Abstract
Background
Young adolescents with perinatally acquired human immunodeficiency virus (HIV) are at risk for poor care outcomes. We examined whether universal antiretroviral treatment (ART) eligibility policies (Treat All) improved rapid ART initiation after care enrollment among 10–14-year-olds in 7 sub-Saharan African countries.
Methods
Regression discontinuity analysis and data for 6912 patients aged 10–14-years were used to estimate changes in rapid ART initiation (within 30 days of care enrollment) after adoption of Treat All policies in 2 groups of countries: Uganda and Zambia (policy adopted in 2013) and Burundi, Democratic Republic of the Congo, Kenya, Malawi, and Rwanda (policy adopted in 2016).
Results
There were immediate increases in rapid ART initiation among young adolescents after national adoption of Treat All. Increases were greater in countries adopting the policy in 2016 than in those adopting it in 2013: 23.4 percentage points (pp) (95% confidence interval, 13.9–32.8) versus 11.2pp (2.5–19.9). However, the rate of increase in rapid ART initiation among 10–14-year-olds rose appreciably in countries with earlier treatment expansions, from 1.5pp per year before Treat All to 7.7pp per year afterward.
Conclusions
Universal ART eligibility has increased rapid treatment initiation among young adolescents enrolling in HIV care. Further research should assess their retention in care and viral suppression under Treat All.
Keywords: adolescents, Treat All, ART eligibility, ART initiation, sub-Saharan Africa, regression discontinuity
Universal antiretroviral treatment (ART) policies led to increases in rapid ART initiation among young adolescents, aged 10–14 years, after enrollment in human immunodeficiency virus care. Immediate increases in rapid ART initiation were observed after policy adoption in some countries.
(See the Editorial Commentary by Zanoni and Haberer, on pages 705–7.)
In 2018, an estimated 599 000 young adolescents, aged 10–14 years, were living with human immunodeficiency virus (HIV), with close to 90% in sub-Saharan Africa [1, 2]. Although data on this age group are limited [3, 4], young adolescents living with HIV are presumed to have acquired HIV perinatally [5, 6]. Studies suggest that adolescents aged 10–14 years may be less likely than younger children and older age groups to be tested for HIV because of slow-progressing disease, HIV-related stigma, parental concerns about disclosing their own status, and HIV testing strategies, including requirements for caregiver consent, that are not tailored toward adolescents [7–13].
Disproportionately high HIV-related mortality rates have been reported for young adolescents, compared with older adolescents who likely acquire HIV later in life [2]. Young adolescents enrolling in HIV care are often underweight and stunted, and they tend to have lower CD4 cell counts and more advanced disease than both older adolescents [14, 15] and younger children with HIV diagnosed earlier after perinatal infection [16]. Research has shown that adolescents are also at greater risk of failing to start antiretroviral treatment (ART), particularly if they are ineligible for treatment when they enroll in HIV care [17].
The extent to which treatment eligibility guidelines have constrained ART initiation for adolescents is unknown. Before 2015, the World Health Organization (WHO) provided consolidated guidance on HIV treatment for both adolescents, aged 10–19 years, and adults that based ART initiation on CD4 cell count and clinical eligibility criteria [18–21]. Although a few countries in sub-Saharan Africa extended HIV treatment to all adolescents aged <15 years in 2013 [22, 23], adolescents became universally eligible for immediate treatment only with the 2015 WHO recommendation to treat all people living with HIV/AIDS (PLHA), irrespective of immunologic or clinical status [18].
Using longitudinal patient data from 7 countries participating in the International Epidemiology Databases to Evaluate AIDS (IeDEA) research consortium, we assessed changes in rates of rapid ART initiation among 10–14-year-old patients newly enrolling in HIV care after national adoption of Treat All policies for young adolescents or Treat All policies for the general population of PLHA.
METHODS
Data Sources and Management
The IeDEA consortium (www.iedea.org) assembles sociodemographic and clinical data on adult and pediatric patients receiving HIV care across 7 regional cohorts [24]. The data represent diverse clinical sites, the majority of which (87%) are public-sector health facilities, including primary (42%), as well as secondary and tertiary level sites (58%) [25]. In the current analysis, we used medical records from 10–14-year-old patients newly enrolling in HIV care from 2010 to 2018 in 7 sub-Saharan African countries in 3 regional IeDEA cohorts. These countries were selected because patient data were available for analysis after the adoption of universal treatment eligibility policies (Central Africa cohort: Burundi, Democratic Republic of the Congo [DRC], and Rwanda; East Africa cohort: Kenya and Uganda; Southern Africa cohort: Malawi and Zambia). Before data analysis, each region’s data were standardized by regional data managers in accordance with IeDEA data definitions and formatting standards (available at www.iedeades.org).
For each country, we identified the date when ART eligibility was first extended to all patients aged 10–14 years, either as part of a pediatric Treat All policy (for patients aged <15 years), or a general Treat All policy (covering patients of all ages). If a country first adopted a pediatric Treat All policy and subsequently adopted a general Treat All policy, we recorded both dates. Tymejczyk et al [26] have previously described our systematic search for current and historical ART eligibility guidelines based on publicly available policy documents, published literature, and input from in-country experts. If the exact date of expansion was unknown, expansion was assumed to have occurred on the first day of the month in which the policy was adopted. Data were deidentified before sharing and approved for use by local research ethics committees in each of the IeDEA regions included in the study.
Inclusion Criteria
Patients
Patients had to be 10–14 years of age at the time of enrollment into HIV care, with ≥30 days of possible follow-up between enrollment and database closure. Patients were excluded if they were known to have transferred to an IeDEA site from another clinic or were known to be ART experienced at enrollment.
Sites
Sites had to have patient data available for the period between care enrollment and ART initiation (ie, pre-ART data) for both ART initiators and noninitiators (ie, those dying or dropping out of care before starting treatment). Sites with data only from the period after ART initiation were excluded.
Outcome and Exposure
The outcome of interest was “rapid” ART initiation, defined as initiation of treatment within 30 days of enrollment in HIV care, which is consistent with our group’s prior analyses in adults [27]. This definition differs from the 2017 WHO definition of rapid ART initiation, which is ART initiation occurring within 7 days of HIV diagnosis [28]. The exposure was period of enrollment in HIV care, as defined by the relationship to the calendar date of country-level ART eligibility expansion to Treat All.
Other Definitions
ART was defined as treatment with any regimen of ≥3 antiretroviral drugs, excluding antiretrovirals taken solely for the prevention of mother-to-child transmission. As a measure of HIV disease severity, pretreatment CD4 cell count was defined as the count closest to the enrollment date within a 90-day window (before or after), but no later than 1 week after ART initiation.
Study Design
Patient characteristics, including sex, age, availability of pretreatment CD4 test results, and median CD4 cell count, were described for each country where a pediatric or general Treat All policy extended ART eligibility to all children aged 10–14 years, and characteristics were aggregated by time period of policy change. The proportion of patients starting ART rapidly in the year before and after Treat All adoption was calculated for each country and for the Central Africa region (ie, Burundi, DRC, and Rwanda) because of small sample sizes available for the individual countries.
Effect of ART Eligibility Expansion to Treat All on Rapid ART Initiation
The effect of enrollment in HIV care under Treat All on the proportion of young adolescents initiating ART rapidly was assessed using a regression discontinuity design. This approach takes advantage of local randomness in a continuous eligibility assignment variable (calendar time of HIV care enrollment), relative to a cutoff threshold (date of country-level adoption of pediatric or general Treat All). In this quasi-experimental condition, as long as there is no evidence that values of the assignment variable are being manipulated, patients enrolling in care directly before and after the cutoff date are considered exchangeable. Accordingly, there should be no systematic differences in measured and unmeasured characteristics between the groups, other than the higher probability of treatment eligibility among those enrolling after Treat All policy adoption. If these assumptions are met, observed effects can be interpreted causally, as intention-to-treat estimates [29, 30].
To assess whether there were systematic differences between patients enrolling in HIV care on either side of the threshold, as well as nonrandom enrollment before or after the Treat All adoption date, we used covariate balance tests and plots of the date of enrollment in HIV care. Because complete information about each patient’s true ART eligibility status at enrollment before Treat All adoption was not known (owing to missing information on HIV stage, comorbid conditions, pregnancy, and/or special population status), the study is an intention-to-treat analysis using a “sharp” regression discontinuity design [29, 30].
We examined the association between calendar time of enrollment in HIV care and rapid ART initiation for 2 groups of countries: those where a pediatric Treat All policy extended ART eligibility to all children 10–14 years old in 2013, and those where a general Treat All policy extended ART eligibility to this age group along with adults in 2016. A discontinuity at the date of each country’s Treat All policy adoption allowed for different slopes before and after the cutoff, or threshold, date. Local linear regression models [31] were used to estimate predicted outcomes and risk differences at the Treat All threshold date, as follows:
where Yi is the patient-level outcome (rapid ART initiation), Zi is the number of days between a patient’s enrollment date and national Treat All policy adoption date (negative if patient enrolled before the policy was adopted), and 1[Zi ≥ 0)] indicates whether a patient enrolled after the policy was adopted or not.
Data-driven Imbens-Kalyanaraman bandwidths [32] were used to define windows of time around the date of Treat All adoption within which predicted outcomes and risk differences at the Treat All threshold date were estimated. All observations within the bandwidth were weighted equally. Sensitivity analyses were completed using 3 other bandwidth sizes, ranging from 150 to 450 days.
In the countries with a general Treat All expansion to all ages after a pediatric Treat All policy, an additional regression discontinuity analysis for the general Treat All adoption was completed to assess whether further expansions of eligibility criteria to encompass older age groups affected rapid ART initiation among already-eligible 10–14-year-olds. Such effects could be positive (increase in rapid ART initiation because of, for example, stigma reduction) or negative (decrease in rapid ART initiation because of, for example, facility capacity constraints).
Trends in Rapid ART Initiation Before and After Treat All Adoption
To characterize trends in rapid ART initiation after enrollment into HIV care, slopes from linear regression models for the period before and after the date of Treat All adoption were compared, and expressed as percentage point (pp) change in rapid ART initiation per year (ie, average annual rate of increase). Analyses were completed using SAS 9.4 and Stata/MP software, version 15.1.
RESULTS
Sample Characteristics
Longitudinal data were available for 7296 patients aged 10–14 years who enrolled in HIV care between 2010 and 2018, including 7239 (99.2%) with ≥30 days of possible follow-up. Of these patients, 6912 (95.5%) had no evidence of transfer from another site or ART before enrollment.
Among the 7 countries in the analysis, 5 (Burundi, DRC, Kenya, Malawi, Rwanda) adopted general Treat All policies that extended treatment eligibility to young adolescents aged 10–14 years in 2016. Two countries (Uganda, Zambia) adopted pediatric Treat All policies in 2013, which extended treatment to all children <15 years old, and adopted general Treat All policies in 2016 (Supplementary Table 1).
Among the 6912 patients who met study inclusion criteria, 3592 (52.0%) were in countries where pediatric Treat All was adopted in 2013, and 3320 (48.0%) in countries where general Treat All was adopted in 2016. The median age at enrollment in HIV care was 12 years (interquartile range, 11–13 years), with no significant age differences before and after Treat All adoption (Supplementary Table 2); 58.3% of patients were female. The availability of pretreatment CD4 test results varied across countries and before vs. after Treat All policy adoption. Few patients had pretreatment CD4 test results after the adoption of Treat All policies, particularly in countries that introduced the policy in 2016 (20.1% overall, and only 3% among patients from Malawi). Among patients with a pretreatment CD4 test result, the median cell count before Treat All adoption was 315/µL (interquartile range, 124–551/µL) in countries adopting general Treat All policies in 2016 and 363/µL (193–589/µL) in countries that adopted a pediatric Treat All policy in 2013 (Table 1).
Table 1.
Characteristics of Adolescents Aged 10–14 Years of Age Enrolling in Human Immunodeficiency Virus Care (n = 6912), 2010–2018
| Characteristic | Adolescents, No. (%)a | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| General Treat All Adopted in 2016 | Pediatric Treat All Adopted in 2013 | ||||||||
| Burundi | DRC | Kenya | Malawi | Rwanda | Overall | Uganda | Zambia | Overall | |
| Treat All adoption date | September 2016 | September 2016 | July 2016 | May 2016 | July 2016 | 2016 | December 2013 | December 2013 | 2013 |
| Total enrollments | 178 (2.6) | 123 (1.8) | 1752 (25.3) | 1168 (16.9) | 99 (1.4) | 3320 (48.0) | 461 (6.7) | 3131 (45.3) | 3592 (52.0) |
| Period of enrollment | |||||||||
| Before Treat All adoption | 154 (86.5) | 110 (89.4) | 1548 (88.4) | 1067 (91.4) | 82 (82.8) | 2961 (89.2) | 257 (55.7) | 1635 (52.2) | 1892 (52.7) |
| After Treat All adoption | 24 (13.5) | 13 (10.6) | 204 (11.6) | 101 (8.6) | 17 (17.2) | 359 (10.8) | 204 (44.3) | 1496 (47.8) | 1700 (47.3) |
| Sex | |||||||||
| Male | 73 (41.0) | 67 (54.5) | 702 (40.1) | 511 (43.8) | 50 (50.5) | 1403 (42.3) | 186 (40.3) | 1294 (41.3) | 1480 (41.2) |
| Female | 105 (59.0) | 56 (45.5) | 1050 (59.9) | 657 (56.3) | 49 (49.5) | 1917 (57.7) | 275 (59.7) | 1837 (58.7) | 2112 (58.8) |
| Age, median (IQR), y | 12 (11–13) | 12 (11–13) | 12 (11–13) | 12 (11–13) | 12 (11–13) | 12 (11–13) | 12 (11–13) | 12 (11–13) | 12 (11–13) |
| Pretreatment CD4 cell count | |||||||||
| Before Treat All adoption | |||||||||
| Count available | 42 (27.3) | 79 (71.8) | 1073 (69.3) | 235 (22.0) | 66 (80.5) | 1495 (50.5) | 167 (65.0) | 1090 (66.7) | 1257 (66.4) |
| Count, median (IQR), cells/µL | 417 (220–762) | 297 (93–499) | 315 (120–574) | 308 (145–456) | 400 (176–675) | 315 (124–551) | 377 (197–671) | 359 (193–579) | 363 (193–589) |
| After Treat All adoption | |||||||||
| Count available | 5 (20.8) | 1 (7.7) | 53 (26.0) | 3 (3.0) | 10 (58.8) | 72 (20.1) | 106 (52.0) | 704 (47.1) | 1700 (47.7) |
| Count, median (IQR), cells/µL | NA | NA | 397 (233–558) | NA | NA | NA | 408 (194–579) | 343 (173–545) | 347 (173–554) |
Abbreviations: DRC, Democratic Republic of the Congo; IQR, interquartile range; NA, not available.
aData represent no. (%) of adolescent unless otherwise specified.
Distributions of baseline characteristics among newly enrolling patients were similar just before and just after Treat All adoption (Supplementary Table 2). No major discontinuity was observed in the number of new enrollments around the date of Treat All adoption (Supplementary Figure 1).
Rapid ART Initiation Before and After Treat All Adoption (Descriptive Analysis)
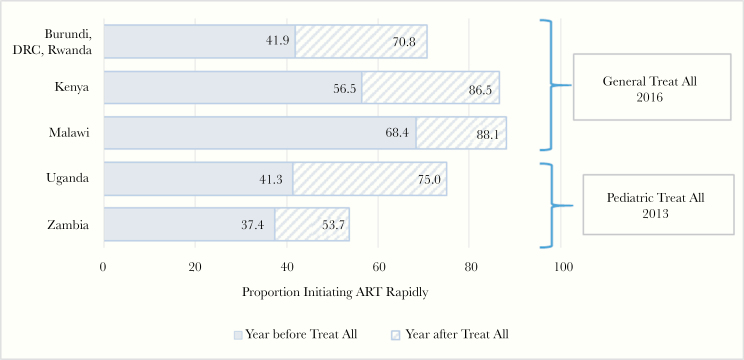
There were appreciable increases in rapid ART initiation among young adolescents in the year after Treat All adoption in all 7 countries. Increases ranged from 16.3pp in Zambia (from 37.4% in the year before to 53.7% in the year after) to 33.7pp in Uganda (from 41.3% to 75.0%, respectively). The proportion of young adolescents rapidly initiating ART in the year after Treat All adoption was highest in Malawi and Kenya (88.1% and 86.5%, respectively), both of which adopted a general Treat All policy in 2016. (Figure 1).
Figure 1.
Proportions of adolescents 10–14 years old initiating antiretroviral treatment (ART) rapidly (within 30 days of enrollment in human immunodeficiency virus care) in the years before and after Treat All adoption, by country or region.
Effect of Treat All Adoption on Rapid ART Initiation (Regression Discontinuity Analysis)
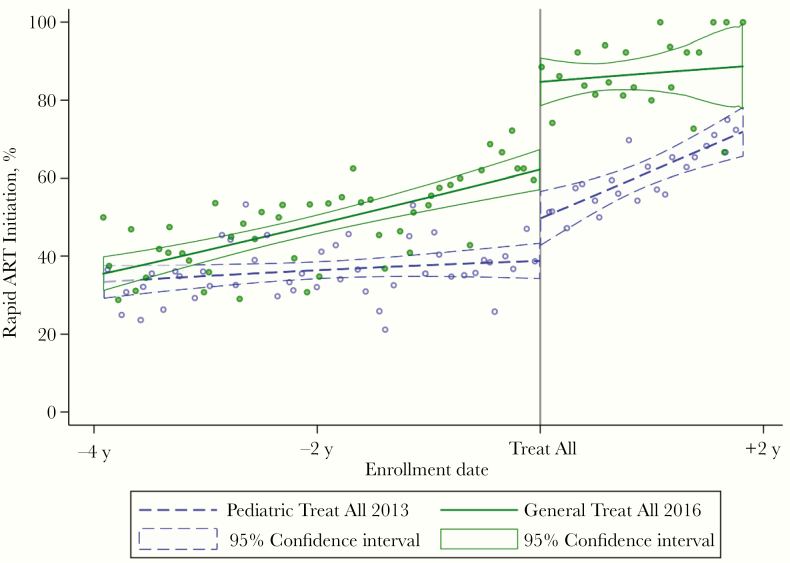
Statistically significant increases in rapid ART initiation among young adolescents were observed immediately after national Treat All policies expanded treatment eligibility for this age group. A larger absolute effect was observed in the group of countries that adopted a general Treat All policy in 2016 (Burundi, DRC, Kenya, Malawi, and Rwanda): 23.4pp (95% confidence interval, 13.9–32.8), compared with the countries with a 2013 pediatric Treat All policy (Uganda and Zambia), where there was an 11.2pp increase in rapid ART initiation (2.5–19.9). In the 2016 Treat All group, 85.4% of young adolescents enrolling immediately after Treat All adoption initiated ART rapidly (up from 62.0% immediately before), compared with, 50.2% in the 2013 group (up from 39.0% immediately before) (Table 2 and Figure 2).
Table 2.
Effect of Antiretroviral Treatment (ART) Eligibility Expansion to Treat All Adolescents 10–14 Years Old and Trends in Rapid ART Initiation Before and After Treat All Adoption
| Outcomes | Countries by Expansion Date and Type | ||
|---|---|---|---|
| Burundi, DRC, Kenya, Malawi, and Rwanda | Uganda and Zambia | ||
| 2016: General Treat All | 2013: Pediatric Treat All | 2016: General Treat All | |
| Risk difference at Treat All adoption thresholda | 23.4 | 11.2 | −1.1 |
| 95% CI | 13.9–32.8 | 2.5–19.9 | −13.9 to 11.7 |
| P value | <.001 | .01 | .86 |
| Imbens-Kalyanaraman bandwidth, d | 681 | 780 | 252 |
| No. within bandwidth | 970 | 1937 | 665 |
| Predicted outcome at Treat All threshold, %a | |||
| Enrollment just before Treat All adoption | 62.0 | 39.0 | 77.7 |
| Enrollment just after Treat All adoption | 85.4 | 50.2 | 76.6 |
| Relative change after Treat All adoption | 37.7 | 28.7 | −1.4 |
| Slopes before and after Treat All adoption, rate of change in rapid ART initiation, pp/yb | |||
| Before Treat All adoption | 4.1 | 1.5 | 5.8 |
| After Treat All adoption | 2.0 | 7.7 | 4.4 |
| P value for difference in slopes | .69 | <.001 | .93 |
Abbreviations: ART, antiretroviral treatment; CI, confidence interval; DRC, Democratic Republic of the Congo.
aRisk difference and predicted outcomes at the Treat All threshold are from regression discontinuity analyses. Effects are calculated at the guideline expansion threshold of 1 day before versus 1 day after Treat All adoption.
bSlope comparison is from separate linear regression models comparing the periods before and after Treat All adoption.
Figure 2.
Trends in rapid antiretroviral treatment (ART) initiation before and after Treat All adoption among adolescents 10–14 years old, by year of adoption.
There was no statistically significant change in rapid ART initiation among young adolescents immediately after general Treat All policies were adopted in Uganda and Zambia in 2016 (Table 2). Pediatric Treat All policies were already in place in these 2 countries, and the proportion of young adolescents initiating ART rapidly was 77.7% immediately before the general Treat All policy, versus 76.6% immediately afterward. Results of sensitivity analyses using other bandwidths were consistent with the findings based on the data-driven Imbens-Kalyanaraman bandwidth (Supplementary Table 3).
Trends in Rapid ART Initiation Before and After Treat All Adoption (Slope Comparison)
The average annual rate of increase in rapid ART initiation among young adolescents rose in countries where a pediatric Treat All policy was adopted in 2013, from 1.5pp per year before Treat All adoption to 7.7pp afterward (Table 2). However, no statistically significant change in the annual rate of increase in rapid ART initiation was observed in countries that adopted a general Treat All policy in 2016. In addition, no rate change was observed in Uganda and Zambia after the expansion of pediatric Treat All policies to include all PLHA in 2016.
DISCUSSION
Whether part of a pediatric Treat All policy or a general Treat All policy, expansions of HIV treatment eligibility to those <15 years old were followed by significant and substantial increases in ART initiation among 10–14-year-olds within 30 days of enrollment in HIV care. Increases in rapid ART initiation were particularly large after national adoptions of a general Treat All policy (ie, for all ages) in 2016 in Burundi, DRC, Kenya, Malawi, and Rwanda. Observed increases in the proportion of young adolescents rapidly initiating ART after national adoption of Treat All policies may have substantial clinical importance, given evidence indicating that young adolescents with perinatally acquired HIV often enroll in care late and do not start ART until they are at advanced stages of disease [14, 15, 33–35].
Pretreatment CD4 test restuls were not available for a large proportion of patients, making it difficult to ascertain clinical eligibility for treatment among those enrolling before Treat All policies were adopted. However, among the 56.7% of young adolescents with pretreatment CD4 cell count data available before Treat All adoption, median counts for each country in our study were well below 500/µL, the previous immunologic threshold for treatment eligibility. This suggests that there were gaps in rapid ART initiation for young adolescents when CD4 cell count–based eligibility criteria were in effect. These findings are in accordance with previous research highlighting a range of social and structural barriers to HIV care and treatment for adolescents, whose needs may not be adequately met by service delivery strategies designed for younger children or adult HIV patients [6, 7, 36, 37].
The absolute effect of treatment eligibility expansions for young adolescents was greater under general Treat All policies than under earlier pediatric Treat All policies. This was noteworthy, given that large numbers of adult patients, newly eligible under Treat All, could potentially strain HIV service provision, leading to the crowding out of vulnerable groups, such as young adolescents, for whom tailored interventions and services are recommended [11, 15, 38–40]. These results lend support for the supposition that general Treat All policies, with harmonized treatment recommendations for different population groups, are easier to implement in real-world treatment settings than prior policies targeting specific groups. Age-agnostic guidelines may help simplify the provision of HIV treatment in low-resourced health systems in ways that lead to efficiency gains in service delivery [41, 42].
Given the 3-year interval between the pediatric Treat All expansions of 2013 and the general Treat All expansions of 2016, findings may also reflect temporal trends in provider preparedness, health system capacities to rapidly implement expanded treatment guidelines, and improved strategies for initiating ART, despite decreases in donor funding for HIV during the period [43, 44].
Although immediate increases in rapid ART initiation among 10–14-year-olds were smaller after national adoptions of pediatric Treat All policies, the average annual rate of change in rapid ART initiation increased significantly after the adoption of these policies in 2013. Consistent with the smaller immediate effect observed at the Treat All adoption threshold, this may reflect a gradual roll-out or delayed implementation of the policy. In contrast, the lack of a statistically significant change in annual rates of rapid ART initiation after general Treat All policy adoptions in 2016 may be due to the high rates of rapid ART initiation achieved immediately after the policy (85.4%), with limited space for further increases above this level, as well as regression to the mean.
A concerning finding was the large decrease in pretreatment CD4 testing, as reflected by an increased proportion of young adolescents with no CD4 test results before treatment initiation. This finding is not unique to our study population [26, 45], and it reflects a larger trend of abandoning the use of CD4 cell count monitoring altogether in sub-Saharan Africa, driven by combinations of cost and laboratory supply chain issues, as well as prioritization of viral load over CD4 testing [46, 47]. Treat All policies have negated the need for pretreatment CD4 testing to assess eligibility for treatment, and routine CD4 monitoring after ART initiation is generally not necessary in virally suppressed patients in settings with routine viral load testing [46]. However, pretreatment CD4 testing remains important for identifying severely immunodeficient individuals who need enhanced clinical services, such as treatment of opportunistic infections [46] and for monitoring progress toward achieving the public health goals of HIV care and treatment scale-up [48].
A strength of this analysis is the use of a regression discontinuity design with real-world service delivery data from diverse settings in 7 sub-Saharan African countries that adopted Treat All policies at 2 points in time. This quasi-experimental design provides support for the causal interpretation of the association between expanded ART eligibility under Treat All and increases in rapid ART uptake among young adolescents newly enrolling into HIV care. The use of a data-driven Imbens-Kalyanaraman bandwidth [32] and sensitivity analyses with 3 other bandwidths enabled us to generate robust effect estimates with minimal risk of researcher bias.
A limitation of our study is lack of complete data on treatment eligibility criteria for patients enrolling before national Treat All policy adoption (eg, pretreatment CD4 cell counts, WHO staging, and coinfection with tuberculosis). Such data would have allowed us to adjust for differences in ART eligibility in the pre–Treat All sample.
In addition, the limited availability of data on patient characteristics beyond age, sex, and pretreatment CD4 cell count restricted our ability to assess whether patients on each side of the regression discontinuity threshold were similar with respect to other pretreatment covariates. We also lacked data on service delivery strategies, including tailored services for adolescents, and supply side constraints, such as drug stockouts, that may influence rapid ART initiation among young adolescents enrolling into care. Moreover, though we know the dates when Treat All policies were adopted in each country, lags in site-level implementation likely varied across sites and countries included in this analysis [49]. Finally, the use of a 30-day rapid ART initiation window, intended to enable comparisons with this group’s prior work [27], limits the comparability of findings to WHO’s rapid ART initiation estimates defined by a 7-day window after confirmation of HIV diagnosis.
These limitations notwithstanding, our results suggest that expanded treatment eligibility under Treat All has benefited 10–14-year-olds by getting them treated more rapidly after enrollment in care. Because there are few age-disaggregated data related to the HIV care continuum for this age group, the current study fills an important gap, indicating that an increasing share of young adolescents may be initiating ART rapidly under Treat All policies. Although these results are encouraging, further research is needed on effective strategies for enrolling children with perinatally acquired HIV in HIV care earlier and improving care retention and ART adherence among adolescent patients to support sustained viral suppression among this vulnerable population.
Supplementary Material
Notes
Acknowledgments. We thank patients, providers, and administrative staff at participating International Epidemiology Databases to Evaluate AIDS (IeDEA) facilities. We also are grateful for information on antiretroviral treatment eligibility expansions contributed by Edith Apondi, Lastone Chitembo, Nathan Ford, John Humphrey, Olivia Keiser, Yee Yee Kuhn, Malango Msukwa, Martina Penazzato, and Ellon Twinomuhwezi.
Disclaimer. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health (grants U01AI096299 to IeDEA Central Africa and U01AI069924 to IeDEA Southern Africa), the Eunice Kennedy Shriver National Institute of Child Health and Human Development, the National Institute on Drug Abuse, the National Cancer Institute, and the National Institute of Mental Health of the National Institutes of Health (grant U01AI069911 to IeDEA East Africa).
Potential conflicts of interest. A. H. S. reports grants from ViiV Healthcare outside of submitted work. All other authors report no potential conflicts.
References
- 1. United Nations Children’s Fund (UNICEF). Children, HIV and AIDS: The world in 2030. New York, NY: UNICEF 2018. [Google Scholar]
- 2. Slogrove AL, Sohn AH. The global epidemiology of adolescents living with HIV: time for more granular data to improve adolescent health outcomes. Curr Opin HIV AIDS 2018; 13:170–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Slogrove AL, Mahy M, Armstrong A, Davies M-A. Living and dying to be counted: what we know about the epidemiology of the global adolescent HIV epidemic. J Int AIDS Soc 2017; 20:4–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Idele P, Gillespie A, Porth T, et al. . Epidemiology of HIV and AIDS among adolescents: current status, inequities, and data gaps. J Acquir Immune Defic Syndr 2014; 66(suppl 2):S144–53. [DOI] [PubMed] [Google Scholar]
- 5. Ng’eno BN, Kellogg TA, Kim AA, et al. . Modes of HIV transmission among adolescents and young adults aged 10-24 years in Kenya. Int J STD AIDS 2018; 29:800–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lowenthal ED, Bakeera-Kitaka S, Marukutira T, Chapman J, Goldrath K, Ferrand RA. Perinatally acquired HIV infection in adolescents from sub-Saharan Africa: a review of emerging challenges. Lancet Infect Dis 2014; 14:627–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Enane LA, Davies MA, Leroy V, et al. . Traversing the cascade: urgent research priorities for implementing the ‘treat all’ strategy for children and adolescents living with HIV in sub-Saharan Africa. J Virus Erad 2018; 4:40–6. [PMC free article] [PubMed] [Google Scholar]
- 8. Davies MA, Kalk E. Provider-initiated HIV testing and counselling for children. PLoS Med 2014; 11:e1001650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chikwari CD, Dringus S, Ferrand RA. Barriers to, and emerging strategies for, HIV testing among adolescents in sub-Saharan Africa. Curr Opin HIV AIDS 2018; 13:257–64. [DOI] [PubMed] [Google Scholar]
- 10. Govindasamy D, Ferrand RA, Wilmore SM, et al. . Uptake and yield of HIV testing and counselling among children and adolescents in sub-Saharan Africa: a systematic review. J Int AIDS Soc 2015; 18:20182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lightfoot M, Dunbar M, Weiser SD. Reducing undiagnosed HIV infection among adolescents in sub-Saharan Africa: provider-initiated and opt-out testing are not enough. PLoS Med 2017; 14:e1002361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zanoni BC, Elliott RJ, Neilan AM, Haberer JE. Screening for HIV and linkage to care in adolescents: insights from a systematic review of recent interventions in high- versus low- and middle-income settings. Adolesc Health Med Ther 2018; 9:211–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wong VJ, Murray KR, Phelps BR, Vermund SH, McCarraher DR. Adolescents, young people, and the 90-90-90 goals: a call to improve HIV testing and linkage to treatment. AIDS 2017; 31(suppl 3):191–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Koech E, Teasdale CA, Wang C, et al. . Characteristics and outcomes of HIV-infected youth and young adolescents enrolled in HIV care in Kenya. AIDS 2014; 28:2729–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Apondi E, Humphrey JM, Sang E, et al. . Trends over time for adolescents enrolling in HIV care in Kenya, Tanzania, and Uganda from 2001–2014. J Acquir Immune Defic Syndr 2018; 79:164–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Westerlund E, Jerene D, Mulissa Z, Hallström I, Lindtjørn B. Pre-ART retention in care and prevalence of tuberculosis among HIV-infected children at a district hospital in southern Ethiopia. BMC Pediatr 2014; 14:250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Desmonde S, Tanser F, Vreeman R, et al. . Access to antiretroviral therapy in HIV-infected children aged 0–19 years in the International Epidemiology Databases to Evaluate AIDS (IeDEA) Global Cohort Consortium, 2004–2015: a prospective cohort study. PLoS Med 2018; 15:e1002565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. World Health Organization. Guideline on when to start antiretroviral therapy and on pre-exposure prophylaxis for HIV. Geneva, Switzerland: World Health Organization, 2015. [PubMed] [Google Scholar]
- 19. World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. 2nd ed. Geneva, Switzerland: World Health Organization, 2016. [PubMed] [Google Scholar]
- 20. World Health Organization. Antiretroviral therapy for HIV infection in adults and adolescents: recommendations for a public health approach. (2010 revision). Geneva, Switzerland: World Health Organization, 2010. [PubMed] [Google Scholar]
- 21. World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. Geneva, Switzerland: World Health Organization, 2013. [PubMed] [Google Scholar]
- 22. Ministry of Health (Republic of Uganda). Addendum to the antiretroviral treatment guidelines for Uganda. Kampala, Uganda: Ministry of Health, 2013. [Google Scholar]
- 23. Ministry of Health (Government of the Republic of Zambia). Zambia consolidated guidelines for the prevention and treatment of HIV infection. Lusaka, Zambia: Ministry of Health, 2013. [Google Scholar]
- 24. IeDEA. International Epidemiologic Databases to Evaluate AIDS https://www.iedea.org/ Accessed 1 June 2018.
- 25. Fritz CQ, Blevins M, Lindegren ML, et al. . Comprehensiveness of HIV care provided at global HIV treatment sites in the IeDEA consortium: 2009 and 2014. J Int AIDS Soc 2017; 20:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tymejczyk O, Brazier E, Yiannoutsos C, et al. ; IeDEA Collaboration HIV treatment eligibility expansion and timely antiretroviral treatment initiation following enrollment in HIV care: A metaregression analysis of programmatic data from 22 countries. PLoS Med 2018; 15:e1002534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tymejczyk O, Brazier E, Yiannoutsos CT, et al. ; IeDEA consortium Changes in rapid HIV treatment initiation after national “treat all” policy adoption in 6 sub-Saharan African countries: regression discontinuity analysis. PLoS Med 2019; 16:e1002822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. World Health Organization. Guidelines for managing advanced HIV disease and rapid initiation of antiretroviral therapy, July 2017. Geneva, Switzerland: World Health Organization, 2017. [PubMed] [Google Scholar]
- 29. Moscoe E, Bor J, Bärnighausen T. Regression discontinuity designs are underutilized in medicine, epidemiology, and public health: a review of current and best practice. J Clin Epidemiol 2015; 68:122–33. [DOI] [PubMed] [Google Scholar]
- 30. Bor J, Fox MP, Rosen S, et al. . Treatment eligibility and retention in clinical HIV care: A regression discontinuity study in South Africa. PLoS Med 2017; 14:e1002463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hahn J, Todd P, Van der Klaauw W. Identification and estimation of treatment effects with a regression‐discontinuity design. Econometrica 2001; 69:201–9. [Google Scholar]
- 32. Imbens G, Kalyanaraman K. Optimal bandwidth choice for the regression discontinuity estimator. Rev Econ Stud 2012; 79:933–59. [Google Scholar]
- 33. Kariminia A, Law M, Davies MA, et al. ; IeDEA Mortality and losses to follow-up among adolescents living with HIV in the IeDEA global cohort collaboration. J Int AIDS Soc 2018; 21:e25215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Koller M, Patel K, Chi BH, et al. . Immunodeficiency in children starting antiretroviral therapy in low-, middle-, and high-income countries. J Acquir Immune Defic Syndr 2015; 68:62–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Slogrove AL, Schomaker M, et al. . The Collaborative Initiative for Paediatric HIV Education Research (CIPHER) The epidemiology of adolescents living with perinatally acquired HIV: a cross-region global cohort analysis. PLoS Med 2018; 15:e1002514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. World Health Organization. HIV and adolescents: guidance for HIV testing and counselling and care for adolescents living with HIV: recommendations for a public health approach and considerations for policy-makers and managers. Geneva, Switzerland: World Health Organization, 2013. [PubMed] [Google Scholar]
- 37. Mark D, Armstrong A, Andrade C, et al. . HIV treatment and care services for adolescents: a situational analysis of 218 facilities in 23 sub-Saharan African countries. J Int AIDS Soc 2017; 20:21591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kose J, Tiam A, Ochuka B, et al. . Impact of a comprehensive adolescent-focused case finding intervention on uptake of HIV testing and linkage to care among adolescents in Western Kenya. J Acquir Immune Defic Syndr 2018; 79:367–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Simms V, Dauya E, Dakshina S, et al. . Community burden of undiagnosed HIV infection among adolescents in Zimbabwe following primary healthcare-based provider-initiated HIV testing and counselling: a cross-sectional survey. PLoS Med 2017; 14:e1002360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. MacPherson P, Munthali C, Ferguson J, et al. . Service delivery interventions to improve adolescents’ linkage, retention and adherence to antiretroviral therapy and HIV care. Trop Med Int Health 2015; 20:1015–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hontelez JA, Chang AY, Ogbuoji O, de Vlas SJ, Bärnighausen T, Atun R. Changing HIV treatment eligibility under health system constraints in sub-Saharan Africa: investment needs, population health gains, and cost-effectiveness. AIDS 2016; 30:2341–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ford N, Penazzato M, Vitoria M, et al. . The contribution of observational studies in supporting the WHO ‘treat all’ recommendation for HIV/AIDS. J Virus Erad 2018; 4:5–8. [PMC free article] [PubMed] [Google Scholar]
- 43. Kates J, Wexler A, Leif E.. Donor government funding for HIV in low- and middle-income countries in 2016. Menlo Park, CA: Kaiser Family Foundation and UNAIDS, 2017. [Google Scholar]
- 44. Kates J, Wexler A, Lief E.. Financing the response to HIV in low- and middle-income countries: international assistance from donor governments in 2015. Menlo Park, CA: Kaiser Family Foundation and UNAIDS, 2016. [Google Scholar]
- 45. The IeDEA and COHERE Cohort Collaborations. Global trends in CD4 cell count at the start of antiretroviral therapy: collaborative study of treatment programs. Clin Infect Dis 2018; 66:893–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ford N, Meintjes G, Vitoria M, Greene G, Chiller T. The evolving role of CD4 cell counts in HIV care. Curr Opin HIV AIDS 2017; 12:123–8. [DOI] [PubMed] [Google Scholar]
- 47. Tenforde MW, Walker AS, Gibb DM, Manabe YC. Rapid antiretroviral therapy initiation in low- and middle-income countries: a resource-based approach. PLoS Med 2019; 16:e1002723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Nash D, Robertson M. How to evolve the response to the global HIV epidemic with new metrics and targets based on pre-treatment CD4 counts. Curr HIV/AIDS Rep 2019; 16:304–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Brazier E, Maruri F, Duda SN, et al. ; IeDEA Consortium Implementation of “Treat-all” at adult HIV care and treatment sites in the Global IeDEA Consortium: results from the Site Assessment Survey. J Int AIDS Soc 2019; 22:e25331. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.