Abstract
Objective
Changing Behavior through Physical Therapy (CBPT), a cognitive-behavioral–based program, has been shown to improve outcomes after lumbar spine surgery in patients with a high psychosocial risk profile; however, little is known about potential mechanisms associated with CBPT treatment effects. The purpose of this study was to explore potential mediators underlying CBPT efficacy after spine surgery.
Methods
In this secondary analysis, 86 participants were enrolled in a randomized trial comparing a postoperative CBPT (n = 43) and education program (n = 43). Participants completed validated questionnaires at 6 weeks (baseline) and 3 and 6 months following surgery for back pain (Brief Pain Inventory), disability (Oswestry Disability Index), physical health (12-Item Short-Form Health Survey), fear of movement (Tampa Scale for Kinesiophobia), pain catastrophizing (Pain Catastrophizing Scale), and pain self-efficacy (Pain Self-Efficacy Questionnaire). Parallel multiple mediation analyses using Statistical Package for the Social Sciences (SPSS) were conducted to examine whether 3- and 6-month changes in fear of movement, pain catastrophizing, and pain self-efficacy mediate treatment outcome effects at 6 months.
Results
Six-month changes, but not 3-month changes, in fear of movement and pain self-efficacy mediated postoperative outcomes at 6 months. Specifically, changes in fear of movement mediated the effects of CBPT treatment on disability (indirect effect = −2.0 [95% CI = −4.3 to 0.3]), whereas changes in pain self-efficacy mediated the effects of CBPT treatment on physical health (indirect effect = 3.5 [95% CI = 1.2 to 6.1]).
Conclusions
This study advances evidence on potential mechanisms underlying cognitive-behavioral strategies. Future work with larger samples is needed to establish whether these factors are a definitive causal mechanism.
Impact
Fear of movement and pain self-efficacy may be important mechanisms to consider when developing and testing psychologically informed physical therapy programs.
Chronic daily pain affects over 40 million individuals in the United States, with approximately 10.6 million people reporting high-impact pain that results in significant activity limitation or participation restriction.1 Cognitive-behavioral therapy (CBT) is a well-established and effective strategy for reducing pain and disability in chronic pain populations.2 Meta-analytical reviews have found small to medium effects on pain and disability when comparing CBT with usual care and small effects of CBT on disability relative to other active treatments.2,3 CBT focuses on altering negative thoughts or feelings about the pain experience. In particular, CBT has been developed to target psychosocial risk factors for persistent pain, including elevated fear of movement and pain catastrophizing.4
Robust evidence supports the importance of psychosocial risk factors in the rehabilitation setting. Subsequently, studies have tested the effectiveness of combining CBT with physical therapy treatment to improve patient outcomes by addressing psychosocial risk factors of fear of movement, pain catastrophizing, and pain self-efficacy.5–7 However, few studies have examined the mechanisms by which CBT for pain works, that is, what the mediators of treatment effects are in people with pain.8 Mansell et al9 observed reductions in fear of movement mediated the effects of a multidisciplinary intervention delivered by psychologists and physical therapists on disability in individuals with chronic low back pain (LBP). In contrast, Stevens et al10 did not find fear to be a mediator of a combined workplace intervention of CBT, ergonomics, and physical training on LBP-related outcomes. Smeets et al11 found that decreased pain catastrophizing mediated the relationship between active therapy (including CBT, physical treatment, or combined treatment) and improvements in pain and disability in adults with chronic back pain, suggesting pain catastrophizing may not be a specific mediator of CBT but rather a common mechanism of active treatments. In contrast, pain self-efficacy—a positive psychosocial characteristic—may play a larger role in mediating CBT effects than factors like pain catastrophizing.12,13 Currently, there is limited evidence on whether pain self-efficacy mediates CBT-based physical therapy interventions after spine surgery. Further examination of specific mediating factors of CBT-based rehabilitation interventions could improve theoretical models and aid the development of effective and efficient therapies.
Changing Behavior through Physical Therapy (CBPT) is a brief CBT12,14,15 and self-management program16,17 delivered by physical therapists over the phone. Archer et al18 demonstrated the clinical efficacy of CBPT in adults with chronic pain and high psychosocial risk undergoing spine surgery. Relative to participants assigned to an education group (eg, time and attention control), participants who participated in the CBPT program showed significantly greater reductions in pain and disability and improvements in physical health at 6 months after spine surgery.18 The overall hypothesis for the development of the CBPT program was that postoperative outcomes would improve through reductions in fear of movement and pain catastrophizing and increases in pain self-efficacy (Fig. 1). This hypothesis was informed by the fear-avoidance model of musculoskeletal pain, which posits that certain individuals exhibiting heightened fear of movement or pain catastrophizing or lower pain self-efficacy may be at risk for persistent pain and disability and worse physical health.19,20 Archer and colleagues21,22 and others23–25 have found high fear of movement to be an independent predictor of poor outcomes related to pain, disability, and physical health after lumbar spine surgery, and pain catastrophizing and self-efficacy have been found to be associated with these outcomes in individuals with persistent musculoskeletal pain.26–29
Figure 1.
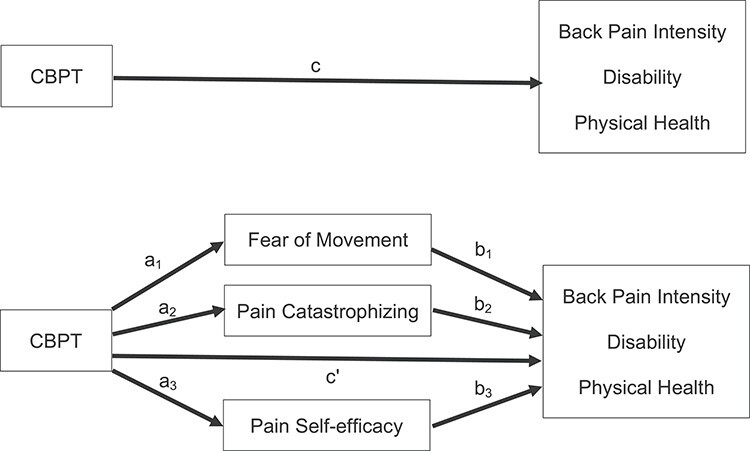
Graphical representation of conceptual mediation model.
The purpose of this study was to examine whether the CBPT program improved postoperative pain, disability, and physical health through changes in fear of movement, pain catastrophizing, and pain self-efficacy. Multiple mediation analyses were conducted to test whether pre- to post-treatment changes at 3 and 6 months in pain-related cognitive factors were associated with improvement in outcomes at 6 months following surgery. We hypothesized changes in fear of movement, pain catastrophizing, and pain self-efficacy would mediate the effects of CBPT on 6-month outcomes.
Methods
Participants
Participants provided informed consent and were enrolled into a randomized trial (NCT01131611) comparing a postoperative CBPT intervention with an education control. Participants were recruited from a single academic medical center and included English-speaking adults 21 years of age or older who were undergoing lumbar spine surgery for a degenerative condition and reported high preoperative fear of movement based on a score 39 or greater on the Tampa Scale for Kinesiophobia (TSK). This score has been used as an indicator of individuals with dysfunctional pain beliefs and those at risk for poor outcomes after spine surgery.21,30 Study inclusion criteria were having a laminectomy with or without arthrodesis for spinal stenosis, spondylosis with or without myelopathy, or degenerative spondylolisthesis; back and/or lower extremity pain for longer than 6 months; and no history of neurological movement disorder or psychotic disease. Exclusion criteria included having microsurgical techniques and surgery for spinal deformity, trauma, infection, or tumor. The Institutional Review Board at Vanderbilt University Medical Center approved the study.
Procedures
Participants were enrolled at a preoperative clinic visit, and their eligibility was confirmed after surgery. Participants completed an intake form and validated questionnaires at 6 weeks (baseline), 3 months (post-intervention), and 6 months following surgery to measure pain, disability, physical health, fear of movement, pain catastrophizing, and pain self-efficacy. Participants unable to return to the clinic for routine follow-up visits were asked to complete questionnaires at home and return in a self-addressed stamped envelope. Follow-up assessments were obtained by personnel unaware of randomized group status.
Interventions
Participants were randomized to (1) CBPT intervention, or (2) education control. The CBPT intervention and education control programs both lasted 6 weeks and consisted of weekly postoperative sessions with a trained study physical therapist (S.W.V.). In both groups, the first session was conducted in person at a standard 6-week postoperative clinic visit, and the remaining sessions were delivered over the telephone. All sessions were 30 minutes in length, except the first session, which was approximately 1 hour. Participants in both the CBPT and education groups received standard of care based on the discretion of their surgeon. Standard of care included postoperative advice to stay active, lifting restrictions, and referral to traditional physical therapy.
The CBPT intervention was developed based on brief CBT12,14,15 and self-management programs.16,17 CBPT included empirically supported strategies for behavioral self-management, problem solving, cognitive restructuring, and relaxation training.14,15,31 Components of CBPT included cognitive-behavioral pain education, a graded activity plan, and weekly activity and walking goals. The study physical therapist helped participants identify distraction strategies, replace negative thoughts with positive thoughts, find balance between rest and activity, and manage setbacks by recognizing high-risk situations. Both the physical therapist and participant utilized an intervention manual that outlined the CBPT intervention (Suppl. Tab. 1).
The education control consisted of rehabilitation topics including the benefits of physical therapy, proper postoperative biomechanics, importance of daily exercise, ways to promote healing, and educational material on stress reduction, sleep, energy management, health provider communication, and injury prevention. Similar to the CBPT group, an education manual was used to guide topics. To ensure therapist fidelity and protocol adherence, all sessions were audio recorded and then reviewed. Additional details of the CBPT intervention and education control can be found in our previously published work.18,32
Mediators
The proposed psychosocial mediators of fear of movement, pain catastrophizing, and pain self-efficacy were investigated. These mediators were chosen a priori based on our underlying theoretical model (fear avoidance model of musculoskeletal pain). All mediators were assessed at 6 weeks (baseline), 3 months, and 6 months after surgery.
Fear of movement
Fear of movement is the fear or anxiety a person experiences during physical movement or activity.33 The 17-item TSK was used to measure fear of movement.33 Participants rate items using a 4-point scale from “strongly disagree” to “strongly agree.” Total scores range from 17 to 68, with higher scores indicating greater fear of movement. The TSK has good psychometric properties and has been validated in the lumbar spine surgery population and individuals with various musculoskeletal conditions.30,34,35
Pain catastrophizing
Pain catastrophizing is an exaggerated and negative outlook associated with actual or anticipated pain-related events.29 The 13-item Pain Catastrophizing Scale (PCS) was used to assess catastrophic thinking associated with pain.36 Participants rate items on a 5-point scale from “not at all” to “all the time.” Total scores range from 0 to 52. Scores greater than 24 differentiate “catastrophizers” and “noncatastrophizers.”37 The PCS has demonstrated strong internal consistency, high test-retest reliability, and validity through associations with pain, disability, negative affect, and pain-related fear.36,38,39
Pain self-efficacy
Pain self-efficacy is a person’s belief or judgement in his/her ability to accomplish a range of activities despite pain.40 The Pain Self-Efficacy Questionnaire (PSEQ) was used to measure pain self-efficacy.41 The PSEQ is a 10-item questionnaire where confidence is rated on a 7-point scale from “not at all confident” to “completely confident.” Total scores range from 0 to 60, with scores greater than 40 indicating high self-efficacy.42 The PSEQ is a reliable measure of self-efficacy and demonstrates correlations with other psychosocial constructs such as depression, anxiety, coping strategies, pain ratings, and work-related tasks in individuals with chronic pain.42
Outcomes
Outcomes included back pain intensity, disability, and physical health. All outcomes were measured at 6 weeks (baseline) and 6 months after surgery.
Back pain intensity
The Brief Pain Inventory was used to measure back pain intensity.43 The back pain intensity scale includes 4 items assessing current, worst, least, and average pain. Participants rate each item on an 11-point scale, with 0 meaning “no pain at all” and 10 meaning “pain as bad as can imagine.” The Brief Pain Inventory is a reliable and valid pain measure used in surgical patients and individuals with chronic LBP.44–46
Disability
The Oswestry Disability Index (ODI) was used to measure low back-related disability.47 The 10-item ODI assesses 10 aspects of daily living, including pain intensity, lifting, sitting, standing, walking, sleeping, hygiene, traveling, and social and sex life. Participants rate each item using a 6-point scale ranging from 0 (high functioning) to 5 (low functioning), and a percentage score for disability is obtained. The ODI is a reliable measure of condition-specific disability and demonstrates moderate to high correlations with other disability measures.48,49
Physical health
The 12-Item Short-Form Health Survey (SF-12) was used to measure physical health.50 The physical component scale (PCS) of the SF-12 assesses 4 subdomains: physical functioning, role-physical, bodily pain, and general health. The PCS subscale score ranges from 0 to 100. Higher scores represent better physical health, and in the general population, the PCS scores have a mean of 50 with an SD of 10. The SF-12 PCS is a reliable, valid, and responsive measure in both generalized and various patient populations.50–52
Data Analysis
All analyses were intent-to-treat and conducted using IBM SPSS Statistics version 26.0 (IBM Corp., Armonk, NY, USA). Pre- to post-treatment changes at 3 and 6 months were calculated for the proposed psychosocial mediators. Pearson correlations were performed to assess strength of association between mediators at each time point and between post-treatment change scores. Absolute correlations less than 0.3 were considered weak, between 0.3 and 0.5 were moderate, and greater than 0.5 were strong.53
Multiple mediation analysis using ordinary least squares path analysis was performed to test whether 3-month and 6-month changes in fear of movement, pain catastrophizing, and pain self-efficacy mediate the effects of CBPT on pain, disability, and physical health at 6 months following surgery. Figure 1 illustrates the model guiding our mediation analysis. Multiple mediation provides estimates of an overall mediation effect (total indirect effect) and indirect effects for each mediator holding constant all other mediators in the model. Multiple mediation was performed using the PROCESS macro developed by Preacher and Hayes for SPSS.54,55 A parallel mediator model was chosen as we did not have a priori hypotheses related to the serial relationship between proposed mediators and the effects of CBPT. We generated separate models for testing mediation involving 3-month and 6-month changes in psychosocial variables on each 6-month outcome. All models controlled for the baseline score of the outcome. Indirect effects (unstandardized path coefficients) and bias-corrected 95% CI for overall mediation and individual mediators were estimated based on 5000 bootstrap samples. Bootstrapping has been recommended as a superior approach for inferences in studies with small sample sizes and non-normally distributed variables.56–58 Indirect effects with bias-corrected CIs not containing 0 were interpreted as evidence for mediation.
Role of the Funding Source
The funders played no role in the design, conduct, or reporting of this study.
Results
Participants
Participants (N = 86, mean [SD] age = 57.6 [12.2] years, N [%] females = 48 [55.8]) were randomly assigned to CBPT (n = 43) or education (n = 43). A Consolidated Standards of Reporting Trials (CONSORT) flow diagram and sample characteristics were previously published.18 Groups did not differ in demographic and clinical characteristics and baseline pain intensity, disability, and psychosocial measures (Tab. 1). A total of 93% of CBPT participants completed all treatment sessions compared with 95% of education participants. Follow-up rates at 6 months after surgery were similar across groups for participant-reported measures (CBPT: 88%; education: 97%). There were no significant differences in baseline characteristics between participants with and without complete follow-up data.
Table 1.
Baseline Characteristics of Samplea
| Characteristic | CBPT Intervention (n = 43) | Education Control (n = 43) |
|---|---|---|
| Demographic | ||
| Age, years | 56.9 (11.1) | 58.4 (13.3) |
| Sex, N (%) female | 25 (58.1) | 23 (53.5) |
| Race, N (%) White | 36 (83.7) | 33 (76.7) |
| Education, N (%) some college or more | 30 (69.8) | 32 (74.4) |
| Employment, N (%) working | 21 (48.8) | 18 (41.9) |
| Clinical | ||
| Fusion surgery, N (%) yes | 29 (67.4) | 31 (72.1) |
| Prior spine surgery, N (%) yes | 17 (39.5) | 17 (39.5) |
| Psychosocial scores 6 weeks after surgery | ||
| Fear of movement, mean (SD) | 40.2 (7.3) | 38.7 (6.5) |
| Pain catastrophizing, mean (SD) | 14.5 (11.9) | 11.5 (10.8) |
| Pain self-efficacy, mean (SD) | 36.0 (16.8) | 41.5 (15.2) |
| Outcome scores 6 weeks after surgery | ||
| Back pain intensity, mean (SD) | 3.0 (2.2) | 2.8 (2.0) |
| Disability, mean (SD) | 38.8 (17.3) | 34.0 (16.7) |
| Physical health, mean (SD) | 29.8 (20.5) | 32.1 (9.8) |
a Fear of movement measured by Tampa Scale for Kinesiophobia; pain catastrophizing measured by Pain Catastrophizing Scale; pain self-efficacy measured by Pain Self-Efficacy Questionnaire; back pain intensity measured by Brief Pain Inventory; disability measured by Oswestry Disability Index; physical health measured by physical component scale of the 12-Item Short-Form Health Survey.
At baseline (6 weeks after surgery), the mean (SD) score for fear of movement was 39.4 (6.9), for pain catastrophizing was 13.0 (11.4), and for pain self-efficacy was 38.8 (16.2) (Tab. 2). The proportion of participants at baseline reporting high fear of movement was 53.5% (n = 46), high pain catastrophizing was 14.0% (n = 12), and low self-efficacy was 50.0% (n = 43).
Table 2.
Descriptive Statistics for Mediators of Fear of Movement, Pain Catastrophizing, and Pain Self-Efficacy Assessed After Spine Surgerya
| 6 Weeks | 3 Months | 6 Months | ||||
|---|---|---|---|---|---|---|
| Mean (SD) | Min–Max | Mean (SD) | Min–Max | Mean (SD) | Min–Max | |
| Fear of movement | 39.4 (6.9) | 24–61 | 36.9 (7.1) | 23–53 | 35.1 (8.3) | 18–61 |
| Pain catastrophizing | 13.0 (11.4) | 0–50 | 10.6 (10.7) | 0–40 | 10.2 (11.2) | 0–42 |
| Pain self-efficacy | 38.8 (16.2) | 6–60 | 43.4 (16.8) | 8–60 | 46.0 (15.5) | 1–60 |
a Fear of movement measured by Tampa Scale for Kinesiophobia; pain catastrophizing measured by Pain Catastrophizing Scale; pain self-efficacy measured by Pain Self-Efficacy Questionnaire.
Association Between Proposed Mediators
Baseline (ie, 6 week) fear of movement was moderately associated with baseline pain self-efficacy (r = −0.47, P < .001). All other associations between psychosocial factors were strong (Tab. 3).
Table 3.
Associations Between Fear of Movement, Pain Catastrophizing, and Pain Self-Efficacy at 6 Weeks, 3 Months, and 6 Months After Surgerya
| 6-Week Associations | 3-Month Associations | 6-Month Associations | ||||
|---|---|---|---|---|---|---|
| Pain Self-Efficacy | Pain Catastrophizing | Pain Self-Efficacy | Pain Catastrophizing | Pain Self-Efficacy | Pain Catastrophizing | |
| Fear of movement | −0.47 | 0.62 | −0.62 | 0.64 | −0.67 | 0.70 |
| Pain catastrophizing | −0.56 | — | −0.74 | — | −0.73 | — |
aValues are Pearson correlation coefficients and all are significant at P < .001.
Change in pain self-efficacy from baseline to 3 months was not associated with 3-month change in fear of movement (r = −0.13, P = .25) or 3-month change in pain catastrophizing (r = −0.18, P = .11). Three-month change in fear of movement and 3-month change in pain catastrophizing were moderately associated (r = 0.37, P = .001). Change in pain self-efficacy from baseline to 6 months was not associated with 6-month change in pain catastrophizing (r = −0.10, P = .39). Change in fear of movement from baseline to 6 months was associated to a weak degree with 6-month change in pain self-efficacy (r = −0.28, P = .01) and to a moderate degree with 6-month change in pain catastrophizing (r = 0.38, P < .001).
Mediation analyses
Multiple mediation analyses for CBPT effects on 6-month back pain intensity, disability, and physical health through changes in psychosocial variables at 3 months did not show evidence of mediation based on indirect effects (Tab. 4). Similar results were observed for the multiple mediation model involving 6-month psychosocial change variables and back pain intensity (Tab. 5).
Table 4.
Multiple Mediation Analyses Testing Indirect Effects of CBPT on 6-Month Back Pain Intensity, Disability, and Physical Health Through 3-Month Changes in Fear of Movement, Pain Catastrophizing, and Pain Self-Efficacya
| Back Pain Intensity | Disability | Physical Health | ||||
|---|---|---|---|---|---|---|
| Effects of: | Coefficient | Bias-Corrected 95% CI | Coefficient | Bias-Corrected 95% CI | Coefficient | Bias-Corrected 95% CI |
| CBPT on outcome (total effect, c) | −0.8 | −1.5 to −0.2 | −9.1 | −14.6 to −3.6 | 6.3 | 2.3 to 10.3 |
| CBPT on outcome (direct effect, c’) | −0.7 | −1.3 to −0.1 | −7.5 | −12.8 to −2.3 | 5.2 | 1.4 to 9.0 |
| CBPT on fear of movement (a1) | −0.6 | −2.8 to 1.6 | −0.6 | −2.8 to 1.6 | −0.5 | −2.8 to 1.7 |
| CBPT on pain catastrophizing (a2) | −1.6 | −5.3 to 2.0 | −1.5 | −5.2 to 2.1 | −1.7 | −5.4 to 1.9 |
| CBPT on pain self-efficacy (a3) | 4.2 | −0.7 to 9.1 | 4.4 | −0.5 to 9.3 | 3.7 | −1.2 to 8.6 |
| Fear of movement on outcome (b1) | 0.08 | 0.01 to 0.15 | 0.6 | 0.1 to 1.2 | −0.5 | −0.9 to −0.1 |
| Pain catastrophizing on outcome (b2) | 0.003 | −0.04 to 0.04 | 0.2 | −0.1 to 0.6 | 0.1 | −0.2 to 0.3 |
| Pain self-efficacy on outcome (b3) | −0.01 | −0.04 to 0.02 | −0.2 | −0.4 to 0.1 | 0.3 | 0.1 to 0.4 |
| Effects of CBPT on outcome through: | ||||||
| Fear of movement (a1b1) | −0.05 | −0.2 to 0.1 | −0.4 | −1.9 to 1.3 | 0.3 | −1.1 to 1.4 |
| Pain catastrophizing (a2b2) | −0.004 | −0.1 to 0.2 | −0.4 | −1.6 to 1.1 | −0.2 | −1.3 to 0.2 |
| Pain self-efficacy (a3b3) | −0.06 | −0.2 to 0.1 | −0.8 | −3.2 to 0.7 | 1.0 | −0.2 to 3.0 |
| All mediators (a1b1 + a2b2 + a3b3) | −0.1 | −0.4 to 0.2 | −1.5 | −4.5 to 1.4 | 1.1 | −0.9 to 3.3 |
a CBPT = Changing Behavior through Physical Therapy. Items in parentheses indicate mediation paths from conceptual model. Mediation models control for baseline outcome score.
Table 5.
Multiple Mediation Analyses Testing Indirect Effects of CBPT on 6-Month Back Pain Intensity, Disability, and Physical Health Through 6-Month Changes in Fear of Movement, Pain Catastrophizing, and Pain Self-Efficacya
| Back Pain Intensity | Disability | Physical Health | ||||
|---|---|---|---|---|---|---|
| Effects of: | Coefficient | Bias-Corrected 95% CI | Coefficient | Bias-Corrected 95% CI | Coefficient | Bias-Corrected 95% CI |
| CBPT on outcome (total effect, c) | −0.8 | −1.5 to −0.2 | −9.1 | −14.6 to −3.6 | 6.3 | 2.3 to 10.3 |
| CBPT on outcome (direct effect, c’) | −0.4 | −1.1 to 0.2 | −4.9 | −10.1 to 0.2 | 2.8 | −1.3 to 6.9 |
| CBPT on fear of movement (a1) | −3.7 | −6.5 to −1.0 | −3.9 | −6.6 to −1.1 | −3.8 | −6.6 to −1.1 |
| CBPT on pain catastrophizing (a2) | −1.2 | −5.1 to 2.7 | −1.0 | −5.0 to 2.9 | −1.4 | −5.3 to 2.6 |
| CBPT on pain self-efficacy (a3) | 9.9 | 4.7 to 15.2 | 9.9 | 4.6 to 15.2 | 9.3 | 4.2 to 14.5 |
| Fear of movement on outcome (b1) | 0.1 | 0.002 to 0.1 | 0.5 | 0.1 to 0.9 | −0.3 | −0.6 to 0.05 |
| Pain catastrophizing on outcome (b2) | 0.02 | −0.02 to 0.06 | 0.5 | 0.2 to 0.8 | −0.05 | −0.3 to 0.2 |
| Pain self-efficacy on outcome (b3) | −0.02 | −0.04 to 0.01 | −0.2 | −0.4 to 0.03 | 0.2 | 0.08 to 0.4 |
| Effects of CBPT on outcome through: | ||||||
| Fear of movement (a1b1) | −0.2 | −0.6 to 0.007 | −2.0 | −4.3 to −0.3 | 1.1 | −0.2 to 2.7 |
| Pain catastrophizing (a2b2) | −0.02 | −0.2 to 0.1 | −0.5 | −2.6 to 1.5 | 0.07 | −0.7 to 0.6 |
| Pain self-efficacy (a3b3) | −0.2 | −0.6 to 0.07 | −1.7 | −5.3 to 0.5 | 2.3 | 0.5 to 4.7 |
| All mediators (a1b1 + a2b2 + a3b3) | −0.4 | −1.1 to 0.03 | −4.2 | −9.6 to 0.3 | 3.5 | 1.2 to 6.1 |
a CBPT = Changing Behavior through Physical Therapy. Items in parenthesis indicate mediation paths from conceptual model. Mediation models control for baseline outcome score.
For disability at 6 months, results from multiple mediation demonstrate that changes in psychosocial variables at 6 months revealed a significant indirect effect (Tab. 5; Suppl. Fig. 1). The CBPT intervention resulted in greater reductions in fear of movement (path a1 coefficient = −3.9) and improvements in pain self-efficacy at 6 months (path a3 coefficient = 9.9). Participants with greater reductions in fear of movement (path b1 coefficient = 0.5) and pain catastrophizing at 6 months (path b2 coefficient = 0.5) had lower disability at 6 months. Only changes in fear of movement were observed to be a mediator as evidenced through its indirect effect (path a1b1 coefficient = −2.0) and bias-corrected CI (−4.3 to −0.3).
Results from multiple mediation for CBPT effects on 6-month physical health through changes in psychosocial variables at 6 months revealed a significant indirect effect (Tab. 5; Suppl. Fig. 2). Similar to the disability model, the CBPT intervention resulted in greater reductions in fear of movement (path a1 coefficient = −3.8) and improvements in pain self-efficacy at 6 months (path a3 coefficient = 9.3). Participants with greater improvements in pain self-efficacy at 6 months had higher physical health at 6 months (path b3 coefficient = 0.2). Changes in pain self-efficacy were observed to be a mediator as evidenced through its indirect effect (path a1b1 coefficient = 2.3) and bias-corrected CI (0.5 to 4.7).
Discussion
Beneficial effects on pain, disability, and general health were observed at 6 months after lumbar spine surgery in participants receiving a 6-week physical therapist-delivered CBPT program; this study was designed to examine the potential psychosocial mechanisms underlying these outcomes. In a multiple mediation model, 6-month changes in fear of movement mediated the effects of CBPT on 6-month disability, while 6-month changes in pain self-efficacy mediated the effects of CBPT on 6-month physical health. Pain catastrophizing was not found to be a mediator following the CBPT program.
The results of our mediation analyses do not provide definitive evidence that fear of movement and pain self-efficacy are causal mechanisms for CBPT effects on disability or physical health. This perspective is based on the finding that we did not observe a change in psychosocial variables at 3 months to have indirect effects on 6-month outcomes. For a mediator to be considered in the “causal path,” it is expected that a change in the mediator should precede a change in the outcome variable. In the current study, only 6-month changes in fear of movement and pain self-efficacy mediated the effects of CBPT on 6-month disability and physical health, respectively. Our findings may be explained by a latency effect. A greater effect over time may occur because of the time needed to adopt new skills or strategies for coping or changing behavior. DasMahapatra et al59 reported greater mediation effects at a 6-month follow-up compared with 1 or 3 months after an online CBT self-management program in participants with chronic pain. Bergbom et al60 and Sullivan and colleagues61,62 also highlighted the importance of both early (eg, sequential) and late (eg, concurrent) changes in psychosocial constructs for predicting pain-related outcomes. Longer-term follow-up is needed for the CBPT intervention to determine if psychosocial changes from baseline to 6 months mediate participant-reported outcomes at 1-year following spine surgery.
Our mediation analyses focused on our theoretical psychosocial constructs of fear of movement, pain catastrophizing, and pain self-efficacy. As has been suggested by prior work,63 these factors are thought to represent distinct yet overlapping constructs (supported by our correlation findings). While these psychosocial factors showed substantial correlations with each other at each time point, their longitudinal change (our mediating variable) did not. The lack of strong correlation in change may alleviate concerns for multicollinearity in our statistical modeling. This finding suggests psychosocial changes after treatment are not redundant, especially when considering the association between changes in pain self-efficacy with the other negative psychosocial factors. Fear of movement, pain catastrophizing, and pain self-efficacy have been studied in the context of chronic pain and are known determinants of persistent disability.20,64,65 Often, studies examine these factors in isolation; however, Wideman et al66 have shown that simultaneous consideration of these factors within statistical models may be more pertinent for risk estimation. Multidimensional screening tools have been developed to account for various contributions of psychosocial constructs on risk and for comprehensive monitoring over a course of treatment.67–70 Moreover, studies aimed at examining multiple treatment effects may show a dominant construct(s), which has implications for how treatment is applied.13,71
Psychosocial constructs are expected to remain stable postoperatively in the absence of targeted therapy.21,22 Studies by Abbott et al72 and Monticone et al73 suggest that postoperative, in-person, cognitive-behavioral interventions can improve fear immediately after spinal fusion surgery above what is expected by standard postoperative exercise alone. While we did not observe an immediate benefit following CBPT on fear of movement, we did see greater reductions at the 6-month time point that mediated the effect on 6-month disability. This was similar to the effects we observed on pain self-efficacy and 6-month physical health. Across multiple psychosocial variables, including fear, Turner et al13 found self-efficacy to have a unique mediating role following CBT. The results of the current study suggest potential disability and health benefits of postoperative rehabilitation strategies that not only reduce negative psychosocial characteristics such as fear of movement but also increase positive characteristics such as pain self-efficacy. For example, targeted application of treatment may need to consider strategies such as graded activity or exposure in vivo for fear of movement, and goal-setting, problem solving, or mindfulness training for pain self-efficacy.74–78
The hypothesis that pain catastrophizing would be a mediator was not supported. Preliminary work has shown pain catastrophizing to have a negative influence on pain-related disability in patients after lumbar spine surgery.26,79 Monticone et al73 found pain catastrophizing to be a potential mediator when testing a combined cognitive-behavioral and exercise intervention after spinal fusion. In participants with chronic LBP, earlier CBT studies by Smeets et al11 and Spinhoven et al80 reported a mediating role of pain catastrophizing. Burns and colleagues81 reported a lack of treatment-specific change in pain catastrophizing following CBT for chronic pain. Our findings for pain catastrophizing may be due to the limited variability and low values at the postoperative baseline visit. Targeting CBPT to individuals with high levels of pain catastrophizing may have yielded a more robust effect. Another explanation may be the CBPT program’s greater focus on graded activity and goal setting relative to cognitive restructuring techniques, which are commonly used to address pain catastrophizing.77 This may have resulted in CBPT being more conducive to altering fear of movement and pain self-efficacy compared with pain catastrophizing.
The current study included pre-specified a priori mediation analyses from a randomized trial. However, the study may not be adequately powered to detect mediation effects, and results need to be interpreted with caution.82 The results of our study should be viewed as preliminary and exploratory. Future mediation analyses should involve larger samples to validate findings from the current study and possibly disentangle more complex relationships between the proposed mediators. Our study enrolled participants with high preoperative fear of movement. However, individuals with varying levels of pain self-efficacy and lower levels of pain catastrophizing were included. The CBPT intervention may be more efficacious for individuals with higher levels across all targeted mediators. As mentioned, our results demonstrate concurrent mediation only, that is, a change in mediators did not appear to precede a change in clinical outcomes. To establish a more definitive causal path, designs involving lagged or cross-lagged analyses could be considered. Only 6-month outcome data are included in the current study. It is possible that the effects of CBPT may be reduced at longer term follow-up.83 The current analyses are limited to only those cognitive mediators included within the mediation model. It is possible that other factors—unmeasured or not included in modeling—may influence our findings. Other potential psychosocial mediators of CBT include social support, acceptance, and coping as well as measures of behavioral change (ie, activity participation, adherence). Non-specific or contextual factors involving the patient-therapist relationship or expectations may also underlie the therapeutic effects of CBPT. Future trials should consider how additional constructs mediate the effects of CBT-based interventions.
The results of our exploratory mediation analyses suggest fear of movement (for disability) and pain self-efficacy (for physical health) may be important mediators of CBPT. Future work with the CBPT intervention should establish whether psychosocial changes in the first 6 months after surgery mediate long-term patient-reported outcomes following spine surgery. Fear of movement and pain self-efficacy may be important mechanisms to consider when developing and testing psychologically informed physical therapy programs.
Supplementary Material
Contributor Information
Rogelio A Coronado, Department of Orthopaedic Surgery, Department of Physical Medicine and Rehabilitation, and Center for Musculoskeletal Research, Vanderbilt University Medical Center, Nashville, Tennessee.
Dawn M Ehde, Department of Rehabilitation Medicine, University of Washington, Seattle, Washington.
Jacquelyn S Pennings, Department of Orthopaedic Surgery, and Center for Musculoskeletal Research, Vanderbilt University Medical Center.
Susan W Vanston, Department of Orthopaedic Surgery, Vanderbilt University Medical Center.
Tatsuki Koyama, Department of Biostatistics, Vanderbilt University Medical Center.
Sharon E Phillips, Department of Biostatistics, Vanderbilt University Medical Center.
Shannon L Mathis, Department of Kinesiology, University of Alabama, Huntsville, Alabama.
Matthew J McGirt, Carolina Neurosurgery and Spine Associates, Charlotte, North Carolina.
Dan M Spengler, Department of Orthopaedic Surgery, Vanderbilt University Medical Center.
Oran S Aaronson, Howell Allen Clinic, Saint Thomas Medical Partners, Nashville, Tennessee.
Joseph S Cheng, Department of Neurosurgery, University of Cincinnati School of Medicine, Cincinnati, Ohio.
Clinton J Devin, Department of Orthopaedic Surgery, Vanderbilt University Medical Center; and Steamboat Orthopaedic and Spine Institute, Steamboat Springs, Colorado.
Stephen T Wegener, Department of Physical Medicine and Rehabilitation, Johns Hopkins Medicine, Baltimore, Maryland.
Kristin R Archer, Department of Orthopaedic Surgery, Center for Musculoskeletal Research, Vanderbilt University Medical Center, 1215 21st Avenue S, Medical Center East - South Tower, Suite 4200, Nashville, TN 37232 (USA); Department of Physical Medicine and Rehabilitation, Osher Center for Integrative Medicine, Vanderbilt University Medical Center.
Author Contributions
Concept/idea/research design: R.A. Coronado, D.M. Ehde, S.W. Vanston, T. Koyama, M.J. McGirt, D.M. Spengler, O.S. Aaronson, J.S. Cheng, C.J. Devin, S.T. Wegener, K.R. Archer
Writing: R.A. Coronado, D.M. Ehde, T. Koyama, D.M. Spengler, C.J. Devin, S.T. Wegener, K.R. Archer
Data collection: S.W. Vanston, S.L. Mathis, S.T. Wegener
Data analysis: J.S. Pennings, T. Koyama, S.E. Phillips
Project management: S.L. Mathis, K.R. Archer
Fund procurement: K.R. Archer
Providing institutional liaisons: S.T. Wegener
Clerical/secretarial support: R.A. Coronado
Consultation (including review of manuscript before submitting): R.A. Coronado, D.M. Ehde, J.S. Pennings, S.W. Vanston, T. Koyama, S.E. Phillips, S.L. Mathis, M.J. McGirt, D.M. Spengler, O.S. Aaronson, J.S. Cheng, C.J. Devin, S.T. Wegener, K.R. Archer
Ethics Approval
This study was approved by the Institutional Review Board at Vanderbilt University Medical Center.
Funding
This study was supported by a research grant (R21AR062880) from the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health, and a Magistro Family Foundation grant from the Foundation for Physical Therapy Research. This study used REDCap as the secure database, which was supported by a CTSA award (UL1TR000445) from the National Center for Advancing Translational Sciences.
During manuscript development and submission, Dr Coronado was supported by a Vanderbilt Faculty Research Scholars award.
Disclosures and Presentations
The authors completed the ICMJE Form for Disclosure of Potential Conflicts of Interest and reported no conflicts of interest.
Data from this study were presented at the American Physical Therapy Association Combined Sections Meeting, February 16, 2017, San Antonio, Texas, and posted online at: Archer K, Coronado RA, Ehde D, et al. Fear of Movement and Pain Self-Efficacy Mediate Outcomes Following a Targeted Rehabilitation Intervention After Spine Surgery. Abstract OPL4. Orthopaedic Section Platform Presentations (Abstracts OPL1–OPL64). J Ortho Sports Phys Ther. 2016;47:A1-A29. https://www.jospt.org/doi/10.2519/jospt.2017.47.1.A1#_i129.
References
- 1. Pitcher MH, Von Korff M, Bushnell MC, Porter L. Prevalence and profile of high-impact chronic pain in the United States. J Pain. 2019;20:146–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Williams AC, Eccleston C, Morley S. Psychological therapies for the management of chronic pain (excluding headache) in adults. Cochrane Database Syst Rev. 2012;11:CD007407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hoffman BM, Papas RK, Chatkoff DK, Kerns RD. Meta-analysis of psychological interventions for chronic low back pain. Health Psychol. 2007;26:1–9. [DOI] [PubMed] [Google Scholar]
- 4. Morley S. Efficacy and effectiveness of cognitive behaviour therapy for chronic pain: progress and some challenges. Pain. 2011;152:S99–S106. [DOI] [PubMed] [Google Scholar]
- 5. Archer KR, Coronado RA, Wegener ST. The role of psychologically informed physical therapy for musculoskeletal pain. Curr Phys Med Rehabil Rep. 2018;6:15–25. [Google Scholar]
- 6. Coronado RA, Patel AM, McKernan LC, Wegener ST, Archer KR. Preoperative and postoperative psychologically informed physical therapy: a systematic review of randomized trials among patients with degenerative spine, hip, and knee conditions. J Appl Biobehav Res. 2019;24:e12159. [Google Scholar]
- 7. Silva Guerrero AV, Maujean A, Campbell L, Sterling M. A systematic review and meta-analysis of the effectiveness of psychological interventions delivered by physiotherapists on pain, disability and psychological outcomes in musculoskeletal pain conditions. Clin J Pain. 2018;34:838–857. [DOI] [PubMed] [Google Scholar]
- 8. Vlaeyen JW, Morley S. Cognitive-behavioral treatments for chronic pain: what works for whom? Clin J Pain. 2005;21:1–8. [DOI] [PubMed] [Google Scholar]
- 9. Mansell G, Hill JC, Main CJ, Von Korff M, van der Windt D. Mediators of treatment effect in the back in action trial: using latent growth modeling to take change over time into account. Clin J Pain. 2017;33:811–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Stevens ML, Boyle E, Hartvigsen J, et al. Mechanisms for reducing low back pain: a mediation analysis of a multifaceted intervention in workers in elderly care. Int Arch Occup Environ Health. 2019;92:49–58. [DOI] [PubMed] [Google Scholar]
- 11. Smeets RJ, Vlaeyen JW, Kester AD, Knottnerus JA. Reduction of pain catastrophizing mediates the outcome of both physical and cognitive-behavioral treatment in chronic low back pain. J Pain. 2006;7:261–271. [DOI] [PubMed] [Google Scholar]
- 12. Turner JA, Mancl L, Aaron LA. Short- and long-term efficacy of brief cognitive-behavioral therapy for patients with chronic temporomandibular disorder pain: a randomized, controlled trial. Pain. 2006;121:181–194. [DOI] [PubMed] [Google Scholar]
- 13. Turner JA, Holtzman S, Mancl L. Mediators, moderators, and predictors of therapeutic change in cognitive-behavioral therapy for chronic pain. Pain. 2007;127:276–286. [DOI] [PubMed] [Google Scholar]
- 14. Williams AC, McCracken LM. Cognitive-behavioral therapy for chronic pain: an overview with specific references to fear and avoidance. In: Asmundson GG, Vlaeyen JW, Crombez G, eds., Understanding and Treating Fear of Pain. London, UK: Oxford University Press; 2004: 293–312. [Google Scholar]
- 15. Woods MP, Asmundson GJ. Evaluating the efficacy of graded in vivo exposure for the treatment of fear in patients with chronic back pain: a randomized controlled clinical trial. Pain. 2008;136:271–280. [DOI] [PubMed] [Google Scholar]
- 16. Lorig K, Holman H. Arthritis self-management studies: a twelve-year review. Health Educ Q. 1993;20:17–28. [DOI] [PubMed] [Google Scholar]
- 17. Lorig KR, Ritter P, Stewart AL, et al. Chronic disease self-management program: 2-year health status and health care utilization outcomes. Med Care. 2001;39:1217–1223. [DOI] [PubMed] [Google Scholar]
- 18. Archer KR, Devin CJ, Vanston SW, et al. Cognitive-behavioral-based physical therapy for patients with chronic pain undergoing lumbar spine surgery: a randomized controlled trial. J Pain. 2016;17:76–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Leeuw M, Goossens ME, Linton SJ, Crombez G, Boersma K, Vlaeyen JW. The fear-avoidance model of musculoskeletal pain: current state of scientific evidence. J Behav Med. 2007; 30:77–94. [DOI] [PubMed] [Google Scholar]
- 20. Woby SR, Urmston M, Watson PJ. Self-efficacy mediates the relation between pain-related fear and outcome in chronic low back pain patients. Eur J Pain. 2007;11:711–718. [DOI] [PubMed] [Google Scholar]
- 21. Archer KR, Wegener ST, Seebach C, et al. The effect of fear of movement beliefs on pain and disability after surgery for lumbar and cervical degenerative conditions. Spine. 2011;36: 1554–1562. [DOI] [PubMed] [Google Scholar]
- 22. Archer KR, Seebach CL, Mathis SL, Riley LH 3rd, Wegener ST. Early postoperative fear of movement predicts pain, disability, and physical health six months after spinal surgery for degenerative conditions. Spine J. 2014;14:759–767. [DOI] [PubMed] [Google Scholar]
- 23. Abbott AD, Tyni-Lenne R, Hedlund R. The influence of psychological factors on pre-operative levels of pain intensity, disability and health-related quality of life in lumbar spinal fusion surgery patients. Physiotherapy. 2010;96:213–221. [DOI] [PubMed] [Google Scholar]
- 24. den Boer JJ, Oostendorp RA, Beems T, Munneke M, Evers AW. Continued disability and pain after lumbar disc surgery: the role of cognitive-behavioral factors. Pain. 2006;123:45–52. [DOI] [PubMed] [Google Scholar]
- 25. Johansson AC, Linton SJ, Rosenblad A, Bergkvist L, Nilsson O. A prospective study of cognitive behavioural factors as predictors of pain, disability and quality of life one year after lumbar disc surgery. Disabil Rehabil. 2010;32:521–529. [DOI] [PubMed] [Google Scholar]
- 26. Coronado RA, George SZ, Devin CJ, Wegener ST, Archer KR. Pain sensitivity and pain catastrophizing are associated with persistent pain and disability after lumbar spine surgery. Arch Phys Med Rehabil. 2015;96:1763–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Martinez-Calderon J, Jensen MP, Morales-Asencio JM, Luque-Suarez A. Pain catastrophizing and function in individuals with chronic musculoskeletal pain a systematic review and meta-analysis. Clin J Pain. 2019;35:279–293. [DOI] [PubMed] [Google Scholar]
- 28. Nicholas MK, Asghari A, Corbett M, et al. Is adherence to pain self-management strategies associated with improved pain, depression and disability in those with disabling chronic pain? Eur J Pain. 2012;16:93–104. [DOI] [PubMed] [Google Scholar]
- 29. Quartana PJ, Campbell CM, Edwards RR. Pain catastrophizing: a critical review. Expert Rev Neurother. 2009;9:745–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Archer KR, Phelps KD, Seebach CL, Song Y, Riley LH 3rd, Wegener ST. Comparative study of short forms of the Tampa Scale for Kinesiophobia: fear of movement in a surgical spine population. Arch Phys Med Rehabil. 2012;93:1460–1462. [DOI] [PubMed] [Google Scholar]
- 31. Turner JA, Mancl L, Aaron LA. Brief cognitive-behavioral therapy for temporomandibular disorder pain: effects on daily electronic outcome and process measures. Pain. 2005;117: 377–387. [DOI] [PubMed] [Google Scholar]
- 32. Archer KR, Motzny N, Abraham CM, et al. Cognitive- behavioral-based physical therapy to improve surgical spine outcomes: a case series. Phys Ther. 2013;93:1130–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kori SH, Miller RP, Todd DD. Kinesiophobia: a new region of chronic pain behavior. Pain Manage. 1990;3:35–43. [Google Scholar]
- 34. French DJ, France CR, Vigneau F, French JA, Evans RT. Fear of movement/(re)injury in chronic pain: a psychometric assessment of the original English version of the Tampa Scale for Kinesiophobia (TSK). Pain. 2007;127:42–51. [DOI] [PubMed] [Google Scholar]
- 35. Roelofs J, Goubert L, Peters ML, Vlaeyen JW, Crombez G. The Tampa Scale for Kinesiophobia: further examination of psychometric properties in patients with chronic low back pain and fibromyalgia. Eur J Pain. 2004;8:495–502. [DOI] [PubMed] [Google Scholar]
- 36. Sullivan M, Bishop S, Pivik J. The Pain Catastrophizing Scale: development and validation. Psychol Assess. 1995;7:524–532. [Google Scholar]
- 37. Van Wyngaarden JJ, Noehren B, Archer KR. Assessing psychosocial profile in the physical therapy setting. J Appl Behav Res. 2019;24:e12165. [Google Scholar]
- 38. Osman A, Barrios FX, Gutierrez PM, Kopper BA, Merrifield T, Grittman L. The Pain Catastrophizing Scale: further psychometric evaluation with adult samples. J Behav Med. 2000;23:351–365. [DOI] [PubMed] [Google Scholar]
- 39. Van Damme S, Crombez G, Bijttebier P, Goubert L, Van Houdenhove B. A confirmatory factor analysis of the Pain Catastrophizing Scale: invariant factor structure across clinical and non-clinical populations. Pain. 2002;96:319–324. [DOI] [PubMed] [Google Scholar]
- 40. Bandura A. Self-efficacy: toward a unifying theory of behavioral change. Psychol Rev. 1977;84:191–215. [DOI] [PubMed] [Google Scholar]
- 41. Nicholas MK. The pain self-efficacy questionnaire: taking pain into account. Eur J Pain. 2007;11:153–163. [DOI] [PubMed] [Google Scholar]
- 42. Miles CL, Pincus T, Carnes D, Taylor SJ, Underwood M. Measuring pain self-efficacy. Clin J Pain. 2011;27:461–470. [DOI] [PubMed] [Google Scholar]
- 43. Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23: 129–138. [PubMed] [Google Scholar]
- 44. Keller S, Bann CM, Dodd SL, Schein J, Mendoza TR, Cleeland CS. Validity of the Brief Pain Inventory for use in documenting the outcomes of patients with noncancer pain. Clin J Pain. 2004;20:309–318. [DOI] [PubMed] [Google Scholar]
- 45. Mendoza TR, Chen C, Brugger A, et al. The utility and validity of the modified Brief Pain Inventory in a multiple-dose postoperative analgesic trial. Clin J Pain. 2004;20:357–362. [DOI] [PubMed] [Google Scholar]
- 46. Zalon ML. Comparison of pain measures in surgical patients. J Nurs Meas. 1999;7:135–152. [PubMed] [Google Scholar]
- 47. Fairbank JC, Pynsent PB. The Oswestry Disability Index. Spine. 2000;25:2940–2952 Discussion 2952. [DOI] [PubMed] [Google Scholar]
- 48. Davidson M, Keating JL. A comparison of five low back disability questionnaires: reliability and responsiveness. Phys Ther. 2002;82:8–24. [DOI] [PubMed] [Google Scholar]
- 49. Pratt RK, Fairbank JC, Virr A. The reliability of the shuttle walking test, the Swiss Spinal Stenosis Questionnaire, the Oxford Spinal Stenosis Score, and the Oswestry Disability Index in the assessment of patients with lumbar spinal stenosis. Spine. 2002;27:84–91. [DOI] [PubMed] [Google Scholar]
- 50. Ware J Jr, Kosinski M, Keller SD. A 12-item short-form health survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–233. [DOI] [PubMed] [Google Scholar]
- 51. Jenkinson C, Layte R, Jenkinson D, et al. A shorter form health survey: can the SF-12 replicate results from the SF-36 in longitudinal studies? J Public Health Med. 1997;19:179–186. [DOI] [PubMed] [Google Scholar]
- 52. Luo X, George ML, Kakouras I, et al. Reliability, validity, and responsiveness of the short form 12-item survey (SF-12) in patients with back pain. Spine. 2003;28:1739–1745. [DOI] [PubMed] [Google Scholar]
- 53. Cohen J. Statistical Power Analysis for the Behavioral Sciences. Vol. 2. Hillsdale, NJ, USA: L. Erlbaum Associates; 1988. [Google Scholar]
- 54. Hayes AF. Introduction to Mediation, Moderation, and Conditional Process Analysis: A Regression-Based Approach. New York, NY, USA: The Guilford Press; 2013. [Google Scholar]
- 55. Williamson W, Kluzek S, Roberts N, et al. Behavioural physical activity interventions in participants with lower-limb osteoarthritis: a systematic review with meta-analysis. BMJ Open. 2015;5:e007642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. MacKinnon DP, Lockwood CM, Hoffman JM, West SG, Sheets V. A comparison of methods to test mediation and other intervening variable effects. Psychol Methods. 2002;7:83–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav Res Methods. 2008;40:879–891. [DOI] [PubMed] [Google Scholar]
- 58. Shrout PE, Bolger N. Mediation in experimental and nonexperimental studies: new procedures and recommendations. Psychol Methods. 2002;7:422–445. [PubMed] [Google Scholar]
- 59. DasMahapatra P, Chiauzzi E, Pujol LM, Los C, Trudeau KJ. Mediators and moderators of chronic pain outcomes in an online self-management program. Clin J Pain. 2015;31: 404–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Bergbom S, Boersma K, Linton SJ. Both early and late changes in psychological variables relate to treatment outcome for musculoskeletal pain patients at risk for disability. Behav Res Ther. 2012;50:726–734. [DOI] [PubMed] [Google Scholar]
- 61. Sullivan MJ, Adams H, Thibault P, Corbiere M, Stanish WD. Initial depression severity and the trajectory of recovery following cognitive-behavioral intervention for work disability. J Occup Rehabil. 2006;16:63–74. [DOI] [PubMed] [Google Scholar]
- 62. Wideman TH, Adams H, Sullivan MJ. A prospective sequential analysis of the fear-avoidance model of pain. Pain. 2009;145: 45–51. [DOI] [PubMed] [Google Scholar]
- 63. George SZ, Valencia C, Beneciuk JM. A psychometric investigation of fear-avoidance model measures in patients with chronic low back pain. J Orthop Sports Phys Ther. 2010; 40:197–205. [DOI] [PubMed] [Google Scholar]
- 64. Crombez G, Eccleston C, Van Damme S, Vlaeyen JW, Karoly P. Fear-avoidance model of chronic pain: the next generation. Clin J Pain. 2012;28:475–483. [DOI] [PubMed] [Google Scholar]
- 65. Wertli MM, Burgstaller JM, Weiser S, Steurer J, Kofmehl R, Held U. Influence of catastrophizing on treatment outcome in patients with nonspecific low back pain: a systematic review. Spine (Phila Pa 1976). 2014;39:263–273. [DOI] [PubMed] [Google Scholar]
- 66. Wideman TH, Sullivan MJ. Development of a cumulative psychosocial factor index for problematic recovery following work-related musculoskeletal injuries. Phys Ther. 2012;92: 58–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Hill JC, Dunn KM, Main CJ, Hay EM. Subgrouping low back pain: a comparison of the STarT back tool with the Örebro Musculoskeletal Pain Screening Questionnaire. Eur J Pain. 2010;14:83–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Hill JC, Dunn KM, Lewis M, et al. A primary care back pain screening tool: identifying patient subgroups for initial treatment. Arthritis Rheum. 2008;59:632–641. [DOI] [PubMed] [Google Scholar]
- 69. Beneciuk JM, Lentz TA, He Y, Wu SS, George SZ. Prediction of persistent musculoskeletal pain at 12 months: a secondary analysis of the Optimal Screening for Prediction of Referral and Outcome (OSPRO) validation cohort study. Phys Ther. 2018; 98:290–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Lentz TA, Beneciuk JM, Bialosky JE, et al. Development of a yellow flag assessment tool for orthopaedic physical therapists: results from the Optimal Screening for Prediction of Referral and Outcome (OSPRO) cohort. J Orthop Sports Phys Ther. 2016;46:327–343. [DOI] [PubMed] [Google Scholar]
- 71. Day MA, Thorn BE. The mediating role of pain acceptance during mindfulness-based cognitive therapy for headache. Complement Ther Med. 2016;25:51–54. [DOI] [PubMed] [Google Scholar]
- 72. Abbott AD, Tyni-Lenne R, Hedlund R. Early rehabilitation targeting cognition, behavior, and motor function after lumbar fusion: a randomized controlled trial. Spine. 2010;35:848–857. [DOI] [PubMed] [Google Scholar]
- 73. Monticone M, Ferrante S, Teli M, et al. Management of catastrophising and kinesiophobia improves rehabilitation after fusion for lumbar spondylolisthesis and stenosis. A randomised controlled trial. Eur Spine J. 2014;23:87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Jensen MP. Psychosocial approaches to pain management: an organizational framework. Pain. 2011;152:717–725. [DOI] [PubMed] [Google Scholar]
- 75. George SZ, Wittmer VT, Fillingim RB, Robinson ME. Comparison of graded exercise and graded exposure clinical outcomes for patients with chronic low back pain. J Orthop Sports Phys Ther. 2010;40:694–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Loeser JD, Bonica JJ. Bonica's Management of Pain. 3rd ed. Philadelphia, PA, USA: Lippincott Williams & Wilkins; 2001. [Google Scholar]
- 77. Thorn BE. Cognitive Therapy for Chronic Pain: A Step-by-Step Guide. New York, NY, USA: Guilford Press; 2004. [Google Scholar]
- 78. Day MA, Thorn BE. Mindfulness-based cognitive therapy for headache pain: an evaluation of the long-term maintenance of effects. Complement Ther Med. 2017;33:94–98. [DOI] [PubMed] [Google Scholar]
- 79. Abbott AD, Tyni-Lenne R, Hedlund R. Leg pain and psychological variables predict outcome 2-3 years after lumbar fusion surgery. Eur Spine J. 2011;20:1626–1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Spinhoven P, Ter Kuile M, Kole-Snijders AM, Hutten Mansfeld M, Den Ouden DJ, Vlaeyen JW. Catastrophizing and internal pain control as mediators of outcome in the multidisciplinary treatment of chronic low back pain. Eur J Pain. 2004;8: 211–219. [DOI] [PubMed] [Google Scholar]
- 81. Burns JW, Day MA, Thorn BE. Is reduction in pain catastrophizing a therapeutic mechanism specific to cognitive-behavioral therapy for chronic pain? Transl Behav Med. 2012;2:22–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Fritz MS, Mackinnon DP. Required sample size to detect the mediated effect. Psychol Sci. 2007;18:233–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Rolving N, Nielsen CV, Christensen FB, Holm R, Bunger CE, Oestergaard LG. Does a preoperative cognitive-behavioral intervention affect disability, pain behavior, pain, and return to work the first year after lumbar spinal fusion surgery? Spine. 2015;40:593–600. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


