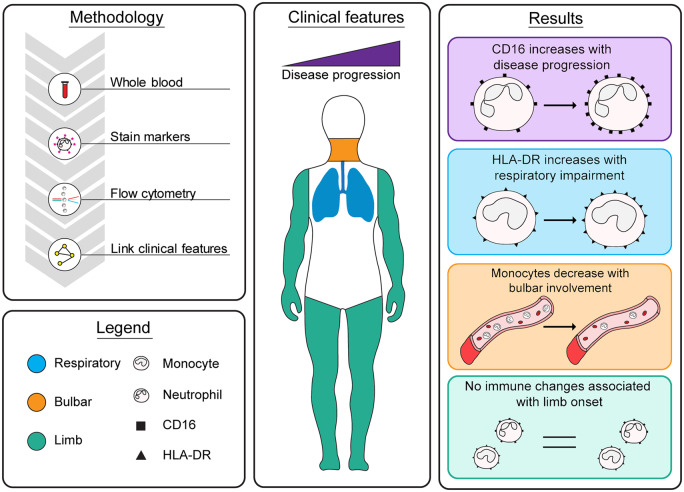
Abstract
Immunity has emerged as a key player in neurodegenerative diseases such as amyotrophic lateral sclerosis, with recent studies documenting aberrant immune changes in patients and animal models. A challenging aspect of amyotrophic lateral sclerosis research is the heterogeneous nature of the disease. In this study, we investigate the associations between peripheral blood myeloid cell populations and clinical features characteristic of amyotrophic lateral sclerosis. Peripheral blood leukocytes from 23 healthy controls and 48 patients with amyotrophic lateral sclerosis were analysed to measure myeloid cell alterations. The proportion of monocytes (classical, intermediates and non-classical subpopulations) and neutrophils, as well as the expression of select surface markers, were quantitated using flow cytometry. Given the heterogeneous nature of amyotrophic lateral sclerosis, multivariable linear analyses were performed to investigate associations between patients’ myeloid profile and clinical features, such as the Revised Amyotrophic Lateral Sclerosis Functional Rating Scale, bulbar subscore of the Revised Amyotrophic Lateral Sclerosis Functional Rating Scale, change in Revised Amyotrophic Lateral Sclerosis Functional Rating Scale over disease duration and respiratory function. We demonstrate a shift in monocyte subpopulations in patients with amyotrophic lateral sclerosis, with the ratio of classical to non-classical monocytes increased compared with healthy controls. In line with this, patients with greater disease severity, as determined by a lower Revised Amyotrophic Lateral Sclerosis Functional Rating Scale score, had reduced non-classical monocytes. Interestingly, patients with greater bulbar involvement had a reduction in the proportions of classical, intermediate and non-classical monocyte populations. We also revealed several notable associations between myeloid marker expression and clinical features in amyotrophic lateral sclerosis. CD16 expression on neutrophils was increased in patients with greater disease severity and a faster rate of disease progression, whereas HLA-DR expression on all monocyte populations was elevated in patients with greater respiratory impairment. This study demonstrates that patients with amyotrophic lateral sclerosis with distinct clinical features have differential myeloid cell signatures. Identified cell populations and markers may be candidates for targeted mechanistic studies and immunomodulation therapies in amyotrophic lateral sclerosis.
Keywords: amyotrophic lateral sclerosis, monocyte, neutrophil, bulbar and respiratory
Monocyte and neutrophil frequencies and their expression markers were associated with bulbar symptoms, disease severity, rate of disease progression and respiratory impairment in patients with amyotrophic lateral sclerosis. Our study reveals that aberrant peripheral immunity is related to amyotrophic lateral sclerosis patient phenotype and supports the need for tailored therapies to modify the immune profile.
Graphical Abstract
Introduction
Amyotrophic lateral sclerosis (ALS) is a debilitating neurodegenerative disease with documented immune abnormalities (Zhang et al., 2005; Babu et al., 2008; Zhao et al., 2013; Zondler et al., 2017). Several studies have demonstrated that peripheral myeloid cell populations are functionally altered in patients with ALS and influence survival in animal models of ALS (Graves et al., 2004; Henkel et al., 2004; Butovsky et al., 2012; Zondler et al., 2016; Zhao et al., 2017; Zondler et al., 2017). A challenging aspect of research and clinical trials in ALS is the heterogeneity of clinical features. In any given study cohort, patients vary in disease severity, rate of progression, degree of upper and lower motor neuron involvement, site of onset, bulbar involvement and degree of respiratory impairment (Brown and Al-Chalabi, 2017). Indeed, some previous studies have included disease severity and rate of progression into data analysis and found that the frequency and activation of immune cell populations such as T cells, monocytes and neutrophils have been associated with disease severity and rate of disease progression (Zhang et al., 2005; Mantovani et al., 2009; Murdock et al., 2016; Murdock et al., 2017). These findings reveal that the immunological changes observed in ALS are dynamic and associated with clinical presentation and may have implications in understanding the disease pathophysiology, therapeutic targets and timing of immunomodulation treatment. Clinical features such as the degree of bulbar involvement and respiratory impairment may also provide additional insight as patients with greater bulbar involvement or respiratory impairment have a worse prognosis (Czaplinski et al., 2006; Brown and Al-Chalabi, 2017). Exploring shared and distinct phenotypes in ALS relative to well-defined clinical features may further reveal novel pathological drivers that might be suitable targets for drug discovery applications or serve as biomarkers in clinical trials.
In the present study, we quantified myeloid cell populations and the expression of key markers in peripheral blood samples from patients with ALS and healthy controls. We then assessed whether there were associations between immune profiles and clinical features of ALS. Our data demonstrate that the frequencies of myeloid cell populations and expression of select surface markers are associated with disease severity, rate of disease progression, bulbar symptoms and degree of respiratory impairment.
Materials and methods
Study participants
The study cohort included 23 healthy controls and 48 participants with ALS (demographics in Table 1). The number of participants was chosen based on the study size of previous literature. Patients who met a diagnosis for ALS based on the revised El Escorial criteria (Brooks et al., 2000) and controls were enrolled from the Royal Brisbane and Women’s Hospital multidisciplinary Motor Neuron Disease Clinic and The University of Queensland, between March 2017 and August 2018. Participants who met the following criteria at the time of enrolment were excluded: use of gastrostomy tube, forced vital capacity (FVC) <60%, use of immunotherapy or a history of diabetes. Control and patient samples were collected across The University of Queensland Centre for Clinical Research and The University of Queensland. This study was approved by the Royal Brisbane and Women’s Hospital, The Wesley Hospital and The University of Queensland Human Research Ethics Committees. All participants were informed of the study outline and provided written consent.
Table 1.
Participant demographics and clinical features of patients with ALS
| Patients with ALS | Controls | |
|---|---|---|
| Age (years), mean ± SD | 59 ± 9.22 | 56 ± 10.29 |
| Sex | ||
| Females (%) | 16 (33) | 11 (48) |
| Site of onset | ||
| Spinal (%) | 32 (67) | |
| Bulbar (%) | 14 (29) | |
| Respiratory (%) | 1 (2) | |
| Mixed (%) | 1 (2) | |
| Disease duration (years)a, mean ± SD (range) | 2.33 ± 1.66 (0.67–8.00) | |
| ALSFRS-Rb, mean ± SD (range) | 36.46 ± 5.53 (21–46) | |
| ALSFRS-R Bulbarc, mean ± SD (range) | 9.94 ± 1.95 (4–12) | |
| ΔALSFRS-Rd, mean ± SD (range) | 0.59 ± 0.44 (0.08–1.99) | |
| SNIPe (% of predicted), mean ± SD (range) | 60.10 ± 19.64 (25–99) | |
| FVCf (% of predicted), mean ± SD (range) | 85.12 ± 20.15 (34–116) | |
Duration calculated from the time of symptom onset to the collection of blood sample.
ALSFRS-R scored at the time of blood collection.
ALSFRS-R bulbar subscore ranges from 0 to 12, with lower scores indicating a greater severity of bulbar symptoms.
Rate of disease progression was calculated as the change in ALSFRS-R from the date of symptom onset to sample collection.
SNIP, sniff nasal inspiratory pressure.
FVC, forced vital capacity.
SD, standard deviation.
Clinical assessment
For participants with ALS, the Revised Amyotrophic Lateral Sclerosis Functional Rating Scale (ALSFRS-R) (Cedarbaum et al., 1999) was used to assess functional impairment. Given that patients with bulbar ALS tend to have a poor prognosis (Brown and Al-Chalabi, 2017), the ALSFRS-R bulbar subscore and site of onset were also recorded. Rate of disease progression was calculated as the change in ALSFRS-R divided by the duration from symptom onset to blood collection. Respiratory function was assessed as part of routine clinical care. We therefore included FVC (% of predicted) and sniff nasal inspiratory pressure (% of predicted) as reference measures for lung function (Pinto and de Carvalho, 2018).
Peripheral blood immunophenotyping by flow cytometry
To understand the contribution of peripheral immune myeloid cells in disease pathology, we utilized multi-parameter flow cytometry to investigate the relative proportions of myeloid cell populations and the expression of select surface markers, specifically, CD14, CD16 and HLA-DR surface proteins. Previous studies investigating frequencies and marker expression have found conflicting findings; some authors report differences, while others report no significant changes or changes in the opposite direction (Zhang et al., 2005; Mantovani et al., 2009; Butovsky et al., 2012; Murdock et al., 2016; Zondler et al., 2016; Gustafson et al., 2017; Murdock et al., 2017), which may be due to discrepancies in experimental study designs. In this study, we therefore followed recommendations for immunophenotyping blood myeloid cell populations as per the International Union of Immunological Societies Nomenclature Committee (Lundahl et al., 1995; Ziegler-Heitbrock et al., 2010). Blood samples from healthy controls and patients with ALS were collected into K2EDTA tubes to inhibit the activation of the complement system (Harboe et al., 2011) and the subsequent alteration of surface receptors (BD Vacutainer; BD Biosciences, San Jose, CA, USA). Unprocessed whole blood was also used in our ex vivo analysis to minimize the activation of leukocytes (Ziegler-Heitbrock et al., 2010). The following panel of monoclonal fluorophore-conjugated antibodies was used: CD14-PerCP/Cy5.5, CD16-APC/Cy7 and HLA-DR-BV785. All reagents were sourced from BioLegend (San Diego, CA, USA).
Briefly, whole blood was blocked with mouse IgG (Abacus) and then stained with our panel of antibodies, as per manufacturer’s guidelines. Erythrocytes were lysed, and leukocytes were fixed with FACS Lysing Solution (BD Biosciences). The final leukocyte suspensions were analysed on a flow cytometer within 2 h (BD LSRII; BD Biosciences). For each sample, a total of 200 000 events were acquired. Appropriate unstained, fluorescence-minus-one and single-stain tubes were used for internal controls, colour compensation and gating guidance. A novel three-parameter approach was used to determine the gating strategy for the three main monocyte subpopulations: classical, intermediate and non-classical monocytes (see Supplementary Fig. 1). Incorporating HLA-DR expression into the typical two-parameter (CD14 versus CD16) cytometric plot enabled accurate distinction between the monocyte subpopulations that cannot be validated with the previous standard method. Neutrophils were identified as SSChi and CD16hi (see Supplementary Fig. 2). Analysis of data was performed using the FlowJo 10.0 software (BD Biosciences, San Jose, CA, USA). Histograms validating each monoclonal fluorophore-conjugated antibody with appropriate isotype controls are presented in Supplementary Fig. 3. Finally, representative histograms displaying the relative expression of CD14, CD16 and HLA-DR on the three monocyte subpopulations are presented in Supplementary Fig. 4.
Statistical analysis
Demographic factors and clinical features were summarized using the mean, standard deviation and range for continuous variables or number and proportion for categorical variables. The analysis was completed after the acquisition of all the immune and clinical data. Statistical analyses were conducted using StataSE 15 (StataCorp. 2015. Stata Statistical Software: Release 15; StataCorp LP, College Station, TX, USA). To allow adjustment for age and sex, differences between two groups were evaluated using a multivariable linear regression model with 95% confidence intervals; all data represented as mean ± SEM. Multivariable linear regression models with 95% confidence intervals, and adjustment for age and sex, were used to assess associations between two continuous variables. Results were considered statistically significant at P-value <0.05. A Grubbs’ test for outliers was undertaken (alpha = 0.05); data points excluded are outlined in Supplementary Tables 1 and 2. Any clinical information for patients with ALS that was unavailable is outlined in Supplementary Table 3. Graphing was performed using GraphPad Prism 8.0 (GraphPad Software, Inc., La Jolla, CA, USA).
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Results
Participant demographics and patient clinical features
The study cohort included 23 healthy controls [11 females (48%) and 12 males (52%); 56 years ± 10.29] and 48 patients with ALS [16 females (33%) and 32 males (47%); 59 years ± 9.22]. Participants with ALS had a mean ALSFRS-R score of 36.46 ± 5.53 and the disease duration of 2.33 ± 1.66 years. The demographics of our participant cohort and the clinical features of patients are outlined in Table 1.
Ratio of classical to non-classical monocytes is elevated in patients with ALS
Peripheral myeloid cell populations were analysed ex vivo using flow cytometry to measure the frequencies and expression of select surface markers. Healthy controls and patients with ALS had comparable frequencies of monocyte subpopulations and neutrophils circulating in peripheral blood (Fig. 1A–E). While all analyses adjusted for sex and age, it should be noted that there was a slight difference in the sex ratio between healthy controls and patients with ALS (Table 1). The frequency of classical monocytes was the only immune metric with an interaction with sex (95% CI −1.76 to −0.10; P = 0.029); male participants in both the control and patient group had higher levels of classical monocytes, which has been widely reported in previous literature (Bain, 1996; Bouman et al., 2004). An additional indicator of immune dysfunction is the ratio of monocyte subpopulations within each participant (Zondler et al., 2016). We found that patients in our cohort had a greater ratio of classical to non-classical monocytes compared with healthy controls (95% CI 0.45–5.39; P = 0.021) (Fig. 1D). When we investigated the associations between myeloid cell population frequencies and clinical features, we found that this shift in monocyte subpopulations may be driven by a reduction in non-classical monocytes with disease progression: patients with a greater disease severity (lower ALSFRS-R score) had a reduction in non-classical monocytes (95% CI 0.002–0.03; P = 0.024) (Fig. 1H). This association equated to a 13% reduction in the proportion of non-classical monocytes for every 5-point loss on the ALSFRS-R scale. There were no statistically significant associations between other population frequencies and disease severity or rate of progression (Fig. 1F–J). Collectively, our data report a shift in the proportion of classical to non-classical monocyte subpopulations in ALS, which appears to be driven by a reduction in non-classical monocytes.
Figure 1.
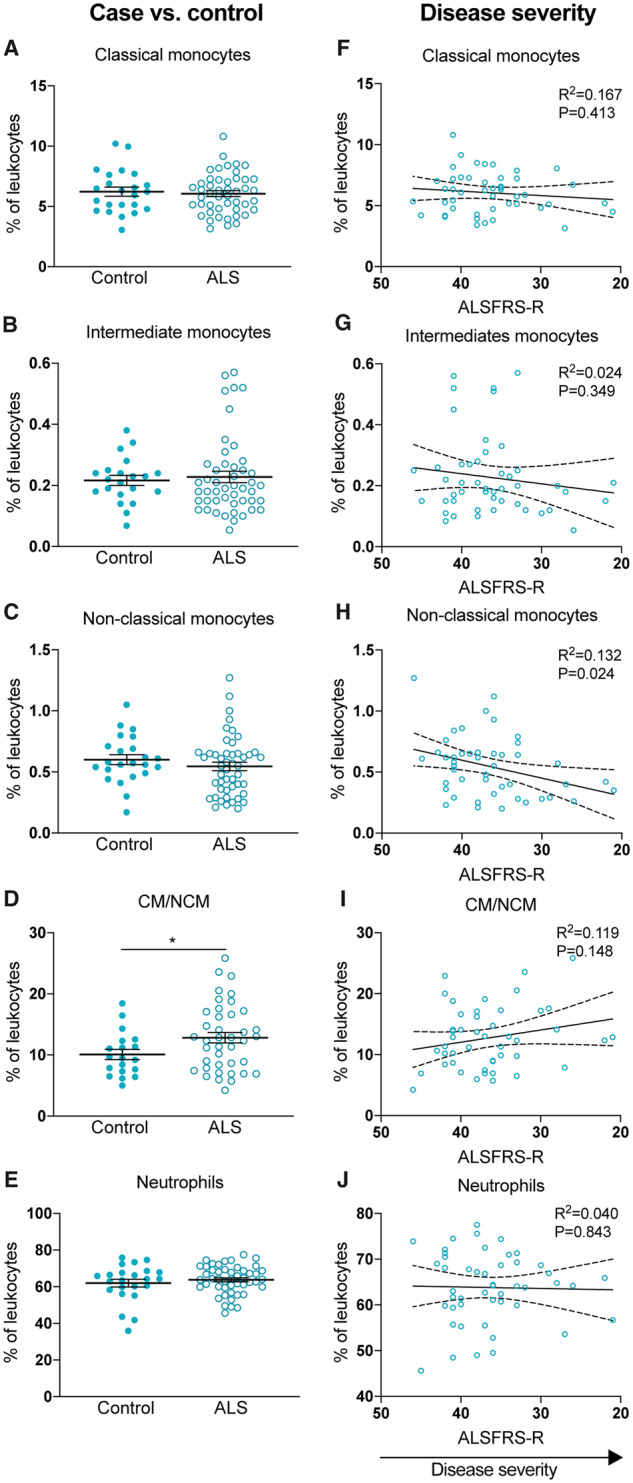
The frequency of monocyte subpopulations shift in patients with ALS. (A–E) The frequency of myeloid cell populations in the peripheral blood from healthy controls and ALS patients. (F–J) The frequency of myeloid cell populations correlated with patients’ ALSFRS-R score at the time of blood collection. ALSFRS-R scores are displayed from high to low to illustrate disease progression. Data are displayed in left plots as mean and SEM or in right plots with the best-fit line and 95% confidence intervals. R2 value and P-value are also shown. Analysis performed with multivariable linear regression models controlling for age and sex, significant at P < 0.05 (*P = 0.021). CM/NCM, ratio of classical to non-classical monocytes.
Circulating monocyte subpopulations are reduced in patients with ALS with greater bulbar involvement
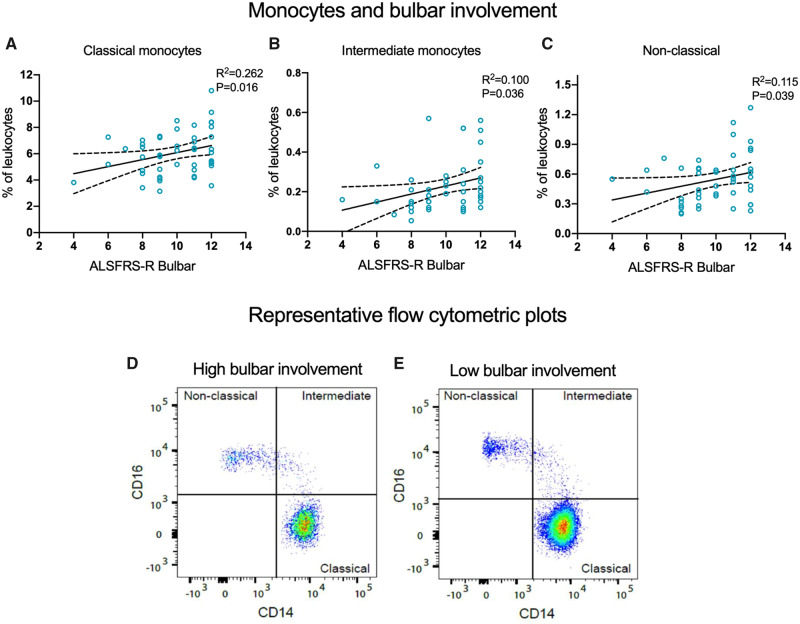
Given the heterogeneous nature of ALS and poor prognosis of bulbar onset, we investigated whether patients with greater bulbar involvement had different frequencies of monocyte subpopulations and neutrophils. We found that patients with a greater severity of bulbar symptoms had a reduction in classical (95% CI 0.06–0.51; P = 0.016), intermediate (95% CI 0.001–0.04; P = 0.036) and non-classical monocytes (95% CI 0.002–0.07; P = 0.039) (Figs 2A and 3A–D). For every 2-point loss on the ALSFRS-R bulbar subscore, there was a 9% reduction in classical, 18% reduction in intermediate and 14% reduction in non-classical monocytes. Neither ratio of classical to non-classical monocytes nor neutrophil frequencies were significantly associated with patients’ degree of bulbar involvement (Fig. 2A). Next, we compared the frequencies of myeloid cell population and respiratory impairment. There were no statistically significant associations between monocyte subpopulations and sniff nasal inspiratory pressure (% predicted) or FVC (% predicted) (Fig. 2). However, a modest positive association between the frequency of neutrophils and FVC (% predicted) was observed (95% CI 0.007–0.27; P = 0.040). This corresponded to a 2% decrease in neutrophils for every 10% drop in FVC (% predicted) (Fig. 2A). Collectively, our findings indicate that a drop in the proportion of peripheral blood monocyte subpopulations may vary considerably relative to the progression of bulbar symptoms.
Figure 2.
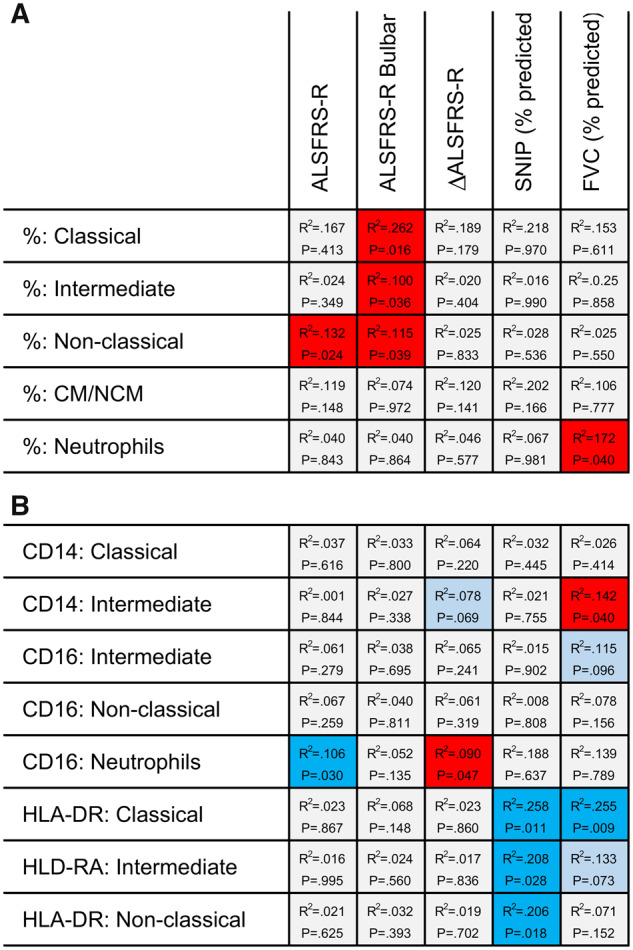
Myeloid cell population frequencies and surface marker expressions are associated with disease features. (A) The frequency of myeloid cell populations in the peripheral blood from patients with ALS, and (B) the expression of select surface markers correlated with disease severity, degree of bulbar involvement, rate of disease progression and respiratory function. R2 value and P-value are also shown. Analysis achieved with multivariable linear regression models controlling for age and sex, significant at P < 0.05. Colour boxes indicate the following: light blue = negative association, not significant (P < 0.1 but >0.05); bright blue = negative association, significant (P < 0.05); bright red = positive association, significant (P < 0.05). ALSFRS-R bulbar, a subscore measuring the degree of bulbar involvement; SNIP, sniff nasal inspiratory pressure.
Figure 3.
Patients with greater bulbar involvement have a reduction in all monocyte subpopulations. (A–C) Frequency of monocyte subpopulations correlated with ALSFRS-R bulbar subscore. (D, E) Peripheral blood monocyte subpopulations from patients with ALS quantitated using flow cytometry. Representative flow cytometric plots demonstrating that patients with ALS with greater bulbar involvement (D; a lower ALSFRS-R bulbar subscore) have a reduced number of monocyte subpopulations compared with patients with low bulbar involvement (E). Data are displayed with the best-fit line and 95% confidence intervals. R2 value and P-value are also shown. Analysis achieved with multivariable linear regression models, adjusted for age and sex, significant at P < 0.05.
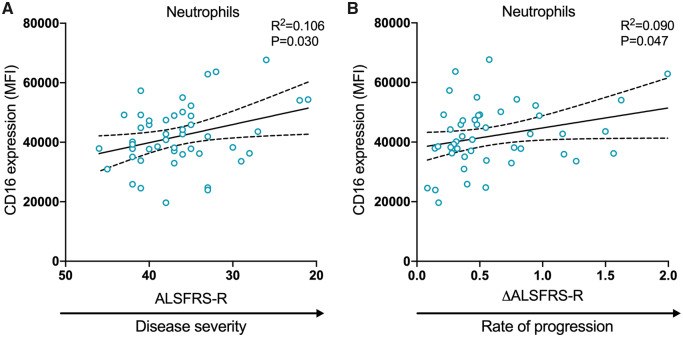
CD16 expression on neutrophils is associated with disease severity and rate of progression in ALS
Similar to the myeloid cell population frequencies, we observed no significant differences in the expression of CD14, CD16 or HLA-DR on myeloid cell populations between controls and our cohort of patients (see Supplementary Table 4). To explore disease heterogeneity, we analysed the expression of CD14, CD16 and HLA-DR relative to clinical feature presented. This revealed that neutrophil CD16 expression was positively associated with both disease severity (95% CI −1164.41 to −63.56; P = 0.030) and rate of progression (95% CI 118.97–15 127.13; P = 0.047) (Fig. 4A and B, respectively), indicating that CD16 expression increased as the disease progressed and in patients with a faster decline in function. No other significant associations were found between the expression of markers and ALSFRS-R, ALSFRS-R Bulbar or ΔALSFRS-R (Fig. 2B).
Figure 4.
CD16 expression on neutrophils is associated with disease severity and rate of progression in ALS. The expression of CD16 on neutrophils was correlated with (A) disease severity and (B) rate of progression. ALSFRS-R scores are displayed from high to low to illustrate disease progression. Data are displayed with the best-fit line and 95% confidence intervals. R2 value and P-value are also shown. Analysis achieved with multivariable linear regression models, adjusted for age and sex, significant at P < 0.05. ΔALSFRS-R, rate of disease progression as measured by the change in ALSFRS-R over disease duration; MFI, median fluorescence intensity.
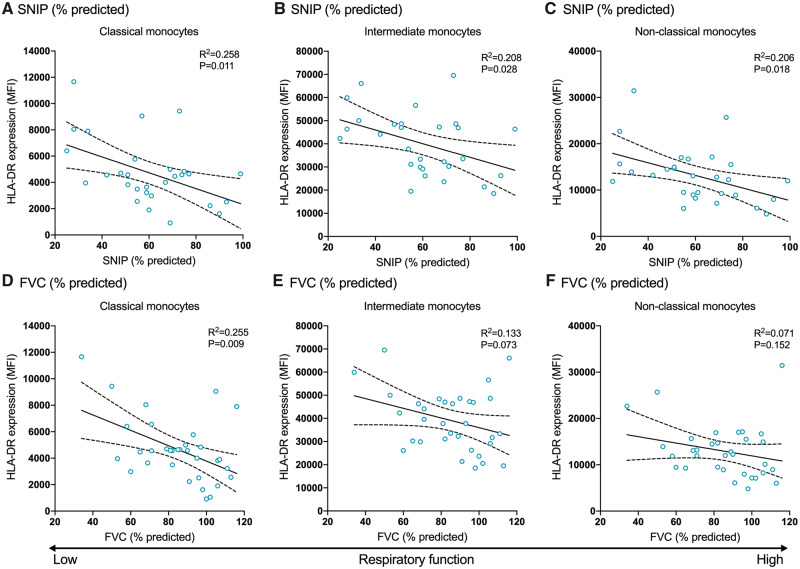
Patients with ALS with greater respiratory impairment have increased HLA-DR expression on monocytes
Finally, we investigated whether there were any changes in CD14, CD16 or HLA-DR expression relative to respiratory impairment. Strikingly, we observed an association between respiratory function as measured by sniff nasal inspiratory pressure (% predicted) and HLA-DR expression on classical (95% CI −104.25 to −14.56; P = 0.011), intermediate (95% CI −544.19 to −33.91; P = 0.028) and non-classical monocytes (95% CI −246.99 to −25.21; P = 0.018) (Fig. 5A–C), where marker expression increased as patients’ respiratory function decreased. The same relationship was found between HLA-DR expression on classical monocytes and FVC (% predicted) (95% CI −105.79 to −16.27; P = 0.009; Fig. 5D); however, the other monocyte subpopulations were not significantly associated (Fig. 5E and F). We also found that CD14 expression was reduced on intermediate monocytes in patients with lower than predicted FVC (95% CI 1.46–56.15; P = 0.040) (Fig. 2B). Overall, these results suggest that patients with ALS with greater disease severity, rate of disease progression and lower respiratory function have increased expression of distinct myeloid cell surface proteins, in particular, CD16 and HLA-DR.
Figure 5.
Patients with ALS with impaired respiratory function have increased HLA-DR expression on monocyte subpopulations. HLA-DR expression on monocyte subpopulations was correlated with (A–C) SNIP (% predicted) and (D–F) FVC (% predicted). Data are displayed with the best-fit line and 95% confidence intervals. R2 value and P-value are also shown. Analysis achieved with multivariable linear regression models, adjusted for age and sex, significant at P < 0.05. MFI, median fluorescence intensity; SNIP, sniff nasal inspiratory pressure.
Discussion
Evidence from human and animal studies has demonstrated that the peripheral immune system is altered in ALS and can play a role in disease progression (Zhang et al., 2005; Babu et al., 2008; Mantovani et al., 2009; Zhang et al., 2011; Butovsky et al., 2012; Murdock et al., 2016; Zondler et al., 2016; Murdock et al., 2017; Zhao et al., 2017; Zondler et al., 2017). However, previous literature reporting on immune cell frequencies and marker expression has not revealed consistent findings, which could be due to the heterogeneity of patients and methodological variations. This study, therefore, set out to carefully re-examine myeloid cell populations and to consider myeloid cell profiles relative to clinical features of the disease. Our results show that myeloid cell population frequencies and their expression markers are associated with bulbar symptoms, the degree of respiratory impairment, disease severity and rate of disease progression. These findings indicate that the immune features of ALS vary with disease features and highlight the importance of addressing disease heterogeneity in research studies and clinical trials that focus on immunopathology in ALS.
Healthy controls and patients with ALS had comparable frequencies of circulating monocyte subpopulations and neutrophils in the blood. These findings are in agreement with previous studies that also found no alterations (Zhang et al., 2005; Mantovani et al., 2009; Murdock et al., 2016; Gustafson et al., 2017), but at odds with others who reported significant differences (Zhang et al., 2005; Mantovani et al., 2009; Butovsky et al., 2012; Murdock et al., 2017). These conflicts in the literature may be due to discrepancies in sample processing and gating strategies for subpopulations. Importantly, our study adopted methodologies adapted from guidelines on immunophenotyping in whole blood (Lundahl et al., 1995; Ziegler-Heitbrock et al., 2010; Harboe et al., 2011) and employed a novel three-parameter approach to identify monocyte subpopulations to minimize cell activation and maximize accuracy (see Supplementary Fig. 1). An additional indicator of immune dysfunction is the ratio of classical to non-classical monocytes. Patients with ALS in our cohort had a greater ratio of classical to non-classical monocytes compared with healthy controls, which supports prior literature (Zondler et al., 2016). This observed shift in monocyte subpopulations may be driven by a reduction in non-classical monocytes, as we found that patients with greater disease severity (lower ALSFRS-R score) had a reduction in non-classical monocytes. This change is of importance because prior experimental studies identified that classical monocytes could exacerbate disease progression, while non-classical monocytes appear to have a protective role (Butovsky et al., 2012; Zondler et al., 2016; Zondler et al., 2017). A key study found that blockade of the murine analogue of classical monocytes reduced motor neuron loss and prolonged survival in a mouse model of ALS (Butovsky et al., 2012). Concurrently, the treatment shifted this high ratio of classical to non-classical monocytes to wild-type proportions. Therefore, our observed subpopulation shift in patients supports strategies that therapeutically target these monocyte populations in ALS (Butovsky et al., 2012; Miller et al., 2015).
A novel finding from our study was the association between monocyte frequencies and the degree of functional impairment of bulbar muscles in patients with ALS. Patients with greater bulbar involvement had a reduction in all monocyte subpopulations. Currently, it is not known whether these changes are contributing to disease progression or a result of it. Given that patients with bulbar ALS have a worse prognosis (Brown and Al-Chalabi, 2017), it does raise questions as to how monocytes may play a role in this subset of patients. Prior studies have speculated that a reduction in peripheral blood monocytes could be due to the recruitment and infiltration of these cells into the CNS (Mantovani et al., 2009; Butovsky et al., 2012). Indeed, studies investigating differential CNS pathology in patients with greater bulbar involvement have reported atypical neurofibrillary tangles and basophilic inclusions, a distinct metabolic state, greater microstructural damage to cerebral white matter regions and impaired cognition (Schreiber et al., 2005; Cistaro et al., 2012; Shellikeri et al., 2017; Trojsi et al., 2017). While only speculative, our findings in addition to current literature could indicate that bulbar patients with ALS with differential CNS pathology have greater monocyte infiltration. It would be of interest for future studies to assess monocyte infiltration in post-mortem brain and spinal cord tissue from patients with ALS and correlate this with bulbar symptoms and associated pathology.
Surface marker expression was investigated to determine whether myeloid cell populations were differentially activated. No overall changes in CD14, HLA-DR or CD16 marker expression on myeloid cell populations were observed when comparing patients and controls. Importantly, however, the expression of these markers did change within the ALS patient cohort relative to key features of the disease. The relationship between CD14 expression and disease characteristics in ALS is of particular interest given a monoclonal anti-CD14 antibody is undergoing a Phase 2 clinical trial in patients with ALS (identifier: NCT03474263). Our study identified reduced CD14 expression on intermediate monocytes in patients with respiratory impairment. In addition, while not statistically significant, patients with a faster rate of disease progression had reduced CD14 expression on intermediate monocytes, which could possibly be attributed to monocyte activation. Specifically, upon activation, monocytes can shed their membrane-bound CD14, resulting in reduced surface expression and a concomitant increase in soluble CD14 levels (Shive et al., 2015; Leveque et al., 2017).
Another measure of monocyte activation is HLA-DR expression. Patients in our cohort with impaired respiratory function also had elevated levels of HLA-DR on all monocyte subpopulations. The underlying mechanism driving this association between HLA-DR expression and respiratory impairment remains unexplored. Given the observational nature of this study, these findings can only be interpreted as correlational. The lack of association between HLA-DR expression and disease progression in this study suggests that the increased expression is related to specifically to respiratory impairment. Future studies could investigate this link between respiratory function and monocyte activation via HLA-DR cell expression and soluble CD14 in plasma.
In addition to our observed alterations in monocytes with ALS disease features, we found that CD16 expression on neutrophils was associated with both disease severity and rate of disease progression. This strong association suggests that neutrophils have greater activation in patients experiencing severe functional impairment and deterioration. We can only speculate as to whether increased CD16 expression on neutrophils is directly exacerbating the disease, or a downstream indirect response to the pernicious effects of apoptotic motor neurons and muscle atrophy. What is known, however, is that CD16 expression on neutrophils is linked to their oxidative burst and phagocytic activity (Chinda et al., 2003), It is therefore possible that chronic neutrophil activation leads to reactive oxygen species production that exacerbates motor neuron degeneration. Indeed, neutrophils have been implicated in other neurodegenerative diseases such as AD (Zenaro et al., 2015; Dong et al., 2018) and produce greater levels of reactive oxygen species in these patients (Cruz Hernandez et al., 2019). While it is plausible that the functionally deleterious role of neutrophils in AD could be mirrored in ALS, future studies aimed at investigating the oxidative burst and phagocytic ability in neutrophils from patients with ALS and animal models of ALS are needed to determine their contribution to disease pathophysiology.
Limitations
The observational and explorative nature of this study served to address disease heterogeneity while opening avenues to identify future research questions. Further research is needed to validate these findings in separate cohorts of patients with ALS.
Conclusions
This study aimed to explore distinct immunological abnormalities relative to clinical phenotypes and to uncover novel pathological drivers that might be suitable targets for drug discovery applications or biomarker studies in clinical trials. Monocyte and neutrophil frequencies and their marker expression were associated with bulbar symptoms, disease severity, rate of disease progression and respiratory impairment. Our study thus reveals that immune dysfunction is related to patient phenotype and may suggest the need for tailored therapies to modify the immune profile in patients with ALS.
Supplementary Material
Acknowledgements
We thank patients with ALS and healthy control individuals for donating their time to research and Megan McStea for statistical support.
Abbreviations
- ALS =
amyotrophic lateral sclerosis
- ALSFRS-R =
Revised Amyotrophic Lateral Sclerosis Functional Rating Scale
- FVC =
forced vital capacity
Funding
This study was supported by funding from the Queensland Government (Advance Queensland Innovation Partnership AQIP01216), FightMND (02_TRG_2017), Wesley Medical Research (2016-31) and the National Health and Medical Research Council (1082271). T.M.W. is supported by a National Health and Medical Research Council Career Development Fellowship (1105420).
Competing interests
The authors report no competing interests.
References
- Babu GN, Kumar A, Chandra R, Puri SK, Kalita J, Misra UK.. Elevated inflammatory markers in a group of amyotrophic lateral sclerosis patients from northern India. Neurochem Res 2008; 33: 1145–9. [DOI] [PubMed] [Google Scholar]
- Bain BJ. Ethnic and sex differences in the total and differential white cell count and platelet count. J Clin Pathol 1996; 49: 664–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouman A, Schipper M, Heineman MJ, Faas MM.. Gender difference in the non-specific and specific immune response in humans. Am J Reprod Immunol 2004; 52: 19–26. [DOI] [PubMed] [Google Scholar]
- Brooks BR, Miller RG, Swash M, Munsat TL; World Federation of Neurology Research Group on Motor Neuron Diseases. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord 2000; 1: 293–9. [DOI] [PubMed] [Google Scholar]
- Brown RH, Al-Chalabi A.. Amyotrophic lateral sclerosis. N Engl J Med 2017; 377: 162–72. [DOI] [PubMed] [Google Scholar]
- Butovsky O, Siddiqui S, Gabriely G, Lanser AJ, Dake B, Murugaiyan G, et al. Modulating inflammatory monocytes with a unique microRNA gene signature ameliorates murine ALS. J Clin Invest 2012; 122: 3063–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cedarbaum JM, Stambler N, Malta E, Fuller C, Hilt D, Thurmond B, et al. The ALSFRS-R: a revised ALS functional rating scale that incorporates assessments of respiratory function. BDNF ALS Study Group (Phase III). J Neurol Sci 1999; 169: 13–21. [DOI] [PubMed] [Google Scholar]
- Chinda D, Nakaji S, Umeda T, Shimoyama T, Kurakake S, Okamura N, et al. A competitive marathon race decreases neutrophil functions in athletes. Luminescence 2003; 18: 324–9. [DOI] [PubMed] [Google Scholar]
- Cistaro A, Valentini MC, Chio A, Nobili F, Calvo A, Moglia C, et al. Brain hypermetabolism in amyotrophic lateral sclerosis: a FDG PET study in ALS of spinal and bulbar onset. Eur J Nucl Med Mol Imaging 2012; 39: 251–9. [DOI] [PubMed] [Google Scholar]
- Cruz Hernandez JC, Bracko O, Kersbergen CJ, Muse V, Haft-Javaherian M, Berg M, et al. Neutrophil adhesion in brain capillaries reduces cortical blood flow and impairs memory function in Alzheimer’s disease mouse models. Nat Neurosci 2019; 22: 413–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czaplinski A, Yen AA, Appel SH.. Forced vital capacity (FVC) as an indicator of survival and disease progression in an ALS clinic population. J Neurol Neurosurg Psychiatry 2005; 77: 390–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y, Lagarde J, Xicota L, Corne H, Chantran Y, Chaigneau T, et al. Neutrophil hyperactivation correlates with Alzheimer’s disease progression. Ann Neurol 2018; 83: 387–405. [DOI] [PubMed] [Google Scholar]
- Graves MC, Fiala M, Dinglasan LA, Liu NQ, Sayre J, Chiappelli F, et al. Inflammation in amyotrophic lateral sclerosis spinal cord and brain is mediated by activated macrophages, mast cells and T cells. Amyotroph Lateral Scler Other Motor Neuron Disord 2004; 5: 213–9. [DOI] [PubMed] [Google Scholar]
- Gustafson MP, Staff NP, Bornschlegl S, Butler GW, Maas ML, Kazamel M, et al. Comprehensive immune profiling reveals substantial immune system alterations in a subset of patients with amyotrophic lateral sclerosis. PLoS One 2017; 12: e0182002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harboe M, Thorgersen EB, Mollnes TE.. Advances in assay of complement function and activation. Adv Drug Deliv Rev 2011; 63: 976–87. [DOI] [PubMed] [Google Scholar]
- Henkel JS, Engelhardt JI, Siklos L, Simpson EP, Kim SH, Pan T, et al. Presence of dendritic cells, MCP-1, and activated microglia/macrophages in amyotrophic lateral sclerosis spinal cord tissue. Ann Neurol 2004; 55: 221–35. [DOI] [PubMed] [Google Scholar]
- Leveque M, Simonin-Le Jeune K, Jouneau S, Moulis S, Desrues B, Belleguic C, et al. Soluble CD14 acts as a DAMP in human macrophages: origin and involvement in inflammatory cytokine/chemokine production. FASEB J 2017; 31: 1891–902. [DOI] [PubMed] [Google Scholar]
- Lundahl J, Hallden G, Hallgren M, Skold CM, Hed J.. Altered expression of CD11b/CD18 and CD62L on human monocytes after cell preparation procedures. J Immunol Methods 1995; 180: 93–100. [DOI] [PubMed] [Google Scholar]
- Mantovani S, Garbelli S, Pasini A, Alimonti D, Perotti C, Melazzini M, et al. Immune system alterations in sporadic amyotrophic lateral sclerosis patients suggest an ongoing neuroinflammatory process. J Neuroimmunol 2009; 210: 73–9. [DOI] [PubMed] [Google Scholar]
- Miller RG, Block G, Katz JS, Barohn RJ, Gopalakrishnan V, Cudkowicz M, et al. Randomized phase 2 trial of NP001-a novel immune regulator: safety and early efficacy in ALS. Neurol Neuroimmunol Neuroinflamm 2015; 2: e100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdock BJ, Bender DE, Kashlan SR, Figueroa-Romero C, Backus C, Callaghan BC, et al. Increased ratio of circulating neutrophils to monocytes in amyotrophic lateral sclerosis. Neurol Neuroimmunol Neuroinflamm 2016; 3: e242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdock BJ, Zhou T, Kashlan SR, Little RJ, Goutman SA, Feldman EL.. Correlation of peripheral immunity with rapid amyotrophic lateral sclerosis progression. JAMA Neurol 2017; 74: 1446–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto S, de Carvalho M.. Sniff nasal inspiratory pressure (SNIP) in amyotrophic lateral sclerosis: relevance of the methodology for respiratory function evaluation. Clin Neurol Neurosurg 2018; 171: 42–5. [DOI] [PubMed] [Google Scholar]
- Schreiber H, Gaigalat T, Wiedemuth-Catrinescu U, Graf M, Uttner I, Muche R, et al. Cognitive function in bulbar- and spinal-onset amyotrophic lateral sclerosis. A longitudinal study in 52 patients. J Neurol 2005; 252: 772–81. [DOI] [PubMed] [Google Scholar]
- Shellikeri S, Karthikeyan V, Martino R, Black SE, Zinman L, Keith J, et al. The neuropathological signature of bulbar-onset ALS: a systematic review. Neurosci Biobehav Rev 2017; 75: 378–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shive CL, Jiang W, Anthony DD, Lederman MM.. Soluble CD14 is a nonspecific marker of monocyte activation. AIDS 2015; 29: 1263–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trojsi F, Caiazzo G, Di Nardo F, Fratello M, Santangelo G, Siciliano M, et al. High angular resolution diffusion imaging abnormalities in the early stages of amyotrophic lateral sclerosis. J Neurol Sci 2017; 380: 215–22. [DOI] [PubMed] [Google Scholar]
- Zenaro E, Pietronigro E, Della Bianca V, Piacentino G, Marongiu L, Budui S, et al. Neutrophils promote Alzheimer’s disease-like pathology and cognitive decline via LFA-1 integrin. Nat Med 2015; 21: 880–6. [DOI] [PubMed] [Google Scholar]
- Zhang R, Gascon R, Miller RG, Gelinas DF, Mass J, Hadlock K, et al. Evidence for systemic immune system alterations in sporadic amyotrophic lateral sclerosis (sALS). J Neuroimmunol 2005; 159: 215–24. [DOI] [PubMed] [Google Scholar]
- Zhang R, Hadlock KG, Do H, Yu S, Honrada R, Champion S, et al. Gene expression profiling in peripheral blood mononuclear cells from patients with sporadic amyotrophic lateral sclerosis (sALS). J Neuroimmunol 2011; 230: 114–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W, Beers DR, Appel SH.. Immune-mediated mechanisms in the pathoprogression of amyotrophic lateral sclerosis. J Neuroimmune Pharmacol 2013; 8: 888–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W, Beers DR, Hooten KG, Sieglaff DH, Zhang A, Kalyana-Sundaram S, et al. Characterization of gene expression phenotype in amyotrophic lateral sclerosis monocytes. JAMA Neurol 2017; 74: 677–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler-Heitbrock L, Ancuta P, Crowe S, Dalod M, Grau V, Hart DN, et al. Nomenclature of monocytes and dendritic cells in blood. Blood 2010; 116: e74–80. [DOI] [PubMed] [Google Scholar]
- Zondler L, Feiler MS, Freischmidt A, Ruf WP, Ludolph AC, Danzer KM, et al. Impaired activation of ALS monocytes by exosomes. Immunol Cell Biol 2017; 95: 207–14. [DOI] [PubMed] [Google Scholar]
- Zondler L, Muller K, Khalaji S, Bliederhauser C, Ruf WP, Grozdanov V, et al. Peripheral monocytes are functionally altered and invade the CNS in ALS patients. Acta Neuropathol 2016; 132: 391–411. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.