Abstract
Dermacentor variabilis is the predominant tick species in Nebraska and is presumed to be the primary vector of Rickettsia rickettsii associated with cases of Rocky Mountain spotted fever (RMSF). Interestingly, RMSF cases in Nebraska have increased on a year-to-year basis, yet the prevalence of R. rickettsii in D. variabilis ticks has not been established for Nebraska. Here we sought to set a baseline for the prevalence of R. rickettsii and other spotted fever group (SFG) rickettsiae harbored by D. variabilis ticks. Over a 3-yr period, D. variabilis were collected along the Platte River in south central Nebraska. Individual tick DNA was analyzed using endpoint PCR to identify ticks carrying SFG rickettsiae. In total, 927 D. variabilis were analyzed by PCR and 38 (4.1%) ticks tested positive for SFG rickettsiae. Presumptive positives were sequenced to identify the Rickettsia species, of which 29 (76%) were R. montanensis, 5 (13%) were R. amblyommatis, 4 (11%) were R. bellii, and R. rickettsii was not detected. These data indicate that R. rickettsii is likely at a low prevalence in south central Nebraska and spillover of R. amblyommatis into D. variabilis is likely occurring due to the invasive lone star tick (Amblyomma americanum). In addition, our data suggest that R. montanensis and R. amblyommatis could be associated with the increase in SFG rickettsiae infections in Nebraska. This information will be of value to clinicians and the general public for evaluating diagnosis of disease- and risk-associated environmental exposure, respectively.
Keywords: vector-borne diseases, surveillance, prevention
The American dog tick, Dermacentor variabilis, is found throughout most of the United States east of the Rocky Mountains and in a defined area along the west coast including California (Minigan et al. 2018). To maintain tick populations, D. variabilis must feed on three hosts to complete the lifecycle (Oliver 1989). Adult females prefer large mammals for the final feeding to provide the energy required to produce an egg mass while the adult males take a brief bloodmeal to mate with the engorging females. It is generally during this third feeding that the adult ticks come into contact with humans, which potentially exposes these individuals to a tick-borne disease. In the United States, D. variabilis is a vector of both spotted fever group (SFG) rickettsiae and Francisella tularensis (Burgdorfer 1975). Infections with SFG rickettsiae are caused by Rickettsia rickettsii, Rickettsia parkeri, Rickettsia massiliae, Rickettsia akari, and Rickettsia 364D, now designated as Rickettsia philipii (Openshaw et al. 2010). Interestingly, there is suspicion that infections with other SFG rickettsiae may be misdiagnosed as the more severe disease Rocky Mountain spotted fever (RMSF), which would account for the increased percentage of RMSF cases reported in parts of the United States where R. rickettsii are not detected in field-collected D. variabilis ticks (Pagac et al. 2014, Delisle et al. 2016, Blanton 2019).
Infections with SFG rickettsiae have been on the rise in the United States for the past 20 yr (Dumler 2010, Openshaw et al. 2010, Biggs et al. 2016). From 2008 to 2012, the majority of SFG rickettsiae infections were reported in five states in a narrow area from Oklahoma in the west to North Carolina in the east (Drexler et al. 2016), but cases were reported in 48 contiguous states (Openshaw et al. 2010; Drexler et al. 2016). There were 8.9 cases reported per million people in the United States in 2012 (Biggs et al. 2016; Drexler et al. 2016). Nebraska is on the northern edge of the range and had between 9 and 25 cases between 2012 and 2015 (Adams et al. 2014, Adams et al. 2015, Adams et al. 2016, Adams et al. 2017). However, the SFG rickettsiae transmitted by D. variabilis and their prevalence is poorly characterized for Nebraska. The objective of this study was to use molecular methods to determine the identities and prevalence of SFG rickettsiae present in D. variabilis ticks collected along the Platte River in south central Nebraska. These data can help clinicians to know the prevalence of R. rickettsii and properly diagnose suspected cases of SFG rickettsiae in central Nebraska.
Methods
Tick Collection Sites
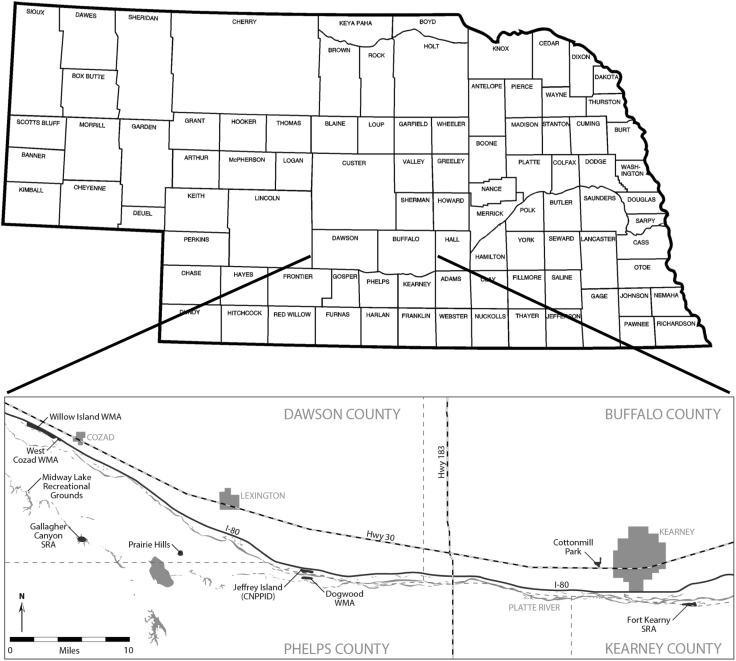
Ticks were collected over three summers (2014–2016) April–August in three counties along the Platte River in south central Nebraska on primarily publicly owned land (Fig. 1). Cases of RMSF have previously been reported in Buffalo and Dawson counties, whereas no confirmed cases of RMSF have been reported in Phelps county (Biggs et al. 2016). Study sites were selected based on ease of public access and the likelihood of citizens encountering potentially infected ticks while participating in outdoor recreation activities. In Buffalo county, ticks were collected at Cottonmill Park and Fort Kearney State Recreation Area (SRA), whereas in Dawson county, ticks were collected at Dogwood Wildlife Management Area (WMA), Jeffrey Island owned by Central Nebraska Public Power and Irrigation District (CNPPID), Prairie Hills bluff owned by Prairie Hills Retrievers, Overton WMA, Gallagher Canyon SRA, Willow Island WMA, Cozad WMA, and West Cozad WMA. In Phelps county, ticks were collected on one private property due south of Overton.
Fig. 1.
Map of collection sites in Buffalo, Dawson, and Phelps counties along the Platte River in Nebraska.
Tick Collection Methods
Dermacentor variabilis ticks were collected by dragging white felt cloth, approximately 1 m wide by 2 m long, through grass or by using carbon dioxide traps consisting of a perforated Styrofoam cooler filled with dry ice and placed on white felt cloth. The species of collected ticks was determined using morphological keys and ticks were then sorted by sex before being stored at −20°C. Dermacentor variabilis ticks were the most prevalent species collected during our study period and adults were selected for use in this study primarily due to the ability to isolate sufficient amounts of DNA from individual ticks for analysis for tick-borne bacterial pathogens without the need to pool ticks from the larval or nymphal stages.
DNA Extraction
Individual adult ticks were thawed at room temperature and surface sterilized by washing in 75% ethanol for 30 s. Once dry, the ticks were transferred to 2-ml tubes containing 2.8-mm stainless steel beads and crushed using Beadbug Microtube Homogenizer (Benchmark Scientific, Edison, NJ) at 3,200 rpm for 6 cycles of 30 s. DNA extraction from individual tick samples was carried out using the Wizard Genomic DNA Purification kit (Promega, Madison, WI) per the manufacturer's instructions for harvesting genomic DNA from yeast. Harvested DNA was quantified using a Nanodrop One (Thermo Fisher Scientific, Waltham, MA), and stored at −20°C to await further analysis.
Identification of SFG Rickettsiae
The harvested DNA from the ticks was screened for SFG rickettsiae DNA using end point PCR. Reactions consisted of Accustart II PCR ToughMix (Quantabio, Beverly, MA), 500 nM primers (Table 1), at least 50 ng of DNA, and molecular grade water to a total volume of 20 µl. PCR cycling conditions were: 3 min at 95°C, 39 cycles of: 10 s at 95°C, 20 s at 62°C, 30 s at 72°C; 5 min at 72°C; and a final holding temperature of 12°C. PCR products were visualized using 5 µl of the reaction in a 1.7% agarose gel stained with ethidium bromide. PCR reactions without DNA were used as negative controls. Potential positive PCR samples were purified using GeneJET PCR purification kit (Thermo Fisher Scientific), and samples sent for Sanger sequencing to the Genomics Core Facility at the University of Nebraska Medical Center (Omaha, NE). Samples PCR products producing low-quality sequences were cloned into pCDNA4.0 using TOPO TA cloning kit (Invitrogen, Carlsbad, CA) per the manufacturer's instructions. Plasmids were purified using the GeneJET Plasmid Miniprep kit (Thermo Fisher Scientific) and sent back for sequencing. Identities were determined by nucleotide BLAST analysis of the sequences against all genomes of the genus Rickettsia in the NCBI database.
Table 1.
Primers
| Organism | Primer Name | ORF | Sequence (5′ ≥ 3′) | Amplicon Size | Reference |
|---|---|---|---|---|---|
| Rickettsia spp. | PR-F | RRM_05200* | ATTCATAACGTCTCACTATCC | 385 bp | This study |
| PR-R | TTTAGAAAAGCTCATAAGGGTAG | ||||
| Rickettsia rickettsii | RR-F | RRM_04030* | TTCTCAATTCGGTAAGGGC | 246 bp | This study |
| RR-R | ATATTGACCAGTGCTATTTC | ||||
| Rickettsia rickettsii | Rr190k.71p | ompA | TGGCGAATATTTCTCCAAAA | 532 bp | (Regnery et al. 1991) |
| Rr190k.620n | AGTGCAGCATTCGCTCCCCCT |
*Rickettsia rickettsii str. Morgan, complete genome (GenBank: CP006010.1).
Statistical Analysis
Statistical comparisons were done using the χ 2 test of independence with the free calculator at socialsciencestatistics.com. P values were set at 0.05.
Results
Over the study period, a total of 927 D. variabilis were collected from three counties along the Platte River in south central Nebraska. Of the 927 ticks tested, 38 (4.1%) tested positive for a SFG rickettsiae (Table 2). Of the 437 males and 490 females collected, 17 males (3.9%) and 21 females (4.3%) were positive for SFG rickettsiae. A χ 2 test of independence was used to compare the occurrence of positive infection between males and females. No significant differences were found in the prevalence of SFG rickettsiae found in males and female ticks, X2(2, n = 927) = 0.0919, P = 0.761731.
Table 2.
No. of adult D. variabilis male and female ticks infected with SFG Rickettsia
| By county | By sex | |||||
|---|---|---|---|---|---|---|
| Buffalo | Dawson | Phelps | Male | Female | Total | |
| Positive | 24 | 13 | 1 | 17 | 21 | 38 |
| Negative | 488 | 365 | 74 | 437 | 490 | 927 |
| % Infected | 3.6 | 4.9 | 1.6 | 3.9 | 4.3 | 4.1 |
This study included ticks collected from three counties along the Platte River: Buffalo, Dawson, and Phelps. In Buffalo county, 488 ticks were collected with 24 ticks (4.9%) positive for Rickettsia spp. (Table 2). Thirteen (3.6%) of the 365 ticks collected in Dawson county were positive, and only 1 (1.4%) of 74 ticks collected in Phelps county was positive for Rickettsia spp. When comparing counties using the χ 2 test of independence, no significant difference was found in the prevalence of SFG rickettsiae found in ticks, X2(2, n = 927) = 2.5219, P = 0.283381.
This study examined the prevalence of SFG rickettsiae in ticks collected over three summers: 2014, 2015, and 2016. Of the 213 ticks collected in 2014, 11 (5%) were positive for SFG rickettsiae, whereas 17 (3%) of the 522 ticks collected in 2015 tested positive. In 2016, 10 (5%) of the 192 ticks collected were positive for SFG rickettsiae. No significant differences in the prevalence of SFG rickettsiae in ticks were found when comparing years using the χ 2 test of independence, X2(2, n = 927) = 2.158, P = 0.339936.
All of the positives PCR products were sent for DNA sequencing. Twenty-nine (76%) of the positive samples were R. montanensis, 4 (11%) were R. bellii, and 5 (13%) were R. amblyommatis. No samples were found to be positive for R. rickettsii in either sex in any year.
Discussion
Infections caused by SFG rickettsiae represent a major public health concern as the incidence rate from 2008 to 2012 increased by 5.8 cases per million person-years (Drexler et al. 2016). Determining the etiological agents responsible for SFG rickettsiae infections is challenging due to the low prevalence of R. rickettsii in resident tick populations along with the misdiagnosis of some patients with RMSF due the close relatedness of R. rickettsii to other SFG rickettsiae (Sumner et al. 2008, McQuiston et al. 2012, Barrett et al. 2014, Drexler et al. 2016). In addition, with the potential for range expansion of ticks and accompanying transmissible disease-causing agents (Sonenshine 2018), native ticks might act as a secondary vector for SFG rickettsiae that are not previously known to be present in specific areas (Henning et al. 2014a, Wright et al. 2015). The results of this study support efforts to provide diligent active surveillance of tick species and subsequent determination of SFG rickettsiae species present in native tick populations to provide the public and clinicians with information about endemic species of SFG rickettsiae that may result in positive diagnoses of RMSF in this region of Nebraska.
Over the course of the study, we identified two SFG rickettsiae species, R. montanensis and R. amblyommatis, and the basal group rickettsiae, R. bellii, at a prevalence of 3.1%, 0.4%, and 0.5%, respectively. Moreover, year to year prevalence of D. variabilis positive for a SFG rickettsiae remained consistent suggesting these Rickettsia spp. are being maintained in the environment in this area of Nebraska. The overall prevalence of these Rickettsia spp. in D. variabilis ticks is similar to what has been observed for the entire United States (Stromdahl et al. 2011, St John et al. 2016). However, the prevalence is lower than 5% and 4% reported for R. amblyommatis (5%) and R. montanensis (4%) in D. variabilis ticks collected in northeast Missouri (Hudman and Sargentini 2016) and for R. amblyommatis (4.7%) in D. variabilis ticks collected in Kansas (Telford et al. 2011). This is likely due to the higher numbers of the lone star tick, Amblyomma americanum in these states (Moncayo et al. 2010). A recent study has shown that A. americanum ticks are expanding their range into south central Nebraska (Cortinas and Spomer 2013). As A. americanum becomes established in south central Nebraska, it is expected that there will be increased spillover of R. amblyommatis into the D. variabilis population, which has similarly been observed for R. parkeri spilling over into D. variabilis and A. americanum from the primary vector the Gulf Coast tick Amblyomma maculatum in the eastern United States (Henning et al. 2014b, Wright et al. 2015).
Despite Nebraska having a high incidence of greater than 10.3 cases of SFG rickettsiae infections per million people in 2016, we did not identify any ticks positive for R. rickettsii or R. parkeri, which are the primary SFG rickettsiae species associated with human disease in the Midwest (Goldsmith et al. 2004, Chen and Sexton 2008). However, this is not surprising since R. rickettsii has rarely been detected in D. variabilis populations (Dergousoff et al. 2009, Moncayo et al. 2010, Stromdahl et al. 2011, Sutton et al. 2018). Moreover, A. maculatum have not been identified in Nebraska (Spomer and Cortinas 2014) nor has it been determined if A. americanum ticks carry R. parkeri in south central Nebraska (unpublished data). This further suggests that cases of RMSF in Nebraska may be attributed to infections with R. montanensis and R. amblyommatis as both have been associated with human infections (Billeter et al. 2007, Apperson et al. 2008, McQuiston et al. 2012). Although unlikely to account for the RMSF cases in Nebraska due to its low prevalence, R. bellii has a potential role in human disease since it has been shown to produce eschars in animals (Ogata et al. 2006). It is possible SFG rickettsiae are transmitted by A. americanum ticks in this area of Nebraska, but further research on the prevalence of SFG rickettsiae is required to determine the public health impact associated with this invasive tick species.
Acknowledgments
This publication was made possible by grants from the University of Nebraska at Kearney's Research Service Council and the National Institute for General Medical Science (NIGMS) (5P20GM103427), a component of the National Institutes of Health (NIH) and its contents are the sole responsibility of the authors and do not necessarily represent the official views of NIGMS or NIH.
References Cited
- Adams D. A., Jajosky R. A., Ajani U., Kriseman J., Sharp P., Onwen D. H., Schley A. W., Anderson W. J., Grigoryan A., Aranas A. E.,. et al. ; Centers for Disease Control and Prevention (CDC). 2014. Summary of notifiable diseases–United States, 2012. MMWR. Morb. Mortal. Wkly. Rep. 61: 1–121. [PubMed] [Google Scholar]
- Adams D., Fullerton K., Jajosky R., Sharp P., Onweh D., Schley A., Anderson W., Faulkner A., and Kugeler K... 2015. Summary of notifiable infectious diseases and conditions - United States, 2013. MMWR. Morb. Mortal. Wkly. Rep. 62: 1–122. [DOI] [PubMed] [Google Scholar]
- Adams D. A., Thomas K. R., Jajosky R. A., Foster L., Sharp P., Onweh D. H., Schley A. W., and Anderson W. J.; Nationally Notifiable Infectious Conditions Group 2016. Summary of notifiable infectious diseases and conditions - United States, 2014. MMWR. Morb. Mortal. Wkly. Rep. 63: 1–152. [DOI] [PubMed] [Google Scholar]
- Adams D. A., Thomas K. R., Jajosky R. A., Foster L., Baroi G., Sharp P., Onweh D. H., Schley A. W., and Anderson W. J.; Nationally Notifiable Infectious Conditions Group 2017. Summary of notifiable infectious diseases and conditions - United States, 2015. MMWR. Morb. Mortal. Wkly. Rep. 64: 1–143. [DOI] [PubMed] [Google Scholar]
- Apperson C. S., Engber B., Nicholson W. L., Mead D. G., Engel J., Yabsley M. J., Dail K., Johnson J., and Watson D. W... 2008. Tick-borne diseases in North Carolina: is “Rickettsia amblyommii” a possible cause of rickettsiosis reported as Rocky Mountain spotted fever? Vector Borne Zoonotic Dis. 8: 597–606. [DOI] [PubMed] [Google Scholar]
- Barrett A., Little S. E., and Shaw E... 2014. “Rickettsia amblyommii” and R. montanensis infection in dogs following natural exposure to ticks. Vector Borne Zoonotic Dis. 14: 20–25. [DOI] [PubMed] [Google Scholar]
- Biggs H. M., Behravesh C. B., Bradley K. K., Dahlgren F. S., Drexler N. A., Dumler J. S., Folk S. M., Kato C. Y., Lash R. R., Levin M. L.,. et al. 2016. Diagnosis and management of tickborne rickettsial diseases: rocky mountain spotted fever and other spotted fever group rickettsioses, ehrlichioses, and anaplasmosis - United States. MMWR. Recomm. Rep. 65: 1–44. [DOI] [PubMed] [Google Scholar]
- Billeter S. A., Blanton H. L., Little S. E., Levy M. G., and Breitschwerdt E. B... 2007. Detection of Rickettsia amblyommii in association with a tick bite rash. Vector Borne Zoonotic Dis. 7: 607–610. [DOI] [PubMed] [Google Scholar]
- Blanton L. S. 2019. The rickettsioses: a practical update. Infect. Dis. Clin. North Am. 33: 213–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgdorfer W. 1975. A review of Rocky Mountain spotted fever (tick-borne typhus), its agent, and its tick vectors in the United States. J. Med. Entomol. 12: 269–278. [DOI] [PubMed] [Google Scholar]
- Chen L. F., and Sexton D. J... 2008. What's new in Rocky Mountain spotted fever? Infect. Dis. Clin. North Am. 22: 415–32, vii. [DOI] [PubMed] [Google Scholar]
- Cortinas R., and Spomer S... 2013. Lone star tick (Acari: Ixodidae) occurrence in Nebraska: historical and current perspectives. J. Med. Entomol. 50: 244–251. [DOI] [PubMed] [Google Scholar]
- Delisle J., Mendell N. L., Stull-Lane A., Bloch K. C., Bouyer D. H., and Moncayo A. C... 2016. Human infections by multiple spotted fever group rickettsiae in tennessee. Am. J. Trop. Med. Hyg. 94: 1212–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dergousoff S. J., Gajadhar A. J., and Chilton N. B... 2009. Prevalence of rickettsia species in canadian populations of dermacentor andersoni and D. variabilis. Appl. Environ. Microbiol. 75: 1786–1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drexler N. A., Dahlgren F. S., Heitman K. N., Massung R. F., Paddock C. D., and Behravesh C. B... 2016. National surveillance of spotted fever group rickettsioses in the United States, 2008-2012. Am. J. Trop. Med. Hyg. 94: 26–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumler J. S. 2010. Fitness and freezing: vector biology and human health. J. Clin. Invest. 120: 3087–3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsmith C. S., Comer J. A., Sumner J. W., Zaki S. R., Paddock C. D., Goddard J., McLellan S. L. F., Tamminga C. L., and Ohl C. A... 2004. Rickettsia parkeri: a newly recognized cause of spotted fever rickettsiosis in the United States. Clin. Infect. Dis. 38: 805–811. [DOI] [PubMed] [Google Scholar]
- Henning T. C., Orr J. M., Smith J. D., Arias J. R., and Norris D. E... 2014. Spotted fever group rickettsiae in multiple hard tick species from Fairfax County, Virginia. Vector Borne Zoonotic Dis. 14: 482–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudman D. A., and Sargentini N. J... 2016. Detection of Borrelia, Ehrlichia, and Rickettsia spp. in ticks in northeast Missouri. Ticks Tick. Borne. Dis. 7: 915–921. [DOI] [PubMed] [Google Scholar]
- McQuiston J. H., Zemtsova G., Perniciaro J., Hutson M., Singleton J., Nicholson W. L., and Levin M. L... 2012. Afebrile spotted fever group Rickettsia infection after a bite from a Dermacentor variabilis tick infected with Rickettsia montanensis. Vector Borne Zoonotic Dis. 12: 1059–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minigan J. N., Hager H. A., Peregrine A. S., and Newman J. A... 2018. Current and potential future distribution of the American dog tick (Dermacentor variabilis, Say) in North America. Ticks Tick. Borne. Dis. 9: 354–362. [DOI] [PubMed] [Google Scholar]
- Moncayo A. C., Cohen S. B., Fritzen C. M., Huang E., Yabsley M. J., Freye J. D., Dunlap B. G., Huang J., Mead D. G., Jones T. F.,. et al. 2010. Absence of Rickettsia rickettsii and occurrence of other spotted fever group rickettsiae in ticks from Tennessee. Am. J. Trop. Med. Hyg. 83: 653–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogata H., La Scola B., Audic S., Renesto P., Blanc G., Robert C., Fournier P. E., Claverie J. M., and Raoult D... 2006. Genome sequence of Rickettsia bellii illuminates the role of amoebae in gene exchanges between intracellular pathogens. PLoS Genet. 2: e76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver J. H., Jr 1989. Biology and Systematics of Ticks (Acari:Ixodida). Ann. Rev. Ecol. Syst. 20: 397–430. [Google Scholar]
- Openshaw J. J., Swerdlow D. L., Krebs J. W., Holman R. C., Mandel E., Harvey A., Haberling D., Massung R. F., and McQuiston J. H... 2010. Rocky mountain spotted fever in the United States, 2000-2007: interpreting contemporary increases in incidence. Am. J. Trop. Med. Hyg. 83: 174–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagac B. B., Miller M. K., Mazzei M. C., Nielsen D. H., Jiang J., and Richards A. L... 2014. Rickettsia parkeri and Rickettsia montanensis, Kentucky and Tennessee, USA. Emerg. Infect. Dis. 20: 1750–1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regnery R. L., Spruill C. L., and Plikaytis B. D... 1991. Genotypic identification of rickettsiae and estimation of intraspecies sequence divergence for portions of two rickettsial genes. J. Bacteriol. 173: 1576–1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonenshine D. E. 2018. Range expansion of tick disease vectors in North America: implications for spread of tick-borne disease. Int. J. Environ. Res. Public Health 15: 478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spomer S. M., and Cortinas R... 2014. Occurrence and county-level distribution of ticks (Acari: Ixodoidea) in nebraska using passive surveillance. J. Med. Entomol. 51: 352–359. [DOI] [PubMed] [Google Scholar]
- St John H. K., Adams M. L., Masuoka P. M., Flyer-Adams J. G., Jiang J., Rozmajzl P. J., Stromdahl E. Y., and Richards A. L... 2016. Prevalence, distribution, and development of an ecological niche model of dermacentor variabilis ticks positive for Rickettsia montanensis. Vector Borne Zoonotic Dis. 16: 253–263. [DOI] [PubMed] [Google Scholar]
- Stromdahl E. Y., Jiang J., Vince M., and Richards A. L... 2011. Infrequency of Rickettsia rickettsii in Dermacentor variabilis removed from humans, with comments on the role of other human-biting ticks associated with spotted fever group Rickettsiae in the United States. Vector Borne Zoonotic Dis. 11: 969–977. [DOI] [PubMed] [Google Scholar]
- Sumner J. W., Zaki S. R., Paddock C. D., Schrodt B. J., Lane C. C., Tamminga C. L., Ohl C. A., McLellan S. L. F., Openshaw J. J., Eremeeva M. E.,. et al. 2008. Rickettsia parkeri Rickettsiosis and Its Clinical Distinction from Rocky Mountain Spotted Fever. Clin. Infect. Dis. 47: 1188–1196. [DOI] [PubMed] [Google Scholar]
- Sutton H., Kakumanu M. L., Apperson C. S., Ponnusamy L., Meshnick S. R., and Nicholson W. L... 2018. Prevalence of rickettsia species (Rickettsiales: Rickettsiaceae) in Dermacentor variabilis Ticks (Acari: Ixodidae) in North Carolina. J. Med. Entomol. 55: 1284–1291. [DOI] [PubMed] [Google Scholar]
- Telford S. R. III, Goethert H. K., Cunningham J., and Berrada Z. L... 2011. Rickettsia rickettsii (Rickettsiales: Rickettsiaceae) in Amblyomma americanum (Acari: Ixodidae) From Kansas. J. Med. Entomol. 48: 461–467. [DOI] [PubMed] [Google Scholar]
- Wright C. L., Sonenshine D. E., Gaff H. D., and Hynes W. L... 2015. Rickettsia parkeri transmission to Amblyomma americanum by cofeeding with Amblyomma maculatum (Acari: Ixodidae) and potential for spillover. J. Med. Entomol. 52: 1090–1095. [DOI] [PubMed] [Google Scholar]