Abstract
Objectives
We estimate life expectancy with and without dementia for Americans 65 years and older by education and race to examine how these stratification systems combine to shape disparities in later-life cognitive health.
Method
Based on the Health and Retirement Study (2000–2014), we use a multivariate, incidence-based life table approach to estimate life expectancy by cognitive health status for race–education groups. The models also simulate group differences in the prevalence of dementia implied by these rates.
Results
The life table results document notable race–education differences in dementia and dementia-free life expectancy, as well as stark differences in implied dementia prevalence. At each education level, blacks can expect to live more years with dementia and they have significantly higher rates of dementia prevalence. This distribution of disparities in the older population is anchored by 2 groups—blacks without a high school diploma and whites with some college or more.
Discussion
Dementia experience and dementia burden differ dramatically along race–education lines. Race and education combine to exaggerate disparities and they both have enduring effects. Future research should explicitly consider how race and education combine to influence dementia in the older American population.
Keywords: Dementia-free life expectancy, Race disparities, Education, Dementia prevalence
Older black Americans are especially hard hit by dementia. Recent national estimates document that blacks aged 50 years and older are two to three times more likely than whites to have dementia (e.g., Garcia et al., 2017). Life expectancy with dementia is also significantly higher among older blacks compared with whites (Garcia et al., 2017), for example, 1.6 years for white females compared with 3.9 years for black females. Related research on the race gap in the incidence of moderate/severe cognitive impairment documents that the risk of acquiring dementia is 2.5 times higher among older blacks compared with older whites (Zhang, Hayward, & Yu, 2016).
Here, we build on this line of work by examining racial disparities in dementia-free life expectancy by levels of educational attainment. Education is a critical indicator of cognitive reserve. Through education, individuals acquire the skills and resources necessary to gain a greater degree of control over their environment and maximize their potential for a longer, healthier life via the adoption of healthier lifestyles, early adoption of health enhancing technologies, and improved control over risk factors like hypertension and cholesterol—all factors identified as important contributors to cognitive reserve (Richards & Sacker, 2010). Recent research also confirms the importance of educational attainment in differentiating the cognitive health of older Americans (Crimmins et al., 2018). Among males in 2010, 0.7% of males 65–69 with a college education had dementia compared with 9.4% of men with less than a high school education. The educational gradient for women is similar, and the gradients for both men and women persist into the oldest ages. The educational gradients in dementia life expectancy are also evident. Life expectancy with dementia for men with more than a high school education is 0.65–0.77 years while dementia life expectancy for men with less than a high school education is 2.57–2.96 years. A similar educational gradient in dementia life expectancy characterizes women aged 65 years and older.
Educational attainment and race are both critical components of population health and have been cast as fundamental causes of disease (Link & Phelan, 1995; Phelan & Link, 2015). In the context of the historical experiences of the HRS cohorts considered here, it is especially important to understand how these fundamental social causes of health combine to influence population heterogeneity in dementia experience. Older black HRS respondents were largely educated in segregated schools, typically grew up in the South, and faced decades of discrimination and challenges in translating educational attainment into health benefits (Hayward, Miles, Crimmins, & Yang, 2000; Williams & Sternthal, 2010). Blacks growing up in the South not only went to schools with lower levels of funding and poorer quality than those attended by whites, but blacks also spent fewer days in school. Glymour and Manly (2008) reported that school terms were 50–100% longer for whites compared with blacks in segregated states.
Although we are unable to explicitly incorporate these racialized educational experiences into our analysis, they nonetheless point to the importance of documenting how race and education combine to differentiate the dementia experience for the major race–education groups. How do the educational gradients in dementia life expectancy differ for black and white Americans? How does racial inequality in dementia life expectancy change when persons have higher levels of education? Prior research on mortality and physical health often suggests that racial differences in health are greater at higher levels of education (Crimmins, Hayward, & Saito, 1996; Crimmins & Saito, 2001). Other research suggests that educational gradients in health are less steep for blacks compared with whites, because of difficulties in translating higher education into social advantage (Albano et al., 2007; Braveman, Cubbin, Egerter, Williams, & Pamuk, 2010; Montez, Hummer, Hayward, Woo, & Rogers, 2011; Schoendorf, Hogue, Kleinman, & Rowley, 1992). Do we observe similar patterns for dementia?
Given higher prevalence rates of dementia among black Americans at all levels of education, we expect that blacks with less than a high school education may be the most disadvantaged group in terms of life with and without dementia. Highly educated whites are expected to anchor the other end of the continuum with fewer years in poor cognitive health and more years cognitively healthy. By documenting how these two major types of fundamental causes of disease coincide to shape later life cognitive health, this study provides new evidence on how disparities arise for a component of population health that has largely been ignored in the disparities literature.
Method
Data
Our assessment of cognitive status life expectancy is based on the Health and Retirement Study (HRS) from 2000 to 2014 for the U.S. black and white population aged 65 years and older. The Health and Retirement Survey (HRS) is a nationally representative longitudinal survey of the U.S. population over the age of 50. The survey began in 1992, and the survey waves are approximately 2 years apart. Response rates for people re-interviewed are greater than 85% for every year.
In this study, we include only the sample waves from 2000 to 2014 because consistent cognitive information for both community-dwelling and nursing home residents first became available in 2000. The age-eligible sample included all blacks and whites, in 2000, 65 years of age and older, as well as HRS respondents who became age-eligible from 2002 to 2012. HRS respondents may “age” into the analytic sample when they become 65 years of age. The analytic sample included 16,113 white respondents and 2,822 black respondents aged 65 years and older (Table 1). The education distributions differ greatly for blacks and whites. 51.2% of blacks did not have a high school diploma compared with 28.5% for whites. 24.7% of blacks had a high school diploma compared with 33% for whites. 24.1% of blacks had some college or more compared with 38.5% for whites. In general, whites had higher levels of education. Nursing home residency was roughly equal between the two groups: 3.2% for whites and 3.4% for blacks. Blacks had a much higher prevalence of dementia (17.5%) compared with whites (7.5%). In 2004 and 2010, the HRS added refreshment cohorts to replenish the younger older adults in the survey to make the HRS representative of the older American population over age 50 years.
Table 1.
Descriptive Information for Analytic Sample of Black and White HRS Respondents (2000–2014)
| Whites, % | Blacks, % | |
|---|---|---|
| Demographic variable | (N = 16,113) | (N = 2,822) |
| Male | 44 | 39 |
| Education | ||
| GED or without high school diploma | 28.55 | 51.24 |
| High school diploma | 32.95 | 24.70 |
| Some college or more | 38.50 | 24.06 |
| Percent living in nursing home | 3.15 | 3.40 |
| Percent with dementia over total study period | 7.53 | 17.53 |
Note: Analytic sample includes age-eligible blacks and whites in 2000 and black and white respondents who became age eligible from 2002 to 2014.
Cognitive Status
For self-respondents, a battery of tests was used to assess cognitive function. For proxy-based respondents, cognitive function was determined from proxy, interviewer assessments, and functional ability. From their scores, respondents were classified in this study into two cognitive statuses: with and without dementia. The dementia classification has been verified using clinical diagnoses and survey scores from a HRS subsample in the ADAMS—the Aging Demographics, and Memory Study (Crimmins, Kim, Langa, & Weir, 2011).
Self-respondent scores ranged from 0 to 27. These scores are based on the following tests: immediate recall of 10 words, delayed recall of the same 10 words, 5 trials of serial 7 s, and backward counting. These continuous measures were then classified into states of cognitive functioning, with respondents who scored 0–6 being classified as having dementia. Proxy respondents’ scores were based on a direct assessment of memory, limitation on five instrumental activities of daily living, and an interviewer assessment of difficulty completing the survey. Higher scores across all measures indicated poor performance. The scores were then summed and proxy respondents were classified as having dementia if their scores ranged 6–11.
Our assessment of dementia and dementia-free life expectancy is based on changes between interviews in cognitive status and between cognitive status and mortality for the 2000–2014 period. Our estimates are based on dementia onset and recovery from dementia as well as mortality among those with and without dementia. Table 2 provides information on the distribution of observations by dementia at the beginning and state at the end of an observation interval from 2000 to 2014 (N = 80,746 observation intervals provided by 18,935 persons in the analytic sample), broken down by race and education. Because of the specific estimation approach used in our analysis (described below), the interval length need not be uniform but is taken into account, bidirectional changes are allowed between the alive states, and the algorithm adjusts for attrition (Lièvre, Brouard, & Heathcote, 2003). Vital status is based on information from the National Death Index and information provided by survivors for persons lacking NDI information.
Table 2.
Distribution of Observations by State at Beginning and End of Intervals, by Education and Race, HRS (2000–2014)
| Whites without a high school diploma | Whites with high school diploma | Whites with some college or more | Blacks without a high school diploma | Blacks with high school diploma | Blacks with some college or more | Total | |
|---|---|---|---|---|---|---|---|
| Beginning state to end state | N | N | N | N | N | N | N |
| Dementia-free at both interviews | 12,961 | 21,322 | 29,061 | 2,225 | 1,667 | 1,875 | 69,111 |
| Dementia-free to dementia | 1,137 | 734 | 580 | 391 | 115 | 59 | 3,016 |
| With dementia to dementia-free | 531 | 218 | 148 | 247 | 76 | 26 | 1,246 |
| Dementia-free to dead | 1,370 | 1,524 | 1,660 | 220 | 113 | 99 | 4,986 |
| Dementia to dead | 920 | 593 | 497 | 281 | 61 | 35 | 2,387 |
| Total | 16,919 | 24,391 | 31,946 | 3,364 | 2,032 | 2,094 | 80,746 |
The transition intervals presented in Table 2 provide a rough characterization of the amount of change that occurred over the 2000–2014 period. The number of transition intervals beginning dementia-free and ending with dementia is 3,016. We also observe 1,246 transition from dementia to being dementia-free. Although it is clear in our data that there is a general decline in cognitive functioning with age, transitions from dementia to dementia-free likely reflect the inconsistencies between the neuropathology of dementia and cognitive functioning (Plassman et al., 2011). In addition, sometimes people with and without dementia overlap in the neuropathology due to improvements in cognitive function after recovery from stroke or surgery or better control over insulin.
Covariates
We created three categories of education based on reported years of education: without a high school diploma (which also includes respondents with a GED), with a high school diploma, some college, or more. Although it is desirable to distinguish college-educated from some college, small cell sizes for blacks prohibit this level of detail (Table 1). Race was based on self-response: non-Hispanic black or non-Hispanic white.
Life Table Approach
We use a multivariate, multistate life table approach based on eight waves of data from the HRS (2000–2014) to estimate dementia and dementia-free life expectancy for persons aged 65 years and older (Lièvre et al., 2003). Multistate life table models are a useful approach in understanding health disparities at the population level because they allow for an assessment of how long, on average, individuals live with and without a health problem (e.g., dementia) over their lifetime. A substantive interpretation of the state expectancies is the average individual’s lifetime burden of having (or not having) a health problem.
The specific approach used here, IMaCh, draws on the longitudinal nature of the data to estimate transition probabilities as the input for the life table models. This approach is especially advantageous because we are able to circumvent some of challenges associated with prevalence-based multistate models. For example, early life circumstances have a strong impact on the initial race difference in dementia at older ages, which may not reflect changes in cognitive status at older ages. In contrast, however, cognitive status life expectancies for the race groups in our study are based on the cognitive changes that occur after age 65, and thus clarify how race differences in dementia experience emerge at older ages.
IMaCh is an interpolated Markov Chain (IMaCh) approach to estimate multistate life tables developed by Brouard and Lièvre (Lièvre et al., 2003). As described above, the data for IMaCh are a sequence of health states at various time points, for example, dementia-free, dementia, and dead. IMaCh estimates the likelihood of what is observed in terms of the higher probabilities of the embedded Markov chain, that is, it allows for multiple transitions to occur within an observation interval. Multinomial logistic regression is used to estimate age-specific transition probabilities. The probabilities are the input to calculate the multistate life tables.
The models used to estimate the transition probabilities are race-specific, and include a continuous measure of age and categorical variable for education level (Additionally, we produced sex specific models. We do not include them in the text for parsimony. The substantive findings remain unchanged for men and women. See Supplementary Table 1). The race-stratified models allow the effect of education to vary freely across race groups. This specification is important given our question of documenting educational gradients for older blacks and whites.
As shown in the results, we provide estimates of dementia life expectancy and dementia-free life expectancy for each race–education group and life expectancy, along with standard errors. IMaCh also computes another valuable indicator of dementia burden, the implied prevalence of dementia. Implied prevalence simulates the prevalence of dementia in the life table population based on the estimated transition probabilities. Similar to our life expectancy estimates, these simulated prevalence rates are based only on changes in cognitive state and mortality for individuals after 65. The implied age-specific prevalence rates are shown for the race–education groups to assess group differences in the burden of dementia and how the disparities in burden change with age.
Results
The cognitive status life expectancies (with and without dementia) presented in Table 3 confirm our hypothesis that blacks with less than a high school education bear the greatest lifetime burden of dementia. Compared with all other groups, at 65 years of age, blacks without a high school diploma can expect to spend the fewest years dementia-free (10.59) and the greatest number of years with dementia (3.77). The group differences in cognitive health status expectancies not only show that blacks at the low end of the education distribution die younger, but also that the process of dementia occurs sooner, prolonging their time spent with a cognitive disability. For example, on average, a black person without a high school degree could expect to live to 79 years and live almost 4 years with dementia. Whites with a college degree, on the other hand, can expect to live to 83 years of age (4 years longer than blacks) and live approximately 1 year with dementia (3 years less than blacks). The stark disparity between the most vulnerable and most advantageous groups depicts how the disproportionate lifetime burden on blacks with lower levels of education.
Table 3.
Total Life Expectancy, Dementia Life Expectancy, and Dementia-Free Life Expectancy by Race and Education at 65, HRS 2000–2014 (Markov Chain Estimation: IMaCh Software)
| Blacks | Without a high school diploma | With a high school diploma | Some college or more |
|---|---|---|---|
| Total e(x) | 14.36 | 15.84 | 17.53 |
| (SE = .304) | (SE = .49) | (SE = .59) | |
| DemLE(x) | 3.77 | 2.31 | 1.65 |
| (SE = .159) | (SE = .19) | (SE = .2) | |
| DemFLE(x) | 10.59 | 13.52 | 15.88 |
| (SE = .23) | (SE = .42) | (SE = .54) | |
| Whites | Without a high school diploma | With a high school diploma | Some college or more |
| Total e(x) | 15.76 | 17.77 | 18.07 |
| (SE = .18) | (SE = .17) | (SE = .17) | |
| DemLE(x) | 2.15 | 1.34 | 1.00 |
| (SE = .068) | (SE = .05) | (SE = .04) | |
| DemFLE(x) | 13.61 | 16.44 | 17.83 |
| (SE = .15) | (SE = .16) | (SE = .17) |
Note: DemFLE(x) = dementia-free life expectancy, DemLE(x) = dementia life expectancy.
The race disparity in dementia-free and dementia life expectancy is present at every education level. In each group, blacks trail whites in dementia life expectancy, ranging from 1.62 years for those without a high school diploma to 0.65 years for those with some college or more. The difference in dementia life expectancy suggests that whites experience a compression of morbidity due to their consistently fewer years spent with dementia, but also that race disparity attenuates at higher levels of education. The race disparity in dementia life expectancy is greatest at lower levels of education and smallest at higher levels of education. Findings from dementia-free life expectancy mirror these patterns.
In contrast to other health-related findings that the education gradient is smaller for blacks than for whites (Albano et al., 2007; Braveman et al., 2010; Montez et al., 2011), our findings point to a greater education gradient for blacks than for whites with respect to cognitive health. For blacks, dementia life expectancy shrunk 2.3 years with greater education (3.77 for without a high school diploma to 1.65 for some college or more). Largely, this reflects the poor cognitive health of less educated blacks. Whites in contrast only had a 1.15-year difference. Put differently, blacks have almost double the range of whites, showing a substantially greater gradient. Dementia-free life expectancy on the other hand was greater for blacks but not to the same extent as dementia life expectancy. For blacks, the difference between no high school diploma and some college is 5.29 years, whereas for whites it is 4.2 years. The difference between the gradients is only 1 year.
In terms of the proportion of life without dementia, blacks at each education level spend a lower proportion of life without dementia than whites. At age 65 for blacks, the proportion of life that is free of dementia is 73% and 90% for the lowest and highest education categories. For whites, the range is 86% to 95%. Table 3’s results also make clear that persons with higher levels of education are able to experience longer lives free of dementia than their less educated counter parts. The proportions of the population in the each education is not uniform across race groups. The extremely low proportion of life spent without dementia for blacks with no high school diploma is cause for concern because this group represents 52% of the sample.
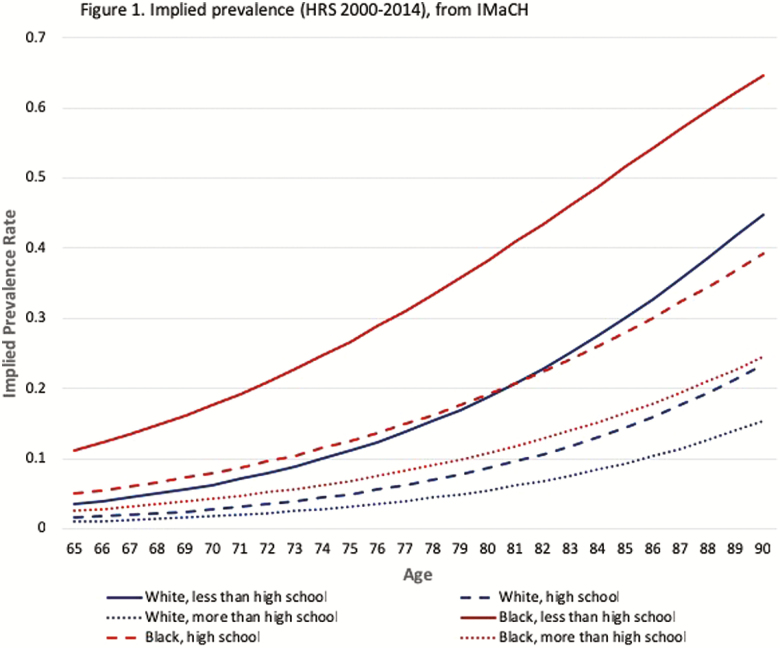
Figure 1 presents the results on implied prevalence—the prevalence rates implied by the life table transition probabilities. Not surprisingly, dementia prevalence increases with age for all groups. The levels of implied prevalence, however, vary greatly across the education and race groups. Results show a couple of noteworthy patterns. First, similar to the differences in cognitive status life expectancies, education drastically lowers dementia prevalence for both race groups, especially at older ages when dementia becomes more common. However, the education gradient for implied prevalence among blacks stands out. Blacks without a high school diploma fair dramatically worse. Their implied prevalence rates are consistently 2–4 times the rates of all other groups across older ages. Even the youngest age (65) when all other groups are indistinguishable from each other, with rates lower than 3%, the implied prevalence for blacks without a high school diploma already reaches 14%. The early burden supports the idea that the dementia process is faster and begins sooner for this group than all the others. Furthermore, the differences in timing between blacks at different education levels is also noteworthy. For example, the prevalence of dementia for blacks without a high school diploma at age 65 is not reached by blacks with a high school education until approximately 75 years of age. The differences in equivalent ages across the most disadvantaged and advantaged groups is vast. The prevalence of dementia among blacks without a high school diploma at age 65 is not reached among whites with some college or more until age 85. Additionally, and parallel to the results for the cognitive status life expectancies, the race gaps in prevalence diminish at higher levels of education. Education appears to be especially important in reducing race differences in dementia prevalence.
Figure 1.
Implied prevalence (HRS 2000–2014) from IMaCH.
Discussion
Prior studies have documented striking disparities in cognitive status life expectancy for race and education groups (Crimmins, Kim, Sasson, & Hayward, 2018; Garcia et al., 2017; Tom et al., 2015). Blacks bear a greater burden of dementia than whites and highly educated older Americans have much lower expectancy of living with dementia than low educated Americans (Langa et al., 2008). Our study extends this work, showing that the stratification systems of race and socioeconomic status combine in ways that exacerbate disparities in cognitive health in later life. Combining these stratification systems makes clear that cognitive health disparities defined by both race and education are enormous, and that highly educated whites and less educated blacks anchor the tail-ends of the cognitive health distribution. For example, highly educated, 65-year-old whites can expect to live free of dementia more than 17 years—an expectancy even greater than the total life expectancy for less educated blacks. Less educated, 65-year-old blacks, on the other hand, can only expect 10.59 years of life without dementia. The 7-year difference in life expectancy without dementia shows how stark these cognitive health inequalities can be. This difference is even more striking when considering the educational composition of the race groups: more than 50% of older blacks in our sample do not have a high school diploma, while about 38% of whites have more than a high school education. The educational distributions differ dramatically by race.
In combining race and education to examine cognitive health, we also are able to assess two important and related questions. First, where in the education distribution are race differences in cognitive health the greatest? As is evident in our results, the race gap narrows at higher levels of education, depicting what appears to be declining racial disparities associated with higher levels of education. For example, the race gap in DemLE is greatest among the lowest educated and smallest among the highest educated group. Second, how does the educational gradient in cognitive health differ for blacks and whites? The results point to a substantially larger educational gradient for blacks compared with whites, due largely to the very high levels of dementia life expectancy and dementia prevalence among less educated blacks compared with well-educated blacks.
The finding that race differences narrowed at higher levels of education was surprising. Prior studies on health and mortality, for example, document very small race gaps at lower levels of education and widening race differences with higher levels of education (e.g., Boen, 2016; Sasson, 2016; Williams, Priest, & Anderson, 2016). Researchers have argued that the larger racial gap in health at higher levels of education reflects blacks’ inability to translate their socioeconomic resources into health at the same level for whites (Boen, 2016; Pearson, 2008). Blacks exposure to neighborhood disadvantage, racial discrimination, and stress associated with higher educational attainment have been offered as possible mechanisms (Boen, 2016). At least for the case of dementia, however, education appears to be critically important for reducing race disparities. Moreover, at the opposite end of the education distribution, blacks are extraordinarily disadvantaged.
Part of the explanation for this pattern may be the critical role that education and other early life experiences play in developing and maintaining cognitive reserve. As we noted earlier, the majority of older black HRS respondents in this study were largely educated in segregated schools, typically grew up in the South, and faced decades of discrimination. Blacks growing up in the South not only went to schools with lower levels of funding and poorer quality than those attended by whites, but blacks also spent many fewer days in school. This suggests that especially at the low end of the educational distribution, blacks and whites had highly divergent educational experiences, perhaps contributing to less educated blacks’ extended dementia life expectancy and unusually high rates of dementia prevalence.
The extreme disadvantages for blacks with less than a high school education may also be contributing to the accentuated educational gradient in cognitive health for blacks compared with whites. Disadvantaged blacks are largely driving the educational gradient. This does not mean, however, that well educated blacks are able to erase their disadvantage in cognitive health compared with whites. Education may still have worse cognitive “payoffs” among educated blacks compared with whites because of a variety of life course experiences that contribute differentially to the cognitive reserve of blacks and whites. Future work will examine more carefully the life course mechanisms that are driving the race gap in dementia within educational groups.
We were able to assess whether the distribution of years of schooling differed between blacks and whites within the education groups, possibly contributing to why less educated blacks fared so poorly. We were concerned that among less educated blacks that the distribution of schooling might be shifted to the lower tail of the distribution compared with whites. It was not, suggesting that it is the actual combination of without a high school diploma and being black that puts blacks at much greater risk of dementia compared with all other groups.
Limitations
While viewing race and education as co-determinants of cognitive health status is an important step in understanding how these two types of stratification intersect to differentiate lifetime experience with dementia, it is also important to take note of several limitations. First, our measure of educational attainment does not capture fine-grained differences by years of education or the effect of credentialing. For example, previous studies found that compared with people who took college courses but never attained a degree, people who receive a college degree have an even lower risk of cognitive impairment and lower rates of dementia prevalence (Albert, 1995; Crimmins, Kim, Sasson, & Hayward, 2018; Lièvre, Alley, & Crimmins, 2008). Due to the small number of cases (given in Table 1), primarily among black respondents at older ages with some college, we are unable to provide estimates for college credentialed compared with noncredentialed. Nonetheless, our estimates document notable declines in dementia life expectancy for higher levels of educational attainment for both blacks and whites.
Second, while our study clearly documents the association between education and cognitive status life expectancy for blacks and whites, one should not construe the education effect as causal. Education is associated other early life factors and well as adult mechanisms which are important for understanding disparities in cognitive status in later life (e.g., Glymour & Manly 2008; Zhang, Gu, & Hayward 2008; Zhang et al., 2016). Here, education serves as a powerful marker of these life course influences.
Third, while our study does provide evidence for stark race disparities, the experience of blacks should not be extrapolated to other minority status persons. A few studies have included Hispanics in an effort to document their lifetime dementia experience (Garcia et al., 2017). We chose to exclude Hispanics given the large proportion of foreign-born Hispanics in the HRS and also their low levels of education that is usually acquired in their country of origin.
Conclusion
This analysis makes clear that dementia is at epidemic levels for some segments of the older U.S. population, especially less educated black Americans. At the same time, our findings point to the potential value of education both for reducing overall dementia burden in the older population and also for decreasing race disparities. Neither of these arguments, however, would have surfaced had we not combined race and education in this analysis. Increasingly, researchers are recognizing that it is difficult to simply examine race differences in health without considering socioeconomic status. Similarly, researchers examining socioeconomic disparities increasingly acknowledge the importance of considering race simultaneously. Racial and socioeconomic stratification systems are not isomorphic (Kawachi, Kennedy, Lochner, & Prothrow-Stith, 1997; Phelan & Link, 2015; Williams et al., 2016). They define distinct, yet sometimes overlapping life course exposures that have enduring associations with health and mortality. When race and socioeconomic status are combined in empirical research, they intersect in ways that typically exaggerate disparities because of the distinct racialized and class based exposures that affect health outcomes (Phelan & Link, 2015; Williams et al., 2016). This is evident in our results. Race and education combine to exaggerate disparities in cognitive health, while identifying an incredibly vulnerable group where dementia has reached epidemic levels. Future research on cognitive health status of older Americans should take an integrative approach to further explore the combined effects of race and education.
Funding
This work was supported by the Eunice Kennedy Shriver National Institute of Child Health and Development (RO1 HD053696, R24 HD042849, P2CHD042849, and T32 HD007081) and the National Institute of Aging (R24 AG045061, P30 AG17265, and U01 AG009740).
Author Contributions
M. P. Farina wrote the article, conducted analyses, and planned the study. M. D. Hayward edited the article, wrote sections of the article, and planned the study. E. M. Crimmins edited the article and planned the study. J. K. Kim helped plan the study and provided guidance on the analysis.
Conflict of Interest
None reported.
Supplementary Material
References
- Albano J. D., Ward E., Jemal A., Anderson R., Cokkinides V. E., Murray T., Henley J., Liff J., & Thun M. J (2007). Cancer mortality in the United States by education level and race. JNCI: Journal of the National Cancer Institute, 99(18), 1384–1394. doi:10.1016/j.amepre.2007.09.017 [DOI] [PubMed] [Google Scholar]
- Albert M. (1995). How does education affect cognitive functioning. Annals of Epidemiology, 5(1), 76–78. doi:10.1016/1047–2797(94)00044-T [DOI] [PubMed] [Google Scholar]
- Boen C. (2016). The role of socioeconomic factors in black–white health inequalities across the life course: Point-in-time measures, long-term exposures, and differential health returns. Social Science and Medicine, 170, 63–76. doi:10.1016/j.socscimed.2016.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braveman P. A., Cubbin C., Egerter S., Williams D. R., & Pamuk E (2010). Socioeconomic disparities in health in the United States: What the patterns tell us. American Journal of Public Health, 100, S186–S196. doi:10.2105/AJPH.2009.166082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crimmins E. M., Hayward M. D., & Saito Y (1996). Differentials in active life expectancy in the older population of the United States. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 51(3), 111–120. doi:10.1093/geronb/51B.3.S111 [DOI] [PubMed] [Google Scholar]
- Crimmins E. M., & Saito Y (2001). Trends in healthy life expectancy in the United States, 1970–1990: Gender, racial, and educational differences. Social Science and Medicine, 52(11), 1629–1641. doi:10.1016/S0277-9536(00)00273-2 [DOI] [PubMed] [Google Scholar]
- Crimmins E. M., Kim J. K., Langa K., & Weir D (2011). Assessment of cognition using surveys and neuropsychological assessment: The Health and Retirement Study and Aging, Demographics, and Memory Study. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 66(S1), i162–i171. doi:10.1093/geronb/gbr048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crimmins E. M., Saito Y., Kim J. K., Zhang Y. S., Sasson I., & Hayward M. D (2018). Educational differences in the prevalence of dementia and life expectancy with dementia: Changes from 2000 to 2010. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 73, 20–28. doi:10.1093/geronb/gbx135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia M., Downer B., Chiu C., Saenz J., Rote S., & Wong R (2017). Racial/ethnic and nativity differences in cogntive life expectancies among older adults in the United States. The Gerontologist, 59(2), 1–9. doi:10.1093/geront/gnx142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glymour M. M., & Manly J. J (2008). Lifecourse social conditions and racial and ethnic patterns of cognitive aging. Neuropsychology Review, 18(3), 223–254. doi:10.1007/s11065-008-9064-z [DOI] [PubMed] [Google Scholar]
- Hayward M. D., Miles T. P., Crimmins E. M., & Yang Y (2000). The significance of socioeconomic status in explaining the racial gap in chronic health conditions. American Sociological Review, 65(6), 910–930. doi:10.2307/2657519 [Google Scholar]
- Kawachi I., Kennedy B., Lochner K., & Prothrow-Stith D (1997). Social capital, income inequality, and mortality. American Journal of Public Health, 87, 1491–1498. doi:10.2105/AJPH.87.9.1491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langa K., Larson E., Karlawish J., Cutler D., Kabeto M., Kim S., et al. (2008). Trends in the prevalence and mortality impairment in the United States: Is their evidence of a compression of cognitive morbidity? Alzheimer’s & Dementia: The Journal of Alzheimer’s Association, 4, 134–144. doi:10.1016/j.jalz.2008.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lièvre A., Brouard N., & Heathcote C (2003). The estimation of health expectancies from cross-longitudinal surveys. Mathematical Studies, 10(4), 211–248. doi:10.1080/713644739 [Google Scholar]
- Lièvre A., Alley D., & Crimmins E. M (2008). Education differentials in life expectancy with cognitive impairment among the elderly in the United States. Journal of Aging and Health, 20(4), 456–477. doi:10.1177/0898264308315857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Link P., & Phelan J (1995). Social conditions as fundamental causes of diseases. Journal of Health and Social Behavior, 80–94. doi:10.2307/2626958 [PubMed] [Google Scholar]
- Montez J. K., Hummer R., Hayward M. D., Woo H., & Rogers R. G (2011). Trends in the education gradient of U.S. adult mortality from 1986 through 2006 by race, gender and age group. Research on Aging, 33(2), 145–171. doi:10.1177/0164027510392388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson J. A. (2008). Can’t buy my whiteness: New lessons from the Titanic on race, ethnicity, and health. Du Bois Review: Social Science Research on Race, 5(1), 27–47. doi:10.1017/S1742058X0808003X [Google Scholar]
- Phelan J., & Link B (2015). Is racism a fundamental cause of inequalities in health? Annual Review of Sociology, 41, 311–330. doi:10.1146/annurev-soc-073014-112305 [Google Scholar]
- Plassman B. L., Langa K. M., McCammon R. J., Fisher G. G., Potter G. G., Burke J. R.,…Wallace R. B (2011). Incidence of dementia and cognitive impairment, not dementia in the united states. Annals of Neurology, 70, 418–426. doi:10.1002/ana.22362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards M., & Sacker A (2010). Lifetime antecedents of cognitive reserve. Journal of Clinical and Experimental Neuropsychology, 25(5), 614–624. doi:10.1076/jcen.25.5.614.14581 [DOI] [PubMed] [Google Scholar]
- Sasson I. (2016). Diverging trends in cause-specific mortality and life years lost by educational attainment: Evidence from United States vital statistics data, 1990–2010. PLoS ONE, 11, e0163412. doi:10.1371/journal.pone.0163412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoendorf K. C., Hogue C. J., Kleinman J. C., & Rowley D (1992). Mortality among infants of black as compared with white college-educated parents. The New England Journal of Medicine, 326, 1522–1526. doi:10.1056/NEJM199206043262303 [DOI] [PubMed] [Google Scholar]
- Tom S. E., Hubbard R. A., Crane P. K., Haneuse S. J., Bowen J., McCormick W. C.,…Larson E. B (2015). Characterization of dementia and Alzheimer’s disease in an older population: Updated incidence and life expectancy with and without dementia. American Journal of Public Health, 105, 408–413. doi:10.2105/AJPH.2014.301935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D. R., & Sternthal M (2010). Understanding racial-ethnic disparities in health: Sociological contributions. Journal of Health and Social Behavior, 51, S15–S27. doi:10.1177/0022146510383838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D. R., Priest N., & Anderson N. B (2016). Understanding associations among race, socioeconomic status, and health: Patterns and prospects. Health Psychology, 35, 407–411. doi:10.1037/hea0000242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z., Gu D., & Hayward M. D (2008). Early life influences of cognitive impairment among oldest old Chinese. The Journals of Gerontology, Series B: Psychological Sciences and Social Sciences, 63, S25–S33. doi:10.1093/geronb/63.1.S25 [DOI] [PubMed] [Google Scholar]
- Zhang Z., Hayward M. D., & Yu Y. L (2016). Life course pathways to racial disparities in cognitive impairment among older Americans. Journal of Health and Social Behavior, 57(2), 184–199. doi:10.1177/0022146516645925 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.