Abstract
The prevalence of dementia and other age-associated cognitive disorders is steadily increasing worldwide. With no cure after diagnosis, successful treatment likely requires maximum adherence to preventative countermeasures. Many potential risk factors are modifiable through exercise. Specifically, mounting evidence suggests that long-term resistance training (RT) can help maintain cognitive abilities with aging and have additional benefits to overall brain health. Physical therapists are uniquely positioned to administer such clinical interventions designed to slow disease progression. However, a neuroscientific foundation for these benefits must be established to justify the integration of RT for brain health into practice. The mechanisms of cognitive decline are commonly linked to fundamental processes of aging. Even healthy older adults experience decreases in physical capacity, vascular function, brain structure and function, glucose regulation, inflammation, mood, and sleep quality. Yet, clinical trials involving RT in older adults have consistently demonstrated improvements in each of these systems with concomitant enhancement of cognitive performance. Beneficial adaptations may indirectly or directly mediate benefits to brain function, and understanding this relationship can help us develop optimal intervention strategies for the aging population.
Keywords: Brain, Exercise, Aging, Strength, Dementia, Mental Health
Populations around the world are living longer and healthier lives. But with this change, elderly individuals are increasingly susceptible to nonfatal progressive disorders. Dementia is one such age-related disease caused by neurodegeneration and characterized by significant cognitive decline that interferes with independent living. In the United States alone, the total monetary cost of dementia care and treatment was estimated to be $277 billion in 2018, with an additional family caregiver burden valued at more than $232 billion.1 The development and severity of dementia is strongly influenced by factors occurring years to decades earlier.2 Because irreversible brain damage often occurs before patients present to clinic with demonstrable cognitive dysfunction, successful treatment likely requires presymptomatic neuroprotective therapies as opposed to disease reversal.
Exercise is one of the most promising strategies to slow or prevent the progression of cognitive decline and dementia. However, the direct mechanisms remain relatively unknown. While animal exercise studies have identified molecular pathways with overlapping or redundant effects on brain function,3 human translational studies have been less definitive and generally underpowered.4 But exercise also targets fundamental aging processes that share common pathways with brain function. Thus, gaining knowledge of the underlying neural and physiological processes of cognitive enhancement requires an awareness of the accompanying adaptations related to cognitive changes. Understanding the factors that interact with exercise-induced cognitive enhancement will help us design optimal treatment strategies for the aging population.
There are 2 primary types of chronic exercise, aerobic training and resistance training (RT), that have common and distinct physiological benefits.5,6 Exercise interventions involving one or both types improve fluid cognitive function in older adults.7–9 Previous reviews of potential mechanisms have focused on physical activity in general or aerobic exercise exclusively.10,11 Meanwhile, there is growing interest in the distinct mechanisms of RT.
In this perspective, we will briefly discuss the positive changes in cognition after RT interventions in older adults, then analyze the supramolecular physiological and psychological benefits that may be related to cognitive enhancement. Potential mechanisms include improvements in physical capacity, vascular function, brain structure and function, glucose regulation, inflammation, mood, and sleep quality. These RT-induced adaptations may indirectly or directly mediate benefits to brain function in older adults. We propose mechanistic studies to investigate the contribution of each adaptation to overall cognitive performance. Understanding these relationships will help establish a neuroscientific foundation for the effects of RT on cognition and develop optimal intervention strategies to maintain cognitive health in the aging population.
Cognitive Changes
With aging, even healthy older adults (ie, without major medical or neuropsychiatric disorders) experience declines in fluid cognitive functions (ie, the capacity to reason and solve novel problems). Decreased performance is typical in normal aging on tests involving processing speed, attention, memory, and executive function. But there is strong evidence to suggest that higher levels of physical activity reduce the rate of cognitive decline and risk of dementia.12 Compared with general physical activity, specific forms of exercise like RT may have greater implications for cognitive health in older adults.7
Many clinical trials have investigated the effects of RT on fluid cognition in older adults. Both a systematic review and a meta-analysis have recently been published to summarize study characteristics and provide a quantitative examination of RT efficacy.8,9 We will discuss the results of studies that have the greatest influence on this rapidly expanding line of research. These well-designed randomized controlled trials (RCTs) have large sample sizes and active control groups to reduce the confounding effects of attention. At the end of this article, we discuss future directions and recommend specific study characteristics to produce new high-quality RCTs. Overall, RT appears to have positive effects on fluid cognition.
In healthy older adults with normal cognitive aging, the greatest improvements after RT interventions are observed in fluid cognition composite scores and executive function.13 Moderate improvements to working memory have also been observed after RT,14 but changes to this subdomain are generally less common than after aerobic interventions.15 Long-term benefits have also been demonstrated in healthy older adults. After 1-year follow-up, twice-weekly RT promoted executive function (d = 0.31) and memory (d = 0.45) relative to a balance-and-toning control.16 Once-weekly RT also promoted executive function (d = 0.48), but not memory. Thus, it appears that healthy older adults with normal age-related cognitive decline can achieve positive and lasting cognitive benefits from RT interventions.
Recent attention has turned towards populations with mild cognitive impairment (MCI), as these groups have the highest risk of dementia.1 People with MCI show cognitive decline greater than expected for their age and education level but are still capable of performing everyday activities. A study in older women with probable MCI demonstrated that twice-weekly RT improved executive function and associative memory compared with a balance and toning control group,17 even though exercise compliance was reported to be low. Similarly, a RT trial in patients diagnosed with MCI had to reduce training from 3 d/wk to 2 d/wk to minimize burden/transportation difficulties.18 The study still demonstrated improved global cognition on the Alzheimer's Disease Assessment Scale-Cognitive scale and improved executive function subdomain. Notably, both studies were 6 months in duration in contrast to the 12-month duration of the studies on which the training protocols were based, suggesting that improvements may be detectable earlier in MCI. However, clinicians should consider the lower compliance rates in these populations when designing therapeutic interventions.
While more high-quality RCTs are still needed to accurately estimate the efficacy of RT on cognition, the above findings provide supportive evidence for the positive effects both in older adults with normal cognitive aging and in MCI. These cognitive changes could be a direct response to practicing a goal-oriented motor skill that involves force production, muscle coordination, kinesthetic memory, and sensory processing of a mechanical load. Thus, RT itself could have independent effects. Cognitive changes could also be driven by molecular growth factor cascades within the brain. But as mentioned earlier, these are difficult to measure in humans. Because epidemiological studies link cognitive decline with other fundamental processes of aging and RT is known to benefit overall health, indirect mechanisms have been proposed to explain how RT improves cognition. Evaluating the rationale of these potential mechanisms can help justify the need for future mechanistic studies.
Physiological Factors
Physical Capacity
RT is well known for its effects on muscle mass and physical capacity. The expected strength increase for older adults participating in an RT program (mean duration of 17.6 weeks and mean frequency of 2.7 d/wk) is about 24–33%, with higher intensities associated with greater improvement.19 Epidemiological studies link muscular strength with rate of cognitive decline and incident dementia.20 However, RCTs are necessary to investigate the causality of this relationship.
While improvements in cognition after aerobic training are associated with increases in aerobic capacity (ie, VO2max),21 this has not been demonstrated after RT. Instead, improvements in fluid cognition and executive function after RT appear to be mediated by increases in muscular strength.22,23 With the known dose-response relationship between RT and muscular strength,19 a high-intensity RT program designed specifically to maximize strength gains may also optimize improvements in cognition. However, studies have failed to demonstrate greater cognitive improvements in participants who undergo higher RT frequency or intensity compared with those undergoing a lower “dose.”13,14 Determining how to maximize cognitive improvements is an important consideration for future investigations to help therapists make recommendations for exercise prescription. The link between strength and cognition may be 1 source of RT’s unique benefits to brain function.
At the supraspinal level, neural mechanisms of muscular strength development remain unclear to date, partly due to varying methodologies. Very short-term RT (<4 weeks), thought to elicit neural adaptations without muscular hypertrophy, has been demonstrated to alter white matter microstructure, reduce intracortical inhibition, and increase corticospinal excitability.24–26 Because there is evidence to suggest that the balance between cortical inhibition and excitation is related to cognitive performance,27 these neural adaptations may explain the statistical mediation of cognitive improvements by muscular strength gains.22,23 Thus, changes in strength and cognition could share supraspinal neural pathways in response to RT.
Additional practical benefits of RT have been demonstrated. For example, even simple home RT programs involving only body mass or elastic bands for resistance can improve executive function and memory in older adults.28,29 These types of interventions demonstrate positive correlations between changes in global cognition and functional mobility.30 Similarly with machine-based RT, enhancements in executive function are associated with increased gait speed.13 Thus, RT can be a practical solution for both physical and cognitive decline in older adults. And like the lasting benefits for executive function, changes in muscle strength and muscle power can persist at 1 year following 52 weeks of twice weekly RT.16,31
We have highlighted the positive link between physical and cognitive function in older adults after training, but an equally strong association is present in their declines with aging. Whether improving or worsening, physical and cognitive function are more likely to change in concert than in different directions.32 Because changes in one do not precede changes in the other, this association is likely not causal but rather due to a shared neural or vascular substrate.33 Therefore, having a mechanistic understanding may aid the protection of both. RT may be a vital form of exercise to maintain both the physical and cognitive capacities necessary for functional independence in older adults.
Vascular Function
The cerebrovascular (CV) system secures adequate blood supply to the brain in dynamic and resting conditions. Disturbances to this system have long been suspected to play a role in the development of dementia. In principle, CV dysfunction can involve any of the multiple etiologies of stroke such as hypoperfusion, small vessel disease, or cardioembolism.34 Recent evidence suggests that CV dysfunction may contribute to, and even precede, neuronal dysfunction and cognitive impairment.35 For example, in a population-based study of older adults in the Netherlands, cerebral hypoperfusion at baseline was associated with accelerated cognitive decline and a higher risk of dementia at the median 6.9-year follow-up.36 CV function is now recognized as a top research priority in all national plans to address dementia.
Epidemiological and aerobic intervention studies suggest that improved CV function is a primary mechanism of exercise-induced cognitive enhancement.37 For example, resting cerebral blood flow (CBF) is improved after only 12 weeks of aerobic training in healthy older adults and independently explains improvements in episodic memory.21 While this mechanism has not been tested after RT directly, accumulating evidence supports the use of RT for vascular adaptations.
Resistance training is known to improve systemic vascular function and cardiovascular health.38 A recent meta-analysis of RCTs ≥4 weeks in duration reported a positive relationship between RT frequency and improvements in endothelial function.39 These observations are likely due to exercise-induced elevations in skeletal muscle blood flow, providing a site-specific hemodynamic stimulus. It is likely that a similar phenomenon occurs in the brain but is currently unclear. In healthy adults, global CBF increases during aerobic exercise in an intensity-dependent manner but fluctuates with arterial blood pressure at higher exercise intensities and during resistance exercise.40 While this may serve as a protective response against blood brain barrier disruption, the transient fluctuation between hyperperfusion and hypoperfusion may also stimulate hypoxemia-related mechanisms for vascular adaptation. In addition, regional CBF, which reflects the blood flow response to increased neural activity, is augmented during muscle contraction and dependent on force production levels.41 The many factors affecting CBF during intense exercise are complex but seem to present an adaptive stimulus to the CV system. In support of this, healthy older women who engage in RT have greater resting global cerebral perfusion than women who do not.42
The promise of CV plasticity after RT can significantly impact how clinicians treat their elderly patients. Because of the proven value of cardiovascular risk factors for the prevention of heart disease, measures of CV function are proposed to have a similarly positive impact on the prevention of vascular forms of dementia.43 Acute improvements in cognitive performance after a single bout of exercise do not seem to be influenced by CBF.44 Instead, measuring the relationship between CV function and cognition may be more informative in exercise intervention studies due to long-term adaptations at rest. This supports the hypothesis that the neural consequences of CV dysfunction are mediated by long-term hypoxia, which in turn stimulates neurodegeneration, inflammation, and formation of plaques and neurofibrillary tangles.45
Brain Structure and Function
Morphometric imaging results support RT-induced brain plasticity with concomitant improvements in cognitive function. For example, after 6 months of RT in patients with probable MCI, expanded gray matter in the posterior cingulate was associated with improvements in cognitive function.18 Thus, RT may help improve cognition by reversing salient Alzheimer’s Disease processes like gray matter atrophy. While reports following aerobic training consistently demonstrate structural plasticity in the hippocampus, no such evidence has been found for RT, suggesting that different training modalities produce distinct patterns of structural plasticity in older adults.15,46 White matter hyperintensities are structural markers of cerebral small vessel disease that are common in older adults and strongly implicated in the pathogenesis of vascular cognitive impairment and dementia.47 In studies of both healthy older adults and patients with MCI, RT has been demonstrated to reverse the normal progression of white matter hyperintensities.18,48 However, it is unclear whether this reduction of white matter hyperintensity progression aids cognitive enhancement. Demyelination and axonal damage may persist despite the reversal of interstitial fluid accumulations represented by hyperintensities.
Regional neural activation during cognitive tasks is an important indicator of brain function that can be assessed using changes in blood flow during functional MRI (fMRI). Twice-weekly RT has been demonstrated to positively alter hemodynamic activity during task-based fMRI after 12 months in healthy older women and after 6 months in older women with probable MCI.17,49 These hemodynamic changes occurred with improved performance in executive function tasks. While this functional plasticity after RT is associated with response inhibition processes, aerobic training appears to promote selective attention.50 Changes in resting state fMRI have also been noted after RT with correlations between increased hippocampal-superior frontal connectivity and improved memory.18 Previous reports indicate that MCI patients have reduced hippocampal-superior frontal connectivity,51 and therefore strengthening this resting-state network may represent a promising mechanism that counteracts cognitive dysfunction.
In sum, structural and functional brain plasticity may have direct influence on cognitive improvements. However, narrowing down plasticity to a few specific structures may not fully capture the complex nature of human brain function. Therefore, multimodal imaging would improve our understanding of RT’s effect and help us determine how specific patient populations could benefit.
Glucose Regulation
Diabetes is considered a major risk factor for the development of dementia. The link between these 2 increasingly prevalent diseases is primarily attributed to hyperglycemia-induced neuroinflammation via oxidative stress and mitochondrial production of free radicals.52 Hyperglycemia often manifests in CV pathology,53 making the above discussion on vascular function relevant to this section as well. Further damage by hyperglycemia can also lead to insulin resistance in the brain, an early event in the development of dementia.54 Thus, the profound evidence collected to date suggesting that RT is an effective strategy for the prevention and treatment of diabetes may also provide support for its effectiveness in the prevention and treatment of cognitive impairment.
RT improves glycemic control by decreasing visceral fat, lowering glycosylated hemoglobin, and increasing insulin sensitivity.55 In addition, RT increases lean body mass in older adults by about 1.1 kg (mean duration of 20.5 weeks), with higher training volumes associated with greater hypertrophy.56 Gains in muscle mass raise resting metabolism and provide additional capacity for glucose uptake and storage. However, there is currently little evidence for a direct association between these changes and benefits to brain function. Serum levels of insulin-like growth factor play a major role in glucose regulation and have been demonstrated to increase following RT intervention, associated with improved cognitive performance.14 But insulin-like growth factor-1 is involved in numerous processes, including insulin sensitivity, muscular hypertrophy, neurogenesis, and angiogenesis.3 Additional studies are necessary to test the hypothesis that improved glycemic control is associated with RT-induced cognitive enhancement. Overall, these adaptations could promote cognition by reversing cellular impairments or CV dysfunction induced by poor glucose regulation.
Inflammation
Aging significantly affects immune function. The resulting systemic inflammation has been described as an important contributor to cognitive impairment.57 Increased inflammation is characterized by an increase in pro-inflammatory cytokines such as interleukin (IL)-1 beta (IL-1β), IL-6, C-reactive protein (CRP), and tumor necrosis factor-alpha (TNF-α), and/or a decrease in antiinflammatory cytokines such as IL-4, IL-10, and IL-13. These circulating markers can induce a neuroinflammatory response in the central nervous system via active transport across the blood brain barrier. Higher levels of systemic inflammation are associated with lower cognitive function and greater risk of cognitive decline and dementia.58 Even at midlife, higher levels of CRP are associated with increased risk of dementia 25 years later.59
General exercise is recognized as an effective countermeasure to chronic systemic inflammation. RT has been found to reduce resting levels of the inflammatory markers TNF-α and CRP.60,61 In cognitively impaired older women, RT has been demonstrated to increase antiinflammatory cytokine concentrations with concomitant improvements in global cognition.30 IL-10 increased in the RT group, whereas TNF-α and CRP increased in the age-matched control group. Like proinflammatory cytokines, white blood cell counts also exhibit a negative relationship with cognitive function.62 Barring the effect of acute infection, regular exercise can lower white blood cell counts in previously sedentary older adults.63 A recent study in cognitively impaired older women demonstrated that RT decreased the total number of leukocytes and lymphocytes in the blood.30 Furthermore, decreased granulocyte counts were associated with increased cognition.
Taken together, the above evidence supports the hypothesis that RT can improve cognitive function by creating a more antiinflammatory environment. While the mechanisms are still unclear, it is postulated that exercise induces acute IL-6 production, which suppresses other proinflammatory cytokines and stimulates the release of antiinflammatory cytokines, resulting in lower chronic systemic inflammation over time.64 This may quell the activation of microglia and ensuing cognitive damage caused by neuroinflammation.65 Ultimately, the reduction in systemic inflammation can have a widespread effect, aiding in the protection against dementia and various other chronic noncommunicable diseases.
Psychological Factors
Mood
Although rapid cognitive decline is the hallmark of dementia, behavioral and psychological symptoms such as anxiety, depression, and apathy often dominate the presentation of disease. These neuropsychiatric alterations can occur across all stages of dementia with fluctuating prominence and are detectable even before the MCI stage. Because dementia is not a specific disease but an overall term that describes a wide range of symptoms of cognitive decline, there is no clear distinction between its causes and effects. There is strong evidence that these psychological disorders act more as modifiable risk factors than symptoms of dementia. For example, depression is not only a symptom but also among the top 5 most important risk factors for dementia that can be reduced with clinical intervention.66 Thus, countermeasures to these disorders are necessary for both the prevention and treatment of dementia.
Higher levels of late-life anxiety are associated with poorer cognitive function and predictive of cognitive decline, especially in memory domains.67 Theories from performance literature suggest that anxiety can affect cognition through over-arousal or by occupying storage and preprocessing resources within the working memory system. In support of this, anxiety has shown a curvilinear relationship with cognition in older adults.68 Specifically, mild anxiety symptoms are associated with better cognitive performance, whereas severe anxiety symptoms are negatively associated with specific cognitive domains such as learning and delayed recall. A moderate effect (0.54) on anxiety reduction is often achieved with regular RT in healthy older adults.69 This may in part be due to decreased self-attentiveness,31 which is known to enhance psychological well-being, perhaps by freeing up cognitive resources. Some of these findings occurred with concomitant improvements in cognitive function,14 but associations were not tested.
In contrast to the curvilinear relationship above, symptoms of depression consistently exhibit negative correlations with cognitive function in older adults.68 Furthermore, late-life depressive symptoms can predict incident cognitive decline, especially in those who already have MCI, while earlier life depression is associated with an over 2-fold increased risk of dementia.70 Thus, depression, particularly at clinical levels, is considered a modifiable risk factor that contributes to the onset and course of cognitive decline in old age rather than a consequence of cognitive impairment. Further studies are needed to determine whether treatment of depression alone can delay or prevent dementia. The benefits of RT have been demonstrated, with large improvements (effect size >1.0) in patients diagnosed with depression and small to moderate effects on depressive symptoms in healthy older adults.69 At least 1 study has demonstrated decreased symptoms of depression in older adults with concomitant improvements in cognitive function.71 However, changes in fluid cognition were not associated with changes in depression. Clearly, further investigations are necessary if we are to determine the efficacy of targeting depression to prevent dementia. These countermeasures might need to be undertaken sooner than what is commonly believed due to the association between earlier life depression and risk of dementia.
Sleep Quality
Sleep has a well-known relationship with cognitive performance. Sleep deprivation can result in daytime dysfunction and deficits in specific cognitive domains, including executive function, attention, and working memory.72 Even without the sensation of sleepiness, repeated partial sleep deprivation has cognitive consequences akin to total sleep deprivation. Although older adults are less susceptible than young adults to the acute cognitive effects of a poor night’s sleep, age-related changes in habitual sleep patterns are apparent even in normal healthy aging. The most prevalent changes include decreased sleep duration, worsening sleep efficiency, and more frequent awakenings.73 These changes are associated with cognitive decline and greater risk of cognitive impairment.74 Thus, improving sleep in older adults may enhance cognitive performance and reduce the risk of dementia.
A recent systematic review concluded that chronic RT improves subjective assessments of both sleep duration and sleep quality in older adults, with small-to-moderate and moderate-to-large effect sizes, respectfully.75 These results have been reported in a range of populations from older patients diagnosed with depression to healthy older adults characterized as good sleepers.76 Furthermore, the effect sizes on sleep quality tended to be larger in studies using higher loads and/or higher frequencies (3 d/wk) of RT. A high-intensity RT program may therefore be an important part of behavioral therapies targeting sleep quality. Unfortunately, to the authors’ knowledge, no such studies have tested for associations between improvements in sleep and cognition after RT. It is plausible that RT improves cognitive function partially through improved sleep quality. Future studies are warranted to test this hypothesis.
Discussion
Conceptual Relationships
We have highlighted the paucity of research directly establishing mechanisms of cognitive enhancement after RT. We outlined potential factors and provided evidence to illustrate how each of these factors could contribute to late-life cognitive improvements. The overall theme involves a reversal of well-characterized, age-related changes—that is, decreased muscle mass and strength, vascular senescence, and sleep deprivation—all of which have been shown to improve after RT. RT, and exercise in general, affect fundamental processes of aging that result in pleiotropic benefits to the individual.
There are different possibilities for the relationship between cognition and the factors proposed above. RT could cause adaptations that subsequently promote brain health. For example, improved CV function could promote neurovascular coupling and provide resources for neuronal integrity, thus mediating the effects of RT on cognition (Fig. 1, Panel A). Alternatively, RT could directly stimulate mutually beneficial adaptations independently (Fig. 1, Panel B). For example, RT could improve both mood and cognition. Then, lower anxiety allows for increased cognitive performance, while clearer thinking brings about a positive attitude. Along these lines, RT could also directly improve both cognition and mediating variables (Fig. 1, Panel C). For example, RT could directly improve both sleep and cognition, and the improved sleep quality further benefits cognitive performance. Finally, RT could have multiple independent effects, causing simultaneous changes with no ensuing interaction (Fig. 1, Panel D). Thus, some physiological or psychological benefits of RT may not necessarily be required for cognitive enhancement.
Figure 1.
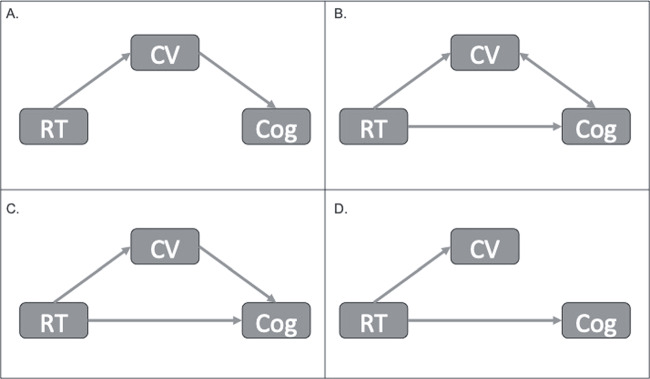
Potential relationships between resistance training (RT) and cognitive enhancement (Cog). Cerebrovascular (CV) function is used as an example but can be replaced with any of the factors described above (ie, muscular strength, brain structure and function, glucose regulation, inflammation, mood, and sleep).
The relationship between cognitive enhancement and what is actually changing at the cellular and molecular levels remain unclear in humans.4 Improvements may be mediated by neurophysiological changes that are best assessed at the micro-scale, such as by tissue biomarker expression, electrophysiology in single neurons, or acute brain slice methods.3 These are not measurable in humans in vivo using current technology. For example, serum levels of neurotrophic factors are promising molecular signals of neuroplasticity, but there are numerous variables that affect their concentrations in blood and cerebral spinal fluid. Thus, cross-species studies still play a vital role in translational neuroscience, bridging molecular, systems, and cognitive neuroscience research.
Future Directions
We advocate the need for future studies to reproduce the effects of RT on fluid cognition and investigate the potential mechanisms proposed here. While established guidelines for RCTs should always be followed to reduce the risk of bias, we have additional recommendations for this line of research specifically. We acknowledge that the following study characteristics increase trial costs and complexity, but the benefits toward reproducibility have made them the standard for key RCTs.
First, it is widely accepted that RT arms should be compared with active control arms to accurately estimate the effects of training. Having the control arm participants perform light stretching or low-intensity exercise with equal frequency and duration as the RT group reduces the confounding effects of attention, social interaction, practice, scheduling, and study commitment.77 A small but nonsignificant cognitive outcome effect size is generally expected in these active control groups. Second, the variety of cognitive measures and effect sizes in the literature highlights the need to use standardized performance-based measures of cognition.9 Measurement standardization will ensure that assessment methods and results can be compared across existing and future studies. One final characteristic for high-quality RCTs is an appropriate level of statistical power. With moderate effect sizes, larger samples are needed for reliability. Null findings have typically been associated with small sample sizes (<20 participants per group)78 or high attrition rates (>25%).79 In addition, studies have been published with low adherence to training protocols,80 which may also provide only conservative estimates of efficacy.
These issues highlight some of the challenges involved in conducting RT interventions. For example, supervised RT, often used to maximize the effectiveness of RT for physical performance,81 requires a high level of commitment from both participants and study personnel. Trainers must be qualified to oversee proper exercise technique, ensure appropriate adjustment of exercise loads, and monitor participant safety. Furthermore, attention provides key motivational aspects of participation, including the perception of encouragement and expertise from trainers, the feelings of exercise competence, and the recognition of personal positive health benefits.82
Over the last 15 years, the results of our RT studies with older adult populations have benefited from high compliance rates (>98%). This might be due to random factors such as study population or facility location, or to strategic factors such as hiring experienced trainers and providing greater attention with one-on-one personal sessions. Compared with other forms of exercise, RT offers the distinct advantage of allowing time for conversation during rest periods between sets. This time can be used to inspire and educate participants. In addition, RT has a goal-oriented skill component that requires concentration and facilitates learning and problem solving. Investigators with physical therapy or strength and conditioning backgrounds are therefore uniquely equipped with the knowledge and interpersonal skills necessary to improve the reliability of these outcomes.
It is important to note that participants often perceive health benefits earlier than what is supported by functional scores. Although these perceptions may not accurately reflect actual abilities, these perceptions do drive behavior.82 We can anecdotally confirm that many participants will comment on improvements in muscular strength and cognitive abilities in as little as 2 weeks of training. Those participants who perceive the greatest benefits are more inclined to challenge themselves in future training sessions. While some investigators recommend blinding participants and trainers to study hypotheses, we have found that to be impractical. Participants frequently ask about study hypotheses to justify their time and effort, and there is enough media coverage of this research that participants and trainers will often assume benefits to cognition. Over the course of our current intervention study, we have received several news articles from participants highlighting the benefits of exercise for brain function. Therefore, we support the transparency of cognitive benefits in future mechanistic studies. However, other safeguards should be taken to reduce bias during testing such as blinding assessors to group assignments.
Innovations in neuroimaging technology, assessment techniques, and biomarker analyses are frequently being developed, setting the stage for rapid advancements in dementia prevention strategies. Future progress will require collaboration between neuroscientists and exercise physiologists to develop optimal interventions for the aging population. Proof-of-concept clinical trials are a critical first step toward accelerating this field, providing a practical approach to test key hypotheses while minimizing the risks in conducting larger, more expensive trials.
Conclusions
While the importance of RT for overall health in older adults is well established, this new and exciting evidence for cognitive improvements furthers the impact that therapists can have on their patients. Our long-term goal is to help establish a neuroscientific foundation of RT’s benefit to cognition to promote its therapeutic use in both treatment and prevention strategies. Fully understanding the indirect and direct mechanisms will help clinicians develop comprehensive interventions for their elderly patients. In doing so, we can make informed recommendations that provide the most real-world benefit for the individual. At a time when social factors and longer lifespans are increasing the incidence of noncommunicable progressive disorders like dementia, the role of the physical therapist will necessarily involve treatment of the underlying factors related to their patients’ cognitive challenges. RT may be the preferred prescription. The improvements in physical capacity, vascular function, brain structure and function, glucose regulation, inflammation, mood, and sleep quality offer significant health benefits in addition to those that may contribute specifically to cognitive processes. Therefore, even with moderate benefit to a specific disease like dementia, the global impact of increased RT prescription on healthcare would be substantial.
Author Contributions
Concept/idea/research design: T.R. Macaulay, B.E. Fisher, E.T. Schroeder
Writing: T.R. Macaulay, B.E. Fisher, E.T. Schroeder
Fund procurement: E.T. Schroeder
Providing participants: E.T. Schroeder
Providing facilities/equipment: E.T. Schroeder
Providing institutional liaisons: E.T. Schroeder
Consultation (including review of manuscript before submitting): B.E. Fisher, E.T. Schroeder
Funding
This study was supported by the Southern California Clinical and Translational Science Institute grant UL1TR001855.
Disclosures
The authors completed the ICMJE Form for Disclosure of Potential Conflicts of Interest and reported no conflicts of interest.
References
- 1. Alzheimer's Association 2018 Alzheimer's disease facts and figures. Alzheimers Dement. 2018;14:367–429. [Google Scholar]
- 2. Cavedo E, Lista S, Khachaturian Z, et al. The road ahead to cure Alzheimer’s disease: development of biological markers and neuroimaging methods for prevention trials across all stages and target populations. J Prev Alzheimers Dis. 2014;1:181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cotman CW, Berchtold NC, Christie L-A. Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci. 2007;30:464–472. [DOI] [PubMed] [Google Scholar]
- 4. Voss MW, Vivar C, Kramer AF, van Praag H. Bridging animal and human models of exercise-induced brain plasticity. Trends Cogn Sci. 2013;17:525–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wanderley FA, Moreira A, Sokhatska O, et al. Differential responses of adiposity, inflammation and autonomic function to aerobic versus resistance training in older adults. Exp Gerontol. 2013;48:326–333. [DOI] [PubMed] [Google Scholar]
- 6. Cassilhas R, Lee K, Fernandes J, et al. Spatial memory is improved by aerobic and resistance exercise through divergent molecular mechanisms. Neuroscience. 2012;202:309–317. [DOI] [PubMed] [Google Scholar]
- 7. Liu-Ambrose T, Best JR. Exercise is medicine for the aging brain. Kinesiol Rev. 2017;6:22–29. [Google Scholar]
- 8. Li Z, Peng X, Xiang W, Han J, Li K. The effect of resistance training on cognitive function in the older adults: a systematic review of randomized clinical trials. Aging Clin Exp Res. 2018;30:1259–1273. [DOI] [PubMed] [Google Scholar]
- 9. Landrigan J-F, Bell T, Crowe M, Clay OJ, Mirman D. Lifting cognition: a meta-analysis of effects of resistance exercise on cognition. Psychol Res. 2019;1–17. [DOI] [PubMed] [Google Scholar]
- 10. Taubert M, Villringer A, Lehmann N. Endurance exercise as an “endogenous” neuro-enhancement strategy to facilitate motor learning. Front Hum Neurosci. 2015;9:692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kennedy G, Hardman RJ, Macpherson H, Scholey AB, Pipingas A. How does exercise reduce the rate of age-associated cognitive decline? A review of potential mechanisms. J Alzheimers Dis. 2017;55:1–18. [DOI] [PubMed] [Google Scholar]
- 12. Sofi F, Valecchi D, Bacci D, et al. Physical activity and risk of cognitive decline: a meta-analysis of prospective studies. J Intern Med. 2011;269:107–117. [DOI] [PubMed] [Google Scholar]
- 13. Liu-Ambrose T, Nagamatsu LS, Graf P, Beattie BL, Ashe MC, Handy TC. Resistance training and executive functions: a 12-month randomized controlled trial. Arch Intern Med. 2010;170:170–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cassilhas RC, Viana VA, Grassmann V, et al. The impact of resistance exercise on the cognitive function of the elderly. Med Sci Sports Exerc. 2007;39:1401. [DOI] [PubMed] [Google Scholar]
- 15. Erickson KI, Gildengers AG, Butters MA. Physical activity and brain plasticity in late adulthood. Dialogues Clin Neurosci. 2013;15:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Best JR, Chiu BK, Hsu CL, Nagamatsu LS, Liu-Ambrose T. Long-term effects of resistance exercise training on cognition and brain volume in older women: results from a randomized controlled trial. J Intern Neuropsychol Soc. 2015;21:745–756. [DOI] [PubMed] [Google Scholar]
- 17. Nagamatsu LS, Handy TC, Hsu CL, Voss M, Liu-Ambrose T. Resistance training promotes cognitive and functional brain plasticity in seniors with probable mild cognitive impairment. Arch Intern Med. 2012;172:666–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Suo C, Singh MF, Gates N, et al. Therapeutically relevant structural and functional mechanisms triggered by physical and cognitive exercise. Mol Psychiatry. 2016;21:1633–1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Peterson MD, Rhea MR, Sen A, Gordon PM. Resistance exercise for muscular strength in older adults: a meta-analysis. Ageing Res Rev. 2010;9:226–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Boyle PA, Buchman AS, Wilson RS, Leurgans SE, Bennett DA. Association of muscle strength with the risk of Alzheimer disease and the rate of cognitive decline in community-dwelling older persons. Arch Neurol. 2009;66:1339–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Maass A, Düzel S, Goerke M, et al. Vascular hippocampal plasticity after aerobic exercise in older adults. Mol Psychiatry. 2015;20:585–593. [DOI] [PubMed] [Google Scholar]
- 22. Mavros Y, Gates N, Wilson GC, et al. Mediation of cognitive function improvements by strength gains after resistance training in older adults with mild cognitive impairment: outcomes of the study of mental and resistance training. J Am Geriatr Soc. 2017;65:550–559. [DOI] [PubMed] [Google Scholar]
- 23. Forte R, Boreham CA, Leite JC, et al. Enhancing cognitive functioning in the elderly: multicomponent vs resistance training. Clin Interv Aging. 2013;8:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Palmer HS, Håberg A, Fimland MS, et al. Structural brain changes after 4 wk of unilateral strength training of the lower limb. J Appl Physiol. 2013;115:167–175. [DOI] [PubMed] [Google Scholar]
- 25. Giboin LS, Weiss B, Thomas F, Gruber M. Neuroplasticity following short-term strength training occurs at supraspinal level and is specific for the trained task. Acta Physiologica. 2018;222:e12998. [DOI] [PubMed] [Google Scholar]
- 26. Fisher BE, Southam AC, Kuo Y-L, Lee Y-Y, Powers CM. Evidence of altered corticomotor excitability following targeted activation of gluteus maximus training in healthy individuals. Neuroreport. 2016;27:415–421. [DOI] [PubMed] [Google Scholar]
- 27. Morris T, Shafi M, Bartres-Faz D, et al. Intracortical inhibition of the parietal cortex is associated with cognitive function in older adults: a TMS-EEG study. Brain Stimul. 2019;12:456–457. [Google Scholar]
- 28. Ikudome S, Mori S, Unenaka S, Kawanishi M, Kitamura T, Nakamoto H. Effect of long-term body-mass-based resistance exercise on cognitive function in elderly people. J Appl Gerontol. 2017;36:1519–1533. [DOI] [PubMed] [Google Scholar]
- 29. Lachman ME, Neupert SD, Bertrand R, Jette AM. The effects of strength training on memory in older adults. J Aging Phys Act. 2006;14:59–73. [DOI] [PubMed] [Google Scholar]
- 30. Chupel MU, Direito F, Furtado GE, et al. Strength training decreases inflammation and increases cognition and physical fitness in older women with cognitive impairment. Front Physiol. 2017;8:377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Peig-Chiello P, Perrig WJ, Ehrsam R, Staehelin HB, Krings F. The effects of resistance training on well-being and memory in elderly volunteers. Age Ageing. 1998;27:469–475. [DOI] [PubMed] [Google Scholar]
- 32. Qualls C, Waters D, Vellas B, et al. Reversible states of physical and/or cognitive dysfunction: a 9-year longitudinal study. J Nutr, Health Aging. 2017;21:271–275. [DOI] [PubMed] [Google Scholar]
- 33. Mendonca GV, Pezarat-Correia P, Vaz JR, Silva L, Heffernan KS. Impact of aging on endurance and neuromuscular physical performance: the role of vascular senescence. Sports Med. 2017;47:583–598. [DOI] [PubMed] [Google Scholar]
- 34. Dichgans M, Leys D. Vascular cognitive impairment. Circ Res. 2017;120:573–591. [DOI] [PubMed] [Google Scholar]
- 35. Montagne A, Nation DA, Pa J, Sweeney MD, Toga AW, Zlokovic BV. Brain imaging of neurovascular dysfunction in Alzheimer’s disease. Acta Neuropathol. 2016;131:687–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wolters FJ, Zonneveld HI, Hofman A, et al. Cerebral perfusion and the risk of dementia: a population-based study. Circulation. 2017;36:719–728. [DOI] [PubMed] [Google Scholar]
- 37. Barnes JN. Exercise, cognitive function, and aging. Adv Physiol Educ. 2015;39:55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Williams MA, Haskell WL, Ades PA, et al. Resistance exercise in individuals with and without cardiovascular disease: 2007 update. Circulation. 2007;116:572–584. [DOI] [PubMed] [Google Scholar]
- 39. Ashor AW, Lara J, Siervo M, et al. Exercise modalities and endothelial function: a systematic review and dose–response meta-analysis of randomized controlled trials. Sports Med. 2015;45:279–296. [DOI] [PubMed] [Google Scholar]
- 40. Smith KJ, Ainslie PN. Regulation of cerebral blood flow and metabolism during exercise. Exp Physiol. 2017;102:1356–1371. [DOI] [PubMed] [Google Scholar]
- 41. Dettmers C, Fink GR, Lemon RN, et al. Relation between cerebral activity and force in the motor areas of the human brain. J Neurophysiol. 1995;74:802–815. [DOI] [PubMed] [Google Scholar]
- 42. Xu X, Jerskey BA, Cote DM, et al. Cerebrovascular perfusion among older adults is moderated by strength training and gender. Neurosci Lett. 2014;560:26–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Corriveau RA, Bosetti F, Emr M, et al. The science of vascular contributions to cognitive impairment and dementia (VCID): a framework for advancing research priorities in the cerebrovascular biology of cognitive decline. Cell Mol Neurobiol. 2016;36:281–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ogoh S, Tsukamoto H, Hirasawa A, Hasegawa H, Hirose N, Hashimoto T. The effect of changes in cerebral blood flow on cognitive function during exercise. Physiol Rep. 2014;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zhang X, Le W. Pathological role of hypoxia in Alzheimer's disease. Exp Neurol. 2010;223:299–303. [DOI] [PubMed] [Google Scholar]
- 46. Firth J, Stubbs B, Vancampfort D, et al. Effect of aerobic exercise on hippocampal volume in humans: a systematic review and meta-analysis. Neuroimage. 2018;166:230–238. [DOI] [PubMed] [Google Scholar]
- 47. Prins ND, Scheltens P. White matter hyperintensities, cognitive impairment and dementia: an update. Nat Revs Neurol. 2015;11:157. [DOI] [PubMed] [Google Scholar]
- 48. Bolandzadeh N, Tam R, Handy TC, et al. Resistance training and white matter lesion progression in older women: exploratory analysis of a 12-month randomized controlled trial. J Am Geriatr Soc. 2015;63:2052–2060. [DOI] [PubMed] [Google Scholar]
- 49. Liu-Ambrose T, Nagamatsu LS, Voss MW, Khan KM, Handy TC. Resistance training and functional plasticity of the aging brain: a 12-month randomized controlled trial. Neurobiol Aging. 2012;33:1690–1698. [DOI] [PubMed] [Google Scholar]
- 50. Colcombe SJ, Kramer AF, Erickson KI, et al. Cardiovascular fitness, cortical plasticity, and aging. Proc Natl Acad Sci USA. 2004;101:3316–3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wang Z, Liang P, Jia X, et al. Baseline and longitudinal patterns of hippocampal connectivity in mild cognitive impairment: evidence from resting state fMRI. J Neurol Sci. 2011;309:79–85. [DOI] [PubMed] [Google Scholar]
- 52. Spielman LJ, Little JP, Klegeris A. Inflammation and insulin/IGF-1 resistance as the possible link between obesity and neurodegeneration. J Neuroimmunol. 2014;273:8–21. [DOI] [PubMed] [Google Scholar]
- 53. Pruzin JJ, Schneider JA, Capuano AW, et al. Diabetes, hemoglobin A1C, and regional Alzheimer's disease and infarct pathology. Alzheimer Dis Assoc Disord. 2017;31:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Talbot K, Wang H-Y, Kazi H, et al. Demonstrated brain insulin resistance in Alzheimer’s disease patients is associated with IGF-1 resistance, IRS-1 dysregulation, and cognitive decline. J Clin Invest. 2012;122:1316–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Westcott WL. Resistance training is medicine: effects of strength training on health. Curr Sports Med Rep. 2012;11:209–216. [DOI] [PubMed] [Google Scholar]
- 56. Peterson MD, Sen A, Gordon PM. Influence of resistance exercise on lean body mass in aging adults: a meta-analysis. Med Sci Sports Exerc. 2011;43:249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Trollor JN, Smith E, Baune BT, et al. Systemic inflammation is associated with MCI and its subtypes: the Sydney Memory and Aging Study. Dement Geriatr Cogn Disord. 2010;30:569–578. [DOI] [PubMed] [Google Scholar]
- 58. Schram MT, Euser SM, De Craen AJ, et al. Systemic markers of inflammation and cognitive decline in old age. J Am Geriatr Soc. 2007;55:708–716. [DOI] [PubMed] [Google Scholar]
- 59. Schmidt R, Schmidt H, Curb JD, Masaki K, White LR, Launer LJ. Early inflammation and dementia: a 25-year follow-up of the Honolulu-Asia Aging Study. Annals Neurol. 2002;52:168–174. [DOI] [PubMed] [Google Scholar]
- 60. Ogawa K, Sanada K, Machida S, Okutsu M, Suzuki K. Resistance exercise training-induced muscle hypertrophy was associated with reduction of inflammatory markers in elderly women. Mediators Inflamm. 2010;2010:171023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Phillips MD, Flynn MG, McFarlin BK, Stewart LK, Timmerman KL. Resistance training at eight-repetition maximum reduces the inflammatory milieu in elderly women. Med Sci Sports Exerc. 2010;42:314–325. [DOI] [PubMed] [Google Scholar]
- 62. Kao TW, Chang YW, Chou CC, Hu J, Yu YH, Kuo HK. White blood cell count and psychomotor cognitive performance in the elderly. Eur J Clin Invest. 2011;41:513–520. [DOI] [PubMed] [Google Scholar]
- 63. de Gonzalo-Calvo D, Fernández-García B, de Luxán-Delgado B, et al. Long-term training induces a healthy inflammatory and endocrine emergent biomarker profile in elderly men. Age. 2012;34:761–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Mathur N, Pedersen BK. Exercise as a mean to control low-grade systemic inflammation. Mediators Inflamm. 2008;2008:109502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Colton CA. Heterogeneity of microglial activation in the innate immune response in the brain. J Neuroimmune Pharmacol. 2009;4:399–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Deckers K, Boxtel MP, Schiepers OJ, et al. Target risk factors for dementia prevention: a systematic review and Delphi consensus study on the evidence from observational studies. Intern J Geriatr Psychiatry. 2015;30:234–246. [DOI] [PubMed] [Google Scholar]
- 67. Wetherell JL, Reynolds CA, Gatz M, Pedersen NL. Anxiety, cognitive performance, and cognitive decline in normal aging. J Gerontol B Psychol Sci Soc Sci. 2002;57:P246–P255. [DOI] [PubMed] [Google Scholar]
- 68. Bierman E, Comijs H, Jonker C, Beekman A. Effects of anxiety versus depression on cognition in later life. Am J Geriatr Psychiatry. 2005;13:686–693. [DOI] [PubMed] [Google Scholar]
- 69. O'Connor PJ, Herring MP, Caravalho A. Mental health benefits of strength training in adults. Am J Lifestyle Med. 2010;4:377–396. [Google Scholar]
- 70. Byers AL, Yaffe K. Depression and risk of developing dementia. Nat Rev Neurol. 2011;7:323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Brown AK, Liu-Ambrose T, Tate R, Lord S. The effect of group-based exercise on cognitive performance and mood in seniors residing in intermediate care and self-care retirement facilities: a randomized controlled trial. Br J Sports Med. 2009;43:608–614. [DOI] [PubMed] [Google Scholar]
- 72. Durmer JS, Dinges DF. Neurocognitive consequences of sleep deprivation. Seminars in Neurology. 2005;25:117–129. [DOI] [PubMed] [Google Scholar]
- 73. Buysse DJ, Monk TH, Carrier J, Begley A. Circadian patterns of sleep, sleepiness, and performance in older and younger adults. Sleep. 2005;28:1365–1376. [DOI] [PubMed] [Google Scholar]
- 74. Miller MA. The role of sleep and sleep disorders in the development, diagnosis, and management of neurocognitive disorders. Front Neurol. 2015;6:224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Kovacevic A, Mavros Y, Heisz JJ, Singh MAF. The effect of resistance exercise on sleep: a systematic review of randomized controlled trials. Sleep Medicine Rev. 2017. [DOI] [PubMed] [Google Scholar]
- 76. Ferris LT, Williams JS, Shen CL, O'Keefe KA, Hale KB. Resistance training improves sleep quality in older adults a pilot study. J Sports Sci Med. 2005;4:354. [PMC free article] [PubMed] [Google Scholar]
- 77. Krogh-Madsen R, Nagamatsu LS, Smoliga JM, Davis JC, Liu-Ambrose TY. Commentaries on viewpoint: control arms in exercise training studies: transitioning from an era of intervention efficacy to one of comparative clinical effectiveness research. J Appl Physiol. 2011;111:949–950. [DOI] [PubMed] [Google Scholar]
- 78. Tsutsumi T, Don BM, Zaichkowsky LD, Delizonna LL. Physical fitness and psychological benefits of strength training in community dwelling older adults. Appl Hum Sci. 2001;16:257–266. [DOI] [PubMed] [Google Scholar]
- 79. Kimura K, Obuchi S, Arai T, et al. The influence of short-term strength training on health-related quality of life and executive cognitive function. J Physiol Anthropol. 2010;29:95–101. [DOI] [PubMed] [Google Scholar]
- 80. Ansai JH, Rebelatto JR. Effect of two physical exercise protocols on cognition and depressive symptoms in oldest-old people: a randomized controlled trial. Geriatr Gerontol Int. 2015;15:1127–1134. [DOI] [PubMed] [Google Scholar]
- 81. Lacroix A, Kressig RW, Muehlbauer T, et al. Effects of a supervised versus an unsupervised combined balance and strength training program on balance and muscle power in healthy older adults: a randomized controlled trial. Gerontol. 2016;62:275–288. [DOI] [PubMed] [Google Scholar]
- 82. Whaley DE, Schrider AF. The process of adult exercise adherence: self-perceptions and competence. Sport Psychologist. 2005;19:148–163. [Google Scholar]


