This pooled analysis of 2 randomized clinical trials examines the efficacy and safety of oxymetazoline hydrochloride, 0.1%, ophthalmic solution in participants with acquired ptosis.
Key Points
Question
Is administration of a single drop of oxymetazoline hydrochloride, 0.1%, ophthalmic solution (oxymetazoline, 0.1%) for 6 weeks associated with improved superior visual field and reduced upper eyelid droop in acquired blepharoptosis?
Findings
In a pooled analysis from 2 randomized, double-masked, placebo-controlled, multicenter phase 3 clinical trials, treatment with oxymetazoline, 0.1%, was associated with improved superior visual field and upper eyelid elevation vs vehicle after instillation at 1 and 14 days.
Meaning
Oxymetazoline, 0.1%, may be a promising treatment option for patients with acquired blepharoptosis, although further study is needed to assess the clinical relevance of this effect beyond 2 weeks.
Abstract
Importance
Treatment of acquired blepharoptosis (ptosis) is currently limited to surgical intervention.
Objective
To examine the efficacy and safety of oxymetazoline hydrochloride, 0.1%, ophthalmic solution (oxymetazoline, 0.1%) in participants with acquired ptosis.
Design, Setting, and Participants
This pooled analysis of 2 randomized, double-masked, placebo-controlled, multicenter phase 3 clinical trials included participants 9 years and older with acquired ptosis and superior visual field deficit. The 2 studies were conducted across 16 and 27 sites in the United States. Patients were enrolled from May 2015 to April 2019. Analyses for the individual trials were initiated after database lock and completed on September 6, 2017, and May 16, 2019. Pooled analysis was completed on August 25, 2019.
Interventions
Participants (randomized 2:1) received oxymetazoline, 0.1%, or vehicle, self-administered as a single drop per eye, once daily, for 42 days.
Main Outcomes and Measures
The primary efficacy end point was change from baseline in the number of points seen on the Leicester Peripheral Field Test, a test to detect superior visual field deficits due to ptosis, on days 1 (6 hours after instillation) and 14 (2 hours after instillation). The secondary end point, change from baseline in marginal reflex distance 1, was assessed at the same time points.
Results
In total, 304 participants were enrolled (mean [SD] age, 63.8 [13.8] years; 222 women [73%]). Overall, 97.5% (198 of 203) of participants receiving oxymetazoline, 0.1%, and 97.0% (98 of 101) of participants receiving vehicle completed the studies. Oxymetazoline, 0.1%, was associated with a significant increase in the mean (SD) number of points seen on the Leicester Peripheral Field Test vs vehicle (day 1: 5.9 [6.4] vs 1.8 [4.1]; mean difference, 4.07 [95% CI, 2.74-5.39]; P < .001; day 14: 7.1 [5.9] vs 2.4 [5.5]; mean difference, 4.74 [95% CI, 3.43-6.04]; P < .001). Oxymetazoline, 0.1%, also was associated with a significant increase in marginal reflex distance 1 from baseline (mean [SD]: day 1: 0.96 [0.89] mm vs 0.50 [0.81] mm; mean difference, 0.47 mm [95% CI, 0.27-0.67]; P < .001; day 14: 1.16 [0.87] mm vs 0.50 [0.80] mm; mean difference, 0.67 mm [95% CI, 0.46-0.88]; P < .001). Treatment-emergent adverse events (TEAEs) occurred in 31.0% (63 of 203) of participants receiving oxymetazoline, 0.1%, and 35.6% (36 of 101) of participants receiving vehicle. Among participants receiving oxymetazoline, 0.1%, with a TEAE, 81% (51 of 63) had a maximum TEAE intensity of mild, and 62% (39 of 63) had no TEAE suspected of being treatment related.
Conclusions and Relevance
Oxymetazoline, 0.1%, was associated with positive outcomes and was well tolerated in phase 3 trials after instillation at days 1 and 14, demonstrating its potential promise for the treatment of acquired ptosis, although further study is needed to elucidate the clinical relevance of these findings beyond 6 weeks.
Introduction
Blepharoptosis (or ptosis) is an abnormal drooping of the upper eyelid margin with the eye in primary gaze.1 In addition to the characteristic asymmetric or sleepy appearance caused by ptosis, obstruction of the pupil by the upper eyelid can lead to superior visual field deficits.2 Reduced visual function can diminish independence in activities of daily living,3,4,5 and patients with ptosis report elevated levels of anxiety, depression, and appearance-related distress.6 Current evidence identifies ptosis as a common disorder of the eyelid, particularly among elderly patients.7,8,9
Ptosis is broadly categorized as either congenital or acquired,10 with the acquired form classified based on underlying etiology as involutional (aponeurotic), neurogenic, myogenic, traumatic, or mechanical.11 Transient or persistent ptosis can also occur following ocular procedures, such as glaucoma or cataract surgery,12,13 and has been associated with contact lens wear.14,15,16
Standard of care is surgery, often targeting the upper eyelid retractor muscles.3,17,18 While effective in improving visual function and quality-of-life measures,3 there are risks associated with surgical intervention, including asymmetry, overcorrection/undercorrection, bleeding, lagophthalmos, and infection.11 An effective, noninvasive, pharmacologic treatment option might enable treatment of a wider range of affected individuals.
The superior tarsal, or Müller, muscle arises from the levator muscle and inserts on the tarsus, where it helps maintain upper eyelid elevation provided by the levator, while providing 2 to 3 mm of additional elevation.19,20 Therefore, Müller muscle is a common surgical target.3,17,18 Because it expresses adrenergic receptors,21 Müller muscle is also a target for pharmacologic intervention. A limited number of studies have described the use of adrenergic drugs (phenylephrine, apraclonidine) for ptosis treatment.22,23,24,25,26,27
Oxymetazoline hydrochloride, 0.1%, ophthalmic solution (oxymetazoline, 0.1%; RVL Pharmaceuticals Inc) is a novel pharmacologic agent approved for the treatment of acquired ptosis. Oxymetazoline is a potent, direct-acting α-adrenergic receptor agonist that binds the α1 and α2 subtypes.28,29 It is used, at low concentrations, as a topical nasal decongestant, and ocular administration of less than 0.025% oxymetazoline has been shown to reduce hyperemia.30
It is hypothesized that oxymetazoline, 0.1%, stimulates α-adrenergic receptors on Müller muscle,21 causing muscle contraction and upper eyelid lift. The objective of this analysis was to examine the efficacy and safety of oxymetazoline, 0.1%, for the treatment of acquired ptosis, when administered once daily as a single drop in each eye after instillation at 1 and 14 days, and then followed up with continued treatment for 6 weeks in 2 randomized clinical trials.
Methods
Studies
This analysis combines data from 2 randomized, double-masked, placebo-controlled, multicenter phase 3 clinical trials (study RVL-1201-201 [NCT02436759] and study RVL-1201-202 [NCT03565887]). Study RVL-1201-201 recruited participants from May 2015 to October 2016, and study RVL-1201-202 recruited participants from June 2018 to April 2019. Both studies were conducted In the United States and in accordance with the principles of the Declaration of Helsinki31 and Good Clinical Practice and International Conference on Harmonisation guidelines. Protocols and informed consent forms were approved by a central institutional review board (Alpha IRB, San Clemente, California) prior to study initiation. All study participants provided written informed consent at screening and were compensated financially. Participant information and data were handled per Health Insurance Portability and Accountability Act provisions.
Studies RVL-1201-201 and RVL-1201-202 enrolled 140 participants across 16 sites and 164 participants across 27 sites, respectively. Both studies revealed significant effects of oxymetazoline, 0.1%, in participants with acquired ptosis (eTable 2 in the Supplement). Data were pooled based on consistency in study design, inclusion/exclusion criteria, treatment regimen, duration, and end points. Study rationale, methodology, results, and conclusions are reported in accordance with Consolidated Standards of Reporting Trials (CONSORT) reporting guideline.
Study Participants
The key inclusion criteria for both studies were acquired ptosis and a superior visual field deficit in at least 1 eye at screening (3-7 days before treatment day 1) and baseline (treatment day 1). The presence of acquired ptosis was confirmed based on 2 criteria. First was the participant’s inability to detect at least 8 of 17 points in the top 2 rows on the Leicester Peripheral Field Test (LPFT; a validated visual field test using a Humphrey visual field [HVF] analyzer, designed to assess superior visual field loss due to ptosis), and the ability to detect at least 9 of 35 total points in the top 4 rows.32 Second was marginal reflex distance 1 (MRD-1; distance from the central pupillary light reflex to the central inferior margin of the upper eyelid, ≤2 mm).
Before treatment initiation, performance on the LPFT was tested twice at screening (hour 0 and hour 6) and once at baseline (hour 0) to ensure the participant’s ability to perform the test reliably. Performance was tested no more than twice in a visit to help mitigate learning effects that might introduce false-positive measures. Inclusion required 4 or less points’ variability between LPFT assessments at screening and baseline. For all LPFT assessments through treatment day 14, reliability was assessed in an automated manner, by the test apparatus (HVF analyzer). If the HVF analyzer detected loss of fixation or a false positive/negative during testing, then the test was automatically flagged as unreliable, at which point it could be retaken at most once. For enrolled participants, both eyes were evaluated, and the eye with the smaller MRD-1 was defined as the study eye (eMethods in the Supplement).
Individuals were excluded from either study if they had congenital ptosis, Horner syndrome, myasthenia gravis, mechanical ptosis, or visual field loss from any cause other than ptosis. Participants with pseudoptosis or substantial dermatochalasis (redundant eyelid skin occurring within 3 mm of the upper eyelid margin) in the study eye were also excluded. Study RVL-1201-201 required that participants be 18 years or older. Study RVL-1201-202 required that patients be 9 years or older.
Randomization and Treatment
Participants in both studies were randomized 2:1 to treatment with oxymetazoline, 0.1%, or vehicle. Randomization schemes were created by an independent biostatistician using a block design (eMethods in the Supplement). Participants, investigators, staff, and study management personnel were masked to the identity of treatment until after final database lock. Treatments were self-administered by participants as a single drop per eye, once daily in the morning, for 42 days. Study visits occurred on treatment days 1, 14, and 42.
Efficacy Outcome Measures
The primary efficacy end point in both studies was the change from baseline in the number of points seen in the top 4 rows on the LPFT on day 1 (6 hours after instillation) and day 14 (2 hours after instillation). These times were selected to assess duration (6 hours) and onset (2 hours) of effect.
The LPFT, performed using an HVF analyzer, is a validated test for the measurement of superior visual field loss due to ptosis.32 It is an automated, observer-independent, static perimetry test in which the center of fixation is shifted 15 ° inferiorly to allow for maximum superior field testing, more natural eyelid positioning, and to prevent compensatory brow elevation. The LPFT examines a 48 ° range in the superior visual field, using a 35-point grid comprising the top 4 rows of the field. Fourteen points are also tested in the inferior visual field as a reference. The LPFT demonstrates high sensitivity (98.8%), specificity (92.5%), positive predictive value (96.6%), and negative predictive value (97.4%) in detecting ptosis.32
The secondary efficacy end point, change from baseline in MRD-1 on day 1 (6 hours) and day 14 (2 hours), was measured using digital photographs taken at the study sites. Care was taken to ensure consistent ambient lighting for photography at all sites and time points. A standardized self-adhesive millimeter-scale ruler was placed vertically on each participant’s forehead, centered above the eyebrows, and used by investigators at each site to take standardized MRD-1 measurements from digital photographs using calipers. Photographs were also used to measure pupil diameter and palpebral fissure distance (study RVL-1201-201 only). Compliance was measured by comparing the numbers of returned and dispensed treatment vials.
Safety Outcome Measures
Treatment-emergent adverse events (TEAEs) were monitored and reported throughout both studies and evaluated with respect to severity and relationship to treatment. Vital signs, Snellen visual acuity, pupil diameter, slitlamp examination, and corneal fluorescein staining were examined at all study visits. Intraocular pressure tonometry and dilated ophthalmoscopy/fundus examinations were conducted at screening and day 42.
Statistical Analysis
Analyses were conducted using the combined intent-to-treat population (participants receiving ≥1 treatment dose). Efficacy was analyzed using data from the study eye, while safety was analyzed for both eyes. Because less than 5% of data points were missing in both treatment groups, with no evidence of either group being particularly subject to missing data, efficacy analysis used the intent-to-treat population, with the last observation carried forward for missing data.
Descriptive statistics of the observed and change from baseline for efficacy end points were tabulated by visit and treatment group, using common time points across studies. Differences between treatment groups were compared using an analysis of covariance model with treatment as a fixed factor and baseline score as a covariate. Treatments were compared using pairwise comparison from the model. Statistical tests were 2-sided, with P < .05 considered statistically significant for the primary outcome. Analyses for the individual trials were initiated after database lock and completed on September 6, 2017, and May 16, 2019. Pooled analysis was completed on August 25, 2019.
Results
Participant Disposition
In total, 304 participants were enrolled and randomized to receive oxymetazoline, 0.1% (203 [66.8%]), or vehicle (101 [33.2%]) (Table 1 and Figure 1). Demographic characteristics were similar across treatment groups (Table 1). Study completion rates (oxymetazoline, 0.1%: 198 of 203 [97.5%]; vehicle: 98 of 101 [97.0%]) and treatment compliance (mean [SD]: oxymetazoline, 0.1%: 98.1% [8.7%]; vehicle: 97.2% [10.7%]) were high, and mean treatment exposure was 42 days in both groups (Table 1).
Table 1. Participant Disposition and Demographicsa.
| Characteristic | No. (%) | |
|---|---|---|
| Oxymetazoline, 0.1% (n = 203) | Vehicle (n = 101) | |
| Enrolled, No. | 203 | 101 |
| Completed all visits | 198 (97.5) | 98 (97.0) |
| Compliance with treatment, mean (SD), %b | 98.1 (8.7) | 97.2 (10.7) |
| Treatment exposure, mean (SD), d | 42.1 (4.6) | 42.2 (5.3) |
| Age, mean (SD) [range], y | 64.1 (13.4) [20-92] | 63.3 (14.7) [14-85] |
| Sex | ||
| Female | 151 (74.4) | 71 (70.3) |
| Male | 52 (25.6) | 30 (29.7) |
| Race | ||
| White | 177 (87.2) | 92 (91.1) |
| Black | 18 (8.9) | 6 (5.9) |
| Asian | 6 (3.0) | 3 (3.0) |
| American Indian | 2 (1.0) | 0 |
| Ethnicity | ||
| Not Hispanic/Latino | 170 (83.7) | 84 (83.2) |
| Hispanic/Latino | 33 (16.3) | 17 (16.8) |
| Baseline points seen, top 4 rows, LPFT | ||
| Mean (SD) | 17.3 (4.7) | 17.3 (5.3) |
| Median (range) | 17.0 (8-27) | 17.0 (2-26) |
| Baseline MRD-1, mm | ||
| Mean (SD) | 1.09 (0.70) | 1.05 (0.69) |
| Median (range) | 1.00 (0-3.0) | 1.00 (0-2.0) |
Abbreviations: LPFT, Leicester Peripheral Field Test; MRD-1, marginal reflex distance.
Data were similar in the individual studies (eTable 1 in the Supplement).
Percentage of opened vials returned relative to the number of vials that should have been used during the treatment period.
Figure 1. Participant Disposition Flowchart: Study RVL-1201-201, Study RVL-1201-202, and Pooled Analysis.
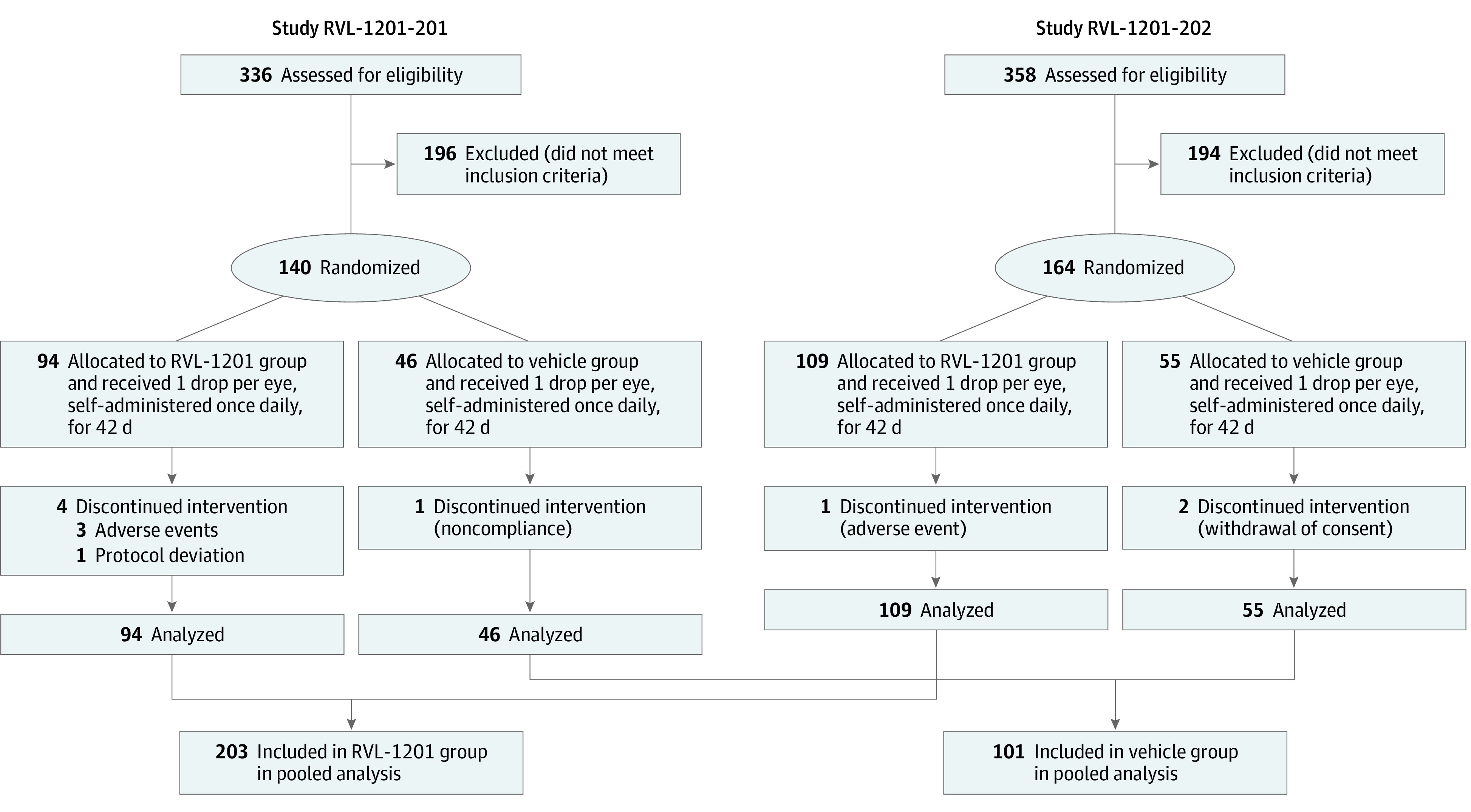
In study RVL-1201-201, the first participant was enrolled on May 29, 2015, and the last participant completed on October 24, 2016. In study RVL-1201-202, first enrollment was on June 20, 2018, and last completion was on April 10, 2019. Study RVL-1201-201’s sample size was defined to establish more than 99% power to detect an effect size of 1.0 using a 2-group t test with a .05 two-sided significance level. Study RVL-1201-202’s sample size was based on establishing 90% power to detect a difference of 3.50 on the primary end point using a 2-group t test with a .05 two-sided significance level.
Primary Efficacy: LPFT
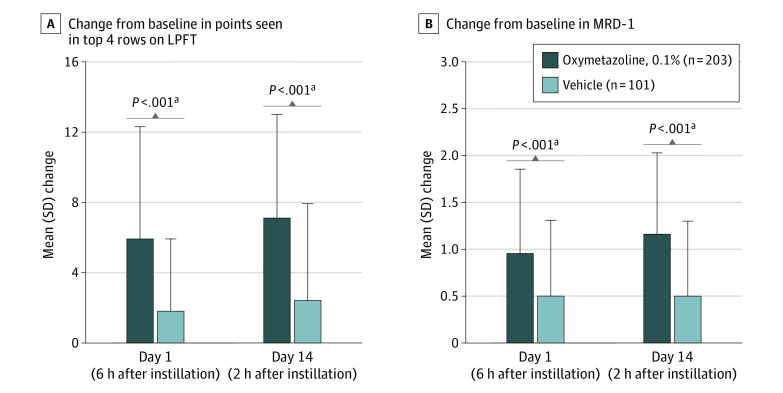
At baseline, the mean (SD) number of points seen in the top 4 rows on the LPFT was the same across treatment groups (oxymetazoline, 0.1%: 17.3 [4.7]; vehicle: 17.3 [5.3]; Table 1), suggesting an equivalent superior visual field deficit. On day 1 (6 hours), the mean (SD) number of points seen increased to 23.1 (6.5) in the oxymetazoline, 0.1%, group and 19.1 (6.1) in the vehicle group, with a significantly greater mean change in the oxymetazoline, 0.1% group (mean [SD], 5.9 [6.4] vs 1.8 [4.1]; mean difference, 4.07 [95% CI, 2.74-5.39]; P < .001; Figure 2A). Two hours after instillation of oxymetazoline, 0.1%, or vehicle on day 14, the mean (SD) numbers of points seen were 24.3 (6.0) and 19.5 (6.0), respectively, with a significantly greater mean (SD) change from baseline in the oxymetazoline, 0.1%, group (7.1 [5.9] vs 2.4 [5.5]; mean difference, 4.74 [95% CI, 3.43-6.04]; P < .001; Figure 2A).
Figure 2. Mean Change on the Leicester Peripheral Field Test (LPFT) and Marginal Reflex Distance 1 (MRD-1) After Instillation of 1 Drop Oxymetazoline, 0.1%, or Vehicle.
Error bars represent standard deviation of the mean change from baseline.
aP < .001 vs vehicle, from analysis of covariance model with treatment as a fixed factor and baseline score as a covariate. In both studies included in the pooled analysis, oxymetazoline, 0.1%, treatment resulted in improvement on the LPFT and in MRD-1 (eTable 2 in the Supplement).
Secondary Efficacy: MRD-1
Mean (SD) baseline MRD-1 in the study eye was similar across treatment groups (oxymetazoline, 0.1%: 1.09 [0.70] mm; vehicle: 1.05 [0.69] mm; Table 1). Mean (SD) change from baseline on day 1 (6 hours) was 0.96 (0.89) mm with oxymetazoline, 0.1%, and 0.50 (0.81) mm with vehicle (mean difference, 0.47 mm [95% CI, 0.27-0.67]; P < .001; Figure 2B and Figure 3). On day 14 (2 hours), mean (SD) change from baseline in MRD-1 was 1.16 (0.87) mm and 0.50 (0.80) mm in the oxymetazoline, 0.1%, and vehicle groups, respectively (mean difference, 0.67 mm [95% CI, 0.46-0.88]; P < .001; Figure 2B and Figure 3).
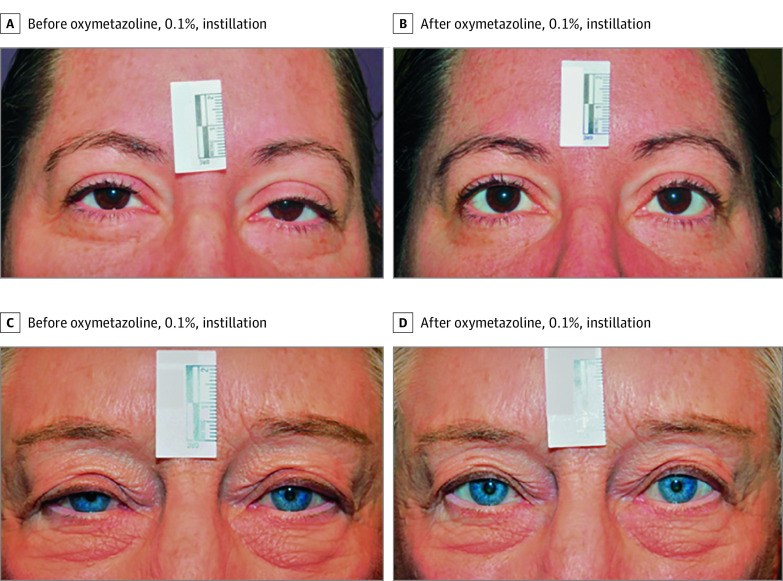
Figure 3. Representative Images Demonstrating Lifting of the Upper Eyelid After Instillation of 1 Drop of Oxymetazoline, 0.1%.
A 44-year-old woman at baseline (A) and 2 hours after oxymetazoline, 0.1%, instillation (B) on treatment day 1 and a 78-year-old woman at baseline (C) and 6 hours after oxymetazoline, 0.1%, instillation (D) on treatment day 1. Participants pictured here had postinstillation improvements in marginal reflex distance and points seen in the top 4 rows of the Leicester Peripheral Field Test, compared with baseline.
Safety
There were 129 TEAEs in 63 participants (31.0%) receiving oxymetazoline, 0.1%, and 73 TEAEs in 36 participants (35.6%) receiving vehicle (Table 2). Among participants receiving oxymetazoline, 0.1%, and reporting a TEAE, 51 of 63 (81.0%) had a maximum TEAE intensity of mild, and 39 of 63 (61.9%) had no TEAE suspected of being treatment related. Among participants receiving vehicle with a reported TEAE, 25 of 36 (69.4%) had a maximum TEAE intensity of mild, and 27 of 36 (75.0%) had no TEAE suspected of being treatment related. Two participants (1.0%) receiving oxymetazoline, 0.1%, reported serious TEAEs (hyperparathyroidism; cerebrovascular accident), and 1 participant (1.0%) receiving vehicle had a serious TEAE (lower gastrointestinal hemorrhage). No serious TEAE was suspected of being treatment related, and all were resolved. Three participants (1.5%) in the oxymetazoline, 0.1%, group and 2 participants (2.0%) in the vehicle group discontinued owing to a TEAE.
Table 2. Summary of TEAEsa.
| Characteristic | No. (%) | |
|---|---|---|
| Oxymetazoline, 0.1% (n = 203) | Vehicle (n = 101) | |
| Participants reporting any TEAE | 63 (31.0) | 36 (35.6) |
| Total TEAEs reported, No. | 129 | 73 |
| Participants reporting TEAE, No. of TEAEs | ||
| 0 | 140 (69.0) | 65 (64.4) |
| 1 | 23 (11.3) | 18 (17.8) |
| >1 | 40 (19.7) | 18 (17.8) |
| Participants reporting TEAE by maximum intensityb | ||
| Mild | 51 (25.1) | 25 (24.8) |
| Moderate | 10 (4.9) | 11 (10.9) |
| Severe | 2 (1.0) | 0 |
| Participants reporting TEAE by association with study drugc | ||
| Not suspected | 39 (19.2) | 27 (26.7) |
| Suspected | 24 (11.8) | 9 (8.9) |
| Participants reporting any serious TEAE | 2 (1.0) | 1 (1.0) |
| Participants reporting any TEAE leading to discontinuation | 3 (1.5)d | 2 (2.0)e |
| TEAEs reported for ≥2% of participants, by MedDRA preferred term, events | ||
| Punctate keratitis | 19:11 (5.4) | 3:3 (3.0) |
| Vision blurred | 12:7 (3.4) | 0:0 |
| Conjunctival hyperemia | 10:6 (3.0) | 2:1 (1.0) |
| Instillation site pain | 11:6 (3.0) | 0:0 |
Abbreviations: MedDRA, Medical Dictionary for Regulatory Activities; TEAE, treatment-emergent adverse events.
TEAEs were coded by system organ class and preferred term in accordance with MedDRA. The individual studies were similar with respect to overall TEAE rates, as well as TEAE severity and association with treatment (eTable 3 in the Supplement).
Participants reporting ≥1 TEAE counted once at the maximum intensity of all reported TEAEs.
Participants reporting the same TEAE at more than 1 association counted at the greatest association.
Includes 1 participant with mild eyelid edema, 1 with mild headache and instillation site pain, and 1 with mild ocular discomfort.
Includes 1 participant with mild iritis and 1 with moderate colitis, hematochezia, lower gastrointestinal hemorrhage, diverticulum, and hemorrhoids.
TEAEs occurring in 2% or more of participants in either group were punctate keratitis (oxymetazoline, 0.1%: 11 of 203 [5.4%]; vehicle: 3 of 101 [3.0%]), blurred vision (oxymetazoline, 0.1%: 7 of 203 [3.4%]; vehicle: 0), conjunctival hyperemia (oxymetazoline, 0.1%: 6 of 203 [3.0%]; vehicle: 1 of 101 [1.0%]), and instillation site pain (oxymetazoline, 0.1%: 6 of 203 [3.0%]; vehicle: 0) (Table 2). Punctate keratitis, blurred vision, and conjunctival hyperemia were each suspected of being treatment related in 2 participants (1.0%) receiving oxymetazoline, 0.1%. Instillation site pain was suspected of being treatment related in all 6 participants reporting it.
There were no mean shifts from baseline in vital signs, or intraocular pressure, Snellen visual acuity, pupil diameter, slitlamp or ophthalmoscopy/fundus examination results in either eye judged to be clinically relevant. Mean (SD) changes from baseline in the right pupil diameter with oxymetazoline, 0.1%, and vehicle, respectively, were 0.4 (0.8) mm and 0 (0.6) mm on day 1, 0.1 (0.7) mm and −0.1 (0.8) mm on day 14, and −0.1 (0.8) mm and −0.2 (0.8) mm on day 42.
Discussion
These clinical study results support the efficacy and safety of oxymetazoline, 0.1%, for the treatment of acquired ptosis, a potentially important finding given the lack of other approved pharmacologic options for this condition, as well as the barriers to and potential risks11 associated with surgery.
Reliable visual field testing is essential to accurately assessing the effect of any potential therapy for ptosis. While the LPFT used for the primary outcome in these trials has been shown to detect functional disability (superior visual field loss) in patients with ptosis with high specificity, owing to the wide superior visual field tested and inferior shift in the target of fixation, which may help prevent compensatory behaviors, the clinical relevance of the effects identified in this study is not known, nor were the efficacy or safety beyond 6 weeks determined by this trial. The LPFT is an automated, observer-independent test, which may help limit concerns about observer-dependence or repeatability.32 Thus, the positive change from baseline on the LPFT among participants who received oxymetazoline, 0.1%, may be a meaningful functional improvement of the aspect of the visual field directly affected by ptosis after instillation at day 1 and day 14, the primary outcome assessment times for these trials. Supporting this observed functional improvement, MRD-1 analysis provides evidence of a significant effect of oxymetazoline, 0.1%, on the characteristic appearance of ptosis by reducing the amount of upper eyelid droop.
The safety data from these 6-week studies are likewise promising. Most TEAEs in participants receiving oxymetazoline, 0.1%, were mild and nontreatment related, and there were only 3 total TEAEs (all treatment unrelated) in the oxymetazoline, 0.1%, group resulting in discontinuation. Among TEAEs occurring in 2% or more of participants, 3 were ocular: punctate keratitis, blurred vision, and conjunctival hyperemia. Although punctate keratitis (reported in 5.4% and 3.0% of participants receiving oxymetazoline, 0.1%, and vehicle, respectively) was judged by investigators to be treatment related in only 2 participants, the presence of this TEAE suggests a potential risk of corneal irritation. Lagophthalmos was not reported in conjunction with the upper eyelid elevation resulting from oxymetazoline, 0.1%, treatment, but longer-duration safety studies should examine this potential concern. Further, tear production was not assessed but warrants examination in future safety studies, given that a lower concentration of topical oxymetazoline (0.026%) has been observed to reduce tear volume and flow for up to 6 hours.33 Dry eye was uncommon in these studies, occurring in 2 of 203 participants (1.0%) receiving oxymetazoline, 0.1%. Dry eye was noted in the medical history of 48.3% and 39.6% of participants who received oxymetazoline, 0.1%, and vehicle, respectively, suggesting a potential susceptibility to corneal irritation in the study population. α-Adrenergic agonists applied to the eye can cause pupil dilation.34 The absence of a clinically significant mean change in pupil diameter (along with no reports of mydriasis) in participants receiving oxymetazoline, 0.1%, demonstrates that there was no effect on pupil size with this formulation.
Limitations
The present analysis is not without limitations. While improvement on the LPFT was greater with oxymetazoline, 0.1%, use after instillation on day 1 and day 14, the broader clinical implications of this improvement require further evaluation, given the lack of literature independently validating the LPFT’s clinical relevance as used in this study.32 Future studies of longer duration of the effect of oxymetazoline, 0.1%, on activities of daily living and psychosocial factors typically affected by ptosis in conjunction with improvements on the LPFT might support the clinical benefit of oxymetazoline, 0.1%, as might comparative assessment of the LPFT and previously developed visual field tests that currently are used routinely to evaluate ptosis prior to eyelid surgery. Like the LPFT findings, a more detailed evaluation of MRD-1 in conjunction with known markers of positive clinical outcomes (eg, validated patient questionnaires) might further elucidate the potential benefits of oxymetazoline, 0.1%, in the clinic. It is also important to note that while MRD-1 measurement procedures and instrumentation were standardized to provide consistency across sites, some minor variability might be expected and should be considered with respect to the observed change from baseline, although variation (SD) was similar across treatment groups and days.
While the data support the efficacy of oxymetazoline, 0.1%, at 2 hours and 6 hours after instillation at day 1 and day 14, respectively, this analysis also has limitations related to the period evaluated. Given the once-daily dosing regimen of oxymetazoline, 0.1%, it will be important to further evaluate both its onset of action and its duration of action beyond 6 hours after instillation. Similarly, the current analysis is limited to evaluation of efficacy over 14 days, and as such, it is unclear whether oxymetazoline, 0.1%, maintains its efficacy in the context of longer-term use. From a clinical practice perspective, it is important that future analyses define the efficacy of oxymetazoline, 0.1%, beyond 14 days of use.
The safety findings over 6 weeks of oxymetazoline, 0.1%, use are encouraging; however, longer-term safety evaluation is necessary. Chronic ophthalmic use of tetrahydrozoline (an α-adrenergic agonist) can result in rebound vasodilation and chronic red eyes.35,36 Similarly, chronic use of oxymetazoline nasal spray can cause tachyphylaxis and rebound congestion.37,38 Neither tachyphylaxis nor rebound hyperemia was observed over 42 days of oxymetazoline, 0.1%, use in these studies. Conjunctival hyperemia was uncommon and occurred during, rather than following, treatment. Still, understanding the longer-term safety profile of oxymetazoline, 0.1%, is essential for future clinical studies. Therefore, the results of a 12-week randomized safety study enrolling 234 participants (NCT03536949) will be essential in helping to further evaluate the safety of once-daily oxymetazoline, 0.1%, use. Further, an assessment of outcomes with oxymetazoline, 0.1%, treatment vs surgical intervention might be achieved via comparative study.
Conclusions
The efficacy and safety of oxymetazoline, 0.1%, ophthalmic solution after instillation at day 1 and day 14 demonstrated in 2 randomized phase 3 trials suggest that this novel pharmacologic agent may represent a promising treatment option for acquired ptosis. However, the clinical relevance of oxymetazoline, 0.1%, effects in clinical practice are not yet known, and the results of these studies can be used to generate hypotheses for future clinical evaluation of this treatment. Given the lack of other noninvasive treatment options, oxymetazoline, 0.1%, may potentially allow for more expansive treatment of acquired ptosis, including patients who may not qualify for or desire ptosis repair surgery if benefits and safety beyond 6 weeks can be demonstrated.
eMethods
eTable 1. Subject disposition and demographics, individual studies included in pooled analysis
eTable 2. Primary (mean change from baseline in the number of points seen in the top 4 rows on the Leicester Peripheral Field Test (LPFT)) and secondary (mean change from baseline in Marginal Reflex Distance 1 (MRD-1)) efficacy outcomes, individual studies included in pooled analysis
eTable 3. Summary of treatment-emergent adverse events (TEAEs), individual studies included in pooled analysis
References
- 1.Fausett BV, Nerad JA. Upper eyelid ptosis and retraction. In: Fay A, Dolman PJ, eds. Diseases and Disorders of the Orbit and Ocular Adnexa: Expert Consult. 1st ed. Elsevier; 2016. [Google Scholar]
- 2.Cahill KV, Burns JA, Weber PA. The effect of blepharoptosis on the field of vision. Ophthalmic Plast Reconstr Surg. 1987;3(3):121-125. doi: 10.1097/00002341-198703030-00001 [DOI] [PubMed] [Google Scholar]
- 3.Cahill KV, Bradley EA, Meyer DR, et al. Functional indications for upper eyelid ptosis and blepharoplasty surgery: a report by the American Academy of Ophthalmology. Ophthalmology. 2011;118(12):2510-2517. doi: 10.1016/j.ophtha.2011.09.029 [DOI] [PubMed] [Google Scholar]
- 4.Battu VK, Meyer DR, Wobig JL. Improvement in subjective visual function and quality of life outcome measures after blepharoptosis surgery. Am J Ophthalmol. 1996;121(6):677-686. doi: 10.1016/S0002-9394(14)70634-8 [DOI] [PubMed] [Google Scholar]
- 5.McKean-Cowdin R, Varma R, Wu J, Hays RD, Azen SP; Los Angeles Latino Eye Study Group . Severity of visual field loss and health-related quality of life. Am J Ophthalmol. 2007;143(6):1013-1023. doi: 10.1016/j.ajo.2007.02.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Richards HS, Jenkinson E, Rumsey N, et al. The psychological well-being and appearance concerns of patients presenting with ptosis. Eye (Lond). 2014;28(3):296-302. doi: 10.1038/eye.2013.264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sridharan GV, Tallis RC, Leatherbarrow B, Forman WM. A community survey of ptosis of the eyelid and pupil size of elderly people. Age Ageing. 1995;24(1):21-24. doi: 10.1093/ageing/24.1.21 [DOI] [PubMed] [Google Scholar]
- 8.Hashemi H, Khabazkhoob M, Emamian MH, et al. The prevalence of ptosis in an Iranian adult population. J Curr Ophthalmol. 2016;28(3):142-145. doi: 10.1016/j.joco.2016.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim MH, Cho J, Zhao D, et al. Prevalence and associated factors of blepharoptosis in Korean adult population: the Korea National Health and Nutrition Examination Survey 2008-2011. Eye (Lond). 2017;31(6):940-946. doi: 10.1038/eye.2017.43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Custer PL. Blepharoptosis. In: Yanoff M, Duker JS, eds. Ophthalmology. 3rd ed. Elsevier; 2008. [Google Scholar]
- 11.Finsterer J. Ptosis: causes, presentation, and management. Aesthetic Plast Surg. 2003;27(3):193-204. doi: 10.1007/s00266-003-0127-5 [DOI] [PubMed] [Google Scholar]
- 12.Wang Y, Lou L, Liu Z, Ye J. Incidence and risk of ptosis following ocular surgery: a systematic review and meta-analysis. Graefes Arch Clin Exp Ophthalmol. 2019;257(2):397-404. doi: 10.1007/s00417-018-4130-6 [DOI] [PubMed] [Google Scholar]
- 13.Godfrey KJ, Korn BS, Kikkawa DO. Blepharoptosis following ocular surgery: identifying risk factors. Curr Opin Ophthalmol. 2016;27(1):31-37. doi: 10.1097/ICU.0000000000000218 [DOI] [PubMed] [Google Scholar]
- 14.Hwang K, Kim JH. The risk of blepharoptosis in contact lens wearers. J Craniofac Surg. 2015;26(5):e373-e374. doi: 10.1097/SCS.0000000000001876 [DOI] [PubMed] [Google Scholar]
- 15.Kitazawa T. Hard contact lens wear and the risk of acquired blepharoptosis: a case-control study. Eplasty. 2013;13:e30. [PMC free article] [PubMed] [Google Scholar]
- 16.Thean JHJ, McNab AA. Blepharoptosis in RGP and PMMA hard contact lens wearers. Clin Exp Optom. 2004;87(1):11-14. doi: 10.1111/j.1444-0938.2004.tb03139.x [DOI] [PubMed] [Google Scholar]
- 17.Fasanella RM, Servat J. Levator resection for minimal ptosis: another simplified operation. Arch Ophthalmol. 1961;65:493-496. doi: 10.1001/archopht.1961.01840020495005 [DOI] [PubMed] [Google Scholar]
- 18.Putterman AM, Urist MJ. Müller muscle-conjunctiva resection. Technique for treatment of blepharoptosis. Arch Ophthalmol. 1975;93(8):619-623. doi: 10.1001/archopht.1975.01010020595007 [DOI] [PubMed] [Google Scholar]
- 19.Beard C. Müller’s superior tarsal muscle: anatomy, physiology, and clinical significance. Ann Plast Surg. 1985;14(4):324-333. doi: 10.1097/00000637-198504000-00005 [DOI] [PubMed] [Google Scholar]
- 20.Putterman AM, Fett DR. Müller’s muscle in the treatment of upper eyelid ptosis: a ten-year study. Ophthalmic Surg. 1986;17(6):354-360. [PubMed] [Google Scholar]
- 21.Esmaeli-Gutstein B, Hewlett BR, Pashby RC, Oestreicher J, Harvey JT. Distribution of adrenergic receptor subtypes in the retractor muscles of the upper eyelid. Ophthalmic Plast Reconstr Surg. 1999;15(2):92-99. doi: 10.1097/00002341-199903000-00005 [DOI] [PubMed] [Google Scholar]
- 22.Kirkpatrick CA, Shriver EM, Clark TJE, Kardon RH. Upper eyelid response to topical 0.5% apraclonidine. Ophthalmic Plast Reconstr Surg. 2018;34(1):13-19. doi: 10.1097/IOP.0000000000000843 [DOI] [PubMed] [Google Scholar]
- 23.Grace Lee N, Lin LW, Mehta S, Freitag SK. Response to phenylephrine testing in upper eyelids with ptosis. Digit J Ophthalmol. 2015;21(3):1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scheinfeld N. The use of apraclonidine eyedrops to treat ptosis after the administration of botulinum toxin to the upper face. Dermatol Online J. 2005;11(1):9. [PubMed] [Google Scholar]
- 25.Steinsapir KD, Groth MJ, Boxrud CA. Persistence of upper blepharoptosis after cosmetic botulinum toxin type A. Dermatol Surg. 2015;41(7):833-840. doi: 10.1097/DSS.0000000000000386 [DOI] [PubMed] [Google Scholar]
- 26.Wijemanne S, Vijayakumar D, Jankovic J. Apraclonidine in the treatment of ptosis. J Neurol Sci. 2017;376:129-132. doi: 10.1016/j.jns.2017.03.025 [DOI] [PubMed] [Google Scholar]
- 27.Garibaldi DC, Hindman HB, Grant MP, Iliff NT, Merbs SL. Effect of 0.5% apraclonidine on ptosis in Horner syndrome. Ophthalmic Plast Reconstr Surg. 2006;22(1):53-55. doi: 10.1097/01.iop.0000196322.05586.6a [DOI] [PubMed] [Google Scholar]
- 28.Haenisch B, Walstab J, Herberhold S, et al. Alpha-adrenoceptor agonistic activity of oxymetazoline and xylometazoline. Fundam Clin Pharmacol. 2010;24(6):729-739. doi: 10.1111/j.1472-8206.2009.00805.x [DOI] [PubMed] [Google Scholar]
- 29.Sugden D, Anwar N, Klein DC. Rat pineal α 1-adrenoceptor subtypes: studies using radioligand binding and reverse transcription-polymerase chain reaction analysis. Br J Pharmacol. 1996;118(5):1246-1252. doi: 10.1111/j.1476-5381.1996.tb15530.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Duzman E, Anderson J, Vita JB, Lue JC, Chen CC, Leopold IH. Topically applied oxymetazoline: ocular vasoconstrictive activity, pharmacokinetics, and metabolism. Arch Ophthalmol. 1983;101(7):1122-1126. doi: 10.1001/archopht.1983.01040020124022 [DOI] [PubMed] [Google Scholar]
- 31.World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 32.Ho SF, Morawski A, Sampath R, Burns J. Modified visual field test for ptosis surgery (Leicester Peripheral Field Test). Eye (Lond). 2011;25(3):365-369. doi: 10.1038/eye.2010.210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Göbbels MJ, Achten C, Spitznas M. Effect of topically applied oxymetazoline on tear volume and tear flow in humans. Graefes Arch Clin Exp Ophthalmol. 1991;229(2):147-149. doi: 10.1007/BF00170547 [DOI] [PubMed] [Google Scholar]
- 34.McAuliffe-Curtin D, Buckley C. Review of alpha adrenoceptor function in the eye. Eye (Lond). 1989;3(Pt 4):472-476. doi: 10.1038/eye.1989.71 [DOI] [PubMed] [Google Scholar]
- 35.Soparkar CN, Wilhelmus KR, Koch DD, Wallace GW, Jones DB. Acute and chronic conjunctivitis due to over-the-counter ophthalmic decongestants. Arch Ophthalmol. 1997;115(1):34-38. doi: 10.1001/archopht.1997.01100150036004 [DOI] [PubMed] [Google Scholar]
- 36.Spector SL, Raizman MB. Conjunctivitis medicamentosa. J Allergy Clin Immunol. 1994;94(1):134-136. doi: 10.1016/0091-6749(94)90081-7 [DOI] [PubMed] [Google Scholar]
- 37.Ramey JT, Bailen E, Lockey RF. Rhinitis medicamentosa. J Investig Allergol Clin Immunol. 2006;16(3):148-155. [PubMed] [Google Scholar]
- 38.Vaidyanathan S, Williamson P, Clearie K, Khan F, Lipworth B. Fluticasone reverses oxymetazoline-induced tachyphylaxis of response and rebound congestion. Am J Respir Crit Care Med. 2010;182(1):19-24. doi: 10.1164/rccm.200911-1701OC [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods
eTable 1. Subject disposition and demographics, individual studies included in pooled analysis
eTable 2. Primary (mean change from baseline in the number of points seen in the top 4 rows on the Leicester Peripheral Field Test (LPFT)) and secondary (mean change from baseline in Marginal Reflex Distance 1 (MRD-1)) efficacy outcomes, individual studies included in pooled analysis
eTable 3. Summary of treatment-emergent adverse events (TEAEs), individual studies included in pooled analysis