Abstract
In recent years, genome-wide association studies have shed light on the genetics of early growth and its links with later-life health outcomes. Large-scale datasets and meta-analyses, combined with recently developed analytical methods, have enabled dissection of the maternal and fetal genetic contributions to variation in birth weight. Additionally, longitudinal approaches have shown differences between the genetic contributions to infant, childhood and adult adiposity. In contrast, studies of adult height loci have shown strong associations with early body length and childhood height. Early growth-associated loci provide useful tools for causal analyses: Mendelian randomization (MR) studies have provided evidence that early BMI and height are causally related to a number of adult health outcomes. We advise caution in the design and interpretation of MR studies of birth weight investigating effects of fetal growth on later-life cardiometabolic disease because birth weight is only a crude indicator of fetal growth, and the choice of genetic instrument (maternal or fetal) will greatly influence the interpretation of the results. Most genetic studies of early growth have to date centered on European-ancestry participants and outcomes measured at a single time-point, so key priorities for future studies of early growth genetics are aggregation of large samples of diverse ancestries and longitudinal studies of growth trajectories.
Introduction
For decades, researchers have accumulated strong evidence of associations between in utero and early growth and health throughout life. Recently, studies investigating the genetic variation associated with early growth have brought these lifelong health links into focus, both in terms of individual genetic loci associated with early growth traits and later health outcomes and when comparing the genome-wide association landscape between traits using genetic correlation analyses (1). In addition, genetic studies can be informative for uncovering key biological pathways and in determining the causal effects of genetic and environmental exposures. Here, we review major advances of the past 2 years and highlight areas where studies should place additional focus in the near future.
Studies of Birth Weight Taking Maternal Genotype into Account
Over the last decade, studies of increasing size and diversity have investigated the genetic underpinnings of birth weight. In the last 2 years, a major advance is in our ability to estimate, for a genome-wide set of single nucleotide polymorphism (SNPs), the direct fetal effects on birth weight and the independent maternal genetic effects that act indirectly via the uterine environment. This advance was made possible by (i) aggregation of genome-wide association studies (GWAS) with maternal genotypes and offspring birth weight (2) in addition to studies with own (fetal) genotype and own birth weight and (ii) the development of a new genome-wide method to estimate independent maternal and fetal effects on birth weight in the absence of large numbers of genotyped mother–child pairs (3). The most recent GWAS of birth weight estimated independent maternal and fetal effects at 190 genome-wide significant loci (4). Variance in birth weight explained by these loci showed a greater fetal than maternal component, with more than three-quarters of the variance attributable to direct fetal genotype effects. While causal genes and underlying functional variants remain largely unknown, there was a strong overlap of fetal genetic loci with the genetics of childhood and adult height (Fig. 1) and enrichment for imprinted genes and those involved in insulin-like growth factor and insulin signaling.
Figure 1.
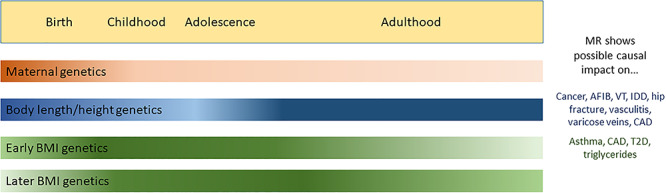
A schematic of genetic effects on growth traits, with color gradients showing the approximate timing of genetic effects based on current knowledge. Some questions remain, for example, the extent to which maternal effects persist to adulthood and which height variants act during postnatal, but not fetal, growth. In MR studies, adult height-associated variants have typically been used to create genetic instruments to test causality with health outcomes. For BMI, more work has been done using childhood BMI-associated variants (with some example outcomes shown here). AFIB, atrial fibrillation; VT, venous thromboembolism; IDD, intervertebral disc disorder; CAD, coronary artery disease; T2D, type 2 diabetes.
Many identified loci showed evidence of independent contributions from both mother and fetus: of the 138 lead SNPs that could be confidently classified as having maternal and/or fetal effects, 30% showed evidence of both a direct fetal and an indirect maternal effect. For one-third of those, the maternal and fetal effects were in opposite directions. Examples of loci with opposite maternal and fetal effects on birth weight include those at established glycemic trait loci, such as G6PC2, ADCY5 and CDKAL1. At those loci, fetal insulin is a likely mediator, given the paradigm maternal and fetal birth weight effects of rare mutations in the glucokinase gene (5). However, for most loci, the underlying mechanism is unknown, and current evidence suggests that fetal growth attributable to fetal effects at most birth weight-associated loci is not insulin-mediated (6).
Early Growth Traits Assessed by Longitudinal Approaches
Recent genetic studies have included postnatal longitudinal approaches to discovering novel loci. Many longitudinal studies have focused on changes in adiposity and body mass index (BMI). For example, two studies investigating early life adiposity found similar associations at the LEPR locus prior to 5 years of age, with different genetic loci influencing later childhood and adult BMI (7,8). In a separate study, application of a genetic risk score (GRS) based on adult BMI to a pediatric cohort showed similar patterns of association from infancy to later childhood: the adult BMI-derived GRS was not associated with birth weight, but became increasingly associated with BMI across childhood (9). However, a study of normal variation in childhood BMI assessed between the ages of 2 and 10 years old observed a genetic correlation with both birth weight and adult BMI (10). Collectively, these studies suggest that although some loci influence weight from birth through to adulthood, the mechanisms influencing body mass shift during early childhood, after which they remain relatively constant throughout adolescence and into adulthood (Fig. 1). Additionally, there is some evidence that BMI-associated variants may have varying effects across adulthood, as reported in a candidate study of the association of 282 variants with BMI at age 20 and change in BMI during adulthood in the Japanese (11).
In addition to 25 loci associated with childhood BMI (10), assessing obesity as a dichotomous trait [with cases defined as having BMI ≥95th percentile and controls being consistently <50th percentile] in youth aged 2–18 years revealed a novel locus and fine-mapped many previously reported loci primarily due to the inclusion of African, North/South American and East Asian samples (12). Still, the largest genetic studies of BMI continue to be carried out in European-only samples, such as the recent GIANT study, which reported 941 nearly independent loci for adult BMI (13). While several small studies of childhood and adult BMI have been published in non-European samples (e.g. (14–16)), future studies including large sample sizes from diverse ancestral backgrounds will yield additional discoveries.
Insights into Height Growth from Genetic Studies
In recent years, it has become clear that the genetic determinants of size at birth and height throughout childhood are correlated, with positive genetic correlations beginning with birth length and childhood height to final adult stature (4). Additionally, there is an enrichment of GWAS signals for adult height in DNAseI hypersensitivity sites in human embryonic stem cells (17), showing that these signals may act in early development, with an impact on growth throughout the life course. However, the correlation between the genetic determinants of birth weight and height is weaker during puberty (4), which may be explained by the impact of differences in the timing of puberty on height growth. Still, these findings suggest that many of the same genetic determinants of body length, in contrast to BMI, act throughout life (Fig. 1).
While few studies have been published recently investigating the genetic determinants of specific height growth phases, the GIANT consortium released a GWAS of adult stature including ~700 000 individuals and reporting 3200 loci associated with the trait (13). This data is providing insights into the mechanisms underlying growth; using expression quantitative trait locus data, 610 genes were prioritized as potentially causal for variation in height, and pathway analysis subsequently identified enrichment for skeletal growth and cartilage and connective tissue development. Further, height-associated GWAS signals were enriched in noncoding regulatory regions in the growth plates of long bones (18), which is intuitively plausible as a key effector tissue for mediating differences in stature. Other studies have noted enrichment in pathways relevant to growth, such as the transforming growth factor beta and Hedgehog pathways in growth plate development (19), as well as growth hormone regulation (20). A Japanese study subsequently noted in a pathway comparison with the GIANT European study that the biological mechanisms regulating height growth are largely shared across populations (21), indicating that additional genetic insights could be gained by trans-ancestry approaches.
Another axis becoming clear is the relationship between the genetic determinants of height and other skeletal traits, such as bone size, bone mineral density and osteoarthritis (22,23). These studies suggest that the genetic determinants affecting variation in height also impact other aspects of endochondral ossification and bone biology (24,25), with implications for therapeutic development. Specific loci with potential clinical applications for later health include the GDF5 locus, associated with early growth, body size and developmental traits (infant length (26), height at age 10 (UK Biobank (http://www.nealelab.is/uk-biobank/)), bone size (22) and age at menarche (27)). This locus was originally associated with osteoarthritis in Asian populations (28) and adult height (29). In the past 2 years, however, additional GWAS and large-scale biobank phenome-wide association studies have revealed a breadth of health outcomes across the life course associated with the GDF5 locus, including skeletal site-specific osteoarthritis (23,30), congenital hip dysplasia (31), internal derangement of the knee, monoarthritis, connective tissue disease, bunions (SAIGE (32)) and knee pain (33). Furthermore, the causal SNP has been identified as well the mechanism by which noncoding variants differentially affect DNA methylation, transcription factor binding and subsequent GDF5 expression level, with differential methylation between knee osteoarthritis samples when compared to other skeletal sites and non-osteoarthritis samples (34,35). GDF5 may thus play a role in tissue repair and remodeling (36) and is currently under development as a therapeutic target for osteoarthritis and cartilage regeneration (30,37). For most genetic association loci, much less is known about the causal variants, genes and mechanisms by which they act, but recent developments in identifying lifelong effects for childhood growth loci such as GDF5 suggest that in the coming years, additional loci should yield insights of clinical relevance.
Impact of Early Growth on Lifelong Health Outcomes
In addition to specific loci, GWAS summary results from across the genome can be informative for uncovering the causal relationships between early growth traits and future health outcomes. In the past few years, causal inference methods utilizing genetic variants, collectively termed Mendelian randomization (MR), have begun to investigate these associations. Briefly, to test for a causal relationship between an exposure and outcome of interest, MR uses genetic variants associated with the exposure as proxies (‘instruments’) for that exposure. In contrast correlations between the measured exposure and outcome, a genetic association between the exposure instruments and the outcome of interest is unlikely to be subject to confounding or reverse causality due to the random shuffling of alleles that occurs between parents and offspring and can thus provide evidence supportive of causality, subject to various assumptions (38).
While MR is a potentially powerful tool for determining causal factors that influence health-relevant outcomes, these studies need to be carried out with care, especially when aiming to examine causal relationships between fetal growth and later health outcomes. This is because (i) fetal growth is influenced by both maternal and fetal genetic effects (4,39) and (ii) available instruments are for birth weight, which is a crude proxy for fetal growth. Recently, studies using maternal genetic variants as instruments for in utero environmental exposures that lower birth weight (independent of fetal genetic effects) found no evidence that they are causally associated with adverse cardiometabolic health outcomes in adulthood, contrary to observational epidemiological associations (4,40). Explicit understanding of the precise exposure captured by genetic instruments is crucial for appropriate interpretation of such MR studies. For example, studies using fetal genotype effects on birth weight as instruments for fetal growth have, in contrast, observed associations with later adverse outcomes such as type 2 diabetes (41,42). Such associations may reflect (i) direct effects of an individual’s own genotype on their birth weight and on later health, as expected under the fetal insulin hypothesis (43); (ii) direct effects of the individual’s genotype on their own later health and correlation with maternal genotype effects on birth weight, as seen recently for blood pressure (4); or (iii) potentially, health effects secondary to fetal growth. However, under such study designs with fetal genotype instruments, it is not possible to separate possibility (iii) from (i) or (ii).
While many studies have used MR to investigate the causal impact of adult stature on health outcomes, very few studies to date have assessed the causal impact of distinct childhood growth phases. One study investigated both childhood height and adult height and found a separate influence of each on osteosarcoma risk (45). Indeed, in the past 2 years alone, large studies taking a broad approach have implicated taller height as a causal factor for many health outcomes (24), whether increasing risk (such as for diagnosis with cancer and death from any cancer (45,46) and specific cancers (ovarian cancer (47); head and neck cancer (48); melanoma (49)), varicose veins (50), atrial fibrillation, venous thromboembolism, intervertebral disc disorder, hip fracture, and vasculitis (46). Taller stature also appears to have protective effects for other traits, in particular coronary artery disease (46,51). These relationships are sometimes complex, such as findings that taller height was causally related to ovarian cancer (46) in the general population, but not among premenopausal BRCA1/2 carriers (52). These divergent findings could also be due to specific limitations of each study, and further work is needed to gain clarity. Additionally, while very few have been carried out to date, studies are beginning to look at the impact of height on pediatric outcomes, such as ocular biometry at age 6 years (53), and in non-European populations, e.g. on pulmonary traits in Latinos (54).
Future Priorities for Genetic Studies of Early Growth Traits: Diversity and Mechanism
To date, genetic investigations of birth weight have been performed predominantly in European ancestry samples, but low birth weight and associated adverse outcomes are often higher in non-Europeans, e.g. in South Asian or African ancestry (55); the reasons for these differences are not fully understood. The key priority for future GWAS efforts is to target large studies of other ancestral backgrounds, as these genetic studies will inform about both the heritable and environmental effects on birth weight. Indeed, a recent study examined birth weight, length and ponderal index across geographic regions and found that while the contribution of genetic factors was smaller than environmental factors, genetic variance was similar across geographic regions (56).
Aside from increasing diversity in future genetic studies of birth weight, there are further priorities for data aggregation and analysis that will improve our understanding of mechanisms. First, a limitation of the most recent GWAS of birth weight was that most samples did not have information on gestational duration (4), so further large studies with both birth weight and gestational duration data will be necessary to understand which loci primarily influence birth timing versus fetal growth. While initial indications are that only a few loci influence birth weight through a primary effect on gestational duration (4,57), while most fetal alleles that increase fetal growth result in shorter gestation, but larger studies are needed to confirm these findings. Second, the integration of epigenetic data will help to inform on mechanisms. For example, DNA methylation may be influenced by environmental exposures and may in turn influence gene expression. A recent epigenome-wide association study (EWAS) of birth weight in 8825 individuals identified more than 900 differentially methylated associated sites in neonatal blood (58). Another smaller EWAS (n = 301) identified 15 differentially methylated sites in the placenta (59). By integrating such findings with GWAS data, future studies will be able to assess evidence of causal effects, and examination of relevant cells and tissues will also be important (60). Third, longitudinal antenatal studies are needed to gain a clearer understanding of the genetics of fetal growth. Multilevel models can be applied to ultrasound scan data to model fetal growth trajectories (61), and both the heritability of fetal growth measures (62) and the contribution of a fetal genetic score for birth weight to fetal growth are higher in later gestation (63). Applying GWAS to antenatal phenotypes should improve understanding of links between different growth trajectories and pregnancy outcomes.
For childhood and adolescent growth, studies are needed to examine whether specific growth phases or trajectories more closely relate to adverse health outcomes. For instance, pubertal timing is correlated with many health outcomes, and variation in pubertal timing impacts the total amount and velocity of growth during adolescence (64). When dealing with anthropometric traits such as pubertal timing and height growth, BMI should also be taken into account, due to its close relationship with pubertal timing (e.g. in assessing the causal impact of prepubertal BMI and pubertal timing on cardiometabolic outcomes (65)). Furthermore, taking BMI into account as a mediator or confounder can even reverse causal relationships; for example, age at menarche had an inverse direct causal effect on risk of breast cancer independent of BMI, while BMI itself had a positive indirect effect (66,67).
Perhaps of most direct societal impact is the increasing understanding that elevated BMI impacts adult health beginning in childhood, with growing numbers of genetic studies also supporting the idea that efforts should be made to reduce childhood obesity rates to improve public health. In the past 2 years, MR approaches have implicated childhood BMI in risk for asthma (68) and coronary artery disease and type 2 diabetes (69,70), as well as childhood abdominal obesity on plasma triglycerides and other cardiometabolic risk factors (71). Importantly, there is some evidence that the impact of childhood adiposity can be mitigated by weight loss later in life (72). Here, also, there is a need for studies in non-European population groups; standard obesity thresholds are not equally valid across ethnicities, with some ethnic group having higher disease risks at lower BMI thresholds than Europeans (73), and rates of adverse health outcomes, including BMI and cardiometabolic traits, vary by ethnicity (74).
Conclusions
Genetic studies of early growth phenotypes have recently made great progress in identifying underlying genetic loci, and analyses using these loci have begun to illuminate links with lifelong health. To further improve understanding in this field, key future priorities are the examination of growth trajectories through longitudinal study designs and a redoubled effort to study samples of diverse ancestries.
Acknowledgements
D.L.C. is currently employed by GSK (after July 16, 2020).
Conflict of Interest statement. The authors declare that they have no conflicts of interest.
Contributor Information
Diana L Cousminer, Division of Human Genetics, Children’s Hospital of Philadelphia, Philadelphia, PA 19104, USA; Department of Genetics, University of Pennsylvania, Philadelphia, PA 19104, USA; Center for Spatial and Functional Genomics, Children’s Hospital of Philadelphia, Philadelphia, PA 19104, USA.
Rachel M Freathy, Institute of Biomedical and Clinical Science, University of Exeter Medical School, University of Exeter, Exeter EX2 5DW, UK.
Funding
Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health (Award Number K99HD099330 to D.L.C.). Wellcome Trust and Royal Society Sir Henry Dale Fellowship (104150/Z/14/Z to R.M.F.).
References
- 1. Middeldorp C.M., Felix J.F., Mahajan A., EArly Genetics Lifecourse Epidemiology (EAGLE) consortium, Early Growth Genetics (EGG) consortium, McCarthy M.I. (2019) The Early Growth Genetics (EGG) and EArly Genetics and Lifecourse Epidemiology (EAGLE) consortia: design, results and future prospects. Eur. J. Epidemiol., 34, 279–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Beaumont R.N., Warrington N.M., Cavadino A., Tyrrell J., Nodzenski M., Horikoshi M., Geller F., Myhre R., Richmond R.C., Paternoster L. et al. (2018) Genome-wide association study of offspring birth weight in 86 577 women identifies five novel loci and highlights maternal genetic effects that are independent of fetal genetics. Hum. Mol. Genet., 27, 742–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Warrington N.M., Freathy R.M., Neale M.C. and Evans D.M. (2018) Using structural equation modelling to jointly estimate maternal and fetal effects on birthweight in the UK Biobank. Int. J. Epidemiol., 47, 1229–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Warrington N.M., Beaumont R.N., Horikoshi M., Day F.R., Helgeland Ø., Laurin C., Bacelis J., Peng S., Hao K., Feenstra B. et al. (2019) Maternal and fetal genetic effects on birth weight and their relevance to cardio-metabolic risk factors. Nat. Genet., 51, 804–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hattersley A.T., Beards F., Ballantyne E., Appleton M., Harvey R. and Ellard S. (1998) Mutations in the glucokinase gene of the fetus result in reduced birth weight. Nat. Genet., 19, 268–270. [DOI] [PubMed] [Google Scholar]
- 6. Hughes A.E., Nodzenski M., Beaumont R.N., Talbot O., Shields B.M., Scholtens D.M., Knight B.A., Lowe W.L., Hattersley A.T. and Freathy R.M. (2018) Fetal genotype and maternal glucose have independent and additive effects on birth weight. Diabetes, 67, 1024–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Helgeland Ø., Vaudel M., Juliusson P.B., Holmen O.L., Juodakis J., Bacelis J., Jacobsson B., Lindekleiv H., Hveem K., Lie R.T. et al. (2019) Genome-wide association study reveals dynamic role of genetic variation in infant and early childhood growth. Nat. Commun., 10, 4448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Couto Alves A., De Silva N.M.G., Karhunen V., Sovio U., Das S., Taal H.R., Warrington N.M., Lewin A.M., Kaakinen M., Cousminer D.L. et al. (2019) GWAS on longitudinal growth traits reveals different genetic factors influencing infant, child, and adult BMI. Sci. Adv., 5, eaaw3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Khera A.V., Chaffin M., Wade K.H., Zahid S., Brancale J., Xia R. Distefano M., Senol-Cosar O.,Haas M.E., Bick A. et al. (2019) Polygenic prediction of weight and obesity trajectories from birth to adulthood. Cell, 177, 587–596.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vogelezang S., Bradfield J.P., Ahluwalia T.S., Curtin J.A., Lakka T.A., Grarup N., Scholz M., van der Most P.J., Monnereau C., Stergiakouli E. et al. Novel loci for childhood body mass index and shared heritability with adult cardiometabolic traits. PLoS Genet. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Iwase M., Matsuo K., Nakatochi M., Oze I., Ito H., Koyanagi Y., Ugai T., Kasugai Y., Hishida A., Takeuchi K. et al. (2020) Differential effect of polymorphisms on body mass index across the life course of Japanese: the Japan multi-institutional collaborative cohort study. J. Epidemiol. doi: 10.2188/jea.JE20190296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bradfield J.P., Vogelezang S., Felix J.F., Chesi A., Helgeland Ø., Horikoshi M., Karhunen V., Lowry E., Cousminer D.L., Ahluwalia T.S. et al. (2019) A trans-ancestral meta-analysis of genome-wide association studies reveals loci associated with childhood obesity. Hum. Mol. Genet., 28, 3327–3338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yengo L., Sidorenko J., Kemper K.E., Zheng Z., Wood A.R., Weedon M.N., Frayling T.M., Hirschhorn J., Yang J., Visscher P.M. and the GIANT Consortium (2018) Meta-analysis of genome-wide association studies for height and body mass index in ∼700000 individuals of European ancestry. Hum. Mol. Genet., 27, 3641–3649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chiang K.M., Chang H.C., Yang H.C., Chen C.H., Chen H.H., Lee W.J. and Pan W.H. (2019) Genome-wide association study of morbid obesity in Han Chinese. BMC Genet., 20, 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Giri A.K., Prasad G., Bandesh K., Parekatt V., Mahajan A., Banerjee P., Kauser Y., Chakraborty Y., Rajashekar D., INDICO et al. (2020) Multifaceted genome-wide study identifies novel regulatory loci in SLC22A11 and ZNF45 for body mass index in Indians. Mol. Gen. Genom., 295, 1013–1026. [DOI] [PubMed] [Google Scholar]
- 16. Costa-Urrutia P., Colistro V., Jiménez-Osorio A.S., Cárdenas-Hernández H., Solares-Tlapechco J., Ramirez-Alcántara M., Granados J., Ascencio-Montiel I.J. and Rodríguez-Arellano M. (2019) Genome-wide association study of body mass index and body fat in Mexican-Mestizo children. Genes, 10, 945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Trynka G., Westra H.-J., Slowikowski K., Hu X., Xu H., Stranger B.E., Klein R.J., Han B. and Raychaudhur S. (2015) Disentangling the effects of Colocalizing genomic annotations to functionally prioritize non-coding variants within complex-trait loci. Am. J. Hum. Genet., 97, 139–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Guo M., Liu Z., Willen J., Shaw C.P., Richard D., Jagoda E., Doxey A.C., Hirschhorn J. and Capellini T.D. (2017) Epigenetic profiling of growth plate chondrocytes sheds insight into regulatory genetic variation influencing height. elife, 6, e29329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Samsa W.E., Zhou X. and Zhou G. (2017) Signaling pathways regulating cartilage growth plate formation and activity. Semin. Cell Dev. Biol., 62, 3–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Baron J., Sävendahl L., De Luca F., Dauber A., Phillip M., Wit J.M. and Nilsson O. (2015) Short and tall stature: a new paradigm emerges. Nat. Rev. Endocrinol., 11, 735–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Akiyama M., Ishigaki K., Sakaue S., Momozawa Y., Horikoshi M., Hirata M., Matsuda K., Ikegawa S., Takahashi A., Kanai M., Suzuki S. and Matsui D. (2019) Characterizing rare and low-frequency height-associated variants in the Japanese population. Nat. Commun., 10, 4393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Styrkarsdottir U., Stefansson O.A., Gunnarsdottir K., Thorleifsson G., Lund S.H., Stefansdottir L., Juliusson K., Agustsdottir A.B., Zink F., Halldorsson G.H. et al. (2019) GWAS of bone size yields twelve loci that also affect height, BMD, osteoarthritis or fractures. Nat. Commun., 10, 2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Styrkarsdottir U., Lund S.H., Thorleifsson G., Zink F., Stefansson O.A., Sigurdsson J.K., Juliusson K., Bjarnadottir K., Sigurbjornsdottir S., Jonsson S. et al. (2018) Meta-analysis of Icelandic and UK data sets identifies missense variants in SMO, IL11, COL11A1 and 13 more new loci associated with osteoarthritis. Nat. Genet., 50, 1681–1687. [DOI] [PubMed] [Google Scholar]
- 24. Zhu Z., Zheng Z., Zhang F., Wu Y., Trzaskowski M., Maier R., Robinson M.R., McGrath J.J., Visscher P.M., Wray N.R. and Yang J. (2018) Causal associations between risk factors and common diseases inferred from GWAS summary data. Nat. Commun., 9, 224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Liang X., Wu C., Zhao H., Liu L., Du Y., Li P., Wen Y., Zhao Y., Ding M., Cheng B. et al. (2018) Assessing the genetic correlations between early growth parameters and bone mineral density: a polygenic risk score analysis. Bone, 116, 301–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Valk R.J.P., Kreiner-Møller E., Kooijman M.N., Guxens M., Stergiakouli E., Sääf A., Bradfield M.N., Geller F., Hayes MG.., Cousminer D.L. et al. (2015) A novel common variant in DCST2 is associated with length in early life and height in adulthood. Hum. Mol. Genet., 24, 1155–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kichaev G., Bhatia G., Loh P.-R., Gazal S., Burch K., Freund M.K., Schoech A., Pasaniuc B. and Price A.L. (2019) Leveraging polygenic functional enrichment to improve GWAS power. Am. J. Hum. Genet., 104, 65–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Miyamoto Y., Mabuchi A., Shi D., Kubo T., Takatori Y., Saito S., Fujioka M., Sudo A., Uchida A., Yamamoto S. et al. (2007) A functional polymorphism in the 5′ UTR of GDF5 is associated with susceptibility to osteoarthritis. Nat. Genet., 39, 529–533. [DOI] [PubMed] [Google Scholar]
- 29. Sanna S., Jackson A.U., Nagaraja R., Willer C.J., Chen W.-M., Bonnycastle L.L., Shen H., Timpson N., Lettre G., Usala G. et al. (2008) Common variants in the GDF5-UQCC region are associated with variation in human height. Nat. Genet., 40, 198–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tachmazidou I., Hatzikotoulas K., Southam L., Esparza-Gordillo J., Haberland V., Zheng J., Johnson T., Koprulu M., Zengini E., Steinberg J. et al. (2019) Identification of new therapeutic targets for osteoarthritis through genome-wide analyses of UK biobank data. Nat. Genet., 51, 230–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hatzikotoulas K., Roposch A., DDH Case Control Consortium, Shah K.M., Clark M.J., Bratherton S., Limbani V., Steinberg J., Zengini E., Warsame K. et al. (2018) Genome-wide association study of developmental dysplasia of the hip identifies an association with GDF5. Commun. Biol., 1, 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gagliano Taliun S.A., VandeHaar P., Boughton A.P., Welch R.P., Taliun D., Schmidt E.M., Zhou W., Nielsen J.B., Willer C.J., Lee S. et al. (2020) Exploring and visualizing large-scale genetic associations by using PheWeb. Nat. Genet., 52, 550–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Meng W., Adams M.J., Palmer C.N.A., The 23andMe Research Team, Sh J., Auton A., Ryan K.A., Jordan J.M., Mitchell B.D., Jackson R.D. et al. (2019) Genome-wide association study of knee pain identifies associations with GDF5 and COL27A1 in UK biobank. Commun. Biol., 2, 321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Reynard L.N., Bui C., Canty-Laird E.G., Young D.A. and Loughlin J. (2011) Expression of the osteoarthritis-associated gene GDF5 is modulated epigenetically by DNA methylation. Hum. Mol. Genet., 20, 3450–3460. [DOI] [PubMed] [Google Scholar]
- 35. Reynard L.N., Bui C., Syddall C.M. and Loughlin J. (2014) CpG methylation regulates allelic expression of GDF5 by modulating binding of SP1 and SP3 repressor proteins to the osteoarthritis susceptibility SNP rs143383. Hum. Genet., 133, 1059–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kania K., Colella F., Riemen A.H.K., Wang H., Howard K.A., Aigner T., Dell'Accio F., Capellini T.D., Roelofs A.J. and De Bari C. (2020) Regulation of Gdf5 expression in joint remodelling, repair and osteoarthritis. Sci. Rep., 10, 157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sun Y., You Y., Jiang W., Zhai Z. and Da K. (2019) 3D-bioprinting a genetically inspired cartilage scaffold with GDF5-conjugated BMSC-laden hydrogel and polymer for cartilage repair. Theranostics, 9, 6949–6961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Davey Smith G. and Ebrahim S. (2003) ‘Mendelian randomization’: can genetic epidemiology contribute to understanding environmental determinants of disease?*. Int. J. Epidemiol., 32, 1–22. [DOI] [PubMed] [Google Scholar]
- 39. Lawlor D.A., Richmond R., Warrington N., McMahon G., Smith G.D., Bowden J. and Evans D.M. (2017) Using Mendelian randomization to determine causal effects of maternal pregnancy (intrauterine) exposures on offspring outcomes: sources of bias and methods for assessing them. Wellcome Open Res., 2, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Moen G.-H., Brumpton B., Willer C., Aasvold B.O., Birkeland K., Neale M.C., Freathy R.M., Smith G.D., Lawlor D.A., Kirkpatrick R.M., Warrington N.M, Evans D.M. (2020) Do maternal intrauterine environmental influences that lower offspring birthweight causally increase offspring cardiometabolic risk factors in later life? A Mendelian randomization study of 45,849 genotyped parent offspring pairs in the HUNT study. https://www.medrxiv.org/content/10.1101/2020.05.04.20091173v2. [Google Scholar]
- 41. Zanetti D., Tikkanen E., Gustafsson S., Priest J.R., Burgess S. and Ingelsson E. (2018) Birthweight, type 2 diabetes mellitus, and cardiovascular disease: addressing the barker hypothesis with Mendelian randomization. Circ. Genom. Precis. Med., 11, e002054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. BIRTH-GENE (BIG) Study Working Group, Huang T., Wang T., Zheng Y., Ellervik C., Li L., Gao M., Fang Z., Chai J.-F., Ahluwalia T.V.S. et al. (2019) Association of birth weight with type 2 diabetes and glycemic traits: a Mendelian randomization study. JAMA Netw. Open, 2, e1910915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hattersley A.T. and Tooke J.E. (1999) The fetal insulin hypothesis: an alternative explanation of the association of low bir thweight with diabetes and vascular disease. Lancet, 353, 1789–1792. [DOI] [PubMed] [Google Scholar]
- 44. Zhang C., Morimoto L.M., Smith A.J., Hansen H.M., Gonzalez-Maya J., Endicott A.A., Smirnov I.V., Metayer C., Wei Q., Eward W.C., Wiemels J.L. and Walsh K.M. et al. (2018) Genetic determinants of childhood and adult height associated with osteosarcoma risk: height genetics and osteosarcoma. Cancer, 124, 3742–3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ong J.-S., An J., Law M.H., Whiteman D.C., Neale R.E., Gharahkhani P. and MacGregor S. (2018) Height and overall cancer risk and mortality: evidence from a Mendelian randomisation study on 310,000 UK Biobank participants. Br. J. Cancer, 118, 1262–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lai F.Y., Nath M., Hamby S.E., Thompson J.R., Nelson C.P. and Samani N.J. (2018) Adult height and risk of 50 diseases: a combined epidemiological and genetic analysis. BMC Med., 16, 187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Dixon-Suen S.C., Nagle C.M., Thrift A.P., Pharoah P.D.P., Ewing A., Pearce C.L., Zheng W., Australian Ovarian Cancer Study Group, Chenevix-Trench G., Fasching F.A. et al. (2018) Adult height is associated with increased risk of ovarian cancer: a Mendelian randomisation study. Br. J. Cancer, 118, 1123–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Pastorino R., Puggina A., Carreras-Torres R., Lagiou P., Holcátová I., Richiardi L., Kjaerheim K., Agudo A., Castellsagué X., Macfarlane T.V. et al. (2018) Genetic contributions to the association between adult height and head and neck cancer: a Mendelian randomization analysis. Sci. Rep., 8, 4534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Dusingize J.C., Olsen C.M., An J., Pandeya N., Law M.H., Thompson B.S., Goldstein A.M., Iles M.M., Webb P.M., Neale O.R. et al. (2020) Body mass index and height and risk of cutaneous melanoma: Mendelian randomization analyses. Int. J. Epidemiol. doi: 10.1093/ije/dyaa009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Fukaya E., Flores A.M., Lindholm D., Gustafsson S., Zanetti D., Ingelsson E. and Leeper N.J. (2018) Clinical and genetic determinants of varicose veins: prospective, community-based study of ≈500 000 individuals. Circulation, 138, 2869–2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Marouli E., Del Greco M.F., Astley C.M., Yang J., Ahmad S., Berndt S.I., Caulfield M.J., Evangelou E., McKnight B., Medina-Gomez C. et al. (2019) Mendelian randomisation analyses find pulmonary factors mediate the effect of height on coronary artery disease. Commun. Biol., 2, 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Qian F., Rookus M.A., Leslie G., Risch H.A., Greene M.H., Aalfs C.M., Adank M.A., Adlard J., Agnarsson B.A., Ahmed M. et al. (2019) Mendelian randomisation study of height and body mass index as modifiers of ovarian cancer risk in 22,588 BRCA1 and BRCA2 mutation carriers. Br. J. Cancer, 121, 180–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Tideman J.W.L., Polling J.R., Jaddoe V.W.V., Vingerling J.R.. and Klaver C.C.W. (2019) Growth in foetal life, infancy, and early childhood and the association with ocular biometry. Ophthalmic Physiol. Opt., 39, 245–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Sofer T., Moon J.-Y., Isasi C.R., Qi Q., Shah N.A., Kaplan R.C. and Kuniholm M.H. (2018) Relationship of genetic determinants of height with cardiometabolic and pulmonary traits in the Hispanic Community Health Study/Study of Latinos. Int. J. Epidemiol., 47, 2059–2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kelly Y., Panico L., Bartley M., Marmot M., Nazroo J. and Sacker A. (2008) Why does birthweight vary among ethnic groups in the UK? Findings from the Millennium Cohort Study. J. Public Health, 31, 131–137. [DOI] [PubMed] [Google Scholar]
- 56. Yokoyama Y., Jelenkovic A., Hur Y.-M., Sund R., Fagnani C., Stazi M.A., Brescianini S., Ji F., Ning F., Pang Z. et al. (2018) Genetic and environmental factors affecting birth size variation: a pooled individual-based analysis of secular trends and global geographical differences using 26 twin cohorts. Int. J. Epidemiol., 47, 1195–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zhang G., Feenstra B., Bacelis J., Liu X., Muglia L.M., Juodakis J., Miller D.E., Litterman N., Jiang P.P., Russell L. et al. (2017) Genetic associations with gestational duration and spontaneous preterm birth. N. Engl. J. Med., 377, 1156–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Küpers L.K., Monnereau C., Sharp G.C., Yousefi P., Salas L.A., Ghantous A., Page C.M., Reese S.E., Wilcox A.J., Czamara D. et al. (2019) Meta-analysis of epigenome-wide association studies in neonates reveals widespread differential DNA methylation associated with birthweight. Nat. Commun., 10, 1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Tekola-Ayele F., Zeng X., Ouidir M., Workalemahu T., Zhang Z., Delahaye F. and Wapner R. (2020) DNA methylation loci in placenta associated with birthweight and expression of genes relevant for early development and adult diseases. Clin. Epigenetics, 12, 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Herzog E.M., Eggink A.J., Willemsen S.P., Slieker R.C., Felix J.F., Stubbs A.P., van der Spek P.J., van Meurs J.B.J., Heijmans B.T. and Steegers-Theunissen R.P.M. (2020) The tissue-specific aspect of genome-wide DNA methylation in newborn and placental tissues: implications for epigenetic epidemiologic studies. J. Dev. Orig. Health Dis., 1–11. [DOI] [PubMed] [Google Scholar]
- 61. Brand J.S., Gaillard R., West J., McEachan R.R.C., Wright J., Voerman E., Felix J.F., Tilling K. and Lawlor D.A. (2019) Associations of maternal quitting, reducing, and continuing smoking during pregnancy with longitudinal fetal growth: findings from Mendelian randomization and parental negative control studies. PLoS Med., 16, e1002972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Workalemahu T., Grantz K.L., Grewal J., Zhang C., Louis G.M.B., Tekola-Ayele F. (2018) Genetic and environmental influences on fetal growth vary during sensitive periods in pregnancy. Sci. Rep., 8, 7274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Vermeulen M.J., Gaillard R., Miliku K., Reiss I., Steegers E.A.P., Jaddoe V., Felix J. (2020) Influence of genetic variants for birth weight on fetal growth and placental haemodynamics. Arch. Dis. Child. Fetal Neonatal Ed., 105, 393–398. [DOI] [PubMed] [Google Scholar]
- 64. Soliman A., De Sanctis V., Elalaily R. and Bedair S. (2014) Advances in pubertal growth and factors influencing it: can we increase pubertal growth? Indian J. Endocrinol. Metab., 18, S53–S62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Bell J.A., Carslake D., Wade K.H., Richmond R.C., Langdon R.J., Vincent E.E., Holmes M.V., Timpson N.J. and Davey Smith J. (2018) Influence of puberty timing on adiposity and cardiometabolic traits: a Mendelian randomisation study. PLoS Med., 15, e1002641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Burgess S., Thompson D.J., Rees J.M.B., Day F.R., Perry J.R. and Ong K.K. (2017) Dissecting causal pathways using Mendelian randomization with summarized genetic data: application to age at menarche and risk of breast cancer. Genetics, 207, 481–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Day F.R., Thompson D.J., Helgason H., Chasman D.I., Finucane H., Sulem P., Ruth K.S., Whalen S., Sarkar A.K., Albrecht E. et al. (2017) Genomic analyses identify hundreds of variants associated with age at menarche and support a role for puberty timing in cancer risk. Nat. Genet., 49, 834–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Chen Y.-C., Fan H.-Y., Huang Y.-T., Huang S.Y., Liou T.H. and Lee Y.L. (2019) Causal relationships between adiposity and childhood asthma: bi-directional Mendelian randomization analysis. Int. J. Obes., 43, 73–81. [DOI] [PubMed] [Google Scholar]
- 69. Geng T., Smith C.E., Li C. and Huang T. (2018) Childhood BMI and adult type 2 diabetes, coronary artery diseases, chronic kidney disease, and cardiometabolic traits: a Mendelian randomization analysis. Diabetes Care, 41, 1089–1096. [DOI] [PubMed] [Google Scholar]
- 70. Tekola-Ayele F., Lee A., Workalemahu T. and Sánchez-Pozos K. (2019) Shared genetic underpinnings of childhood obesity and adult cardiometabolic diseases. Hum. Genomics, 13, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Viitasalo A., Schnurr T.M., Pitkänen N., Hollensted M., Nielsen T.R.H., Pahkala K., Atalay M., Lind M.V., Heikkinen S., Frithioff-Bøjsøe N. et al. (2019) Abdominal adiposity and cardiometabolic risk factors in children and adolescents: a Mendelian randomization analysis. Am. J. Clin. Nutr., 110, 1079–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Richardson T.G., Sanderson E., Elsworth B. et al. (2019) Can the impact of childhood adiposity on disease risk be reversed? A Mendelian randomization study. medRxiv, 19008011. [Google Scholar]
- 73. Chiu M., Austin P.C., Manuel D.G., Shah B.R. and Tu J.V. (2011) Deriving ethnic-specific BMI cutoff points for assessing diabetes risk. Diabetes Care, 34, 1741–1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Yaghootkar H., Whitcher B., Bell J.D. and Thomas E.L. (2020) Ethnic differences in adiposity and diabetes risk – insights from genetic studies. J. Intern. Med. doi: 10.1111/joim.13082. [DOI] [PubMed] [Google Scholar]


