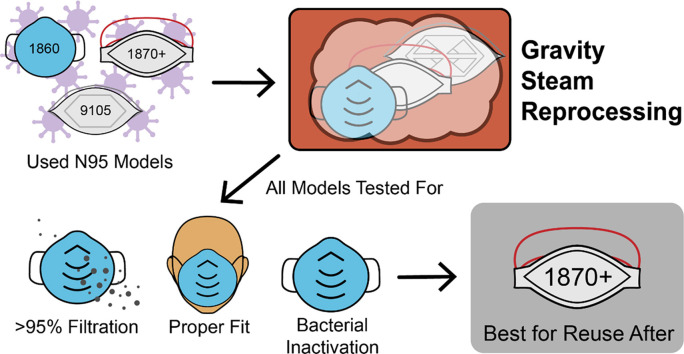
Abstract
Background
Coronavirus disease 2019 (COVID-19) has significantly impacted the health of millions of people around the world. The shortage of personal protective equipment, including N95 respirators, in hospital facilities has put frontline healthcare professionals at high risk for contracting this virus.
Aim
To develop a reproducible and safe N95 respirator reprocessing method that satisfies all presented regulatory standards and that can be directly implemented by hospitals using existing available equipment.
Methods
A non-toxic gravity steam reprocessing method has been developed for the reuse of N95 respirators consisting of 30 min of steam treatment at 121°C followed by 30 min of heat drying. Samples of model number 1860, 1860s, 1870+, and 9105 N95 respirators were either collected from hospitals (for microbiology testing) or purchased new (for functionality testing), with all functionality tests (i.e. filter efficiency, fit evaluation, and strap integrity) performed at the Centers for Disease Control and Prevention using standard procedures established by the National Institute for Occupational Safety and Health.
Findings
All tested models passed the minimum filter efficiency of 95% after three cycles of gravity steam reprocessing. The 1870+ N95 respirator model is the most promising model for reprocessing based on its efficient bacterial inactivation coupled with the maintenance of all other key functional respirator properties after multiple reprocessing steps.
Conclusions
The gravity steam method can effectively reprocess N95 respirators over at least three reprocessing cycles without negatively impacting the functionality requirements set out by regulators. Enabling the reuse of N95 respirators is a crucial tool for managing both the current pandemic and future healthcare crises.
Keywords: N95 respirators, COVID-19, Reprocessing, Steam, Reuse, Filtration
Graphical abstract
Introduction
The coronavirus disease 2019 (COVID-19) pandemic has ravaged the global economy and society, with 29.7 million cases and 939,422 deaths worldwide as of September 16th, 2020 [1]. The disease has put immense strain on hospital facilities and frontline workers, leading to a shortage of personal protective equipment (PPE) including face shields, gowns, and filtering facepiece respirators (FFRs, including N95s) used to prevent aerosolized droplet disease transmission [2,3]. Typically, respirators are not designed to be used more than once because they retain contaminants within their filtering layers after exposure and are thus difficult to clean or disinfect [4]. However, Health Canada and the US Food and Drug Administration (FDA) have previously approved the reuse and/or reprocessing of some single-use medical devices, including balloon angioplasty catheters, implanted fusion pumps, and laparoscopic equipment, provided that disinfection or sterilization processes are performed under strict regulations [[5], [6], [7]]. In light of the current shortages of FFRs worldwide and the imperative to ensure frontline workers are adequately protected in the short-to-medium term of these expected shortages, FFR reuse protocols are now being promoted by organizations such as the World Health Organization and the Centers for Disease Control and Prevention (CDC) [2,5,8]. Reprocessing of medical devices is based on the use of validated processes to render a previously used device fit for at least one more use. Such processes are designed to accomplish two main goals: (i) to remove soil and contaminants by cleaning; and (ii) to inactivate micro-organisms by disinfection or sterilization [9]. Effective cleaning of masks is not feasible due to their intrinsic filtering fabric structure that, by design, retains particulate species. As such, it is not possible to achieve ‘sterile’ status for FFRs following reprocessing. However, given the pandemic stress on respirator supplies, reprocessing without cleaning is now being accepted as an alternative provided that respirators are returned to their original user. This regulatory relaxation has led to the CDC giving Emergency Use Authorization (EUA) to disinfection procedures that meet certain standards set out by the National Institute for Occupational Safety and Health (NIOSH) [10].
Several reprocessing methods have been tested and used in various laboratories and hospitals in an effort to safely reuse N95 respirators, including the use of ultraviolet germicidal irradiation (UVGI), vaporous hydrogen peroxide (VHP), liquid hydrogen peroxide (LHP), dry and moist heat, steam, autoclave treatment, immersion in 70% isopropyl alcohol or ethanol, and microwave irradiation [[11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24]]. Some of these methods have been found to be unsuitable for FFR disinfection, with the CDC explicitly stating that the use of autoclaving, 160°C dry heat, 70% isopropyl alcohol, microwave irradiation, and soap/water can degrade FFR filters to the point they no longer meet NIOSH standards [10]. Furthermore, Lin et al. found that ethanol treatment significantly increased aerosol penetration of disinfected respirators, thus compromising filtration capability [23]. LHP disinfection has been noted by the CDC as a promising option, although to date there has not been appropriate data collected on FFR fit and disinfection efficacy post treatment to warrant full support [10]. Ultimately, the CDC has highlighted UVGI, VHP, moist heat, and steam methods as showing the most promise since they have performed well in three of the most relevant categories in which disinfection methods are typically tested after repeated treatment: to kill pathogens, to maintain proper fit, and to preserve filtration capability.
UVGI involves the use of short-wavelength UVC radiation to inactivate micro-organisms by causing DNA damage and preventing replication [11]. Previous studies have shown UVGI to maintain filtration performance and fit while efficiently disinfecting respirators against severe acute respiratory syndrome (SARS), Middle East respiratory syndrome (MERS), and influenza viruses when exposed to doses of ∼1 J/cm2 [11,17,24]. However, Lindsley et al. showed that exposure of various respirator models to high doses of UV irradiation – more than 100 times greater than a typical dose – greatly diminished the strength of the respirator material and reduced filtration capacity by up to 1.25% [11]. Though disinfection with lower levels of UV irradiation would not produce such drastic results after one treatment, the ability for respirators to endure repeated UVGI disinfections is nevertheless limited by the potential for UV irradiation to degrade polymers. Furthermore, given that the efficacy of the method depends on an appropriate dose of UV radiation reaching all parts of the respirator, shadowing effects may prevent complete disinfection of FFRs by UVGI methods [12].
VHP was used prior to the COVID-19 pandemic in hospitals to disinfect medical equipment and rooms, and the CDC has given EUAs to multiple VHP disinfection systems for use on N95 respirators [10,15,25]. VHP has a strong ability to kill pathogens and to be reapplied to FFRs without degrading filtering or fit quality [10,16]. However, a severe limitation of these hydrogen peroxide-based methods is the risk of toxic chemical residues persisting post treatment [4,14], mandating additional aeration in a ventilated room prior to use to ensure hydrogen peroxide vapour exposure below OSHA and NIOSH limits of 1 ppm [26]. Though proper ventilation should reduce the risk of using any respirators disinfected in this manner, the fact that this additional aeration step is required – coupled with the (albeit low) residual risk of hydrogen peroxide inhalation – limits the widespread applicability of this technique.
In comparison to UVGI and VHP, disinfection of FFRs using moist heat and steam is highly attractive since these procedures do not rely on toxic materials (thus avoiding any potential exposures to personnel or patients), have rapid turnaround times, and are already broadly used in healthcare settings [27]. Wet treatments are generally more effective than dry heat for viral inactivation on FFR surfaces and can be implemented either as moist heat (i.e. temperatures of 60–70°C and humidity levels of 80–85%) or steam treatments performed at temperatures above the boiling point of water (most commonly using microwavable steam bags) [17,28,29]. Moist heat of 60°C at 80% relative humidity has demonstrated minimal loss of filtration and fit performance after three cycles of FFR application while also enabling an average >4 log10 reduction of H1N1 and H5N1 influenza virus strains; however, some moist heat methods have also resulted in slight separation of the inner nose foam cushion from the FFR body and cannot fully deactivate highly thermostable pathogens [9,17,24,[28], [29], [30]]. Alternately, Fisher et al. used microwave-based steam treatment to decontaminate six FFR models with 99.9% inactivation of an MS2 bacteriophage [31]. Filtration and fit performance were adequate to NIOSH certification requirements, consistent with findings from Bergman et al. using a similar approach [17]. Microwave-based steam techniques are, however, limited by the inconsistent generation of steam depending on the available microwave power rating and the potential for sparking due to the presence of the metal nosebands in most FFRs that represents a safety concern for scale-up [10]. Other methods of steam disinfection of N95 respirators include steaming on boiling water and steam sterilizer autoclaves [20]; however, all these methods either do not scale well or have not been tested under relevant regulations for respirator reprocessing [21]. A thoroughly tested steam disinfection procedure for FFR disinfection would provide a quicker non-toxic alternative to VHP and UVGI methods.
In comparing the results from FFR reprocessing experiments (e.g. to assess the feasibility of novel reprocessing methods) it is necessary to ensure consistent testing for validating the efficacy of the treatments beyond simple viricidal abilities. For example, Health Canada, the FDA, and the CDC have introduced new regulations that place companies aiming to reprocess and distribute N95 respirators to healthcare facilities under the same regulations as N95 respirator manufacturers, requiring the disclosure of full information on material compatibility, filtration performance, respirator fit, and contamination presence among other criteria [8,32]. In terms of reprocessing, processes must reduce pathogenic burden with bacterial sporicidal testing demonstrating a sterility assurance level of 10−6 and viral inactivation for a broad range of viruses [8]. Bacterial spores were shown to be the most resistant to sterilization processes and represent the worst-case scenario, making validation of reprocessing efficacy for killing spores indicative of broader-scale performance for disinfection [9]. In addition, N95 respirators must specifically adhere to the testing requirements outlined by NIOSH and the FDA, including >95% particle filtration efficiency [33]. However, many recent publications do not mention adherence to such standardized testing protocols, with crucial information about respirator fit and filtration capabilities post treatment often omitted; furthermore, many other articles report results on only specific segments of respirators that may or may not provide an accurate depiction of the performance of the FFR as a whole. Results collected using such unregulated laboratory testing standards, while useful for rapidly advancing knowledge in this critical field given the urgency of the pandemic situation, cannot be promoted or supported by health organizations for clinically proven safety, making such reports of limited actionable utility to hospitals looking to provide their workers with urgently needed personal protective equipment.
The aim of this study was to investigate a non-toxic gravity steam reprocessing method that can be implemented easily in hospitals and that is rigorously tested against current regulatory standards.
Methods
Materials
Four models of 3M N95 respirators were tested in this study: 3M Health Care Particulate Respirator and Surgical Mask 1860, 3M Health Care Particulate Respirator and Surgical Mask 1860s (small), 3M Health Care Particulate Respirator and Surgical Mask 1870+, and 3M VFlex Particulate Respirator 9105. The different models were selected based on the different construction of the different respirators. The 1860 respirator is a traditional cup-shaped model (with the 1860s model simply a smaller version of the 1860 model), whereas the 1870+ respirator is a three-fold panel model, and the 9105 respirator has V-shaped pleats that flex and expand to better accommodate breathing and talking. As such, assessing the effects of a disinfection protocol on all three of these respirator categories will provide a broad-based assessment of the efficacy of gravity steam reprocessing of N95 respirators. Sterilization reproducibility was assessed using a 3M Attest Rapid 5 Steam-Plus Test Pack 41382F containing a 3M Attest 1292 Rapid Readout Biological Indicator and a 3M Comply SteriGage 1243 Chemical Indicator, ensuring that the targeted temperature/steam conditions are achieved during each cycle performed.
Gravity steam reprocessing cycle
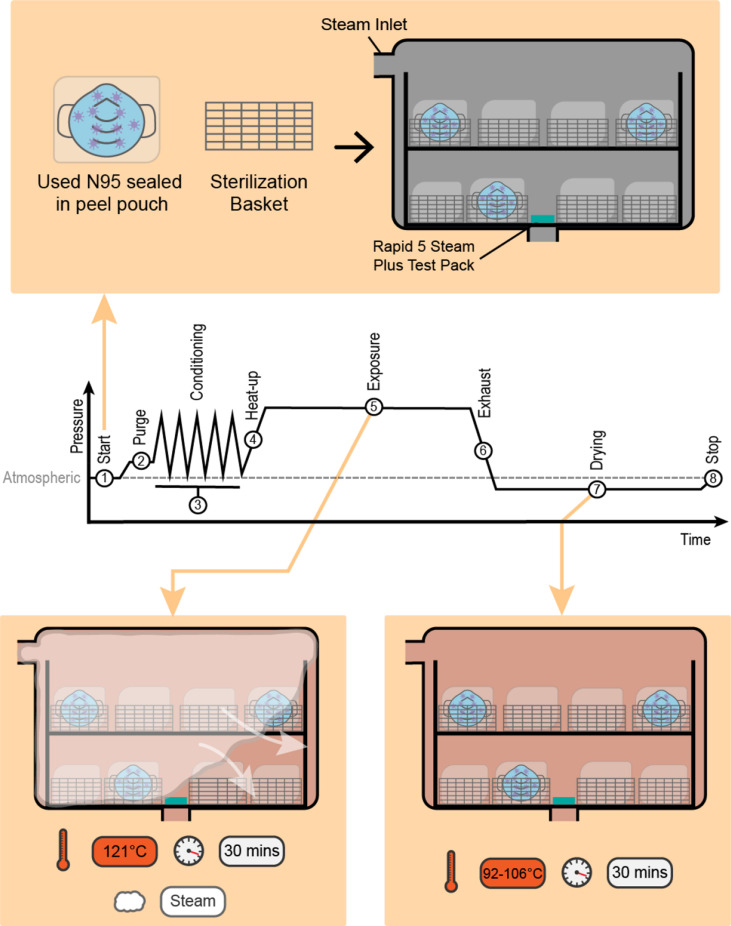
The reprocessing cycle was executed with a Getinge Steam Sterilizer (Model Number 633) using a gravity steam cycle consisting of an exposure temperature of 121°C and an exposure time of 30 min followed by 30 min of subsequent drying (Figure 1 ).
Figure 1.
Proposed gravity steam reprocessing (packaged medical devices in sealed peel pouches not containing N95 respirators are not shown). (1) Start: the sterilizer is loaded, the chamber door is sealed, and program timer is started. (2) Purge: steam flows through the chamber to displace air and preheat the load. (3) Conditioning: trapped air within the load is removed through a series of five positive pressure pulses. (4) Heat-up: the chamber/load is heated to the exposure temperature. (5) Exposure: the temperature is maintained at the exposure temperature for a set time (121°C, 30 min). (6) Exhaust: the chamber pressure is reduced to atmospheric pressure or below. (7) Drying: samples are left to dry for the selected drying time (30 min). (8) Stop: end of reprocessing process.
The reprocessing cycle was designed to deliver the treatment at a lower temperature and pressure compared to the widely performed pre-vacuum steam cycle that uses a 132°C exposure temperature, aiming to reduce the potential for respirator deformation during reprocessing that can alter fit. The reprocessed load was composed of eight sterilization baskets, with all baskets not containing respirator samples loaded with packaged surgical instruments to simulate a full load. Given the ongoing supply restrictions on the N95 respirators, the packaged surgical instruments were used only for the purpose of simulating a full reprocessing load; this choice should not be interpreted as a recommendation to mix a reprocessing load with respirators with stainless steel surgical instruments.
The baskets were placed on a loading cart with four baskets on the top shelf and the other four baskets on the bottom shelf (Figure 1). All samples were packaged using Wipak Steriking heat seal peel pouches (Famos Heat Sealer, Model Number: F108). All pouches were placed in a vertical orientation (i.e. on edge) with the transparent surface of the pouch facing the non-transparent surface of the adjacent pouch to facilitate air removal, steam contact and evaporation of condensate, following standard CSA Z314-18 [36]. A 3M Attest Rapid 5 Steam-Plus Test Pack 41382F was placed on the bottom shelf and over the drain of the sterilizer in each load. Dryness of the respirators was assessed by visual inspection to verify the presence of moisture following the standard ANSI/AAMI ST79: 2017: Comprehensive guide to steam sterilization and sterility assurance in healthcare facilities [37]. Wet packaging is considered contaminated.
Reprocessing cycle efficacy testing
The effectiveness of the reprocessing cycle was assessed based on the ISO 17665-1:2006 standard [38]. Annex D describes a widely used process to validate a sterilization cycle for reusable medical devices and is based on the inactivation of reference micro-organisms by delivering a treatment that exceeds the conditions required to achieve sterility (30 min). The reprocessing cycle was thus also executed at a reduced level of treatment following the Partial Cycle Approach to assess the efficacy of the cycle at half of the exposure time (15 min).
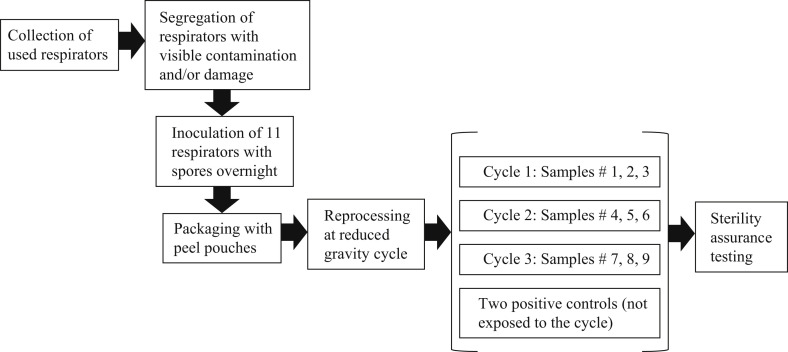
Microbial testing was performed based on the flowchart in Figure 2 . Used 1860, 1860s, 9105 and 1870+ 3M respirators were collected from hospitals to simulate the real clinical use of the respirator. The collected samples were visually inspected for damage and/or contamination, and then inoculated with a Geobacillus stearothermophilus spore suspension (Crosstex product number VGS-106, cell line 7953, lot number AR633, concentration 2.6 × 106/0.1 mL, expiry date 2022-02-28). This spore suspension was selected since it demonstrates high resistance to moist heat and thus represents a worst-case scenario to the gravity steam reprocessing strategy (ISO 17665-1:2006) [38].
Figure 2.
Process chart for microbiology testing on used N95 respirators.
Each respirator was inoculated with 1 mL of the spore inoculum and left to dry overnight. The inoculation sites were chosen to best simulate real use and thus comprised (i) the locations determined to be the most difficult to disinfect and (ii) the areas that face the mouth and nose (Figure 3 ). The inner layers of the respirators represent a particular challenge to the disinfection process since steam must penetrate the outer surfaces of the respirators to reach them; as such, a syringe and needle were used to inject the spore suspension into the layers of the respirators at multiple points and on areas that face the mouth and nose of the user. The inoculated respirators were then exposed to a partial gravity steam cycle (121°C, 15 min of steam exposure followed by 30 min of drying). The reprocessing cycle was executed in triplicate using three samples in each cycle to demonstrate reproducibility.
Figure 3.
Inoculation sites for the tested respirator models; 1860, 1860s, 1870, and 9105.
Bacterial inactivation testing was performed on all treated samples and two positive controls that were inoculated but not exposed to the steam cycle. Residual bacteria growth was assessed using an incubation time of 14 days at a temperature of 55–60°C by fully immersing the respirators in soybean–casein digest broth as the test media. Microbial identification (ID) was performed on any samples that showed microbial growth using the Vitek system, a fully automated system that uses fluorogenic methodologies and compares growth in the presence of various reagents to its extensive database [39]. An ID was run on the positive control respirator concurrently to compare the growth in the samples to the organism used as the inoculum.
Respirator functionality testing
A total of 32 respirators (21 samples and 11 controls) that were unworn and had not been exposed to any pathogenic micro-organisms was evaluated for functionality based on the flow chart in Figure 4 . The 1860s respirator model was excluded from this study due to its resemblance to the larger respirator model 1860 that was subjected to functionality testing. All respirator samples were packaged individually in peel pouches and exposed to three gravity steam cycles consisting of steam exposure at 121°C for 30 min followed by 30 min of drying. Functionality testing of the reprocessed N95 respirators was then conducted by The National Personal Protective Technology Laboratory (NPPTL, the research centre within NIOSH/CDC) following procedure TEB-APR-STP-0059, revision 3.2, but omitting the inhalation and exhalation tests (as per NIOSH recommendation). Filtration efficiency was tested using a TSI Automated Filter Tester model 8130A using a sodium chloride aerosol to confirm that the particulate filtering efficiency met the minimum certification standards set forth in 42 CFR, Part 84 Subpart K, 84.181 [33]. The flow rate was set to 85.0 ± 4.0 L/min, with the aerosol concentration not exceeding 200 mg/m3; the particle size distribution of the aerosol was 0.075 ± 0.020 μm with a geometric standard deviation not exceeding 1.86. Each respirator was tested for 10 min, with the maximum aerosol penetration recorded for each individual respirator. For laboratory fit evaluation, a static manikin headform was used to quantify changes in manikin fit factor using a TSI PortaCount® PRO+ 8038 instrument operating in ‘N95 Enabled’ mode. For strap integrity testing, the tensile strength of both the top and bottom straps was measured using an Instron® 5943 Tensile Tester. Straps were sectioned into 10 cm segments, with an additional ∼15 mm maintained on each side to enable clamping. Straps were pulled at 1 cm/s until 200% strain (30 cm sample length) was reached. This ‘pre-stretching’ position was held for 2 min. Straps were then returned to their original position for 5 min, and the new segment length was measured. Straps were subsequently pulled at 1 cm/s until reaching 150% strain relative to the new length, holding the position for 30 s, and recording the residual tensile force.
Figure 4.
Process chart for functionality testing of used N95 respirators.
Statistical analysis
All data sets were tested for statistical significance using a two-sample t-test with unequal variances at 95% confidence. Based on NIOSH standard, the functionality testing assessment was based on convenience sampling, a non-probability sampling technique whereby samples are drawn from the population based on their availability. The selection of nine samples for the microbiology testing is based on ISO 17665-1:2006 [38].
Results
Reprocessing cycle efficacy testing
To assess the efficacy of gravity steam reprocessing for inactivating bacteria on used N95 respirators, respirators were pre-inoculated with thermophilic G. stearothermophilus bacteria spores, reprocessed, and then tested for bacterial regrowth in triplicate, the results of which are shown in Table I .
Table I.
Microbiology testing results following inoculation of a spore suspension of Geobacillus stearothermophilus
| Cycle no. | Sample no. | Respirator model |
|||
|---|---|---|---|---|---|
| 1860 | 1860s | 1870+ | 9105 | ||
| 1 | 1 | – | – | – | – |
| 2 | – | – | – | – | |
| 3 | – | – | – | BG | |
| Rapid 5 Steam-Plus test pack | – | – | – | – | |
| 2 | 4 | – | – | – | – |
| 5 | – | – | – | – | |
| 6 | – | – | – | – | |
| Rapid 5 Steam-Plus test pack | – | – | – | – | |
| 3 | 7 | – | – | – | – |
| 8 | – | – | – | – | |
| 9 | – | – | – | BG | |
| Rapid 5 Steam-Plus test pack | – | – | – | – | |
| Control 1 (inoculated, not reprocessed) | BG | BG | BG | BG | |
| Control 2 (inoculated, not reprocessed) | BG | BG | BG | BG | |
BG, bacterial growth observed; –, no bacterial growth observed.
The treated 1860, 1860s and 1870+ respirator models did not show any bacterial growth, indicating that the partial steam reprocessing cycle (15 min as opposed to 30 min) is successful at inactivating heat-resistant bacterial spores. However, the 9105 respirator model showed bacterial growth in samples 3 and 9 even following gravity steam reprocessing and thus cannot be reliably reprocessed using this protocol.
Filtration efficiency
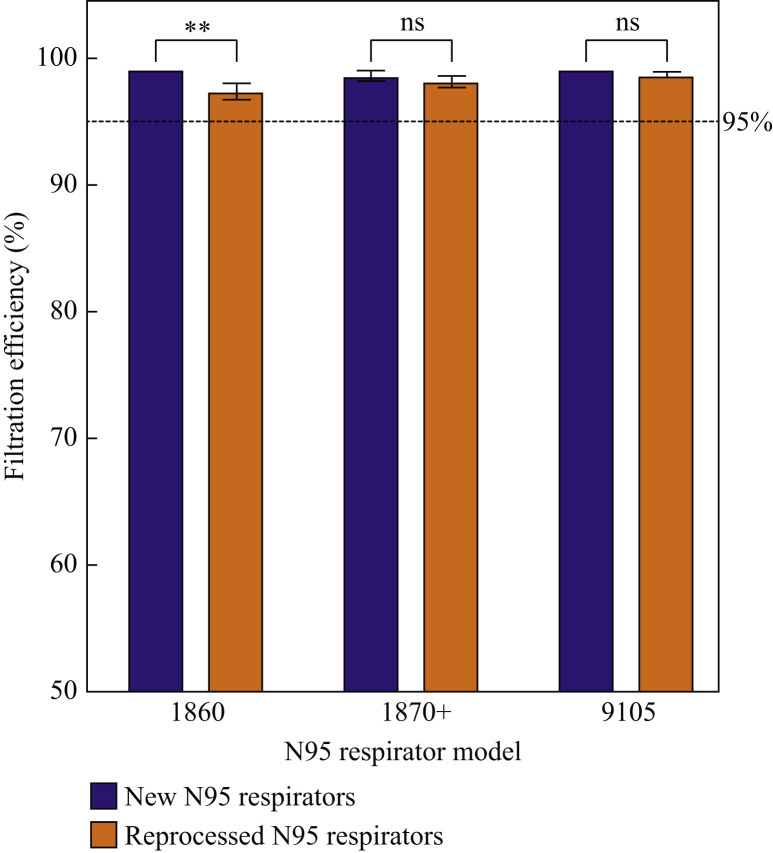
Three N95 respirator models (1860, 1870+, 9105, all new and unused) were tested for filtration efficiency after three full gravity steam reprocessing cycles and compared to the control respirators. Figure 5 shows the filtration efficiency of the respirators against sodium chloride aerosols before and after reprocessing.
Figure 5.
Filtration efficiency of three N95 respirator models before and after three full cycles of gravity steam reprocessing. ∗∗P < 0.004; ns, not significant.
All treated N95 respirators maintained the minimum requirement of 95% filtration efficiency following three full cycles of gravity steam reprocessing, with the 1870+ and the 9105 models showing no significant reduction whatsoever in filtration efficiency following reprocessing, whereas the 1860 model showed only a small (albeit statistically significant) filtration efficiency reduction of <2% (P = 0.004). Thus, the functional filtration performance of the respirators following multiple cycles of gravity steam reprocessing can be maintained at or above the minimum specifications.
Manikin fit evaluation
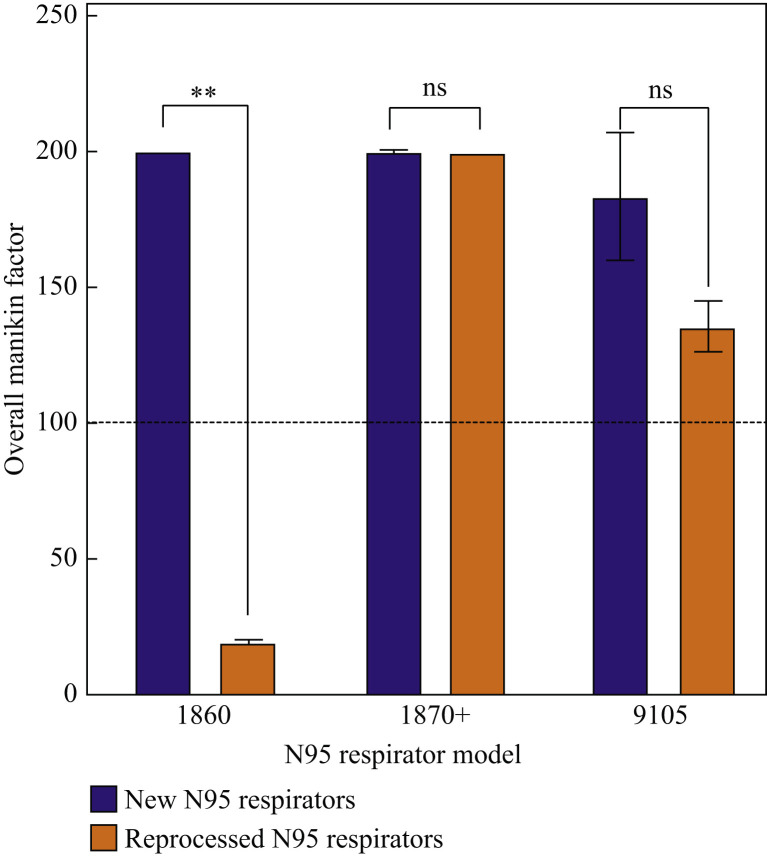
The overall manikin fits of the three reprocessed N95 respirator models (1860, 1870+, 9105) were evaluated and compared to the pre-processed samples, the results of which are shown in Figure 6 .
Figure 6.
Overall manikin fit factors for three N95 respirator models before and after three full cycles of gravity steam reprocessing. ∗∗P < 0.004; ns, not significant.
The treated 1870+ and 9105 N95 respirator models showed no significant decreases in fit compared to the control respirators (P = 0.5 and 0.2 respectively). However, the treated 1860 model suffered a significant decrease in the overall manikin fit factor after three gravity steam cycles (P = 0.004).
Strap integrity evaluation
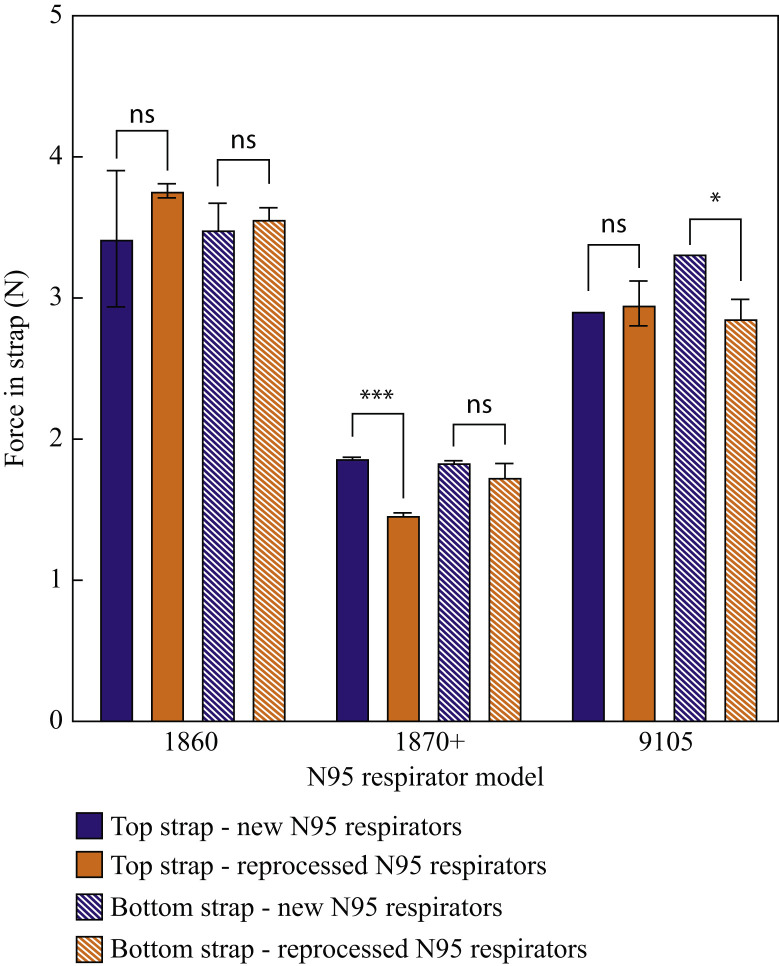
The integrity of both the top and bottom straps was evaluated using tensile testing on both the reprocessed N95 models (1860, 1870+, 9105) and the untreated control respirators (Figure 7 ).
Figure 7.
Strap integrity (based on tensile force exerted by the strap following a pre-stretch protocol) for the top strap and the bottom strap for three N95 respirator models before and after three full cycles of gravity steam reprocessing. ∗P = 0.027; ∗∗∗P = 0.000022; ns, not significant.
Whereas significant variation was observed in the tensile force exerted by the straps of different N95 respirator models, reprocessing had no or very minor effects on the strap properties. The tensile force exerted by both the top and bottom respirator straps of the 1860 respirator model remained unchanged following three cycles of reprocessing whereas the 1870+ and 9105 respirator models showed only small, albeit statistically significant, decreases in strap tensile force in the top strap for the 1870+ respirator (P = 0.000022) and the bottom strap for the 9105 respirator (P = 0.027).
Discussion
The gravity steam cycle demonstrated herein to effectively reprocess single layer N95 respirators is already widely utilized at hospitals in North America and worldwide for other disinfection/sterilization protocols and is thus highly adaptable to existing equipment with high throughputs (∼75 min total per cycle accounting for pre-treatment, heat/cool, reprocessing, and drying times combined). The gravity cycle uses lower pressures and temperatures than common pre-vacuum steam cycles, making the process significantly less aggressive to the polymeric components of the respirators and thus preserving respirator shape and function beyond what can be achieved with conventional steam treatment methods.
The efficacy of the reprocessing cycle was tested following standard test procedures for validating the reprocessing efficacy of reusable medical devices. The ISO 17665-1, Annex D process is conservative by nature, with the treatment determined by extrapolation to correspond to a predicted probability of survival of 10−6 or better [38]. Furthermore, ISO 14937 describes the challenge to the sterilization process (i.e. the load/types of pathogens to be deactivated) as difficult to define, while pre-processing treatments such as cleaning are difficult to control in healthcare facilities [40]. Therefore, sterilization processes applied for respirator reprocessing should be conservative and employ a treatment that exceeds that needed to achieve the specified requirements for sterility, defined conservatively as twice that found to be required to achieve the targeted bacterial deactivation in the microbiology test. Gravity steam cycles were executed using a simulated load that includes the test samples and other packaged devices to mimic real case situations in hospitals. The test samples were placed across the load, in between the other packaged devices and on both top and bottom shelves of the load cart. The 3M Attest Rapid 5 Steam-Plus Test Pack was placed on the bottom shelf and over the drain as this area is considered the most challenging area in the chamber for steam to access due to the circulation patterns in the instrument [36].
A worst-case contaminated respirator was simulated by utilizing respirators that were used in clinical settings and collected from hospitals. These respirators were inoculated with bacterial spores that were shown to be the most resistant to sterilization processes and also represent the worst-case scenario for effective reprocessing; whereas bacterial spores have high heat resistance (>95°C), SARS-CoV-2 is killed at only 56°C at ∼10,000 units per 15 min according to the World Health Organization and even faster at higher relative humidity [[41], [42], [43], [44], [45], [46]]. Furthermore, the cycle effectiveness for inactivating the reference micro-organisms was assessed using a 15 min partial cycle as opposed to a 30 min full cycle. As such, the consistent bacterial inactivation of the 1860 and 1870+ model N95 respirators still achieved under these multi-component challenge conditions is expected only to further improve when a full reprocessing cycle is used.
The shape of the respirator is critical for the effectiveness of the reprocessing procedure because it directly impacts the exposure to, and penetration of, steam for all parts of the device. The traditional cup-shaped 1860/1860s model respirators and the flat design with minimal fold of the 1870+ model respirator allow for optimal steam exposure and penetration, consistent with the high degree of bacterial inactivation observed upon reprocessing. However, the pleats of the 9105 respirators represent a challenging multilayer structure that may result in inadequate/inconsistent penetration of steam between the pleats/multilayers. Note that all Challenge Packs included in the reprocessing loads successfully passed both the Chemical and Biological Indicators tests, indicating that the cycles were successfully executed. As such, the bacterial growth observed on samples 3 and 9 of the 9105 respirators (Figure 4) is likely related to the geometry of that specific respirator rather than to an inherent limitation of the gravity steam process. As such, we would advise caution in extrapolating these results beyond the reprocessing of the respirator models tested herein, particularly when a different model has pleats.
3M (the manufacturer of the tested N95 respirators) has determined that filtration and fit are the two main criteria for successful reprocessing of respirators [36]. In a recent Technical Bulletin, 3M states that ‘3M does not, at this time, recommend the use of High Temperatures above 75°C, such as Autoclave or Steam due to significant filter degradation’ [36]. The results of this work, however, demonstrate that the filtration capacity of all tested gravity steam reprocessed respirators is minimally if at all affected and remains well within the acceptable limit for N95 respirators (Figure 5).
Whereas reprocessed 1870+ and 9105 N95 respirator models pass the fit test, the 1860 N95 respirator model fit parameter was below the acceptable limit following reprocessing (Figure 6). These differences are related to the different structures of the three respirators, with the more rigid cup structure of the 1860 respirator being more susceptible to mild deformation (and thus fit inconsistencies) upon gravity steam reprocessing than the other two models that contain multiple folds and pleats that can better restore fit after reprocessing. In addition, the straps of all tested N95 models proved resilient to reprocessing (Figure 7), with no or minimal reductions in the tensile strength of the straps observed following three full reprocessing cycles. Thus, the 1870+ respirator model in particular is a clear candidate to be reprocessed using a gravity steam process since high bacteria inactivation can be achieved without significantly altering the filtration capacity, fit, or strap integrity of the respirator.
Although the reprocessing cycle proposed in this study was tested against challenging worst-case scenario conditions, it is still recommended that reprocessed respirators are only reused by the original user. Adequate cleaning through the removal of contamination and bodily fluids must be performed before the execution of a full sterilization step. Since the respirators, as of now, cannot be cleaned effectively, the full sterility of the respirators cannot be guaranteed, and cross-infection would remain a risk if different healthcare workers use the respirator after reprocessing. Whereas some blurring of the manufacturer-printed text may be observed following gravity steam reprocessing, permanent marker labelling of the respirator's owner was observed to remain clear following all repeated cycles, allowing easy redistribution to the original user. The relatively short process time (∼75 min from start to finish), the lack of any toxic residual chemicals, the relatively low cost, and the wide availability of suitable equipment at hospital sites that can be repurposed (or copurposed with other routine disinfection tasks required) all offer key advantages of gravity steam reprocessing versus other proposed respirator reprocessing techniques.
In conclusion, gravity steam reprocessing enables the safe and effective reprocessing of N95 respirators over multiple reuse cycles. In particular, the 1870+ model shows high bacterial deactivation while maintaining high filtration capacity, good fit, and consistent strap integrity over at least three cycles of gravity steam reprocessing, with further optimization of the method to better promote steam penetration into the folds of the 9105 model likely also to overcome the occasional failure of this model in the microbiology test protocol. However, the fit quality of the more rigid 1860 model appears to be significantly affected by the gravity steam process and, as such, this protocol is not recommended for this respirator model. It should be emphasized that all these tests were conducted by regulatory agencies governing respirator reuse protocols, making this protocol amenable to practical and immediate adoption by hospitals and other healthcare facilities in the context of current guidelines, while observing the local regulatory requirements. Furthermore, the facile adaptability of this process to existing steam sterilizers available in multiple healthcare settings (unlike most other processes currently being investigated for reprocessing of N95 respirators) makes this process of particular practical utility toward addressing the current widespread shortages of respirators, as is essential to keep healthcare workers safer during the COVID-19 pandemic as well as future health emergencies.
Acknowledgements
The National Personal Protective Technology Laboratory (NPPTL) laboratory at the Centers for Disease Control is gratefully acknowledged for performing all of the reported functionality testing. Humber River Hospital is gratefully acknowledged for providing the used respirators for the reprocessing cycle efficacy testing. SteriPro Canada team is gratefully acknowledged for providing the facility and support required to complete the studies.
Conflict of interest statement
A.A. and A.J. are employees of SteriPro Canada, Inc., which offers medical device reprocessing services to hospitals and has a financial interest in this work. A.J. has indirect equity interest in SteriPro Canada, Inc. E.M., D.A.A., and T.H. have no conflict of interest in this work.
Funding sources
None.
References
- 1.Johns Hopkins Coronavirus Resource Center. COVID-19 map. Available at: https://coronavirus.jhu.edu/map.html [last accessed September 2020].
- 2.Centers for Disease Control and Prevention. COVID-19: strategies for optimizing the supply of PPE. Available at: https://www.cdc.gov/coronavirus/2019-ncov/hcp/ppe-strategy/index.html [last accessed July 2020].
- 3.Vogel L. Canada’s PPE crisis isn’t over yet, say doctors. Can Med Assoc J. 2020:192. doi: 10.1503/cmaj.1095868. E563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ultra Clean Systems, Inc. Reprocessing of single-use items (you know, masks). Available at: https://www.ultracleansystems.com/reprocessing-of-single-use-items-you-know-masks/[last accessed July 2020].
- 5.World Health Organization. Rational use of personal protective equipment for coronavirus disease (COVID-19) and considerations during severe shortages. Available at: https://www.who.int/publications/i/item/rational-use-of-personal-protective-equipment-for-coronavirus-disease-(covid-19)-and-considerations-during-severe-shortages [last accessed July 2020].
- 6.Government of Canada. Update: notice to stakeholders – Health Canada’s regulatory approach to commercial reprocessing of medical devices originally labelled for single use. Available at: https://www.canada.ca/en/health-canada/services/drugs-health-products/medical-devices/activities/announcements/update-notice-stakeholders-regulatory-approach-commercial-reprocessing-medical-devices-originally-labelled-single-use.html [last accessed July 2020].
- 7.Canadian Agency for Drugs and Technologies in Health. Repro-cessing of single-use medical devices: a 2015 update. Available at: https://www.cadth.ca/reprocessing-single-use-medical-devices-2015-update [last accessed July 2020].
- 8.Government of Canada. Important regulatory considerations for the reprocessing of single use N95 respirators during the COVID-19 response: notice. Available at: https://www.canada.ca/en/health-canada/services/drugs-health-products/medical-devices/activities/announcements/covid19-notice-reprocessing-n95-respirators.html [last accessed July 2020].
- 9.US Food and Drug Administration. Reprocessing medical devices in health care settings: validation methods and labeling. Available at: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/reprocessing-medical-devices-health-care-settings-validation-methods-and-labeling [last accessed July 2020].
- 10.Centers for Disease Control and Prevention . 2020. Decontamination and reuse of filtering facepiece respirators.https://www.cdc.gov/coronavirus/2019-ncov/hcp/ppe-strategy/decontamination-reuse-respirators.html Available at: [last accessed July 2020] [Google Scholar]
- 11.Lindsley W.G., Martin S.B., Thewlis R.E., Sarkisian K., Nwoko J.O., Mead K.R. Effects of ultraviolet germicidal irradiation (UVGI) on N95 respirator filtration performance and structural integrity. J Occup Environ Hyg. 2015;12:509–517. doi: 10.1080/15459624.2015.1018518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mills D., Harnish D.A., Lawrence C., Sandoval-Powers M., Heimbuch B.K. Ultraviolet germicidal irradiation of influenza-contaminated N95 filtering facepiece respirators. Am J Infect Control. 2018;46:e49–e55. doi: 10.1016/j.ajic.2018.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamzavi I.H., Lyons A.B., Kohli I., Narla S., Parks-Miller A., Gelfand J.M. Ultraviolet germicidal irradiation: possible method for respirator disinfection to facilitate reuse during the COVID-19 pandemic. J Am Acad Dermatol. 2020;82:1511–1512. doi: 10.1016/j.jaad.2020.03.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oral E., Wannomae K.K., Connolly R., Gardecki J., Leung H.M., Muratoglu O. Vapor H2O2 sterilization as a decontamination method for the reuse of N95 respirators in the COVID-19 emergency. MedRxiv. 2020 doi: 10.1101/2020.04.11.20062026. [DOI] [Google Scholar]
- 15.National Institute of Standards and Technology. Tool for evaluation of vaporized hydrogen peroxide disinfection of N95 masks in small rooms. Available at: https://www.nist.gov/publications/tool-evaluation-vaporized-hydrogen-peroxide-disinfection-n95-masks-small-rooms [last accessed July 2020].
- 16.Kenney P., Chan B.K., Kortright K., Cintron M., Havill N., Russi M. Hydrogen peroxide vapor sterilization of N95 respirators for reuse. MedRxiv. 2020 doi: 10.1101/2020.03.24.20041087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bergman M.S., Viscusi D.J., Heimbuch B.K., Wander J.D., Sambol A.R., Shaffer R.E. Evaluation of multiple (3-cycle) decontamination processing for filtering facepiece respirators. J Eng Fibers Fabrics. 2010;5 doi: 10.1177/155892501000500405. [DOI] [Google Scholar]
- 18.Li D.F., Cadnum J.L., Redmond S.N., Jones L.D., Donskey C.J. It’s not the heat, it’s the humidity: effectiveness of a rice cooker-steamer for decontamination of cloth and surgical face masks and N95 respirators. Am J Infect Control. 2020;48:854–855. doi: 10.1016/j.ajic.2020.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lore M., Heimbuch B., Brown T., Wander J., Hinrichs S. Effectiveness of three decontamination treatments against influenza virus applied to filtering facepiece respirators. Ann Occup Hyg. 2012;56:92–101. doi: 10.1093/annhyg/mer054. [DOI] [PubMed] [Google Scholar]
- 20.Carrillo I.O., Floyd A.C.E., Valverde C.M., Tingle T.N., Zabaneh F.R. Immediate-use steam sterilization sterilizes N95 masks without mask damage. Infect Control Hosp Epidemiol. 2020;41:1104–1105. doi: 10.1017/ice.2020.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ma Q.-X., Shan H., Zhang C.-M., Zhang H.-L., Li G.-M., Yang R.-M. Decontamination of face masks with steam for mask reuse in fighting the pandemic COVID-19: experimental supports. J Med Virol. 2020:1–4. doi: 10.1002/jmv.25921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim H.P., Jo M.S., Kim C.H., Choi J.S., Yu I.J. Re-use of health masks after autoclaving. NanoImpact. 2020;19:100231. doi: 10.1016/j.impact.2020.100231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin T.H., Chen C.C., Huang S.H., Kuo C.W., Lai C.Y., Lin W.Y. Filter quality of electret masks in filtering 14.6–594 nm aerosol particles: effects of five decontamination methods. PLoS One. 2017;12 doi: 10.1371/journal.pone.0186217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Viscusi D., Bergman M., Eimer B., Shaffer R. Evaluation of five decontamination methods for filtering facepiece respirators. Ann Occup Hyg. 2009;53:815–827. doi: 10.1093/annhyg/mep070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.US Food and Drug Administration. Decontamination systems for personal protective equipment EUAs. Available at: https://www.fda.gov/medical-devices/coronavirus-disease-2019-covid-19-emergency-use-authorizations-medical-devices/decontamination-systems-personal-protective-equipment-euas [last accessed July 2020].
- 26.State of New Jersey. Right to know hazardous substance fact sheets. https://www.nj.gov/health/workplacehealthandsafety/right-to-know/hazardous-substances/[last accessed July 2020].
- 27.Association for the Advancement of Medical Instrumentation. TIR12:2010 (AAMI TIR 12:2010). Designing, testing and labeling reusable medical devices for reprocessing in health care facilities: a guide for medical device manufacturers. Available at: https://webstore.ansi.org/standards/aami/aamitir122010tir12 [last accessed July 2020].
- 28.Viscusi D.J., Bergman M.S., Novak D.A., Faulkner K.A., Palmiero A., Powell J. Impact of three biological decontamination methods on filtering facepiece respirator fit, odor, comfort, and donning ease. J Occup Environ Hyg. 2011;8:426–436. doi: 10.1080/15459624.2011.585927. [DOI] [PubMed] [Google Scholar]
- 29.Heimbuch B.K., Wallace W.H., Kinney K., Lumley A.E., Wu C.Y., Woo M.H. A pandemic influenza preparedness study: use of energetic methods to decontaminate filtering facepiece respirators contaminated with H1N1 aerosols and droplets. Am J Infect Control. 2011;39 doi: 10.1016/j.ajic.2010.07.004. e1–9. [DOI] [PubMed] [Google Scholar]
- 30.McNally J, O’Hearn K, Gertsman S, Sampson M, Sikora L, Agarwal A. Microwave- and heat-based decontamination for facemask personal protective equipment (PPE): protocol for a systematic review. OSF Preprints. Available at: https://osf.io/4se6b/[last accessed July 2020].
- 31.Fisher E.M., Williams J.L., Shaffer R.E. Evaluation of microwave steam bags for the decontamination of filtering facepiece respirators. PLoS One. 2011;6 doi: 10.1371/journal.pone.0018585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.US Food and Drug Administration. Coronavirus (COVID-19) update: FDA reissues emergency use authorizations revising which types of respirators can be decontaminated for reuse. Available at: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-reissues-emergency-use-authorizations-revising-which-types [last accessed July 2020].
- 33.Centers for Disease Control and Prevention. 42 CFR Part 84. Respiratory protective devices. Available at: https://www.cdc.gov/niosh/npptl/topics/respirators/pt84abs2.html [last accessed July 2020].
- 36.Standards Council of Canada. CAN/CSA-Z314-18. Conseil canadien des normes. https://www.scc.ca/en/standardsdb/standards/29301 [last accessed July 2020].
- 37.ANSI/AAMI ST79:2017 – Comprehensive guide to steam sterilization and sterility assurance in health care facilities. n.d. Available at: https://webstore.ansi.org/standards/aami/ansiaamist792017 [last accessed September 2020].
- 38.ISO 17665-1:2006(en). Sterilization of health care products – Moist heat – Part 1: Requirements for the development, validation and routine control of a sterilization process for medical devices. https://www.iso.org/obp/ui/#iso:std:iso:17665:-1:ed-1:v1:en [last accessed September 2020].
- 39.Nakasone I., Kinjo T., Yamane N., Kisanuki K., Shiohira C.M. Laboratory-based evaluation of the colorimetric VITEK-2 Compact system for species identification and of the Advanced Expert System for detection of antimicrobial resistances: VITEK-2 Compact system identification and antimicrobial susceptibility testing. Diagn Microbiol Infect Dis. 2007;58:191–198. doi: 10.1016/j.diagmicrobio.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 40.ISO – ISO 14937:2009 – Sterilization of health care products – General requirements for characterization of a sterilizing agent and the development, validation and routine control of a sterilization process for medical devices. Available at: https://www.iso.org/standard/44954.html [last accessed September 2020].
- 41.Block S.S. Lippincott Williams & Wilkins; 2001. Disinfection, sterilization, and preservation. [Google Scholar]
- 42.Centers for Disease Control and Prevention. Disinfection & sterilization guidelines. 2019. Available at: https://www.cdc.gov/infectioncontrol/guidelines/disinfection/efficacy.html [last accessed September 2020].
- 43.Wells-Bennik M.H.J., Janssen P.W.M., Klaus V., Yang C., Zwietering M.H., Den Besten H.M.W. Heat resistance of spores of 18 strains of Geobacillus stearothermophilus and impact of culturing conditions. Int J Food Microbiol. 2019;291:161–172. doi: 10.1016/j.ijfoodmicro.2018.11.005. [DOI] [PubMed] [Google Scholar]
- 44.Watanabe T., Furukawa S., Hirata J., Koyama T., Ogihara H., Yamasaki M. Inactivation of Geobacillus stearothermophilus spores by high-pressure carbon dioxide treatment. Appl Environ Microbiol. 2003;69:7124–7129. doi: 10.1128/AEM.69.12.7124-7129.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.World Health Organization. First data on stability and resistance of SARS coronavirus compiled by members of WHO laboratory network. Available at: https://www.who.int/csr/sars/survival_2003_05_04/en/[last accessed September 2020].
- 46.Chan K.H., Peiris J.S.M., Lam S.Y., Poon L.L.M., Yuen K.Y., Seto W.H. The effects of temperature and relative humidity on the viability of the SARS coronavirus. Adv Virol. 2011;2011 doi: 10.1155/2011/734690. [DOI] [PMC free article] [PubMed] [Google Scholar]