Graphical abstract
Keywords: Respiratory viruses, SARS-CoV-2, Diagnosis, Epidemiology, Pandemic, Molecular assays, Culture
Highlights
-
•
In this study, the viral aetiology was explored in patients with respiratory infections.
-
•
In this study, adult males were the most infected by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
-
•
In this study, children were the most infected by non-SARS-CoV-2 respiratory viruses.
Abstract
Objectives
The aim of this study was to determine the prevalence of respiratory virus infections, including severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), during the winter period December 2019 to March 2020, via a tertiary care hospital-based survey in Parma, Northern Italy.
Methods
A total of 906 biological samples from the respiratory tract were analysed by both conventional assays (including culture) and molecular assays targeting nucleic acids of SARS-CoV-2 and other respiratory viruses.
Results
Overall, 474 samples (52.3%) were positive for at least one virus, with a total of 583 viruses detected. Single infections were detected in 380 (80.2%) samples and mixed infections were detected in 94 (19.8%). Respiratory syncytial virus (138/583, 23.7%) and rhinovirus (130/583, 22.3%) were the most commonly identified viruses, followed by SARS-CoV-2 (82/583, 14.1%). Respiratory syncytial virus predominated until February, with 129 detections; it then decreased drastically in March to only nine detections. SARS-CoV-2 was absent in the study area until February 26, 2020 and then reached 82 detections in just over a month. SARS-CoV-2 was found in mixed infections in only three cases, all observed in children younger than 1 year old.
Conclusions
This study showed a completely different trend between SARS-CoV-2 and the ‘common’ respiratory viruses: the common viruses mostly affected children, without any distinction according to sex, while SARS-CoV-2 mostly affected adult males.
Introduction
Viral infections of the upper and lower respiratory tracts are among the most common illnesses in humans. They occur mainly in children and infants, who can experience up to five to six episodes in any given year (Berry et al., 2015). For this reason, acute respiratory infections (ARIs) represent a persistent public health problem. Although the majority of ARIs remain confined to the upper respiratory tract (rhinosinusitis, pharyngitis, laryngitis, and tracheitis), they can cause severe manifestations when they affect the lower respiratory tract (bronchitis, bronchiolitis, and pneumonia) (Bicer et al., 2013, Tregoning and Schwarze, 2010, Zappa et al., 2008).
Although influenza virus (FLU) causes considerable morbidity and mortality worldwide despite the availability of FLU vaccines and antiviral agents (Jeon et al., 2019), this is not the only virus responsible for ARIs. As matter of fact, the viruses primarily associated with ARIs are rhinoviruses (RV), enteroviruses (EV), adenoviruses (ADV), parainfluenza viruses (PIV), influenza viruses (FLU), respiratory syncytial viruses (RSV), and coronaviruses (CoV) (Berry et al., 2015, De Conto et al., 2019). Over the last few years, new emerging viruses such as human metapneumovirus (MPV), bocavirus (BoV), and four new human coronaviruses have been reported to be involved in ARIs (Berry et al., 2015, De Conto et al., 2019); the new coronaviruses include severe acute respiratory syndrome coronavirus (SARS-CoV), Middle East respiratory syndrome coronavirus (MERS-CoV), human coronavirus NL63 (CoV-NL63), and human coronavirus HKU1 (CoV-HKU1).
In December 2019, the existence of patients with pneumonia of an unknown aetiology was reported to the World Health Organization (WHO) by the national authorities in China. The causative agent was officially identified as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and the coronavirus-associated acute respiratory disease was labelled coronavirus disease 2019 (COVID-19) (Chen and Yu, 2020, Liu et al., 2020, Hasöksüz et al., 2020).
At the time of writing the WHO had reported 14 765 256 cases of SARS-CoV-2 infection globally and 612 057 deaths attributable to this virus (World Health Organization, 2020). In particular, 244 752 cases with 35 073 deaths had been reported in Italy (World Health Organization, 2020).
The aim of this study was to determine the prevalence of respiratory virus infections, including the emerging SARS-CoV-2, over a 4-month period (December 2019 to March 2020) during the pandemic, via a tertiary care hospital-based survey in Parma, Northern Italy.
Materials and methods
Study setting
The study was conducted in the Virology Unit of the Department of Medicine and Surgery, University of Parma – University Hospital of Parma in Northern Italy. This is a 1044-bed tertiary care centre with more than 46 000 admissions per year from the city and surrounding area, covering approximately 450 000 inhabitants (Azienda Ospedaliero-Universitaria di Parma, 2020, Provincia di Parma, 2020). Laboratory diagnosis was performed upon medical request. Patient identities and medical information were protected.
During the period considered, all patients with respiratory symptoms were included, without any exclusion. From December 1, 2019 to March 31, 2020, 906 samples were collected from 574 children (63.4%) and 332 adults (36.6%), of whom 374 were female (41.3%) and 532 were male (58.7%). Samples collected included bronchoalveolar lavage (BAL), bronchial aspirate (BAS), nasopharyngeal aspirate (NPA), nasal swab (NS), sputum (SP), and throat swab (TS). NPA, NS, and TS samples were stored in viral transport medium (De Conto et al., 2018) and all samples were kept at 4 °C until submitted to laboratory procedures (within 2 h of sampling).
During the study period, all viruses were prospectively searched for. SARS-CoV-2 was prospectively searched for only from February 24, 2020, after the first case of local transmission was identified in Italy (dated February 21, 2020) (Spiteri et al., 2020). In samples that arrived before that date, SARS-CoV-2 was retrospectively searched for, depending on the availability of the sample.
Respiratory virus identification
Aliquots of the respiratory samples were used to detect the nucleic acids of adenovirus, influenza A virus H1 and H3, influenza B virus, parainfluenza virus types 1–4, respiratory syncytial virus types A and B, metapneumovirus, bocavirus types 1–4, rhinovirus types A, B, and C, enterovirus, and coronavirus types 229E, NL63, and OC43, with the one-step real-time reverse transcription PCR (rRT-PCR) Allplex Respiratory Panels 1–3 assays (Seegene Inc., Seoul, Korea), as described previously (Calderaro et al., 2020).
Real-time PCR for the detection of the nucleic acid of SARS-CoV-2
The qualitative detection of SARS-CoV-2 nucleic acid was performed according to the ‘real-time RT-PCR panel’ for the detection of 2019-Novel Coronavirus, which was published on February 4, 2020 by the US Centers for Disease Control and Prevention (Centers for Disease Control and Prevention, 2020), with some modifications, as described previously (Calderaro et al., 2020).
Briefly, the nucleic acid was extracted from 200 μL of the specimen using the NucliSENS easyMAG extraction assay (BioMérieux, France). The nucleic acid amplification was conducted on an Applied Biosystems 7500 Fast DX Thermal Cycler (Applied Biosystems, USA) at 25 °C for 2 min for Uracil-N-glycosylase incubation, and then at 50 °C for 15 min for the reverse transcription step, followed by the enzyme activation step at 95 °C for 2 min. Then, the amplification was conducted for 45 cycles (3 s at 95 °C and 30 s at 55 °C).
In the prospective analysis, a specimen was considered negative for SARS-CoV-2 if marker N1 and N3 cycle threshold growth curves did not cross the threshold and the RNase P growth curve crossed the line. Conversely, if the marker cycle threshold growth curves crossed the threshold line, the specimen was considered positive for SARS-CoV-2. In the retrospective analysis, only N1 marker was used.
Immunofluorescence assay for RSV antigen detection in sample cells
NPA, NS, and TS samples were centrifuged (1000 rpm, 10 min, 4 °C) and the pellets resuspended in phosphate-buffered saline (PBS, pH 7.4; 7 mM Na2HPO4, 1.5 mM KH2PO4, 137 mM NaCl, 2.7 mM KCl). An aliquot of 200 μL of the pellet was cytocentrifuged onto a glass slide at 200 × g for 5 min. After drying, the slide was fixed with cold acetone for 10 min and then incubated with a fluorescein isothiocyanate-conjugated anti-RSV monoclonal antibody (Imagen; Oxoid) for 15 min at 37 °C, in a humid chamber. After incubation, the slide was washed three times with PBS. Stained cells were observed under a fluorescence microscope (Leica DMLB, Germany) (De Conto et al., 2019).
Respiratory virus identification by rapid cell culture technique
Madin–Darby canine kidney cells sialyltransferase 1 (MDCK-SIAT1, a kind gift from the Microbiology Institute, Sacred Heart University, Rome, Italy), Hep-2 and Vero cell (“Istituto Sperimentale Zooprofilattico della Lombardia e dell'Emilia-Romagna”, Brescia, Italy) monolayers grown in chamber slides using Earle’s modified minimum essential medium, supplemented with 1% l-glutamine, 1% foetal bovine serum (FBS), and antibiotics (100 U/mL penicillin, 100 μg/mL streptomycin) (MEM 1%) were inoculated with the sample supernatant (50 μL of supernatant/each cell line) obtained as described above and incubated for 1 h 15 min at 37 °C, 5% CO2. Subsequently, the inoculum was removed, the monolayer was washed twice with PBS, and MEM without FBS (MEM-S) was added. After an incubation time of 24 h at 37 °C, 5% CO2, the medium was removed, following which the cells were fixed with cold acetone for 10 min and then incubated with fluorescein isothiocyanate-conjugated monoclonal antibodies anti-FLUA, anti-FLUB, anti-PIV1, anti- PIV2, anti-PIV3, and anti-RSV antigens (Imagen, Oxoid) for 30 min at 37 °C, in a humid chamber. After incubation, the slide was washed three times with PBS. Stained cells were observed under a fluorescence microscope (Leica DMLB) (De Conto et al., 2019, Calderaro et al., 2020).
Viral isolation by conventional cell culture
Intestine 407, Hep-2, Vero, LLC-MK2 (“Istituto Sperimentale Zooprofilattico della Lombardia e dell'Emilia-Romagna”, Brescia, Italy), MRC5 embryonic lung fibroblasts (ATCC CCL-171), and MDCK-SIAT1 monolayers grown in 24-well plates using MEM 1% medium were inoculated with the sample supernatant (200 μL of supernatant/each cell line) obtained as described above and incubated for 1 h 15 min at 37 °C, 5% CO2. Subsequently, the inoculum was removed and the monolayer was washed twice with PBS and MEM-S was added.
The inoculated cell monolayers were observed daily under an inverted optical microscope in order to check cellular morphology modifications possibly related to virus replication (cytopathic effect) (De Conto et al., 2019, Calderaro et al., 2020).
Electron microscopy
The supernatant of cell monolayers showing a cytopathic effect was collected under Biosafety Level 3 and biocontainment facilities, and first inactivated with 1% formaldehyde in PBS for 1 h. It was then ultracentrifuged at 25 000 rpm (SW41 rotor, Beckman Coulter Optima XE ultracentrifuge) (Calderaro et al., 2020). The resulting pellet was used to prepare a standard (50 μL) drop to be put in contact with a 400-mesh plastic (formvar)-coated copper grid. After negative staining using an aqueous solution of phosphotungstic acid (2%, pH 6.4), sample observation was done using a transmission electron microscope (EM 208S Philips) at 44 000 magnifications (Calderaro et al., 2014).
Statistical analysis
The Chi-square test was used for comparisons of the different categories considered. Statistical significance was set at p < 0.01. In additions the odds ratio (OR) was calculated in order to evaluate the strength of the associations that emerged.
Results
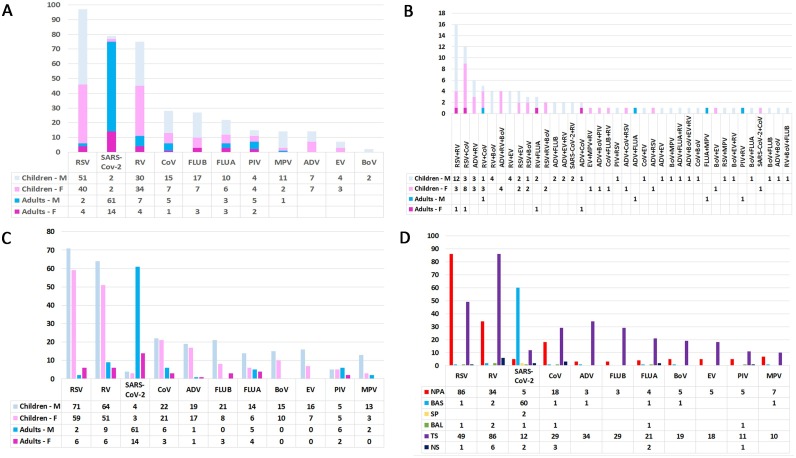
Of the 906 biological samples from the respiratory tract analysed, 474 (52.3%) were positive for at least one virus: single infections were detected in 380 (80.2%) samples and mixed infections were detected in 94 (19.8%) samples (Supplementary Material Table S1). The distributions of single and mixed infections according to age and sex are shown in Figure 1A and B, respectively. Overall, a total of 583 viruses were detected. RSV (138/583, 23.7%) and RV (130/583, 22.3%) were the most commonly identified viruses, followed by SARS-CoV-2 (82/583, 14.1%), CoV (52/583, 8.9%), ADV (38/583, 6.5%), FLUB (32/583, 5.5%), FLUA (29/583, 5%), BoV (25/583, 4.3%), EV (23/583, 3.9%), PIV (18/583, 3.1%), and MPV (18/583, 3.1%). The detection of each viral agent according to age and sex is shown in Figure 1C. Excluding RV, which was the agent most implicated in mixed infections (75 single infections versus 55 mixed infections), SARS-CoV-2 was found to be the second virus involved in single infections (79 infections). The detection of each viral agent in each of the different biological sample types analysed is reported in Figure 1D.
Figure 1.
Infectious agents detected according to age, sex, and biological sample. (A) Distribution of single infections according to age and sex. (B) Distribution of mixed infections according to age and sex. (C) Detection of each viral agent according to age and sex. (D) Detection of each viral agent according to the type of biological sample analysed.
Abbreviations: M, male; F, female; RSV, respiratory syncytial virus; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; RV, rhinovirus; CoV, coronavirus other than SARS-CoV-2; FLU, influenza virus (types B and A); PIV, parainfluenza virus; MPV, metapneumovirus; ADV, adenovirus; EV, enterovirus; BoV, bocavirus; NPA, nasopharyngeal aspirate; BAS, bronchial aspirate; SP, sputum; BAL, bronchoalveolar lavage; TS, throat swab; NS, nasal swab.
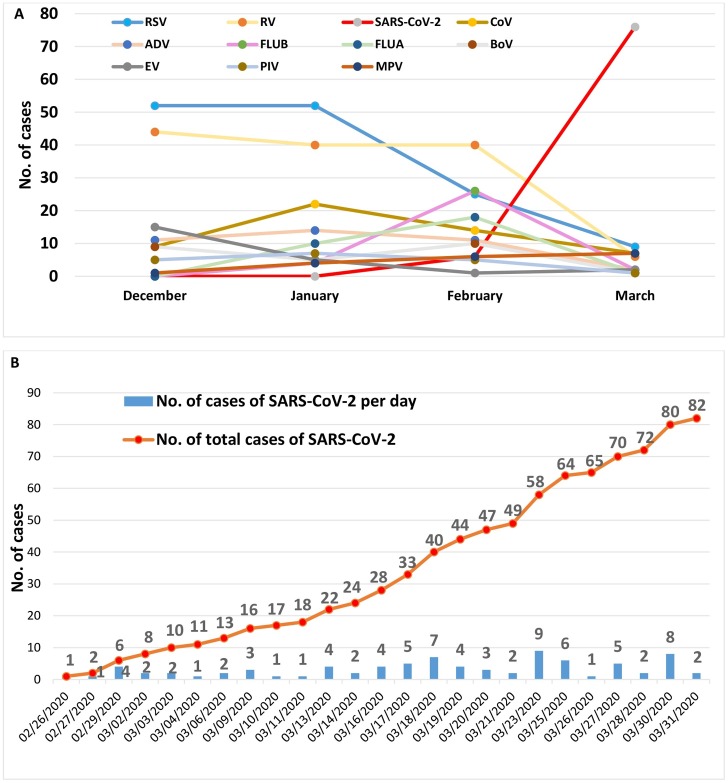
The temporal distribution of detection of each virus is reported in Figure 2A. RSV was found to predominate in December and January, with 108 detections during the period December 2019 to January 2020. The detection of RSV then decreased drastically in March, reaching only nine detections. In contrast, SARS-CoV-2 was absent until February 26, but then 82 detections were documented in just over a month (Figure 2B); in 20 cases it was possible to isolate this virus by cell culture. In addition, it should be underlined that in March, the recovery rate of all respiratory viruses, with the exception of SARS-CoV-2, decreased to below 10 cases each.
Figure 2.
(A) Temporal distribution of the detection of each virus. (B) Temporal distribution of the detection of SARS-CoV-2 alone.
Abbreviations: RSV, respiratory syncytial virus; SARS-CoV-2, Severe acute respiratory syndrome coronavirus 2; RV, rhinovirus; CoV coronavirus other than SARS-CoV-2; FLU, influenza virus (types B and A); PIV, parainfluenza virus; ADV, adenovirus; MPV, metapneumovirus; EV, enterovirus; BoV, bocavirus.
Overall, 43% of males (230/532) and 44.1% of females (165/374) who were tested for respiratory viruses other than SARS-CoV-2 were positive for at least one respiratory virus (p = 0.8444, OR 0.96). Of those tested for SARS-CoV-2, 13.4% of males (65/486) and 5% of females (17/340) were positive (p = 0.0001, OR 2.9) (Table 1 ).
Table 1.
Rates of positive sample detection according to sex and age.
| PCR for respiratory viruses other than SARS-CoV-2 | PCR for SARS-CoV-2 | |||
|---|---|---|---|---|
| Male | Female | Male | Female | |
| Samples tested | 532 | 374 | 486 | 340 |
| Negative samples (%) | 302 (57%) | 209 (55.9%) | 421 (86.6%) | 323 (95%) |
| Positive samples (%) | 230 (43%) | 165 (44.1%) | 65 (13.4%) | 17 (5%) |
| Children | Adults | Children | Adults | |
| Samples tested | 574 | 332 | 513 | 313 |
| Negative samples (%) | 227 (39.5%) | 284 (85.5%) | 506 (98.6%) | 238 (76%) |
| Positive samples (%) | 347 (60.5%) | 48 (14.5%) | 7 (1.4%) | 75 (24%) |
PCR, polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Similarly, 60.5% of the children (347/574) and 14.5% of the adults (48/332) tested were positive for at least one respiratory virus other than SARS-CoV-2 (p < 0.00001, OR 9.04). For SARS-CoV-2, 1.4% of the children tested (7/513) and 24% of the adults tested (75/313) were positive (p < 0.00001, OR 0.04) (Table 1).
As reported in Table 2 the viruses most frequently found in children were RSV (130 cases, 37% of the positive children) and RV (115 cases, 32.7% of the positive children). The least detected virus was SARS-CoV-2 (seven cases, 2% of the positive children). In contrast, SARS-CoV-2 was the most frequent virus detected among adults (75 cases, 61% of the positive adults), followed by RV (15 cases, 12.2% of the positive adults), FLUA (nine cases, 7.3% of the positive adults), and CoV (nine cases, 7.3% of the positive adults).
Table 2.
Rates of respiratory viruses detected according to sex and age.
| Virus | Total | Male | Female | Children | Adults |
|---|---|---|---|---|---|
| RSV | 138 | 73 | 65 | 130 | 8 |
| RV | 130 | 73 | 57 | 115 | 15 |
| SARS-CoV-2 | 82 | 65 | 17 | 7 | 75 |
| CoV | 52 | 28 | 24 | 43 | 9 |
| ADV | 38 | 20 | 18 | 36 | 2 |
| FLUB | 32 | 21 | 11 | 29 | 3 |
| FLUA | 29 | 19 | 10 | 20 | 9 |
| BoV | 25 | 15 | 10 | 25 | 0 |
| EV | 23 | 16 | 7 | 23 | 0 |
| PIV | 18 | 11 | 7 | 10 | 8 |
| MPV | 18 | 15 | 3 | 16 | 2 |
RSV, respiratory syncytial virus; RV, rhinovirus; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; CoV, coronavirus other than SARS-CoV-2; ADV, adenovirus; FLU, influenza virus (types B and A); BoV, bocavirus; EV, enterovirus; PIV, parainfluenza virus; MPV, metapneumovirus.
The viruses most frequently found in males were RSV and RV (73 cases each, 25% of the positive males), followed by SARS-CoV-2 (65 cases, 22.2% of the positive males). Among females, while RSV (65 cases, 36% of the positive females) and RV (57 cases, 31.5% of the positive females) remained in the first two places, SARS-CoV-2 (17 cases, 9.4% of the positive females) was only the fifth most frequently isolated agent.
Discussion
In 2015, Berry et al. highlighted that the etiological agents of 12–39% of lower respiratory tract infections remain unidentified and that these results can depend significantly on the sensitivity of the diagnostic test used, the respiratory site sampled, and the geographical area of the study (Berry et al., 2015). This suggests that many respiratory pathogens may remain undetected.
All novel emergent respiratory viruses have varying but significant impacts on human health and the potential to give rise to outbreaks (Berry et al., 2015). SARS-CoV-2, as seen in these months, has shown its own unique potential to give rise to epidemics worldwide.
In this study, the viral aetiology of ARIs was investigated in 906 patients with acute respiratory tract infections, both outpatients and inpatients, attending the tertiary care University Hospital of Parma, Italy, from December 2019 to March 2020.
Overall, 52.3% of cases were positive for at least one virus; single infections were detected in 80.2% of cases and mixed infections were detected in the remaining 19.8% of cases. This result is consistent with previous observations, including those of a study performed in the same area during the period October 2012 to September 2015 in children, in which single and mixed infections accounted for 80.1% and 19.9% of cases, respectively (De Conto et al., 2019), as well as studies in other areas (Martin et al., 2012, Paranhos-Baccalà et al., 2008). As reported previously, although mixed infections have been widely assessed in ARIs, there is still no clear relationship with prolonged virus shedding, asymptomatic virus persistence, and disease severity (De Conto et al., 2019, Moesker et al., 2016, Seo et al., 2014, Turner et al., 2013).
Despite RSV being the virus most commonly detected in this study (23.7%), the presence of this virus was found to be concentrated in the December to January period and it was ousted in March following the arrival of the new human coronavirus SARS-CoV-2. This result agrees perfectly with those of the previous study performed in the same area (October 2012 to September 2015) in which RSV (27.12%) was the predominant virus isolated (De Conto et al., 2019); moreover, in agreement with the first discovery of SARS-CoV-2 in our area on February 26, 2020 (Calderaro et al., 2020), no samples positive for this virus were found in the retrospective analysis. The high prevalence of SARS-CoV-2, which in only 1 month became the second most isolated virus in the winter season December 2019 to March 2020, if excluding RV, further underlines the strong epidemic power of this virus.
SARS-CoV-2 was found in mixed infections in only three cases (3.7%): with RV in two cases and with another CoV in one case (CoV-NL63); all of these mixed infections were observed in children younger than 1 year old.
As reported in the literature, COVID-19 has affected more men than women, and principally those aged 30–65 years, with around half of cases being older than 50 years of age (de Lusignan et al., 2020). These data agree with those obtained in this study, in which SARS-CoV-2 was detected in 65 males (79.3%) and 17 females (20.7%), suggesting that sex may be a risk factor implicated in the appearance of COVID-19 disease. Similarly, age could represent a risk factor; indeed, SARS-CoV-2 was detected in 75 adults (age range 28 years and 1 month to 86 years, median 59 years and 10 months) and in only seven children (age range 22 days to 13 years and 4 months, median 3 years and 2 months).
This study showed a completely different trend when comparing SARS-CoV-2 to the ‘common’ respiratory viruses, which most often affected children without any distinction by sex.
As expected, with children attending the paediatric wards or outpatient clinics being the major source of respiratory viruses other than SARS-CoV-2, TS and NPA were the most representative specimens. In contrast, the majority of adults were positive for SARS-CoV-2, hospitalized in intensive care units, and BAS was the most representative biological sample.
Although the first detection of SARS-CoV-2 in the study area was dated February 26, 2020 (Calderaro et al., 2020), in Parma, as in other areas of the world (Chan et al., 2020, Ralph et al., 2020, Shang et al., 2020), cases of atypical pneumonia had been observed since December 2019. However, when a retrospective molecular analysis is performed, as was done in the present study in the search for SARS-CoV-2 RNA, several factors related to sample storage may affect the outcome of these investigations. Moreover, it should be noted that members of certain groups, such as children and people living in deprived areas, are almost completely absent or have only been tested when in a serious condition (for example when admitted to the hospital). In such circumstances, when tested, these subjects could be more likely to be positive for SARS-CoV-2 than the general population, representing an incomplete picture of the situation in those months. Moreover, although the RT-PCR assay is the gold standard for SARS-CoV-2 diagnosis, factors such as sampling and the timing relative to symptom onset might reduce sensitivity. Therefore, some SARS-CoV-2 cases could have been missed, particularly among patients with lower viral loads and mostly not subjected to virological investigations.
As demonstrated by the first case of SARS-CoV-2 detected in our area (Calderaro et al., 2020), molecular techniques should be used, in laboratories that have the facility to perform in vitro virus culture, in parallel with viral culture, the only reference laboratory method able to reveal the presence of cytopathogenic viral agents and demonstrate their infectivity in cases of emerging viruses. In this study, all samples were submitted to in vitro culture, resulting in 20 samples positive for SARS-CoV-2, and all of the samples negative by PCR were also negative by culture.
Author contributions
AC designed the study. AC, MCA, FDC, MB, GP, SM, CM, MM, AD, FF, FP, and PM performed the laboratory analysis. AC and CC performed the structural studies. AC, MCA, FDC, MB, GP, and CC collected the data. AC, FDC, and CC analysed the data. AC, MB, and CC prepared the manuscript.
Informed consent
The samples analysed in this study were sent to the University Hospital of Parma for routine diagnostic purposes, and the laboratory diagnosis results were reported in the medical records of the patients in response to a clinical suspicion; ethical approval at the University Hospital of Parma is required only in cases in which the clinical samples are to be used for applications other than diagnosis.
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
This study was supported by the Ministry of University and Scientific Research Grant FIL, Parma, Italy.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.ijid.2020.09.1473.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- Azienda Ospedaliero-Universitaria di Parma. L’ospedale. Web page: https://www.ao.pr.it/chi-siamo/lospedale/. (Accessed 31 July 2020).
- Berry M., Gamieldien J., Fielding B.C. Identification of new respiratory viruses in the new millennium. Viruses. 2015;7:996–1019. doi: 10.3390/v7030996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bicer S., Giray T., Çöl D., Erdağ G.Ç, Vitrinel A., Gürol Y. Virological and clinical characterizations of respiratory infections in hospitalized children. Ital J Pediatr. 2013;39:22. doi: 10.1186/1824-7288-39-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderaro A., Arcangeletti M.C., De Conto F., Buttrini M., Montagna P., Montecchini S., Ferraglia F., Pinardi F., Chezzi C. SARS-CoV-2 infection diagnosed only by cell culture isolation before the local outbreak in an Italian seven-week-old suckling baby. Int J Infect Dis. 2020;96:387–389. doi: 10.1016/j.ijid.2020.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderaro A., Arcangeletti M.C., Rodighiero I., Buttrini M., Gorrini C., Motta F., Germini D., Medici M.C., Chezzi C., De Conto F. Matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrometry applied to virus identification. Sci Rep. 2014;4:6803. doi: 10.1038/srep06803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention . 2020. Coronavirus Disease 2019 (COVID-19) [Google Scholar]
- Chan J.F.W., Kok K.H., Zhu Z., Chu H., To K.K.W., Yuan S. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg Microbes Infect. 2020;9:221–236. doi: 10.1080/22221751.2020.1719902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Yu B. First two months of the 2019 Coronavirus Disease (COVID-19) epidemic in China: real-time surveillance and evaluation with a second derivative model. Glob Heal Res Policy. 2020;5:7. doi: 10.1186/s41256-020-00137-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Conto F., Conversano F., Medici M.C., Ferraglia F., Pinardi F., Arcangeletti M.C., Chezzi C., Calderaro A. Epidemiology of human respiratory viruses in children with acute respiratory tract infection in a 3-year hospital-based survey in Northern Italy. Diagn Microbiol Infect Dis. 2019;94:260–267. doi: 10.1016/j.diagmicrobio.2019.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Conto F., Fazzi A., Razin S.V., Arcangeletti M.C., Medici M.C., Belletti S., Chezzi C., Calderaro A. Mammalian Diaphanous-related formin-1 restricts early phases of influenza A/NWS/33 virus (H1N1) infection in LLC-MK2 cells by affecting cytoskeleton dynamics. Mol Cell Biochem. 2018;437:185–201. doi: 10.1007/s11010-017-3107-9. [DOI] [PubMed] [Google Scholar]
- de Lusignan S., Dorward J., Correa A., Jones N., Akinyemi O., Amirthalingam G. Risk factors for SARS-CoV-2 among patients in the Oxford Royal College of General Practitioners Research and Surveillance Centre primary care network: a cross-sectional study. Lancet Infect Dis. 2020;S1473–3099(20):30371–30376. doi: 10.1016/S1473-3099(20)30371-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasöksüz M., Kiliç S., Saraç F. Coronaviruses and SARS-COV-2. Turkish J Med Sci. 2020;50:549–556. doi: 10.3906/sag-2004-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon J.H., Han M., Chang H.E., Park S.S., Lee J.W., Ahn Y.J., Hong D.J. Incidence and seasonality of respiratory viruses causing acute respiratory infections in the Northern United Arab Emirates. J Med Virol. 2019;91:1378–1384. doi: 10.1002/jmv.25464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R., Han H., Liu F., Lv Z., Wu K., Liu Y., Feng Y., Zhu C. Positive rate of RT-PCR detection of SARS-CoV-2 infection in 4880 cases from one hospital in Wuhan, China, from Jan to Feb 2020. Clin Chim Acta. 2020;505:172–175. doi: 10.1016/j.cca.2020.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin E.T., Kuypers J., Wald A., Englund J.A. Multiple versus single virus respiratory infections: viral load and clinical disease severity in hospitalized children. Influenza Other Respi Viruses. 2012;6:71–77. doi: 10.1111/j.1750-2659.2011.00265.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moesker F.M., van Kampen J.J.A., van Rossum A.M.C., de Hoog M., Koopmans M.P.G., Osterhaus A.D.M.E. Viruses as Sole Causative Agents of Severe Acute Respiratory Tract Infections in Children. PLoS One. 2016;11 doi: 10.1371/journal.pone.0150776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paranhos-Baccalà G., Komurian-Pradel F., Richard N., Vernet G., Lina B., Floret D. Mixed respiratory virus infections. J Clin Virol. 2008;43:407–410. doi: 10.1016/j.jcv.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provincia di Parma. Cresce la popolazione del Parmense. Web page: http://www.provincia.parma.it/notizie/cresce-la-popolazione-del-parmense. (Accessed 31 July 2020).
- Ralph R., Lew J., Zeng T., Francis M., Xue B., Roux M. 2019-nCoV (Wuhan virus), a novel Coronavirus: human-to-human transmission, travel-related cases, and vaccine readiness. J Infect Dev Ctries. 2020;2020(14):3–17. doi: 10.3855/jidc.12425. [DOI] [PubMed] [Google Scholar]
- Seo Y.B., Song J.Y., Kim I.S., Yang T.U., Hong K.W., Cheong H.J., Kim W.J. Etiology and clinical outcomes of acute respiratory virus infection in hospitalized adults. Infect Chemother. 2014;46:67–76. doi: 10.3947/ic.2014.46.2.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang W., Yang Y., Rao Y., Rao X. The outbreak of SARS-CoV-2 pneumonia calls for viral vaccines. NPJ Vaccines. 2020;5:18. doi: 10.1038/s41541-020-0170-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiteri G., Fielding J., Diercke M., Campese C., Enouf V., Gaymard A. First cases of coronavirus disease 2019 (COVID-19) in the WHO European Region, 24 January to 21 February 2020. Euro Surveill. 2020;25(9) doi: 10.2807/1560-7917.ES.2020.25.9.2000178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tregoning J.S., Schwarze J. Respiratory viral infections in infants: causes, clinical symptoms, virology, and immunology. Clin Microbiol Rev. 2010;23:74–98. doi: 10.1128/CMR.00032-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner P., Turner C., Watthanaworawit W., Carrara V., Cicelia N., Deglise C., Phares C., Ortega L., Nosten F. Respiratory virus surveillance in hospitalised pneumonia patients on the Thailand-Myanmar border. BMC Infect Dis. 2013;13:434. doi: 10.1186/1471-2334-13-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . 2020. Coronavirus disease: Situation Report – 184. [Google Scholar]
- Zappa A., Perin S., Amendola A., Bianchi S., Pariani E., Ruzza M. Epidemiological and molecular surveillance of influenza and respiratory syncytial viruses in children with acute respiratory infections (2004/2005 season) Microbiol Medica. 2008:23. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.