Key Points
Question
What is the clinical antitumor activity and safety of dostarlimab for patients with deficient mismatch repair endometrial cancer?
Findings
In this nonrandomized phase 1 clinical trial, the confirmed objective response rate was 42%; 13% of patients had a confirmed complete response, and 30% of patients had a confirmed partial response. Anemia (3%), colitis (2%), and diarrhea (2%) were the most common grade 3 or higher treatment-related adverse events.
Meaning
Dostarlimab was associated with clinically meaningful and durable antitumor activity with an acceptable safety profile for patients with deficient mismatch repair endometrial cancers that have progressed after prior platinum-based chemotherapy.
Abstract
Importance
Deficient mismatch mutation repair mechanisms may sensitize endometrial cancers to anti–programmed death 1 (PD-1) therapies. Dostarlimab (TSR-042) is an investigational anti–PD-1 antibody that binds with high affinity to the PD-1 receptor.
Objective
To assess the antitumor activity and safety of dostarlimab for patients with deficient mismatch repair endometrial cancer.
Design, Setting, and Participants
This ongoing, open-label, single-group, multicenter study began part 1 on March 7, 2016, and began enrolling patients with deficient mismatch mutation repair endometrial cancer on May 8, 2017. Median follow-up was 11.2 months (range, 0.03 [ongoing] to 22.11 [ongoing] months; based on radiological assessments). Statistical analysis was performed July 8 to August 9, 2019.
Interventions
Patients received 500 mg of dostarlimab intravenously every 3 weeks for 4 doses, then 1000 mg every 6 weeks until disease progression, treatment discontinuation, or withdrawal.
Main Outcomes and Measures
The primary end point was objective response rate and duration of response by blinded independent central review using Response Evaluation Criteria in Solid Tumors, version 1.1.
Results
As of the data cutoff, 104 women (median age, 64.0 years [range, 38-80 years]) with deficient mismatch mutation repair endometrial cancers were enrolled and treated with dostarlimab. Of these, 71 had measurable disease at baseline and at 6 months or more of follow-up and were included in the analysis. There was a confirmed response in 30 patients (objective response rate, 42.3%; 95% CI, 30.6%-54.6%); 9 patients (12.7%) had a confirmed complete response, and 21 patients (29.6%) had a confirmed partial response. Responses were durable; the median duration of response was not reached (median follow-up was 11.2 months). The estimated likelihood of maintaining a response was 96.4% at 6 months and 76.8% at 12 months. Anemia (3 of 104 [2.9%]), colitis (2 of 104 [1.9%]), and diarrhea (2 of 104 [1.9%]) were the most common grade 3 or higher treatment-related adverse events.
Conclusions and Relevance
In this nonrandomized trial, dostarlimab was associated with clinically meaningful and durable antitumor activity with an acceptable safety profile for patients with deficient mismatch mutation repair endometrial cancers after prior platinum-based chemotherapy.
Trial Registration
ClinicalTrials.gov identifier: NCT02715284
This open-label phase 1 study assesses the antitumor activity, tolerability, and safety of dostarlimab for patients with recurrent or advanced deficient mismatch repair endometrial cancer that has progressed after platinum-containing chemotherapy.
Introduction
Approximately 15 000 patients in the United States and 11 000 patients in the European Union are diagnosed annually with either advanced or recurrent endometrial cancer (EC).1 Early-stage EC can be successfully treated by surgery alone or surgery with adjuvant radiotherapy or chemotherapy (usually platinum-based doublet chemotherapy). The prognosis for patients with a diagnosis of advanced or recurrent EC is poor, and, to our knowledge, there are no accepted consensus-based guidelines for standard of care after the disease progresses while undergoing or after treatment with a platinum-containing regimen. Patients in this setting generally receive salvage care with single-agent chemotherapy or hormone therapy, with limited clinical activity; response rates range from 7% to 14%, and median overall survival (OS) is less than 1 year.2,3,4,5,6
Endometrial cancer is a tumor type associated with high rates of the microsatellite instability–high condition and DNA mismatch repair–deficiency (MSI-H/dMMR). A 2017 report by Le et al7 evaluated 12 019 tumor samples, representing 32 distinct tumor types for MSI-H/dMMR, and identified EC as one of the cancers with the highest rate of MSI-H/dMMR (approximately 30%), varying by EC histologic type and tumor grade.7,8 These results confirmed preliminary data from the Cancer Genome Atlas Research Network that identified 34% of cases of EC as MSI-H and 40% of cancers with endometrioid histologic characteristics as MSI-H.9 Although some reports have found MSI-H/dMMR EC to be exclusively type I (endometrioid histologic characteristics), there are reports that have found type II EC (especially serous and clear cell histologic characteristics) can also be MSI-H/dMMR.10,11 Because of their inability to repair DNA replication errors, MSI-H/dMMR tumors are associated with a 100-fold to 1000-fold increase in mutation rates and express high levels of neoantigens, making the tumor immunogenic7; patients with MSI-H/dMMR tumors may represent a population primed to respond to anti–programmed death 1 (PD-1) and anti–programmed death-ligand 1 (PD-L1) agents.12,13
Dostarlimab (TSR-042) is an investigational humanized anti–PD-1 immunoglobulin G4 monoclonal antibody that binds with high affinity to the PD-1 receptor and effectively blocks the interaction with PD-L1 and PD-L2.14 The GARNET trial (NCT02715284) was designed to assess the safety, tolerability, and antitumor activity of dostarlimab monotherapy for patients with advanced solid tumors. Here, we report preliminary data from patients with recurrent or advanced dMMR EC with disease progression after treatment with a platinum-containing chemotherapy regimen.
Methods
Study Design and Conduct
This ongoing multicenter, open-label, single-group, multicohort study evaluating the safety and efficacy of dostarlimab monotherapy that began March 7, 2016, was conducted in 2 parts. Part 1 was a dose-escalation study to evaluate weight-based doses of dostarlimab monotherapy. Part 2A was an extension of part 1 to evaluate the safety of non–weight-based fixed doses of dostarlimab. Part 2B enrolled patients into 4 expansion cohorts based on tumor type and mutation status (cohort A1, dMMR EC; cohort A2, proficient MMR EC; cohort E, non–small cell lung cancer; and cohort F, MSI-H/dMMR nonendometrial solid tumors) to assess the antitumor activity and safety of dostarlimab. Here, we report a prespecified analysis of one of the expansion cohorts with patients with recurrent or advanced dMMR EC (cohort A1) that has progressed after treatment with a platinum-containing chemotherapy regimen. Patients provided written informed consent. The trial was performed in accordance with the principles of the Declaration of Helsinki,15 Good Clinical Practices, and all local laws. Part 2B of the study was overseen by an independent data and safety monitoring committee. The study protocol (Supplement 1) and/or other relevant documents received approval by the institutional ethics committee, institutional review board, and/or relevant competent authorities at each site (eAppendix in Supplement 2).
Eligibility Criteria
Eligible patients were aged 18 years or older with histologically or cytologically proven recurrent or advanced EC with measurable lesion(s) per Response Evaluation Criteria in Solid Tumors, version 1.1 (RECIST v1.1). Patients could be screened on the basis of local MSI and/or MMR testing results, including dMMR as assessed by immunohistochemistry or MSI-H as assessed by polymerase chain reaction or next-generation sequencing performed in a certified local laboratory. The protocol was amended on May 10, 2019, to use only the results of the immunohistochemistry MMR test for classifying patients. This analysis, as prespecified, is based on patients who were identified by local immunohistochemistry testing as having dMMR tumors. Patients must have demonstrated disease progression while undergoing or after platinum-based doublet chemotherapy and should have received no more than 2 lines of therapy for advanced or recurrent disease. Full eligibility criteria are provided in the study protocol (Supplement 1).
Treatment, End Points, Assessments, and Safety
All patients with dMMR EC were treated with a 30-minute infusion of intravenous dostarlimab, 500 mg, once every 3 weeks for 4 doses, then 1000 mg once every 6 weeks until disease progression, treatment discontinuation due to toxic effects, or patient withdrawal of consent. The primary objective of this analysis was to evaluate the antitumor activity of dostarlimab in patients with recurrent or advanced dMMR EC, with the assessment of the objective response rate (ORR), defined as the proportion of patients with confirmed complete or partial response by blinded independent central review (BICR) using RECIST v1.1, and duration of response (DOR), defined as the time from first documented evidence of complete or partial response until the first documented sign of disease progression or death from any cause, whichever occurred first. Radiographic evaluations were conducted at week 12 after the first dose of dostarlimab, then every 6 weeks (±10 days) or as clinically indicated until month 12, and then every 12 weeks thereafter. Secondary end points included the disease control rate, defined as the proportion of patients with an objective response or stable disease lasting 12 weeks or longer based on BICR using RECIST v1.1; immune-related ORR (irORR) and immune-related DOR (irDOR) based on investigator assessment using immune-related RECIST (irRECIST); progression-free survival (PFS), defined as the time from the first dose of study medication to the first documented disease progression based on BICR using RECIST v1.1; immune-related PFS (irPFS) based on investigator assessment using irRECIST; and OS, defined as the time from the date of the first dose of study medication to the date of death from any cause. Adverse events (AEs) were coded according to the Medical Dictionary for Regulatory Activities, version 20.016 and graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.03.17
Statistical Analysis
Statistical analysis was performed July 8 to August 9, 2019. Demographic characteristics, baseline characteristics, safety, and efficacy results were summarized descriptively. All patients with dMMR EC who received at least 1 dose of dostarlimab were included in the safety analysis. All patients with dMMR EC who received at least 1 dose of dostarlimab had at least 1 BICR-confirmed measurable lesion at baseline and had the opportunity to be followed up for at least 6 months as of the data cutoff date were included in the efficacy analysis, regardless of whether the patient had a postbaseline tumor assessment.
Point estimates and exact Clopper-Pearson 95% CIs were provided for ORR and irORR; DOR, irDOR, PFS, irPFS, and OS were evaluated with the Kaplan-Meier method. Patients who did not achieve a confirmed response were excluded from the DOR and irDOR analysis, and those who did not experience a PFS, irPFS, or OS event were censored at their last assessment. Statistical analyses were performed with SAS, version 9.4 (SAS Institute Inc).
Cohort A1 was designed as a single-stage cohort, with no interim futility analysis planned before 65 patients were enrolled. The sample size of 65 was determined to have 92% power to rule out an ORR of 20% or less (null hypothesis) when the true ORR is 40% at the 2.5% type I error rate (1-sided). The results presented here are from the prespecified analysis based on the original power calculations. Enrollment in this cohort has been extended based on encouraging clinical activity; the results from the full cohort will be presented when data are mature.
Results
Patients
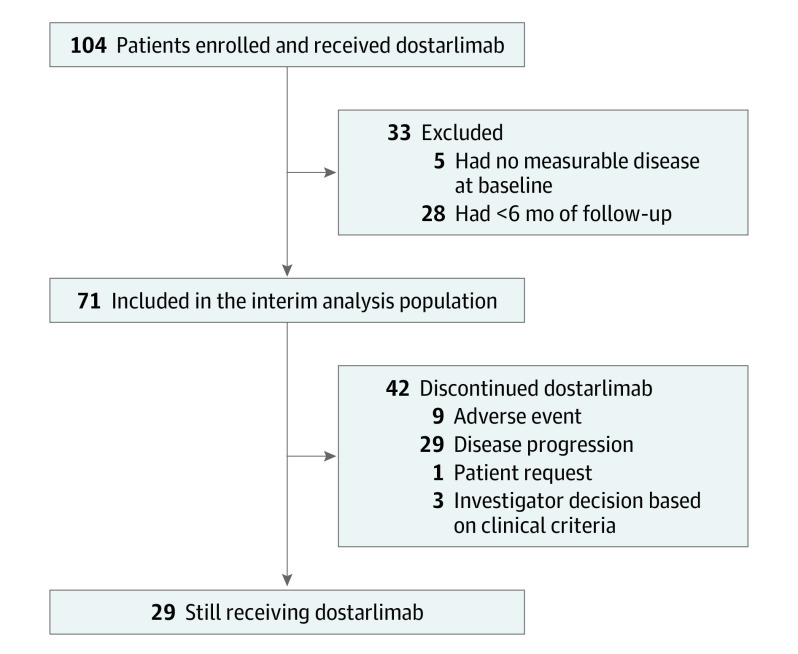
As of July 8, 2019, 104 patients with dMMR EC were enrolled and treated with dostarlimab (Figure 1). Among these patients, 71 had at least 1 measurable lesion at baseline and 6 months or more of follow-up in the study at the time of the data cutoff and were therefore included in the interim efficacy analysis population; the median follow-up time was 11.2 months (range, 0.03 [ongoing] to 22.11 [ongoing] months; based on radiological assessments) in this population. In the interim analysis population, the median age was 64.0 years (range, 38-80 years), and 35 patients (49.3%) had International Federation of Gynecology and Obstetrics stage III or IV disease at diagnosis, with the most common histologic subtype being type I EC (50 [70.4%]) (eTable 1 in Supplement 2; a further histologic breakdown of patients with type II EC can be found in eTable 2 in Supplement 2). All patients had received at least 1 prior anticancer therapy, 64 of 71 (90.1%) had undergone prior anticancer surgery, and 56 of 71 (78.9%) had undergone prior radiotherapy. Half the patients had prior treatment for metastatic disease. The median progression-free interval from the last platinum-containing anticancer therapy was 6.4 months (range, 1.6-79.6 months).
Figure 1. Enrollment and Outcomes.
Efficacy Analysis
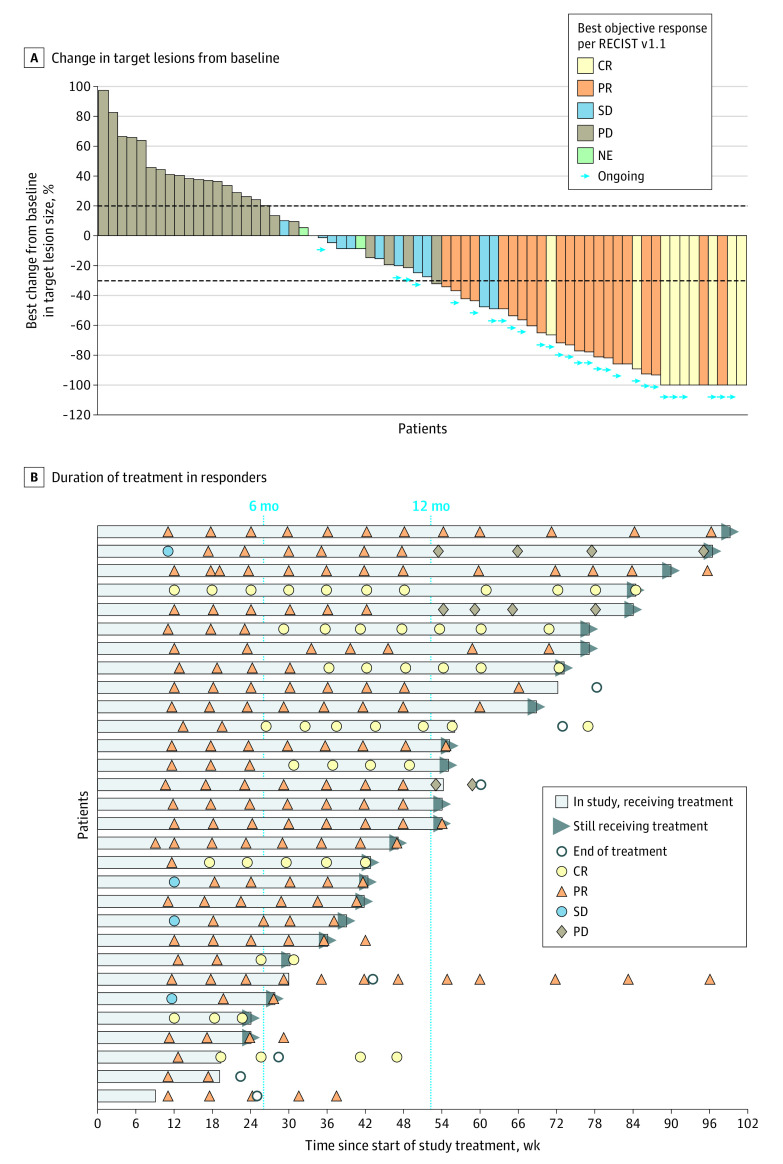
Among the 71 patients in the interim efficacy analysis population, there was an observed response in 30 patients (ORR, 42.3%; 95% CI, 30.6%-54.6%) (Table and Figure 2); 9 patients (12.7%) had a confirmed complete response, and 21 (29.6%) had a confirmed partial response. Among the 30 responders, 18 had received 1 prior line of therapy, and 12 had received 2 or more prior lines of therapy; 15 patients had achieved a response to their last platinum-based chemotherapy. Responses were seen in 20 of 50 patients with type I EC (ORR, 40.0%; 95% CI, 26.4%-54.8%) and 10 of 21 patients with type II EC (ORR, 47.6%; 95% CI, 25.7%-70.2%). All patients with a confirmed complete response remained in response as of the data cutoff date. In post hoc analyses, the ORR benefit of dostarlimab was observed across histologic subtypes, disease stages, and lines of therapy (eFigure 2 in Supplement 2), although subgroup analyses were not powered and should be interpreted with caution.
Table. Tumor Response by RECIST v1.1.
| Characteristic | Cohort A1, dMMR endometrial cancer, No. (%) (n = 71) |
|---|---|
| Best overall response | |
| Complete response | 9 (12.7) |
| Partial response | 21 (29.6) |
| Stable disease | 11 (15.5) |
| Progressive disease | 27 (38.0) |
| Not evaluable | 3 (4.2) |
| Confirmed ORR | |
| No. (%) [95% CI] | 30 (42.3) [30.6-54.6] |
| Response ongoing | 25/30 (83.3) |
| Disease control rate, No. (%) [95% CI] | 41 (57.7) [45.4-69.4] |
| Duration of response, median (95% CI), mo | Not reached |
Abbreviations: dMMR, deficient mismatch mutation repair; ORR, objective response rate; RECIST v1.1, Response Evaluation Criteria in Solid Tumors version 1.1.
Figure 2. Tumor Best Percentage Change in Lesion Size From Baseline in the Efficacy-Evaluable Population (n = 71) and Duration of Treatment in Responders (n = 30).
A, Waterfall plot shows the maximum percentage change in target lesions from baseline, indicated by bar length; best overall response is indicated by color coding of bars and includes assessment of target, nontarget, and new lesions. B, Swimmer lane of duration of treatment for patients with an objective response. Patients were permitted to remain receiving treatment after progression if they were considered to be benefiting from treatment. (Two examples of this can be seen in lanes 2 and 5; in both cases, patients had progressive disease [PD] based on Response Evaluation Criteria in Solid Tumors version 1.1 [RECIST v1.1] but were considered to be still responding per immune-related RECIST and continued receiving dostarlimab. The patient represented in lane 5 had progression based on immune-related RECIST on May 15, 2019.) Computed tomography scans after treatment discontinuation were used to monitor disease for follow-up. All assessments are based on blinded independent centralized review per RECIST v1.1. Ongoing indicates that patients were continuing to receive treatment at time of the data cutoff. CR indicates complete response; NE, not evaluable; PR, partial response; and SD, stable disease.
At the July 8, 2019, data cutoff, the median DOR was not reached, with a median follow-up of 11.2 months. The estimated likelihood of maintaining a response was 96.4% at 6 months and 76.8% at 12 months based on the Kaplan-Meier method (eFigure 1A in Supplement 2).
The disease control rate was 57.7% (95% CI, 45.4%-69.4%), and the median PFS was 8.1 months (95% CI, 3.0-18.0 months) (eFigure 1B in Supplement 2). The median OS was also not reached, with a Kaplan-Meier estimation of 72.7% survival at 12 months after treatment initiation (eFigure 1C in Supplement 2).
Safety
Among the 104 patients included in the safety analysis, most treatment-related AEs (TRAEs) were grade 1 or 2 (eTable 3 in Supplement 2). The most frequently reported TRAEs of any grade (≥10%) were asthenia, diarrhea, fatigue, and nausea (eTable 4 in Supplement 2). The incidence of TRAEs of grade 3 or higher was 11.5% (n = 12), with anemia being the most frequently reported TRAE at 2.9% (n = 3). A total of 10 patients (9.6%) experienced at least 1 serious TRAE. The most frequently reported serious TRAE was colitis (2 [1.9%]). Two patients (1.9%) discontinued the study because of a TRAE (increased levels of transaminase); 1 of these 2 patients also had increased levels of γ-glutamyltransferase. No deaths due to TRAEs were reported.
The incidence of treatment-related immune-related AEs (irAEs) was 23.1% (n = 24) in this cohort; diarrhea (6 [5.8%]) and hypothyroidism (6 [5.8%]) were reported most frequently. Of patients with treatment-related irAEs, 7 (6.7%) had a serious AE, 8 (7.7%) had a grade 3 or higher event, and 2 (1.9%) had an event that led to study treatment discontinuation (the same 2 discontinuations already listed). Pneumonitis was reported in 1 patient, and there were no grade 3 or higher pneumonitis events. Additional safety data are provided in eTable 5 and eTable 6 in Supplement 2.
Discussion
The results of this analysis show that dostarlimab monotherapy was associated with an ORR of 42.3% (95% CI, 30.6%-54.6%) for patients with recurrent or advanced dMMR EC that had progressed after treatment with platinum-based chemotherapy. Responses were durable, and with a median follow-up of 11.2 months, the median DOR was not reached. The safety profile was manageable and consistent with that of other drugs in the anti–PD-1 antibody class. Less than 2% of the patients discontinued treatment because of TRAEs, and no treatment-related deaths were reported. To our knowledge, the results presented here represent the largest data set to date of patients with dMMR EC treated with a PD-1 inhibitor.
One-third of EC tumors show evidence of dMMR.7,9 Because of the DNA repair deficiency and high neoantigen load associated with these genomic alterations, dMMR EC tumors have been hypothesized to be more sensitive to immune checkpoint inhibitors than proficient MMR tumors.8 The first published evidence of such activity was with a PD-1 inhibitor (pembrolizumab) in early-phase umbrella studies.7,18 A recently published phase 2 trial of pembrolizumab showed an ORR of 57.1% (95% CI, 42.2%-71.2%) for 49 patients with dMMR EC.13 The anti–PD-L1 therapy avelumab showed an ORR of 26.7% (95% CI, 7.8%-55.1%) for 15 patients with dMMR EC.19 Activity with the PD-L1 inhibitor durvalumab showed an objective tumor response of 40% (95% CI, 26%-56%) for 35 patients with dMMR EC.20 A direct comparison of the results from other trials with the results from the present study are not appropriate because the patient populations and trial designs differ. However, data from these studies, collectively with the results presented herein, support the activity of the anti–PD-1 and anti–PD-L1 therapeutic drug class in dMMR EC.
Prior to the introduction of anti–PD-1 therapies, single-agent therapies showed ORRs ranging from 13.5% (90% CI, 6.5%-27%) for bevacizumab21 to 27.3% (95% CI, 15%-42.8%) for paclitaxel.22 These trials reflect response rates in a population with EC without biomarker selection criteria. Although cross-trial comparisons cannot be made, the response rates with anti–PD-1 therapies seem to be favorable.
Despite the GARNET trial being a single-group study, the antitumor activity observed in patients with dMMR EC is promising and suggests that dostarlimab has the potential to become a treatment option for this population. The high ORR and long duration of response are encouraging. Because we studied a pretreated metastatic population, an important point is the OS data. More than 74% of patients in the GARNET trial dMMR EC population are still alive 1 year after inclusion. Historical data on doxorubicin in a similar setting showed a median OS of 5.8 months (95% CI, 1.0-15.0 months).23
In addition to the activity of dostarlimab, a unique feature of this therapy is the dosing regimen. The first 4 treatments were administered every 3 weeks, after which treatments were administered every 6 weeks. This dosing regimen was based on receptor occupancy and pharmacokinetic data showing that this dosing regimen provides sufficient serum concentrations to achieve and maintain maximal receptor occupancy throughout both intervals. This unique dosing schedule allows for less frequent clinic visits after 12 weeks of initial treatment with dostarlimab, which benefits both patients and caregivers and also has the potential to reduce health care costs. An analysis of patient-reported outcomes in the GARNET trial is planned at the primary analysis and will provide additional insights into quality of life for patients receiving dostarlimab.
Limitations
This study has some limitations. The main limitation of the GARNET trial is that it is a single-group trial and, therefore, lacks a comparator group from which statistical comparisons vs a standard of care could be drawn. Furthermore, at the time of the data cutoff, the sample size was not sufficient to allow for robust subgroup analyses. Patients in this cohort were selected based on MMR status, currently the factor most reliably associated with checkpoint inhibitor activity in EC. However, other potential biomarkers, including PD-L1 expression level and tumor mutational burden, were not conducted at this time and limit our findings and conclusions. This knowledge may help to further identify patients who benefit the most and/or, conversely, may help to identify the potential mechanisms of resistance to dostarlimab in dMMR tumors. Despite these limitations, dostarlimab has been associated with antitumor activity; future data cutoff dates will provide more information on the benefit of dostarlimab in subgroups, as well as the duration of response and long-term safety. Larger future trials, including the currently enrolling randomized, placebo-controlled RUBY trial (NCT03981796) of dostarlimab in combination with carboplatin-paclitaxel in primary advanced or recurrent EC will be important to better understand the efficacy and safety profile of dostarlimab.
Conclusions
These preliminary results from the GARNET trial dMMR EC cohort demonstrate that dostarlimab monotherapy was associated with meaningful and durable clinical activity and an acceptable safety profile. Considering these results, the protocol has been amended to continue enrolling patients with dMMR EC.
Trial Protocol
eAppendix. Institutions and Collaborators
eTable 1. Patient Demographics and Baseline Characteristics
eTable 2. Histology of Patients With Type II Endometrial Carcinoma
eTable 3. Adverse Event Summary
eTable 4. Safety
eTable 5. Treatment-Related Serious Adverse Events
eTable 6. Immune-Related Adverse Events
eFigure 1. Duration of Response (A), Progression-Free Survival (B), and Overall Survival (C)
eFigure 2. Subgroup Analysis
References
- 1.Boll D, Karim-Kos HE, Verhoeven RHA, et al. Increased incidence and improved survival in endometrioid endometrial cancer diagnosed since 1989 in The Netherlands: a population based study. Eur J Obstet Gynecol Reprod Biol. 2013;166(2):209-214. doi: 10.1016/j.ejogrb.2012.10.028 [DOI] [PubMed] [Google Scholar]
- 2.Muggia FM, Blessing JA, Sorosky J, Reid GC. Phase II trial of the pegylated liposomal doxorubicin in previously treated metastatic endometrial cancer: a Gynecologic Oncology Group study. J Clin Oncol. 2002;20(9):2360-2364. doi: 10.1200/JCO.2002.08.171 [DOI] [PubMed] [Google Scholar]
- 3.Miller DS, Blessing JA, Lentz SS, Waggoner SE. A phase II trial of topotecan in patients with advanced, persistent, or recurrent endometrial carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2002;87(3):247-251. doi: 10.1006/gyno.2002.6804 [DOI] [PubMed] [Google Scholar]
- 4.Fracasso PM, Blessing JA, Molpus KL, Adler LM, Sorosky JI, Rose PG. Phase II study of oxaliplatin as second-line chemotherapy in endometrial carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2006;103(2):523-526. doi: 10.1016/j.ygyno.2006.03.043 [DOI] [PubMed] [Google Scholar]
- 5.Garcia AA, Blessing JA, Nolte S, Mannel RS; Gynecologic Oncology Group . A phase II evaluation of weekly docetaxel in the treatment of recurrent or persistent endometrial carcinoma: a study by the Gynecologic Oncology Group. Gynecol Oncol. 2008;111(1):22-26. doi: 10.1016/j.ygyno.2008.06.013 [DOI] [PubMed] [Google Scholar]
- 6.Dizon DS, Blessing JA, McMeekin DS, Sharma SK, Disilvestro P, Alvarez RD. Phase II trial of ixabepilone as second-line treatment in advanced endometrial cancer: Gynecologic Oncology Group trial 129-P. J Clin Oncol. 2009;27(19):3104-3108. doi: 10.1200/JCO.2008.20.6995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Le DT, Durham JN, Smith KN, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357(6349):409-413. doi: 10.1126/science.aan6733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mittica G, Ghisoni E, Giannone G, Aglietta M, Genta S, Valabrega G. Checkpoint inhibitors in endometrial cancer: preclinical rationale and clinical activity. Oncotarget. 2017;8(52):90532-90544. doi: 10.18632/oncotarget.20042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kandoth C, Schultz N, Cherniack AD, et al. ; Cancer Genome Atlas Research Network . Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497(7447):67-73. doi: 10.1038/nature12113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fountzilas E, Kotoula V, Pentheroudakis G, et al. Prognostic implications of mismatch repair deficiency in patients with nonmetastatic colorectal and endometrial cancer. ESMO Open. 2019;4(2):e000474. doi: 10.1136/esmoopen-2018-000474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nagle CM, O’Mara TA, Tan Y, et al. ; Australian Endometrial Cancer Study Group . Endometrial cancer risk and survival by tumor MMR status. J Gynecol Oncol. 2018;29(3):e39. doi: 10.3802/jgo.2018.29.e39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372(26):2509-2520. doi: 10.1056/NEJMoa1500596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marabelle A, Le DT, Ascierto PA, et al. Efficacy of pembrolizumab in patients with noncolorectal high microsatellite instability/mismatch repair–deficient cancer: results from the phase II KEYNOTE-158 study. J Clin Oncol. 2020;38(1):1-10. doi: 10.1200/JCO.19.02105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laken H, Kehry M, McNeeley P, et al. Identification and characterization of TSR-042, a novel anti-human PD-1 therapeutic antibody. Eur J Cancer. 2016;69(suppl 1):S102. doi: 10.1016/S0959-8049(16)32902-1 [DOI] [Google Scholar]
- 15.World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 16.MedDRA . Support documentation. Accessed August 26, 2020. https://www.meddra.org/how-to-use/support-documentation/english
- 17.National Cancer Institute . Common Terminology Criteria for Adverse Events (CTCAE). Accessed August 26, 2020. https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm
- 18.O’Neil BH, Wallmark JM, Lorente D, et al. Safety and antitumor activity of the anti-PD-1 antibody pembrolizumab in patients with advanced colorectal carcinoma. PLoS One. 2017;12(12):e0189848. doi: 10.1371/journal.pone.0189848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Konstantinopoulos PA, Luo W, Liu JF, et al. Phase II study of avelumab in patients with mismatch repair deficient and mismatch repair proficient recurrent/persistent endometrial cancer. J Clin Oncol. 2019;37(30):2786-2794. doi: 10.1200/JCO.19.01021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Antill YC, Kok PS, Robledo K, et al. Activity of durvalumab in advanced endometrial cancer (AEC) according to mismatch repair (MMR) status: the phase II PHAEDRA trial (ANZGOG1601). J Clin Oncol. 2019;37(15)(suppl):5501. doi: 10.1200/JCO.2019.37.15_suppl.5501 [DOI] [Google Scholar]
- 21.Aghajanian C, Sill MW, Darcy KM, et al. Phase II trial of bevacizumab in recurrent or persistent endometrial cancer: a Gynecologic Oncology Group study. J Clin Oncol. 2011;29(16):2259-2265. doi: 10.1200/JCO.2010.32.6397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lincoln S, Blessing JA, Lee RB, Rocereto TF. Activity of paclitaxel as second-line chemotherapy in endometrial carcinoma: a Gynecologic Oncology Group study. Gynecol Oncol. 2003;88(3):277-281. doi: 10.1016/S0090-8258(02)00068-9 [DOI] [PubMed] [Google Scholar]
- 23.Makker V, Hensley ML, Zhou Q, Iasonos A, Aghajanian CA. Treatment of advanced or recurrent endometrial carcinoma with doxorubicin in patients progressing after paclitaxel/carboplatin: Memorial Sloan-Kettering Cancer Center experience from 1995 to 2009. Int J Gynecol Cancer. 2013;23(5):929-934. doi: 10.1097/IGC.0b013e3182915c20 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eAppendix. Institutions and Collaborators
eTable 1. Patient Demographics and Baseline Characteristics
eTable 2. Histology of Patients With Type II Endometrial Carcinoma
eTable 3. Adverse Event Summary
eTable 4. Safety
eTable 5. Treatment-Related Serious Adverse Events
eTable 6. Immune-Related Adverse Events
eFigure 1. Duration of Response (A), Progression-Free Survival (B), and Overall Survival (C)
eFigure 2. Subgroup Analysis