Abstract
Tracheopulmonary complications following placement of a nasogastric (NG) feeding tube are uncommon but can cause significant morbidity and mortality. In this case report, an 83-year-old woman of American Society of Anesthesiologists class IV with underlying pulmonary disease required placement of an NG feeding tube after surgical treatment of primary squamous cell carcinoma of the tongue. Malpositioning of the NG feeding tube into the right pleural space was confirmed by computed tomography. Removal of the NG feeding tube resulted in a tension pneumothorax that necessitated chest tube placement. Because of the difficulty of blind NG feeding tube placement in this patient, the subsequently placed NG feeding tube was successfully positioned with the aid of a video laryngoscope. This case report illustrates the risk of NG feeding tube malpositioning in a nasally intubated patient undergoing head and neck surgery and discusses improvements in techniques for proper NG feeding tube placement.
Keywords: Nasogastric tube complications, Video laryngoscopy, Nasogastric feeding tube, Enteral feeding tube
Placement of a nasogastric (NG) feeding tube is often a straightforward procedure, but the consequences of malpositioning can be devastating.1 NG feeding tubes are frequently placed in intensive care units to establish early alimentation in the critically ill patient.2 This procedure typically occurs in an awake and cooperative patient. Traditional NG feeding tube insertion is performed blindly.3 The tube is inserted through one naris and gently passed into the pharynx. The patient is then asked to swallow, and the swallow reflex carries the tube into the esophagus and down into the stomach.
Intubated patients present a particular challenge for proper positioning of the NG feeding tube.4 Although the presence of a cuffed endotracheal tube (ETT) may seem to be an obstruction that would prevent passage into the lungs, in reality it can make inadvertent tracheopulmonary intubation with the NG feeding tube even more likely. Most modern ETTs have high-volume, low-pressure cuffs that offer minimal resistance to the passage of a narrow-bore enteric feeding tube. The ETT establishes a straight path to the trachea by preventing the closure of the epiglottis and vocal cords. Furthermore, the presence of an ETT with an inflated cuff can compress the esophagus and impede passage of the NG feeding tube.
Anesthesiologists may encounter the necessity for NG tube placement during a variety of head and neck procedures, most commonly for gastric decompression or as a conduit for enteral feeding in critically ill patients postoperatively. This case report highlights the importance of proper positioning of an NG feeding tube, addresses the unique challenges of proper placement in the nasally intubated patient, and discusses improvements in NG feeding tube placement techniques that would eliminate the morbidity associated with malpositioning in the tracheopulmonary system.
CASE DESCRIPTION
An 83-year-old woman with a diagnosis of primary squamous cell carcinoma of the left lateral tongue (T2N2bM0) presented for left partial glossectomy, left modified radical neck dissection including levels I–V, and local rotational flap reconstruction. She was 172 cm tall, weighed 91 kg, and had a calculated body mass index of 32 kg/m2.
Her medical history was significant for chronic obstructive pulmonary disease (GOLD grade 2), coronary artery disease, aortic stenosis, hypertension, hyperlipidemia, depression, diabetes mellitus type 2, mild obstructive sleep apnea, gastroesophageal reflux disease, osteoarthritis, restless leg syndrome, carpal tunnel syndrome, and prior deep vein thrombosis. Her surgical history was significant for a triple vessel coronary artery bypass graft with bioprosthetic aortic valve replacement, total right knee arthroplasty, total left knee arthroplasty, total left shoulder arthroplasty, total right hip arthroplasty, and a lumbar fusion.
On echocardiography, the prosthetic aortic valve showed residual trace insufficiency. Her left ventricle demonstrated moderately increased wall thickness, mild diastolic dysfunction, and an ejection fraction of 65%. Her hypertension was poorly controlled, with typical systolic blood pressures ranging from 160–190 mmHg. In addition, her preoperative electrocardiogram was notable for sinus rhythm, left axis deviation, first-degree atrioventricular block, right bundle branch block, QTc prolongation (513 ms), and occasional premature ventricular contractions. Her hyperlipidemia and gastroesophageal reflux disease were adequately controlled with oral medications. Her diabetes was well controlled with oral medications, with a preoperative HbA1c of 5.9. The patient experienced chronic dyspnea during mild-to-moderate exertion but denied previous use of supplemental oxygen. She had no history of smoking and was noncompliant with her home continuous positive airway pressure machine.
The patient's medications at the time of presentation were albuterol, aspirin, atorvastatin, clopidogrel, diltiazem, glipizide, losartan, hydrochlorothiazide, metoprolol, omeprazole, sertraline, and tiotropium.
The patient acknowledged significant postoperative nausea and vomiting following her total right hip arthroplasty completed approximately 6 months prior to presentation to the oral and maxillofacial surgeon. She denied any complications from prior anesthetics.
Preoperative physical examination of this American Society of Anesthesiologists IV patient's airway was notable for an edentulous mouth, Mallampati II score, full temporomandibular joint range of motion, and full neck mobility. Of note, significant airway edema was expected because of the nature and location of the planned surgical procedures. Given the anticipated lengthy surgical time, her age, and her underlying pulmonary disease, the patient was deemed to be at high risk for postoperative pulmonary complications. The anesthesia plan included postoperative intubation for 12–48 hours or until the patient could maintain a patent airway.
In the preoperative holding area, a 20-gauge intravenous catheter was placed in the patient's left hand by the nursing staff followed by administration of midazolam (2 mg). Upon arrival to the operating room, standard American Society of Anesthesiologists monitors were placed in the usual fashion consisting of a 5-lead electrocardiogram, noninvasive blood pressure cuff on the left upper arm, pulse oximeter on the right fourth finger, and a bispectral index monitor applied to her forehead. The end-tidal carbon dioxide monitor was attached to the anesthesia circuit, and an axillary temperature probe was placed in the left axilla after induction. The patient was induced with intravenous fentanyl (100 μg) and lidocaine (50 mg), followed by propofol (70 mg) infused over 3 minutes. Continuous infusions of propofol (120 μg/kg/min) and remifentanil (0.1 μg/kg/min) were started. The nares were prepared with oxymetazoline 0.5% topical spray, and the nasal passages were lubricated and serially dilated up to a size 30 French nasopharyngeal airway. A Cormack-Lehane grade 1 laryngoscopic view was obtained upon video laryngoscopy with a C-MAC D blade (Storz, Tuttlingen, Germany). The patient's trachea was intubated with a 7.0 Mallinckrodt nasal RAE cuffed ETT (Covidien, Boulder, Colo) through the right naris. Magill forceps were used to guide the ETT into the glottis, and the cuff was subsequently inflated to prevent any leak. Proper position of the ETT was confirmed by bilateral chest rise, the presence of equal and bilateral breath sounds, and capnography. In addition, a 22-gauge arterial line was placed in the right radial artery after induction. General anesthesia was maintained with a combination of continuous propofol infusion and desflurane titrated to bispectral index monitor values between 40 and 60. The surgical team completed the planned surgical procedures without intraoperative complications in approximately 16 hours.
At the conclusion of the surgical procedures, the decision was made to place an NG feeding tube to allow for early initiation of enteral feeding in the surgical intensive care unit. The length of insertion was estimated to be 56 cm, as established by measuring the distance from the tip of the nose to the earlobe and then to the xiphoid process. While the patient remained nasotracheally intubated under general anesthesia, a senior surgical resident lubricated the distal end of a standard tip, weighted, 12 French Kangaroo NG feeding tube (Covidien, Mansfield, Mass) and advanced it through the left naris with the wire insertion stylet in place. Notably, there was no resistance encountered during advancement of the NG feeding tube to the estimated length. Proper placement was first assessed with low continuous suction, but little return was observed. Next, a bulb syringe was used to assess position by air insufflation, but the sounds heard on auscultation were ambiguous. The patient was transferred to the postanesthesia care unit, where an anteroposterior (AP) chest radiograph was obtained that showed a malpositioned NG feeding tube coursing through the left mainstem bronchus with the tip located in the left lower lung lobe (Figure 1). The NG feeding tube was immediately removed at the bedside in the postanesthesia care unit. The patient remained intubated and deeply sedated while a second senior surgical resident inserted a new NG feeding tube with the aid of a wire insertion stylet, which was secured at 56 cm. A second bedside AP chest radiograph was obtained.
Figure 1.
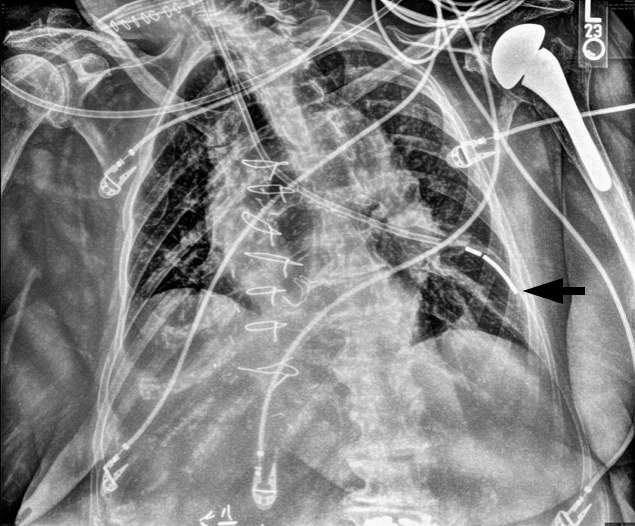
First anteroposterior chest radiograph demonstrating the weighted enteric tube coursing through the left mainstem bronchus with the distal tip projecting into the left lower lung field (arrow).
Following placement of the second NG feeding tube, the patient remained intubated and sedated while transferred to the surgical intensive care unit. Upon review of the most recent AP chest radiograph, there was uncertainty regarding the position of the NG feeding tube (Figure 2). A lateral chest radiograph was obtained, but it was not possible to adequately identify the NG feeding tube on the image. Over the next 24 hours, 2 additional attempts were made to reposition the NG feeding tube by senior surgical intensive care unit residents with no demonstrable change in NG feeding tube position upon radiographic assessment.
Figure 2.
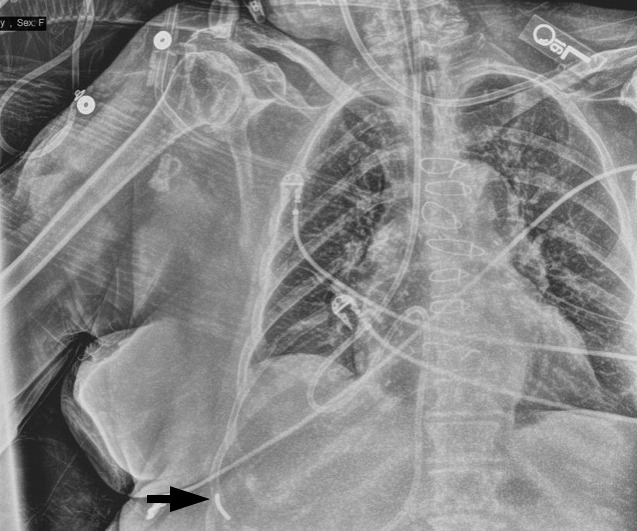
Subsequent anteroposterior chest radiograph demonstrating ambiguous placement of the weighted enteric tube with the distal tip denoted (arrow).
Forty-eight hours after the conclusion of the surgical procedure, the surgical intensive care unit team wanted to initiate enteral feeding. The positioning of the NG feeding tube was again evaluated by an AP chest radiograph (Figure 3). This image demonstrated a small pleural effusion and appeared to show the NG feeding tube located in the right pleural space.
Figure 3.
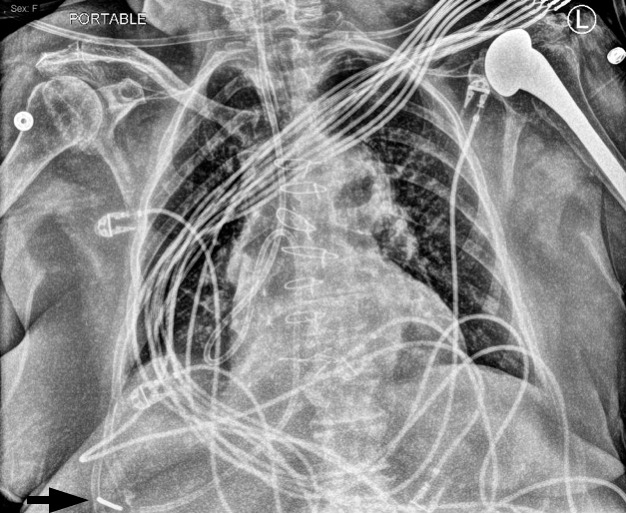
Anteroposterior chest radiograph demonstrating the weighted enteric tube with likely termination in the right pleural space (arrow). In addition, a small pleural effusion can be seen.
At this point, the team obtained a chest computed tomography scan to definitively clarify the position of the tube (Figure 4). The computed tomography scan demonstrated that the NG feeding tube had traveled through the right mainstem bronchus into the right lower lobe, perforated through the lung parenchyma and the pleural membrane, and looped in the posterior pleural space of the right thorax. It is important to note that enteral feeds were never initiated while the NG tube was malpositioned. The cardiothoracic surgery team was consulted for further evaluation of the patient. They prepared for urgent placement of a chest tube drain in anticipation of a possible pneumothorax following bedside removal of the NG feeding tube. As expected, the patient developed a tension pneumothorax upon removal of the NG tube, and a therapeutic chest tube drain was placed immediately. Over the next 24 hours, 300 mL of fluid was collected, with minimal improvement in the patient's respiratory status.
Figure 4.
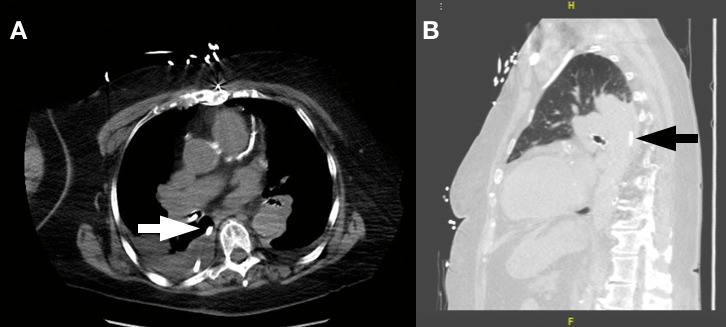
(A) Axial slice of the computed tomography scan showing the radiopaque enteric tube positioned in the right bronchus and looping into the posterior pleural space (arrow). (B) Sagittal slice of the same computed tomography scan with a lung filter demonstrating the enteric tube lying in the posterior pleural space with surrounding lung field atelectasis and effusion (arrow).
The patient continued to fail extubation criteria and was taken to the operating room for a tracheostomy on postoperative day 5. The nasal ETT was removed after establishing and securing the surgical airway with a cuffed tracheostomy tube. While in the operating room, a new 12 French Kangaroo weighted-tip NG feeding tube was placed with the aid of a video laryngoscope (Glidescope, Verathon, Wash). The NG feeding tube was passed through the left naris and into the pharynx until it came into view on the laryngoscope monitor. Upon advancement, the NG feeding tube was observed to passively follow a direct path into the glottis despite manual manipulation efforts to redirect it into the esophagus. The NG tube was withdrawn into the hypopharynx, and Magill forceps were used to successfully redirect the tip of the NG tube posteriorly into the esophagus. Proper position was then confirmed on an AP chest radiograph.
The chest tube drain was removed on postoperative day 12, and the NG feeding tube was removed on postoperative day 15, at which point the patient could tolerate intake by mouth. The patient's tracheostomy was changed to a cuffless tube on postoperative day 17, and she was subsequently decannulated on postoperative day 21, at which point she was able to maintain acceptable hemodynamic, oxygenation, and ventilation parameters unaided. The patient was discharged to a subacute rehabilitation facility on postoperative day 22.
DISCUSSION
Nasogastric tubes are flexible, hollow-bore tubes that are passed through the nose and into the stomach, duodenum, or jejunum for the following indications: enteral feeding, medication administration, stomach lavage, or gastric decompression.5 The widely accepted gold standard for confirming the proper position of any type of NG tube is an AP chest radiograph.5
NG tubes are made of polyvinylchloride, polyurethane, or silicone.5 The type of NG tube depends on the particular indication for use.5 Sump-style tubes made from polyvinylchloride are more rigid and have a larger outer diameter.5 Their dual-lumen tube design allows for effective gastric decompression, but the size and rigidity of the tube can cause significant irritation and increase the risk of aspiration if left in place for more than 48 hours.5 Proper sump-style tube position is confirmed clinically by the return of gastric contents following application of low-continuous suction. Enteral feeding tubes are narrower and more flexible.5 The thin, collapsible walls do not permit effective suctioning, but they can be left in place for up to 4–6 weeks.5 In addition, both tube styles have differing target sites. Sump-style tubes must be placed with the distal tip in the stomach to allow for gastric decompression. Enteral feeding tubes can be placed with the distal tip in the stomach, but placement in the duodenum or beyond is more common to effectively reduce the risk of aspiration.6 NG tube tips can be weighted or nonweighted; the weighted tip allows the tube to be carried more effectively into the gastrointestinal tract via peristaltic motion and gravity.7,8 In addition, the design of modern NG tubes demonstrates improved safety aspects. For example, polyurethane tubes do not stiffen or degrade in vivo, thus reducing the risk of gastric perforation.8
There are both absolute and relative contraindications to NG tube placement. Absolute contraindications to NG tube placement include patients with a bleeding diathesis, esophageal varices, esophageal strictures, or severe mid-face trauma, which carries a risk of intracranial insertion in the presence of a basilar skull fracture.5 Relative contraindications to NG tube placement include recent nose, pharyngeal, or esophageal surgery or any prior surgery involving the esophagus or stomach that may alter the local anatomy, such as esophagectomy, hiatal hernia repair, or gastric bypass.5
In head and neck surgery, a sump-type NG or orogastric tube is commonly used to evacuate blood and other surgical irrigating fluids that may have passed into the stomach during the procedure.5 Continuous low-pressure suction is used in these cases to evacuate stomach contents to diminish postoperative nausea and vomiting and reduce the risk of aspiration. Nasogastric feeding tubes are placed in critically ill patients with compromised oral intake to allow for adequate nutrition.
Traditionally, NG feeding tubes are placed blindly in awake patients.3 While the first-pass success of blind NG tube placement in orally intubated patients ranges from 34–60%,9–11 the success rate of NG tube placement in nasally intubated patients is unknown. Investigators have identified a number of techniques to improve blind placement of NG tubes. Anatomically, the esophagus is a collapsed cylinder. An NG tube must pass posterior or lateral to the glottis to enter the esophagus. Many techniques, such as the use of intubating stylets,12 guidewires,11 frozen NG tubes,13 and slit ETTs,9 aim to encourage posterior positioning of the NG tube. Additional techniques, such as a lateral head turn,10 reverse Sellick's maneuver13 (ie, anterior displacement of the trachea), or neck flexion with lateral neck pressure,11 aim to make the posterolateral path to the esophagus more direct. Each of the above techniques has been shown to achieve a higher rate of first-pass esophageal intubation in comparison with unaided, blind NG tube insertion. There is no consensus regarding the best of the blind techniques, and furthermore, none is foolproof.
AP chest radiography remains the gold standard for confirmation of proper NG tube position.5 The radiograph must be interpreted carefully, with the NG tube being noted to follow the course of the esophagus and avoid the contours of the trachea and bronchi, clearly bisect the carina or bronchi, cross the diaphragm in the midline, and curve to the left as it enters the body of the stomach below the diaphragm.14 In many cases of complications from NG tube malpositioning, the AP chest radiograph is simply misinterpreted.14
In some countries, pH measurement of obtained aspirate is the primary means of confirming the correct positioning of the NG tube.14 Other methods exist (measurement of aspirate bilirubin level8 and lipase activity,15 capnography,16 and ultrasound17) but are used much less widely. Although commonly relied upon as an initial test of gastric placement, air insufflation is often misleading.5
The usual methods of confirming NG tube position, including AP chest radiography, are secondary tests, meaning that the tests do not prevent malpositioning of the NG tube but rather only indicate when such an event has already occurred. Tracheopulmonary malpositioning of the NG tube occurs in approximately 2% of placement attempts overall.18 If the NG tube is malpositioned in the tracheopulmonary system on the first attempt, 36% of these patients may experience a significant tracheopulmonary complication, such as pneumothorax, even if the misplaced NG tube is identified immediately.4
Indeed, in Rassias's4 prospective study of ICU patients who experienced tracheopulmonary malpositioning of NG tubes, all patients received chest radiographs, but the radiographs were misinterpreted in 2 of the 14 cases. Bedside chest radiographs are convenient and have become the mainstay for monitoring the respiratory status of hospital patients, especially those in intensive care units. Unfortunately, proper positioning of the critically ill patient for chest radiographic imaging can be challenging. Poor patient positioning results in poor image quality, increasing the risk of misinterpretation.19
Capnometry,20 electromagnetic guidance,21 fluoroscopy,22 and laryngoscopy23,24 have each been used as a means of primary confirmation of esophageal intubation with NG tubes. Capnometry used a colorimetric device that indicates the presence of CO2. Thus, a color change during NG tube insertion indicates tracheopulmonary malpositioning. The early detection of CO2 allows the NG tube to be withdrawn from the trachea or mainstem bronchus and repositioned before it can perforate into the pleural space and cause a significant complication. Capnometry is especially useful when traditional laryngoscopy is impossible.25 Electromagnetic guidance systems include a specialized transmitting stylet, a receiver, and a video monitor, which provides real-time tracking of the NG tube during insertion. Deflections into a right or left mainstem bronchus, coiling, or other malpositioning can be visually assessed on the monitor. Fluoroscopy can guide placement of the NG tube in special cases, such as with postlaryngectomy patients. Direct or video laryngoscopy allows the clinician to visualize NG tube passage into the esophagus. Each of these 4 techniques is distinguished by the ability to identify proper NG tube positioning in the esophagus as the tube is inserted, thus eliminating the possibility of inadvertent NG feeding tube malpositioning in the tracheopulmonary system.
There have been a few small-scale studies reporting on the efficacy of NG tube insertion using video laryngoscopy. Moharari et al24 reported an 85% first-pass success rate of NG tube placement using a Glidescope and Magill forceps in 40 orally intubated and sedated patients.24 Similarly, Kim et al23 reported a 100% first-pass success rate of NG tube placement using Glidescope and modified Magill forceps in a total of 35 orally intubated and sedated patients.22 In a comparative study, Appukutty9 reported on the efficacy of 3 different NG intubating aids for blind NG tube placement in orally intubated patients. Most notable was the protocol described in that study that allowed for use of a laryngoscope with Magill forceps as a rescue technique after the 2 failed attempts at NG tube placement.
Multiple factors may have contributed to the malpositioning of the NG feeding tube in this case. For instance, the patient was elderly, nasally intubated, and generally anesthetized or deeply sedated. Surgical alteration of local oropharyngeal anatomy with the resultant edema may have complicated proper NG tube positioning. Placement of the NG tube prior to the start of the surgical procedure along with the grade 1 view on video laryngoscopy may have provided better conditions for successful placement of the NG tube than were encountered at the conclusion of the surgical procedure and beyond. Retrospectively, blind placement could have been avoided using one of the aforementioned modalities and should have not been repeated again after the first failure. In addition, difficulty in reading the AP chest radiographs prolonged the amount of time that the NG tube remained incorrectly positioned in the patient's pleural space.
In this case, video laryngoscopy and manipulation with Magill forceps ensured that the NG feeding tube passed into the esophagus. Today, video laryngoscopes are essentially omnipresent in hospital operating rooms. Anesthesiologists' familiarity with video laryngoscopy renders it a safe, efficient, and effective method for placing NG tubes in nasally intubated patients. Given the likelihood of complications even when a misplaced NG tube is recognized promptly, blind placement of NG tubes in nasally intubated patients is not recommended. Primary utilization of video laryngoscopy definitively helps to avoid the complications caused by tracheopulmonary malpositioning.
CONCLUSION
Anesthesiologists must be aware that all nasally intubated patients are at high risk for tracheopulmonary malpositioning of NG feeding tubes. Furthermore, every anesthesia provider has a responsibility to contribute to patient safety by ensuring proper NG tube placement in the head and neck surgery patient. Therefore, use of video laryngoscopy is recommended to visualize and assist with proper NG tube placement.
REFERENCES
- 1.Odocha O, Lowery RC, Mezghebe HM, Siram SM, Warner OG. Tracheopleuropulmonary injuries following enteral tube insertion. J Natl Med Assoc. 1988;81:275–281. [PMC free article] [PubMed] [Google Scholar]
- 2.Blumenstein I, Shastri YM. Stein. Gastroenteric tube feeding: techniques, problems and solutions. World J Gastroenterol. 2014;20:8505–8524. doi: 10.3748/wjg.v20.i26.8505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Metheny NA, Stewart BJ, Mills AC. Blind insertion of feeding tubes in intensive care units: a national survey. Am J Crit Care. 2012;21(5):352–360. doi: 10.4037/ajcc2012549. [DOI] [PubMed] [Google Scholar]
- 4.Rassias AJ, Ball PA, Corwin HL. A prospective study of tracheopulmonary complications associated with the placement of narrow-bore enteral feeding tubes. Crit Care. 1998;2:25–28. doi: 10.1186/cc120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hodin RA, Bordeianou L. Inpatient placement and management of nasogastric and nasoenteric tubes in adults. In: Collins KA, editor. UpToDate. Waltham, MA: UpToDate Inc; Available at: https://www.uptodate.com Accessed April 9, 2019. [Google Scholar]
- 6.Jiyong J, Tiancha H, Huiqin W, Jingfen J. Effect of gastric versus post-pyloric feeding on the induce of pneumonia in critically ill patients: observations from traditional and Bayesian random-effects meta-analysis. Clin Nutr. 2013;32:8–15. doi: 10.1016/j.clnu.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 7.Dobbie RP, Hoffmeister JA. Continuous pump-tube enteric hyperalimentation. Nutrition. 1998;14(3):332–339. doi: 10.1016/s0899-9007(97)00466-8. [DOI] [PubMed] [Google Scholar]
- 8.Pillai JB, Vegas A, Brister S. Thoracic complications of nasogastric tube: review of safe practice. Interact Cardiovasc Thorac Surg. 2005;4:429–433. doi: 10.1510/icvts.2005.109488. [DOI] [PubMed] [Google Scholar]
- 9.Appukutty J, Shroff PP. Nasogastric tube insertion using different techniques in anesthetized patients: a prospective, randomized study. Anesth Analg. 2009;109:832–835. doi: 10.1213/ane.0b013e3181af5e1f. [DOI] [PubMed] [Google Scholar]
- 10.Bong CL, Macachor JD, Hwang NC. Insertion of the nasogastric tube made easy. Anesthesiology. 2004;101:266. doi: 10.1097/00000542-200407000-00058. [DOI] [PubMed] [Google Scholar]
- 11.Kirtania J, Ghose T, Garai D, Ray S. Esophageal guidewire-assisted nasogastric tube insertion in anesthetized and intubated patients: a prospective randomized controlled study. Anesth Analg. 2012;114:343–348. doi: 10.1213/ANE.0b013e31823be0a4. [DOI] [PubMed] [Google Scholar]
- 12.Tsai YF, Luo CF, Illias A, Lin CC, Yu HP. Nasogastric tube insertion in anesthetized and intubated patients: a new and reliable method. BMC Gastroenterol. 2012;12:99. doi: 10.1186/1471-230X-12-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mandal M, Karmakar A, Basu SR. Nasogastric tube insertion in anaesthetised, intubated adult patients: a comparison between three techniques. Indian J Anaesth. 2018;62:609–615. doi: 10.4103/ija.IJA_342_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Healy F, Lyman B. Irvine, CA: Patient Safety Movement Foundation; 2018. Actionable Patient Safety Solution (APSS) #15: Nasogastric Feeding and Drainage Tube Placement and Verification. Available at: https://patientsafetymovement.org/ Accessed April 4, 2019. [Google Scholar]
- 15.Anderson O, Reuben C, Harbinson M, Hanna GB. Development and validation of a lipase nasogastric tube position test. BMJ Open Gastroenterol. 2016;3:e000064. doi: 10.1136/bmjgast-2015-000064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Erzincanli S, Zaybak A, Güler A. Investigation of the efficacy of colorimetric capnometry method used to verify the correct placement of the nasogastric tube. Intensive Crit Care Nurs. 2017;38:46–52. doi: 10.1016/j.iccn.2016.08.005. [DOI] [PubMed] [Google Scholar]
- 17.Zatelli M, Vezzali N. 4-Point ultrasonography to confirm the correct position of the nasogastric tube in 114 critically ill patients. J Ultrasound. 2017;20:53–58. doi: 10.1007/s40477-016-0219-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sparks DA, Chase DM, Coughlin LM, Perry E. Pulmonary complications of 9931 narrow-bore nasoenteric tubes during blind placement: a critical review. J Parenter Enteral Nutr. 2011;35:625–629. doi: 10.1177/0148607111413898. [DOI] [PubMed] [Google Scholar]
- 19.Eisenhuber E, Schaefer-Prokop CM, Prosch H, Schima W. Bedside chest radiography. Respir Care. 2012;57:427–443. doi: 10.4187/respcare.01712. [DOI] [PubMed] [Google Scholar]
- 20.Meyer P, Henry M, Maury E, Baudel JL, Guidet B, Offenstadt G. Colorimetric capnography to ensure correct nasogastric tube position. J Crit Care. 2009;24:231–5. doi: 10.1016/j.jcrc.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 21.Bourgalt AM, Aguirre L, Ibrahim J. Cortrak-assisted feeding tube insertion: a comprehensive review of adverse events in the MAUDE database. Am J Crit Care. 2017;26:149–156. doi: 10.4037/ajcc2017369. [DOI] [PubMed] [Google Scholar]
- 22.Kozin ED, Remenschneider AK, Cunnane ME, Deschler DG. Otolaryngologist-assisted fluoroscopic guided nasogastric tube placement in the postoperative laryngectomy patient. Laryngoscope. 2014;124:916–920. doi: 10.1002/lary.24560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim HJ, Park SI, Cho SY, Cho MJ. The GlideScope with modified Magill forceps facilitates nasogastric tube insertion in anesthetized patients: a randomized clinical study. J Int Med Res. 2018;46:3124–3130. doi: 10.1177/0300060518772719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moharari RS, Fallah AH, Khajavi MR, Khashayar P, Lakeh MM, Najafi A. The Glidescope facilitates nasogastric tube insertion: a randomized clinical trial. Anesth Analg. 2010;110:115–118. doi: 10.1213/ANE.0b013e3181be0e43. [DOI] [PubMed] [Google Scholar]
- 25.Kalava A, Clark K, McIntye J, Yarmush J, Lizardo T. Mistaken endobronchial placement of a nasogastric tube during mandibular fracture surgery. Anesth Prog. 2015;62:114–117. doi: 10.2344/13-00021R1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]


