Abstract
Familial Mediterranean Fever (FMF) and COVID-19 show a remarkable overlap of clinical symptoms and similar laboratory findings. Both are characterized by fever, abdominal/chest pain, elevation of C-reactive protein, and leukocytosis. In addition, colchicine and IL-1 inhibitors treatments that are effective in controlling inflammation in FMF patients have recently been proposed for off-label use in COVID-19 patients. Thus, FMF may resemble a milder recapitulation of the cytokine storm that is a hallmark of COVID-19 patients progressing to severe disease. We analyzed the sequence of the MEFV-encoded Pyrin protein – whose mutations cause FMF- in mammals, bats and pangolin. Intriguingly, although Pyrin is extremely conserved in species that are considered either a reservoir or intermediate hosts for SARS-CoV-2, some of the most common FMF-causing variants in humans are present as wildtype residues in these species. We propose that in humans, Pyrin may have evolved to fight highly pathogenic infections.
Keywords: FMF disease, COVID-19, cytokine storm, pyrin, innate immunity
Introduction
The World Health Organization reported a novel coronavirus on December 30, 2019 as the cause of a cluster of pneumonia cases in the city of Wuhan in the Hubei Province of China. Since then, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has infected nearly 30 million individuals worldwide causing more than 900,000 deaths during the past 6 months of the COVID-19 pandemic. Among European countries, Italy has been severely hit by COVID-19 (1). Since the initial outbreak, a huge body of clinical and scientific information has been accumulated on COVID-19, a multifaceted disease hitting not only lungs, but also other organs, with different defined stages (2, 3). In most cases, SARS-CoV-2 enters the human body through inhaled droplets and aerosols. Although contact with contaminated surfaces has been hypothesized as a second possible infection route, the importance of this alternative mode of infection has not been assessed systematically (4, 5). Upon infection, SARS-CoV-2 enters its target cells via: (a) binding of its spike protein (S) to angiotensin converting enzyme 2 (ACE2); (b) activation through proteolysis of the viral S protein catalyzed by the cellular transmembrane protease serine 2 (TMPRSS2); and (c) fusion of SARS-CoV-2 virus with the host cell membrane. A wide variation in allele frequencies at ACE2 expressions single nucleotide polymorphisms (eSNPs) loci can partly explain the differences in COVID-19 prevalence across different countries (6). Also, differential expression of ACE2 occurs in several human cancers and chronic diseases, possibly influencing COVID-19 susceptibility and severity (7). The involvement of ACE2 and TMPRSS2 in viral cell entry has been exploited to plan experimental therapies based on using protease inhibitors such as camostat mesylate or nafamostat to block SARS-CoV-2 entry into cells (8, 9). Once inside the host cells, the SARS-CoV-2 positive single stranded RNA (ssRNA) genome begins replication and cytoplasmic accumulation. The ssRNA or its double stranded intermediate (dsRNA) are recognized by the innate immune nucleic acid sensing systems whose activation exerts a first antiviral response through the production of type 1 interferons and the secretion of pro-inflammatory cytokines. The immunomodulation at this early stage of infection might determine the growth of the viral load, with most infected individuals still being asymptomatic or paucisymptomatic, while almost 15% of SARS-CoV-2 positive patients develop fever, coughing, occasionally ageusia and anosmia and even gastrointestinal symptoms (3), with or without hepatic involvement (10).
Patients progressing to the second phase show a strong immunological and hyperinflammatory response that is defined as a “cytokine storm” and which may lead to respiratory worsening and bilateral pneumonitis. In this second stage, COVID-19 patients manifest symptoms mimicking those present in patients with auto-inflammatory diseases such as fever, arthralgia, leucopenia and myocarditis (11–13).
The Parallel Worlds of FMF and COVID-19
We were intrigued by the remarkable overlap between these clinical manifestations and some of the typical manifestations of Familial Mediterranean Fever (FMF), a largely recessively inherited monogenic inflammasomopathy (autoinflammatory disorder involving the inflammasome) caused by mutations in the MEFV gene that is particularly prevalent in the Mediterranean basin (14). While the previously mentioned clinical signs are not specific to FMF and shared with other hereditary recurrent fevers and inflammatory diseases, yet they represent an indication of similarities among FMF pathogenesis and the hyperinflammatory response observed in COVID-19 patients. Worthy of note, taste alteration has been reported amongst the prodromal manifestations preceding fever attacks in FMF (15, 16). We should stress that the cytokine storm observed in the inflammatory stage of COVID19, has been reported in other autoinflammatory diseases (AIDs) such as the macrophage activation syndrome (MAS) and adult onset Still’s disease (17). However, authors have often reported genetic heterogeneity and overlap between these AIDs and hereditary recurrent fevers (18–20).
Colchicine, a natural alkaloid from Colchicum autumnale, has a long history as a drug to treat pain and swelling since ancient Egypt. It is nowadays used to treat FMF, gout, Behçet syndrome and recurrent non-infective pericarditis. Unsurprisingly, it is currently being investigated in several COVID-19 therapeutic trials (21–23). Notably, in colchicine-resistant or colchicine-intolerant FMF patients, alternative treatments include biologics that neutralize the pro-inflammatory cytokine interleukin (IL)-1β directly (Canakinumab) or inhibit IL-1β-mediated activation of the IL-1 Receptor (Anakinra and Rilonacept) (24–26). Similarly to colchicine, several ongoing trials are evaluating the use of IL-1 pathway inhibitors to treat COVID-19 patients (27–30). Although first results from COVID-19 patients treated with these repurposed drugs have been conflicting (31–33), the role of Pyrin (the inflammasome sensor protein that is encoded by MEFV) in modulating severity and outcome of COVID-19 is still unknown.
The Pyrin Inflammasome
The Pyrin domain architecture shows intriguing features which may add insights into its possible role in COVID-19 disease. Pyrin has a N-terminal pyrin domain (PYD) that is frequently found in other innate immune pathogen sensors that mount inflammasome responses such as NLRP1, NLRP3, and AIM2. The N-terminal PYD domain engages in homotypic interactions with its PYD counterpart in the adaptor protein apoptosis-associated speck-like protein with a caspase recruitment domain (ASC) to assemble ASC specks, which recruit the inflammatory protease procaspase-1 inducing its self-cleavage and activation (34). Caspase-1 in turn matures the proinflammatory cytokines IL-1β and IL-18 and cleaves gasdermin D to trigger a lytic cell death mode termed pyroptosis that promotes secretion of aforementioned cytokines along with danger-associated molecular patterns (DAMPs) such as IL-1alpha, HMGB1 and ATP (35).
Pyroptosis is a double-edged sword with both antiviral and proviral activities during viral infections (36). In fact, cell death can lead to halting viral replication and infection, frequently at the price of increased inflammation. Conversely, dead cells release a large number of viral particles contributing to viral dissemination.
The MEFV encoded Pyrin sensor contains in its central region three domains: a bZIP domain (aa 370-412), a B-box domain (aa 370-412) and a coiled-coil domain (CC, aa 420-440). The role of these three domains has not been thoroughly investigated and few FMF-causing variants localize to Pyrin’s central region (37). This region may have an autoinhibitory role precluding the PYD domain from interacting with ASC and activating pyroptosis (38). The C-terminal B30.2 (also known as PRY/SPRY) domain is extremely important in FMF pathogenesis since most of the disease-penetrant MEFV variants cluster in this region (39). Although this uneven distribution of FMF-associated MEFV mutations suggests that the B30.2 domain is crucial in regulating Pyrin inflammasome activity, the precise molecular mechanisms by which the B30.2 domain regulates FMF pathogenesis have not been elucidated (40). Likely, FMF-causing mutations in the B30.2 domain may derail intramolecular interactions that keep Pyrin in an autoinhibitory state.
The Curious Family of Pyrin Orthologs
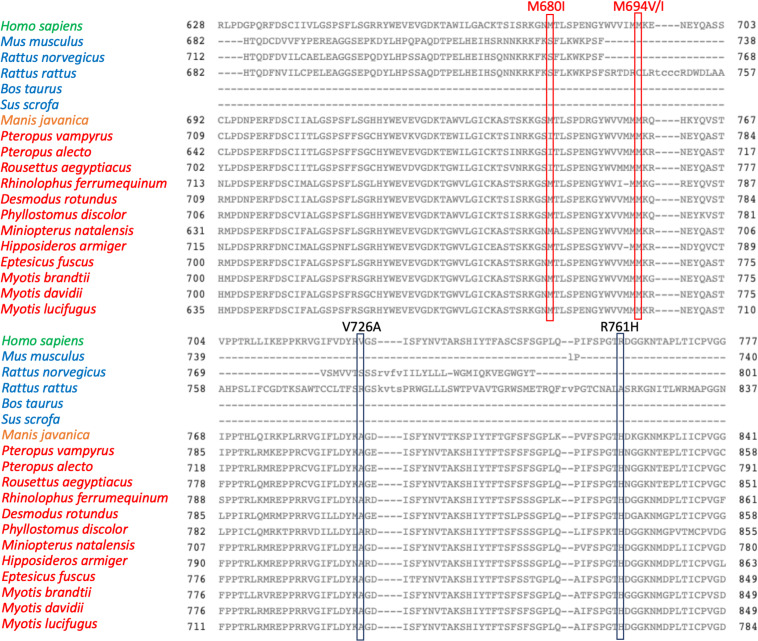
Interestingly, the degree of amino acid conservation along the pyrin protein sequence is rather variable and may offer some insights in the dangerous liaisons between FMF and COVID-19. We aligned the MEFV-encoded Pyrin amino acid sequences from 19 different species including the only pangolin and all bat species sequences available in GenBank (Figure 1). Bats and pangolins have been considered the reservoir and intermediate host, respectively, of SARS-CoV-2 before transmission to humans.
FIGURE 1.
Alignment of 19 MEFV orthologs from top to bottom as follow: Homo sapiens NP_000234.1, Mus musculus NP_001155263.1, Rattus norvegicus XP_017452974.1, Rattus rattus XP_032769237.1, Bos taurus XP_015315767.1, Sus scrofa XP_013851182.1, Manis javanica XP_017515721.1, Pteropus vampyrus XP_011374846.1, Pteropus alecto XP_006913958.1, Rousettus aegyptiacus XP_015977766.1, Rhinolophus ferrumequinum XP_032957625.1, Desmodus rotundus XP_024409044.1, Phyllostomus discolor XP_028366066.1, Miniopterus natalensis XP_016066853.1, Hipposideros armiger XP_019488407.1, Eptesicus fuscus XP_028001472.1, Myotis brandtii XP_014400628.1, Myotis davidii XP_015414020.1, Myotis lucifugus XP_023611041.1. The common names for species analyzed are as follow: M. musculus (mouse), R. norvegicus (Norway or brown rat), R. rattus (black rat), B. taurus (cattle), S. scrofa (pig), M. javanica (Malayan pangolin), P. vampyrus (Large flying fox), P. alecto (black flying fox), R. aegyptiacus (Egyptian rousettes or Egyptian fruit bat), R. ferrumequinum (greater horseshoe bat), D. rotundus (common vampire bat), P. discolor (pale spear-nosed bat), M. natalensis (Natal long-fingered bat), H. armiger (great roundleaf bat), E. fuscus (big brown bat), M. brandtii (Brandt’s bat), M. davidii (David’s myotis), M. lucifugus (little brown bat). Alignment were performed using the Cobalt software (Papadopoulos JS and Agarwala R, Bioinformatics 23:1073-79, 2007). Boxes highlight the amino acid residues corresponding to common FMF-causing variants. In green homo sapiens Pyrin, in blue pyrin from mammal species, in orange pangolin Pyrin, in red bats pyrin proteins.
The alignment of the Pyrin sequences presented unique evolutionary features (Figure 1).
In fact, some of the most prevalent FMF-associated mutations in human Pyrin were present as wild type in all bat species analyzed and in pangolin (V726A, R761H). This resembles previous findings that some FMF-associated mutations are retrieved as wild type in primates, suggesting evolutionary pressure on Pyrin (41). In contrast, other FMF-associated amino acids residues that are largely prevalent in middle eastern populations (M680I, M694I, M694V) were not observed in bats and pangolin.
Worthy of note M680I, M694V, M694I, and R761H were all associated with derailed Pyrin-induced IL-1β secretion in a recently developed blood-based functional test for FMF alleles (42). It is tempting to speculate that FMF patients carrying V726A and R761H variants- which represents the wild type residues in all bats and pangolin sequences- might modulate better their cytokine response to SARS-CoV-2 infection. Further, in a mouse knock-in model the V726A variant causes a more severe FMF phenotype compared to M694V, M680I, and elevated production of multiple cytokines (43). Given that the M680I and M694V/I alleles in patients provoke a hyper-inflammatory response not different from V726A and R761H (at least in FMF), one may hypothesize that they could warrant a comparable attenuation of viral infection. An elaboration on this hypothesis would stem from the increased FMF severity associated to M680I and M694V/I mutations. This raised level of inflammation can either move the balance toward excess inflammation in COVID-19 or be causing an even improved immunomodulation in COVID-19 compared to V726A, R761H. Moreover, historic pandemics and different pathogens may have selected for different MEFV variants in their respective host species and populations. Thus, a pathogen different from coronaviruses (i.e., Yersinia pestis) might have selected the M680I, M694V/I mutations in humans but not in bats. Indeed, confirming this hypothesis, and shortly after the submission of this work, genetic and experimental evidences have been reported linking these MEFV variants with resistance to Yersinia pestis (44, 45).
The E148Q and R202Q MEFV variants, which according to a current consensus are considered neutral polymorphisms, showed a response to colchicine challenge alike normal controls (42). These two variants presented rather divergent evolutionary features. While the glutamic acid at position 148 was strictly conserved in all species analyzed, the arginine at position 202 was conserved in pangolin and in 7 of 13 bat species, and changed to a glutamine in mouse and rats (Figure 2). Therefore, E148Q and R202Q which appear to be neutral in functional assays seem to be under apparently different evolutionary pressures.
FIGURE 2.
Alignment of 19 Pyrin orthologs from amino acid 148 to amino acid 202 (human gene). Alignment order and color coding as in Figure 1.
The Less than Casual Worldwide Distribution of Pyrin Variants
The frequency of MEFV mutations and polymorphisms shows a great variability in countries where FMF has a high frequency. In fact, the M694V and M694I, are prevalent among Turks, non-ashkhenazi Jews, and Arabs, while the M680I is frequent in the Armenian population (46–49). The V726A and R761H are generally associated with a milder phenotype, and generally reported in clusters of FMF patients of Ashkhenazi Jewish origin, and in the western Mediterranean area (50). The E148Q variant, whose pathogenicity is still debated, also presents a wide variation in frequency across different populations (51).
This peculiar distribution of the MEFV variome led to the hypothesis that the MEFV gene has been subjected to a balancing selection and nucleotide variation, particularly in the B30.2 region, is adaptive (52). Thus, the severity of COVID-19 disease in FMF patients, once infected, might be influenced, at least partially, depending on specific MEFV genotypes which shows country-specific differences.
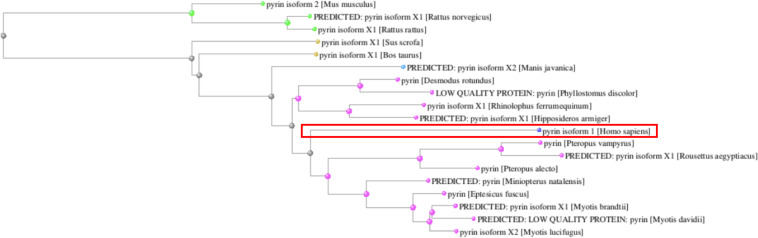
The MEFV gene displays other intriguing evolutionary features. In fact, while the N-terminal PYD domain is conserved across all species, the B30.2 domain appears of more recent origin. Of note, when considering the entire Pyrin amino acid sequence, human Pyrin is more closely related to its bats and pangolin homologs than to other mammal species (Figure 3).
FIGURE 3.
Phylogenetic tree of the Pyrin orthologs reconstructed from the COBALT multiple sequence alignment tool. The human Pyrin is boxed.
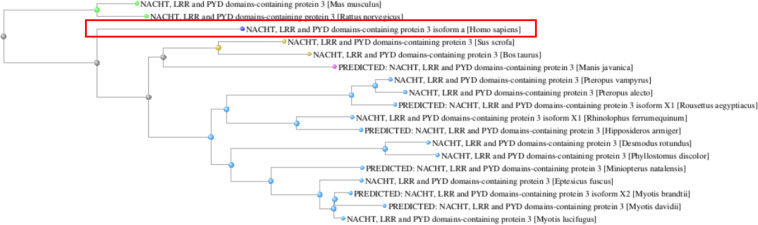
In contrast, the human NLRP3 protein presented a high level of homology in all analyzed species. It clustered with other mammalian NLRP3 proteins in the phylogenetic tree and was more distantly related to bat sequences (Figure 4).
FIGURE 4.
Phylogenetic tree of the NLRP3 orthologs reconstructed from the COBALT multiple sequence alignment tool. The human NLRP3 protein is boxed.
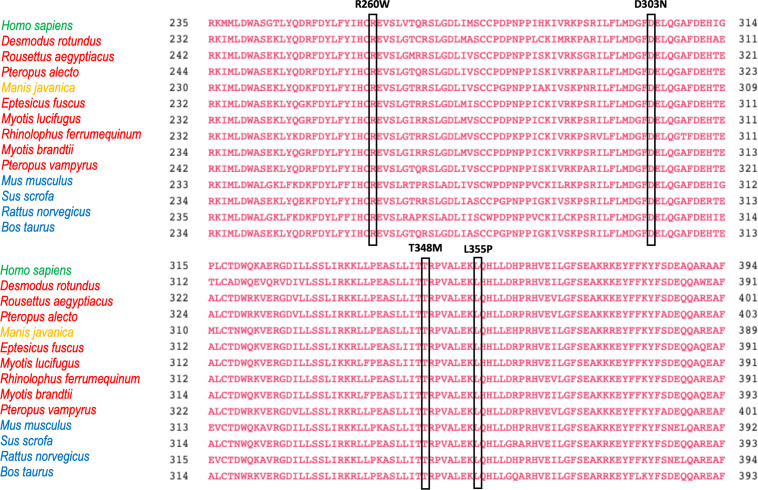
In addition, differently from MEFV, the most common human NLRP3 mutations- R260W, D303N, T348M, and L355P- which represent more than 40% of the total mutation burden for this gene (53, 54), were never present as wild type amino acid residues in all bat species and pangolin (Figure 5).
FIGURE 5.
Alignment of 14 NLRP3 orthologs from amino acid 235 to amino acid 394 (human gene). The four residues whose mutations are responsible of more than 40% of NLRP3 –associated autoinflammatory diseases are boxed.
Therefore, considering the Pyrin inflammasome a passive bystander in SARS-CoV-2 infection could lead to overlooking an important innate immune pathway that coordinates the response to pathogens. In fact, inflammasome-driven pyroptosis is one of the results of the cytokine storm which concurs to the high pathogenicity of both SARS-CoV, and SARS-CoV-2 (55, 56).
The Role of the NLRP3 Inflammasome in Viral Infections
Recent findings demonstrated that the SARS-CoV ORF3a protein can provoke a cytokine storm by activation of the NLRP3 inflammasome through TRAF3-dependent ubiquitination of ASC (57). A recent analysis of 2782 SARS-CoV-2 strains showed that non-synonymous substitutions in the SARS-CoV-2 ORF3a protein may alter virulence and infectivity (58). Additional viral proteins are able to activate the NLRP3 inflammasome. The NLRP3-mediated response to influenza A virus (IAV) has been extensively studied and plays a crucial role in protecting the host while helping in clearing the infection. However, if the inflammatotory response is particularly prolonged and excessive it can increase disease burden (59). The NLRP3 inflammasome plays a critical role in guarding against IAV infection and reducing lung damage consequent to infection (59, 60). Other viruses are capable of activating the NLRP3 inflammasome. The viroporin 2B, released upon human rhinovirus infection (HRV), causes proteolytic activation of procaspase-1 and IL-1ß secretion in a NLRP3 dependent manner (61). Several other viruses can provoke a sustained response from the NLRP3 inflammasome as extensively reviewed (62). Similarly to the MEFV-encoded Pyrin, the N-terminal PYD domain of NLRP3 can recruit ASC via homotypic interaction with its PYD counterpart on ASC to assemble ASC filaments. The assembly of ASC filaments in macromolecular structures known as ASC specks allow the recruitment of procaspase-1 to induce its self-cleavage and activation. Caspase-1 in turn cleaves the proinflammatory cytokines IL-1β/IL-18 and gasdermin D to trigger pyroptosis. Thus, both Pyrin and NLRP3 inflammasomes may compete for ASC binding and ASC oligomerization-dependent caspase-1 activation.
And the Answer Is….
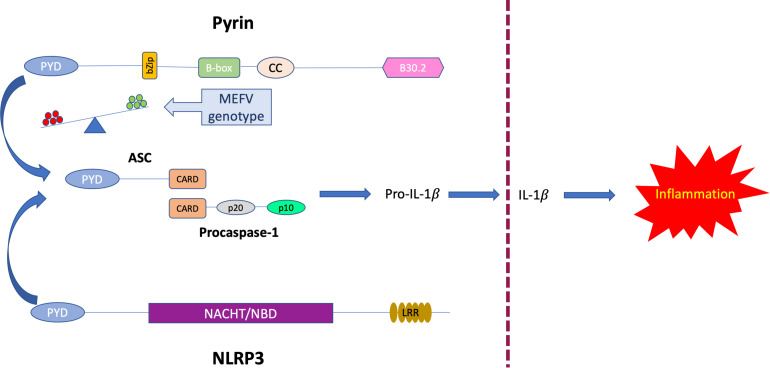
Before the COVID-19 pandemic, a recent report (63) demonstrated that bats, when infected with different zoonotic viruses, can sustain high viral loads while presenting a dampened NLRP3-mediated inflammatory response associated with a decrease in both ASC speck formation and IL-1β secretion. Hence, a dysregulation of the NLRP3 inflammasome can also contribute to the utterly complex individual immune response to viral infections (64). These findings suggest that the modulation of innate immunity rather than an enhanced antiviral defense might shape the different outcome of COVID-19 disease. Thus, we hypothesize that competition between Pyrin and the NLRP3 inflammasomes for ASC recruitment may tilt the balance between a cytokine storm and a finely adjusted protective inflammation (Figure 6).
FIGURE 6.
Proposed model of competitive binding to ASC of MEFV-encoded Pyrin and NLRP3. Abbreviations used: PYD, Pyrin domain, CARD, Caspase recruitment domain, NACHT/NBD NACHT-, Nucleotide binding- domain, LRR, Leucine-rich repeats.
FMF, in which Pyrin activity and consequent ASC oligomerization are increased because of MEFV pathogenic variants, may therefore represent a unique opportunity as a disease model to investigate the regulation of the inflammatory response to novel emerging viruses. We presented here the unique evolutionary features of the MEFV-encoded Pyrin suggesting its putative contribution in shaping the individual risk to develop severe complications consequent to infectious diseases.
Several factors could have contributed to the rapid spreading of COVID-19 pandemic, including access to adequate health care, aging demographic, metabolic dysfunctions, socio-cultural differences. It would be wise not to discard individual genetics, including the MEFV gene, from the mix.
Future investigation on how carriers of different MEFV genotypes have responded to coronavirus infections could help in refining existing and novel therapeutics in development for present and future challenges. In conclusion, the answer to the title question might not be blowing in the wind but could hopefully be found in a deeper knowledge of inflammasome regulation in well-known inflammatory diseases.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/ Supplementary Material.
Author Contributions
AS designed the study and drafted the manuscript. ML and PP drafted the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2020.574593/full#supplementary-material
References
- 1.Di Ciaula A, Palmieri VO, Migliore G, Portincasa P, Group IMC. COVID-19, internists and resilience: the north-south Italy outbreak. Eur J Clin Invest. (2020) 50:e13299. 10.1111/eci.13299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gonzalez-Gay MA, Mayo J, Castaneda S, Cifrian JM, Hernandez-Rodriguez J. Tocilizumab: from the rheumatology practice to the fight against COVID-19, a virus infection with multiple faces. Expert Opin Biol Ther. (2020) 20:717–23. 10.1080/14712598.2020.1770222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smyk W, Janik MK, Portincasa P, Milkiewicz P, Lammert F, Krawczyk M. COVID−19: focus on the lungs but do not forget the gastrointestinal tract. Eur J Clin Invest. (2020) 50:e13276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. (2020) 395:507–13. 10.1016/S0140-6736(20)30211-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. (2020) 395:497–506. 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cao Y, Li L, Feng Z, Wan S, Huang P, Sun X, et al. Comparative genetic analysis of the novel coronavirus (2019-nCoV/SARS-CoV-2) receptor ACE2 in different populations. Cell Discov. (2020) 6:11. 10.1038/s41421-020-0147-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li Y, Xu Q, Ma L, Wu D, Gao J, Chen G, et al. Systematic profiling of ACE2 expression in diverse physiological and pathological conditions for COVID-19/SARS-CoV-2. J Cell Mol Med. (2020) 24:9478–82. 10.1111/jcmm.15607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yamamoto M, Matsuyama S, Li X, Takeda M, Kawaguchi Y, Inoue JI, et al. Identification of nafamostat as a potent inhibitor of middle east respiratory syndrome Coronavirus S Protein-mediated membrane fusion using the split-protein-based cell-cell fusion assay. Antimicrob Agents Chemother. (2016) 60:6532–9. 10.1128/AAC.01043-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoffmann M, Kleine-Weber H, Schroeder S, Kruger N, Herrler T, Erichsen S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. (2020) 181:271–80.e8. 10.1016/j.cell.2020.02.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Portincasa P, Krawczyk M, Machill A, Lammert F, Di Ciaula A. Hepatic consequences of COVID-19 infection. Lapping or biting? Eur J Int Med. (2020) 77:18–24. 10.1016/j.ejim.2020.05.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu K, He J, Wu Y, Xie B, Liu X, Wei B, et al. Dysregulated adaptive immune response contributes to severe COVID-19. Cell Res. (2020) 30:814–6. 10.1038/s41422-020-0391-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qin C, Zhou L, Hu Z, Zhang S, Yang S, Tao Y, et al. Dysregulation of immune response in patients with coronavirus 2019 (COVID-19) in Wuhan, China. Clin Infect Dis. (2020) 71:762–8. 10.1093/cid/ciaa248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hintenberger R, Falkinger A, Danninger K, Pieringer H. Cardiovascular disease in patients with autoinflammatory syndromes. Rheumatol Int. (2018) 38:37–50. 10.1007/s00296-017-3854-7 [DOI] [PubMed] [Google Scholar]
- 14.Ben-Chetrit E, Touitou I. Familial mediterranean Fever in the world. Arthritis Rheum. (2009) 61:1447–53. 10.1002/art.24458 [DOI] [PubMed] [Google Scholar]
- 15.Lidar M, Yaqubov M, Zaks N, Ben-Horin S, Langevitz P, Livneh A. The prodrome: a prominent yet overlooked pre-attack manifestation of familial Mediterranean fever. J Rheumatol. (2006) 33:1089–92. [PubMed] [Google Scholar]
- 16.Lidar M, Livneh A. Familial Mediterranean fever: clinical, molecular and management advancements. Neth J Med. (2007) 65:318–24. [PubMed] [Google Scholar]
- 17.Crayne CB, Albeituni S, Nichols KE, Cron RQ. The immunology of macrophage activation syndrome. Front Immunol. (2019) 10:119. 10.3389/fimmu.2019.00119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sighart R, Rech J, Hueber A, Blank N, Lohr S, Reis A, et al. Evidence for genetic overlap between adult onset Still’s disease and hereditary periodic fever syndromes. Rheumatol Int. (2018) 38:111–20. 10.1007/s00296-017-3885-0 [DOI] [PubMed] [Google Scholar]
- 19.Nonaka F, Migita K, Jiuchi Y, Shimizu T, Umeda M, Iwamoto N, et al. Increased prevalence of MEFV exon 10 variants in Japanese patients with adult-onset Still’s disease. Clin Exp Immunol. (2015) 179:392–7. 10.1111/cei.12463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim JJ, Kim JK, Shim SC, Choe JY, Kim TH, Jun JB, et al. MEFV gene mutations and their clinical significance in Korean patients with adult-onset Still’s disease. Clin Exp Rheumatol. (2013) 31(3 Suppl. 77):60–3. [PubMed] [Google Scholar]
- 21.Deftereos SG, Siasos G, Giannopoulos G, Vrachatis DA, Angelidis C, Giotaki SG, et al. The Greek study in the effects of colchicine in COvid-19 complications prevention (GRECCO-19 study): rationale and study design. Hellenic J Cardiol. (2020) 61:42–5. 10.1016/j.hjc.2020.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen IY, Moriyama M, Chang MF, Ichinohe T. Severe acute respiratory syndrome coronavirus Viroporin 3a activates the NLRP3 inflammasome. Front Microbiol. (2019) 10:50. 10.3389/fmicb.2019.00050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nerlekar N, Beale A, Harper RW. Colchicine–a short history of an ancient drug. Med J Aust. (2014) 201:687–8. 10.5694/mja14.00846 [DOI] [PubMed] [Google Scholar]
- 24.Portincasa P. Colchicine, biologic agents and more for the treatment of familial mediterranean fever. the old, the new, and the rare. Curr Med Chem. (2016) 23:60–86. 10.2174/0929867323666151117121706 [DOI] [PubMed] [Google Scholar]
- 25.Varan O, Kucuk H, Babaoglu H, Atas N, Salman RB, Satis H, et al. Effect of interleukin-1 antagonists on the quality of life in familial Mediterranean fever patients. Clin Rheumatol. (2019) 38:1125–30. 10.1007/s10067-018-4384-8 [DOI] [PubMed] [Google Scholar]
- 26.Sag E, Akal F, Atalay E, Akca UK, Demir S, Demirel D, et al. Anti-IL1 treatment in colchicine-resistant paediatric FMF patients: real life data from the HELIOS registry. Rheumatology. (2020). 10.1093/rheumatology/keaa121 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 27.Curtin N, Banyai K, Thaventhiran J, Le Quesne J, Helyes Z, Bai P. Repositioning PARP inhibitors for SARS-CoV-2 infection (COVID-19); a new multi-pronged therapy for ARDS? Br J Pharmacol. (2020) 177:3635–45. 10.1111/bph.15137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Day JW, Fox TA, Halsey R, Carpenter B, Kottaridis PD. IL−1 blockade with anakinra in acute leukaemia patients with severe COVID−19 pneumonia appears safe and may result in clinical improvement. Br J Haematol. (2020) 190:e80–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pontali E, Volpi S, Antonucci G, Castellaneta M, Buzzi D, Tricerri F, et al. Safety and efficacy of early high-dose IV anakinra in severe COVID-19 lung disease. J Allergy Clin Immunol. (2020) 146:213–5. 10.1016/j.jaci.2020.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ, et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. (2020) 395:1033–4. 10.1016/S0140-6736(20)30628-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jamilloux Y, Henry T, Belot A, Viel S, Fauter M, El Jammal T, et al. Should we stimulate or suppress immune responses in COVID-19? Cytokine and anti-cytokine interventions. Autoimmunity Rev. (2020) 19:102567. 10.1016/j.autrev.2020.102567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cure MC, Kucuk A, Cure E. Colchicine may not be effective in COVID-19 infection; it may even be harmful? Clin Rheumatol. (2020) 39:2101–2. 10.1007/s10067-020-05144-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gendelman O, Amital H, Bragazzi NL, Watad A, Chodick G. Continuous hydroxychloroquine or colchicine therapy does not prevent infection with SARS-CoV-2: insights from a large healthcare database analysis. Autoimmunity Rev. (2020) 19:102566. 10.1016/j.autrev.2020.102566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lamkanfi M, Dixit VM. Mechanisms and functions of inflammasomes. Cell. (2014) 157:1013–22. 10.1016/j.cell.2014.04.007 [DOI] [PubMed] [Google Scholar]
- 35.Lamkanfi M. Emerging inflammasome effector mechanisms. Nature Rev. Immunol. (2011) 11:213–20. 10.1038/nri2936 [DOI] [PubMed] [Google Scholar]
- 36.Danthi P. Viruses and the diversity of cell death. Annu Rev Virol. (2016) 3:533–53. 10.1146/annurev-virology-110615-042435 [DOI] [PubMed] [Google Scholar]
- 37.Stella A, Cortellessa F, Scaccianoce G, Pivetta B, Settimo E, Portincasa P. Familial Mediterranean fever: breaking all the (genetic) rules. Rheumatology. (2019) 58:463–7. 10.1093/rheumatology/key328 [DOI] [PubMed] [Google Scholar]
- 38.Yu JW, Fernandes-Alnemri T, Datta P, Wu J, Juliana C, Solorzano L, et al. Pyrin activates the ASC pyroptosome in response to engagement by autoinflammatory PSTPIP1 mutants. Molecular Cell. (2007) 28:214–27. 10.1016/j.molcel.2007.08.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Accetturo M, D’Uggento AM, Portincasa P, Stella A. Improvement of MEFV gene variants classification to aid treatment decision making in familial Mediterranean fever. Rheumatology. (2020) 59:754–61. 10.1093/rheumatology/kez332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chae JJ, Wood G, Masters SL, Richard K, Park G, Smith BJ, et al. The B30.2 domain of pyrin, the familial Mediterranean fever protein, interacts directly with caspase-1 to modulate IL-1beta production. Proc Natl Acad Sci USA. (2006) 103:9982–7. 10.1073/pnas.0602081103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schaner P, Richards N, Wadhwa A, Aksentijevich I, Kastner D, Tucker P, et al. Episodic evolution of pyrin in primates: human mutations recapitulate ancestral amino acid states. Nature Genet. (2001) 27:318–21. 10.1038/85893 [DOI] [PubMed] [Google Scholar]
- 42.Van Gorp H, Huang L, Saavedra P, Vuylsteke M, Asaoka T, Prencipe G, et al. Blood-based test for diagnosis and functional subtyping of familial Mediterranean fever. Ann Rheum Dis. (2020) 79:960–8. 10.1136/annrheumdis-2019-216701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chae JJ, Cho YH, Lee GS, Cheng J, Liu PP, Feigenbaum L, et al. Gain-of-function Pyrin mutations induce NLRP3 protein-independent interleukin-1beta activation and severe autoinflammation in mice. Immunity. (2011) 34:755–68. 10.1016/j.immuni.2011.02.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Park YH, Remmers EF, Lee W, Ombrello AK, Chung LK, Shilei Z, et al. Ancient familial Mediterranean fever mutations in human pyrin and resistance to Yersinia pestis. Nature Immunol. (2020) 21:857–67. 10.1038/s41590-020-0705-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schnappauf O, Chae JJ, Kastner DL, Aksentijevich I. The pyrin inflammasome in health and disease. Front Immunol. (2019) 10:1745. 10.3389/fimmu.2019.01745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gangemi S, Manti S, Procopio V, Casciaro M, Di Salvo E, Cutrupi M, et al. Lack of clear and univocal genotype-phenotype correlation in familial Mediterranean fever patients: a systematic review. Clin Genet. (2018) 94:81–94. 10.1111/cge.13223 [DOI] [PubMed] [Google Scholar]
- 47.Papadopoulos VP, Giaglis S, Mitroulis I, Ritis K. The population genetics of familial mediterranean fever: a meta-analysis study. Ann Hum Genet. (2008) 72(Pt 6):752–61. 10.1111/j.1469-1809.2008.00471.x [DOI] [PubMed] [Google Scholar]
- 48.Cekin N, Akyurek ME, Pinarbasi E, Ozen F. MEFV mutations and their relation to major clinical symptoms of Familial Mediterranean Fever. Gene. (2017) 626:9–13. 10.1016/j.gene.2017.05.013 [DOI] [PubMed] [Google Scholar]
- 49.Bonfrate L, Scaccianoce G, Palasciano G, Ben-Chetrit E, Portincasa P. A novel cluster of patients with familial Mediterranean Fever (FMF) in southern Italy. Eur J Clin Invest. (2017) 47:622–9. 10.1111/eci.12783 [DOI] [PubMed] [Google Scholar]
- 50.Koshy R, Sivadas A, Scaria V. Genetic epidemiology of familial Mediterranean fever through integrative analysis of whole genome and exome sequences from Middle East and North Africa. Clin Genet. (2018) 93:92–102. 10.1111/cge.13070 [DOI] [PubMed] [Google Scholar]
- 51.Fujikura K. Global epidemiology of Familial Mediterranean fever mutations using population exome sequences. Mol Genet Genomic Med. (2015) 3:272–82. 10.1002/mgg3.140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fumagalli M, Cagliani R, Pozzoli U, Riva S, Comi GP, Menozzi G, et al. A population genetics study of the familial Mediterranean fever gene: evidence of balancing selection under an overdominance regime. Genes Immunity. (2009) 10:678–86. 10.1038/gene.2009.59 [DOI] [PubMed] [Google Scholar]
- 53.Louvrier C, Assrawi E, El Khouri E, Melki I, Copin B, Bourrat E, et al. NLRP3-associated autoinflammatory diseases: phenotypic and molecular characteristics of germline versus somatic mutations. J Allergy Clin Immunol. (2020) 145:1254–61. 10.1016/j.jaci.2019.11.035 [DOI] [PubMed] [Google Scholar]
- 54.Levy R, Gerard L, Kuemmerle-Deschner J, Lachmann HJ, Kone-Paut I, Cantarini L, et al. Phenotypic and genotypic characteristics of cryopyrin-associated periodic syndrome: a series of 136 patients from the Eurofever Registry. Ann Rheum Dis. (2015) 74:2043–9. 10.1136/annrheumdis-2013-204991 [DOI] [PubMed] [Google Scholar]
- 55.Cardone M, Yano M, Rosenberg AS, Puig M. Lessons Learned to Date on COVID-19 Hyperinflammatory Syndrome: considerations for Interventions to Mitigate SARS-CoV-2 Viral Infection and Detrimental Hyperinflammation. Front Immunol. (2020) 11:1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.He L, Ding Y, Zhang Q, Che X, He Y, Shen H, et al. Expression of elevated levels of pro-inflammatory cytokines in SARS-CoV-infected ACE2+ cells in SARS patients: relation to the acute lung injury and pathogenesis of SARS. J Pathol. (2006) 210:288–97. 10.1002/path.2067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Siu KL, Yuen KS, Castano-Rodriguez C, Ye ZW, Yeung ML, Fung SY, et al. Severe acute respiratory syndrome coronavirus ORF3a protein activates the NLRP3 inflammasome by promoting TRAF3-dependent ubiquitination of ASC. FASEB J. (2019) 33:8865–77. 10.1096/fj.201802418R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Issa E, Merhi G, Panossian B, Salloum T, Tokajian S. SARS-CoV-2 and ORF3a: nonsynonymous Mutations, Functional Domains, and Viral Pathogenesis. mSystems. (2020) 5:e266–220. 10.1128/mSystems.00266-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thomas PG, Dash P, Aldridge JR, Jr., Ellebedy AH, Reynolds C, Funk AJ, et al. The intracellular sensor NLRP3 mediates key innate and healing responses to influenza A virus via the regulation of caspase-1. Immunity. (2009) 30:566–75. 10.1016/j.immuni.2009.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Allen IC, Scull MA, Moore CB, Holl EK, McElvania-TeKippe E, Taxman DJ, et al. The NLRP3 inflammasome mediates in vivo innate immunity to influenza A virus through recognition of viral RNA. Immunity. (2009) 30:556–65. 10.1016/j.immuni.2009.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Triantafilou K, Kar S, van Kuppeveld FJ, Triantafilou M. Rhinovirus-induced calcium flux triggers NLRP3 and NLRC5 activation in bronchial cells. Am J Respir Cell Mol Biol. (2013) 49:923–34. 10.1165/rcmb.2013-0032OC [DOI] [PubMed] [Google Scholar]
- 62.Chen IY, Ichinohe T. Response of host inflammasomes to viral infection. Trends Microbiol. (2015) 23:55–63. 10.1016/j.tim.2014.09.007 [DOI] [PubMed] [Google Scholar]
- 63.Ahn M, Anderson DE, Zhang Q, Tan CW, Lim BL, Luko K, et al. Dampened NLRP3-mediated inflammation in bats and implications for a special viral reservoir host. Nat Microbiol. (2019) 4:789–99. 10.1038/s41564-019-0371-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.van den Berg DF, Te Velde AA. Severe COVID-19: NLRP3 inflammasome dysregulated. Front Immunol. (2020) 11:1580. 10.3389/fimmu.2020.01580 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/ Supplementary Material.