Abstract
Objective
The Western Ontario and McMaster Universities Osteoarthritis (WOMAC) pain scale quantifies knee pain severity with activities of daily living, but the potential impact of pain in other body regions on WOMAC pain scores has not been explored using a causal modeling approach. The purpose of this study was to determine if pain in other areas of the body impact WOMAC pain scores, a phenomenon referred to as “crosstalk.”
Methods
Cross-sectional datasets were built from public use data available from the Osteoarthritis Initiative (OAI) and the Multicenter Osteoarthritis Study (MOST). The WOMAC Pain Scale and generic hip, knee, ankle, foot and back pain measures were included. Three nested regression models grounded in causally based classical test theory determined the extent of crosstalk. Improvements in the coefficient of determination across the 3 models were used to determine the presence of crosstalk.
Results
Causal modeling provided evidence of crosstalk in both OAI and MOST datasets. For example, in OAI, multiple statistical models demonstrated significant increases in coefficient of determination values (P < .0001) as additional pain areas were added to the models.
Conclusions
Crosstalk appears to be a clinically important source of error in the WOMAC Pain Scale, particularly for patients with a larger number of painful body regions and when contralateral knee joint pain is more severe.
Impact Statement
This study has important implications for arthritis research. It also should raise clinician awareness of the threat to score interpretation and the need to consider the extent of pain in other body regions when interpreting WOMAC pain scores.
The Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) pain scale is 1 of 3 subscales comprising the WOMAC Index.1 The WOMAC is both a stand-alone measure and also imbedded within the Knee Injury and Osteoarthritis Outcome Score (KOOS).2 Both the WOMAC and KOOS are among the most commonly used and endorsed3 self-report pain and function scales for persons with painful arthritis of the knee or hip.
The WOMAC pain scale contains 5 items that address the following person-level activities: walking, stairclimbing, sitting, lying down, and standing. The stem of the scale asks the patient to focus on the extent of pain in the involved knee/hip during these activities. The extent of pain experienced during each of these activities is reported on a 5-point Likert scale ranging from “none” to “extreme.”
These questions require the patient to judge the extent of pain in the knee or hip of interest while also potentially experiencing pain or increased pain in a variety of other body regions during the performance of these activities. For example, it is possible that low back pain that is worsened while sitting, in a patient treated for knee pain, could artificially inflate this item’s WOMAC pain ratings. We refer to this potential phenomenon as crosstalk, defined by the Oxford English dictionary4 as “unwanted transfer of signals between communication channels.” Crosstalk also could occur if, for example, pain in the left (contralateral) knee and hip of a patient treated for right (involved) knee pain was associated with inflated scores on the walking item of the WOMAC pain scale. Generally, it is preferable for measures to represent a single construct or, in other words, be unidimensional, and most psychometric methods for evaluating measures explicitly make this assumption.5 Evidence of crosstalk would indicate a measure is not unidimensional.
Evidence for an association between WOMAC pain scores and additional bodily pain has been reported,6–9 but these studies examined traditional noncausal associations between pain or function and other joint pain and did not rely on a causal measurement model5 to formally test for crosstalk. If WOMAC pain scores are potentially impacted by pain in other body regions (ie, crosstalk), this has substantial implications for interpreting WOMAC pain scores. For example, if a person treated for unilateral knee arthritis had WOMAC pain scores that are inflated because of coexisting spinal pain and contralateral knee and hip pain, the physical therapist may falsely conclude, based on the inflated WOMAC pain score, that the patient’s involved knee pain during activity is actually worse than it may be. Therefore, investigation of the potential inflation of involved joint pain scores is warranted in light of how these inflated scores could affect prognosis or intervention.
To determine if crosstalk influences scores obtained with the WOMAC pain scale, we used a causal (explanatory) modeling approach to answer the following research questions: (1) Are WOMAC pain scores for an involved knee influenced by pain in other lower extremity joints or the back after accounting for generic verbal pain ratings for the involved knee? (2) Does pain in other lower extremity joints or the back moderate the association between WOMAC pain scores and generic verbal pain rating scores for the involved knee? We hypothesized that (1) pain in other lower extremity joints and the back influence WOMAC pain scores for the involved knee after accounting for generic involved knee verbal pain ratings; and (2) pain in other lower extremity joints or the back moderate the association between WOMAC pain scores and generic involved knee verbal pain rating scores.
Methods
We registered our study on Open Science Framework prior to analyzing the data. The registration is available at https://osf.io/87n5q/. Our study used a cross-sectional design to examine data from 2 publicly available National Institutes of Health–funded cohort studies: the Osteoarthritis Initiative (OAI)10 (cohort #1) and the Multicenter Osteoarthritis Study (MOST)11 (cohort #2). We used 2 independent datasets to allow for comparisons of the stability of the findings between the 2 cohorts. Treatment was not required or standardized as part of either study.
Data Source for Cohort #1
The OAI is a 9-year National Institutes of Health and privately funded cohort study of 4674 participants either with tibiofemoral osteoarthritis or at high risk of developing tibiofemoral OA. Participants between the ages of 45 and 79 years were recruited from communities near University of Maryland, The Ohio State University, University of Pittsburgh, and Memorial Hospital of Rhode Island. Participants provided written informed consent. Enrollment began in 2004 and ended in 2006. For more information, see https://data-archive.nimh.nih.gov/oai. To reduce risk of regression to the mean,12,13 we used data from the 1-year follow-up visit.
Data Source for Cohort #2
We used publicly available data collected for the MOST, a 7-year National Institutes of Health–funded 2-site community-based cohort study of 3026 participants with tibiofemoral osteoarthritis or at high risk for developing tibiofemoral osteoarthritis. For more information, see http://most.ucsf.edu/studyoverview.asp. Participants were 50 to 79 years of age and were recruited from 2 sites (University of Iowa, Iowa City, IA, and the University of Alabama at Birmingham, Birmingham, AL). The human participants institutional review boards of both sites approved the study, and all participants provided written informed consent. Enrollment began in 2003 and ended in 2005. We used data collected at the first follow-up visit (30 months postbaseline) in which all required data were collected.
Participants
Our interest was in the study of participants who reported knee pain in at least 1 knee during the visit of interest. All participants had at least 1 knee with a self-reported verbal pain rating scale >0. The knee with the highest (worst) knee pain rating was designated as the involved knee. If both knees had the same pain intensity (≥1), the involved knee was randomly selected.
Dependent Variable of Interest
For both cohorts, the outcome of interest was the WOMAC pain scale. Participants in both cohorts completed the WOMAC pain scale, Likert version 3.1, for each knee as part of a series of measures obtained at each study visit. The WOMAC pain scale ranges from 0 (no pain with all 5 activities, ie, flat surface walking, stairclimbing, at night, sitting or lying, standing) to 20 (extreme pain with all 5 activities). For the OAI, the timeframe for the WOMAC pain scale was the prior 7 days, while for the MOST the timeframe of interest was the prior 30 days. A substantial body of evidence supports the reliability and validity of the WOMAC pain scale.14,15
Independent Variables of Interest
We used a generic knee pain scale to quantify the extent of involved knee pain. In the OAI, scores for the generic knee pain scale ranged from 0 (no pain) to 10 (pain as bad as you can imagine), and participants were asked to identify the extent of knee pain at its worst over the prior 7 days. In MOST, participants were asked to rate their pain in the involved knee, on average, for the past 30 days on a scale from 0 (no pain) to 100 (pain as bad as it could be). This generic involved knee pain scale served as our criterion indicator of involved knee pain severity because it did not require participants to judge pain severity while recalling the performance of a variety of daily activities as is required when using the WOMAC pain scale. While the generic knee pain rating scale is not a perfect measure (ie, a measure without error), reliability estimates of 0.95 have been reported for persons with painful knee OA.16 These data suggest the error associated with these measures is minimal.
To determine if crosstalk occurred, we used 3 different pain measures: a composite pain measure of lower extremity pain not including the knees, a back pain measure, and a generic contralateral knee pain measure. Our rationale for selecting these measures was that pain in any of these regions beyond the involved knee could be either present during or worsened by the activities included in the WOMAC pain scale and could therefore contribute to crosstalk. First, we created a composite lower extremity pain measure from dichotomous yes/no items that asked participants to report whether they had pain, aching, or stiffness the majority of (or most days, in the case of MOST) days in the past 30 days for the right and left feet and ankles. For hip pain, aching, or stiffness measures, the OAI asked participants to indicate whether they had right or left hip pain more than half of the days of a month in the past year while the timeframe for hip pain questions in MOST was the past 30 days. This composite lower extremity pain score, which to our knowledge has not been previously reported, for right and left hip, foot, and ankle ranged from 0 (no pain, aching, or stiffness in any of the 3 joint areas, bilaterally) to 6 (pain, aching, or stiffness in all 6 joint areas).
Second, we used a 2-part back pain measure from OAI and MOST. Participants were initially asked if they had back pain over the past 30 days, and if they reported yes, they were asked if the back pain was mild, moderate, or severe.
Third, we used a generic pain rating scale to quantify knee pain severity for the contralateral knee. In OAI, participants were asked to rate their pain for each knee at its worst over the past 7 days. Scores ranged from 0 (no pain) to 10 (pain as bad as you can imagine). In MOST, participants were asked to rate their pain in each knee on average for the past 30 days on a scale from 0 (no pain) to 100 (pain as bad as it could be). To describe the demographic characteristics of the samples, we report the following person-level variables: age, sex, race (Black/African American, Caucasian, or other) and comorbidity.17
Data Analysis
Three nested models were fit to the data. Model 1 was a simple linear regression of WOMAC pain regressed on the generic involved knee pain scale. Model 1 established the extent of association between WOMAC pain and generic involved knee pain via the coefficient of determination (R2). To examine evidence for crosstalk, Model 2 built on Model 1 by including the generic knee pain rating score for the contralateral knee, the back pain severity score (none, mild, moderate, or severe), and lower extremity composite pain score (0–6 scale). Lastly, Model 3 was built onto model 2 by including the interaction between each covariate and the generic involved knee pain score. Interactions examined the extent to which lower extremity, contralateral knee, and back pain measures modify the effect of generic involved knee scores on prediction of WOMAC pain scores (Fig. 1).
Figure 1.
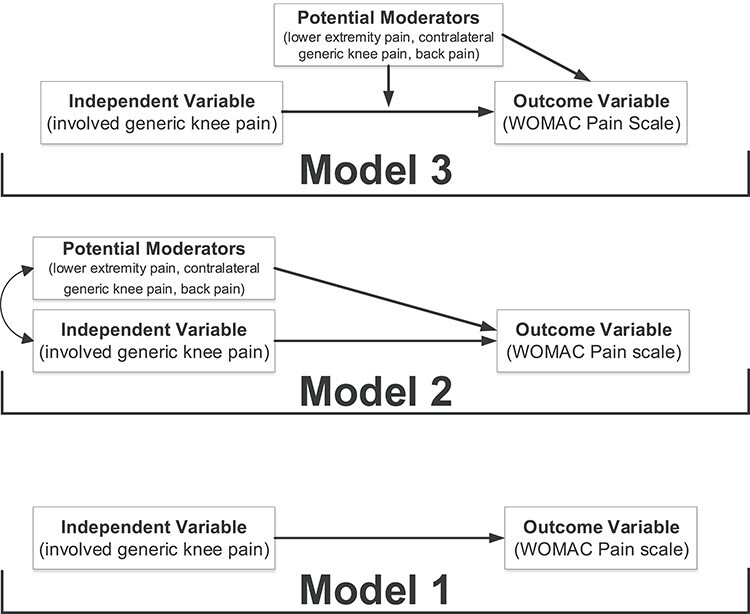
The figure illustrates the 3 models used to test for causal associations between a variety of pain measures and the WOMAC pain score. Model 1 described the association between WOMAC pain and the unidimensional generic pain score for the involved knee. Model 2 included both the involved generic knee pain score and all potential moderators (ie, a composite lower extremity pain score not including both knees, a contralateral generic knee pain score, and a back pain score). Model 3 included the involved generic knee pain score, and interactions among all potential moderators (ie, a composite lower extremity pain score not including both knees, a contralateral generic knee pain score, and a back pain score).
The logic for fitting these models was motivated by classical test theory and the common factor model.5 In this framework, an observed value for a variable (eg, WOMAC pain score) occurs due to its relationship with an unobserved latent variable (ie, a person’s true knee pain with daily activity). Formally, for a given observation, this is given by 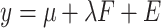 , where
, where 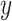 is the observed value,
is the observed value, 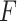 is the unobserved latent variable,
is the unobserved latent variable, 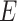 is random measurement error, and
is random measurement error, and 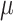 and
and 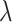 are the intercept and factor loading linking the measurement scale of the latent variable to the scale of the observed variable. In our study, generic involved knee pain was considered as the most pure (ie, unidimensional) measure of self-reported involved knee pain. We suspected that this generic pain measure was unlikely to be influenced by pain in other body regions because no functional context is linked to the measurement. That is, patients are not asked to judge their pain for the generic knee pain scale in the context of daily task performance, like the WOMAC scale. In our study design, we are treating generic involved knee pain as a latent variable even though it also is measured with error. Prior evidence supports the unidimensional nature of generic knee pain measures of pain intensity, particularly for musculoskeletal pain.18
are the intercept and factor loading linking the measurement scale of the latent variable to the scale of the observed variable. In our study, generic involved knee pain was considered as the most pure (ie, unidimensional) measure of self-reported involved knee pain. We suspected that this generic pain measure was unlikely to be influenced by pain in other body regions because no functional context is linked to the measurement. That is, patients are not asked to judge their pain for the generic knee pain scale in the context of daily task performance, like the WOMAC scale. In our study design, we are treating generic involved knee pain as a latent variable even though it also is measured with error. Prior evidence supports the unidimensional nature of generic knee pain measures of pain intensity, particularly for musculoskeletal pain.18
If no other variables influence WOMAC pain scores (after adjustment for involved generic knee pain), the scale would be unidimensional. Non-zero regression values would indicate the observed value is influenced by other factors or is multidimensional, which is not preferable. WOMAC pain scores have been found to be multidimensional using confirmatory factor analysis19 and item response theory.20 Inclusion of interaction terms between other variables and generic involved knee pain can be equated to testing measurement invariance. These terms provide information regarding whether the relationship between generic knee pain and WOMAC knee pain depends on other factors.
Our interest in using classical test theory and the common factor model was in causal explanation and not prediction.21 Our causal theoretical model (ie, the common factor model) was used to test our 2 causal hypotheses.5 Accordingly, while the R2 indicates the strength of association among the variables in our model, the beta coefficients describe the extent to which data align with our causal theoretical model. A change in R2 is a good metric if the focus is on prediction. However, our focus was on explanation (causality). In this case, it is of less concern whether the amount of variance explained (ie, R2) increases. Rather, in causal models the interest is in whether other variables have an influencing role once accounting for what should be the only causal factor (ie, generic knee pain). We therefore rely primarily on the beta coefficients to determine the extent to which the causal model is supported by the data. Beta coefficients also allow for direct clinical interpretation of the causal impact of pain scores other than the involved knee on WOMAC pain scores.
The lower extremity pain variable was included as a continuous predictor after confirming linearity assumptions. The back pain severity variable was treated as categorical. As defined in our protocol, change in the R2 was used to statistically test for evidence of crosstalk. Tests of individual variables were 2-sided and performed only if the change in R2 was significant. An alpha of 0.05 was used for all analyses. No adjustments were made for multiple statistical tests though multiple testing was minimized by reliance on R2 tests prior to testing significance of individual variables. All models were fit separately in the MOST and OAI datasets using R.22
Role of the Funding Source
The funders played no role in the design, conduct, or reporting of this study.
Results
The sample characteristics for OAI and MOST participants are summarized in Table 1. Participants in MOST were approximately 3 years older on average (64.5 years vs 61.8 years in OAI). Participants in MOST had a 1-point higher average WOMAC pain score (ie, 5.1, SD = 3.8) compared with OAI participants (ie, 4.1, SD = 3.6). For both datasets, evidence generally supported the unidimensionality of the generic knee pain scale as indicated by the beta coefficients for generic knee pain scores in Table 2. For OAI data, the generic knee pain beta changed only slightly when progressing from model 1 (1.06) to model 3 (0.88) while for MOST, the beta coefficients stayed the same from Model 1 to Model 3. In all models, multicollinearity was assessed with generalized variance inflation factor and found to be acceptable in all models (1.23–3.4).23
Table 1.
Characteristics of the Samples With Scores for Index WOMAC Pain and Other Pain Variablesa
| OAI (n = 3109) [mean, SD or %] | Missing OAI Data (n) | MOST (n = 2165) [mean, SD or %] | Missing MOST Data | |
|---|---|---|---|---|
| Age | 61.8 (9.1) | 0 | 64.5 (8.0) | 0 |
| Sex (% female) | 58.5 | 0 | 61.8 | 0 |
| Race (% African American) | 19.3 | 0 | 14.8 | 0 |
| Modified Charlson Comorbidity (% ≥ 1)b | 25.6% | 39 | 35.8% | 30 |
| Involved knee WOMAC pain scorec | 4.1 (3.6) | 1 | 5.1 (3.8) | 0 |
| Involved generic pain scored | 4.4 (2.4) | 0 | 29.8 (23.7) | 0 |
| Contralateral generic pain score | 2.0 (2.2) | 0 | 15.6 (19.0) | 0 |
| Summed lower extremity pain scoree | 0.81 (1.3) | 75 | 1.5 (1.6) | 326 |
| Back pain severity score (% for each rating) | 76 | 2 | ||
| None | 34.8 | 30.8 | ||
| Mild | 31.8 | 26.7 | ||
| Moderate | 27.1 | 36.8 | ||
| Severe | 6.3 | 5.7 |
a MOST = Multicenter Osteoarthritis Study; OAI = Osteoarthritis Initiative; WOMAC = Western Ontario and McMaster Universities Osteoarthritis.
b The Modified Charlson Comorbidity Index15 score ranges from 0 to 45 with higher scores equating to higher comorbidity burden.
c The WOMAC Pain score ranges from 0 to 20 with higher scores equating to greater pain with activities of daily living.
d The generic pain scale ranges from 0 to 10 for the OAI and 0 to 100 for MOST, with higher scores equating to greater pain.
e The summed lower extremity pain score ranges from 0 to 10 with higher scores equating to worse pain in lower extremity joints other than the knees.
Table 2.
Nested Regression Analyses Using OAI and MOST Datasets With Index WOMAC Pain as the Dependent Variablea
| OAI | MOST | |||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Estimate | 95% CI (SE) | t | P | Estimate | 95% CI (SE) | t | P |
| Model 1 | R 2 = 0.48 | R 2 = 0.64 | ||||||
| Intercept | −0.56 | −0.76 to −0.37 (0.10) | −5.72 | <.0001 | 1.51 | 1.33 to 1.68 (0.09) | 16.64 | <.0001 |
| Generic knee pain | 1.06 | 1.02 to 1.10 (0.02) | 53.18 | <.0001 | 0.13 | 0.12 to 0.13 (0.00) | 56.96 | <.0001 |
| Model 2 | R 2 = 0.52 | Δ R2 = 0.03 | F = 41.6 | < .0001 | R 2 = 0.66 | Δ R2 = 0.02 | F = 18.2 | < .0001 |
| Intercept | −0.61 | −0.83 to −0.39 (0.11) | −5.46 | <.0001 | 1.10 | 0.86 to 1.35 (0.12) | 8.9 | <.0001 |
| Generic knee pain | 0.94 | 0.89 to 0.99 (0.02) | 39.98 | <.0001 | 0.13 | 0.12 to 0.13 (0.003) | 41.2 | <.0001 |
| Lower extremity pain | 0.38 | −0.30 to .0.45 (0.04) | 9.65 | <.0001 | 0.20 | 0.13 to 0.26 (0.03) | 5.8 | <.0001 |
| Contralateral generic knee pain | 0.08 | 0.03 to 0.13 (0.03) | 3.19 | .0014 | −0.01 | −0.02 to 0.0 (0.004) | −2.5 | .0117 |
| Back pain | F(3, 3004) = 18.3 | <.0001 | F(3, 1831) = 11.0 | <.0001 | ||||
| None (reference) | - | - | - | - | - | - | - | - |
| Mild | −0.09 | −0.31 to 0.13 (0.11) | −0.78 | .4362 | 0.04 | −0.24 to 0.32 (0.14) | 0.29 | .7728 |
| Moderate | 0.14 | −0.10 to 0.37 (0.12) | 1.13 | .2568 | 0.46 | 0.20 to 0.73 (0.13) | 3.45 | .0006 |
| Severe | 1.43 | 1.02 to 1.83 (0.21) | 6.88 | <.0001 | 1.18 | 0.71 to 1.65 (0.24) | 4.96 | <.0001 |
| Model 3 | R 2 = 0.53 | Δ R2 = 0.01 | F = 9.9 | < .0001 | R 2 = 0.66 | Δ R2 = 0.003 | F = 3.8 | .0019 |
| Intercept | −0.28 | −0.61 to 0.06 (0.17) | −1.6 | .1056 | 1.07 | 0.71 to 1.43 (0.18) | 5.89 | <.0001 |
| Generic knee pain | 0.88 | 0.80 to 0.95 (0.04) | 22.9 | <.0001 | 0.13 | 0.12 to 0.14 (0.00) | 25220 | <.0001 |
| Lower extremity pain | 0.17 | 0.0 to 0.33 (0.08) | 2.0 | .0462 | 0.15 | 0.03 to 0.27 (0.06) | 2.39 | .0169 |
| Contralateral knee pain | −0.15 | −0.27 to −0.02 (0.07) | −2.2 | .0254 | 0.02 | 0.00 to 0.03 (0.01) | 2.11 | .0347 |
| Back pain | F(3, 2999) = 18.6 | <.0001 | F(3, 1826) = 11.1 | <.0001 | ||||
| None (reference) | - | - | - | - | - | - | - | - |
| Mild | 0.31 | −0.14 to 0.75 (0.22) | 1.4 | .1724 | −0.21 | −0.67 to 0.26 (0.24) | −0.87 | 0.3821 |
| Moderate | 0.27 | −0.24 to 0.78 (0.26) | 1.0 | .2996 | 0.35 | −0.11 to 0.81 (0.23) | 1.50 | 0.1338 |
| Severe | −0.05 | −1.08 to 0.98 (0.52) | −0.1 | .9239 | 0.38 | −0.51 to 1.28 (0.46) | 0.84 | 0.3999 |
| Generic knee pain x lower extremity pain | 0.04 | 0.01 to 0.07 (0.01) | 2.5 | .0111 | 0.00 | 0.00 to 0.00 (0.00) | 0.79 | 0.4325 |
| Generic knee pain x contralateral knee pain | 0.03 | 0.01 to 0.05 (0.01) | 3.4 | .0006 | 0.00 | 0.00 to 0.00 (0.00) | −3.80 | 0.0001 |
| Generic knee pain x back pain | F(3, 2999) = 4.6 | .0031 | F(3, 1826) = 2.0 | 0.11 | ||||
| None (reference) | - | - | - | - | - | - | - | - |
| Mild | −0.05 | −0.19 to 0.01 (0.05) | −1.8 | .0668 | 0.01 | 0.00 to 0.02 (0.01) | 1.30 | 0.1937 |
| Moderate | −0.02 | −0.12 to 0.08 (0.05) | −0.4 | .6963 | 0.00 | −0.01 to 0.01 (0.01) | 0.56 | 0.5723 |
| Severe | 0.21 | 0.05 to 0.38 (0.08) | 2.6 | .0103 | 0.02 | 0.00 to 0.04 (0.01) | 2.24 | 0.0254 |
MOST = Multicenter Osteoarthritis Study; OAI = Osteoarthritis Initiative; SE = standard error; WOMAC = Western Ontario and McMaster Universities Osteoarthritis.
OAI Findings
The R2 for the association between generic involved knee pain score and WOMAC pain score = 0.48, indicating that 48% of the variance in WOMAC pain was explained by generic involved knee pain. Evidence for crosstalk was found in OAI, based on the statistically significant increase in R2 for Model 2 compared with Model 1 and for Model 3 compared with Model 2 (Tab. 2). While the R2 increases were modest at 3% from Model 1 to Model 2 and 1% from Model 2 to Model 3, these increases were statistically significant at P < .0001.
For Model 2, all variables contributed to prediction of WOMAC pain scores beyond the generic involved knee pain score. For example, the beta estimate for lower extremity pain = 0.38, indicating that for every 1-point increase in lower extremity pain, the WOMAC pain score increased by 0.38 points, on average, after adjustment for all other variables in the model. There was some attenuation of the beta estimate for the generic involved knee pain score in Model 2 (ie, 0.94) compared with Model 1 (ie, 1.06), suggesting that the contralateral knee pain measures were associated with generic involved knee pain scores as well as WOMAC pain scores. While low back pain contributed to crosstalk, the contribution was driven by persons with severe low back pain. The beta estimates for persons with mild and moderate low back pain were nonsignificant.
For model 3, all interactions between the generic involved knee pain score and the contralateral knee pain measures (ie, lower extremity pain, contralateral knee pain, and low back pain measures) were statistically significant. The statistically significant beta coefficients for the interactions were positive, suggesting that the association between generic involved knee pain and WOMAC pain increased as the interacting variable (ie, lower extremity pain) increased. That is, the covariates lower extremity pain, contralateral knee pain, and back pain modified the effect of generic involved knee pain on WOMAC pain.
MOST Findings
The R2 for the association between the generic involved knee pain and WOMAC pain = 0.64, indicating that 64% of the variance in WOMAC pain was explained by generic involved knee pain. The MOST data demonstrated similar though more modest evidence of crosstalk compared with the OAI data as demonstrated by smaller R2 increases in Model 2 (2% increase relative to Model 1) and Model 3 (0.3% increase relative to Model 2). The R2 for both models were significantly higher relative to the prior model (P < .002).
For Model 2, all covariates contributed to WOMAC pain prediction after accounting for generic involved knee pain. Model 3 was statistically significant (P < .0001), but the beta coefficient was rounded to 0, suggesting a statistically significant but clinically insignificant finding.
Discussion
Self-reported outcome measures for patients with lower extremity osteoarthritis have become commonplace in many clinics. Many of these measures have been extensively studied,14,24,25 but we found no evidence that used a study design grounded in classical test theory and explanatory modeling to examine whether pain in other joints influences scores on the outcome measures, a phenomenon we call crosstalk.
Our causal hypotheses were supported because we found evidence for crosstalk in both the OAI and MOST datasets. This is notable because these 2 large datasets used different phrasing and different timeframes in the questions used to measure generic knee pain intensity. For example, MOST measured generic knee pain over a 30-day period while OAI used a 7-day period. Despite these differences in generic pain question format, we found similar evidence for crosstalk and against unidimensionality in both datasets.
Clinical impact is the central focus of our study, and to illustrate the potential clinical impact of crosstalk, we use equations from our Model 2 findings. The regression equation was the following: 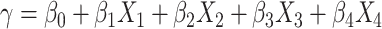 , where
, where 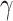 = the predicted WOMAC pain score,
= the predicted WOMAC pain score, 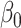 = intercept,
= intercept, 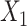 = generic involved knee pain score,
= generic involved knee pain score, 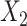 = lower extremity pain score,
= lower extremity pain score, 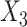 = generic contralateral knee pain score,
= generic contralateral knee pain score, 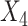 = back pain score, and the corresponding
= back pain score, and the corresponding 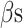 are the beta coefficients for each variable. If we consider a best-case scenario vignette for the OAI data, for example, a patient scores a 6 on the generic involved knee pain scale and a 0 on all other scales, the predicted WOMAC pain score would be 5.0 using the beta estimates from Table 2. If we consider a worst-case scenario vignette with the same generic involved knee pain score of 6 but also with a score of 6 for the contralateral knee, a score of 6 for the lower extremity pain scale, and a score of 3 for the back pain scale, the predicted WOMAC pain score would be 9.2. A vignette that fell approximately midway between a best- and worst-case scenario, for example a patient with a generic involved knee pain score of 6, a lower extremity score of 4, a contralateral knee pain score of 4, and a back pain severity score of 2, would have a predicted WOMAC pain score of 7.0 (Fig. 2). Similar though somewhat lower levels of crosstalk were found using Model 2 for the MOST data. Our data indicate that the greater the musculoskeletal pain burden, as indicated by the lower extremity, back, and contralateral knee pain measures, the greater the crosstalk. As seen in our worst-case and midway scenarios, the extent of crosstalk meets or exceeds the minimal clinically important difference estimate of 2.0 for WOMAC pain.26–28 Clinicians should be most concerned about crosstalk (and inflated WOMAC pain scores) when the patient reports multiple painful areas beyond the knee.
are the beta coefficients for each variable. If we consider a best-case scenario vignette for the OAI data, for example, a patient scores a 6 on the generic involved knee pain scale and a 0 on all other scales, the predicted WOMAC pain score would be 5.0 using the beta estimates from Table 2. If we consider a worst-case scenario vignette with the same generic involved knee pain score of 6 but also with a score of 6 for the contralateral knee, a score of 6 for the lower extremity pain scale, and a score of 3 for the back pain scale, the predicted WOMAC pain score would be 9.2. A vignette that fell approximately midway between a best- and worst-case scenario, for example a patient with a generic involved knee pain score of 6, a lower extremity score of 4, a contralateral knee pain score of 4, and a back pain severity score of 2, would have a predicted WOMAC pain score of 7.0 (Fig. 2). Similar though somewhat lower levels of crosstalk were found using Model 2 for the MOST data. Our data indicate that the greater the musculoskeletal pain burden, as indicated by the lower extremity, back, and contralateral knee pain measures, the greater the crosstalk. As seen in our worst-case and midway scenarios, the extent of crosstalk meets or exceeds the minimal clinically important difference estimate of 2.0 for WOMAC pain.26–28 Clinicians should be most concerned about crosstalk (and inflated WOMAC pain scores) when the patient reports multiple painful areas beyond the knee.
Figure 2.

The figure illustrates the application of formulas to the Osteoarthritis Initiative data for calculation of the extent of crosstalk for 3 different hypothetical clinical scenarios. Note that the differences among the 3 scenarios all meet or exceed the minimal clinically important difference for WOMAC pain and suggest clinicians should be aware that pain in other body regions cause an inflation of WOMAC pain scores, potentially to a clinically important extent.
Evidence for crosstalk was found for both Models 2 and 3 for both the OAI and MOST. However, the improvement in R2 was small, particularly when considering Model 3, which included the interaction terms for generic involved knee pain and the other bodily pain measures. These findings suggest that while evidence for crosstalk via interactions in Model 3 was supported by the statistical tests, the clinical impact of these significant interactions are unlikely. The inclusion of interactions in the MOST, for example, led to only a 0.003 improvement in the R2. We therefore endorse Model 2 for estimating the extent of crosstalk and for estimating the clinical implications of findings related to the potential impact of crosstalk.
Widespread pain is not uncommon in persons with knee OA. Prevalence was 31% in participants recruited to MOST, for example.29 Clinicians are likely to see patients with pain in other body regions and should be aware that pain beyond the involved knee will likely inflate WOMAC pain scores.
Our findings are likely to be generalizable to other instruments designed to estimate the extent of pain in a joint or body region during person-level daily activities. For example, we suspect that the KOOS scale and the Lower Extremity Functional Scale2,30 are potentially vulnerable to crosstalk.
Our study has several strengths, including the pre-analysis registration, large samples, and consistent findings across different datasets with different measures, but there are limitations. Our study should be replicated on active care seekers to confirm findings and on persons with more substantial pain and disability and a more substantial disease spectrum compared with participants in OAI and MOST. Additionally, our focus was on lower extremity and back pain, 2 anatomical areas that are likely to be influenced by the daily activities addressed in WOMAC. However, bodily pain in other areas may also have influenced WOMAC scores. In addition, while we suspect our results likely generalize to other measures, our study should be replicated with instruments other than the WOMAC pain scale. Generic pain scales may also contribute to crosstalk but our design precluded further study of this issue. Our study was cross-sectional in nature and while we suspect our results may also apply to change scores and is consistent with our theoretical model, longitudinal data would better inform this issue. Our generic pain measure was not perfect and contained a small amount of measurement error, and this error could have influenced our findings though we suspect this was minimal given the very high reliability of numeric rating scales.16 Finally, we did not study potential effects of demographic characteristics as causal factors influencing WOMAC pain.
In conclusion, we found causal explanatory evidence for crosstalk when applying the WOMAC pain scale. The extent of crosstalk is greater when bodily pain burden is greater and affects more body regions. Clinicians should be aware of this threat to score interpretation and consider the extent of pain in other body regions when interpreting WOMAC pain scores.
Author Contributions and Acknowledgments
Concept/idea/research design: D.L. Riddle, R. Perera
Writing: D.L. Riddle, R. Perara
Data analysis: D.L. Riddle, R. Perera
Project management: D.L. Riddle
Both authors contributed to the conception and design of the study and drafted and approved the manuscript. D.L. Riddle acquired the data, and both authors analyzed and interpreted the data.
The authors acknowledge Paul Stratford for reviewing an earlier version of the manuscript.
Disclosures and Presentations
The authors completed the ICMJE Form for Disclosure of Potential Conflicts of Interest and reported no conflicts of interest.
Some of the data in this study were scheduled to be presented at the 2020 Osteoarthritis Research Society International (OARSI) World Congress on Osteoarthritis in Vienna, Austria, in May 2020. Following the cancellation of the congress due to the COVID-19 pandemic, the data were presented by the authors in an OARSI online educational webinar.
Funding
The Osteoarthritis Initiative (OAI) is a public-private partnership comprising 5 contracts (N01-AR-2-2258, N01-AR-2-2259, N01-AR-2-2260, N01-AR-2-2261, N01-AR-2-2262) funded by the National Institutes of Health (NIH), a branch of the Department of Health and Human Services, and conducted by the OAI study investigators. Private funding partners include Merck Research Laboratories, Novartis Pharmaceuticals Corporation, GlaxoSmithKline, and Pfizer Inc. Private sector funding for the OAI is managed by the Foundation for the National Institutes of Health. This manuscript was prepared using an OAI public use dataset and does not necessarily reflect the opinions or views of the OAI investigators, the NIH, or the private funding partners.
Multicenter Osteoarthritis Study (MOST) comprises 4 cooperative grants (Felson—AG18820; Torner—AG18832, Lewis—AG18947, and Nevitt—AG19069) funded by NIH, a branch of the Department of Health and Human Services, and conducted by MOST study investigators. This manuscript was prepared using MOST data and does not necessarily reflect the opinions or views of MOST investigators. The investigators of the current study are not part of the OAI or MOST investigative teams, and the funding source played no role in the conduct or submission of the current study.
The authors completed the ICMJE Form for Disclosure of Potential Conflicts of Interest and reported no conflicts of interest.
Contributor Information
Daniel L Riddle, Department of Biostatistics, Virginia Commonwealth University, Richmond, VA 23298–0224 USA.
Robert A Perera, Department of Biostatistics, Virginia Commonwealth University, Richmond, VA 23298–0224 USA.
References
- 1. Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol 1988;15(12):1833–1840. [PubMed] [Google Scholar]
- 2. Roos EM, Toksvig-Larsen S. Knee injury and osteoarthritis outcome score (KOOS) - validation and comparison to the WOMAC in total knee replacement. Health Qual Life Outcomes. 2003;1:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ruyssen-Witrand A, Fernandez-Lopez CJ, Gossec L, Anract P, Courpied JP, Dougados M. Psychometric properties of the OARSI/OMERACT osteoarthritis pain and functional impairment scales: ICOAP, KOOS-PS and HOOS-PS Clin Exp Rheumatol 2011;29(2):231–237. [PubMed] [Google Scholar]
- 4. Oxford English Dictionary. Oxford University Press website. https://en.oxforddictionaries.com/definition/crosstalk. Published 2019. Accessed May 10, 2020. [Google Scholar]
- 5. McDonald RP. Test Theory. A Unified Treatment. Mahwah, NJ: Lawrence Erlbaum Associates; 1999. [Google Scholar]
- 6. Suri P, Morgenroth DC, Kwoh CK, Bean JF, Kalichman L, Hunter DJ. Low back pain and other musculoskeletal pain comorbidities in individuals with symptomatic osteoarthritis of the knee: Data from the osteoarthritis initiative. Arthritis Care Res (Hoboken) 2010;62(12):1715–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Perruccio A V, Power JD, Evans HM, Mahomed SR, Gandhi R, Mahomed NN, et al. Multiple joint involvement in total knee replacement for osteoarthritis: effects on patient-reported outcomes. Arthritis Care Res(Hoboken). 2012;64(6):838–846. [DOI] [PubMed] [Google Scholar]
- 8. Finney A, Dziedzic KS, Lewis M, Healey E. Multisite peripheral joint pain: a cross-sectional study of prevalence and impact on general health, quality of life, pain intensity and consultation behaviour. BMC Musculoskelet Disord 2017;18(1):535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hoogeboom TJ, den Broeder AA, de Bie RA, Van Den Ende CHM. Longitudinal impact of joint pain comorbidity on quality of life and activity levels in knee osteoarthritis: data from the osteoarthritis initiative. Rheumatol (United Kingdom) 2013;52(3):543–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lester G. The osteoarthritis initiative: a NIH public-private partnership. HSS J 2012;8(1):62–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Segal NA, Nevitt MC, Gross KD, Hietpas J, Glass NA, Lewis CE, et al. The multicenter osteoarthritis study: opportunities for rehabilitation research. PM R 2013;5(8):647–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Riddle DL, Kong X, Fitzgerald GK. Psychological health impact on 2-year changes in pain and function in persons with knee pain: data from the osteoarthritis initiative. Osteoarthritis Cart 2011;19(9):1095–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Schilling C, Petrie D, Dowsey MM, Choong PF, Clarke P. The impact of regression to the mean on economic evaluation in quasi-experimental pre–post studies: the example of total knee replacement using data from the osteoarthritis initiative. Heal Econ (United Kingdom) 2017;26(12):e35-e51. [DOI] [PubMed] [Google Scholar]
- 14. Gandek B. Measurement properties of the western Ontario and McMaster universities osteoarthritis index: a systematic review. Arthritis Care Res 2015;67(2):216–229. [DOI] [PubMed] [Google Scholar]
- 15. Stratford PW, Kennedy DM. Does parallel item content on WOMAC’s pain and function subscales limit its ability to detect change in functional status? BMC Musculoskelet Disord 2004;9(5):17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Alghadir AH, Anwer S, Iqbal A, Iqbal ZA. Test-retest reliability, validity, and minimum detectable change of visual analog, numerical rating, and verbal rating scales for measurement of osteoarthritic knee pain. J Pain Res. 2018;11:851–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Katz JN, Chang LC, Sangha O, Fossel AH, Bates DW. Can comorbidity be measured by questionnaire rather than medical record review? Med Care 1996;34(1):73–84. [DOI] [PubMed] [Google Scholar]
- 18. Huber A, Suman AL, Rendo CA, Biasi G, Marcolongo R, Carli G. Dimensions of “unidimensional” ratings of pain and emotions in patients with chronic musculoskeletal pain. Pain 2007;130(3):216–224. [DOI] [PubMed] [Google Scholar]
- 19. Stratford PW, Kennedy DM, Woodhouse LJ, Spadoni GF. Measurement properties of the WOMAC LK 3.1 pain scale. Osteoarthr Cartil 2007;15(3):266–272. [DOI] [PubMed] [Google Scholar]
- 20. Davis AM, Badley EM, Beaton DE, Kopec J, Wright JG, Young NL, et al. Rasch analysis of the western Ontario McMaster (WOMAC) osteoarthritis index: results from community and arthroplasty samples. J Clin Epidemiol 2003;56(11):1076–1083. [DOI] [PubMed] [Google Scholar]
- 21. Shmeuli G. To explain or to predict? Stat Sci 2010;25(3):289–310. [Google Scholar]
- 22. R Development Core Team . R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. [Google Scholar]
- 23. Fox J, Monette G. Generalized collinearity diagnostics. J Am Stat Assoc 1992;87(417):178–183. [Google Scholar]
- 24. Collins NJ, Roos EM. Patient-reported outcomes for total hip and knee arthroplasty: commonly used instruments and attributes of a “good” measure. Clin Geriatr Med 2012;28(3):367–394. [DOI] [PubMed] [Google Scholar]
- 25. Collins NJ, Prinsen CA, Christensen R, Bartels EM, Terwee CB, Roos EM. Knee injury and osteoarthritis outcome score (KOOS): systematic review and meta-analysis of measurement properties. Osteoarthritis Cart 2016;24(8):1317–1329. [DOI] [PubMed] [Google Scholar]
- 26. Angst F, Aeschlimann A, Stucki G. Smallest detectable and minimal clinically important differences of rehabilitation intervention with their implications for required sample sizes using WOMAC and SF-36 quality of life measurement instruments in patients with osteoarthritis of the lower ex. Arthritis Rheum 2001;45(4):384–391. [DOI] [PubMed] [Google Scholar]
- 27. Angst F, Aeschlimann A, Michel BA, Stucki G. Minimal clinically important rehabilitation effects in patients with osteoarthritis of the lower extremities. J Rheumatol 2002;29(1):131–138. [PubMed] [Google Scholar]
- 28. Ehrich EW, Davies GM, Watson DJ, Bolognese JA, Seidenberg BC, Bellamy N. Minimal perceptible clinical improvement with the Western Ontario and McMaster Universities Osteoarthritis Index questionnaire and global assessments in patients with osteoarthritis. J Rheumatol 2000;27(11):2635–2641. [PubMed] [Google Scholar]
- 29. Carlesso LC, Niu J, Segal NA, Frey-Law LA, Lewis CE, Nevitt MC, et al. The effect of widespread pain on knee pain worsening, incident knee osteoarthritis (OA), and incident knee pain: The Multicenter OA (MOST) study. J Rheumatol 2017;44(4):493–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Binkley JM, Stratford PW, Lott SA, Riddle DL. The lower extremity functional scale (LEFS): scale development, measurement properties, and clinical application. North American orthopaedic rehabilitation research network. PhysTher 1999;79(4):371–383. [PubMed] [Google Scholar]


