Key Points
Question
Do atropine, 0.01%, eyedrops slow the myopia progression and axial elongation when compared with a placebo group in Chinese children?
Findings
In this randomized clinical trial, atropine, 0.01%, eyedrops reduced myopia progression and axial elongation compared with placebo treatment after 1 year.
Meaning
While the clinical relevance of the results cannot be determined from this trial, these results support the potential that atropine, 0.01%, eyedrops can slow myopia progression in Chinese children and warrant future studies to determine longer-term results and potential effects on slowing sight-threatening pathologic changes later in life.
Abstract
Importance
Because studies have suggested that atropine might slow the progression of myopia in children, randomized clinical trials are warranted to understand this potential causal relationship.
Objective
To evaluate the efficacy and safety of atropine, 0.01%, eyedrops on slowing myopia progression and axial elongation in Chinese children.
Design, Setting, and Participants
This was a randomized, placebo-controlled, double-masked study. A total of 220 children aged 6 to 12 years with myopia of −1.00 D to −6.00 D in both eyes were enrolled between April 2018 and July 2018 at Beijing Tongren Hospital, Beijing, China. Cycloplegic refraction and axial length were measured at baseline, 6 months, and 12 months. Adverse events were also recorded.
Interventions
Patients were randomly assigned in a 1:1 ratio to atropine, 0.01%, or placebo groups to be administered once nightly to both eyes for 1 year.
Main Outcomes and Measures
Mean changes and percentage differences in myopia progression and axial elongation between atropine, 0.01%, or placebo groups.
Results
Of 220 participants, 103 were girls (46.8%), and the mean (SD) age was 9.64 (1.68) years. The mean (SD) baseline refractive error and axial length were –2.58 (1.39) D and 24.59 (0.87) mm. Follow-up at 1 year included 76 children (69%) and 83 children (75%) allocated into the atropine, 0.01%, and placebo groups, respectively, when mean myopia progression was −0.49 (0.42) D and −0.76 (0.50) D in the atropine, 0.01%, and placebo groups (mean difference, 0.26 D; 95% CI, 0.12-0.41 D; P < .001), with a relative reduction of 34.2% in myopia progression. The mean (SD) axial elongation in the atropine, 0.01%, group was 0.32 (0.19) mm compared with 0.41 (0.19) mm in the placebo group (mean difference, 0.09 mm; 95% CI, 0.03-0.15 mm; P = .004), with relative reduction of 22.0% in axial elongation. Fifty-one percent and 13.2% of children progressed by at least 0.50 D and 1.00 D in the atropine, 0.01%, group, compared with 69.9% and 34.9% in the placebo group. No serious adverse events related to atropine were reported.
Conclusions and Relevance
While the clinical relevance of the results cannot be determined from this trial, these 1-year results, limited by approximately 70% follow-up, suggest that atropine, 0.01%, eyedrops can slow myopia progression and axial elongation in children and warrant future studies to determine longer-term results and potential effects on slowing sight-threatening pathologic changes later in life.
Trial Registration
http://www.chictr.org.cn Identifier: ChiCTR-IOR-17013898
This randomized clinical trial evaluates the efficacy and safety of atropine, 0.01%, eyedrops on slowing myopia progression and axial elongation in Chinese children
Introduction
Myopia has become a critical public health problem among both children and adults, especially in some East and Southeast Asian countries such as China and Singapore.1,2 A 2016 review3 predicted that approximately half of the world’s population will have myopia by 2050, with 10% being high myopia.3 In China, the prevalence of myopia reaches 5.2% in children aged 6 years, nearly 70% in seventh-grade students, and exceeds 80% in university students.4,5,6 Myopia is not only the most common cause of avoidable visual impairment and blindness, but high or pathologic myopia is also associated with increased risk of irreversible blinding conditions, including myopic retinopathy, retinal detachment, choroidal neovascularization, and glaucoma,7,8,9,10 leading to a heavy cost burden on individuals and communities.11
Several randomized clinical trials have been investigated to halt or slow myopic progression including undercorrection, progressive addition lenses, contact lenses, pirenzepine gel, and increased outdoor activity.12,13,14,15,16 However, the results of their effects are disappointing or positive results of marginal clinical significance. At present, topical atropine has been demonstrated to have the strongest clinical effect on slowing the progression of myopia.17,18,19 In 2006, the Atropine for the Treatment of Myopia 1 (ATOM 1) study found that the mean (SD) rate of myopia progression after 2 years was only −0.28 (0.92) D in the atropine, 1%, group compared with −1.20 (0.69) D in the placebo group.18 However, ocular adverse effects induced by atropine, 1%, such as blurred near vision, photophobia cycloplegia, and allergy, have limited its use. Furthermore, there was a greater myopic rebound in eyes that had received atropine, 0.5% and 0.1%, after treatment was discontinued (ATOM 2 study), whereas those receiving a low-dose 0.01% concentration proved sustained and minimal change after treatment cessation.20 Therefore, low concentration of atropine, 0.01%, is increasingly applied to clinical treatment for children with myopia in Asia.
Most studies have been performed to evaluate the efficacy and safety of low concentration of atropine, 0.01%, through nonrandomized controlled trials,21,22,23 but few data are available from randomized clinical trials, with the exception of 2 in Singapore (ATOM 2 study) and Hong Kong, China (Low-Concentration Atropine for Myopia Progression [LAMP] study).19,24 However, the lack of a placebo control group was an acknowledged weakness of the ATOM 2 study. The LAMP study from Hong Kong, China, first provided placebo-compared data of low-concentration atropine eyedrops in slowing myopia progression.24 Thus, the effect of low concentration of atropine, 0.01%, has not been extensively evaluated through placebo-controlled trial. Therefore, we aimed to evaluate the efficacy and safety of low concentrations of atropine, 0.01%, in this randomized, double-masked, placebo-controlled trial in mainland China.
Methods
Study Design
This is a randomized, double-masked, placebo-controlled trial aimed to investigate the efficacy and safety of low concentrations of atropine, 0.01%, in children with low and moderate myopia from April 2018 to July 2020. Two phases were included in this study. All children were recruited and randomized to receive either atropine, 0.01%, or placebo eyedrops in both eyes once daily at an allocation ratio of 1:1 for 1 year in phase 1. At the beginning of the second year, the atropine, 0.01%, group will be crossed over to the placebo group, and the placebo group will be crossed over to the atropine, 0.01%, group for 1 year in phase 2. All eyedrops in monodose preparation were prepared by Shenyang Xingqi Pharmaceutical Co Ltd. Informed written consent was obtained from at least 1 parent as well as verbal assent from each child. No compensation or incentives were offered to the children and parents. This clinical trial adhered to the tenets of the Declaration of Helsinki and was approved by the institutional review board of Beijing Tongren Hospital, Capital Medical University. The trial protocol is available in Supplement 1.
Study Population
We recruited the children with myopia who visited Beijing Tongren Hospital, Beijing, China, between April 2018 and July 2018. All participants met the following inclusion criteria: children aged 6 to 12 years with refractive error of spherical equivalent (SE) range of −1.00 D to −6.00 D in both eyes, astigmatism of −1.50 D or less in both eyes, best-corrected distance visual acuity 0.20 logMAR or better in both eyes, and intraocular pressure (IOP) of less than 21 mm Hg. Exclusion criteria were as follows: children with other combined ocular diseases (eg, amblyopia, strabismus, corneal scar, cataract, glaucoma, or ocular tumor); previous or current treatment with atropine, pirenzepine, contact lenses, bifocals, or progressive addition lenses for myopia; and allergy to atropine, cyclopentolate, or excipients.
Sample Size
Sample size was calculated based on the results from the previous studies.18,19,23 We assume that atropine, 0.01%, reduces the myopia progression rate by at least −0.36 D with standard deviation of 0.70 D, assuming a power of 90% with a 2-sided test of 5%. Thus, this study required 80 participants in each group. Considering a dropout rate of 25%, a total of 220 participants would be adequate.
Randomization and Masking
With the schedule generated by SAS program (SAS Institute Inc), a statistician operated the randomization independently. Every eligible 4 children were randomly allocated into the intervention group or control group according to the priority order the children visited the hospital for treatment. The atropine, 0.01%, and placebo eyedrops were packaged in identical bottles, and thus, investigators and participants were not able to identify the contents. The data analysts were also blinded to minimize observational bias.
Study Procedures
All children who participated in this study underwent the same, standardized examination procedure at the baseline, 6-month, and 12-month visits. Patients were given a calendar to mark out the days when the trial medications were used and, with more than 80% compliance rate, were considered to be included in the results analysis. Children were also provided photochromatic glasses (which darken on exposure to ultraviolet light or sunlight) if they experienced glare or their parents were worried of excessive light exposure or progressive glasses (reading add) if they experienced difficulty with near vision.
Cycloplegic refraction was measured by an autorefractor (HRK7000 A; Huvitz) 3 times consecutively, with average data used for analysis. All 3 readings should be at most 0.25 D apart in both the spherical and cylinder components. During the examination of each patient, 3 drops of cyclopentolate, 1% (Alcon), were administered at a 5-minute interval. Thirty minutes after the last drop, if pupillary light reflex was still present or the pupil size was less than 6.0 mm, a fourth drop of 1% cyclopentolate was administered and the examination was repeated 15 minutes later. Axial length (AL) was measured using the Lenstar LS900 (Haag-Streit), with 5 readings taken and averaged. A noncontact tonometer (HNT-7000; Huvitz) was used to measure the intraocular pressure with 3 repeated measurements.
Additionally, a detailed interviewer-administered questionnaire answered by parents was used to collect the information of their children on the age at myopia onset, number of parents with myopia, and time near work and outdoors activities (hours per day) after school hours.4,25,26
Outcomes
The SE was calculated as the dioptric powers of the sphere and half of the cylinder (sphere + 0.5 × cylinder). Myopia progression defined as the mean change in cycloplegic SE over 1 year. Three levels of myopia progression were defined as less than 0.50 D (mild), between 0.50 D and less than 1.00 D (moderate), and 1.00 D or greater (severe). If the myopia progression was less than 0.50 D over 1 year, children were considered as nonprogressors and otherwise as progressors. The secondary outcomes included AL change over 1 year. Adverse events were recorded based on what patients and parents were asked and examined during the treatment.
Statistical Analyses
For all analyses, SPSS, version 20.0 (SPSS), was used. For continuous variables, the independent t test and analysis of covariance were used to determine statistical significance between the atropine and control groups. The χ2 tests were used to compare the categorized data. To explore potential risk factors, including age at baseline, sex, initial spherical equivalent, intraocular pressure, age at myopia onset, parental myopia, time outdoors, and near work, associated with progressors in atropine, 0.01%, group, the multiple log-binomial regression analysis was performed using those factors as the dependent variable. Analyses were only performed on the right eye. A 2-sided P-value less than .05 was considered statistically significant for the primary outcome.
Results
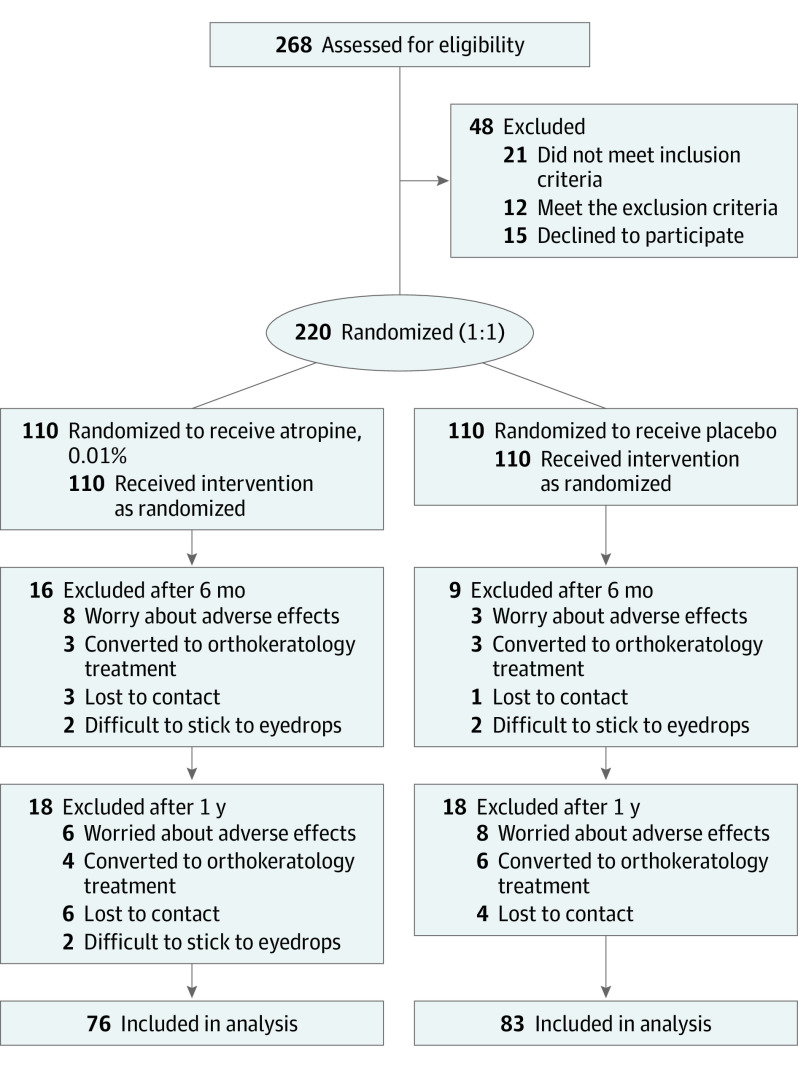
Between April 2018 and July 2019, a total of 268 children were initially assessed in this study; 21 of them did not meet the inclusion criteria, 12 of them met the exclusion criteria, and 15 of them declined to participate in this study, leaving 220 children were enrolled in the study with equal randomization to the atropine, 0.01%, and the placebo-control groups (Figure 1). At baseline, there were no relevant differences identified in demographics, initial SE, initial AL, IOP, age at myopia onset, parental myopia, time outdoors, and near work between 2 groups (Table 1). Twenty-five participants (11.4%) did not complete the first 6 months of the study (16 in the atropine, 0.01%, group and 9 in the placebo group; Figure 1). Finally, a total of 76 children (69%) and 83 children (75%) allocated into the atropine, 0.01%, and placebo groups, respectively, returned for the 1-year primary outcome assessment visit (Figure 1). There was no significant difference in initial SE and AL between completed and discontinued patients in the atropine, 0.01%, group as well as placebo group. Baseline characteristics for those children who completed the 1-year follow-up are shown in eTable 1 in Supplement 2.
Figure 1. Flowchart of Randomized Individuals.
Table 1. Demographics and Baseline Characteristics of Participants.
| Variables | Group, mean (SD) | |
|---|---|---|
| Atropine (n = 110) | Placebo (n = 110) | |
| Age, y, No. (%) | ||
| 6 to <7 | 9 (8.2) | 4 (3.6) |
| 7 to <8 | 11 (10.0) | 7 (6.4) |
| 8 to <9 | 28 (25.5) | 20 (18.2) |
| 9 to <10 | 22 (20.0) | 27 (24.5) |
| 10 to <11 | 20 (18.2) | 23 (20.9) |
| 11 to <12 | 20 (18.2) | 29 (26.4) |
| Mean (SD) | 9.44 (1.80) | 9.84 (1.53) |
| Male/female, No. | 56/54 | 61/49 |
| Initial SE, D | ||
| 6 to <7 y | −2.14 (1.25) | −2.95 (2.43) |
| 7 to <8 y | −1.89 (0.98) | −2.30 (1.69) |
| 8 to <9 y | −2.20 (1.03) | −2.70 (1.22) |
| 9 to <10 y | −2.53 (1.44) | −2.23 (1.29) |
| 10 to <11 y | −2.36 (1.09) | −2.77 (1.44) |
| 11 to <12 y | −3.04 (1.25) | −2.91 (1.60) |
| Mean (SD) | −2.52 (1.33) | −2.64 (1.46) |
| Initial AL, mm | ||
| 6 to <7 y | 24.03 (0.82) | 24.70 (0.50) |
| 7 to <8 y | 24.00 (0.57) | 24.37 (1.24) |
| 8 to <9 y | 24.29 (0.57) | 24.51 (0.73) |
| 9 to <10 y | 24.45 (0.59) | 24.58 (0.94) |
| 10 to <11 y | 24.69 (0.53) | 24.86 (0.98) |
| 11 to <12 y | 24.99 (0.82) | 24.86 (1.11) |
| Mean (SD) | 24.50 (0.76) | 24.69 (0.97) |
| IOP, mm Hg | 15.84 (2.89) | 15.84 (2.76) |
| Age at myopia onset, D | 7.76 (1.70) | 8.07 (1.68) |
| Parental myopia, No. (%) | ||
| None | 5 (4.5) | 7 (6.4) |
| 1 | 42 (38.2) | 34 (30.9) |
| Both | 63 (57.3) | 69 (62.7) |
| Time outdoors, h/d | 1.40 (0.59) | 1.55 (0.57) |
| Near work, h/d | 3.21 (1.42) | 3.37 (1.44) |
Abbreviations: AL, axial length; IOP, intraocular pressure; SE, spherical equivalent.
At the 1-year follow-up, the mean (SD) myopia progression values for the atropine, 0.01%, group and the placebo group were −0.49 (0.42) D and −0.76 (0.50) D (mean difference, 0.26 D; 95% CI, 0.12-0.41 D; P < .001), with relative reduction of 34.2% in myopia progression (Table 2). Nonprogressors had a mean myopia progression of −0.14 (0.23) D compared with −0.83 (0.24) D for progressors (P < .001). The mean 1-year change in spherical equivalent was −0.49 D (95% CI, −0.59 to −0.39 D) for the atropine, 0.01%, group and −0.77 D (95% CI, −0.86 to −0.67 D) for the placebo group (analysis of covariance, mean difference, 0.28 D; 95% CI, 0.14-0.42 D; P < .001) after adjusting for age at baseline. The mean (SD) axial elongation values for the atropine, 0.01%, group and placebo group were 0.32 (0.19) mm and 0.41 (0.19) mm (mean difference, 0.09 mm; 95% CI, 0.03-0.15 mm; P = .004), with a reduction of 22.0% in axial elongation (Table 2). Nonprogressors had a mean (SD) axial elongation of −0.18 (0.14) mm, compared with −0.45 (0.14) mm for progressors (P < .001). The mean 1-year change in axial length was 0.31 mm (95% CI, 0.27-0.35 mm) for the atropine, 0.01%, group and 0.41 mm (95% CI, 0.37-0.45 mm) for the placebo group (analysis of covariance, mean difference, 0.10 mm; 95% CI, 0.05-0.16 mm; P < .001) after adjusting for age at baseline.
Table 2. Mean Change in SE and AL at Follow-up Visits.
| Variables | Group | Difference | 95% CI | P value | |
|---|---|---|---|---|---|
| Atropine (n = 76) | Placebo (n = 83) | ||||
| SE, mean (SD), D | |||||
| Change at 6 mo | −0.21 (0.32) | −0.36 (0.38) | 0.16 (0.06) | 0.05-0.27 | .005 |
| Change at 12 mo | −0.49 (0.42) | −0.76 (0.50) | 0.26 (0.07) | 0.12-0.41 | <.001 |
| AL, mean (SD), mm | |||||
| Change at 6 mo | 0.16 (0.12) | 0.21 (0.11) | 0.05 (0.02) | 0.02-0.09 | .005 |
| Change at 12 mo | 0.32 (0.19) | 0.41 (0.19) | 0.09 (0.03) | 0.03-0.15 | .004 |
Abbreviations: AL, axial length; SE, spherical equivalent.
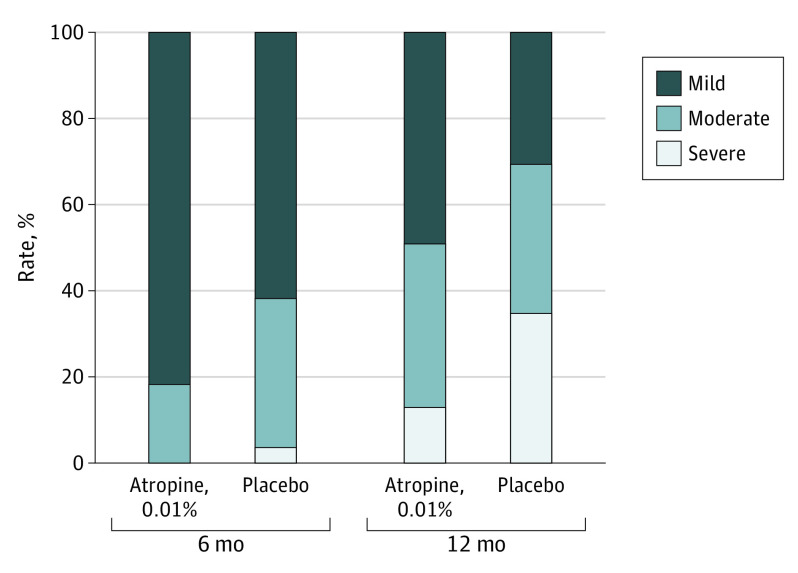
At 6 months, 81.6% of children progressed by less than 0.5 D in the atropine, 0.01%, group compared with 61.5% in the placebo group, whereas no children progressed by 1.00 D or greater, compared with 3.6% in the placebo group (Figure 2). At 12 months, 48.7% of children progressed by less than 0.50 D and 13.2% at least 1.00 D in the atropine, 0.01%, group, compared with 30.1% and 34.9%, respectively, in the placebo group (Figure 2).
Figure 2. Distribution of Mild, Moderate, and Severe Myopia Progression in atropine, 0.01%, and Placebo Groups at 6 and 12 Months.
Myopia progression from baseline if less than 0.50 D (mild), between 0.50 D and less than 1.00 D (moderate), and greater than 1.00 D (severe).
eTable 2 in Supplement 2 presents a comparison of demographics and other characteristics between nonprogressors and progressors in the atropine, 0.01%, group. In multiple log-binomial regression analyses, among the atropine, 0.01%, group, a higher risk of being a progressor was associated with initial SE (relative risk [RR], 1.324; 95% CI, 1.048-1.620; P = .004; eTable 3 in Supplement 2). The adjusted analysis showed that the risk of progressive myopia increased by 32.4% for every 1.0 D less initial SE. However, among the placebo group, no association was found for risk factors with progressors in multiple log-binomial regression analyses (eTable 4 in Supplement 2).
No serious adverse events associated with atropine were reported. Five children (4.5%) reported photophobia in the atropine, 0.01%, group compared with 1 child (0.9%) in the control group. Allergic reactions were uncommon, with 4 children experiencing allergic conjunctivitis; 1 of them was in control group. None of the children in either group reported near-blurred vision.
Discussion
In this study, we found that a once-nightly dose of atropine, 0.01%, eyedrops resulted in reduction of myopia progression by a mean (SD) of 0.26 (0.07) D (34.2% reduction) and axial elongation by 0.09 (0.03) mm (22.0% reduction) compared with placebo treatment. To our knowledge, this study is the first randomized, double-masked, placebo-controlled trial to show the efficacy of atropine, 0.01%, eyedrops in mainland China. However, the clinical relevance of these results cannot be determined from this trial because there was a loss to follow-up of approximately 30%, the follow-up is limited to 1 year, and it is not known whether these findings translate to a slowing of pathologic myopia.
A summary of the randomized clinical trials and case-control studies for atropine, 0.01%, is presented in Table 3. In a retrospective case-control study21 of 52 treated and 50 control European children, the atropine, 0.01%, group had a slower myopia progression of a mean (SD) of 0.54 (0.26) D/y than that of the control group at −1.09 (0.64) D/y. In 2019, the LAMP study24 first provided placebo-compared evidence of low-concentration atropine eyedrops in myopia control. After 1 year, the mean (SD) myopic progression was −0.27 (0.61) D, −0.46 (0.45) D, −0.59 (0.61) D, and −0.81 (0.53) D, in the atropine, 0.05%, 0.025%, and 0.01%, groups and placebo groups, respectively. In our study, the mean (SD) difference between the atropine, 0.01%, and placebo groups in myopia progression was 0.26 (0.07) D (34.2% reduction) at 1 year. It should be noted that the relative reduction of 34.2% in myopia progression in our study was smaller than the study conducted in the Europe.21 We hypothesize that this may owing to the fact that less pigmented eyes, on average, are more sensitive to cycloplegic agents for those of populations of European origin. In addition, the educational systems between Eastern and Western cultures, on average, may be different.27,28 Eastern students, on average, may be more likely to spend more time on their studies, potentially making them more susceptible to myopia progression.26,29,30 Therefore, it may be more difficult to slow the rate of myopia progression for Eastern students.
Table 3. Summary of Design and Key Results From RCTs Case-Control Studies That Include Atropine, 0.01%.
| Source | Design | Duration | Myopia range, D | Treatments | Mean (SD) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age range, y | Size | Duration | Baseline age, y | Average myopia, D | Myopia progression, D | Axial elongation, mm | ||||
| This study, 2020, Mainland China | RCT | 6-12 | 220 | 1 | −0.5 to −6 | A, 0.01% | 9.44 (1.80) | −2.52 (1.33) | −0.49 (0.42) | 0.32 (0.19) |
| Placebo | 9.84 (1.53) | −2.64 (1.46) | −0.76 (0.50) | 0.41 (0.19) | ||||||
| Chia et al,19 2012, Singapore | RCT | 6-12 | 400 | 2 | −2 to −6 | A, 0.5% | 9.7 (1.5) | −4.3 (1.8) | −0.30 (0.60) | 0.27 (0.25) |
| A, 0.1% | 9.7 (1.6) | −4.5 (1.4) | −0.38 (0.60) | 0.28 (0.28) | ||||||
| A, 0.01% | 9.5 (1.5) | −4.5 (1.5) | −0.49 (0.63) | 0.41 (0.32) | ||||||
| Clark et al,23 2015, United States | Retrospective | 6-15 | 60 | 1 | −0.25 to −8.00 | A, 0.01% | 10.2 (2.2) | −2.0 (1.6) | −0.1 (0.6) | NA |
| Placebo | 10.2 (2.2) | −2.0 (1.5) | −0.6 (0.4) | NA | ||||||
| Yam et al,24 2018, Hong Kong, China | RCT | 4-12 | 438 | 1 | At least −1.0 | A, 0.05% | 8.45 (1.81) | −3.98 (1.69) | −0.27 (0.61) | 0.20 (0.25) |
| A, 0.025% | 8.54 (1.71) | −3.71 (1.85) | −0.46 (0.45) | 0.29 (0.20) | ||||||
| A, 0.01% | 8.23 (1.83) | −3.77 (1.85) | −0.59 (0.61) | 0.36 (0.29) | ||||||
| Placebo | 8.42 (1.72) | −3.85 (1.95) | −0.81 (0.53) | 0.41 (0.22) | ||||||
| Sacchi et al,21 2019, Italy | Retrospective | 5-16 | 102 | 1 | NA | A, 0.01% | 9.7 (2.3) | −3.00 (2.23) | −0.54 (0.61) | NA |
| Placebo | 12.1 (2.9) | −2.63 (2.68) | −1.09 (0.64) | NA | ||||||
| Larkin et al,22 2019, United States | Retrospective | 6-15 | 198 | 2 | −0.25 to −8.00 | A, 0.01% | 9.3 (2.1) | −3.1 (1.9) | −0.2 (0.8) | NA |
| Placebo | 9.2 (2.1) | −2.8 (1.6) | −0.6 (0.4) | NA | ||||||
Abbreviations: A, atropine; NA, not applicable; RCT, randomized clinical trial.
The LAMP study24 found 43.8% of patients had myopic progression by less than 0.50 D in the atropine, 0.01%, group over 1 year, which is in agreement with the LAMP study, found 48.7% in our study. Thus, it should be noted that more than 50% of children progressed by at least 0.5 D after 1 year of atropine, 0.01%, treatment. Previous studies have demonstrated a dose-dependent effect of atropine on reducing myopia progression.19,24 It would may be a reasonable strategy for those children with myopia to be treated initially with atropine, 0.01%; if myopia progression was still faster, then change to a higher concentration.31 In a retrospective, case-control study, Lee et al32 found that only 16.7% of patients treated with atropine, 0.05%, eyedrops progressed greater than 0.50 D in 1 year.
A previous study33 has shown that the risk of progressive myopia was associated with younger age at baseline and higher initial SE among the 182 children treated with atropine, 1%, for 1 year. As found in this study, our study observed that the risk of progressive myopia was associated with younger age at baseline in the unadjusted regression analysis. This may be predominantly explained by the fact that younger children experience more myopia progression,34 leading to more progressors in younger age. Thus, it should be noted that younger children with myopia who received atropine, 0.01%, for treatment should be more aware of whether they need to switch to higher-concentration atropine treatment.
Fewer studies have reported the efficacy of the atropine eyedrops in slowing axial elongation, although it is important to reflect the treatment effect. To date, the LAMP study24 has provided the only direct comparison of changes in AL between the atropine, 0.01%, and placebo. This study found that a mean (SD) axial length change was 0.36 (0.29) mm and 0.41 (0.22) mm in the atropine, 0.01%, and placebo groups over 1 year, with reduction of only 12% in axial elongation. Our study showed that mean (SD) axial elongation values for the atropine, 0.01%, group and placebo group were 0.32 (0.19) mm and 0.41 (0.19) mm at 1 year, with reduction of 22.0% in axial elongation. Although the mean progression of axial length was lower in the atropine, 0.01%, group compared with the control group in our study, the reduction of axial elongation by 0.09 mm at 1 year is not of great clinical significance.
Overall, atropine, 0.01%, was generally well tolerated and no serious adverse effects were observed.19,24,35 Similar to our experience, Yam et al24 observed 2 participants (2.1%) had photophobia and 7 participants (6.4%) had allergic conjunctivitis in the atropine, 0.01%, group. The LAMP study reported a small reduction in accommodation by 0.26 D, a dilation of pupils by 0.5 mm in atropine, 0.01%, group, which is not a major clinical issue.24 Cooper et al36 reported atropine, 0.02%, as the threshold dose without producing significant clinical symptoms from accommodation paresis or pupillary dilation by comparing different doses of topical atropine in adolescents.
Strengths and Limitations
The strengths of this study included its randomized, double-masked, and placebo-controlled trial design, the standardized measurement of refractive errors using cycloplegia, and the inclusion of outcome by performing axial length. However, there are several shortcomings in our study. First, we could not avoid the potential for unmasking of the participants attributable to the atropine-induced photophobia and cycloplegia as well as other atropine eyedrops studies. In addition, this study only evaluated the efficacy of low-concentration atropine eyedrops at the level of 0.01%. Thus, further studies should be carried out to compare the efficacy of other lower doses of atropine in mainland China. Other limitations include the potential bias introduced by loss to follow-up of approximately 70% in each group, follow-up only through 1 year, limited information on the clinical relevance of the magnitude of the greater slowing of myopia in the atropine group, and inability to determine whether the atropine effect has relevance for reducing the development or progression of pathologic myopia.
Conclusions
Our study discovered that atropine, 0.01%, can slow the progression of myopia and axial length in children with low and moderate myopia, compared with placebo treatment. A once-nightly dose of atropine, 0.01%, eyedrops was well tolerated without serious adverse events. While the clinical relevance of the results cannot be determined from this trial, these 1-year results, limited by approximately 70% follow-up, suggest that atropine, 0.01%, eyedrops can slow myopia progression and axial elongation in children and warrant future studies to determine longer-term results and potential effects on slowing sight-threatening pathologic changes later in life.
Trial protocol
eTable 1. Demographics and Baseline Characteristics of Those Completing the 1 Year Follow-up
eTable 2. Comparison of Demographics and Other Characteristics between
Nonprogressors and Progressors in atropine, 0.01%, Group
eTable 3. Log-binomial Regression Analysis of Risk Factors for Progressive Myopia in atropine, 0.01%, Group
eTable 4. Log-binomial Regression Analysis of Risk Factors for Progressive Myopia in Placebo Group
Data-sharing statement
References
- 1.Morgan IG, Ohno-Matsui K, Saw SM. Myopia. Lancet. 2012;379(9827):1739-1748. doi: 10.1016/S0140-6736(12)60272-4 [DOI] [PubMed] [Google Scholar]
- 2.Pan CW, Ramamurthy D, Saw SM. Worldwide prevalence and risk factors for myopia. Ophthalmic Physiol Opt. 2012;32(1):3-16. doi: 10.1111/j.1475-1313.2011.00884.x [DOI] [PubMed] [Google Scholar]
- 3.Holden BA, Fricke TR, Wilson DA, et al. Global prevalence of myopia and high myopia and temporal trends from 2000 through 2050. Ophthalmology. 2016;123(5):1036-1042. doi: 10.1016/j.ophtha.2016.01.006 [DOI] [PubMed] [Google Scholar]
- 4.Ma Y, Qu X, Zhu X, et al. Age-specific prevalence of visual impairment and refractive error in children aged 3-10 years in Shanghai, China. Invest Ophthalmol Vis Sci. 2016;57(14):6188-6196. doi: 10.1167/iovs.16-20243 [DOI] [PubMed] [Google Scholar]
- 5.Li SM, Liu LR, Li SY, et al. ; Anyang Childhood Eye Study Group . Design, methodology and baseline data of a school-based cohort study in Central China: the Anyang Childhood Eye Study. Ophthalmic Epidemiol. 2013;20(6):348-359. doi: 10.3109/09286586.2013.842596 [DOI] [PubMed] [Google Scholar]
- 6.Wei S, Sun Y, Li S, et al. Refractive errors in university students in central China: the Anyang University Students Eye Study. Invest Ophthalmol Vis Sci. 2018;59(11):4691-4700. doi: 10.1167/iovs.18-24363 [DOI] [PubMed] [Google Scholar]
- 7.Saw SM, Gazzard G, Shih-Yen EC, Chua WH. Myopia and associated pathological complications. Ophthalmic Physiol Opt. 2005;25(5):381-391. doi: 10.1111/j.1475-1313.2005.00298.x [DOI] [PubMed] [Google Scholar]
- 8.Wong TY, Ferreira A, Hughes R, Carter G, Mitchell P. Epidemiology and disease burden of pathologic myopia and myopic choroidal neovascularization: an evidence-based systematic review. Am J Ophthalmol. 2014;157(1):9-25.e12. [DOI] [PubMed] [Google Scholar]
- 9.Mitchell P, Hourihan F, Sandbach J, Wang JJ. The relationship between glaucoma and myopia: the Blue Mountains Eye Study. Ophthalmology. 1999;106(10):2010-2015. doi: 10.1016/S0161-6420(99)90416-5 [DOI] [PubMed] [Google Scholar]
- 10.Liang YB, Friedman DS, Wong TY, et al. ; Handan Eye Study Group . Prevalence and causes of low vision and blindness in a rural chinese adult population: the Handan Eye Study. Ophthalmology. 2008;115(11):1965-1972. doi: 10.1016/j.ophtha.2008.05.030 [DOI] [PubMed] [Google Scholar]
- 11.Smith TS, Frick KD, Holden BA, Fricke TR, Naidoo KS. Potential lost productivity resulting from the global burden of uncorrected refractive error. Bull World Health Organ. 2009;87(6):431-437. doi: 10.2471/BLT.08.055673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walline JJ, Lindsley K, Vedula SS, Cotter SA, Mutti DO, Twelker JD. Interventions to slow progression of myopia in children. Cochrane Database Syst Rev. 2011;(12):CD004916. doi: 10.1002/14651858.CD004916.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Siatkowski RM, Cotter SA, Crockett RS, Miller JM, Novack GD, Zadnik K; U.S. Pirenzepine Study Group . Two-year multicenter, randomized, double-masked, placebo-controlled, parallel safety and efficacy study of 2% pirenzepine ophthalmic gel in children with myopia. J AAPOS. 2008;12(4):332-339. doi: 10.1016/j.jaapos.2007.10.014 [DOI] [PubMed] [Google Scholar]
- 14.Li SM, Kang MT, Wu SS, et al. Studies using concentric ring bifocal and peripheral add multifocal contact lenses to slow myopia progression in school-aged children: a meta-analysis. Ophthalmic Physiol Opt. 2017;37(1):51-59. doi: 10.1111/opo.12332 [DOI] [PubMed] [Google Scholar]
- 15.Wu PC, Tsai CL, Wu HL, Yang YH, Kuo HK. Outdoor activity during class recess reduces myopia onset and progression in school children. Ophthalmology. 2013;120(5):1080-1085. doi: 10.1016/j.ophtha.2012.11.009 [DOI] [PubMed] [Google Scholar]
- 16.Li SM, Li SY, Liu LR, et al. Full correction and undercorrection of myopia evaluation trial: design and baseline data of a randomized, controlled, double-blind trial. Clin Exp Ophthalmol. 2013;41(4):329-338. doi: 10.1111/j.1442-9071.2012.02884.x [DOI] [PubMed] [Google Scholar]
- 17.Li SM, Wu SS, Kang MT, et al. Atropine slows myopia progression more in Asian than white children by meta-analysis. Optom Vis Sci. 2014;91(3):342-350. doi: 10.1097/OPX.0000000000000178 [DOI] [PubMed] [Google Scholar]
- 18.Chua WH, Balakrishnan V, Chan YH, et al. Atropine for the treatment of childhood myopia. Ophthalmology. 2006;113(12):2285-2291. doi: 10.1016/j.ophtha.2006.05.062 [DOI] [PubMed] [Google Scholar]
- 19.Chia A, Chua WH, Cheung YB, et al. Atropine for the treatment of childhood myopia: safety and efficacy of 0.5%, 0.1%, and 0.01% doses (Atropine for the Treatment of Myopia 2). Ophthalmology. 2012;119(2):347-354. doi: 10.1016/j.ophtha.2011.07.031 [DOI] [PubMed] [Google Scholar]
- 20.Chia A, Chua WH, Wen L, Fong A, Goon YY, Tan D. Atropine for the treatment of childhood myopia: changes after stopping atropine 0.01%, 0.1% and 0.5%. Am J Ophthalmol. 2014;157(2):451-457.e1. doi: 10.1016/j.ajo.2013.09.020 [DOI] [PubMed] [Google Scholar]
- 21.Sacchi M, Serafino M, Villani E, et al. Efficacy of atropine 0.01% for the treatment of childhood myopia in European patients. Acta Ophthalmol. 2019;97(8):e1136-e1140. doi: 10.1111/aos.14166 [DOI] [PubMed] [Google Scholar]
- 22.Larkin GL, Tahir A, Epley KD, Beauchamp CL, Tong JT, Clark RA. Atropine 0.01% eye drops for myopia control in American children: a multiethnic sample across three US sites. Ophthalmol Ther. 2019;8(4):589-598. doi: 10.1007/s40123-019-00217-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clark TY, Clark RA. Atropine 0.01% eyedrops significantly reduce the progression of childhood myopia. J Ocul Pharmacol Ther. 2015;31(9):541-545. doi: 10.1089/jop.2015.0043 [DOI] [PubMed] [Google Scholar]
- 24.Yam JC, Jiang Y, Tang SM, et al. Low-Concentration Atropine for Myopia Progression (LAMP) study: a randomized, double-blinded, placebo-controlled trial of 0.05%, 0.025%, and atropine, 0.01%, eye drops in myopia control. Ophthalmology. 2019;126(1):113-124. doi: 10.1016/j.ophtha.2018.05.029 [DOI] [PubMed] [Google Scholar]
- 25.Li SM, Li H, Li SY, et al. ; Anyang Childhood Eye Study Group . Time outdoors and myopia progression over 2 years in Chinese children: the Anyang Childhood Eye Study. Invest Ophthalmol Vis Sci. 2015;56(8):4734-4740. doi: 10.1167/iovs.14-15474 [DOI] [PubMed] [Google Scholar]
- 26.Li SM, Li SY, Kang MT, et al. ; Anyang Childhood Eye Study Group . Near work related parameters and myopia in Chinese children: the Anyang Childhood Eye Study. PLoS One. 2015;10(8):e0134514. doi: 10.1371/journal.pone.0134514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morgan IG, French AN, Rose KA. Intense schooling linked to myopia. BMJ. 2018;361:k2248. doi: 10.1136/bmj.k2248 [DOI] [PubMed] [Google Scholar]
- 28.Morgan IG, Rose KA. Myopia and international educational performance. Ophthalmic Physiol Opt. 2013;33(3):329-338. doi: 10.1111/opo.12040 [DOI] [PubMed] [Google Scholar]
- 29.Rosner M, Belkin M. Intelligence, education, and myopia in males. Arch Ophthalmol. 1987;105(11):1508-1511. doi: 10.1001/archopht.1987.01060110054030 [DOI] [PubMed] [Google Scholar]
- 30.Wu PC, Chen CT, Lin KK, et al. Myopia prevention and outdoor light intensity in a school-based cluster randomized trial. Ophthalmology. 2018;125(8):1239-1250. doi: 10.1016/j.ophtha.2017.12.011 [DOI] [PubMed] [Google Scholar]
- 31.Wu PC, Chuang MN, Choi J, et al. Update in myopia and treatment strategy of atropine use in myopia control. Eye (Lond). 2019;33(1):3-13. doi: 10.1038/s41433-018-0139-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee JJ, Fang PC, Yang IH, et al. Prevention of myopia progression with 0.05% atropine solution. J Ocul Pharmacol Ther. 2006;22(1):41-46. doi: 10.1089/jop.2006.22.41 [DOI] [PubMed] [Google Scholar]
- 33.Loh KL, Lu Q, Tan D, Chia A. Risk factors for progressive myopia in the atropine therapy for myopia study. Am J Ophthalmol. 2015;159(5):945-949. doi: 10.1016/j.ajo.2015.01.029 [DOI] [PubMed] [Google Scholar]
- 34.Saw SM, Tong L, Chua WH, et al. Incidence and progression of myopia in Singaporean school children. Invest Ophthalmol Vis Sci. 2005;46(1):51-57. doi: 10.1167/iovs.04-0565 [DOI] [PubMed] [Google Scholar]
- 35.Gong Q, Janowski M, Luo M, et al. Efficacy and adverse effects of atropine in childhood myopia: a meta-analysis. JAMA Ophthalmol. 2017;135(6):624-630. doi: 10.1001/jamaophthalmol.2017.1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cooper J, Eisenberg N, Schulman E, Wang FM. Maximum atropine dose without clinical signs or symptoms. Optom Vis Sci. 2013;90(12):1467-1472. doi: 10.1097/OPX.0000000000000037 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol
eTable 1. Demographics and Baseline Characteristics of Those Completing the 1 Year Follow-up
eTable 2. Comparison of Demographics and Other Characteristics between
Nonprogressors and Progressors in atropine, 0.01%, Group
eTable 3. Log-binomial Regression Analysis of Risk Factors for Progressive Myopia in atropine, 0.01%, Group
eTable 4. Log-binomial Regression Analysis of Risk Factors for Progressive Myopia in Placebo Group
Data-sharing statement