Abstract
Telomerase reverse transcriptase (TERT) promoter mutations are frequently found in tumors or urine from patients with urothelial carcinoma (UC). TERT promoter mutations are also detected in urine from patients with no evidence of cancer but are associated with subsequent UC development. Mutations in the TERT promoter are thought to be present in nonmalignant urothelium (NMU) during early stages of tumor formation prior to pathological change, but this has not been proven directly. In this proof‐of‐concept study, we investigated the clinical utility of TERT promoter mutation analysis in NMU of patients with non‐muscle‐invasive bladder cancer (NMIBC). This single‐institute study included 53 primary tumors and 428 systematic bladder biopsy specimens from 54 patients with NMIBC. All patients underwent systematic random biopsy and transurethral resection of the bladder tumor. Genomic DNA was analyzed for TERT C228T and C250T mutations using droplet digital PCR (ddPCR). The association between TERT promoter mutation of NMU and bladder recurrence was examined by the Kaplan–Meier method and Cox proportional hazards model. Of the 54 patients, 16 (29.6%) had a TERT C228T mutation and three (5.6%) had a TERT C250T mutation in NMU. Of 428 biopsy specimens, the TERT C228T mutation was detected in 9% (31/364) of normal urothelium, 27% (4/15) of urothelial dysplasia (UD), 50% (9/18) of UD suspicious for carcinoma in situ (CIS), and 58% (18/31) of CIS. During follow‐up (median: 3.7 years), 22 (40.7%) patients experienced bladder recurrence and five (9.3%) experienced disease progression. Cox proportional hazard analysis showed that TERT C228T mutation in NMU was significantly associated with bladder recurrence after adjustment for cofounding factors (P = 0.0128). Thus, TERT C228T mutation was detected in NMU, which was a reliable independent prognostic factor of bladder tumor recurrence.
Keywords: bladder cancer, field change, prognosis, TERT promoter
Telomerase reverse transcriptase (TERT) C228T mutation was detected in normal urothelium and urothelial dysplasia of patients with nonmuscle invasive bladder cancer (NMIBC) and may thus be a novel and useful prognostic factor for risk stratification of NMIBC. Analysis of systematic random biopsies for TERT promoter mutations in nonmalignant urothelium may guide selection of the optimal treatment strategy.
![]()
Abbreviations
- BCG
Bacillus Calmette–Guérin
- CI
confidence interval
- CIS
carcinoma in situ
- EORTC
European Organisation for Research and Treatment of Cancer
- HR
hazard ratio
- NMIBC
non‐muscle‐invasive bladder cancer
- TERT
telomerase reverse transcriptase
- TURBT
transurethral resection of bladder tumor
- UBC
urothelial bladder cancer
- UC
urothelial carcinoma
1. Introduction
Urothelial bladder cancer (UBC) is the most common malignancy of the urinary tract. Approximately 70% of patients with UBC have non‐muscle‐invasive bladder cancer (NMIBC) at initial diagnosis [1], which can be cured by a transurethral resection of bladder tumor (TURBT) and/or intravesical therapy. Although the overall survival of patients with NMIBC is generally good, 50–80% have intravesical recurrence after TURBT and 10–15% experience disease progression [2, 3]. For this reason, patients need to be monitored for recurrence at regular intervals for a couple of years using cystoscopy, urine cytology, and upper tract imaging. Currently, there are several risk stratification systems for predicting the probability of disease recurrence and progression, but these systems are not optimal for stratifying risk of disease recurrence or progression in patients with NMIBC [3, 4, 5, 6]. Therefore, novel risk stratification tools are needed in order to improve patient outcome.
Hotspot mutations of the telomerase reverse transcriptase (TERT) promoter are frequently identified in primary tumors from patients with various types of bladder cancer and tumor stages, including precancerous lesions [7, 8, 9, 10, 11, 12, 13]. Mutations in the upstream promoter of TERT predominantly affect two positions, g.1295228 C>T (C228T) and g.1295250 C>T (C250T) [14]. These mutations in the TERT promoter allele alter the binding site and subsequently result in increased TERT expression, which enables tumors to maintain their telomere length and avoid senescence [15]. Furthermore, TERT promoter mutations result in a gradual upregulation of telomerase, which contributes to tumorigenesis by promoting genomic instability [16]. The tumor mutation status of the TERT promoter, including TERT C228T and C250T, is associated with disease recurrence and progression of NMIBC [17, 18]. In contrast to other gene mutations frequently found in urothelial carcinoma (UC), such as tumor protein 53 (TP53) and fibroblast growth factor receptor 3 (FGFR3), TERT promoter mutations are persistently present in both primary and recurrent tumors of most patients with NMIBC [17].
We previously reported that TERT promoter mutations in urinary cell‐free DNA (cfDNA) and urinary cellular DNA could be detected prior to the tumors becoming macroscopically visible using a liquid biopsy technique [19, 20]. It is thought that important cancer‐initiating events such as driver gene mutations could occur in pathologically nonmalignant tissues as a consequence of ‘field change’ [21]. TERT promoter mutations were detected in precancerous lesions in patients with hepatocellular carcinoma or malignant melanoma [22, 23]. For these reasons, we hypothesize that cfDNA or urinary cellular DNA containing TERT promoter mutations might be released from precancerous cells with a nonmalignant appearance as a field change. In the current study, we examined the profile of TERT promoter mutations in nonmalignant urothelium (NMU) of systematic biopsy samples collected at the time of TURBT and analyzed the association of the mutations with intravesical recurrence of NMIBC.
2. Methods
2.1. Clinical samples
We randomly selected NMIBC patients who received TURBT and systematic bladder biopsy at Osaka University Hospital, Suita, Japan, from 2013 to 2018 and provided written informed consent. The study was approved by the Institutional Review Board of Osaka University (IRB #668‐3) and conformed with the Declaration of Helsinki. To exclude concurrent carcinoma in situ (CIS), systematic random biopsy was performed for all patients who received TURBT for the first time or who had positive urine cytology in our institution. We analyzed 428 systematic bladder biopsy specimens obtained from the trigone, posterior wall, bladder dome, anterior wall, right wall, left wall, bladder neck, or prostatic urethra (male only) and 53 primary tumors (one tumor was missing) from 54 patients with NMIBC.
2.2. Pathological diagnosis
Histological diagnoses were determined by at least two experienced pathologists. Biopsy specimens were diagnosed as normal urothelium (NU), urothelial dysplasia (UD), UD suspicious for CIS, or CIS. NMU was defined as either NU or UD as previously described [24]. Tumor stage and grade were determined according to the American Joint Committee on Cancer 8th Edition Cancer Staging Manual [25], and the tumors were graded according to the World Health Organization 2016 criteria [26].
2.3. DNA extraction
DNA extraction from the clinical specimens was performed using a GeneRead DNA FFPE Kit (Qiagen, Hilden, Germany) according to the manufacturer's protocol. All DNA extractions were performed using ten–fifteen 10‐µm sections from each biopsy sample and two or three 10‐µm sections from each tumor sample. DNA concentrations were measured using a Qubit dsDNA High Sensitivity Assay Kit (Thermo Fisher Scientific, Waltham, MA, USA).
2.4. Droplet digital PCR
For mutation detection, the QX100 ddPCR System (Bio‐Rad Laboratories, Hercules, CA, USA) along with primers and probes (FAM, mutant‐type, and HEX, wild‐type) and ddPCR Supermix for probes (No dUTP) from Bio‐Rad Laboratories were employed according to the manufacturer's protocol. Details on the primers and probes for TERT promoter mutants C228T and C250T used in the ddPCR analyses are shown in Table S1. Droplets were generated using a droplet generator (Bio‐Rad Laboratories). The PCR cycling parameters included 10 min at 95 °C, 50 cycles of 94 °C for 30 s and 55 °C for 1 min, and one cycle of 98 °C for 10 min followed by a 12 °C hold. Droplet fluorescence was assessed using a droplet reader. Analysis of the ddPCR data for allele calling and the calculation of absolute copy numbers were performed using quantasoft software, version 1.7.4 (Bio‐Rad Laboratories). The samples were considered true positive for targeted mutations when the following two criteria were met. First, they contained at least three droplets in the positive area of the FAM signal. Second, the mutant allele frequency (MAF) was > 5.0% to exclude false‐positive reactions induced by DNA degradation due to the fixation process. MAF was defined as the proportion of mutant‐type copies relative to the total number of copies including both mutant‐type and wild‐type alleles as determined by the ddPCR analyses.
2.5. Statistical analysis
Statistical analysis was performed using jmp pro 14.0.0 software (SAS Institute Inc., Cary, NC, USA). Cox regression analyses were performed to assess the relative contributions of various factors and TERT promoter mutations. Because of the limited sample size, we applied two multivariate models to investigate factors associated with bladder recurrence. Differences were considered statistically significant when the P‐value was < 0.05.
3. Results
3.1. TERT promoter mutations in nonmalignant urothelium
Fifty‐four patients with NMIBC who underwent TURBT and simultaneous systematic random biopsy were included in the analysis. The median age of the patients was 73 years (range, 33–87). The association of clinicopathological characteristics and TERT promoter mutations is shown in Table 1 and Fig. 1. Among the 54 patients, 18 (33.3%) had TERT promoter mutations in NMU; 16 (29.6%) had a TERT C228T mutation; and three (5.6%) had a TERT C250T mutation. There was no difference in age between patients with and without TERT C228T mutation in NMU (P = 0.348). Among 28 patients with TERT C228T‐positive primary tumors, 13 (46.4%) were also TERT C228T‐positive in NMU. On the other hand, among 25 patients with TERT C228T‐negative primary tumors, three (12.0%) were TERT C228T‐positive in NMU. Representative microscopic images for each pathological diagnosis with the TERT C228T mutation are shown in Fig. 2. The frequency of TERT promoter mutations for each biopsy specimen and the primary tumors are shown in Table 2. Of 428 biopsy specimens, the TERT C228T mutation was detected in 9% (31/364) of NU, 27% (4/15) of UD, 50% (9/18) of UD suspicious for CIS, and 58% (18/31) of biopsy‐proven CIS.
Table 1.
Characteristics of 54 patients with NMIBC.
|
Overall (n = 54) |
TERT C228T‐positive in NMU (n = 16) |
TERT C228T‐negative in NMU (n = 38) |
|
|---|---|---|---|
| No. | No. (%) | No. (%) | |
| Gender | |||
| Male | 39 | 13 (33) | 26 (67) |
| Female | 15 | 3 (20) | 12 (80) |
| Age, years | |||
| Median (range) | 73 (33–87) | 72 (59–86) | 73 (33–87) |
| Number of tumors | |||
| Single | 28 | 7 (25) | 21 (75) |
| 2–7 | 25 | 9 (36) | 16 (64) |
| ≥ 8 | 1 | 0 (0) | 1 (100) |
| Tumor diameter | |||
| < 3 cm | 46 | 12 (26) | 34 (74) |
| ≥ 3 cm | 8 | 4 (50) | 4 (50) |
| Prior recurrence rate | |||
| Primary | 43 | 12 (28) | 31 (72) |
| ≤ 1 recurrence/year | 6 | 2 (33) | 4 (67) |
| > 1 recurrence/year | 5 | 2 (40) | 3 (60) |
| Pathological T stage | |||
| pTa | 26 | 6 (23) | 20 (77) |
| pT1 | 25 | 10 (40) | 15 (60) |
| Concurrent CIS | 12 | 4 (33) | 8 (67) |
| ≥ pT2 | 0 | ||
| No malignancy | 0 | ||
| Grade | |||
| G1 | 0 | ||
| G2 | 27 | 8 (30) | 19 (70) |
| G3 | 27 | 8 (30) | 19 (70) |
Fig. 1.

Association of clinical and pathological features and TERT promoter mutations. N/A, not applicable; THP, pirarubicin.
Fig. 2.
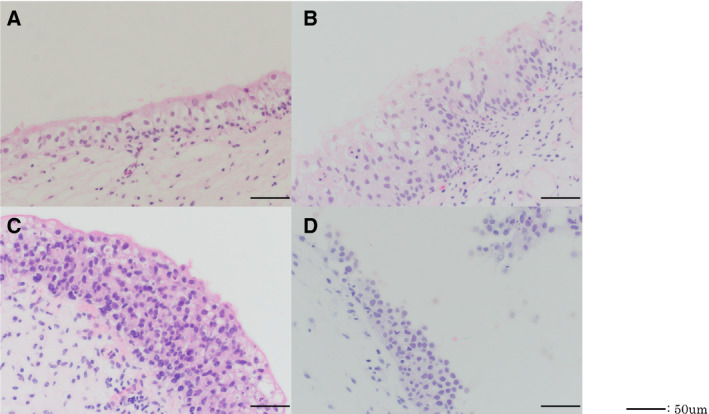
Representative hematoxylin and eosin staining of TERT C228T‐positive lesions. (A) NU. (B) Urothelial dysplasia. (C) Urothelial dysplasia suspicious for CIS. (D) CIS. Black bar indicates 50 μm.
Table 2.
Frequency of TERT promoter mutations in biopsy specimens and primary tumors.
| Biopsy specimens (n = 428) | Primary tumors (n = 53) | |||||
|---|---|---|---|---|---|---|
| NU | UD | UD suspicious for CIS | CIS | pTa or pTis | pT1 | |
| TERT C228T mutation | 9% (31/364) | 27% (4/15) | 50% (9/18) | 58% (18/31) | 52% (15/29) | 54% (13/24) |
| TERT C250T mutation | 1% (4/364) | 0% (0/15) | 0% (0/18) | 0% (0/31) | 10% (3/29) | 0% (0/24) |
3.2. Association of TERT promoter mutations with intravesical tumor recurrence
The median follow‐up period was 3.7 years (range, 0.8–4.8). Based on the European Organisation for Research and Treatment of Cancer (EORTC) recurrence scoring system, 29 (53.7%) patients had a score of 1–4 (intermediate‐low risk), 20 (37.0%) had a score of 5–9 (intermediate‐high risk), and 5 (9.3%) had a score of 10 (high risk). Fifty‐three patients (98.1%) received immediate postoperative intravesical instillation therapy with pirarubicin and 18 (33.3%) received intravesical Bacillus Calmette–Guérin (BCG) induction therapy (Fig. 1). BCG maintenance therapy is not administered in our institution. BCG therapy was recommended for patients with CIS lesions or a pT1 tumor in our institution. During the follow‐up period, 22 (40.7%) patients experienced NMIBC recurrence and five (9.3%) experienced disease progression including muscle‐invasive disease or distant metastasis. There was no significant difference in the bladder recurrence rates among the groups according to the EORTC recurrence risk classification (Fig. 3A). Patients with the TERT C228T mutation in NMU had a significantly greater chance of bladder recurrence compared to that of patients without the TERT C228T mutation in NMU (P = 0.005; Fig. 3B). This association was not observed for the TERT C228T status in primary tumors (P = 0.265; Fig. 3C). When TERT C228T status in NMU was stratified by BCG instillation therapy, patients with TERT C228T mutation in NMU without BCG induction therapy had a significantly worse prognosis for bladder recurrence than patients with TERT C228T mutation with BCG therapy (P = 0.024; Fig. 3D). On the other hand, for patients without TERT C228T mutation in NMU, there was no difference in bladder recurrence between the patients with and without BCG therapy (Fig. 3E).
Fig. 3.
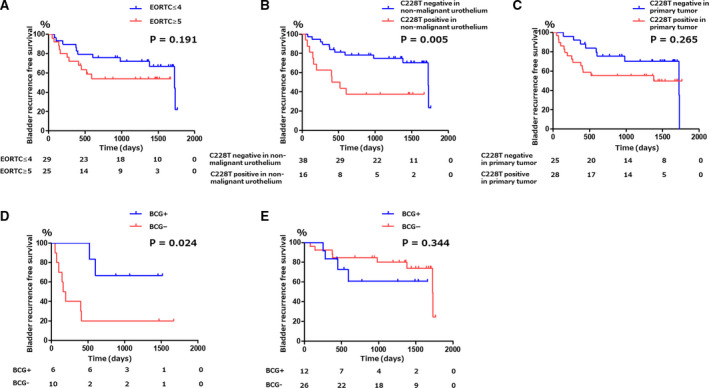
Kaplan–Meier curves for bladder recurrence‐free survival. (A) Stratified by EORTC recurrence score. (B) Stratified by TERT C228T mutation status in NMU. (C) Stratified by TERT C228T mutation status in primary tumors. (D) Stratified by BCG induction therapy for patients with TERT C228T mutation in NMU. (E) Stratified by BCG induction therapy for patients without TERT C228T mutation in nonmalignant urothelium.
Finally, we performed univariate Cox proportional hazard analysis. The number of tumors [hazard ratio (HR): 3.1, 95% confidence interval (CI): 1.3–8.2] and the presence of the TERT C228T mutation in NMU (HR: 3.2, 95% CI: 1.3–7.6) were associated with bladder recurrence (Table 3). Multivariate Cox proportional hazard analysis revealed that the number of tumors (HR: 2.8, 95% CI: 1.2–7.5, P = 0.020) and the presence of the TERT C228T mutation in NMU (HR: 3.1, 95% CI: 1.3–7.6, P = 0.014) were significantly and independently associated with bladder recurrence (multivariate model 1). The TERT C228T mutation was also found to be an independent factor associated with bladder recurrence after adjustment for BCG instillation therapy, EORTC score, and age (P = 0.018; multivariate model 2).
Table 3.
Univariate and multivariate analysis of factors associated with bladder recurrence
| Univariate | Multivariate model 1 | Multivariate model 2 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P‐value | HR | 95% CI | P‐value | HR | 95% CI | P‐value | |
| Age (≥ 73 vs ≤ 72) | 0.6 | 0.2–1.3 | 0.223 | 0.4 | 0.2–1.1 | 0.085 | |||
| pT stage | |||||||||
| pT1 vs pTa | 1.0 | 0.4–2.4 | 0.917 | ||||||
| CIS | |||||||||
| Positive vs negative | 1.4 | 0.5–3.4 | 0.522 | ||||||
| Number of tumors | |||||||||
| Multiple vs solitary | 3.1 | 1.3–8.2 | 0.010 | 2.8 | 1.2–7.5 | 0.020 | |||
| Size | |||||||||
| ≥ 3 cm vs < 3 cm | 2.0 | 0.6–5.4 | 0.250 | ||||||
| EORTC (≥ 5 vs ≤ 4) | 2.1 | 0.9–5.0 | 0.142 | 1.9 | 0.7–5.6 | 0.227 | |||
| 2nd TUR (yes vs no) | 0.9 | 0.3–2.1 | 0.871 | ||||||
| BCG (yes vs no) | 0.9 | 0.3–2.2 | 0.873 | 0.4 | 0.1–1.1 | 0.064 | |||
| TERT C228T in NMU‐positive vs urothelium‐negative | 3.2 | 1.3–7.6 | 0.024 | 3.1 | 1.3–7.6 | 0.014 | 3.3 | 1.2–9.2 | 0.018 |
4. Discussion
In this proof‐of‐concept study, we report for the first time that the TERT C228T mutation was detected in NMU that was pathologically diagnosed as NU or UD, and that the mutation in NMU was associated with bladder recurrence after TURBT in patients with NMIBC. Therefore, analysis for the presence of the TERT C228T mutation in NMU may offer a more precise risk stratification of patients with NMIBC than do current stratifications. Furthermore, a positive result for the TERT C228T mutation in NMU might be a novel indication for BCG instillation therapy. Though certain mutational signatures are associated with patient age at cancer diagnosis [27], no difference was observed in age between patients with and without TERT C228T mutation in NMU.
Malignant tumors evolve from microscopic precursor lesions such as dysplasia; however, there may be cancer‐initiating cells with pathologically normal appearances in tumorigenesis as a consequence of field change caused by exposure to environmental carcinogens [21]. In bladder cancer, Majewski et al. [24] reported that DNA methylation was detected as one of the initial field changes, but little is actually known about the association between field change and TERT promoter mutations. To the best of our knowledge, there are currently no reports regarding clinical outcome for field change and malignant tumors.
Telomeres, which protect the ends of human chromosomes, are shortened by cell division during aging. TERT is a catalytic subunit of telomerase, which lengthens telomeres in DNA strands exceeding the Hayflick limit. In UC, the TERT C228T mutation is more frequently detected than the TERT C250T mutation [20]. Interestingly, the TERT C228T mutation increases TERT expression more than the TERT C250T mutation [16]. TERT promoter mutations may also be detected in urinary liquid biopsies from patients with no obvious evidence of cancer. When detected, this is associated with the development of UC. TERT promoter mutations overcome the proliferative barrier imposed by telomere shortening and thereby play a crucial role early in tumorigenesis by promoting immortalization and genomic instability. TERT promoter mutations are not detected in benign proliferative urothelial lesions [28]. Though these mutations are thought to be present in NMU during early stages of tumor formation prior to pathological change [29], the presence of these mutations has not been proven directly. Taken together, we hypothesize that TERT promoter mutations, especially TERT C228T, exist in urothelium that appears pathologically normal as a field change and play an important role in UC tumorigenesis.
EORTC has developed a scoring system and risk tables, which are widely recommended in various clinical guidelines [3, 5]. However, many researchers have reported that the EORTC risk tables cannot fully stratify risk for both disease recurrence and progression in external validation cohorts of patients with NMIBC [4, 6]. Therefore, a novel tool for improving the current risk stratification system is needed for patients with NMIBC.
There have been several reports regarding the molecular analysis of NMU of patients with UBC [30, 31, 32, 33]. Although field change in DNA methylation or loss of chromosome 9 heterogeneity may initiate bladder tumorigenesis, our study for the first time demonstrated an association between TERT promoter mutations and field change and also showed their clinical utility as prognostic biomarkers for bladder cancer recurrence.
The current study had several limitations, including its small sample size, its retrospective nature, and the fact that maintenance of BCG intravesical therapy was not administered. Because of its retrospective nature, the treatment strategy following TURBT varied, even in patients with similar clinical backgrounds. However, our results indicated that BCG instillation therapy may improve the recurrence rate of UBC after TURBT for patients with the TERT C228T mutation in NMU. Larger‐scale multi‐institutional and prospective studies are needed to validate this finding.
To diagnose concurrent CIS, we performed systematic random biopsies for all patients who had primary UBC and had received TURBT for the first time. However, it remains controversial whether random biopsies should be performed to identify CIS in mucosa that appears normal. An EORTC retrospective review found that 10% of random biopsy specimens were positive and proposed that when concurrent CIS is highly suspected, random biopsies are indicated only in instances of multiple tumors or positive cytology [34]. At our institution, the indication for random biopsy is widely applied to patients with UBC compared with that proposed by clinical guidelines. Our current findings demonstrated significant clinical utility of systematic random biopsies and their analysis for TERT promoter mutations. Although a prospective clinical trial is necessary, the analysis of TERT promoter mutation in NMU might be a novel indication for BCG instillation after TURBT, and the patients with TERT promoter mutation in NMU may benefit from BCG instillation for the prevention of bladder recurrence.
5. Conclusions
The TERT C228T mutation was detected in NU and UD of patients with NMIBC and may thus be a novel and useful prognostic factor for risk stratification of NMIBC. Analysis of systematic random biopsies for TERT promoter mutations in NMU may guide selection of the optimal treatment strategy for patients with NMIBC.
Conflict of interests
The authors declare no conflict of interest.
Author contributions
YH conceptualized the data, involved in formal analysis and methodology, investigated the study, and wrote the original draft. KF conceptualized the study, supervised the data, and wrote, reviewed, and edited the manuscript. SN and EM supervised and analyzed the data. ET, MM, KN, YK, CW, and YI investigated the data. TK, KH, AK, TU, MU, RI, and NN supervised the data.
Supporting information
Table S1. ddPCR assay list.
Acknowledgements
We thank all other researchers in our laboratory. This study was supported by Osaka University Grant and JSPS KAKENHI Grant Number JP 20K18139.
Data accessibility
References
- 1. Babjuk M, Böhle A, Burger M, Capoun O, Cohen D, Compérat EM, Hernández V, Kaasinen E, Palou J, Rouprêt M et al (2017) EAU guidelines on non–muscle‐invasive urothelial carcinoma of the bladder update 2016. Eur Urol 71, 447–461. [DOI] [PubMed] [Google Scholar]
- 2. Prout GR Jr, Barton BA, Griffin PP & Friedell GH(1992) Treated history of noninvasive grade 1 transitional cell carcinoma. The National Bladder Cancer Group. J Urol 148, 1413–1419. [DOI] [PubMed] [Google Scholar]
- 3. Sylvester RJ, van der Meijden AP, Oosterlinck W, Witjes JA, Bouffioux C, Denis L, Newling DWW & Kurth K(2006) Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables a combined analysis of 2596 patients from seven EORTC trials. Eur Urol 49, 466–477. [DOI] [PubMed] [Google Scholar]
- 4. Xylinas E, Kent M, Kluth L, Pycha A, Comploj E, Svatek RS, Lotan Y, Trinh QD, Karakiewicz PI, Holmang S et al (2013) Accuracy of the EORTC risk tables and of the CUETO scoring model to predict outcomes in non‐muscle‐invasive urothelial carcinoma of the bladder. Br J Cancer 109, 1460–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fernandez‐Gomez J, Madero R, Solsona E, Unda M, Martinez‐Piñeiro L, Gonzalez M, Portillo J, Ojea A, Pertusa C, Rodriguez‐Molina J et al (2009) Predicting nonmuscle invasive bladder cancer recurrence and progression in patients treated with Bacillus Calmette‐Guerin the CUETO scoring model. J Urol 182, 2195–2203. [DOI] [PubMed] [Google Scholar]
- 6. Ieda T, Muto S, Shimizu F, Taguri M, Yanada S, Kitamura K, Terai K, Saito K, Ogishima T, Nagata M et al (2016) Development and validation of a novel recurrence risk stratification for initial non‐muscle invasive bladder cancer in Asia. EBioMedicine 12, 98–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cowan ML, Springer S, Nguyen D, Taheri D, Guner G, Mendoza Rodriguez MA, Wang Y, Kinde I, Del Carmen Rodriguez Pena Maria, VandenBussche CJ et al. (2016) Detection of TERT promoter mutations in primary adenocarcinoma of the urinary bladder. Hum Pathol 53, 8–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cowan M, Springer S, Nguyen D, Taheri D, Guner G, Rodriguez MA, Wang Y, Kinde I, VandenBussche CJ, Olson MT et al (2016) High prevalence of TERT promoter mutations in primary squamous cell carcinoma of the urinary bladder. Mod Pathol 29, 511–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Killela PJ, Reitman ZJ, Jiao Y, Bettegowda C, Agrawal N, Diaz LA Jr, Friedman AH, Friedman H, Gallia GL, Giovanella BC et al (2013) TERT promoter mutations occur frequently in gliomas and a subset of tumors derived from cells with low rates of self‐renewal. Proc Natl Acad Sci USA 110, 6021–6026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nguyen D, Taheri D, Springer S, Cowan M, Guner G, Mendoza Rodriguez MA, Wang Y, Kinde I, VandenBussche CJ, Olson MT et al (2016) High prevalence of TERT promoter mutations in micropapillary urothelial carcinoma. Virchows Arch 469, 427–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Palsgrove DN, Taheri D, Springer SU, Cowan M, Guner G, Mendoza Rodriguez MA, Rodriguez Pena MDC, Wang Y, Kinde I, Ricardo BFP et al (2019) Targeted sequencing of plasmacytoid urothelial carcinoma reveals frequent TERT promoter mutations. Hum Pathol 85, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rodriguez Pena MDC, Tregnago AC, Eich M‐L, Springer S, Wang Y, Taheri D, Ertoy D, Fujita K, Bezerra SM, Cunha IW et al(2017) Spectrum of genetic mutations in de novo PUNLMP of the urinary bladder. Virchows Arch 471, 761–767. [DOI] [PubMed] [Google Scholar]
- 13. Zheng X, Zhuge J, Bezerra SM, Faraj SF, Munari E, Fallon JT 3rd, Yang XJ, Argani P, Netto GJ & Zhong M(2014) High frequency of TERT promoter mutation in small cell carcinoma of bladder, but not in small cell carcinoma of other origins. J Hematol Oncol 7, 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kinde I, Munari E, Faraj SF, Hruban RH, Schoenberg M, Bivalacqua T, Allaf M, Springer S, Wang Y, Diaz LA Jr et al (2013) TERT promoter mutations occur early in urothelial neoplasia and are biomarkers of early disease and disease recurrence in urine. Cancer Res 73, 7162–7167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Min J & Shay JW(2016) TERT promoter mutations enhance telomerase activation by long‐range chromatin interactions. Cancer Discov 6, 1212–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chiba K, Lorbeer FK, Shain AH, McSwiggen DT, Schruf E, Oh A, Ryu J, Darzacq X, Bastian BC & Hockemeyer D(2017) Mutations in the promoter of the telomerase gene TERT contribute to tumorigenesis by a two‐step mechanism. Science 357, 1416–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Eich ML, Rodriguez Pena MDC, Springer SU, Taheri D, Tregnago AC, Salles DC, Bezerra SM, Cunha IW, Fujita K, Ertoy D et al (2019) Incidence and distribution of UroSEEK gene panel in a multi‐institutional cohort of bladder urothelial carcinoma. Mod Pathol 32, 1544–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Leão R, Lee D, Figueiredo A, Hermanns T, Wild P, Komosa M, Lau I, Mistry M, Nunes NM, Price AJ et al (2019) Combined genetic and epigenetic alterations of the TERT promoter affect clinical and biological behavior of bladder cancer. Int J Cancer 144, 1676–1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hayashi Y, Fujita K, Matsuzaki K, Matsushita M, Kawamura N, Koh Y, Nakano K, Wang C, Ishizuya Y, Yamamoto Y et al (2019) Diagnostic potential of TERT promoter and FGFR3 mutations in urinary cell‐free DNA in upper tract urothelial carcinoma. Cancer Sci 110, 1771–1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Springer SU, Chen CH, Rodriguez Pena MDC, Li L, Douville C, Wang Y, Cohen JD, Taheri D, Silliman N, Schaefer J et al (2018) Non‐invasive detection of urothelial cancer through the analysis of driver gene mutations and aneuploidy. Elife 7, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Curtius K, Wright NA & Graham TA(2017) An evolutionary perspective on field cancerization. Nat Rev Cancer 18, 19–32. [DOI] [PubMed] [Google Scholar]
- 22. Nault JC, Mallet M, Pilati C, Calderaro J, Bioulac‐Sage P, Laurent C, Laurent A, Cherqui D, , Balabaud C & Zucman‐Rossi J(2013) High frequency of telomerase reverse‐transcriptase promoter somatic mutations in hepatocellular carcinoma and preneoplastic lesions. Nat Commun. 4, 2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shain AH, Yeh I, Kovalyshyn I, Sriharan A, Talevich E, Gagnon A, Dummer R, North J, Pincus L, Ruben B et al (2015) The genetic evolution of melanoma from precursor lesions. N Engl J Med 373, 926–1936. [DOI] [PubMed] [Google Scholar]
- 24. Majewski T, Yao H, Bondaruk J, Chung W, Lee S, Lee JG, Zhang S, Cogdell D, Yang G, Choi W et al (2019) Whole‐organ genomic characterization of mucosal field effects initiating bladder carcinogenesis. Cell Rep [Internet] 26, 2241–2256. [DOI] [PubMed] [Google Scholar]
- 25. Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE & Brookland RK (2017) American Joint Committee on Cancer (2016): AJCC Cancer Staging Manual, 8th edn Springer International Publishing, New York, NY. [Google Scholar]
- 26. Moch H, Humphrey PA, Ulbright TM & Reuter VE (2016) International Agency for Research on Cancer: WHO Classification of Tumours of Urinary System and Male Genital Organs, 4th edn World Health Organization, Geneva. [Google Scholar]
- 27. Alxandrov LB, Nik‐Zainal S, Wedge DC, Aparicio SA, Behjati S, Biankin AV, Bignell GR, Bolli N, Borg A, Børresen‐Dale AL et al (2013) Signatures of mutational processes in human cancer. Nature 500, 15–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Junker K, Boerner D, Schulze W, Utting M, Schubert J & Werner W(2003) Analysis of genetic alterations in normal bladder urothelium. Urology 62, 1134–1138. [DOI] [PubMed] [Google Scholar]
- 29. Gunes C, Wezel F, , Southgate J & Bolenz C (2018) Implications of TERT promoter mutations and telomerase activity in urothelial carcinogenesis. Nat Rev Urol 15, 86–393. [DOI] [PubMed] [Google Scholar]
- 30. Obermann EC, Meyer S, Hellge D, Zaak D, Filbeck T, Stoehr R, Hofstaedter F, Hartmann A & Knuechel R(2004) Fluorescence in situ hybridization detects frequent chromosome 9 deletions and aneuploidy in histologically non‐malignant urothelium of bladder cancer patients. Oncol Rep 11, 745–751. [PubMed] [Google Scholar]
- 31. Chow NH, Cairns P, Eisenberger CF, Schoenberg MP, Taylor DC, Epstein JI & Sidransky D(2000) Papillary urothelial hyperplasia is a clonal precursor to papillary transitional cell bladder cancer. Int J Cancer 89, 514–518. [PubMed] [Google Scholar]
- 32. Hartmann A, Moser K, Kriegmair M, Hofstetter A, Hofstaedter F & Knuechel R (1999) Frequent genetic alterations in simple urothelial hyperplasias of the bladder in patients with papillary urothelial carcinoma. Am J Pathol 154, 721–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. van Oers JM, Adam C, Denzinger S, Stoehr R, Bertz S, Zaak D, Stief C, Hofstaedter F, Zwarthoff EC, van der Kwast TH et al (2006) Chromosome 9 deletions are more frequent than FGFR3 mutations in flat urothelial hyperplasias of the bladder. Int J Cancer 119, 1212–1215. [DOI] [PubMed] [Google Scholar]
- 34. Musser JE, O'Shaughnessy MJ, Kim PH & Herr HW(2015) Bladder biopsy of normal‐appearing mucosa is not helpful in patients with unexplained positive cytology after nonmuscle invasive bladder cancer. J Urol 193, 48–52. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. ddPCR assay list.


