Abstract
Objective. Evidence to date, while sparse, suggests that patients taking long-term opioids require special considerations and protections to prevent potential iatrogenic harms from opioid de-prescribing, such as increased pain or suffering. Following this study protocol, the EMPOWER study seeks to address multiple unmet needs of patients with chronic pain who desire to reduce long-term opioid therapy, and provide the clinical evidence on effective methodology. Methods. EMPOWER applies patient-centered methods for voluntary prescription opioid reduction conducted within a comprehensive, multi-state, 3-arm randomized controlled comparative effectiveness study of three study arms (1) group cognitive behavioral therapy for chronic pain; (2) group chronic pain self-management; and (3) usual care (taper only). Specialized electronic data capture systems collect patient reported symptoms and satisfaction data weekly and monthly during the taper, with real-time clinical alerts and electronic feedback loops informing, documenting, and steering needed care actions. Conclusion. The EMPOWER study seeks to provide granular evidence on patient response to voluntary opioid tapering, and will provide evidence to inform clinical systems changes, clinical care, patient satisfaction, and patient outcomes for opioid reduction.
Keywords: Opioids, chronic pain, taper, EMPOWER, CHOIR
Introduction
Chronic pain erodes the health and quality of life of up to one-third of US population [1,2] and causes tremendous burdens for patients, families, and caregivers. In older adults, the prevalence of chronic pain may be as high as 40% [3]. Furthermore, an estimated 20 million live with high-impact chronic pain, with substantially restricted work, social, and self-care activities [4]. Opioids are one of the most commonly used treatments for chronic pain. An estimated 11 million Americans were prescribed long-term opioids in 2014, 3.4% of the US adult population [5]. Long-term opioid use carries significant health risks, including addiction and accidental overdose [6–8]. High daily opioid consumption is associated with enhanced risk. Patients taking a ≥200 morphine equivalent (MEDD) daily dose have three times the risk of death from accidental overdose compared with patients taking lower doses [8].
Lower daily doses of opioids are safer [9]. However, opioid de-prescribing (tapering) also confers health risk, suggesting that improved taper methods are important for safety and efficacy. Opioid dose changes—either increases or decreases in dose—were associated with enhanced risk for unintentional overdose [10], clearly refuting a common misperception that opioid reduction uniformly reduces health risks. Furthermore, poor tapering practices, such as poor patient selection, forced tapering, rapid tapering, and tapering to a prespecified dose, expose patients to serious iatrogenic risks including withdrawal symptoms, increased pain and distress, risk of overdose, and psychiatric destabilization, including worsening mood, suicidal ideation, and suicide [11–14]. Although multiple stakeholder groups have denounced poor opioid tapering practices and called for evidence-based opioid deprescribing [13–18], best practices guidance and systems for safe, effective, and supportive pain and opioid reduction are lacking. The evidence to date, while sparse, suggests that patients taking long-term opioids require special considerations and protections to prevent the above iatrogenic harms from opioid de-prescribing and ensure that the taper does not increase pain or suffering.
Intensive, interdisciplinary, inpatient, and outpatient opioid and pain reduction programs successfully reduce opioids and pain concomitantly (e.g., [19]). Such programs involve daily patient contact, ongoing management of symptoms, and intensive behavioral treatment, as well as psychological and social support. While successful, these programs are costly and inaccessible to the vast majority of patients with chronic pain.
Our voluntary, community-based, outpatient, patient-centered opioid tapering study [20] revealed that a low-cost patient-centered opioid tapering method yielded good patient engagement. Sixty-two percent of the eligible patients joined the study. At four months, we found roughly a 50% average reduction in MEDD without increased pain. However, psychosocial variables remained static over the study period, suggesting that integrated behavioral treatment may enhance multidimensional outcomes in voluntary patient-centered opioid tapering.
Patient-centered principles and “whole-person” pain care [21] stipulate that the focus should never be solely on opioid reduction. The biopsychosocial model of pain treatment should be applied to facilitate not only goals for pain and opioid reduction but improved function and quality of life. Cognitive behavioral therapy for chronic pain (pain-CBT) and the Chronic Pain Self-Management Program (CPSMP) are two evidence-based treatments for chronic pain, but neither treatment has been studied within the context of active opioid reduction. This need for patient-centered, biopsychosocial approaches to voluntary opioid weaning motivated our Effective Management of Pain and Opioid-Free Ways to Enhance Relief (EMPOWER) study.
The EMPOWER study seeks to address multiple unmet needs of patients with chronic pain who desire to reduce long-term opioid therapy. Patient and broad stakeholder voices were carefully integrated into the study design, including the methods, choice of outcomes, title, and logo. EMPOWER applies patient-centered methods to a comprehensive multistate three-arm randomized controlled comparative effectiveness study. The arms consist of 1) group pain-CBT, 2) group CPMSP, and 3) usual care (Taper Only) within the context of voluntary prescription opioid reduction. Additional information may be sourced at the study website (https://www.empower.stanford.edu; ClinicalTrials.gov Identifier: NCT03308188).
Methods
Study Design
This is a pragmatic prospective longitudinal, multicenter, three-arm, cluster-randomized controlled clinical trial conducted in 865 patients receiving daily prescription opioids in primary care and pain clinics at EMPOWER study clinics (see Table 1 for a list of current study sites).
Table 1.
Current EMPOWER study sites*
| Study Site | Setting | Payer System | Type | Location |
|---|---|---|---|---|
| Veteran’s Affairs Health Care | Primary care | Closed network | Veterans Affairs | Phoenix, AZ |
| Stanford Pain Management | Pain clinic | Open | Academic | Redwood City, CA |
| Stanford Primary Care | Primary care | Open | Academic | Palo Alto, CA |
| Stieg Pain Clinic | Pain clinic | Open | Private practice | Frisco, CO |
| Stieg Pain Clinic | Pain clinic | Open | Private practice | Edwards, CO |
| MedNOW Clinics | Primary care | Open | Private practice | Denver metro area, CO |
| Intermountain Health | Primary care | Closed network | Closed Network (civilian) | Layton, UT |
EMPOWER = Effective Management of Pain and Opioid-Free Ways to Enhance Relief study.
New study sites may be added over the project period.
Inclusion Criteria
Participants were adults 18–85 years of age with chronic noncancer pain (six or more months in duration) receiving prescription opioids (≥10 MEDD) for three or more months, interested in participating in a 12-month patient-centered opioid reduction program, willing to be randomized to one of the three study arms and participate in behavioral treatment if assigned. Patients referred or self-referred to the study from an outside clinic had to be able to have all opioid prescribing transferred to an EMPOWER-trained prescriber for the 12-month study period.
Exclusion Criteria
Exclusion criteria included cognitive impairment, pregnancy, lack of English fluency, inability to provide informed consent, active suicidal ideation, behavioral concerns that could negatively impact the group treatment process, and moderate to severe opioid use disorder (OUD).
Opioid Use Disorder Screening Protocol
Our study team clinical consensus was that in our study settings opioid tapering was acceptable in patients with mild OUD but was contraindicated for those with moderate to severe OUD. Patients taking long-term daily opioids who also had moderate to severe OUD required addiction medicine assessment and alternate treatment pathways than those presented here; this is a subject that will be discussed in a separate manuscript. As such, the EMPOWER investigator team developed an OUD/Opioid Tapering Suitability Protocol that reflects consensus from a national team of eight chronic pain clinician experts (one pain psychologist and seven pain physicians who also prescribe and taper opioids in patients with chronic pain). Four of the consensus team physicians are addiction medicine trained and/or are dually board certified in addictionology and pain medicine.
The EMPOWER Opioid Tapering Suitability Protocol includes two steps: 1) OUD screening using three items from the validated online screening tool Tobacco, Alcohol, Prescription Medication and other Substance use Tool (TAPS) [22]; 2) patients who screen positive for OUD based on the three TAPS items will be administered the DSM-5 Criteria Checklist for Opioid Use Disorder (OUD) [23] to assess OUD severity (see the Supplementary Data for the EMPOWER Opioid Tapering Suitability Protocol). Patients with no OUD or mild OUD will be offered enrollment in EMPOWER; patients with moderate or severe OUD will be referred for addiction medicine evaluation.
Specific Aims
Aim 1
To reduce or contain prescription opioid use while maintaining pain control.
Hypothesis 1a
At one year, 40% of patient participants with no behavioral treatment (Taper Only) will successfully reduce opioids (≥50% reduction in MEDD), but pain intensity and pain interference and role functioning will not worsen.
Hypothesis 1 b
Success with patient-centered opioid tapering will be unrelated to starting dose.
Aim 2
Compare the effectiveness of 1) pain-CBT or 2) CPSMP or 3) Taper Only for all patients receiving voluntary patient-centered opioid tapering.
Hypothesis 2a
More patient participants receiving behavioral interventions (pain-CBT or CPSMP) will reduce their opioid dose than those who received Taper Only.
Hypothesis 2 b
There will be no differences in dosage reduction or percentage of participants reducing dosage between those receiving pain-CBT and CPSMP.
Hypothesis 2c
Those receiving pain-CBT will have less pain, less pain interference, and decreased depression compared with those receiving CPSMP or Taper Only.
Hypothesis 2d
Those receiving CPSMP will have greater improvement in role functioning and self-efficacy compared with pain-CBT or no behavioral treatment (Taper Only).
Exploratory Hypothesis 2e
There will be less opioid escalation for patients in the behavioral intervention groups than for those not receiving behavioral intervention (Taper Only).
Study Procedures
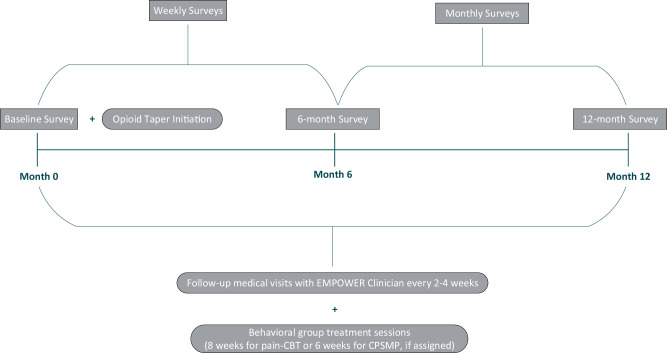
See Figure 1 following screening for OUD, patients will provide informed consent, complete baseline study measures, and will be assigned to one of the three study treatment arms (pain-CBT, CPSMP, Taper Only). The opioid taper program will be initiated per the description below. Patients will receive follow-up medical visits every two to four weeks during active opioid tapering and will remain in the study for 12 months, regardless of the status of their opioid taper. Patients will receive up to $220 for completing study surveys.
Figure 1.
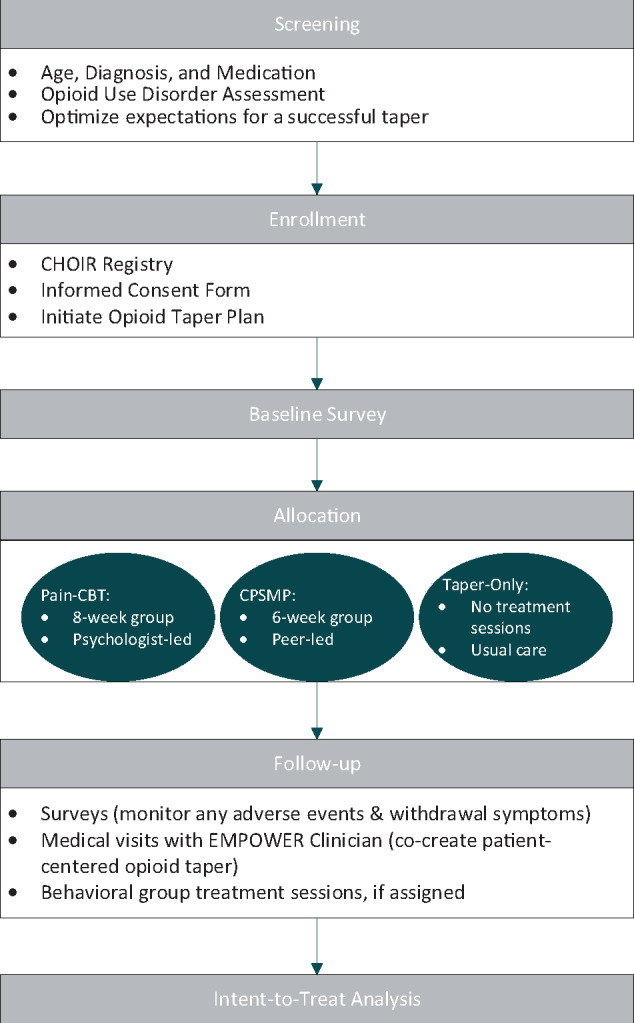
Study flow diagram.
Informatics Platform
The Collaborative Health Outcomes Information Registry (CHOIR; https://choir.stanford.edu/) is an electronic informatics platform that serves multiple study functions. CHOIR collects patient-reported data at each time point using surveys that are tailored to the study and common across all study sites. Additional CHOIR functions include obtaining online consent, automated post-enrollment randomization, deployment of tailored surveys based on treatment arm assignment, a patient-reported outcomes data catchment system that minimizes response burden through use of computer-automated testing for National Institutes of Health Patient-Reported Outcomes Measurement Information System (NIH PROMIS) [24] measures, a centralized study database, an automated electronic payment system following survey completion, display of patient progress available to study clinicians for clinical decision supports, generation of a personalized opioid tapering plan (see below), monitoring for patient adverse events, and deployment of alerts sent to various stakeholders (to address patient risks, minimize missing data, address patient participant payments). Clinicians may view individual patient progress and longitudinal data in real time. Paper surveys are mailed to patients who lack access to e-mail, computers, or smartphone texting, and study staff input data into the electronic database.
Voluntary Patient-Centered Opioid Tapering
All enrolled patients participate in a voluntary patient-centered opioid tapering program tailored to their individual needs. The EMPOWER taper program may only be administered by a prescriber clinician trained in the EMPOWER methods. Training entails a four- to six-hour training on the program methods and support systems provided to clinicians and patients. Training strongly emphasizes communication with patients to align with collaborative, patient-centered care. Methods emphasize assessing patient motivation to participate in voluntary opioid reduction, supporting patients in their decision-making, validating patient concerns, minimizing nocebo related to opioid reduction, and optimizing placebo (expectations) for positive outcomes.
The basic patient-centered opioid taper protocol mirrors the pilot methods described in Darnall et al. [20]. Importantly, the taper goal is only complete opioid cessation if the patient chooses that goal. The recommended taper speed is 5% reduction of the starting dose, with a one-dose decrease monthly until the patient is stable, without physical discomfort or psychological distress. Patients are assessed at each visit; dose decreases and increased taper speed occur only after consultation with and consent from patients. Patients have a choice in the pace of their taper, may pause their taper, and are free to drop out of their taper or the study at any time. The taper is not unidirectional: EMPOWER does not constrain against needed opioid increases for acute pain. The study does not constrain against increasing opioids in cases where patients have poor response to the taper. Participation in the study does not prevent patients from receiving any needed medical care. Patients are not removed from the study if they increase opioid dose; to the contrary, we are interested in following these patients and understanding their experience, their responses, and their unmet treatment needs.
The protocol encourages use of adjuvants within the scope of normal clinical practice and training and does not constrain other analgesic prescribing, such as initiation of selective norepinephrine reuptake inhibitor medication. The protocol provides guidance on short- and long-acting opioid reduction, and it is recommended that clinicians only taper one medication at a time. As such, concurrent tapering of other medications is discouraged.
CHOIR Opioid Taper Tool
EMPOWER includes an electronic taper tool that allows clinicians to input the patient’s current dose, number of daily doses, desired taper time frame, desired taper goal, and desired taper pace. The output provides clinicians and patients with precise guidance on doses/pills per dose daily over the course of months. Because all elements of a taper are flexible, the opioid taper tool may be used multiple times to re-calculate a patient’s taper plan based on new circumstances, such as desired taper pause or patient desire to increase or decrease the speed of their taper.
Weekly CHOIR Surveys
All patients receive, via e-mail, weekly CHOIR e-surveys to assess for any taper discomfort symptoms or other problems, with the explicit purpose of informing clinical care, symptom triage, and any needed adjustment to their taper plan. Taper-related symptoms of moderate severity trigger an automated e-alert to the prescriber, study site director, study site coordinator, and study manager. Patients receive automatic e-response messages alerting to possible and recommended courses of action, including calling the clinic for telephone triage or an in-clinic visit.
At each follow-up visit, taper discomfort symptoms are reviewed and confirmed by the prescribing clinician to either be related or unrelated to the taper. Moderate and severe symptoms are reported to the local institutional review board (IRB), the Data Safety and Monitoring Board (DSMB), and the study sponsor.
Monthly CHOIR Surveys
Patients are assessed monthly for satisfaction with the study, current opioid use (confirmed via chart review), hospital or emergency medical visits, depressive symptoms, suicidality, and other factors that may indicate worsening of symptoms and require follow-up. Suicidality triggers e-alerts to the prescribing clinician, the site director, the site coordinator, the study manager, and the overall principal investigator. Site-specific messages are sent to the patient with instructions for nonemergency triage (e.g., call the clinic for telephone or in-person follow-up) and emergency triage in their community (e.g., 911), rapid contact with clinic staff, and crisis hotlines.
Weekly and monthly surveys provide careful patient monitoring, triaging of problems, and close connections with study staff and the prescribing provider so that the taper can be rapidly adjusted as needed and any additional needs addressed. Study clinicians may access EMPOWER CHOIR via a web browser for secure review of patient responses and to coordinate patient participant activities. Larger survey batteries are administered at baseline, six months, and 12 months (Supplementary Data). See Figure 2 for display of survey time points.
Figure 2.
Participant study timeline and procedures.
Study Arms
1) Cognitive Behavioral Therapy for pain (pain-CBT) [25] is a behavioral treatment that reduces chronic pain and its impacts through patient education, skills acquisition, and social support. Pain-CBT research suggests benefits for reducing pain intensity, pain catastrophizing, depression, and the social impact of pain [26]. CBT is considered the gold-standard psychobehavioral treatment for pain, with other behavioral treatments demonstrating noninferiority but not superiority [27,28]. A general pain-CBT therapist manual and corresponding patient workbook were developed for the EMPOWER study (EMPOWER CBT). A relaxation MP3 audiofile accompanies the patient workbook. Consistent with general pain-CBT, the EMPOWER CBT protocol includes interactive discussion, pain education, relaxation training, goal setting, cognitive restructuring, problem solving, and action planning, with home exercises incorporated into each session. Participants learn how to best manage their pain and symptoms while moving toward achieving the goals that matter to them. A mental health therapist trained in the EMPOWER CBT protocol will deliver the eight-week group intervention to cohorts of patients (N = 8–18) assigned to this treatment arm. Treatment sessions are delivered weekly and are two hours in length. For consistency across all study sites and cohorts, therapists will complete fidelity checklists at every EMPOWER CBT session to ensure that the session content adheres to the EMPOWER CBT manual.
Participants assigned to this treatment arm cannot receive the CPSMP during the study period. No other aspects of pain care are constrained.
2) The Chronic Pain Self-Management Program (CPSMP) [29,30] is an evidence-based behavioral treatment that is delivered by two certified health care clinicians or peer co-leaders who have lived experience in successful pain self-management. Leader certification requires 24 hours of training received over four days. The CPSMP manualized protocol includes patient education about pain and ways to effectively self-manage pain, its impacts, and other symptoms. It is based on self-efficacy theory and delivered in six weekly 2.5-hour group sessions (N = 8–18). All participants receive a book on pain self-management with an exercise CD. Similar to pain-CBT, treatment fidelity checklists are completed by certified instructors at each session to ensure that the administration of CPSMP is consistent across all study sites and cohorts. Similar to pain-CBT, CPSMP incorporates interactive discussion, relaxation training, action planning, and home exercises into each session. Patients learn how to live better with chronic pain by making daily choices that support better health and function. Participants assigned to this treatment arm will not receive group pain-CBT during the study period. No other aspects of pain care are constrained, including receipt of individual psychological treatment; we are tracking receipt of nonstudy treatments.
Prior research has shown that pain-CBT and CPSMP have both shared and distinct effects, suggesting that individuals may have a better response to one treatment vs the other, though no evidence exists to guide a best-practice choice between the two. Although pain-CBT and CPSMP are effective for chronic pain, they have not been tested within an active opioid reduction protocol. We hypothesize that these behavioral treatments will help patients reduce opioids and associated risks, manage pain, and restore function in ways superior to opioid reduction alone.
3) In Taper Only (usual care) arm, participants are involved in the voluntary EMPOWER patient-centered opioid tapering program and do not receive either group pain-CBT or CPSMP during the 12-month study period. No other aspects of pain care are constrained.
The study will be conducted in accordance with the common protocol and procedures that are approved through local IRBs at all study sites.
Randomization
Each site will conduct a site-specific RCT using a cluster randomized design to allow for rapid accrual of patient cohorts (N = 8–18 per cohort) assigned to the same treatment arm and timely delivery of the behavioral treatments for the patients assigned to them. As such, patients who enter the study are automatically assigned to the currently open treatment arm. Patients and clinicians are blinded to the ordering of the treatment arms and are unable to choose or direct group assignment. Study coordinators are unblinded and trained to maintain clinician and patient blinding. After enrollment and completion of baseline measures, group assignment is immediately revealed to the patient participant. Unique randomization schemes were created for each study site by a statistician with no direct involvement with any patient participants. The randomization scheme allows for relatively quick receipt of behavioral treatment for patients who are assigned to one of the behavioral treatment arms (ideally within two to 10 weeks of taper initiation). Timing of behavioral treatment receipt is recorded.
Data Protections
In CHOIR, data transport is SSL encrypted, and data storage and access are HIPAA compliant (requiring two-factor authentication). E-mail notifications for CHOIR surveys will not contain Protected Health Information (PHI). Electronic data will be maintained in a secure database, on servers behind two firewall layers, each accessible only via two-factor authentication. Extensive IP address whitelisting ensures controlled data access. Data validity will be maintained by validity criteria in the database and error-checking procedures. Data will be sent securely (double encrypted) to the study manager with a unique identifier and will be linked to each participant’s CHOIR data file. Multiple data checks will ensure data integrity and accuracy of data linkage.
Ongoing Patient Feedback and Systems for Improvement
Patients may provide narrative feedback in their monthly surveys, and we provide electronic systems for anonymous patient suggestions and complaints. Annually we conduct focus groups with patients at each study site to learn about the patient experience. All of these data sources allow the research team to calibrate study procedures based on patient feedback. Our study advisory board consists of individuals not currently enrolled in the study who serve as patient representatives, individuals who are themselves patients with lived experience with opioid reduction, family members, and professionals who treat and research chronic pain. On an ongoing basis, we will utilize an EMPOWER virtual national patient advisory board to collect broad patient feedback from people with diverse backgrounds who are not enrolled in the study.
Variable Measurement and Data Reporting
See the Supplementary Data for a description of all study time points and instruments. Data source adequacy is assured by our emphasis on patient-reported outcomes to characterize the patient experience, with additional objective data types and sources listed below. We use the PROMIS measures and other reliable and valid measures that are largely considered gold standard in the field and follow the IMMPACT Guidelines [31]. We follow reporting guidelines described at http://www.equator-network.org/.
Additional Data Types and Sources
Opioid medications and MEDD at baseline, month 6, and month 12 (extracted from medical records).
Prescription drug monitoring program data.
Behavioral class attendance (sourced from class attendance sheets) and any patient withdrawals from treatment (patient or clinician initiated).
Insurance type and copayment requirements for medical visits.
Number of medical visits (quantified at six and 12 months for preceding six months).
Appropriate addiction medicine referrals ensure patient protections.
Adverse events (medical chart review, in addition to the patient-reported adverse events).
Power Calculation
We will enroll 865 patients and expect that the statistical analyses can be conducted based on 250 patients per study arm. We assume 250 patients will be allocated to each arm with key outcomes for the final analysis. To achieve this goal, we will enroll 815 patients assuming a retention rate of 92%, which is defined as the proportion of patients whose key outcomes are available at the end of the study to allow ITT analysis (based on completion of the final survey; this rate does not refer to behavioral treatment adherence).
The power consideration is primarily based on the aims for comparative effectiveness. In total, we will randomize using cluster randomization with variable cluster sizes, stratified by site, a minimum 750 patients into one of three groups: 1) CBT, 2) CPSMP, or 3) Taper Only (no behavioral group treatment) with equal probabilities. We also assume an annual dropoff rate of <8%, and we will include all dropouts in our ITT analyses.
Analytic Plan
Aim 1
To reduce or contain prescription opioid use while maintaining pain control.
Hypothesis 1a
We will estimate the proportion of patients with patient-centered opioid taper success and test if the proportion is >40% (estimate for treatment success derived from Darnall et al. [32]. We will estimate the binomial proportion of patients with opioid taper success, using the exact test to test if the proportion is >40%.
Hypothesis 1 b
We will compare the success rates of opioid tapering between patients in different initial opioid dose categories: low (10–49 MEDD), moderate (50–89 MEDD), high (90–199 MEDD), and super high (>200 MEDD).
Aim 2
We will examine the balance in key patient characteristics such as initial opioid dose, pain intensity, and functional measures separately for the three treatment arms. We will use the chi-square test (or Fisher exact test) to compare the success rates between different opioid dose groups.
Hypothesis 2a
We will compare the treatment success rate between 500 patients receiving behavioral interventions (pain-CBT and CPSMP) and 250 patients receiving patient-centered opioid tapering only (no behavioral treatment; Taper Only). We will use the chi-square test to compare the success rate between the group of patients assigned to a behavioral treatment arm and the Taper Only group. The logistic regression will be used to adjust for baseline covariates.
Hypothesis 2b
We will estimate the difference in the treatment success rate between the pain-CBT and CPSMP arms using an intent-to-treat procedure. We will estimate the difference in the treatment success rate between the two arms and construct the 95% confidence interval.
Hypothesis 2c
We will compare the change in pain intensity, pain interference, and depression scores (baseline to 12 months) between pain-CBT and Taper Only, as well as between pain-CBT and CPSMP, using a two-sample t test. We will use a two-sample t test to compare the 12-month change in pain intensity, interference, and depression scores between the pain-CBT and Taper Only arms and between the pain-CBT and CPSMP arms. The analysis of covariance based on multiple regression will be used to adjust for baseline covariates as well.
Exploratory Hypothesis 2e
We will estimate the probability of opioid escalation by treatment arm (pain-CBT, CPSMP, and Taper Only) separately.
Heterogeneity of treatment effects will be explored for each treatment arm, considering gender, age, race, initial opioid dose, baseline depression, anxiety level, insurance type, copayment burden, and medical comorbidities.
Access and Treatment Participation
Comparison across treatments will be performed according to the intent-to-treat principle. Additional comparisons according to the actual treatment received will be conducted as a sensitivity analysis. We will also study the effect of treatment participation and treatment payment burdens on treatment effect. To this end, we will perform regression to examine the association of the success rate for opioid weaning and other outcomes with session attendance for the CBT and CPSMP arms, separately, while adjusting for identified confounding factors affecting compliance. We will also investigate how the baseline factors such as insurance type, copayment amount, and social economic status affect the compliance level. We will assess reasons for missed sessions to determine the impact of financial burden on treatment participation and subsequent treatment effects to inform practical implementation of our results.
Sensitivity Analysis
We will perform sensitivity analyses to account for missing data and noncompletion of treatments. In addition, we will consider an alternative definition of treatment success response, with the 50% reduction in opioid dose being replaced by a 30% reduction in opioid dose; a 30% change has been cited as a benchmark for moderately clinically important difference in chronic pain literature [33]. Similar analyses will be performed with this new end point as part of sensitivity analyses.
Study Timeline
Enrollment remains open until November 2021.
Correspondence to: Beth D. Darnall, PhD, Department of Anesthesiology, Perioperative and Pain Medicine, Psychiatry and Behavioral Sciences (by courtesy), Stanford University School of Medicine, 1070 Arastradero Road, Suite 200, MC5596, Palo Alto, CA 94304, USA. Fax: 650-725-9642; E-mail: bdarnall@stanford.edu.
Funding sources: This work was supported through a Patient-Centered Outcomes Research Institute (PCORI) Project Program Award (OPD-1610–37 007; PI, Darnall).
Disclaimer: All statements in this report are solely those of the authors and do not necessarily represent the views of the Patient-Centered Outcomes Research Institute (PCORI), its Board of Governors, or Methodology Committee.
Supplementary Material
References
- 1.National Institutes of Health Interagency Pain Research Coordinating Committee. National Pain Strategy. 2015. Available at: http://iprcc.nih.gov/National_Pain_Strategy/NPS_Main.htm (accessed June 2019).
- 2.Institute of Medicine. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington, DC: The National Academies Press; 2011. [PubMed] [Google Scholar]
- 3. Johannes CB, Le TK, Zhou X, Johnston JA, Dworkin RH.. The prevalence of chronic pain in United States adults: Results of an Internet-based survey. J Pain 2010;11(11):1230–9. [DOI] [PubMed] [Google Scholar]
- 4. Dahlhamer J, Lucas J, Zelaya C, et al. Prevalence of chronic pain and high-impact chronic pain among adults—United States, 2016. MMWR Morb Mortal Wkly Rep 2018;67(36):1001–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mojtabai R. National trends in long-term use of prescription opioids. Pharmacoepidemiol Drug Saf 2018;27(5):526–34. [DOI] [PubMed] [Google Scholar]
- 6. Gomes T, Mamdani MM, Dhalla IA, Paterson JM, Juurlink DN.. Opioid dose and drug-related mortality in patients with nonmalignant pain. Arch Intern Med 2011;171(7):686–91. [DOI] [PubMed] [Google Scholar]
- 7. Dhalla IA, Mamdani MM, Gomes T, Juurlink DN.. Clustering of opioid prescribing and opioid-related mortality among family physicians in Ontario. Can Fam Physician 2011;57(3):e92–96. [PMC free article] [PubMed] [Google Scholar]
- 8. Gomes T, Juurlink D, Moineddin R, et al. Geographical variation in opioid prescribing and opioid-related mortality in Ontario. Healthc Q 2011;14(1):22–4. [DOI] [PubMed] [Google Scholar]
- 9. Edlund MJ, Martin BC, Fan MY, Devries A, Braden JB, Sullivan MD.. Risks for opioid abuse and dependence among recipients of chronic opioid therapy: Results from the TROUP study. Drug Alcohol Depend 2010;112(1–2):90–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Glanz JM, Binswanger IA, Shetterly SM, Narwaney KJ, Xu S.. Association between opioid dose variability and opioid overdose among adults prescribed long-term opioid therapy. JAMA Netw Open 2019;2(4):e192613.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Demidenko MI, Dobscha SK, Morasco BJ, Meath THA, Ilgen MA, Lovejoy TI.. Suicidal ideation and suicidal self-directed violence following clinician-initiated prescription opioid discontinuation among long-term opioid users. Gen Hosp Psychiatry 2017;47:29–35. [DOI] [PubMed] [Google Scholar]
- 12. Dowell D, Haegerich T, Chou R.. No shortcuts to safer opioid prescribing. N Engl J Med 2019;380(24):2285–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.US Food and Drug Administration Safety Communication. FDA identifies harm reported from sudden discontinuation of opioid pain medicines and requires label changes to guide prescribers on gradual, individualized tapering. Available at: https://wwwfdagov/drugs/drug-safety-and-availability/fda-identifies-harm-reported-sudden-discontinuation-opioid-pain-medicines-and-requires-label-changes (accessed May 2019).
- 14.Human Rights Watch. Not allowed to be compassionate: Chronic pain, the overdose crisis, and unintended harms in the US. 2018. Available at: https://www.hrw.org/sites/default/files/report_pdf/hhr1218_web.pdf (accessed June 2019).
- 15.US Department of Health and Human Services. Pain Management Best Practices Inter-Agency Task Force report. Available at: https://www.hhs.gov/sites/default/files/pmtf-final-report-2019-05-23.pdf2019 (accessed June 2019).
- 16.Alford DP, Dart RC, JDeMicco J, Kertesz SG, Satel S. Health professionals for patients in pain (letter to the CDC). Available at: https://healthprofessionalsforpatientsinpain.org/the-letter-1. 2019. (accessed June 2019).
- 17. Darnall B. Applause for the CDC opioid guideline authors. Available at: https://thehill.com/opinion/healthcare/440837-applause-for-the-cdc-opioid-guideline-authors (accessed June 2019).
- 18. Darnall BD. The national imperative to align practice and policy with the actual CDC opioid guideline. Pain Med 2019; (doi.org/10.1093/pm/pnz152). [DOI] [PubMed] [Google Scholar]
- 19. Murphy JL, Clark ME, Banou E.. Opioid cessation and multidimensional outcomes after interdisciplinary chronic pain treatment. Clin J Pain 2013;29(2):109–17. [DOI] [PubMed] [Google Scholar]
- 20. Darnall BD, Ziadni MS, Stieg RL, Mackey IG, Kao MC, Flood P.. Patient-centered prescription opioid tapering in community outpatients with chronic pain. JAMA Intern Med 2018;178(5):707–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Darnall B. To treat pain, study people in all their complexity. Nature 2018;557(7703):7.. [DOI] [PubMed] [Google Scholar]
- 22.National Institute on Drug Abuse. New clinician screening tool available for substance use; 2018. Available at: https://www.drugabuse.gov/news-events/news-releases/2018/06/new-clinician-screening-tool-available-substance-use (accessed June 2019).
- 23. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- 24. Cella D, Yount S, Rothrock N, et al. The Patient-Reported Outcomes Measurement Information System (PROMIS): Progress of an NIH Roadmap cooperative group during its first two years. Med Care 2007;45(5 Suppl 1):S3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. McCracken LM, Turk DC.. Behavioral and cognitive-behavioral treatment for chronic pain: Outcome, predictors of outcome, and treatment process. Spine (Phila Pa 1976) 2002;27(22):2564–73. [DOI] [PubMed] [Google Scholar]
- 26. Stewart MO, Karlin BE, Murphy JL, et al. National dissemination of cognitive-behavioral therapy for chronic pain in veterans: Therapist and patient-level outcomes. Clin J Pain 2015;31(8):722–9. [DOI] [PubMed] [Google Scholar]
- 27. Williams AC, Eccleston C, Morley S.. Psychological therapies for the management of chronic pain (excluding headache) in adults. Cochrane Database Syst Rev 2012;11:CD007407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Turner JA, Anderson ML, Balderson BH, Cook AJ, Sherman KJ, Cherkin DC.. Mindfulness-based stress reduction and cognitive behavioral therapy for chronic low back pain: Similar effects on mindfulness, catastrophizing, self-efficacy, and acceptance in a randomized controlled trial. Pain 2016;157(11):2434–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. LeFort SM, Gray-Donald K, Rowat KM, Jeans ME.. Randomized controlled trial of a community-based psychoeducation program for the self-management of chronic pain. Pain 1998;74(2):297–306. [DOI] [PubMed] [Google Scholar]
- 30. Lorig K, Holman H.. Arthritis self-management studies: A twelve-year review. Health Educ Q 1993;20(1):17–28. [DOI] [PubMed] [Google Scholar]
- 31. Dworkin RH, Turk DC, Peirce-Sandner S, et al. Research design considerations for confirmatory chronic pain clinical trials: IMMPACT recommendations. Pain 2010;149(2):177–93. [DOI] [PubMed] [Google Scholar]
- 32.Darnall BD, Ziadni MS, Stieg RL, Mackey IG, Flood P. Patient-centered prescription opioid tapering in community outpatients with chronic pain. JAMA Intern Med 2018;17:707–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dworkin RH, Turk DC, McDermott MP, et al. Interpreting the clinical importance of group differences in chronic pain clinical trials: IMMPACT recommendations. Pain 2009;146(3):238–44. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.