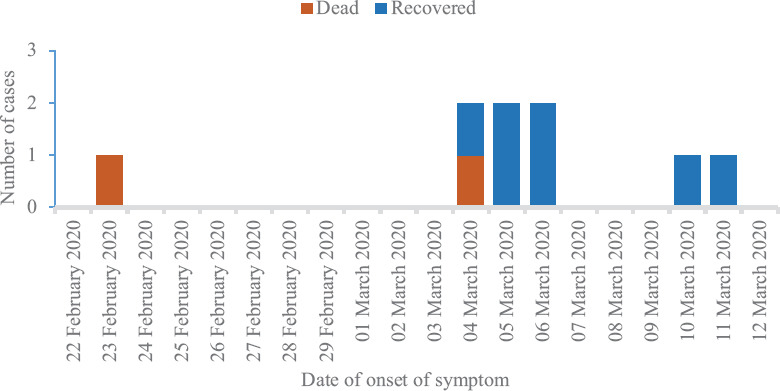
Distribution of COVID-19 cases in the Italian cluster by date of onset of symptoms (n=9).
Our study cluster showed a higher attack rate than that reported in existing literature such as in Diamond Princess Cruise ship (19.2%) and in Grand Princess Cruise ship (16.6%)13. This may be due to the closed environment, high and persistent exposure to index case during their tour travel (average of six hours daily for eight days). Except for the index case, all other cases were asymptomatic at the time of testing and nearly half of the positive cases remained asymptomatic throughout the illness. Proactive COVID-19 testing of close contacts led to the identification and isolation of the asymptomatic and pre-symptomatic cases, thus preventing further transmission.
Older patients with chronic comorbidities progressed to severe/critical illness as reported in other studies14,15. The proportion of symptomatic patients progressing to severe/critical illness in the cluster was high (3/9, 33.3%). The case fatality ratio (CFR) in the affected cluster was 11.5 per cent. The possible reasons for the CFR being on the higher side could be higher median age group of patients and presence of comorbidities.
Laboratory parameters of severely/critically ill patients showed leucocytosis with neutrophilic predominance, thrombocytopenia and increased CRP levels with normal liver and kidney function tests. Two of the three severely/critically ill patients had an elevated CRP level during their pre-symptomatic period and all severely/critically ill patients had an increased NLR during illness. Prognostic value of NLR and CRP has been documented in other studies16,17,18.
Our study had two limitations. First, all the three severe/critical patients and two patients with mild symptoms were started on lopinavir/ritonavir. However, its effects on mortality and viral clearance could not be determined. Second, the exact days of viral clearance could not be ascertained since patients were not serially sampled on daily basis and this might be the reason for the higher median duration for PCR negativity in the cluster.
In conclusion, our study reconfirms higher rates of transmission among close contacts and therefore, public health measures such as physical distancing, personal hygiene and infection control measures are necessary to prevent transmission. Laboratory testing of close contacts identified infection in pre-symptomatic and asymptomatic cases. Hence, the strategy to trace and test close contacts is crucial for early identification and isolation of positive patients and thereby prevent community transmission.
Acknowledgment
Authors acknowledge Drs Dushyant and Arvind Bhushan for their contribution in data and specimen collection.
Footnotes
Financial support & sponsorship: None.
Conflicts of Interest: None.
References
- 1.Lu H, Stratton CW, Tang YW. Outbreak of pneumonia of unknown etiology in Wuhan, China: The mystery and the miracle. J Med Virol. 2020;92:401–2. doi: 10.1002/jmv.25678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. Naming the coronavirus disease (COVID-19) and the virus that causes it WHO. 2020. [accessed on April 18, 2020]. Available from: https://wwwwhoint/emergencies/diseases/novel-coronavirus-2019/technical-guidance/naming-the-coronavirus-disease-(covid-2019)-and-the-virus-that-causes-it">https://wwwwhoint/emergencies/diseases/novel-coronavirus-2019/technical-guidance/naming-the-coronavirus-disease-(covid-2019)-and-the-virus-that-causes-it .
- 4.World Health Organization. Coronavirus disease 2019 (COVID-19) Situation Report - 51. Geneva: WHO; 2020. [Google Scholar]
- 5.World Health Organization. Coronavirus disease 2019 (COVID-19) Situation Report – 101. Geneva: WHO; 2020. [Google Scholar]
- 6.Ministry of Health & Family Welfare, Government of India. COVID-19 India update. [accessed on May 1, 2020]. Available from: https://wwwmohfwgovin/">https://wwwmohfwgovin/
- 7.Chan JF, Yuan S, Kok KH, To KK, Chu H, Yang J, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: A study of a family cluster. Lancet. 2020;395:514–23. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382:1199–207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gupta N, Potdar V, Praharaj I, Giri S, Sapkal G, Yadav P, et al. Laboratory preparedness for SARS-CoV-2 testing in India: Harnessing a network of Virus Research & Diagnostic Laboratories. Indian J Med Res. 2020;151:216–25. doi: 10.4103/ijmr.IJMR_594_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu P, Zhu J, Zhang Z, Han Y. Familial cluster of infection associated with the 2019 novel coronavirus indicating possible person-to-person transmission during the incubation period. Infect Dis. 2020;221:1757–61. doi: 10.1093/infdis/jiaa077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization. Coronavirus Disease 2019 (COVID-19) Situation Report - 32. Geneva: WHO; 2020. [Google Scholar]
- 12.Porcheddu R, Serra C, Kelvin D, Kelvin N, Rubino S. Similarity in case fatality rates (CFR) of COVID-19/SARS-COV-2 in Italy and China. J Infect Dev Ctries. 2020;14:125–8. doi: 10.3855/jidc.12600. [DOI] [PubMed] [Google Scholar]
- 13.Moriarty LF, Plucinski MM, Marston BJ, Kurbatova EV, Knust B, Murray EL, et al. Public health responses to COVID-19 outbreaks on cruise ships – Worldwide, February-March 2020. MMWR Morb Mortal Wkly Rep. 2020;69:347–52. doi: 10.15585/mmwr.mm6912e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–9. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Epidemiology Working Group for NCIP Epidemic Response, Chinese Center for Disease Control and Prevention. The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China. Zhonghua Liu Xing Bing Xue Za Zhi. 2020;41:145–51. doi: 10.3760/cma.j.issn.0254-6450.2020.02.003. [DOI] [PubMed] [Google Scholar]
- 16.Liu F, Li L, Xu M, Wu J, Luo D, Zhu Y, et al. Prognostic value of interleukin-6, C-reactive protein, and procalcitonin in patients with COVID-19. J Clin Virol. 2020;127:104370. doi: 10.1016/j.jcv.2020.104370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang AP, Liu JP, Tao WQ, Li HM. The diagnostic and predictive role of NLR, d-NLR and PLR in COVID-19 patients. Int Immunopharmacol. 2020;84:106504. doi: 10.1016/j.intimp.2020.106504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang L. C-reactive protein levels in the early stage of COVID-19. Med Mal Infect. 2020 doi: 10.1016/j.medmal.2020.03.007. pii: S0399-077X(20)30086-X. [DOI] [PMC free article] [PubMed] [Google Scholar]


