Abstract
BACKGROUND
Intraoperative cone-beam computed tomography (iCBCT) allows for rapid 3-dimensional imaging. However, it is currently unknown whether this imaging technique offers sufficient accuracy for stereotactic registration during deep brain stimulation (DBS) procedures.
OBJECTIVE
To determine the accuracy of iCBCT, with the O-arm O2 (Medtronic), for stereotactic registration by comparing this modality to stereotactic magnetic resonance imaging (MRI).
METHODS
All DBS patients underwent a preoperative non-stereotactic 3 Tesla MRI, stereotactic 1.5 Tesla MRI, stereotactic O-arm iCBCT, postimplantation O-arm iCBCT, and postoperative conventional multidetector computed tomography (CT) scan. We compared stereotactic (X, Y, and Z) coordinates of the anterior commissure (AC), the posterior commissure (PC), and midline reference (MR) between stereotactic MRI and iCBCT. For localisation comparison of electrode contacts, stereotactic coordinates of electrode tips were compared between the postoperative multidetector CT and iCBCT.
RESULTS
A total of 20 patients were evaluated. The average absolute difference in stereotactic coordinates of AC, PC, and MR was 0.4 ± 0.4 mm for X, 0.4 ± 0.4 mm for Y, and 0.7 ± 0.5 mm for Z. The average absolute difference in X-, Y-, and Z-coordinates for electrode localisation (N = 34) was 0.3 ± 0.3 mm, 0.6 ± 0.3 mm, and 0.6 ± 0.6 mm. These differences were small enough not to be considered clinically relevant.
CONCLUSION
Stereotactic MRI and O-arm iCBCT yield comparable coordinates in pre- and postoperative imaging. Differences found are below the threshold of clinical relevance. Intraoperative O-arm CBCT offers rapid stereotactic registration and evaluation of electrode placement. This increases patient comfort and neurosurgical workflow efficiency.
Keywords: O-arm, Accuracy, Stereotactic planning, Deep brain stimulation
ABBREVIATIONS
- AC
anterior commissure
- CT
computed tomography
- DBS
deep brain stimulation
- FOV
field of view
- HD
high definition
- iCBCT
intraoperative cone-beam computed tomography
- kV
kilo voltage
- MPRAGE
magnetization-prepared rapid gradient-echo
- MR
midline reference
- MRI
magnetic resonance imaging
- PC
posterior commissure
- PD
Parkinson's disease
- SD
standard deviation
- TE
echo time
- TR
repetition time
- TSE
turbo spin echo
Deep brain stimulation (DBS) is a surgical technique applied for both movement disorders and psychiatric disorders.1-4 Therapy success is closely linked to accuracy of electrode placement. Placement accuracy is determined by the total error of individual contributing procedural components. In a contemporary DBS workflow, at least the following contributing factors must be taken into account: the platform used for placement (stereotactic frame), stereotactic imaging, co-registration, and intraoperative imaging.5 Due to imaging advancements, DBS teams have the possibility to adapt these factors. Although improvement of surgical workflow and patient comfort is pursued by these
adaptations, stringent evaluation before definite implementation is mandatory.
We recently implemented the use of cone-beam computed tomography (O-arm O2 Imaging System; Medtronic Inc, Dublin, Ireland) during DBS procedures. In recent years, the O-arm system has shown to provide sufficient accuracy for intraoperative electrode localisation.6-8 However, stereotactic imaging using the O-arm for frame-based procedures was hampered by the narrow field of view (FOV) of 20 cm, not allowing capturing of the complete localising box. The new generation O-arm Imaging System, the O-arm O2, is equipped with a FOV of 40 cm. This enables conducting stereotactic registration, potentially offering rapid stereotactic acquisition.8 So far, the accuracy of O-arm for stereotactic imaging during frame-based procedures has not been reported. To address this issue, we compared our conventional workflow using preoperative stereotactic magnetic resonance imaging (MRI) with that of intraoperative stereotactic O-arm scanning. We compared the localization of key structures in stereotactic space between the two imaging modalities and the differences in workflow.
METHODS
Patients
Prospective data were collected from all patients who underwent DBS surgery at our institution between July 2017 and February 2018 and for whom a complete set of preoperative 1.5 Tesla and 3.0 Tesla MRI and intraoperative O-arm scanning was performed. The surgical technique was discussed with all patients, and all gave informed consent. The Medical Ethics Committee of our institution reviewed the study protocol and confirmed that the Dutch Medical Research Involving Human Subjects Act (WMO) is not applicable. Access to an X-ray shielded operating theatre was obligatory for O-arm imaging and was available to a limited extent during data collection. A complete set for the current study consisted of preoperative volumetric T1-weighted 3 Tesla MRI, stereotactic T1-weighted 1.5 Tesla MRI, and stereotactic O-arm imaging, postimplantation intraoperative cone-beam computed tomography (iCBCT), and postoperative multidetector computed tomography (CT) scan. Electrode placement was done using stereotactic MRI. Stereotactic O-arm iCBCT was conducted additionally to enable current postoperative comparison. The rationale for implementing O-arm imaging for intraoperative electrode localisation during our DBS procedures was discussed with all patients.
Surgical Procedure
Our surgical procedure of DBS placement is described in detail in previous work.1,9,10 Patients were operated under local anesthesia or general anesthesia depending on their indication for undergoing DBS, necessity of intraoperative macrostimulation, and the capability to withstand DBS under local anesthesia. Both the Leksell® Coordinate Frame G (Elekta AB, Stockholm, Sweden) and Leksell® VantageTM (Elekta) system were used. All patients received direct placement of the infraclavicular stimulator under general anesthesia.
DBS Using Stereotactic MRI
The stereotactic frame was placed either with local anesthesia on the neurosurgical ward or under general anesthesia in the operating theatre. The patient was then transported to the 1.5 Tesla MRI to perform stereotactic imaging. The stereotactic 1.5 Tesla MRI was co-registered with the preoperative 3 Tesla MRI with the use of SurgiPlan (Elekta AB, Stockholm, Sweden). The 2 sequences and parameters used with the 3T MRI (Philips, Eindhoven, The Netherlands) are (a) 3D sagittal T1-weighted magnetization-prepared rapid acquisition gradient echo (MPRAGE), slice thickness: 0.9 mm, FOV: 256 × 256 mm, SENS: 2.5, repetition time (TR): 8,8 ms, echo time (TE): 4.0 ms, and acquisition time: 4.18 min, and (b) T2-weighted 2D axial and coronal Turbo spin echo (TSE), slice thickness: 2.2 mm, FOV: 230 × 230 mm, TR: 5014 ms, TE: 88 ms, and acquisition time: 3.83 min. For the 1.5T MRI (Siemens, Malvern, Pennsylvania), the parameters included the following: (a) 3D sagittal T1-weighted MPRAGE, slice thickness 1.0 mm, FOV: 256 × 256 mm, TR: 1900 ms, and TE: 2.92 ms, and (b) T2 axial and coronal 2D TSE, slice thickness: 2.0 mm, TR: 5750 ms, TE: 99 ms, FOV: 256 × 256 mm, and acquisition time: 4.43 min. In Surgiplan, each non-stereotactic imaging set is to be co-registered separately to the stereotactic imaging set.
Two members of the neurosurgical team visually inspected the co-registrations, at least one of whom was a DBS surgeon (P.R.S. or P.v.d.M). Multiple structures were used for verification, including the anterior commissure (AC) and posterior commissure (PC), ventricles, outlines of the mesencephalon, gyri, and sulci, and the course of blood vessels. Planning performed on 3 Tesla imaging was then verified on 1.5 Tesla imaging, and frame coordinates of the targets and entry point were calculated. The patient was then transported to the operating theatre. During surgery, electrode implantation was verified with anterior-posterior and lateral O-arm fluoroscopy. On the postoperative day, a multidetector CT scan was performed. This CT scan was co-registered with the intraoperative stereotactic 1.5 Tesla MRI to evaluate electrode position. Co-registration was visually inspected using the cranium for verification.
DBS Using Stereotactic O-arm
After the stereotactic MRI and transport to the operating room, a stereotactic iCBCT was performed using the O-arm O2. For this, the ‘stereotactic mode’ was applied (40 cm FOV, 192 slices, 120 kilovolt [kV] and 150 milliampere [mAs]). The gantry was orientated in line with horizontal plane of the localising box. The head is fixated to the operating room table with an angle varying between 10 to 30 degrees to the horizontal, depending on the comfort of the patient during awake surgery. The Leksell® Coordinate Frame G is fixated to the table using a custom-build 3-point fixation. The Leksell® VantageTM frame is fixated to the table using the Leksell® VantageTM Frame Holder and the DORO® (Pro Med Instruments GmbH, Freiburg im Breisgau, Germany) base unit and swivel adaptor. The stereotactic iCBCT was co-registered with the preoperative 3 Tesla MRI. The assessment of registration accuracy is described in the previous paragraph. After placement of both electrodes, a second iCBCT was performed in ‘high definition (HD)’ mode (20 cm FOV, 192 slices, 120 kV, and 150 mAs). The gantry was orientated in line with the cranium, enabling complete depiction. The HD iCBCT was co-registered with the stereotactic iCBCT.
Comparison of Stereotactic Registration
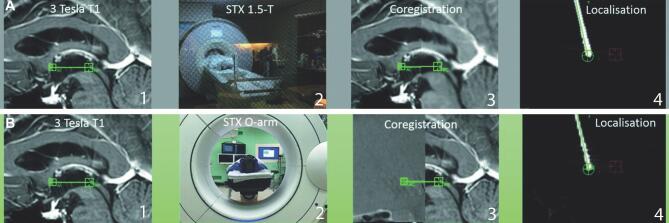
The O-arm registration accuracy was verified by comparison of stereotactic coordinates defined by MRI and O-arm in Surgiplan. The X-, Y-, and Z-coordinates of the AC, PC, and midline reference (MR) were used for comparison. These reference points were visually defined based on anatomy using preoperative T1 weighted 3 Tesla MRI. The 3 Tesla MRI was then (separately) co-registered to the stereotactic 1.5 Tesla MRI and stereotactic O-arm iCBCT. The definition of these points and the co-registration was done by the neurosurgeon performing the DBS surgery (P.R.S. or P.v.d.M.). In this way, stereotactic coordinates resulting from both stereotactic techniques could be compared. An example of the workflow implemented for current comparison is shown in Figure 1.
FIGURE 1.
Imaging workflow in four stages for DBS using stereotactic MRI A and DBS using O-arm iCBCT B, from left to right: A, (1) Definition of AC-PC line on T1-weighted 3 Tesla MRI; (2) stereotactic 1.5 Tesla MRI; (3) co-registration of stereotactic imaging to T1-weighted 3 Tesla MRI; and (4) localisation of the electrode tip after co-registration of the postoperative multidetector CT scan with preoperative stereotactic imaging. B, (1) Definition of AC-PC line on T1-weighted 3 Tesla MRI; (2) stereotactic iCBCT; (3) co-registration of stereotactic imaging to T1-weighted 3 Tesla MRI; and (4) localisation of the electrode tip after co-registration of the HD iCBCT with preoperative stereotactic imaging.
Comparison of Electrode Localisation
Comparison of electrode localisation described both registration accuracy as co-registration accuracy of postimplantation imaging. For this, the postoperative multidetector CT scan was co-registered to the stereotactic 1.5 Tesla MRI and the HD iCBCT was co-registered to the stereotactic iCBCT. The X-, Y-, and Z-coordinates of the electrode tip were defined for both techniques, enabling comparison. An example of determination of the electrode tip is shown in Figure 1. The determination of these points and the co-registration was done by the neurosurgeon who performed the DBS surgery (P.R.S. or P.v.d.M.). All were reviewed by the first author and M.B.
Euclidean Distance
For all complete imaging sets, the differences between the AC, PC, and MR coordinates based on the stereotactic MRI and stereotactic iCBCT were calculated. These differences (errors) were used to calculate the Euclidean distance:
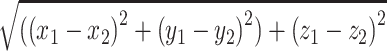 |
Statistical Analysis
The average, standard deviation (SD), minimum, and maximum differences between AC, PC, MR, and electrode tip coordinates were calculated for absolute, directional, and Euclidean differences. A paired students’ T test or Wilcoxon Signed Rank test was used accordingly in order to compare the difference in stereotactic registration and between electrode localisation. Results with a P < .05 were considered statistically significant.
RESULTS
After conducting 20 DBS cases (40 electrodes), the current analysis was performed. The average age in the sample of 12 males and 8 females was 59 (range 23–76) yr. The target for DBS was the subthalamic nucleus for Parkinson's disease (PD) (N = 16), the internal globus pallidus for dystonia (N = 2), the ventral intermediate nucleus for essential tremor (ET) (N = 1), and the ventral part of the anterior limb of internal capsule for Obsessive Compulsive Disorder (N = 1). Awake surgery was performed in 11 cases (1 ET, 10 PD), and implantation under general anesthesia was performed in nine cases. Fifteen patients were operated using the Leksell® Coordinate Frame G (Elekta) and five patients with the Leksell® VantageTM (Elekta) frame.
Comparison of Stereotactic Registration
The average absolute differences in X-, Y-, and Z-coordinates for reference points AC, PC, and MR between the frame-based MRI and frame-based O-arm iCBCT were all less than 1 mm, as shown in Table 1. The average absolute difference ± SD in X-, Y-, and Z-coordinates of all reference points together was 0.4 ± 0.4 mm, 0.4 ± 0.4 mm, and 0.7 ± 0.5 mm. Euclidean distances (±SD) were 1.0 ± 0.6 mm, 0.9 ± 0.5 mm, and 1.3 ± 0.6 mm for AC, PC, and MR, respectively.
TABLE 1.
Average Differences ± SD in Stereotactic Coordinates for AC, PC, and MR
| Reference point | Coordinate | Absolute distance ± SD (mm) | Range (mm) | P valuea |
|---|---|---|---|---|
| AC | X | 0.3 ± 0.2 | 0.0-0.7 | >.85 |
| Y | 0.3 ± 0.3 | 0.0-1.0 | >.75 | |
| Z | 0.8 ± 0.6 | 0.0-2.2 | .006 | |
| Euclidean | 1.0 ± 0.6 | 0.1-2.5 | .031 | |
| PC | X | 0.3 ± 0.2 | 0.0-0.9 | .086 |
| Y | 0.4 ± 0.3 | 0.0-1.1 | >.64 | |
| Z | 0.7 ± 0.5 | 0.0-1.7 | .053 | |
| Euclidean | 0.9 ± 0.5 | 0.2-2.1 | .057 | |
| MR | X | 0.6 ± 0.5 | 0.0-1.9 | >.56 |
| Y | 0.6 ± 0.5 | 0.2-1.9 | .018 | |
| Z | 0.7 ± 0.5 | 0.1-1.7 | .038 | |
| Euclidean | 1.3 ± 0.6 | 0.6-2.8 | .024 |
AC: anterior commissure; PC: posterior commissure; MR: midline reference; SD: standard deviation.
aPaired T test or Wilcoxon Signed Rank test used accordingly.
A statistically significant difference is found between the AC Z-coordinates (P = .006) and Euclidean distance (P = .031). Furthermore, a significant difference is found between MR Y-coordinates (P = .018), Z-coordinates (P = .038), and Euclidean distance (P = .024).
Electrode Localisation
In 17 cases, a postimplantation iCBCT and a multidetector CT scan was made for comparison. In one awake DBS case for PD, the postimplantation iCBCT was not made due to patient fatigue. In the last 2 cases, adequate electrode placement was confirmed on the iCBCT and, at the discretion of the surgeon, the multidetector CT scan on the postoperative day was not performed. The average absolute differences (±SD) in X-, Y-, and Z-coordinates of electrode tips were 0.3 ± 0.3 mm, 0.6 ± 0.3 mm, and 0.6 ± 0.6 mm, respectively. All absolute differences are shown in Table 2.
TABLE 2.
Average Differences ± SD in Stereotactic Coordinates for Electrode Tips
| Electrode | Coordinate | Absolute distance ± SD (mm) | Range (mm) | P valuea |
|---|---|---|---|---|
| Left | X | 0.3 ± 0.3 | 0.1-1.1 | >.37 |
| Y | 0.7 ± 0.4 | 0.1-1.4 | .030 | |
| Z | 0.7 ± 0.5 | 0.0-1.7 | .005 | |
| Euclidean | 1.1 ± 0.5 | 0.4-2.2 | .055 | |
| Right | X | 0.3 ± 0.6 | 0.0-1.3 | >.38 |
| Y | 0.6 ± 0.3 | 0.0-1.0 | .060 | |
| Z | 0.6 ± 0.6 | 0.0-2.1 | .003 | |
| Euclidean | 1.0 ± 0.5 | 0.3-2.3 | .032 |
aPaired T test or Wilcoxon Signed Rank test used accordingly.
A statistically significant difference is found between the Y-coordinates (0.030) and Z-coordinates (P = .005) of the left electrode. Furthermore, a significant difference is found between the Z-coordinates (P = .003) and Euclidean distance (P = .032) of the right electrodes.
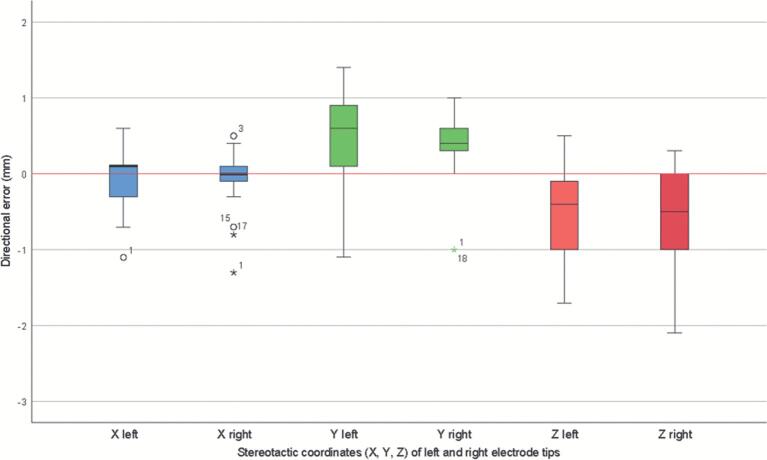
The directional error in electrode tip differences, calculated by subtracting the iCBCT coordinates from the multidetector CT coordinates, is shown in Figure 2. The largest difference is found in the Z-axis, the craniocaudal direction: electrode tips are more inferiorly defined on the postimplantation iCBCT in comparison to the postoperative multidetector CT. Furthermore, electrode tips are more anteriorly defined on the iCBCT in comparison to the multidetector CT.
FIGURE 2.
Directional electrode tip differences. Boxplot showing the median, interquartile range, confidence interval (whiskers), outliers (dots), and extreme cases of individual variables (stars).
DISCUSSION
We compared stereotactic registration using MRI and O-arm iCBCT in frame-based DBS procedures and showed that both techniques deliver a very similar coordinate space after stereotactic registration. O-arm stereotactic registration together with 3 Tesla co-registration provides sufficient accuracy for performing DBS surgery. To our knowledge, this is the first study comparing these different DBS workflows.
Absolute mean differences in stereotactic registration points in our study were ≤0.8 mm. Placement error of the Leksell® (Elekta) system exceeds found differences; hence, we feel that these differences in registration, despite statistical significance, are not clinically relevant.9,11 Foremost, any clinically important differences are corrected by the micro-electrode recordings performed before placement of the definitive electrode is accomplished. Absolute mean differences in lead representation were ≤0.7 mm. As mentioned before, despite found statistical significance, we consider these differences not clinically relevant.
DBS Workflow
Intraoperative stereotactic O-arm CBCT enables DBS surgery to start immediately after co-registration of MRI-based non-stereotactic planning. This offers advantages for both awake and asleep DBS. For awake DBS, patient endurance is optimally used due to the immediate start of DBS surgery after stereotactic registration. For both awake and asleep DBS, the surgical workflow is simplified by omitting transport to the MRI or CT suite for stereotactic imaging.
Postimplantation Imaging
When adding the step of co-registration of postimplantation imaging, the equivalent accuracy between the two techniques persists. The O-arm provides sufficient accuracy for electrode evaluation. Other groups evaluating O-arm for electrode localisation found similar results.6-8,11-16 Intraoperative evaluation enables direct electrode adjustments when indicated. During awake DBS, intraoperative evaluation of electrode placement offers additional anatomical insights; for asleep DBS, this is essential.
Asleep DBS
Asleep DBS is increasingly being performed, and it is the way forward in terms of patient comfort and optimizing neurosurgical workflow efficacy. The O-arm can be well implemented in this workflow. In our experience, O-arm implementation accelerated our DBS workflow, currently enabling performing two asleep DBS cases per day. Currently, we are waiting for the results of our randomized controlled trial comparing awake vs asleep DBS in PD patients.10
O-arm Limitations
The dose profile of O-arm is comparable to a multidetector CT scanner. Nevertheless, care must be taken to limit the number of O-arm scans performed, thereby reducing effective radiation exposure.17,18 A disadvantage of the O-arm iCBCT in comparison to the multidetector CT scan is the disability of good soft tissue reference.13 This is further reduced due to scattering of frame pins and electrodes. Therefore, it is suboptimal for detection of ventricle penetration or an intracerebral haemorrhage during DBS procedures.14 An additional multidetector CT is, in our view, indicated in patients with neurological deficit after electrode placement, in order to rule out intracerebral complications.
Study Limitations
The current study has several limitations. First, the group of patients studied was small. However, our group size is comparable to other published reports.6-8,11-16,19 The level of evidence needed to implement the O-arm iCBCT for stereotactic registration is, in our view, sufficiently met by our data. Second, there was considerable heterogeneity among the (small group of) patients with respect to underlying disease, stereotactic target, awake vs asleep surgery, and the choice of stereotactic frame. A more homogeneous population might have been more appropriate for a study with such small sample size. Third, localisation of electrode tips on postimplantation iCBCT and postoperative multidetector CT scan was performed in separate Surgiplan (Elekta) sessions. The observed differences in X-, Y-, and Z- coordinates of electrode tips may have been confounded by this method. Finally, the use of two different stereotactic frames may have influenced the data found. We did not perform any subgroup analyses because no conclusion can be drawn when no statistically significant difference is found in these small subgroups.
CONCLUSION
Stereotactic MRI and O-arm iCBCT produce comparable definition of coordinates in stereotactic coordinate space. Differences found are below the threshold of clinical relevance. Intraoperative O-arm CBCT offers rapid stereotactic registration and evaluation of electrode placement. This increases patient comfort and increases neurosurgical workflow efficacy.
Disclosures
The authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
Notes
The abstract of this manuscript was presented in an oral poster presentation at the 18th biennial meeting of the World Society for Stereotactic and Functional Neurosurgery, 24-27 June 2019, in New York, New York.
Contributor Information
Rozemarije A Holewijn, Department of Neurosurgery, Amsterdam University Medical Centers, Academic Medical Center (AMC), Amsterdam, The Netherlands.
Maarten Bot, Department of Neurosurgery, Amsterdam University Medical Centers, Academic Medical Center (AMC), Amsterdam, The Netherlands.
Pepijn van den Munckhof, Department of Neurosurgery, Amsterdam University Medical Centers, Academic Medical Center (AMC), Amsterdam, The Netherlands.
P Richard Schuurman, Department of Neurosurgery, Amsterdam University Medical Centers, Academic Medical Center (AMC), Amsterdam, The Netherlands.
REFERENCES
- 1. Odekerken VJ, van Laar T, Staal MJ et al.. Subthalamic nucleus versus globus pallidus bilateral deep brain stimulation for advanced Parkinson's disease (NSTAPS study): a randomised controlled trial. Lancet Neurol. 2013;12(1):37-44. [DOI] [PubMed] [Google Scholar]
- 2. Volkmann J, Mueller J, Deuschl G et al.. Pallidal neurostimulation in patients with medication-refractory cervical dystonia: a randomised, sham-controlled trial. Lancet Neurol. 2014;13(9):875-884. [DOI] [PubMed] [Google Scholar]
- 3. Schuurman PR, Bosch DA, Merkus MP, Speelman JD. Long-term follow-up of thalamic stimulation versus thalamotomy for tremor suppression. Mov Disord. 2008;23(8):1146-1153. [DOI] [PubMed] [Google Scholar]
- 4. Denys D, Mantione M, Figee M et al.. Deep brain stimulation of the nucleus accumbens for treatment-refractory obsessive-compulsive disorder. Arch Gen Psychiatry. 2010;67(10):1061-1068. [DOI] [PubMed] [Google Scholar]
- 5. Kloc M, Kosutzka Z, Steno J, Valkovic P. Prevalent placement error of deep brain stimulation electrode in movement disorders (technical considerations). Bratisl Lek Listy. 2017;118(11):647-653. [DOI] [PubMed] [Google Scholar]
- 6. Bot M, van den Munckhof P, Bakay R, Stebbins G, Verhagen Metman L. Accuracy of intraoperative computed tomography during deep brain stimulation procedures: comparison with postoperative magnetic resonance imaging. Stereotact Funct Neurosurg. 2017;95(3):183-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Caire F, Guehl D, Burbaud P, Benazzouz A, Cuny E. Intraoperative 3D imaging control during subthalamic deep brain stimulation procedures using O-arm(R) technology: experience in 15 patients. Neurochirurgie. 2014;60(6):276-282. [DOI] [PubMed] [Google Scholar]
- 8. Holloway K, Docef A.. A quantitative assessment of the accuracy and reliability of O-arm images for deep brain stimulation surgery. Neurosurgery. 2013;72(1 Suppl Operative):47-57. [DOI] [PubMed] [Google Scholar]
- 9. Bot M, van den Munckhof P, Bakay R, Sierens D, Stebbins G, Verhagen Metman L. Analysis of stereotactic accuracy in patients undergoing deep brain stimulation using Nexframe and the Leksell Frame. Stereotact Funct Neurosurg. 2015;93(5):316-325. [DOI] [PubMed] [Google Scholar]
- 10. Holewijn RA, Verbaan D, de Bie RMA, Schuurman PR. General Anesthesia versus Local Anesthesia in StereotaXY (GALAXY) for Parkinson's disease: study protocol for a randomized controlled trial. Trials. 2017;18(1):417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Caire F, Gantois C, Torny F, Ranoux D, Maubon A, Moreau JJ. Intraoperative use of the Medtronic O-arm for deep brain stimulation procedures. Stereotact Funct Neurosurg. 2010;88(2):109-114. [DOI] [PubMed] [Google Scholar]
- 12. Carlson JD, McLeod KE, McLeod PS, Mark JB. Stereotactic Accuracy and surgical utility of the O-arm in deep brain stimulation surgery. Oper Neurosurg. 2017;13(1):96-107. [DOI] [PubMed] [Google Scholar]
- 13. Katisko JP, Kauppinen MT, Koivukangas JP, Heikkinen ER. Stereotactic operations using the O-arm. Stereotact Funct Neurosurg. 2012;90(6):401-409. [DOI] [PubMed] [Google Scholar]
- 14. Servello D, Zekaj E, Saleh C, Pacchetti C, Porta M. The pros and cons of intraoperative CT scan in evaluation of deep brain stimulation lead implantation: A retrospective study. Surg Neurol Int. 2016;7(Suppl 19):S551-556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shahlaie K, Larson PS, Starr PA. Intraoperative computed tomography for deep brain stimulation surgery: technique and accuracy assessment. Neurosurgery. 2011;68(1 Suppl Operative):114-124; discussion 124. [DOI] [PubMed] [Google Scholar]
- 16. Sharma M, Deogaonkar M.. Accuracy and safety of targeting using intraoperative “O-arm” during placement of deep brain stimulation electrodes without electrophysiological recordings. J Clin Neurosci. 2016;27:80-86. [DOI] [PubMed] [Google Scholar]
- 17. Zhang J, Weir V, Fajardo L, Lin J, Hsiung H, Ritenour ER. Dosimetric characterization of a cone-beam O-arm imaging system. J Xray Sci Technol. 2009;17(4):305-317. [DOI] [PubMed] [Google Scholar]
- 18. Dose considerations - O-arm surgical imaging system. In. Available at: https://www.medtronic.com/us-en/healthcare-professionals/products/neurological/surgical-imaging-systems/o-arm/dose-considerations.html. Accessed May 22, 2019.
- 19. Frizon LA, Shao J, Maldonado-Naranjo AL et al.. The safety and efficacy of using the O-arm intraoperative imaging system for deep brain stimulation lead implantation. Neuromodulation. 2018;21(6):588-592. [DOI] [PubMed] [Google Scholar]