Abstract
Fetal growth restriction (FGR) is a significant risk factor for stillbirth, neonatal complications and adulthood morbidity. Compared with those of appropriate weight for gestational age (AGA), FGR babies have smaller placentas with reduced activity of amino acid transporter systems A and L, thought to contribute to poor fetal growth. The amino acids glutamine and glutamate are essential for normal placental function and fetal development; whether transport of these is altered in FGR is unknown. We hypothesised that FGR is associated with reduced placental glutamine and glutamate transporter activity and expression, and propose the mammalian target of rapamycin (mTOR) signaling pathway as a candidate mechanism. FGR infants [individualised birth weight ratio (IBR) < 5th centile] had lighter placentas, reduced initial rate uptake of 14C-glutamine and 14C-glutamate (per mg placental protein) but higher expression of key transporter proteins (glutamine: LAT1, LAT2, SNAT5, glutamate: EAAT1) versus AGA [IBR 20th–80th]. In further experiments, in vitro exposure to rapamycin inhibited placental glutamine and glutamate uptake (24 h, uncomplicated pregnancies) indicating a role of mTOR in regulating placental transport of these amino acids. These data support our hypothesis and suggest that abnormal glutamine and glutamate transporter activity is part of the spectrum of placental dysfunction in FGR.
Subject terms: Physiology, Reproductive biology, Immunoblotting, PCR-based techniques
Introduction
Fetal growth restriction (FGR), which affects approximately 5% of pregnancies, refers to the inability of a fetus to achieve its growth potential and is primarily caused by placental dysfunction1. FGR is a major risk factor for stillbirth and neonatal, childhood and adulthood morbidity2–5. Despite the significant societal and economic burden of FGR6–8 there are currently no approved pharmacological treatments available9. The reticence to develop novel therapies for placental dysfunction in part reflects concerns of potential fetal toxicity but is also influenced by an incomplete understanding of placental function in health and disease10. FGR is often associated with one, or a combination of, the following characteristics: small placental size, abnormal placental structure, abnormal uteroplacental and fetoplacental blood flow, and abnormal function of the syncytiotrophoblast, the transporting epithelium of the placenta1,11–15. Inconsistencies in the definition of FGR prompted a recent Delphi study, which recommended a consensus-based clinical definition of FGR16.
In women, FGR is associated with reduced placental activity (per mg membrane protein) of a number of amino acid transporter systems including systems A and L13–15,17. Furthermore, in a rat model of FGR, reduced placental amino acid transport by system A is evident prior to the reduction in fetal growth18, whilst inhibition of placental system A directly results in reduced fetal growth19, demonstrating that a reduction of placental amino acid provision by system A can cause FGR.
The amino acids glutamine and glutamate are essential for pH homeostasis, nucleotide synthesis and protein anabolism20,21. Glutamine is a non-essential amino acid that becomes conditionally essential during pregnancy as fetal demand exceeds maternal synthesis22,23. Demand is met through interorgan recycling of glutamine and glutamate. Deamination of glutamine in the fetal liver produces glutamate, an important nitrogen resource and precursor of γ-amino butyric acid (GABA), a key inhibitory neurotransmitter23–25. Glutamate is transported across the syncytiotrophoblast microvillous membrane (MVM: maternal facing) and basal membrane (BM: fetal facing) by high affinity Excitatory Amino Acid Transporters (EAATs; system XAG-)26 and is converted to glutamine in the placenta27. Glutamine is a substrate of the amino acid transporter systems A (isoforms SNAT1, 2 and 4), N (SNAT5), L (LAT1 and 2), y+L and ASC21. However, system y+L-mediated glutamine transfer across the MVM is negligible and system ASC isoforms are predominantly localised to the BM28.
There are as yet no studies of placental glutamine and glutamate transport in the placenta in human FGR but, as noted above, substantial evidence to show that system A and system L transporter activity is reduced in this condition13–15,17. The reasons behind the reduction in placental system A and system L activity in FGR are poorly understood, not least because the determinants of appropriate provision of amino acids to the fetus in normal pregnancy have not been adequately defined. However, there is evidence that the mammalian target of rapamycin (mTOR) signaling pathway, specifically mTOR complex 1 (mTORC1), regulates system A and L activity through reduced plasma membrane trafficking of SNAT2 in the case of system A29,30 and that placental mTOR activity, as evidenced by reduction in the important downstream signaling molecule phosphorylated ribosomal S6 kinase (phospho-S6K1), is reduced in FGR29,31. The mTOR pathway, proposed to act as a nutrient sensor, is thus a candidate mechanism by which glutamine and glutamate transport is modulated in normal pregnancy, though this has never been tested.
In the current investigation we tested the hypotheses that (a) placental glutamine and glutamate transporter expression and activity is reduced in FGR infants compared with infants appropriately grown for gestational age (AGA), and (b) inhibiting mTOR activity in placentas from uncomplicated pregnancies would result in a reduction in placental uptake of glutamine and glutamate.
Results
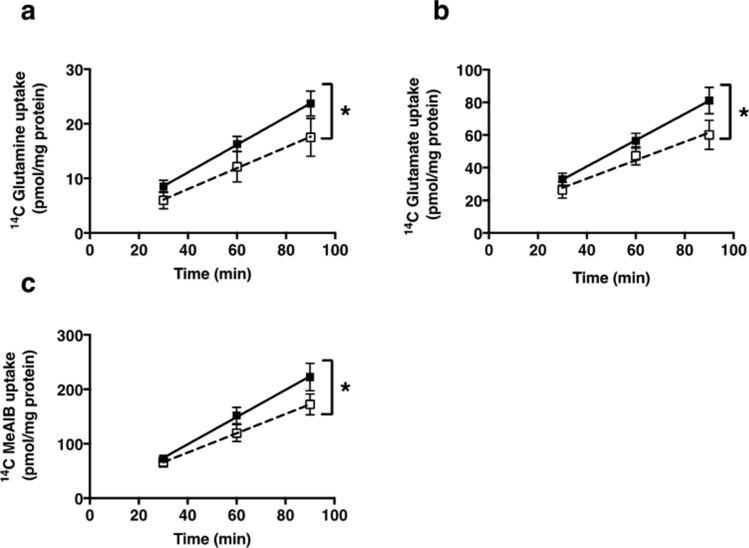
Transporter-mediated uptake of 14C-glutamine, 14C-glutamate and 14C-MeAIB is reduced in FGR pregnancies
A summary of the demographics of study participants is shown in Table 1. FGR infants weighed significantly less than AGA infants (P < 0.001) and had significantly lighter placentas (P < 0.001). There was a difference in the gestational age of FGR babies compared with AGA babies (Table 1; P < 0.05). Transporter-mediated uptake of 14C-glutamine, 14C-glutamate (validation of method: Supplementary Fig. 1) and 14C-MeAIB at initial rate was significantly lower in placentas of FGR babies (IBR < 5th centile) compared with placentas of AGA babies (IBR 20th–80th centile, P < 0.05) (Fig. 1). There was no effect of gestational age on amino acid uptake (Supplementary Fig. 2).
Table 1.
Maternal and fetal demographics from AGA and FGR cohorts.
| AGA (n = 14) | FGR (n = 11) | P value | |
|---|---|---|---|
| Maternal age (years) | 34 (23–40) | 32 (25–39) | 0.946 |
| Body mass index (kg/m2) | 24.9 (18.8–31.2) | 23.9 (19.8–33.9) | 0.442 |
| Birth weight (g) | 3355 (2820–3960) | 1730 (717–2860) | < 0.001 |
| Trimmed placental weight (g) | 498.1 (402.3–719.4) | 301.6 (159.5–429.3) | < 0.001 |
| Individualised birth weight ratio (IBR) | 53.6 (26.4–79.2) | 1 (0–3.5) | < 0.001 |
| Gestation (weeks + days) | 39 + 0 (37 + 1–40 + 2) | 35.2 (29 + 1–40 + 4) | 0.049 |
| Mode of delivery | ELCS (89%) | ELCS (55%) | 0.623 |
| NVD (11%) | EMCS (18%) | ||
| NVD (27%) | |||
| Parity | 1 (0–8) | 1 (0–5) | 0.315 |
| Gravidity | 2 (1–12) | 2 (1–6) | 0.971 |
| Ethnicity | Caucasian (71%) | Caucasian (64%) | 0.999 |
| Smoking status (yes/no) | No (100%) | No (73%) | 0.072 |
Data are median (range) or percentage of total. Analysis by Mann–Whitney test or by Fishers exact test (mode of delivery, ethnicity and smoking status). ELCS elective caesarean section, EMCS emergency caesarean section, NVD normal vaginal delivery.
Figure 1.
Transporter-mediated uptake of 14C-glutamine (a), 14C-glutamate (b) and 14C-MeAIB (c) by placental villous fragments from AGA infants (n = 13–14, solid symbols) and FGR (n = 10–11, hollow symbols). Data are mean ± SEM * P < 0.05 Linear regression analysis showed a significant difference in elevation for glutamine (P < 0.02), glutamate (P < 0.02) and MeAIB (P < 0.03) uptake in FGR versus AGA.
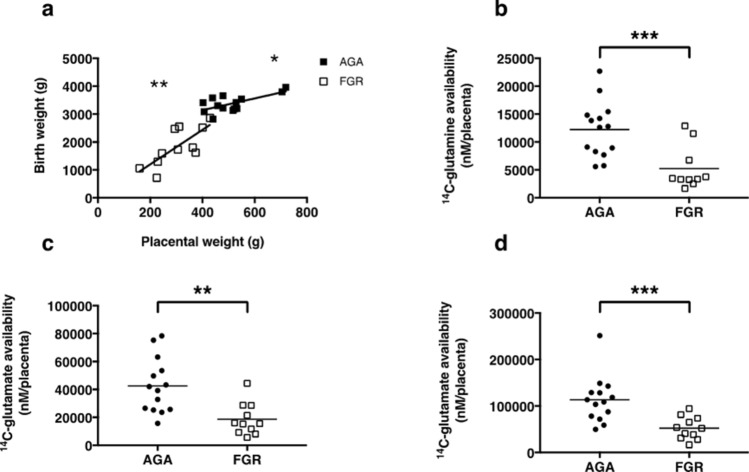
Figure 2.
Correlation between trimmed placental weight and birth weight (a) for AGA (n = 14, solid symbols) and FGR infants (n = 11, hollow symbols) ** P < 0.01, * P < 0.05: Linear regression. Availability of 14C-glutamine (b), 14C-glutamate (c) and 14C-MeAIB (d) for delivery to the fetus in placentas from AGA compared with FGR infants. *** P < 0.001, ** P < 0.01: Mann–Whitney test.
Linear regression analyses fitted to all data indicated that transporter-mediated uptake of 14C-glutamine at 90 min was correlated with uptake of 14C-MeAIB (P < 0.05) and 14C-glutamate (P < 0.001, Supplementary Fig. 3). However, when analyses were fitted to either AGA or FGR groups, we found that the correlation between 14C-glutamine and 14C-MeAIB only remained for placentas from FGR babies (P < 0.05), highlighting the importance of system A in these pregnancies. 14C-glutamine and 14C-glutamate uptake was correlated for both AGA and FGR infants, which suggests that there is an intrinsic relationship between the uptake of these two amino acids that is not altered in FGR.
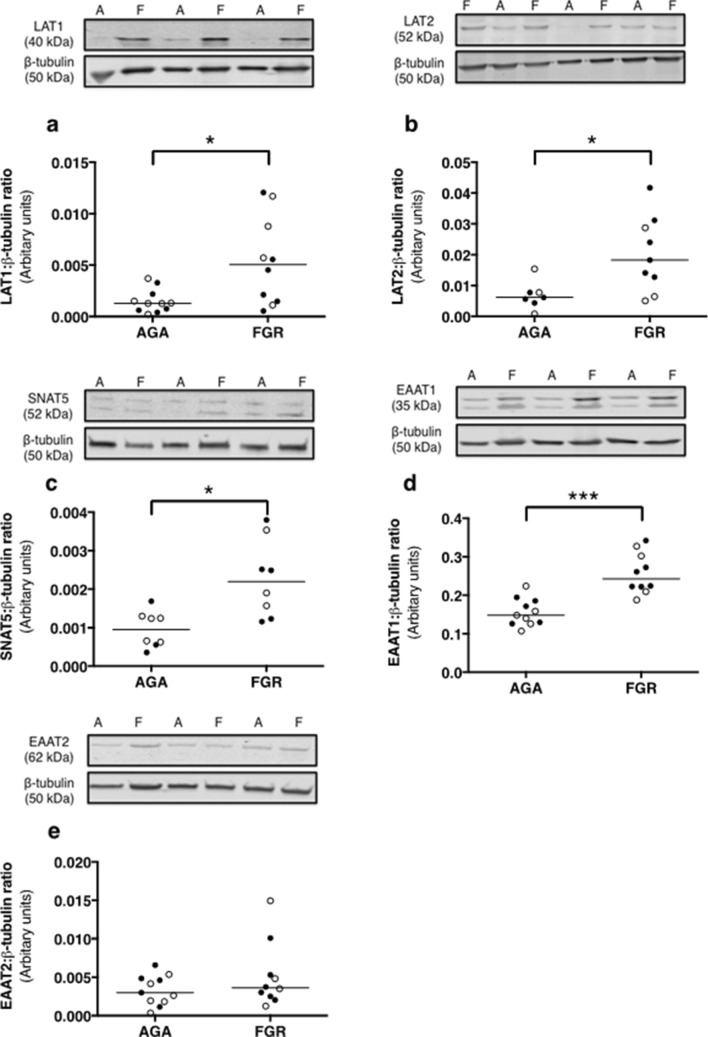
Figure 3.
Expression of glutamine [LAT1 (a), LAT2 (b), SNAT5 (c)] and glutamate [EAAT1 (d), EAAT2 (e)] transporter proteins in membrane-enriched placental homogenates from AGA (n = 7–11) and FGR (n = 8–10) infants. Densitometric analysis is expressed as a ratio of β-tubulin signal. Line denotes median, *** P < 0.001, * P < 0.05: Wilcoxon signed-rank test.
Total amino acid availability for transfer from the placenta to the fetus is reduced in FGR versus AGA infants
Trimmed placental weight and birth weight were correlated for both AGA (P < 0.05) and FGR (P < 0.01) infants (Fig. 2A). A proxy measure of the availability of amino acids (14C-glutamine, 14C-glutamate and 14C-MeAIB) for transfer from the syncytiotrophoblast to the fetus [trimmed placental weight (g) × amino acid uptake at 90 min (per mg placental villous fragment protein)] was significantly lower for placentas of FGR versus AGA infants (Fig. 2B–D).
Placental transporter protein and mRNA expression is altered in FGR versus AGA infants
Expression of glutamine transporter proteins (system L: LAT1, 2, and system N: SNAT5) was significantly higher in placentas from FGR (IBR 0.0–3.5) versus AGA (IBR 27.9–79.1) pregnancies (Fig. 3A–C, P < 0.05). Expression of EAAT1 (glutamate is a substrate) was significantly higher in placentas from FGR versus AGA infants (Fig. 3D, P < 0.001). EAAT2 expression was not different between groups (Fig. 3E). Expression of β-tubulin was not different between AGA and FGR groups. Full-length blots are presented in Supplementary Fig. 4.
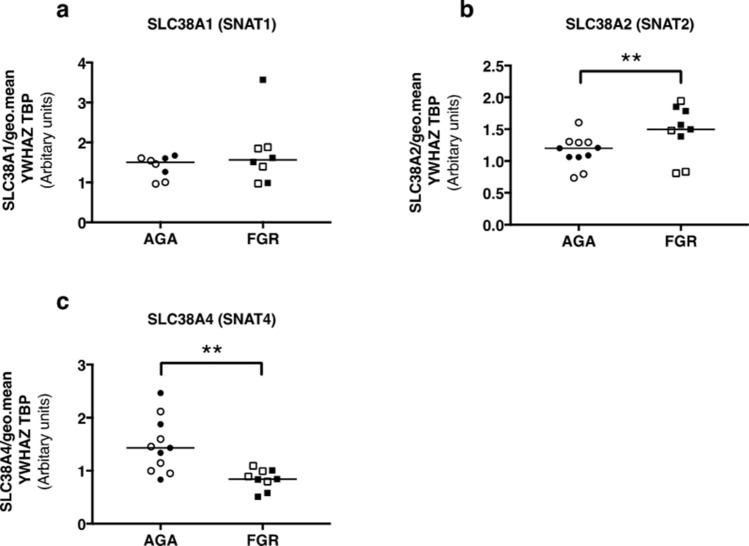
Figure 4.
mRNA expression of SLC38A1 (a), SLC38A2 (b) and SLC38A4 (c) in placentas from AGA (n = 8–11) and FGR (n = 8–9) placentas, normalised to the geometric mean of YWHAZ and TBP. ** P < 0.01: Mann–Whitney test.
It was not possible to assess protein expression of system A transporter isoforms (SNAT1, 2 and 4), as there were no suitably validated commercially available antibodies. However, expression of the gene that encodes the system A isoform SNAT2 (SLC38A2) was significantly higher in placentas from FGR than AGA babies (P < 0.01) (Fig. 4B). Conversely, SLC38A4 mRNA expression (encodes SNAT4) was significantly lower in placentas from FGR versus AGA pregnancies (P < 0.01) (Fig. 4C); expression of SLC38A1 (encodes SNAT1) was not significantly different between placentas of AGA and FGR infants (Fig. 4A).
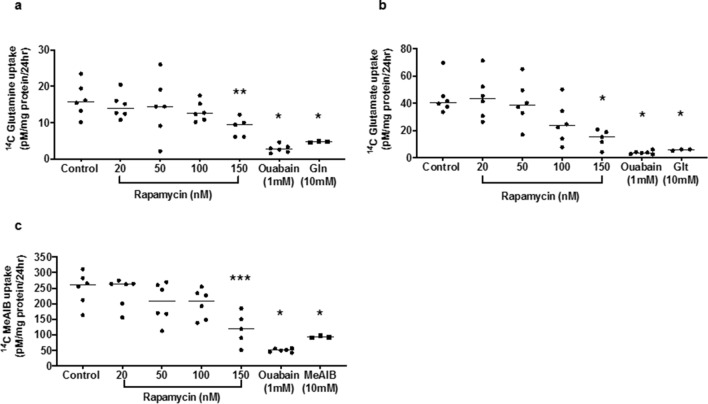
Rapamycin inhibits steady state accumulation of 14C-MeAIB, 14C-glutamine and 14C-glutamate by placental fragments from uncomplicated pregnancies
Rapamycin caused a concentration-dependent inhibition of 14C-MeAIB, 14C-glutamine and 14C-glutamate uptake by placental villous explants from uncomplicated pregnancies (IBR 20–70) over 24 h, which was significantly lower than control at 150 nM (Fig. 5; 14C-MeAIB P < 0.001; 14C-glutamate P < 0.01; 14C-glutamine P < 0.05 versus control). Uptake of the radiolabelled amino acids was reduced to 15–30% (range) of control by the Na+/K+-ATPase inhibitor ouabain, indicating that at least 70% of radiolabel accumulation by the tissue over 24 h could be attributed to amino acid transporter activity, driven by an inwardly directed Na+ gradient. Neither rapamycin nor ouabain altered the uptake of 14C-3-0-methylglucose (3-0MG) over 24 h (Supplementary Fig. 5). The transporter-mediated uptake of 14C-MeAIB, 14C-glutamine and 14C-glutamate was also reduced by a high concentration (10 mM) of unlabelled MeAIB, glutamine and glutamate to 35%, 28% and 13% of control respectively (Fig. 5).
Figure 5.
Effect of rapamycin on 14C-MeAIB (a) 14C-glutamine (b) and 14C-glutamate (c) uptake into placental villous explants (n = 6) over 24 h. *** P < 0.001, ** P < 0.01, * P < 0.05 versus control: ANOVA with Dunn’s multi-comparison post hoc test. 1 mM ouabain (inhibitor of Na+/K+-ATPase) reduced uptake of all radiolabelled amino acids by 70–85% (* P < 0.03: Wilcoxon matched pairs; n = 6 placentas). 10 mM unlabelled amino acids [MeAIB (a) Gln = glutamine (b) Glt = glutamate (c); competing substrate] significantly reduced uptake of the corresponding radiolabelled amino acid (* P < 0.02: Mann–Whitney; n = 3 placentas). Line denotes median.
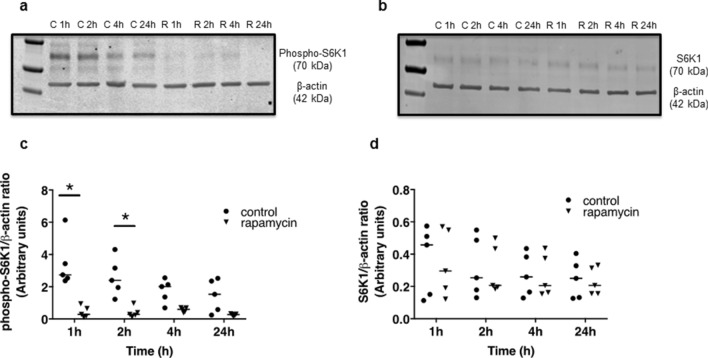
Phospho-S6K1 but not total S6K1 expression is inhibited by rapamycin
Both time and exposure to rapamycin had an overall effect in reducing placental (uncomplicated pregnancies; IBR 37.1–96.6) expression of phosphorylated ribosomal S6 kinase (phospho-S6K1, Fig. 6A,C). When comparing groups directly at each timepoint, expression of phospho-S6K1 was lower in the rapamycin versus control groups at 1 and 2 h (P < 0.05) with a trend towards significance at 4 h (P = 0.07, Fig. 6C). Rapamycin had no effect on total S6K1 expression at any timepoint (Fig. 6B,D). Full-length blots are presented in Supplementary Fig. 6.
Figure 6.
Representative western blots of phospho-S6K1 (a) and total S6K1 (b) protein in placental villous explants treated for 1, 2, 4 and 24 h with (R) or without (control, C) 150 nM rapamycin. Densitometric analysis is expressed as a ratio of β-actin signal. Data are shown in (C) and (D) (n = 5 placentas). Line denotes median, * P < 0.05: 2-way repeated measures ANOVA with Sidak test.
Discussion
In agreement with our hypothesis this study shows that initial rate uptake of glutamine and glutamate, important amino acids for fetal growth and placental metabolism, into placental villous fragments is reduced in FGR. This reduction was not accompanied by reduced expression of transporters utilised by glutamine and glutamate. To the contrary, expression of the transporter proteins, LAT1, LAT2, SNAT5 and EAAT1 were increased in placental homogenates of FGR versus AGA infants. Reasons for this disparity are not known at present but possibilities are discussed below.
In both AGA and FGR, we estimate that approximately twice as much glutamate as glutamine is transported across the MVM (taking into account that different concentrations of these radiolabeled amino acids were used for the initial rate uptake experiments: see Methods). This is probably reflective of the fact that glutamate is readily metabolised to glutamine in the cytosol of the syncytiotrophoblast for subsequent transfer to the fetus and reinforces the importance of glutamate, albeit indirectly, for fetal growth as previously suggested in mice32. Worthy of note is that measures of 14C uptake in these studies are unable to discriminate between glutamine and glutamate should interconversion have taken place. If interconversion did occur over the time course of the experiment (30–90 min), the studies show that there is a reduction in 14C labelled substrates, important for both placental metabolism and fetal growth, in FGR versus AGA.
In the current study we have also shown that, in uncomplicated pregnancies, exposure to rapamycin inhibits placental glutamine and glutamate uptake at steady state. The involvement of mTOR in this effect is evidenced by the reduction in phospho-, but not total, S6K1 expression following rapamycin exposure. Experiments were performed to assess the contribution of transporter-mediated glutamine and glutamate uptake to the total accumulation over 24 h. Using a high concentration of unlabelled substrates to compete for binding sites on the transporters, or ouabain to reduce the activity of transporters primarily- and secondarily-dependent on the intracellular Na+ gradient (latter include exchangers such as system L, which rely on intracellular substrates that are taken up by Na+ dependent transporters), we showed that a maximum of 30% of amino acid uptake over 24 h could be by non-specific diffusion (e.g. through the lipid bilayer or damaged areas of tissue). Accordingly, it is likely that rapamycin reduced MeAIB, glutamine and glutamate uptake by inhibiting the activity of the transporter systems A, L and XAG-. This effect is consistent with our hypothesis and with the regulation of system A and L transporter activity by mTOR reported previously30.
Our data are consistent with existing reports in the literature that FGR fetuses have smaller placentas than AGA babies1 and demonstrate reduced transporter-mediated uptake of 14C-MeAIB (system A activity)13–15,33. Placental uptake of amino acids is important for their subsequent delivery to the fetus and also for modifying the intracellular pool of amino acids available for exchange, for example by system L transporters34. One may expect a smaller placenta (by weight) to have less surface area available for exchange. Indeed in FGR, the surface area of terminal villi and capillaries is decreased35. Ideally, surface area available for nutrient transport would be used as the denominator by which to calculate amino acid transporter activity. However, in the absence of a method to accurately measure syncytiotrophoblast MVM surface area for this assay, placental uptake was measured per mg protein as a proxy measure of placental size. Reduced amino acid uptake (per mg placental protein) in combination with a smaller placenta theoretically contributes to reduced amino acid availability for delivery to the fetus, as illustrated by the proxy measure of total amino acid within the syncytiotrophoblast available for exchange (Fig. 2B–D). It is also postulated that a reduction in initial rate uptake of glutamine and glutamate will lead to a lower intracellular concentration in placentas of FGR compared with AGA pregnancies36. Reductions in amino acid availability thus offer a rationale as to why fetal growth may be compromised in FGR. Further work is required to establish the impact of reduced amino acid uptake in FGR on the composition of amino acid pools within the placenta.
A limitation of the study was the significant difference in the gestation of FGR samples compared with AGA (Table 1). However, analyses performed confirmed no difference in uptake of radiolabeled amino acids from preterm compared to term delivery (Supplementary Fig. 2).
Transporter abundance was assessed as a potential mechanistic explanation for the reduction in amino acid transporter activity in FGR. However, the data presented here show a disparity between transporter activity and expression. In FGR, placental expression of LAT1, LAT2, SNAT5 and EAAT1 transporter proteins was higher compared with AGA. This is in agreement with previous reports that LAT1 expression, as analysed by immunohistochemistry, is increased in the MVM of the syncytiotrophoblast in FGR37. This is the first study to show that placental system N and XAG- (SNAT5 and EAAT1) protein expression is also elevated in FGR.
Assessment of system A transporter proteins by Western blotting was not conducted in the current study due to an absence of commercially available and validated antibodies that reliably produce a specific signal. Instead, we evaluated mRNA expression and found that placental SLC38A2 (SNAT2) expression was higher and SLC38A4 (SNAT4) expression lower in FGR compared with AGA pregnancy. These data conflict with previous observations from a similar study cohort in humans31 (FGR < 3rd centile versus AGA 25th–75th centile, non-customised growth charts) that reported reduced SNAT1 and 2, and no difference in SNAT4, protein expression in MVM isolates from FGR compared with AGA infants; no differences were found when whole homogenates was compared. Furthermore, a study by Malina et al., compared SLC38A1 and SLC38A2 mRNA expression between placentas of women with SGA (< 10th centile) and normal birth weight infants and demonstrated no difference between groups38. Experiments in animal models yield conflicting results. Placental SNAT1 and 2 expression is reduced in the MVM of a protein restricted rat model of FGR but not in a protein-restricted baboon model39,40. In the placental-specific Igf2 knockout mouse, a well characterized model of FGR, placental expression of Slc38a4 is elevated compared with wild-type mice earlier in pregnancy (embryonic day 16) but normalizes near term whilst expression of Slc38a1 and Slc38a2 are no different at either gestational time point41. The mechanisms that underpin these disparate observations remain unclear but likely relate to species differences and also the different perturbations to induce FGR in the animal models. In the current study, SLC38A1, 2 and 4 expression was measured following RNA extraction from villous tissue whereas previous studies determined SNAT1, 2 and 4 protein expression in MVM isolates from human placenta31.This might underlie the different findings between studies. The reduced expression of SLC38A4 in the current study could lead to a reduction in SNAT4 expression but it is unlikely that lower SNAT4 expression exclusively accounts for reduced glutamine uptake in FGR given that it is not considered to be substrate of this isoform42.
The reduction in glutamine and glutamate uptake at initial rate in FGR (Fig. 1) but increase in transporter protein expression (measured in membrane-enriched placental isolates: Fig. 3) could be explained by a reduction in the insertion of the transport proteins into the MVM. Previously, reduced placental system A and L activity in FGR has been attributed to alterations in trafficking of amino acid transporters to the MVM43 as a consequence of a reduction in activity of the mTORC1 signaling pathway. Inhibition of mTORC1 in cytotrophoblast cells activates the ubiquitin ligase Nedd4-231, which increases ubiquitination of specific isoforms of the system A and L transporters causing their removal from the plasma membrane, and a reduction in system A and L activity. This occurs without changing the overall cellular expression of these amino acid transporters. In FGR the activity of mTORC1 is reduced, and Nedd4-2 increased, compared with uncomplicated pregnancy31, which raises the possibility that the reduction in activity of the glutamine and glutamate transporters observed in FGR in the current study could be due to ubiquitination of the transporters and their removal from the MVM. It is possible that the raised transporter protein expression in FGR is an adaptive response in an attempt to facilitate appropriate nutrient delivery to the fetus, but these proteins fail to be inserted into the MVM. To test this hypothesis it is essential that an assessment of protein expression in MVM, rather than the mixed membrane population employed here, be conducted. The reduced glutamine and glutamate uptake following inhibition of mTORC1 by rapamycin observed in the current study shows that the activity of their respective transporters is regulated by mTORC1 in placental villous tissue of normal pregnancy. Further experiments are necessary to determine whether the reduction in glutamine and glutamate uptake in FGR is a consequence of reduced mTORC1 and increased Nedd4-2 activity lowering transporter expression in the MVM.
In conclusion, this study shows that the placental uptake of glutamine and glutamate is reduced in FGR, which may have consequences in terms of modulation of the placental amino acid pool and overall availability for transfer to the fetus. Deprivation of intracellular amino acids also compromises key aspects of syncytiotrophoblast biology such as mitochondrial function and cell renewal44. We also show that system XAG-, which transports glutamate, is regulated by mTOR in normal pregnancy.
These studies contribute to our understanding of how amino acid transport into the placenta is regulated in normal pregnancy, and also add to the wealth of evidence for compromised placental amino acid transport in FGR. Improving placental amino acid transport could be a potential therapy for FGR but further studies are required to fully understand regulatory mechanisms of the different transporters and identify appropriate therapeutic targets.
Methods
Tissue collection
Women who met the inclusion criteria (maternal age > 18 < 40 years; body mass index (BMI) > 19 < 30 kg/m2 at first antenatal appointment) were approached to take part in the study. However, difficulties obtaining tissue during the study from women with a BMI < 30 kg/m2 meant that some over this cut-off were included (n = 1 AGA: BMI 31.2, n = 1 FGR: BMI 33.9). Exclusion criteria were multiple pregnancy, congenital abnormalities or pre-gestational (e.g. hypertension) or gestational maternal disease (e.g. pre-eclampsia, gestational diabetes mellitus). IBR was calculated using GROW software (www.gestation.net). For the purpose of this study we defined FGR as an individualised birth weight ratio (IBR) < 5th centile, in alignment with the widely accepted threshold when this study began. AGA was classified as the 20th–80th centile to reduce the likelihood of including infants that are small or large for gestational age in the study population.
The placenta was collected immediately following delivery and trimmed placental weight (umbilical cord and placental membranes removed) recorded. Villous tissue was sampled according to a systematic sampling protocol and either used immediately for experiments requiring fresh tissue or stored at − 80 °C.
Freshly isolated villous tissue was used to compare initial rate 14C-glutamine and 14C-glutamate uptake in AGA and FGR. The benefit of this technique, compared with MVM vesicles, is the ability to assess amino acid uptake without compromising tissue integrity, intracellular signaling mechanisms and associated driving forces. The uptake of 14C-methylaminoisobutyric acid (MeAIB) was measured alongside as a positive experimental control. MeAIB is a non-metabolisable substrate of system A45 and has been used extensively to assess placental system A activity in human and animal studies15,46,47. Furthermore it is well established that placental system A activity is reduced in FGR versus AGA13–15.
Development of a protocol to assess transporter-mediated uptake of 14C-glutamine and 14C-glutamate
A method is well established to assess system A activity in the MVM by determining Na+-dependent uptake of 14C-MeAIB into villous fragments at initial rate45,48. However, transporter-mediated 14C-glutamine and 14C-glutamate uptake into villous fragments has not been reported previously and a pilot experiment was performed to determine (a) the optimal concentration of radioisotope (b) a strategy to detect the transporter-mediated component of 14C-glutamine and 14C-glutamate uptake, and (c) the time over which glutamine and glutamate transporter activity could be measured at initial rate.
Glutamine transport by syncytiotrophoblast MVM vesicles is mediated by Na+-dependent systems A and N, and the Na+-independent system L28. 14C-glutamine uptake is inhibited by 5 mM histidine (substrate of system N) serine (substrate of system A) and 2-amino-2-norbornanecarboxylic acid (BCH, non-metabolisable analogue, substrate of system L)49. Therefore, the contribution of systems A, N and L to glutamine transport in villous fragments was measured in Tyrode’s buffer in the absence of competitive substrates (total 14C-glutamine uptake comprising non-specific diffusion and transporter-mediated uptake) and in control (i.e. Na+-containing) or Na+-free Tyrode’s buffer [Tyrode’s buffer as described previously50 with equimolar choline chloride to replace 135 mM NaCl (pH 7.4 with KOH)] with 5 mM histidine, 5 mM serine and 5 mM BCH. For 14C-glutamate, 5 mM aspartic acid, a substrate of the Na+-dependent system XAG-28 was used, as described above.
Following tissue collection (described above), villous tissue was maintained in glutamine-free DMEM (1 g/litre glucose, Life Technologies Ltd, Leicestershire, UK) supplemented with 864 μM glutamine and 120 μM glutamic acid mixed 1:1 with Tyrode’s buffer50, for a final concentration of 432 μM and 60 μM, respectively, to mimic the concentration in maternal plasma before beginning the experimental protocol.
To measure uptake, placental villous tissue was exposed to 14C-glutamine (0.066 µCi/ml; 0.24 nmol/ml) and 14C-glutamate (0.13 µCi/ml; 0.5 nmol/ml) for 10–120 min. After the elapsed time period, fragments were vigorously washed for 2 × 15 s in 12 ml ice-cold Tyrode's buffer (control or Na+-free) then suspended in 4 ml water for 18 h. Lysed tissue fragments were incubated in 0.3 M NaOH (37 °C; overnight) and protein determined on the lysate by the Bio-Rad method. Initial rate uptake was expressed per mg fragment protein, using the latter as a proxy measure of fragment size. The transporter-mediated component was determined by subtracting uptake in Na+-free Tyrode’s buffer containing competitive substrates from uptake under control conditions (Supplementary Fig. 1).
Analysis by linear regression demonstrated that transporter activity was at initial rate over 30–90 min. Thus, definitive experiments measured 14C-glutamine, 14C-glutamate and 14C-MeAIB uptake over 30–90 min (n = 14 AGA, 11 FGR) as described above and previously45.
To test the hypothesis that diminished amino acid uptake may lead to decreased amino acid availability for transfer to the fetus, a proxy measure of amino acid availability was calculated as amino acid uptake at 90 min (per mg placental protein) × trimmed placental weight (g).
Effect of rapamycin on amino acid uptake at steady state
Placental villous fragments from uncomplicated pregnancies were dissected and processed under sterile conditions. Fragments were maintained in medium (DMEM 1880028: Gibco) supplemented with alanine (341 µM), glutamine (418 µM), glutamic acid (69 µM) and taurine (44 µM), streptomycin sulphate (100 mg/L), penicillin (60 mg/L) and gentamicin (1 ml/L) on Netwell permeable supports (Corning: 74 µM mesh; in a humidified incubator (37 ºC; 95 % air/5 % CO2)). To measure amino acid uptake at steady state36, fragments were incubated for 24 h with: 0.5 µCi/ml (8.5 nmol/ml) 14C-MeAIB; 0.066 µCi/ml (0.24 nmol/ml) 14C-glutamine; 0.13 µCi/ml (0.5 nmol/ml) 14C-glutamate; or 0.5 µCi/ml (8.5 nmol/ml) 14C-3-0-methylglucose (3-0MG). 3-0MG is a non-metabolised substrate of the GLUT transporters and uptake of 3-0MG was measured to assess whether rapamycin had nonspecific effects on transporters and/or an effect on placental villus tissue integrity.
After 24 h, fragments were washed (2 × 15 s with 25 ml ice cold 0.9% saline) then lysed in 6 ml 0.3 M NaOH at 37 °C overnight to release the accumulated isotope. The radioactivity of the tissue lysate and culture medium was determined as described previously45.
Rapamycin (Tocris, 53123-88-9) (n = 6 placentas) was added to the culture medium containing isotopes at 20–150 nM for 24 h. The effect of rapamycin on 3-0MG uptake was measured to determine whether inhibition of mTOR would affect a non-amino acid facilitated diffusion transporter. Stock rapamycin was prepared in DMSO and diluted in culture medium; equivalent DMSO (0.1%) was added to control medium.
Two approaches were adopted to determine the contribution of transporter-mediated uptake versus non-specific diffusion to the total uptake of radiolabelled amino acids at 24 h. Fragments were treated with 1 mM ouabain to block Na+/K+-ATPase activity, raise intracellular Na+ concentration51 and inhibit the activity of Na+-dependent amino acid transporters (systems A, N and XAG-). Additionally, fragments (n = 3 placentas) were incubated with a high concentration (10 mM) of unlabelled MeAIB, glutamine or glutamate to block uptake of the corresponding radiolabelled amino acids by competitive inhibition of the respective transporter proteins. Uptake in the presence of ouabain, or competing amino acids, is an estimate of non-specific diffusion.
Placental protein expression of glutamine and glutamate transporters
Frozen placental tissue (n = 11 AGA, 10 FGR) was homogenised and centrifuged as previously described52. Membrane-enriched fractions were stored at − 80 °C for later Western blot analysis to detect LAT1, LAT2, SNAT5, EAAT1 and EAAT2. Proteins were separated by SDS-PAGE and transferred to Immobilon-FL PVDF membranes (Millipore UK Ltd., Watford, UK). Primary antibodies were: LAT1 (0.5 µg/ml; KE026; TransGenic Inc, Japan); LAT2 (2 µg/ml; ab75610; Abcam, Cambridge, UK); SNAT5 (1.4 µg/ml; ab72717; Abcam), EAAT1 (1 µg/ml; ab416; Abcam) and EAAT2 (2.69 µg/ml; ab178401; Abcam). β-tubulin (2 µg/ml; ab6046; Abcam) was used as a loading control. Bands detected at the predicted molecular weight (kDa) were validated by the inclusion of positive controls (MVM) during antibody optimisation. Negative controls were by omission of primary antibody. Immunoreactive species were detected with fluorescent-conjugated secondary antibodies (Li-COR Biosciences, Cambridge, UK) and membranes imaged using an Odyssey Sa Infrared Imaging System (Li-COR). Signal density was measured using Image Studio Lite (Li-COR). All signals were in the linear range of detection.
Placental protein expression of phospho-S6K1 and S6K1 following rapamycin exposure
Placental villous explants were set up as described in the steady state measures section (n = 5). At 1, 2, 4 or 24 h, explants were taken and homogenised in RIPA lysis buffer (Sigma-Aldrich, UK, R0278) containing 10% PIC (Sigma-Aldrich, UK P8340) and 10% phosphatase inhibitor cocktails 2 and 3 (Sigma-Aldrich, UK, P5726 and P0044) using the Bullet blender tissue homogeniser (Gold units, Next Advance, USA). The mTOR signaling pathway exerts its effects via the phosphorylation of downstream targets such as ribosomal S6 kinase (S6K1). To assess mTOR activity membranes were probed, as per the Western blotting experiments above, for phospho-S6K1 (Thr-389, #9234, 84.2 ng/ml) and S6K1 (#2708, 48.0 ng/ml, both Cell Signaling Technologies, USA). All blots were re-probed with β-actin as a loading control (Sigma-Aldrich, UK, A5441, 1 µg/ml).
Extraction of total RNA from human placental villous tissue
Total RNA was isolated from AGA (n = 11) and FGR (n = 9) villous tissue samples using the mirVanaTM isolation kit (AM1560, Ambion, LifeTechnologies, UK) following the manufacturer’s protocol. Samples were treated with DNAse using TURBO DNA-freeTM kit (AM1906, Ambion, LifeTechnologies, UK) following the manufacturer’s instructions. RNA purity and concentration were assessed (Nanodrop 2000c, ThermoFisher Scientific, UK; 260/280 ratio of 2.0 ± 0.2 was considered acceptable). Samples were stored at − 80 °C. cDNA was synthesised from 500 ng RNA using an AffinityScript Multiple Temperature cDNA Synthesis Kit (Agilent Technologies, Stratagene, UK). A reference total human placental RNA (1 μg, AM7950, Ambion, Life Technologies, UK) and negative controls (no reverse transcriptase: − RT and no RNA template control: NTC) were also included. 3 μl random primers (0.1 μg/μl) was added to each tube, incubated at 65 °C for 5 min and subsequently cooled at room temperature for 10 min to allow primers to anneal to the RNA. 2 μl 10 × AffinityScript RT Buffer, 0.8 μl dNTP mix (25 mM of each dNTP), 0.5 μl RNase Block Ribonuclease Inhibitor (40 U/μl) and 1 μl AffinityScript Multiple Temperature RT were added to give a final volume of 20 μl. The reaction was incubated at 25 °C for 10 min to extend the primers, 42 °C for 60 min to generate cDNA, then terminated by incubation at 70 °C for 15 min. A pooled cDNA sample was generated by collecting 5 μl of each undiluted cDNA sample. The cDNA pool was diluted 1:4 in PCR H2O then serially diluted to generate samples for a standard curve (1:4–1:256). All samples were stored at − 20 °C. The efficiency of reverse transcription was checked against a housekeeping gene (TATA-box binding protein, TBP).
Quantitative real-time PCR (qRT-PCR) of mRNA
A mastermix for qRT-PCR was prepared per reaction as follows: 0.2 μl PCR H2O, 5 μl 2X SYBR Green QPCR master mix, 0.3 μl ROX (reference dye, diluted 1:500 in PCR H2O) (Brilliant III Ultra-fast SYBR Green Master Mix, Agilent Technologies, Wokingham, UK) and 0.25 μl of each forward and reverse primer (final concentration 300 nM) for the gene of interest. Primer sequences were as follows: SLC38A1 (5′-GTGTATGCTTTACCCACCATTGC-3′ and 3′-GCACGTTGTCATAGAATGTCAAGT-5′), SLC38A2 (5′-ACGAAACAATAAACACCACCTTAA-3′ and 3′-AGATCAGAATTGGCACAGCATA-5′), SLC38A4 (5′-TTGCCGCCCTCTTTGGTTAC-3′ and 3′-GAGGACAATGGGCACAGTTAGT-5′), and TBP (5′-CACGAACCACGGCACTGATT-3′ and 3′-TTTTCTTGCTGCCAGTCTGGAC-5′) used previously45,53. Tyrosine 3- monooxygenase/tryptophan 5-monooxygenase activation protein zeta (YWHAZ) primers (5′-CCTGCATGAAGTCTGTAACTGAG-3′ and 3′-TTGAGACGACCCTCCAAGATG-5′). 4 μl cDNA samples (diluted 1:10 in PCR H2O, 10 ng cDNA) were mixed with 6 μl master mix and measured in duplicate. A standard curve and negative controls (–RT, NTC) were included in duplicate on each plate. Conditions for qPCR (Stratagene MX3005P) were as follows: 95 °C 5 min, then 95 °C 30 s, 60 °C 30 s, 72 °C 30 s (40 cycles, amplification), 95 °C 1 min, 55 °C 30 s then a final increase to 95 °C (in 0.2 °C increments) to generate a dissociation curve; a single peak and an efficiency between 90 and 110% was considered acceptable. Cycle threshold (CT) values were interpolated from the standard curve on each plate, generated from the pooled cDNA sample to calculate mRNA levels. mRNA expression was normalised to the geometric mean of TBP and YWHAZ (stable across samples, data not shown).
Statistical analysis
Data were analysed using GraphPad Prism 7 software; P < 0.05 was considered statistically significant. Normal distribution was determined using D’Agostino & Pearson omnibus normality test. Data from amino acid experiments are expressed as mean ± standard error of the mean (SEM), and least squares linear regression analyses performed to determine whether transporter-mediated amino acid uptake was linearly related to time and whether there were differences in amino acid uptake in AGA versus FGR. Western blot data were analysed using a Mann–Whitney test (transporter proteins) or 2-way repeated measures ANOVA with Sidak test (phospho-S6K1 and total S6K1). mRNA expression was analysed using a Mann–Whitney test. The effect of rapamycin on amino acid uptake was assessed by ANOVA with Dunn's multi-comparison post hoc test with values represented as median. Wilcoxon matched pairs and Mann–Whitney tests were used to assess the effect of ouabain or unlabelled amino acids on uptake, respectively.
Ethical approval and informed consent
This study was performed with local research ethics committee approval (15/NW/0829) and in accordance with relevant guidelines and regulations. Informed written consent from all participants was obtained during pregnancy prior to collection of samples.
Supplementary information
Acknowledgements
K.R.M. conducted this work during her PhD studentship, which was supported by a Medical Research Council Doctoral Training Partnership (1 512 341). This work was also supported by a Career Development Fellowship Award from the Medical Research Council (MR/K024442/1) awarded to M.R.D. The National Institute for Health Research Manchester Biomedical Research Centre and Tommy's charity provided infrastructure support.
Author contributions
C.P.S, S.L.G, M.R.D, M.D and K.R.M designed the experiments. K.R.M, K.M.M.V, C.E.H, X.L, S.L.G and M.R.D performed experimental work. S.L.G, M.R.D and K.R.M analysed data. S.L.G, M.R.D and K.R.M wrote the manuscript. All authors approved the final manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-72930-7.
References
- 1.Mifsud W, Sebire NJ. Placental pathology in early-onset and late-onset fetal growth restriction. Fetal Diagn. Ther. 2014;36:117–128. doi: 10.1159/000359969. [DOI] [PubMed] [Google Scholar]
- 2.Gardosi J, Madurasinghe V, Williams M, Malik A, Francis A. Maternal and fetal risk factors for stillbirth: population based study. BMJ. 2013;346:f108. doi: 10.1136/bmj.f108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barker DJP. The developmental origins of chronic adult disease. Acta Paediatr. Suppl. 2004;93:26–33. doi: 10.1111/j.1651-2227.2004.tb00236.x. [DOI] [PubMed] [Google Scholar]
- 4.Thornton JG, Hornbuckle J, Vail A, Spiegelhalter DJ, Levene M. GRIT II: Infant wellbeing at 2 years of age in the growth restriction intervention trial (GRIT): multicentred randomised controlled trial. Lancet. 2004;364:513–520. doi: 10.1016/S0140-6736(04)16809-8. [DOI] [PubMed] [Google Scholar]
- 5.Veen S, et al. Impairments, disabilities, and handicaps of very preterm and very-low-birthweight infants at five years of age. Lancet. 1991;338:33–36. doi: 10.1016/0140-6736(91)90015-H. [DOI] [PubMed] [Google Scholar]
- 6.Campbell HE, Kurinczuk JJ, Heazell A, Leal J, Rivero-Arias O. Healthcare and wider societal implications of stillbirth: a population-based cost-of-illness study. BJOG. 2018;125:108–117. doi: 10.1111/1471-0528.14972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cantwell R, et al. Saving Mothers’ Lives: reviewing maternal deaths to make motherhood safer: 2006–2008. The eighth report of the confidential enquiries into maternal deaths in the United Kingdom. BJOG. 2011;118(Suppl 1):1–203. doi: 10.1111/j.1471-0528.2010.02847.x. [DOI] [PubMed] [Google Scholar]
- 8.Heazell AEP, et al. Stillbirths: economic and psychosocial consequences. Lancet. 2016;387:604–616. doi: 10.1016/S0140-6736(15)00836-3. [DOI] [PubMed] [Google Scholar]
- 9.Fisk NM, Atun R. Market failure and the poverty of new drugs in maternal health. PLoS Med. 2008;5:e22. doi: 10.1371/journal.pmed.0050022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sibley CP. Treating the dysfunctional placenta. J. Endocrinol. 2017;234:R81–R97. doi: 10.1530/JOE-17-0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baschat AA. Examination of the fetal cardiovascular system. Semin. Fetal Neonatal Med. 2011;16:2–12. doi: 10.1016/j.siny.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 12.Mayhew TM, Charnock-Jones DS, Kaufmann P. Aspects of human fetoplacental vasculogenesis and angiogenesis III. Changes in complicated pregnancies. Placenta. 2004;25:127–139. doi: 10.1016/j.placenta.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 13.Mahendran D, et al. Amino acid (system A) transporter activity in microvillous membrane vesicles from the placentas of appropriate and small for gestational age babies. Pediatr. Res. 1993;34:661–665. doi: 10.1203/00006450-199311000-00019. [DOI] [PubMed] [Google Scholar]
- 14.Shibata E, et al. Placental system A amino acid transport is reduced in pregnancies with small for gestational age (SGA) infants but not in preeclampsia with SGA infants. Placenta. 2008;29:879–882. doi: 10.1016/j.placenta.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glazier JD, et al. Association between the activity of the system A amino acid transporter in the microvillous plasma membrane of the human placenta and severity of fetal compromise in intrauterine growth restriction. Pediatr. Res. 1997;42:514–519. doi: 10.1203/00006450-199710000-00016. [DOI] [PubMed] [Google Scholar]
- 16.Gordijn SJ, et al. Consensus definition of fetal growth restriction: a Delphi procedure. Ultrasound Obstet. Gynecol. 2016;48:333–339. doi: 10.1002/uog.15884. [DOI] [PubMed] [Google Scholar]
- 17.Jansson T, Scholtbach V, Powell TL. Placental transport of leucine and lysine is reduced in intrauterine growth restriction. Pediatr. Res. 1998;44:532–537. doi: 10.1203/00006450-199810000-00011. [DOI] [PubMed] [Google Scholar]
- 18.Jansson N, et al. Down-regulation of placental transport of amino acids precedes the development of intrauterine growth restriction in rats fed a low protein diet. J. Physiol. 2006;576:935–946. doi: 10.1113/jphysiol.2006.116509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cramer S, Beveridge M, Kilberg M, Novak D. Physiological importance of system A-mediated amino acid transport to rat fetal development. Am. J. Physiol. Cell Physiol. 2002;282:C153–C160. doi: 10.1152/ajpcell.2002.282.1.C153. [DOI] [PubMed] [Google Scholar]
- 20.Parimi PS, Kalhan SC. Glutamine supplementation in the newborn infant. Semin. Fetal Neonatal Med. 2007;12:19–25. doi: 10.1016/j.siny.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 21.Pochini L, Scalise M, Galluccio M, Indiveri C. Membrane transporters for the special amino acid glutamine: structure/function relationships and relevance to human health. Front. Chem. 2014;2:61. doi: 10.3389/fchem.2014.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Neu J. Glutamine in the fetus and critically ill low birth weight neonate: metabolism and mechanism of action. J. Nutr. 2001;131:2585S–2589S. doi: 10.1093/jn/131.9.2585S. [DOI] [PubMed] [Google Scholar]
- 23.Tapiero H, Mathé G, Couvreur P, Tew KD., II Glutamine and glutamate. Biomed. Pharmacother. 2002;56:446–457. doi: 10.1016/S0753-3322(02)00285-8. [DOI] [PubMed] [Google Scholar]
- 24.Vaughn PR, et al. Glutamine-glutamate exchange between placenta and fetal liver. Am. J. Physiol. Endocrinol. Metab. 1995;268:E705–711. doi: 10.1152/ajpendo.1995.268.4.E705. [DOI] [PubMed] [Google Scholar]
- 25.Moores RR, et al. Glutamate metabolism in fetus and placenta of late-gestation sheep. Am. J. Physiol. 1994;267:R89–96. doi: 10.1152/ajpregu.1994.267.1.R89. [DOI] [PubMed] [Google Scholar]
- 26.Lofthouse EM, et al. Glutamate cycling may drive organic anion transport on the basal membrane of human placental syncytiotrophoblast. J. Physiol. 2015;593:4549–4559. doi: 10.1113/JP270743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Day PEL, et al. Partitioning of glutamine synthesised by the isolated perfused human placenta between the maternal and fetal circulations. Placenta. 2013;34:1223–1231. doi: 10.1016/j.placenta.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hill C, Greenwood S, Widdows K, D’Souza S, Glazier J. Placental glutamine uptake is mediated by different transport mechanisms; implications for fetal growth. Bjog Int. J. Obstet. Gynaecol. 2014;121:e4–e5. doi: 10.1111/1471-0528.12903. [DOI] [Google Scholar]
- 29.Roos S, et al. Mammalian target of rapamycin in the human placenta regulates leucine transport and is down-regulated in restricted fetal growth. J. Physiol. 2007;582:449–459. doi: 10.1113/jphysiol.2007.129676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roos S, Kanai Y, Prasad PD, Powell TL, Jansson T. Regulation of placental amino acid transporter activity by mammalian target of rapamycin. Am. J. Physiol. Cell Physiol. 2009;296:C142–C150. doi: 10.1152/ajpcell.00330.2008. [DOI] [PubMed] [Google Scholar]
- 31.Chen Y-Y, et al. Increased ubiquitination and reduced plasma membrane trafficking of placental amino acid transporter SNAT-2 in human IUGR. Clin. Sci. 2015;129:1131–1141. doi: 10.1042/CS20150511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McIntyre KR, Hayward CE, Sibley CP, Greenwood SL, Dilworth MR. Evidence of adaptation of maternofetal transport of glutamine relative to placental size in normal mice, and in those with fetal growth restriction. J. Physiol. 2019;597:4975–4990. doi: 10.1113/JP278226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jansson T, Ylvén K, Wennergren M, Powell TL. Glucose transport and system A activity in syncytiotrophoblast microvillous and basal plasma membranes in intrauterine growth restriction. Placenta. 2002;23:392–399. doi: 10.1053/plac.2002.0826. [DOI] [PubMed] [Google Scholar]
- 34.Cleal JK, Lofthouse EM, Sengers BG, Lewis RM. A systems perspective on placental amino acid transport. J. Physiol. 2018 doi: 10.1113/JP274883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mayhew TM, et al. Stereological investigation of placental morphology in pregnancies complicated by pre-eclampsia with and without intrauterine growth restriction. Placenta. 2003;24:219–226. doi: 10.1053/plac.2002.0900. [DOI] [PubMed] [Google Scholar]
- 36.McIntyre K, et al. Cellular accumulation of 14 Cmethylaminoisobutyric acid by system A is lower in placentas of growth restricted compared to normally grown fetuses. Placenta. 2017;57:293. doi: 10.1016/j.placenta.2017.07.219. [DOI] [Google Scholar]
- 37.Aiko Y, et al. Differential levels of amino acid transporters System L and ASCT2, and the mTOR protein in placenta of preeclampsia and IUGR. BMC Pregnancy Childbirth. 2014;14:181. doi: 10.1186/1471-2393-14-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Malina A, Daftary A, Crombleholme W, Markovic N, Roberts JM. Placental system A transporter mRNA is not different in preeclampsia, normal pregnancy, or pregnancies with small-for-gestational-age infants. Hypertens. Pregnancy. 2005;24:65–74. doi: 10.1081/PRG-45780. [DOI] [PubMed] [Google Scholar]
- 39.Rosario FJ, et al. Maternal protein restriction in the rat inhibits placental insulin, mTOR, and STAT3 signaling and down-regulates placental amino acid transporters. Endocrinology. 2011;152:1119–1129. doi: 10.1210/en.2010-1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pantham P, et al. Down-regulation of placental transport of amino acids precedes the development of intrauterine growth restriction in maternal nutrient restricted baboons. Biol. Reprod. 2016;95:98–98. doi: 10.1095/biolreprod.116.141085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Constância M, et al. Adaptation of nutrient supply to fetal demand in the mouse involves interaction between the Igf2 gene and placental transporter systems. Proc. Natl. Acad. Sci. USA. 2005;102:19219–19224. doi: 10.1073/pnas.0504468103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bröer S. The SLC38 family of sodium–amino acid co-transporters. Pflügers Arch. Eur. J. Physiol. 2014;466:155–172. doi: 10.1007/s00424-013-1393-y. [DOI] [PubMed] [Google Scholar]
- 43.Rosario FJ, Kanai Y, Powell TL, Jansson T. Mammalian target of rapamycin signalling modulates amino acid uptake by regulating transporter cell surface abundance in primary human trophoblast cells. J. Physiol. 2013;591:609–625. doi: 10.1113/jphysiol.2012.238014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Desforges M, Whittaker H, Farmer E, Sibley CP, Greenwood SL. Effects of taurine depletion on human placental syncytiotrophoblast renewal and susceptibility to oxidative stress. Adv. Exp. Med. Biol. 2015;803:63–73. doi: 10.1007/978-3-319-15126-7_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hayward CE, et al. Effect of maternal age and growth on placental nutrient transport: potential mechanisms for teenagers’ predisposition to small-for-gestational-age birth? Am. J. Physiol. Endocrinol. Metab. 2012;302:E233–E242. doi: 10.1152/ajpendo.00192.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kusinski LC, Jones CJP, Baker PN, Sibley CP, Glazier JD. Isolation of plasma membrane vesicles from mouse placenta at term and measurement of system A and system beta amino acid transporter activity. Placenta. 2010;31:53–59. doi: 10.1016/j.placenta.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Desforges M, et al. The SNAT4 isoform of the system A amino acid transporter is functional in human placental microvillous plasma membrane. J. Physiol. 2009;587:61–72. doi: 10.1113/jphysiol.2008.161331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Greenwood SL, Sibley CP. In vitro methods for studying human placental amino acid transport placental villous fragments. Methods Mol. Med. 2006;122:253–264. doi: 10.1385/1-59259-989-3:253. [DOI] [PubMed] [Google Scholar]
- 49.Mastroberardino L, et al. Amino-acid transport by heterodimers of 4F2hc/CD98 and members of a permease family. Nature. 1998;395:288–291. doi: 10.1038/26246. [DOI] [PubMed] [Google Scholar]
- 50.Warrander LK, et al. Maternal perception of reduced fetal movements is associated with altered placental structure and function. PLoS ONE. 2012;7:e34851. doi: 10.1371/journal.pone.0034851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Greenwood SL, Clarson LH, Sides MK, Sibley CP. Membrane potential difference and intracellular cation concentrations in human placental trophoblast cells in culture. J. Physiol. 1996;492(Pt 3):629–640. doi: 10.1113/jphysiol.1996.sp021333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dilworth MR, et al. Placental-specific Igf2 knockout mice exhibit hypocalcemia and adaptive changes in placental calcium transport. Proc. Natl. Acad. Sci. USA. 2010;107:3894–3899. doi: 10.1073/pnas.0911710107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Desforges M, et al. SNAT4 isoform of system A amino acid transporter is expressed in human placenta. Am. J. Physiol. Cell Physiol. 2006;290:C305–C312. doi: 10.1152/ajpcell.00258.2005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.