Abstract

The transformation of agrochemicals into herbicidal ionic liquids (HILs) has been suggested as a solution to problems associated with commercial forms of herbicides. The aim of this review was to summarize the latest progress in the field of HILs, including their synthesis as well as physicochemical and biological properties, and to address the areas that require further research in order to ensure their safe commercialization (e.g., data regarding biodegradability, toxicity, and environmental fate). The first part of the review provides an in-depth summary of the current state of knowledge regarding HILs, particularly the anions and cations used for their synthesis. The second part highlights the employed synthesis methods and elucidates their respective advantages and limitations. The third section is focused on the characterization of HILs with emphasis on the methods and factors that are significant in terms of their practical application. Subsequently, the issues associated with the biodegradation and toxic effects of HILs are discussed based on the relevant literature reports. All sections include comprehensively tabulated data in order to enable rapid comparison of utilized approaches. Finally, all the findings are critically analyzed in terms of crucial disadvantages (especially the lack of standardization), which allowed us to establish future recommendations and basic guidelines that are presented in the last section.
Keywords: herbicidal ionic liquids, HILs, volatility, biodegradation, toxicity, leaching
1. Introduction
The use of pesticides in modern agriculture is an absolute necessity in terms of mass and, above all, cheap production of food. This results from the fact that maintaining high productivity of crops is at risk without providing proper crop protection during the growing period as well as storage.1,2 Crops could be affected both by biotic (weeds, pests, pathogens) and abiotic factors (water, temperature, nutrient deficiency, irradiation), which result in substantial losses.1 Hence, in order to avoid a notable decrease of yield, various plant protection products are applied. Only in 1940, 140 t of various pesticides were used, while in 2016 the total amount of pesticides employed for pest control worldwide reached a value of over 4 million metric tons, 35% of which were employed in China alone.2,3 Herbicides are a group of plant protection agrochemicals used for the control of weeds that compete with crop plants.2 The precise mechanism of action of auxins was discovered in the 1930s, and as a result, new active ingredients such as 2,4-D (2,4-dichlorophenoxyactic acid) and MCPA (2-methyl-4-chlorophenoxyacetic acid) revolutionized the field of crop protection in the 1940s.1,2,4 Afterward, in 1967, dicamba (3,6-dichloro-2-methoxybenzoic acid) was introduced,5 followed by glyphosate (N-(phosphonomethyl)glycine) in the 1970s and many others not mentioned here. Hence, herbicides started to be commercially applied on a mass scale, and the recently growing use of genetically modified crops (GMO crops) has favored their extensive usage. However, despite various structures of herbicides, the mode of action of all formulations available on the market is based on a limited number of mechanisms.6
During the period of expansion in the herbicide industry, several notable disadvantages associated with these agrochemicals have been discovered. These substances may be susceptible to volatilization, leaching, and runoff, as well as accumulation in soils, waters, and tissues of organisms; they may also exhibit toxicity toward nontargeted organisms. Additionally, the half-lives of herbicides in various environmental compartments vary greatly, from substances that decompose after days with innocuous degradation products to those that are relatively persistent in the environment.2 Moreover, due to the extensive use of various herbicides, plants exhibit growing resistance toward these formulations.1 This process occurs as a part of natural selection, as well as deliberate introduction of resistance in GMO crops. Introduction of genetically modified organisms that possess genes responsible for herbicide resistance is associated with the risk of horizontal gene transfer to nontarget organisms. Herbicide-resistant weed species can expand rapidly in number and areal coverage and colonize new niches. At present, approximately 250 herbicide-resistant species have been reported, among which at least 34 are resistant to glyphosate only.7 Furthermore, the majority of commercially used herbicidal products include adjuvants in order to improve water solubility of the active ingredient, reduce droplet volatility, increase the adhesion of these formulations to the plant surface, and, as a result, enhance the penetration of the product into plant tissues.8 However, adjuvants (e.g., ethoxylated etheralkylamine, solvent naphtha) are often more hazardous compared to herbicides as they may increase the cytotoxicity of the formulation as much as 1000 times.9,10 Nevertheless, in contrast to herbicidally active substances, adjuvants are subjected to less restrictive registration control.11−15 Due to the above-mentioned issues, modern research in the field of agrochemistry should focus on the minimization of the negative impact of herbicidal formulations and simultaneous maximization of their efficiency. As a result, a novel approach was proposed in 2011, namely, the application of ionic liquids composed of organic cations and herbicidal anions, called herbicidal ionic liquids (HILs).16
Ionic liquids (ILs) are defined as salts composed of discrete ions that occur in the liquid state below 100 °C.16−18 They possess unique properties and various applications. On this basis, ILs were divided into different generations.17 The first generation consists of ILs with unique, tunable physical properties. The second includes ILs with targeted chemical properties (e.g., reactivity, electrochemical window, flammability, chirality, blocking UV rays, or oxygen balance) combined with selected physical properties (such as hydrophobic/hydrophilic character, refractive index, viscosity, density, or thermal stability). The third generation applies to ILs with targeted biological properties combined with selected physical and chemical properties.17,18 The initial attempt was to incorporate active pharmaceutical ingredients (APIs) into ILs and therefore tune their biological functions.17,19 However, it was later discovered that it is possible to apply ILs in agroprotection due to the tunability of these compounds. Hence, ILs based on herbicidal formulations (herbicidal ionic liquids, HILs) with additional surface-active and pesticidal properties (originating from the counterion) were successfully synthesized.16 The proposed compounds exhibited reduced drift and volatility as well as adjustable water solubility and therefore decreased mobility in soils and waters. Additionally, because HILs exhibit surface active properties, the necessity to use adjuvants is eliminated, and they can be applied at lower doses, which results in reduced environmental toxicity.16,20 Furthermore, some HILs may be characterized by prolonged interaction between the plant and the active substance, possibly due to the continuous slow release of active ingredients. Particularly, “esterquat” HILs, comprising the herbicide in the cation bonded via an ester group,21 are sensitive to hydrolysis, which can result in controlled release of active ingredient similarly to the case of polymers based on a coumarin and the 2,4-D herbicide.22 These discoveries were a starting point for further research on HILs, as an alternative for commercially used herbicides.
During recent years, the attention of the scientific community was mostly focused on the synthesis and advantages of novel HIL formulations. However, no article has dealt with the issue of description of this new group of ILs in general or wider discussion regarding the discoveries in this field. Hence, the purpose of this review is to summarize current literature data concerning herbicidal ionic liquids: their synthesis, chemical and biological characteristics, herbicidal efficacy, toxicity, and biodegradability. Additionally, the review presents a critical summary of current limitations regarding understudied areas (e.g., toxicity and biodegradation) that lack environmentally relevant analyses. Finally, future considerations and perspectives are presented.
2. Current State of Knowledge
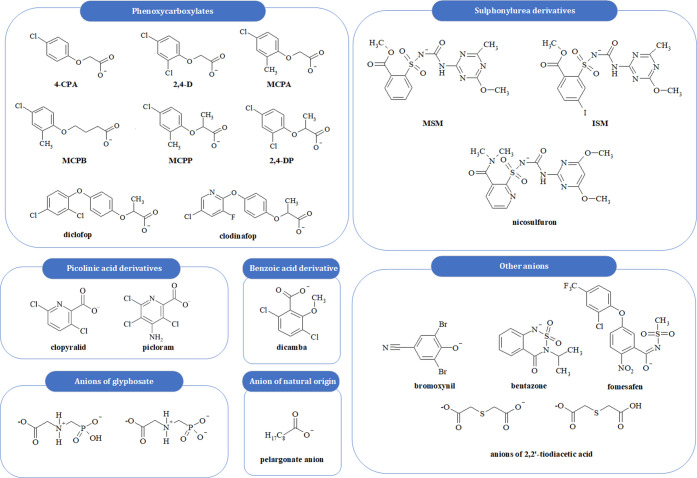
During the initial development of HILs, it was observed that many synthetic auxins occur in the form of anions. Therefore, the idea of combining herbicidal anions with functional cations arose, and soon the first HILs were synthesized.16 Various classes of chemical compounds can be applied as the source of anion (Figure 1), for instance, phenoxycarboxylates,8,16,23−32 benzoic acid derivatives,26 picolinic acid derivatives,27,28 sulfonylurea derivatives,8,29,30 glyphosate,31 anions of natural origin,33,34 or other anions.23,24,32 It is worth noting that most herbicidally active substances (e.g., glyphosate, phenoxyacids, and benzoic or nicotinic acid derivatives) are available on the market in the anionic form, as their introduction into formulations is generally easier than in the case of organic cations. Moreover, herbicides available in the cationic form are known to exhibit different modes of action than commercial formulations in the anionic form. It was also established that herbicidal anions are more effective in selective control of broadleaf weeds.35 Despite the fact that substances such as paraquat or diquat are currently under research regarding their transformation into novel forms, their use is not recommended due to the harmful environmental effects. Interestingly, although paraquat and diquat possess the same mode-of-action, they vary greatly in terms of toxicity. As a result, the European Union withdrew paraquat from its market in July 2007 and recommended the use of less harmful substances.36
Figure 1.
Herbicidal anions employed in HILs.
In order to form HILs, the above-mentioned herbicidal anions are typically paired with cations improving wetting properties, for example, quaternary ammonium, imidazolium, isoquinolinium, morpholinium, phosphonium, piperidinium, pyridinium, pyrrolidinium, quinolinium, or, more recently, DABCO (1,4-diazabicyclo[2.2.2]octane).16,26,34,37 The overview of cations used in HIL syntheses are presented in Table 1.
Table 1. Overview of Cations Used in HILs.
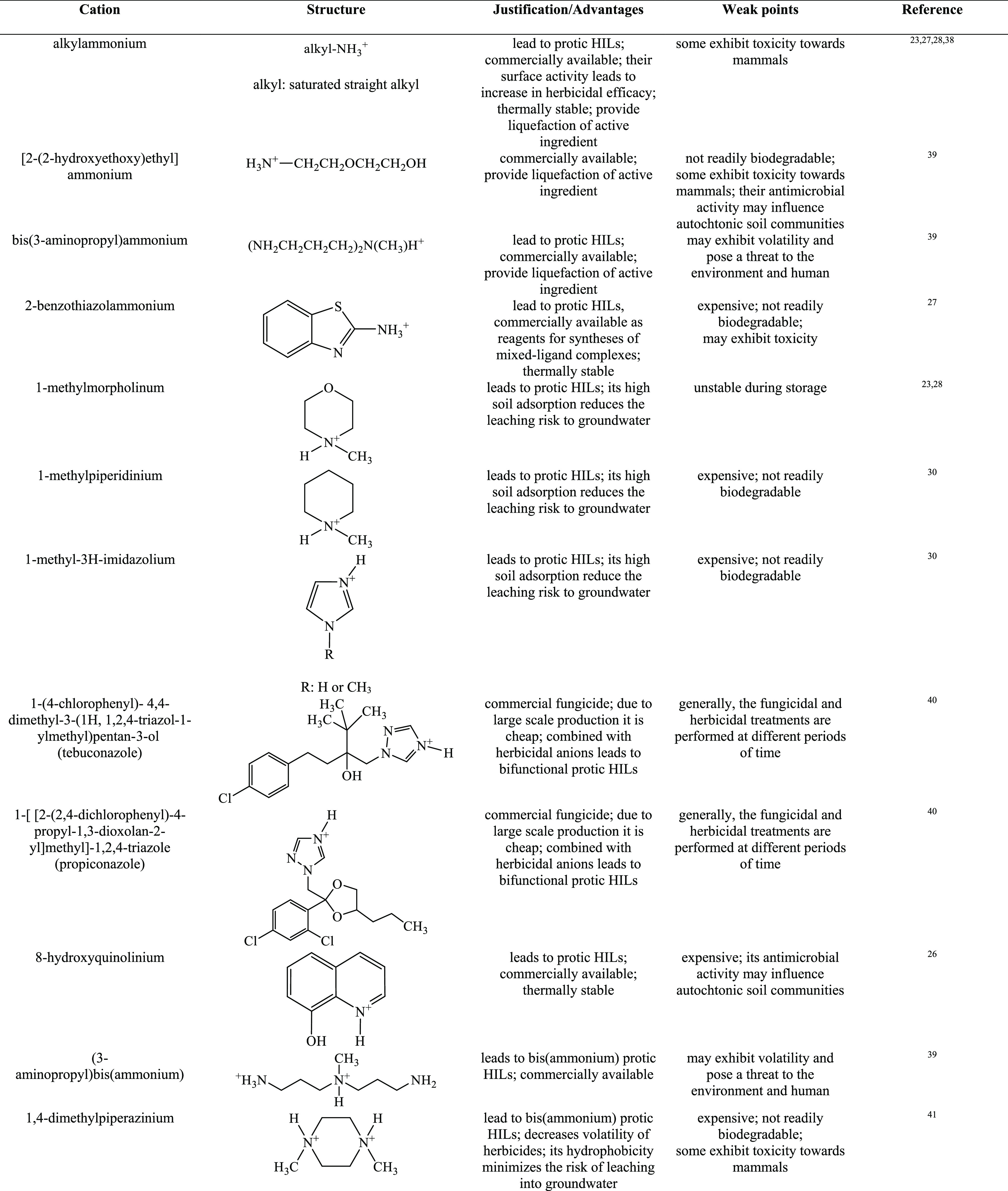
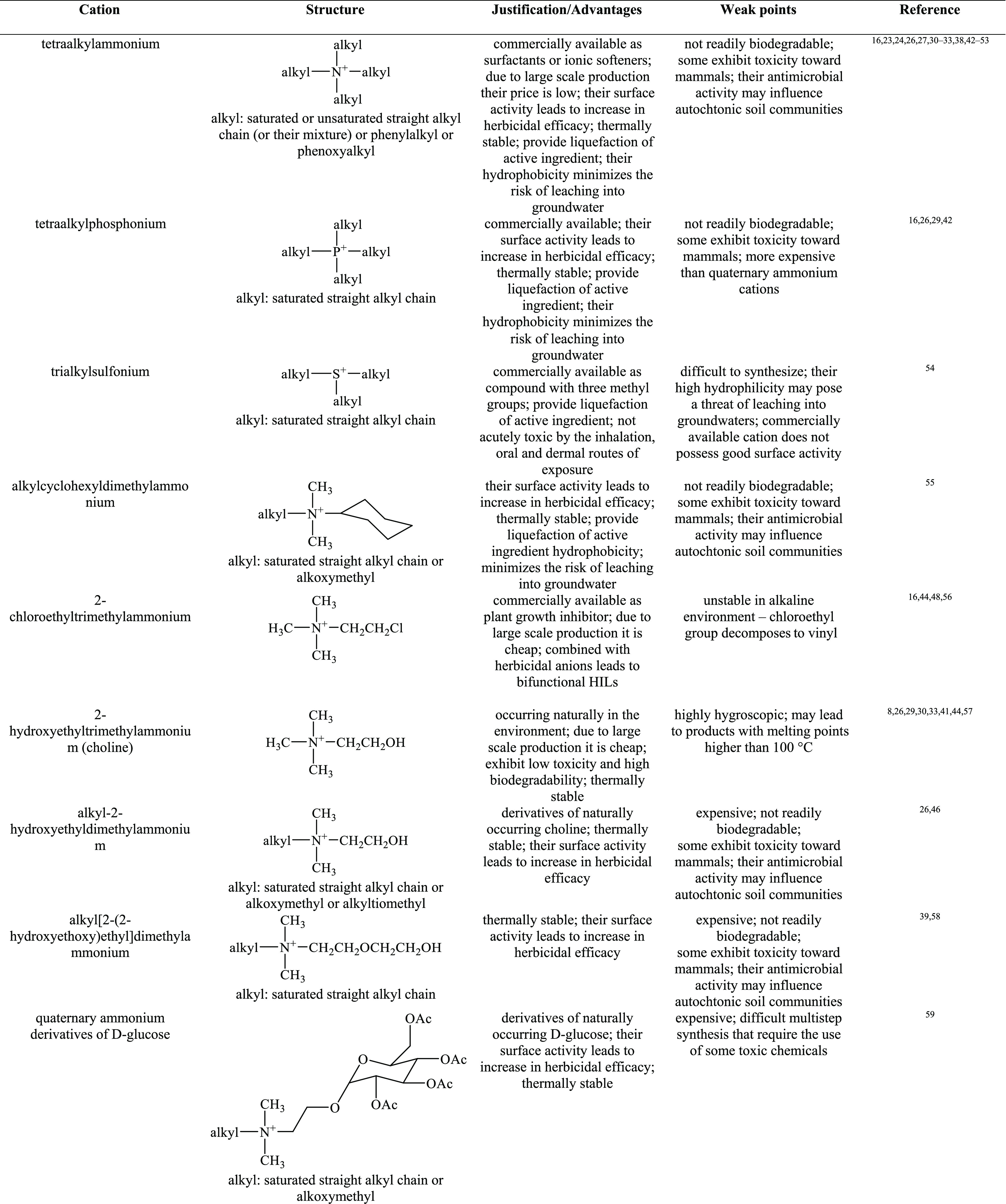
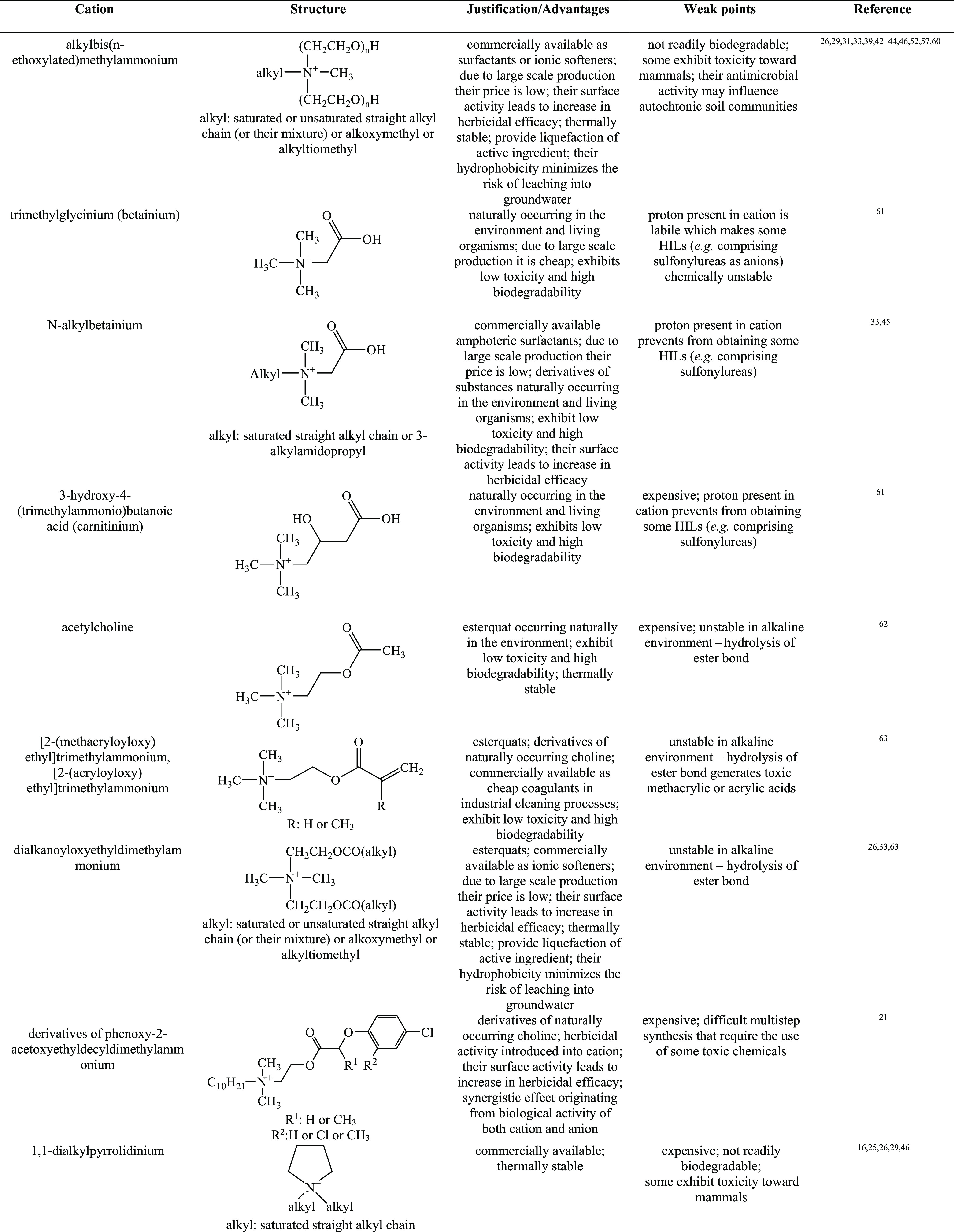
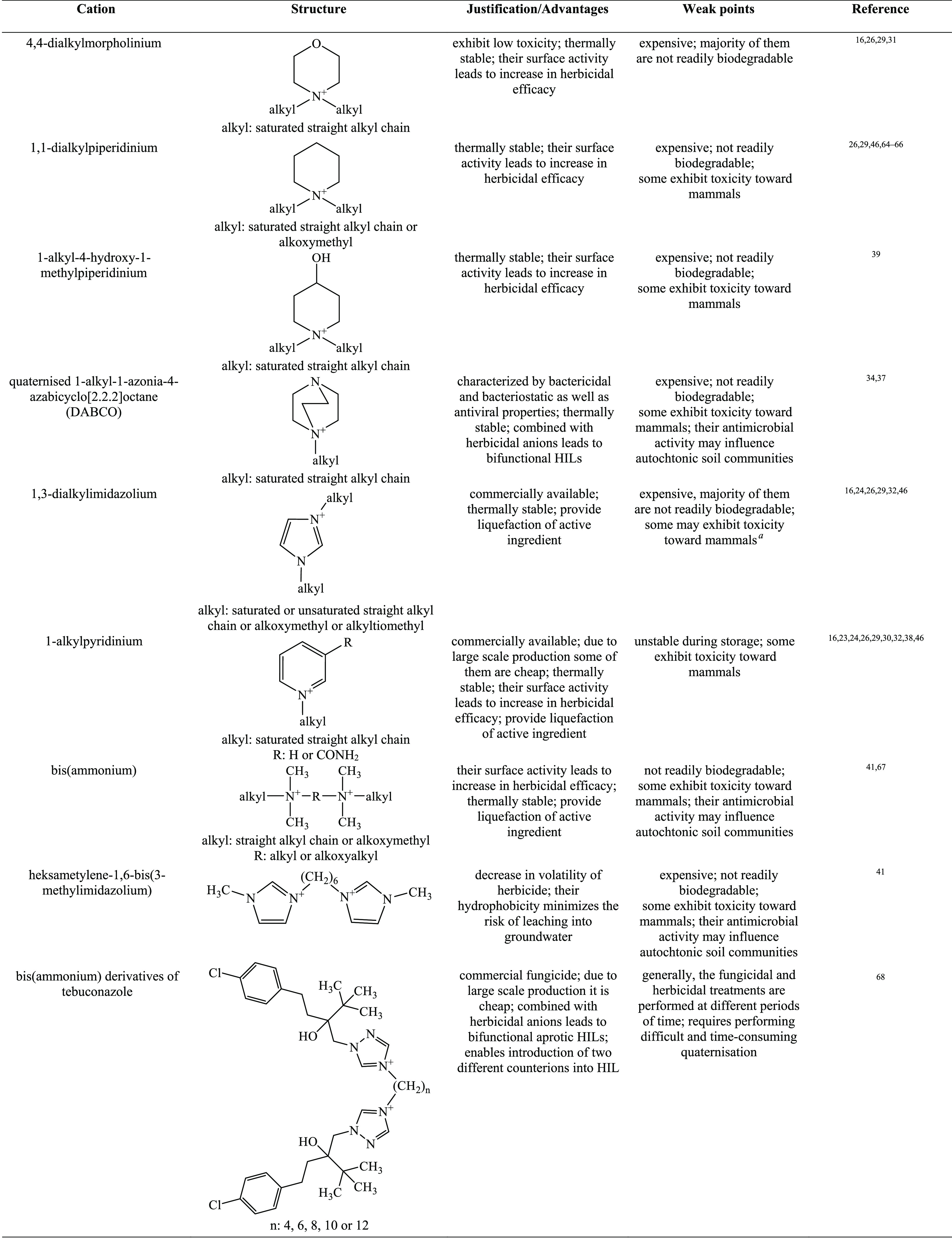
See ref (69).
According to the data presented in Table 1, the majority of studied HILs were based on ammonium cations, whereas phosphonium and sulfonium cations were scarcely used. This may be explained by the fact that quaternary ammonium halides and amines are cheap, readily available, and commercially used as surfactants, disinfectants, or softeners, which makes them attractive candidates for production on a large scale. It should also be emphasized that phosphonium cations are resistant to biodegradation.70 This phenomenon can be caused by formation of phosphine oxides, toxic metabolites that inhibit the biodegradation process and, as a result, may become a potential threat to the environment.71
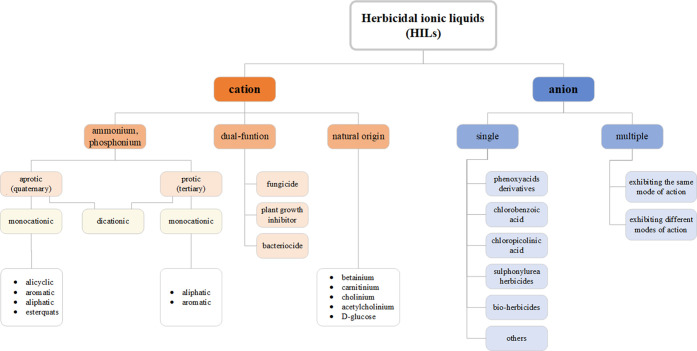
Proper selection of both cations and anions results in an almost unlimited number of herbicides with designable properties. Development in this field was started by pairing various quaternary ammonium cations with herbicidal anions, MCPA, 2,4-D, 2-(4-chloro-2-methylphenoxy)propionic acid (MCPP), and dicamba,16,26,39,46,52 in order to reduce the volatility and dosage of commercially used herbicidal forms as well as to improve their wetting properties. Consequently, due to the possibility to introduce various active compounds into the structure of ILs, experiments have been performed in order to evaluate the relevance of dual function ionic liquids. Hence, the focus was shifted to ILs with chlormequat chloride (2-chloroethyltrimethylammonium chloride, CCC) as the source of cation, with the aim of successful combination of both the herbicidal properties of the anion and the ability to regulate plant growth of the cation.48,56,72 Additionally, the use of fungicides as cations was under research, namely commercially used tebuconazole and propiconazole, as well as morpholine derivatives, often present in various pesticides due to their surface active properties.40,73 Afterward, esterquats, quaternary ammonium surfactants frequently applied in the industry as fabric softeners, were proposed as the cation source. Their function, depending on the study, was either associated with the improvement of surface properties or introduction of herbicidal activity in the cation.63 Another approach was the use of double salt herbicidal ionic liquids (DSHILs), in which two herbicidal anions were used in a single compound.42,58 More recently, the field of bio-HILs was investigated, by searching for renewable or biodegradable cations (e.g., betaine, carnitine, d-glucose, choline, acetylcholine),44,45,59,61,62,74,75 as well as anions (e.g., pelargonate).33,34,53,76,77 Simultaneously, testing of various combinations of herbicidal anions and ammonium-based cations was in progress, resulting in numerous groups of their sources (Figure 2).
Figure 2.
Classification of HILs.
3. Synthesis Methods
The synthesis of HILs is typically conducted via a one- or two-step procedure; however, there are also cases of multistep syntheses. The development of HILs revealed advantages of utilizing cations derived from natural sources, such as betaine, carnitine, choline, or d-glucose.44,59,61 Currently, this direction seems to be the most reasonable approach; although it is necessary to emphasize that the synthesis route of HILs should be relatively simple, efficient, and environmentally friendly. For instance, reports describing HILs derived from glucose59 or esters of choline21 provide highly valuable scientific knowledge; however, their difficult, multistep synthesis gives practically no chance for their commercialization. Additionally, the synthesis of HILs should not result in a significant increase of the overall cost of production. Therefore, considering the lack of necessity for using adjuvants in order to improve the herbicidal activity, HILs may become an attractive alternative to commonly known commercial preparations.
3.1. One-Step Synthesis
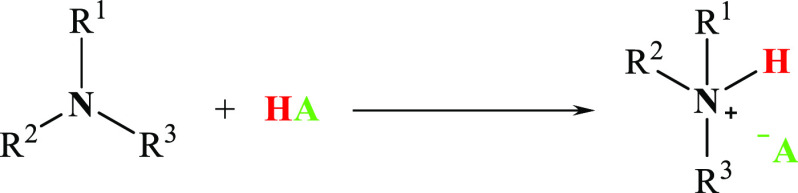
One-step synthesis is a reaction of an amine (or heterocyclic compounds with tertiary nitrogen atom) with a herbicide in the form of an acid (Figure 3).
Figure 3.
One-step synthesis of protonic HILs.
The reaction is usually conducted homogeneously in methanol23,28,39,40 or chloroform78 but also heterogeneously in water27 with further evaporation of the solvent. When necessary, the final product is rinsed with anhydrous hexane40 or anhydrous diethyl ether.41 Using this approach, tebuconazole- and propiconazole-based ILs with MCPA, MCPP, 2,4-D, and dicamba as anions can be obtained.40 Furthermore, protic HILs originating from primary, secondary, and tertiary amines or heterocyclic compounds with tertiary nitrogen atom (e.g., 1-methylimidazole, isoquinoline) may be synthesized using this method, with clopyralid,27 dicamba,39 bromoxynil,23 2,4-D,41 picloram,28 mesotrione,38 2,2′-thioacetate, and 2,2′-thiodiacetate78 as the source of anion. The reported yields of one-step syntheses were in the range of 87–99%. It should be emphasized that to date there is a lack of data regarding the possible byproducts. This aspect is of high importance for future research in order to avoid potential environmental risks, which occurred in the case of 2,4-D and dioxins, for example.
3.2. Two-Step Synthesis
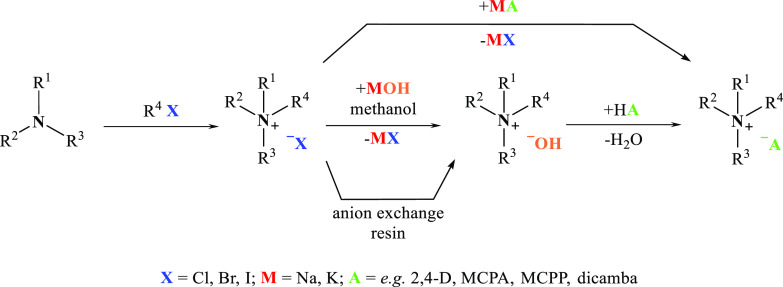
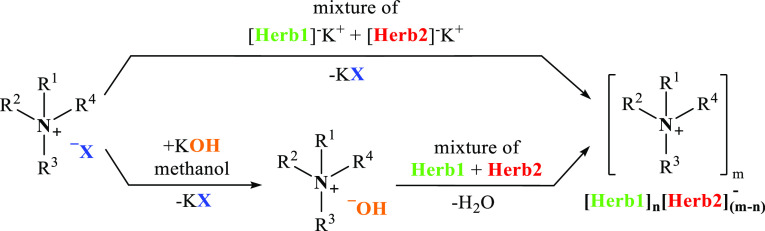
In the first step of this protocol, quaternary chloride or ammonium bromide is obtained as a result of the reaction of tertiary amine (phosphine or heterocyclic compound with tertiary nitrogen atom) with a proper quaternizing agent. In case of alkyl halides, the reaction occurs via the SN2 mechanism and no rearrangement takes place, whereas in the case of other reagents (e.g., chloromethylalkyl ethers), the reaction occurs according to the SN1 mechanism, which is associated with additional hazards related to the formation of byproducts (e.g., increased susceptibility to oxidation or hydrolysis). Subsequently, the halide is substituted with the anion characterized by herbicidal activity through ion exchange. The ion exchange can be carried out either via the metathesis reaction (i.e., double replacement reaction) with sodium or potassium salt of the selected herbicide or via an intermediate quaternary ammonium hydroxide with the herbicide in the form of an acid. Quaternary ammonium hydroxide is obtained using a corresponding quaternary ammonium halide as a product of the reaction of potassium hydroxide with quaternary ammonium halide in anhydrous methanol (or ethanol) or with the use of an ion-exchange resin (Figure 4).
Figure 4.
Two-step synthesis of HILs.
The metathesis reaction in aqueous conditions is well suited for obtaining HILs with cations comprising long chain alkyl substituents. This type of IL with a large hydrophobic cation (e.g., didecyldimethylammonium) and large organic anion (e.g., MCPA)16 can be extracted easily with the use of water-immiscible nonpolar solvents, for example, dichloromethane or chloroform. Multiple rinsing of the organic phase with water results in the elution of sodium halide formed in the reaction as well as the potentially unreacted substrates. However, sometimes it is also possible to elute the IL to a high extent during this step. In such cases, it is necessary to select a different method of product isolation from the postreaction mixture or even a different synthesis method. Another technique of product isolation from the postreaction mixture is extraction using chloroform and its complete evaporation and, afterward, dissolution of the product in anhydrous acetone. Then, the product present in acetone should only be filtered from the residues of inorganic salt and evaporated. This procedure was used to obtain ILs with phenoxycarboxylic anions and 1-alkyl-1-methylpyrrolidinium or 1-alkoxymethyl-1-methylpyrrolidinium cations.25 These compounds, despite their long alkyl substituents (10 or even 12 carbon atoms), exhibited high hydrophilicity and were almost entirely eluted during rinsing of the chloroform phase with water. The described procedure of product isolation from the postreaction mixture (without water rinsing but with anhydrous acetone extraction) provided satisfactory results, as the compounds were obtained with yields exceeding 85% and a cation-active substance content above 91%.
An alternative approach is to conduct the metathesis reaction in methanol instead of water. In this case, potassium chloride or bromide precipitates almost instantly while the IL remains in the organic solvent. However, potassium iodide dissolves in methanol very well. Due to the partial or complete solubility of the inorganic salt in methanol, the IL should be purified after complete evaporation of the alcohol. For this purpose, the crude product needs to be dissolved in acetone or in a mixture of methanol and acetone or acetonitrile when working with substances that exhibit limited solubility (or insolubility) in acetone.61 Then, the KI39 and KCl or KBr residues and possible residues of potassium salt forms of the herbicides should be filtered off. Finally, the solvent should be evaporated, and in the final step, the product should be dried. The latter may be challenging, as some HILs are characterized by hygroscopic properties; therefore the drying should be carried out under vacuum and increased temperature conditions.
It was observed that ILs with the glyphosate anion could not be obtained via the metathesis reaction, regardless of the alkyl chain length in the cation. This most likely results from the fact that glyphosate is a synthetic amino acid that occurs in a stable zwitterion form in the presence of NaOH. In this case, a novel synthesis method was applied, which is also useful for obtaining various HILs with shorter alkyl substituents in cations and different herbicidal anions, for example, MCPA.55 In this method, quaternary ammonium hydroxide is obtained via reaction of potassium hydroxide with quaternary ammonium halide in anhydrous methanol29,31,42,47,55,57,60,67 or ethanol.41 This quaternary ammonium hydroxide reacts with the herbicide in the acidic form, which results in the formation of HIL and water. In this case, the selection of the solvent is an important factor, as it enables the synthesis of quaternary hydroxide because the byproduct (potassium chloride or bromide) precipitates from the solvent. However, the resulting KCl or KBr is partially dissolved in methanol, and hence, an additional purifying procedure with the use of anhydrous acetone extraction is required.29,47,55,60,67 For HILs not soluble in acetone, anhydrous acetonitrile57 or isopropanol31,42 are used as solvents in the extraction procedure.
In the case of HILs composed of glyphosate, it was possible to obtain mono- and dicationic salts depending on the molar ratio of reagents.31
It should be noted that not all HILs can be synthesized with the use of the above presented two-step procedure; namely, it is not valid when their precursors undergo other reactions with strong bases. For instance, esterquats undergo basic hydrolysis, betaine hydrochlorides undergo saponification with simultaneous formation of a zwitterion, and CCC (2-chloroethyltrimethylammonium chloride) undergoes elimination of hydrogen chloride with formation of trimethylvinyl chloride.48,56,63
Another variant of the method in which the herbicide in an acidic form reacts with quaternary ammonium hydroxide is the application of ion-exchange resin. The advantage of this approach is that the resulting ILs do not require further purification from the inorganic salt. Water is preferred solvent for reactions with the ion-exchange resin;26,46,51,64,66 however synthesis in ethanol was also reported.65 After reaction of the quaternary ammonium hydroxide with the herbicide in an acidic form, the solvent is evaporated, and the product is dried. The disadvantage of this method includes excessive foaming of product during vacuum evaporation when compounds with long chain substituents (with 10–12 carbon atoms in alkyl chain) and water as a solvent are used.
The anions used in synthesis of various HILs are presented in Table 2. However, it should be emphasized that the number of herbicides transformed into HILs is relatively low compared to the list of 337 of herbicidally active substances registered in EU Pesticides database (https://ec.europa.eu/food/plant/pesticides/eu-pesticides-database/public/?event=activesubstance.selection&language=EN, accessed 2020-02-04). This is mainly due to the low availability of the majority of herbicides in the form of pure substances suitable for synthesis. As a result, only the active ingredients that are the most popular and common in the USA, Europe, and Asia (particularly glyphosate, dicamba, phenoxyacids (2,4-D and MCPA), metsulfuron-methyl, and bentazone) were transformed into HILs. Moreover, it should be noted that successful conversion of a herbicidally active substance into a HIL is often a difficult task. Generally, the herbicide should be able to form a stable ion (cation or anion), which is associated with the presence of specific functional groups, in order to perform the desired reaction (alkylation, metathesis, or neutralization). Nevertheless, it is certain that the list of herbicides successfully transformed into HILs, provided in Table 3, will be extended by new substances. Taking into consideration the above-mentioned requirements, it can be assumed that the most promising candidates for transformation into HILs in the near future are paraquat (CAS number 1910-42-5), pendimethalin (CAS number 40487-42-1), trifluralin (CAS number 1582-09-8), imazethapyr (CAS number 81335-77-5), metam sodium (CAS number 137-42-8), glufosinate (CAS number 51276-47-2), and chloramben (CAS number 133-90-4).
Table 2. Overview of Anions Used in HIL Synthesis Divided by Reaction Types.
| anion | type of reaction | yield (%) | refs |
|---|---|---|---|
| MCPA | metathesis | 86–99 | (16,25,43,45,50,55,56,58,59,63,73,79) |
| quaternary ammonium hydroxide resulting from KOH in methanol | 86–99 | (37,47,55,60,67,68) | |
| 2,4-D | metathesis | 82–99 | (25,45,46,48,59,63,73) |
| quaternary ammonium hydroxide resulting from KOH in methanol | 89–99 | (47,67) | |
| quaternary ammonium hydroxide resulting from KOH in ethanol | 90–92 | (41) | |
| quaternary ammonium hydroxide with the use of ion-exchange resin | 82–99 | (46) | |
| from commercially available choline hydroxide | 97 | (44) | |
| dicamba | metathesis | 90–99 | (26,39,45,51,58,63,73) |
| quaternary ammonium hydroxide resulting from KOH in methanol | 90–98 | (47,67,68) | |
| quaternary ammonium hydroxide with the use of ion-exchange resin | 74–99 | (26,51) | |
| fomesafen | metathesis | 81–98 | (32) |
| MCPP | metathesis | 90–98 | (25,45,51,63,73) |
| quaternary ammonium hydroxide resulting from KOH in methanol | 92–97 | (47,67) | |
| quaternary ammonium hydroxide with the use of ion-exchange resin | >92 | (51,66) | |
| metsulfuron-methyl (MSM) | metathesis | 91–98 | (29) |
| quaternary ammonium hydroxide resulting from KOH in methanol | 90–99 | (29) | |
| diclofop | metathesis | (8) | |
| clodinafop | metathesis | (8) | |
| bentazone | metathesis | 83–97 | (24) |
| clopyralid | metathesis | (27) | |
| 4-CPA | metathesis | 85–91 | (25) |
| bromoxynil | metathesis | 94–99 | (23) |
| pelargonate | metathesis | 93–99 | (33,62) |
| quaternary ammonium hydroxide resulting from KOH in methanol | 96–99 | (34) | |
| from commercially available choline hydroxide | 98 | (77) | |
| iodosulfuron-methyl (ISM) | metathesis | 88–98 | (62) |
| glyphosate | quaternary ammonium hydroxide resulting from KOH in methanol | 90–98 | (31,42) |
| 2,4-DP | quaternary ammonium hydroxide resulting from KOH in methanol | 89–95 | (57) |
| quaternary ammonium hydroxide with the use of ion-exchange resin | 91–98 | (65) | |
| nicosulfuron | quaternary ammonium hydroxide resulting from KOH in methanol | 86–93 | (30) |
| MCPB | quaternary ammonium hydroxide with the use of ion-exchange resin | 91–96 | (64) |
| mesotrione | quaternary ammonium hydroxide resulting from KOH in methanol | 87–95 | (38) |
Table 3. Physicochemical Properties of HILsa.
| anion | cation | appearance at 25 °Cb | thermal stability and volatilityc | solubility in water | surface tension at CMC (mN/m) | adsorption in soil (%) | KOW | refs |
|---|---|---|---|---|---|---|---|---|
| MCPA | tebuconazole | liquid | 174 °C (T5%) | insoluble | d | d | d | (40) |
| MCPA | propiconazole | liquid | 259 °C (T5%) | insoluble | d | d | d | (40) |
| MCPA | tetraalkylammonium | solids (Tm = 57–90 °C) (alkyltrimethyl-ammonium); solid (Tm = 90 °C) or liquid (dialkyldimethyl-ammonium) | 190–212 °C (T5%) (alkyltrimethyl-ammonium); 205–210 °C (T5%) (dialkyldimethyl-ammonium) | 3.3–10% (w/v) or <3.3% (w/v) (alkyltrimethyl-ammonium); limited (dialkyldimethyl-ammonium) | 28.0–37.6 (alkyltrimethyl-ammonium); 26.2–28.0 (dialkyldimethyl-ammonium) | d | d | (16,47,49,50) |
| MCPA | tetraalkylphosphonium | liquid | 305 °C (T5%) | soluble | d | d | d | (16) |
| MCPA | alkylcyclohexyl-dimethylammonium | liquids or waxes | 150–214 °C (T5%) | d | 31.2–39.4 | d | d | (55) |
| MCPA | 2-chloroethyl-trimethylammonium | solid (Tm = 94–96 °C) | 198 °C (T5%) | soluble | 40.0 | d | d | (16,56) |
| MCPA | alkyl[2-(2-hydroxyethoxy)-ethyl]dimethylammonium | liquids | 187–205 °C (T5%) | >10% (w/v) | 30.7–36.9 | d | –1.00 to 1.38 | (58) |
| MCPA | quaternary ammonium derivatives of d-glucose | liquids or waxes | 164–224 °C (T5%) | d | 32.6–34.4 | d | d | (59) |
| MCPA | alkylbis(n-ethoxylated) methylammonium | solids (Tm = 52–71 °C) | 225–230 °C (T5%) | limited | 34.0–38.5 | d | d | (60) |
| MCPA | betainium | solid (Tm = 64–66 °C) | 234 °C (T5%) | <3.3% (w/v) | d | d | d | (61) |
| MCPA | N-alkylbetainium | solid (Tm = 37–40 °C) or liquid | 159–230 °C (T5%) | <3.3% (w/v) | 31.6–0.31.8 | d | d | (45) |
| MCPA | carnitinium | liquid | 190 °C (T5%) | <3.3% (w/v) | d | d | d | (61) |
| MCPA | acetylcholine | solid (Tm = 90–91 °C) | 183 °C (T5%) | >10% (w/v) | 33.4 | d | –1.38 | (62) |
| MCPA | [2-(methacryloyloxy)-ethyl]trimethylammonium [2-(acryloyloxy)-ethyl]trimethylammonium | liquids | 195–200 °C (T5%) | >10% (w/v) | d | d | d | (63) |
| MCPA | dialkanoyloxyethyl-dimethylammonium | wax | 208 °C (T5%) | <3.3% (w/v) | d | d | d | (63) |
| MCPA | derivatives of phenoxy-2-acetoxyethyl-decyldimethylammonium | liquids | d | d | d | d | d | (21) |
| MCPA | 1,1-dialkylpyrrolidinium | liquids | 128–210 °C (T5%) | >10% (w/v) | 27.4–33.5 | d | d | (25) |
| MCPA | 4,4-dialkylmorpholinium | solid (Tm = 77–79 °C) | 220 °C (T5%) | soluble | d | d | d | (16) |
| MCPA | 1,1-dialkylpiperidinium | solid (Tm = 39–42 °C) | 230 °C (T5%) | soluble | d | d | d | (16) |
| MCPA | quaternized DABCO | waxes | 215–281 °C (T5%) | >10% (w/v) or 3.3–10% (w/v) | 35.6–38.9 | d | d | (37) |
| MCPA | 1-alkylpyridinium | liquids | 200 °C (T5%) | limited | d | d | d | (16) |
| MCPA | bis(ammonium) | waxes | 205–210 °C (T5%) | >10% (w/v) or 3.3–10% (w/v) | d | d | d | (67) |
| MCPA | bis(ammonium) derivatives of tebuconazole | waxes | 209–223 °C (T5%) | d | d | d | d | (68) |
| 2,4-D | tebuconazole | wax | 204 °C (T5%) | insoluble | d | d | d | (40) |
| 2,4-D | propiconazole | liquid | 239 °C (T5%) | insoluble | d | d | d | (40) |
| 2,4-D | 1,4-dimethylpiperazinium | solid (Tm = 98–100 °C) | 3.0% (V) | <30–70% (pH = 5); 70–100% (pH = 7); <30% (pH = 9) | 50.6 | d | 3.08 | (41) |
| 2,4-D | tetraalkylammonium | solids (Tm = 55–74 °C) or waxes (alkyltrimethyl-ammonium); liquids or waxes (dialkyldimethyl-ammonium) | 170–218 °C (T5%) (alkyltrimethyl-ammonium); 204–219 °C (T5%) (alkyltrimethyl-ammonium) | >10% (w/v) or 3.3–10% (w/v) or <3.3% (w/v) (alkyltrimethyl-ammonium); limited (dialkyldimethyl-ammonium) | 28.9–36.8 (alkyltrimethyl-ammonium); 26.6–31.2 (dialkyldimethyl-ammonium) | d | d | (44,46−48,52) |
| 2,4-D | tetraalkylphosphonium | liquid | 260 °C (T5%) | soluble | d | d | d | (16) |
| 2,4-D | 2-chloroethyl-trimethylammonium | solid (Tm = 92–96 °C) | 198 °C (T5%) | soluble | 41.5 | d | d | (44,48) |
| 2,4-D | alkyl-2-hydroxyethyl-dimethylammonium | solid (Tm = 86–88 °C) or wax | d | limited or soluble | d | d | d | (46) |
| 2,4-D | quaternary ammonium derivatives of d-glucose | waxes | 201–221 °C (T5%) | d | 31.4–35.5 | d | d | (59) |
| 2,4-D | alkylbis(n-ethoxylated) methylammonium | solids (Tm = 49–71 °C) liquids or waxes | 222–228 °C (T5%) | >10% (w/v) or 3.3–10% (w/v) | 30.4–37.2 | d | d | (44,46,52,60) |
| 2,4-D | betainium | solid (Tm = 70–72 °C) | 237 °C (T5%) | <3.3% (w/v) | d | d | d | (61) |
| 2,4-D | N-alkylbetainium | solid (Tm = 64–66 °C) or wax | 171–228 °C (T5%) | <3.3% (w/v) | 31.5–31.7 | d | d | (45) |
| 2,4-D | carnitinium | wax | 165 °C (T5%) | <3.3% (w/v) | d | d | d | (61) |
| 2,4-D | acetylcholine | solid (Tm = 95–97 °C) | 189 °C (T5%) | <3.3% (w/v) | 33.0 | d | –1.36 | (62) |
| 2,4-D | [2-(methacryloyloxy)-ethyl]trimethylammonium; [2-(acryloyloxy)-ethyl]trimethylammonium | waxes | 198–200 °C (T5%) | <3.3% (w/v) | d | d | d | (63) |
| 2,4-D | derivatives of phenoxy-2-acetoxyethyl-decyldimethylammonium | liquids | d | d | d | d | d | (21) |
| 2,4-D | 1,1-dialkylpyrrolidinium | liquids | 126–208 °C (T5%) | >10% (w/v) | 28.4–33.2 | d | d | (25,46) |
| 2,4-D | 4,4-dialkylmorpholinium | liquids or waxes | d | >10% (w/v) or limited or soluble | d | d | d | (46,73) |
| 2,4-D | 1,1-dialkylpiperidinium | liquids or waxes | d | soluble | d | d | d | (46) |
| 2,4-D | 1,3-dialkylimidazolium | liquids or waxes | d | soluble | d | d | d | (46) |
| 2,4-D | 1-alkylpyridinium | wax | d | soluble | d | d | d | (46) |
| 2,4-D | bis(ammonium) | waxes | 210–219 °C (T5%) | 3.3–10% (w/v) | d | d | d | (67) |
| 2,4-D | hexamethylene-1,6-bis(3-methylimidazolium) | wax (Tm = 82–84 °C) | 1.9% (V) | <30–70% (pH = 5); freely (pH = 7); 70–100% (pH = 9) | 36.8 | d | 4.21 | (41) |
| MCPP | tebuconazole | liquid | 200 °C (T5%) | insoluble | d | d | d | (40) |
| MCPP | propiconazole | liquid | 245 °C (T5%) | insoluble | d | d | d | (40) |
| MCPP | tetraalkylammonium | liquids or waxes (alkyltrimethyl-ammonium); liquids (dialkyldimethyl-ammonium) | 186–194 °C (T5%) (alkyltrimethyl-ammonium); d (dialkyldimethyl-ammonium) | >10% (w/v) or 3.3–10% (w/v) or <3.3% (w/v) (alkyltrimethyl-ammonium); >10% (w/v) (dialkyldimethyl-ammonium) | 31.0–38.7 (alkyltrimethyl-ammonium); d (dialkyldimethyl-ammonium) | d | d | (16,47,51) |
| MCPP | tetraalkylphosphonium | liquid | 307 °C (T5%) | d | d | d | d | (16) |
| MCPP | betainium | liquid | 218 °C (T5%) | <3.3% (w/v) | d | d | d | (61) |
| MCPP | N-alkylbetainium | liquids | 185–219 °C (T5%) | 3.3–10% (w/v) or <3.3% (w/v) | 31.6–31.7 | d | d | (45) |
| MCPP | carnitinium | liquid | 181 °C (T5%) | <3.3% (w/v) | d | d | d | (61) |
| MCPP | acetylcholine | liquid | 180 °C (T5%) | <3.3% (w/v) | 33.2 | d | –0.81 | (62) |
| MCPP | [2-(methacryloyloxy)-ethyl]trimethylammonium, [2-(acryloyloxy)-ethyl]trimethylammonium | liquids | 190–200 °C (T5%) | >10% (w/v) | d | d | d | (63) |
| MCPP | dialkanoyloxyethyl-dimethylammonium | wax | 200 °C (T5%) | <3.3% (w/v) | d | d | d | (63) |
| MCPP | derivatives of phenoxy-2-acetoxyethyldecyl-dimethylammonium | liquids | d | d | d | d | d | (21) |
| MCPP | 1,1-dialkylpyrrolidinium | liquids | 125–200 °C (T5%) | >10% (w/v) | 28.0–34.4 | d | d | (25) |
| MCPP | 1,1-dialkylpiperidinium | waxes | 192–198 °C (T5%) | 3.3–10% (w/v) | d | d | d | (66) |
| MCPP | 1,3-dialkylimidazolium | liquid | 252 °C (T5%) | soluble | d | d | d | (16) |
| MCPP | bis(ammonium) | waxes | 203–205 °C (T5%) | 3.3–10% (w/v) | d | d | d | (67) |
| dicamba | [2-(2-hydroxyethoxy)ethyl] ammonium | liquid | 0.5% (V); 183 °C (T5%) | >10% (w/v) | lack of CMC | d | d | (39) |
| dicamba | bis(3-aminopropyl)ammonium | liquid | 1.0% (V); 184 °C (T5%) | >10% (w/v) | lack of CMC | d | d | (39) |
| dicamba | tebuconazole | liquid | 218 °C (T5%) | insoluble | d | d | d | (40) |
| dicamba | propiconazole | liquid | 193 °C (T5%) | insoluble | d | d | d | (40) |
| dicamba | 8-hydroxyquinolinium | glass | 190 °C (T5%) | d | d | d | d | (26) |
| dicamba | (3-aminopropyl)bis(ammonium) | solid (Tm = 76–78 °C) | 199 °C (T5%) | >10% (w/v) | lack of CMC | d | d | (39) |
| dicamba | tetraalkylammonium | solids (Tm = 72–76 °C) or waxes (alkyltrimethyl-ammonium); solids (Tm = 86 °C) or liquids or waxes (dialkyldimethyl-ammonium) | 185–187 °C (T5%) (alkyltrimethyl-ammonium); 178 °C (T5%) (dialkyldimethyl-ammonium) | <3.3% (w/v) (alkyltrimethyl-ammonium); >10% (w/v) (dialkyldimethyl-ammonium) | 36.3–37.1 (alkyltrimethyl-ammonium); d (dialkyldimethyl-ammonium) | d | d | (26,47,51) |
| dicamba | alkyl-2-hydroxyethyl-dimethylammonium | liquids or waxes | d | d | d | d | d | (26) |
| dicamba | alkyl[2-(2-hydroxyethoxy)-ethyl]dimethylammonium | liquid or waxes | 0.4% (V); 146–185 °C (T5%) | >10% (w/v) | 30.8–38.6 | d | –2.04 to 0.98 | (39,58) |
| dicamba | alkylbis(n-ethoxylated) methylammonium | liquids or waxes | 189–200 °C (T5%) | <3.3% (w/v) | 36.5 | d | d | (26,39) |
| dicamba | N-alkylbetainium | solid (Tm = 28–30 °C) or liquid | 184–200 °C (T5%) | <3.3% (w/v) | 30.4–32.7 | d | d | (45) |
| dicamba | acetylcholine | liquid | 178 °C (T5%) | 3.3–10% (w/v) | 33.8 | d | 0.67 | (62) |
| dicamba | [2-(methacryloyloxy)-ethyl]trimethylammonium, [2-(acryloyloxy)-ethyl]trimethylammonium | liquids | 187–192 °C (T5%) | >10% (w/v) | d | d | d | (63) |
| dicamba | dialkanoyloxyethyl-dimethylammonium | wax | d | d | d | d | d | (26) |
| dicamba | derivatives of phenoxy-2-acetoxyethyldecyl-dimethylammonium | liquids | d | d | d | d | d | (21) |
| dicamba | 1,1-dialkylpyrrolidinium | liquid | 185 °C (T5%) | d | d | d | d | (26) |
| dicamba | 4,4-dialkylmorpholinium | liquids or waxes | 185 °C (T5%) | d | d | d | d | (26,73) |
| dicamba | 1,1-dialkylpiperidinium | liquids or waxes | 188 °C (T5%) | d | d | d | d | (26) |
| dicamba | 1-alkyl-4-hydroxy-1-methylpiperidinium | solids (Tm = 91–98 °C) or liquids | 0.2–0.3% (V); 186–192 °C (T5%) | >10% (w/v) or 3.3–10% (w/v) | 31.6–38.4 | d | d | (39) |
| dicamba | 1,3-dialkylimidazolium | liquids | d | d | d | d | d | (26) |
| dicamba | 1-alkylpyridinium | liquid | 187 °C (T5%) | d | d | d | d | (26) |
| dicamba | bis(ammonium) | waxes | 184–195 °C (T5%) | >10% (w/v) or 3.3–10% (w/v) | d | d | d | (67) |
| dicamba | bis(ammonium) derivatives of tebuconazole | noncrystalline solids | 201–219 °C (T5%) | d | d | d | d | (68) |
| 2,4-DP | tetraalkylammonium | liquids (alkyltrimethyl-ammonium); liquids (dialkyldimethyl-ammonium); wax (tetrabutyl-ammonium) | 179–189 °C (T5%) (alkyltrimethyl-ammonium); 165–190 °C (T5%) (dialkyldimethyl-ammonium); 172 °C (T5%) (tetrabutyl-ammonium) | <3.3% (w/v) (alkyltrimethyl-ammonium); <3.3% (w/v) (dialkyldimethyl-ammonium); >10% (w/v) (tetrabutyl-ammonium) | 28.0–28.7 (alkyltrimethyl-ammonium); 27.1–28.9 (dialkyldimethyl-ammonium); 29.2 (tetrabutyl-ammonium) | d | 0.73–0.98 (alkyltrimethyl-ammonium); 0.63–0.82 (dialkyldimethyl-ammonium) 0.35 (tetrabutyl-ammonium) | (57) |
| 2,4-DP | alkylbis(n-ethoxylated) methylammonium | liquid | 221 °C (T5%) | <3.3% (w/v) | 29.1 | d | 0.85 | (57) |
| 2,4-DP | acetylcholine | liquid | 184 °C (T5%) | <3.3% (w/v) | 33.4 | d | 0.98 | (62) |
| 2,4-DP | 1,1-dialkylpiperidinium | solids (Tm = 74–87 °C) or liquids or waxes | 191–206 °C (T5%) | <3.3% (w/v) | d | d | –0.57 to 2.15 | (65) |
| 4-CPA | derivatives of phenoxy-2-acetoxyethyldecyl-dimethylammonium | liquids | d | d | d | d | d | (21) |
| 4-CPA | 1,1-dialkylpyrrolidinium | liquids or waxes | 123–209 °C (T5%) | >10% (w/v) | 27.5–35.6 | d | d | (25) |
| clopyralid | alkylammonium | liquids | d | 0.70–49.6% (w/v) (pH = 5); 0.41–46.3% (w/v) (pH = 7); 0.72 to −45.6% (w/v) (pH = 9) | 29.7–58.3 | 21.6–35.6 | d | (27) |
| clopyralid | 2-benzothiazolammonium | wax | d | 0.90% (w/v) (pH = 5); 0.79% (w/v) (pH = 7); 10.3% (w/v) (pH = 9) | 54.6 | 34.8 | d | (27) |
| clopyralid | imidazolium | liquid | d | 4.00% (w/v) (pH = 5); 44.0% (w/v) (pH = 7); 4.54% (w/v) (pH = 9) | 61.0 | 32.2 | d | (27) |
| clopyralid | tetraalkylammonium | liquid | d | 6.0% (w/v) (pH = 5); 11.3% (w/v) (pH = 7); (10.3% (w/v) (pH = 9) | 65.5 | 35.7 | d | (27) |
| clopyralid | derivatives of phenoxy-2-acetoxyethyldecyl-dimethylammonium | liquids | d | d | d | d | d | (21) |
| pelargonate | tetraalkylammonium | waxes (alkyltrimethyl-ammonium); waxes (dialkyldimethyl-ammonium); liquid (tetrabutyl-ammonium) | 171–193 °C (T5%) (alkyltrimethyl-ammonium); 150–180 °C (T5%) (dialkyldimethyl-ammonium); 153 °C (T5%) (tetrabutyl-ammonium) | >10% (w/v) or <3.3% (w/v) (alkyltrimethyl-ammonium); >10% (w/v) or 3.3–10% (w/v) or <3.3% (w/v) (dialkyldimethyl-ammonium); >10% (w/v) (tetrabutyl-ammonium) | d | d | d | (33) |
| pelargonate | choline | wax | 186 °C (T5%) | >10% (w/v) | d | d | d | (33) |
| pelargonate | alkylbis(n-ethoxylated) methylammonium | wax | 196 °C (T5%) | <3.3% (w/v) | d | d | d | (33) |
| pelargonate | N-alkylbetainium | wax | 146 °C (T5%) | <3.3% (w/v) | d | d | d | (33) |
| pelargonate | acetylcholine | liquid | 188 °C (T5%) | >10% (w/v) | 26.8 | d | 1.11 | (62) |
| pelargonate | dialkanoyloxyethyl-dimethylammonium | wax | 156 °C (T5%) | <3.3% (w/v) | d | d | d | (33) |
| pelargonate | quaternized DABCO | liquids | d | >10% (w/v) or 3.3–10% (w/v) or <3.3% (w/v) | 26.1–29.7 | d | d | (34) |
| bentazone | tetraalkylammonium | liquid (alkyltrimethyl-ammonium); wax (dialkyldimethyl-ammonium) | d (alkyltrimethyl-ammonium); d (dialkyldimethyl-ammonium) | 0.22% (w/v) (pH = 5); 0.23% (w/v) (pH = 7); 0.22% (w/v) (pH = 9) (alkyltrimethyl-ammonium); 3.52% (w/v) (pH = 5); 3.55% (w/v) (pH = 7); 3.48% (w/v) (pH = 9) (dialkyldimethyl-ammonium) | 31.3 (alkyltrimethyl-ammonium); 34.8 (dialkyldimethyl-ammonium) | 12.8 (alkyltrimethyl-ammonium); 10.3 (dialkyldimethyl-ammonium) | d | (24) |
| bentazone | 1,3-dialkylimidazolium | liquid | d | 8.59% (w/v) (pH = 5); 8.64% (w/v (pH = 7); 9.00% (w/v) (pH = 9) | 34.3 | 8.7 | d | (24) |
| bentazone | 1-alkylpyridinium | liquid | d | 0.09% (w/v) (pH = 5); 0.09% (w/v) (pH = 7); 0.08% (w/v) (pH = 9) | 30.8 | 10.9 | d | (24) |
| bromoxynil | alkylammonium | wax (Tm = 97–99 °C) | 0.3% (V) | 0.01% (w/v) (pH = 5); 0.02% (w/v) (pH = 7); 0.01% (w/v) (pH = 9) | 36.4 | d | 1.03 | (23) |
| bromoxynil | 4-methylmorpholinum | liquid | 5.4% (V) | 3.63% (w/v) (pH = 5); 3.52% (w/v) (pH = 7); 4.02% (w/v) (pH = 9) | 67.9 | d | 0.59 | (23) |
| bromoxynil | 1-methyl-3H-imidazolium | wax (Tm = 63–65 °C) | 4.0% (V) | 0.87% (w/v) (pH = 5); 1.34% (w/v) (pH = 7); 2.76% (w/v) (pH = 9) | 65.4 | d | 0.45 | (23) |
| bromoxynil | 1-alkylpyridinium | wax (Tm = 89–91 °C) | 0.2% (V) | 0.06% (w/v) (pH = 5); 0.05% (w/v) (pH = 7); 0.05% (w/v) (pH = 9) | 32.7 | d | 0.97 | (23) |
| clodinafop | choline | d (product not isolated from postreaction mixture) | d | d | d | d | d | (8) |
| diclofop | choline | d (product not isolated from postreaction mixture) | d | d | d | d | d | (8) |
| fomesafen | tetraalkylammonium | solid (Tm = 86–87 °C) (alkyltrimethyl-ammonium); liquid (dialkyldimethyl-ammonium) | d (alkyltrimethyl-ammonium); d (dialkyldimethyl-ammonium) | 0.001% (w/v) (pH = 5); 0.002% (w/v) (pH = 7); 0.002% (w/v) (pH = 9) (alkyltrimethyl-ammonium); 0.34% (w/v) (pH = 5); 0.46% (w/v) (pH = 7); 1.13% (w/v) (pH = 9) (dialkyldimethyl-ammonium) | 29.3 (alkyltrimethyl-ammonium); 37.8 (dialkyldimethyl-ammonium) | 15.3 (alkyltrimethyl-ammonium); 13.8 (dialkyldimethyl-ammonium) | 2.88 (alkyltrimethyl-ammonium); 0.64 (dialkyldimethyl-ammonium) | (32) |
| fomesafen | 1,3-dialkylimidazolium | liquid | d | 0.85% (w/v) (pH = 5); 0.97% (w/v) (pH = 7); 1.20% (w/v) (pH = 9) | 34.2 | 13.6 | 0.75 | (32) |
| fomesafen | 1-alkylpyridinium | wax | d | 0.008% (w/v) (pH = 5); 0.008% (w/v) (pH = 7); 0.008% (w/v) (pH = 9) | 31.2 | 14.1 | 2.49 | (32) |
| glyphosate | tetraalkylammonium | solids (Tm = 45 °C) or liquids or waxes | 148–196 °C (T5%) | >10% (w/v) or 3.3–10% (w/v) or <3.3% (w/v) | d | d | d | (31) |
| glyphosate | trialkylsulfonium | liquids | d | d | d | d | d | (84) |
| glyphosate | alkylbis(n-ethoxylated) methylammonium | waxes | 155–208 °C (T5%) | >10% (w/v) or 3.3–10% (w/v) | d | d | d | (31) |
| glyphosate | 4,4-dialkylmorpholinium | wax | 140 °C (T5%) | >10% (w/v) | d | d | d | (31) |
| iodosulfuron-methyl | acetylcholine | solid (Tm = 74–76 °C) | 177 °C (T5%) | >10% (w/v) | 34.1 | d | 0.87 | (62) |
| MCPB | 1,1-dialkylpiperidinium | liquids or waxes (Tm = 40–64 °C) | 154–215 °C (T5%) | >10% (w/v) or 3.3–10% (w/v) or <3.3% (w/v) | 33.5–42.9 | d | d | (64) |
| mesotrione | alkylammonium | solids (Tm = 68–97 °C) or waxes | 140–179 °C (T5%) | 0.13–0.87% (w/v) (pH = 5); 0.51–1.70% (w/v) (pH = 7); 0.59–1.60% (w/v) (pH = 9) | 43.7–57.9 | 4.5–8.0 | 0.92–0.97 | (38) |
| mesotrione | 1-methylpiperidinium | solid (Tm = 91 °C) | 165 °C (T5%) | 1.31% (w/v) (pH = 5); 0.69% (w/v) (pH = 7); 0.88% (w/v) (pH = 9) | 43.2 | 10.0 | 1.39 | (38) |
| mesotrione | 1-methyl-3H-imidazolium | solid (Tm = 96 °C) | 153 °C (T5%) | 1.24% (w/v) (pH = 5); 2.21% (w/v) (pH = 7); 1.83% (w/v) (pH = 9) | 44.6 | 7.7 | 1.42 | (38) |
| mesotrione | tetraalkylammonium | liquids or waxes (Tm = 81–90 °C) | 180–219 °C (T5%) | 0.18–2.42% (w/v) (pH = 5); 0.68–4.20% (w/v) (pH = 7); 0.93–4.01% (w/v) (pH = 9) | 22.8–55.4 | 7.4–21.6 | 1.17–2.37 | (38) |
| mesotrione | 1-alkylpyridinium | solid (Tm = 85 °C) | 202 °C (T5%) | 0.17% (w/v) (pH = 5); 0.45% (w/v) (pH = 7); 0.49% (w/v) (pH = 9) | 27.5 | 22.6 | 2.43 | (38) |
| metsulfuron-methyl | tetraalkylphosphonium | liquids | 194–195 °C (T5%) | >10% (w/v) or <3.3% (w/v) | d | d | d | (29) |
| metsulfuron-methyl | choline | liquid | 150 °C (T5%) | >10% (w/v) | d | d | d | (29) |
| metsulfuron-methyl | alkylbis(n-ethoxylated) methylammonium | liquid | 180 °C (T5%) | >10% (w/v) | d | d | d | (29) |
| metsulfuron-methyl | 1,1-dialkylpyrrolidinium | liquid | 167 °C (T5%) | >10% (w/v) | d | d | d | (29) |
| metsulfuron-methyl | 4,4-dialkylmorpholinium | solid (Tm = 62–64 °C) | 196 °C (T5%) | >10% (w/v) | d | d | d | (29) |
| metsulfuron-methyl | 1,1-dialkylpiperidinium | liquid | 176 °C (T5%) | >10% (w/v) | d | d | d | (29) |
| metsulfuron-methyl | 1,3-dialkylimidazolium | liquids | 182–190 °C (T5%) | >10% (w/v) | d | d | d | (29) |
| metsulfuron-methyl | 1-alkylpyridinium | liquid | 195 °C (T5%) | 3.3–10% (w/v) | d | d | d | (29) |
| nicosulfuron | tetraalkylammonium | liquids or waxes | d | 0.003–0.059% (w/v) (pH = 5); 0.012–0.064% (w/v) (pH = 7); 0.023–0.083% (w/v) (pH = 9) | 14.4–43.9 | d | 0.59–1.13 | (30) |
| nicosulfuron | choline | liquid | d | 0.062% (w/v) (pH = 5); 0.046% (w/v) (pH = 7); 0.051% (w/v) (pH = 9) | 41.2 | d | 0.78 | (30) |
| nicosulfuron | 1-alkylpyridinium | liquid | d | 0.004% (w/v) (pH = 5); 0.003% (w/v) (pH = 7); 0.015% (w/v) (pH = 9) | 19.7 | d | 1.06 | (30) |
| MCPA/dicamba (oligomeric) | alkylbis(n-ethoxylated) methylammonium | liquids or waxes | d | d | d | d | d | (43) |
| MCPA/dicamba (oligomeric) | betainium | wax | 202 °C (T5%) | <3.3% (w/v) | d | d | d | (61) |
| MCPA/glyphosate (oligomeric) | tetraalkylammonium | wax | 200 °C (T5%) | >10% (w/v) | d | d | d | (42) |
| MCPA/glyphosate (oligomeric) | alkylbis(n-ethoxylated) methylammonium | wax | 218 °C (T5%) | >10% (w/v) | d | d | d | (42) |
| MCPA/dicamba (DSHIL) | alkyl[2-(2-hydroxyethoxy)-ethyl]dimethylammonium | liquids | 183–191 °C (T5%) | >10% (w/v) | 29.6–37.7 | d | –1.57 to 1.13 | (58) |
| MCPA/glyphosate (DSHIL) | tetraalkylammonium | solid (Tm = 15, 40 °C) or wax (alkyltrimethyl-ammonium); liquids (dialkyldimethyl-ammonium) | 150–155 °C (T5%) (alkyltrimethyl-ammonium); 159–160 °C (T5%) (dialkyldimethyl-ammonium) | >10% (w/v) or <3.3% (w/v) (alkyltrimethyl-ammonium); >10% (w/v) or 3.3–10% (w/v) (dialkyldimethyl-ammonium) | d | d | d | (42) |
| MCPA/glyphosate (DSHIL) | alkylbis(n-ethoxylated) methylammonium | wax | 215 °C (T5%) | >10% (w/v) | d | d | d | (42) |
| dicamba/glyphosate (DSHIL) | tetraalkylammonium | solid (Tm = 85 °C) | 188 °C (T5%) | >10% (w/v) | d | d | d | (42) |
| dicamba/glyphosate (DSHIL) | tetraalkylphosphonium | liquid | 177 °C (T5%) | <3.3% (w/v) | d | d | d | (42) |
| dicamba/glyphosate (DSHIL) | alkylbis(n-ethoxylated) methylammonium | wax | 213 °C (T5%) | >10% (w/v) | d | d | d | (42) |
Herbicides have been ordered in decreasing number of manuscripts. Table does not include salts with melting points greater than 100 °C. Tm, melting point.
Singular form, only one compound; plural form, more than one compound.
T5%, decomposition temperature of 5% sample; V, volatility rate at 75 °C after 12 h.
Not tested.
Double salt herbicidal ionic liquids (DSHILs)80 can also be obtained via metathesis or reaction with KOH in methanol. In this case, proper molar ratio of the herbicide mixture should be selected (Figure 5). DSHILs with MCPA and dicamba anions58,61 were obtained via metathesis, with the use of KOH in methanol in the case of glyphosate and dicamba anions,42 as well as glyphosate and MCPA.42
Figure 5.
Synthesis of DSHILs
3.3. Multistep Synthesis
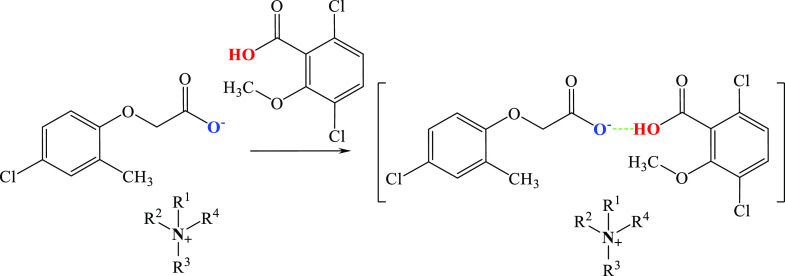
Synthesis of HILs with oligomeric anions (Figure 6) requires an additional third step. In this case, the previously obtained HIL is subjected to a reaction with a second herbicide in an acidic form, which forms a hydrogen bond with the existing HIL. However, it should be considered that the reaction presented in Figure 6 may result in a mixture of products existing in a dynamic equilibrium, consisting of three compounds: two “symmetrical” ones and one with mixed ligands. Further experiments are required to clarify whether the term “oligomeric” is appropriate for such combinations.
Figure 6.
Synthesis of HILs with oligomeric anions.
Syntheses were performed in chloroform43 or methanol.42,61 After mixing the substrates, the solvent was completely evaporated, and the product was rinsed with isopropanol when necessary42 and finally dried under vacuum. With the use of this method, ILs with MCPA anion and dicamba as acid,43,61 as well as glyphosate anion and MCPA as acid42 were synthesized.
Another multistep synthesis of HILs required a series of reactions, which result in the formation of HILs with two active herbicidal centers. An example of this method is the synthesis of herbicidal esterquats with anions of herbicidal mode of action,21 in which the cation contained an ester substituent originating from phenoxyacid (2,4-D, MCPA, MCPP, 4-CPA), while the counterion was a different herbicide from the phenoxyacid group or from a different group (e.g., dicamba, a derivative of benzoic acid, or clopyralid, a derivative of picolinic acid). The synthesis was a 5-step process and required the synthesis of phenoxyacid chloride. Then, aminoester hydrochlorides were obtained as a result of reaction of acid chlorides with 2-dimethylaminoethanol and “freed” from the hydrochloride in subsequent reaction with trimethylamine. The resulting aminoester was subjected to the quaternization reaction with decyl bromide, and finally the bromide anion was substituted with herbicidal anion via metathesis reaction.
Since the first report regarding HILs in 2011,16 a rapid, progressive evolution in the design and optimization of their chemical structure as well as the development of their synthesis routes can be observed. Recently, the scientific community focused their efforts not only on utilization of naturally occurring cations but also on the elaboration of efficient, environmentally friendly methods of HIL preparation. Therefore, initially the most common path of IL synthesis was based on ion exchange in water, followed by product isolation via two-phase extraction using toxic chloroform or dichloromethane, which was finally replaced by acid–base reaction in alcoholic medium. The progress in conducting synthesis via metathesis was based on the replacement of water for low chain alcohol as a reaction medium. This solution allowed substantially shortening of the overall time of synthesis as well as minimization of the difficulties that often appear in two-phase extraction from an aqueous environment, such as the issues with the separation of phases as well as foam formation during evaporation of the solvent.
4. Characterization of HILs
4.1. Physicochemical Tests
The general characterization of HILs consists of spectral analysis (e.g., 1H NMR, 13C NMR, UV, IR) in order to confirm their structure. This is followed by thermal analysis (DSC, differential scanning calorimetry) for determination of thermal transitions and melting points and thermogravimetric analysis (TGA) for establishing the decomposition temperatures and confirmation of thermal stability, which, in turn, leads to the volatility tests, ideally demonstrating the low volatility of HILs due to the presence of an ionic bond.26
It should be emphasized that the issue of volatility of various herbicidal formulations has been studied extensively since the 1970s.81 The so-called “vapor drift”, caused by volatilization of the utilized herbicide after application, may lead to the presence of such chemicals in neighboring areas, severely damaging nontargeted plants as well as trees. Interestingly, reports from 2017 present the drift of a new formulation of dicamba in USA (in the states of Mississippi, Tennessee, and Missouri), advertised as “nonvolatile”, which caused substantial losses in neighboring cultivated plants, particularly in soybeans, as well as tomatoes.82 This case clearly demonstrates that minimizing the potential for off-site movement of many plant protection products is still a significant challenge for modern agriculture. The literature survey provides data demonstrating the volatility of HILs comprising dicamba,26 2,4-D,41 and bromoxynil.23 The volatility of samples was determined by thermogravimetric analysis, wherein the percentage of mass loss of samples was assessed after heating them for 12 h at 75 °C under a nitrogen atmosphere. These studies revealed that transformation of herbicides into HILs can reduce the volatilization rate by up to 20 times compared to the respective herbicide in nonionic form. Therefore, in the case of bromoxynil and 2,4-D, the mass loss of herbicide exceeding 10% was lowered to less than 1%, which underlines the beneficial influence of the utilized quaternary ammonium cations. Additionally, the tested HILs proved to be less volatile than salts comprising tertiary ammonium (protic) cations that are usually applied in commercial formulations.23 This observation may be explained by the fact that in case of the quaternary cations, the risk of deprotonation and subsequent volatilization of both amine and acid does not exist.34 Furthermore, the molecular mass of HILs should also be considered as a factor influencing their volatility. To date, it has been established that the increase in molecular mass of the active ingredient (by combination of 2,4-dichlorophenoxyacetic acid with amines substituted with different length of alkyls) decreased its volatility by reducing its saturated vapor pressure.41
Another important factor is determination of water and halide residues, which notably affect the physicochemical properties of HILs, as they are strictly connected to the synthesis efficiency. HILs are also subjected to additional experiments, for example, testing of their viscosity, density, and refractive index to identify their purity.55 The purity of ILs is generally determined using elemental analysis or the two-phase titration method, coupled with spectral methods, such as NMR, IR, and UV. Nevertheless, these techniques are not appropriate for a precise quantitative determination of the purity of HILs. Therefore, it is recommended to employ chromatographic techniques (e.g., HPLC) in future studies, which proved to be effective in the case of other ILs.83
The crucial variable concerning HILs is their surface tension (surface activity), measured, for example, with the use of a drop shape analyzer.16,55 The results give valuable information concerning HIL wetting properties, since high surface activity indicates enhanced wetting of plants and therefore the possibility of complete reduction of adjuvant use. Solubility tests enable proper solvent selection for analytical purposes as well as optimization of the most effective synthesis method. Additionally, since ILs are known for their tunability, which allows regulation of many factors, the octanol–water partition coefficient is also determined to assess the hydrophobicity of HILs. This factor gives valuable information concerning the environmental risk of these formulations, since its high value illustrates the hazard of bioaccumulation, while low values correspond with the possibility of substance permeation into watercourses.32 The mobility of HILs was tested for the first time in 2015 by Wang et al.24 with the use of soil thin layer chromatography (soil TLC) and was carried out later by other research teams.27,28 Additionally, absorption of HILs by soil was analyzed with high performance liquid chromatography (HPLC), as it is strictly associated with the bioaccumulation of these compounds and essential for further biological tests.24,27,28 The obtained results indicated that the highly lipophilic HILs, comprising long alkyl or cyclic substituents, interacted with organic matter in soils and sediments, thus being rather immobile. On the other hand, the weakly lipophilic HILs were relatively mobile in the soil, which creates the possibility of their permeation into groundwater. HILs with longer chains were found to be easily adsorbed by soil and their adsorption percentage was positively correlated with the length of the carbon substituent. Thus, their high adsorption capacity in soil may be associated with lower risk to algae, snail, and other aquatic organisms compared to commercially available forms of herbicides. However, researchers should also consider the risk of bioaccumulation of lipophilic compounds in soil. This particularly important issue has not been thoroughly considered thus far. Therefore, experiments on HIL soil adsorption should be coupled with biodegradability tests demonstrating whether such compound is fully degraded or mineralized in soil. Otherwise, despite lower risk of contamination of watercourses, the possible soil pollution may influence the community structure of autochthonic bacteria present in soil or even pose a threat to other living organisms, including humans.
Many years of extensive research on HILs revealed their most desirable physicochemical characteristics in terms of their successful commercialization:
-
(a)
HILs should remain in a liquid state at room temperature; thus difficulties associated with the solid state of the majority of produced pesticides (including polymorphic conversion, tendency of the amorphous forms to crystallize spontaneously, low solubility or bioavailability) lead to a decrease or even loss of desired activity.
-
(b)
HIL viscosity should be relatively low, as it would facilitate their synthesis. Additionally, the adsorption of active ingredient as a liquid would be enhanced compared to conventional forms, which may lead to the formation of solid deposits on the surface of leaves.
-
(c)
HILs should be characterized by extremely low volatility, which minimizes the risk of atmospheric pollution as well as poisoning by vapors of chemical substances. However, standardized protocols for analysis of HIL vaporization should be proposed. Additionally, HILs should also be examined in terms of their physical drift from spray solutions.
-
(d)
HILs should exhibit moderate solubility in water. Too high solubility may increase the risk of permeation to groundwaters, while too low affinity toward water may provide difficulties with development of effective formulation and may hinder HIL biodegradation.
-
(e)
Their high surface activity results in enhanced efficiency and allows the required dose of active ingredient per hectare to be significantly reduced, which is directly associated with lower environmental impact of the utilized chemical.
-
(f)
Relatively high adsorption in soil reduces the mobility in soil and enables rapid mineralization, which improves the protection of groundwaters.
-
(g)
The hydrophobicity of HILs, characterized by the value of the logarithm of the octanol–water partition coefficient (log KOW), should vary between 0 and 3. Negative values of the log KOW may result in increased risk of pollution of the hydrosphere; some pesticides can be easily leached from the soil into watercourses. On the other hand, higher KOW values (log KOW > 3) increase the possibility of HIL bioaccumulation in soils, which may pose a threat to the environment or lead to enhanced absorption of harmful pesticides in the interior of cultivated plants.
-
(h)
Multifunctional properties of HILs should result in a reduction of the number of treatments, lowering the costs of protection against pests.
On the basis of the above-mentioned recommendations, the most important physicochemical parameters of HILs known in the literature are listed in Table 3.
4.2. Biological Tests
In most studies, the biological experiments comprise only a few issues. In almost every work concerning HIL synthesis, field experiments are conducted in order to evaluate the herbicidal activity of obtained compounds against weeds, most commonly during 2-year trials with visually evaluated weed control after applying the herbicide. During field studies, crop safety is also assessed visually for injury symptoms. Additionally, biological activity of HILs is evaluated via greenhouse experiments, which can be carried out in both conservatories and growth chambers under controlled conditions. These tests, compiled in Table 4, seem to be the most interesting for HIL designers as they provide valuable information concerning the efficacy of synthesized compounds; however, these alone are insufficient in terms of environmental safety of ILs. Moreover, as it can be clearly seen, plants that were under evaluation, as well as doses of herbicides, vary greatly and do not allow for a direct comparison of the obtained results.
Table 4. Overview of Greenhouse and Field Studies Conducted on HILsa.
| greenhouse studies |
field studies |
|||||
|---|---|---|---|---|---|---|
| anion | cation | amount of AI applied (g/ha) | fresh weight reductionb | amount of AI applied (g/ha) | herbicidal activity | ref |
| MCPA | tebuconazole | 170 | common lambsquarters, 17%; white mustard, 45% | 170 | cornflower, 0–38%; shepherd’s purse, 46–51% | (40) |
| MCPA | propiconazole | 170 | common lambsquarters, 31%; white mustard, 41% | c | (40) | |
| MCPA | tetraalkylammonium | c | 200–500 | field pennycress, 75–100%; larkspur, 54–93%; mayweed, 40–78%; rapeseed, 86–100% | (16) | |
| 400 | common lambsquarters, 51–82%; flixweed, 69–80% | 400 | common lambsquarters, ∼95–99%; field pennycress, ∼82–99% | |||
| MCPA | alkylcyclohexyl- dimethylammonium | 400 | common lambsquarters, ∼21–47%; cornflower, ∼3%–76%; rapeseed, ∼1%−38%; white mustard, ∼19–58% | c | (55) | |
| MCPA | 2-chloroethyl-trimethylammonium | c | field pansy, field pennycress and rapeseed, results similar to the mixture of MCPA-salt and CCC | (16) | ||
| 300 | common lambsquarters, 10–75% | 900 (MCPA-salt), 1450 (CCC) | cornflower, 83–88%; rapeseed, 85–86%; scentless chamomile, 49–67% | (56) | ||
| MCPA | alkyl[2-(2-hydroxyethoxy)-ethyl]dimethylammonium | 400 | common lambsquarters, 41–60%; cornflower, 40–86%; rapeseed, 14–63% | c | (58) | |
| MCPA | quaternary ammonium derivatives of d-glucose | 400 | cornflower, 39–69%; white mustard, 54–87% | c | (59) | |
| MCPA | alkylbis(n-ethoxylated) methylammonium | 400 | common lambsquarters, ∼39–50%; white mustard, ∼15–37% | c | (60) | |
| MCPA | betainium | 400 | common lambsquarters, 21%; white mustard, 38% | 400 | common lambsquarters, 98%; rapeseed, 100% | (61) |
| MCPA | N-alkylbetainium | 400 | common lambsquarters, ∼90–95%; cornflower, ∼98–99%; rapeseed, ∼97–99% | 400 | common lambsquarters, ∼98–100%; rapeseed, 100% | (45) |
| MCPA | carnitinium | 400 | common lambsquarters, 34%; white mustard, 38% | 400 | common lambsquarters, 98%; rapeseed, 100% | (61) |
| MCPA | acetylcholine | 400 | rapeseed, 37.55% | c | (62) | |
| MCPA | [2-(methacryloyloxy)-ethyl]trimethylammonium; [2-(acryloyloxy)-ethyl]trimethylammonium | 400 | white mustard’s efficacy compared to commercial formulation set as 100%; ∼101–108% | c | (63) | |
| MCPA | dialkanoyloxyethyl-dimethylammonium | 400 | white mustard efficacy compared to commercial formulation set as 100%, ∼129% | 400 | common lambsquarters efficacy compared to commercial formulation set as 100%, ∼158% | (63) |
| MCPA | derivatives of phenoxy-2-acetoxyethyl-decyldimethylammonium | 400 | germination index of cornflower, 6.99–15.48%; shoot length, 16.33–27.00 cm; root length, 7.00–11.63 cm | c | (21) | |
| MCPA | 1,1-dialkylpyrrolidinium | 400 | common lambsquarters, 53–67% | c | (25) | |
| MCPA | quaternized DABCO | 400 | cornflower, ∼84–94%; rapeseed, 72–89% | c | (37) | |
| MCPA | bis(ammonium) | 400 | common lambsquarters, 32–60%; white mustard, 33–55% | c | (67) | |
| MCPA | bis(ammonium) derivatives of tebuconazole | 400 | common lambsquarters, 60–87%; rapeseed, 45–55% | c | (68) | |
| 2,4-D | tebuconazole | 170 | common lambsquarters, 10%; white mustard, 19% | 170 | cornflower, 0–38%; shepherd’s purse, 50–58% | (40) |
| 2,4-D | propiconazole | 170 | common lambsquarters, 16%; white mustard, 34% | c | (40) | |
| 2,4-D | 1,4-dimethylpiperazinium | 400 | common amaranth, 73.03%; flixweed, 47.34% | 400 | common lambsquarters, ∼89.30% | (41) |
| 2,4-D | tetraalkylammonium | 400 | cornflower, 80%, field poppy, 44%, flixweed, 51%, rapeseed, 12% | 400 | common lambsquarters, ∼98%; rapeseed, ∼95% | (44) |
| 2,4-D | tetraalkylammonium | 400 | common lambsquarters, 33–42%; flixweed, 42–72% | 400 | common lambsquarters, ∼85–95%; field pennycress, ∼80–98% | (47) |
| 2,4-D | tetraalkylammonium | c | c | (48) | ||
| 2,4-D | tetraalkylammonium | c | 450 | common lambsquarters, 85–100%; field pennycress, 81–100%; shepherd’s purse, 81–100% | (46) | |
| 2,4-D | tetraalkylammonium | c | 450 | cornflower, 75–79%; field pennycress, 74–78%; field poppy, 73–79%; rapeseed, 80–82%; scentless chamomile, 56–57%; shepherd’s purse, 79–82% | (52) | |
| 2,4-D | 2-chloroethyl-trimethylammonium | 400 | Cornflower, 1.047%, field poppy, 4.630%, flixweed, 3.465%, rapeseed, 5.628% | 400 | common lambsquarters, ∼38%; rapeseed, ∼35% | (44) |
| 2,4-D | 2-chloroethyl-trimethylammonium | 220–440 (2,4-D), 158–316 (CCC) | white mustard, 50–75% | 450 | common lambsquarters, 72–95%; cornflower, 94% | (48) |
| 2,4-D | quaternary ammonium derivatives of d-glucose | 400 | cornflower, 23–66%; white mustard, 39–71% | c | (59) | |
| 2,4-D | alkylbis(n-ethoxylated) methylammonium | 400 | common lambsquarters, ∼40–59%; white mustard, ∼21–28% | c | (60) | |
| 2,4-D | alkylbis(n-ethoxylated) methylammonium | 400 | cornflower, 75%, field poppy, 37%, flixweed, 47%, rapeseed, 14% | 400 | common lambsquarters, ∼85–97%; rapeseed, ∼92–95% | (44) |
| 2,4-D | alkylbis(n-ethoxylated) methylammonium | c | 450 | common lambsquarters, 100%; cornflower,100% | (46) | |
| 2,4-D | alkylbis(n-ethoxylated) methylammonium | c | 450 | cornflower, 71%; field pennycress, 77%; field poppy, 75%; scentless chamomile, 52%; shepherd’s purse, 79%; rapeseed, 82% | (52) | |
| 2,4-D | betainium | 400 | common lambsquarters, 27%; white mustard, 32% | 400 | common lambsquarters, 100%; rapeseed, 100% | (61) |
| 2,4-D | N-alkylbetainium | 400 | common lambsquarters, ∼83–90%; cornflower, ∼99–100%; rapeseed, ∼84–97% | 400 | common lambsquarters, 100%; rapeseed, 100% | (45) |
| 2,4-D | carnitinium | 400 | common lambsquarters, 7%; white mustard, 32% | 400 | common lambsquarters, 98%; rapeseed, 100% | (61) |
| 2,4-D | acetylcholine | 400 | rapeseed, 21.12% | c | (62) | |
| 2,4-D | [2-(methacryloyloxy)-ethyl]trimethylammonium; [2-(acryloyloxy)-ethyl]trimethylammonium | 400 | white mustard efficacy compared to commercial formulation set as 100%, ∼72–87% | c | (63) | |
| 2,4-D | dialkanoyloxyethyl-dimethylammonium | 400 | white mustard efficacy compared to commercial formulation set as 100%, ∼108% | 400 | common lambsquarters efficacy compared to commercial formulation set as 100%, ∼154% | (63) |
| 2,4-D | derivatives of phenoxy-2-acetoxyethyl-decyldimethylammonium | 400 | germination index of cornflower, 4.82–7.49% shoot length, 21.00–22.86 cm; root length, 4.14–6.33 cm | c | (21) | |
| 2,4-D | 1,1-dialkylpyrrolidinium | 400 | common lambsquarters, 54–59% | c | (25) | |
| 2,4-D | bis(ammonium) | 400 | common lambsquarters, 27–47%; white mustard, 47–52% | c | (67) | |
| 2,4-D | hexamethylene-1,6-bis(3-methylimidazolium) | 400 | flixweed, 77.15%; common amaranth, 93.57% | 400 | common lambsquarters, 96.78% | (41) |
| MCPP | tebuconazole | 170 | common lambsquarters, 21%; white mustard, 19% | c | (40) | |
| MCPP | propiconazole | 170 | common lambsquarters, 24%; white mustard, 13% | c | (40) | |
| MCPP | tetraalkylammonium | 400 | common lambsquarters, 38–68%; flixweed, 74–86% | 400 | common lambsquarters, ∼87–95%; field pennycress, ∼90–99% | (47) |
| MCPP | betainium | 400 | common lambsquarters, 22%; white mustard, 0% | c | (61) | |
| MCPP | N-alkylbetainium | 400 | common lambsquarters, ∼50–55%; cornflower, ∼90–97%; rapeseed, ∼97–98% | c | (45) | |
| MCPP | carnitinium | 400 | white mustard, 9%; common lambsquarters, 19% | c | (61) | |
| MCPP | acetylcholine | 400 | rapeseed, 56.23% | c | (62) | |
| MCPP | [2-(methacryloyloxy)-ethyl]trimethylammonium, [2-(acryloyloxy)-ethyl]trimethylammonium | 400 | white mustard efficacy compared to commercial formulation set as 100%, ∼88–93% | c | (63) | |
| MCPP | dialkanoyloxyethyl-dimethylammonium | 400 | common lambsquarters efficacy compared to commercial formulation set as 100%, ∼120% | 400 | white mustard efficacy compared to commercial formulation set as 100%, ∼104% | (63) |
| MCPP | derivatives of phenoxy-2-acetoxyethyldecyl-dimethylammonium | 400 | germination index of cornflower, 0–23.45%; shoot length, 0.50–43.22 cm; root length, 0.50–15.67 cm | c | (21) | |
| MCPP | 1,1-dialkylpyrrolidinium | 400 | common lambsquarters, 47–63% | c | (25) | |
| MCPP | 1,1-dialkylpiperidinium | 400 | common lambsquarters, 63–85%; cornflower, 40–88%; white mustard, 50–98%; | c | (66) | |
| MCPP | bis(ammonium) | 400 | common lambsquarters, 37–51%; white mustard, 8–22% | c | (67) | |
| dicamba | [2-(2-hydroxyethoxy)ethyl]ammonium | 200 | common amaranth, ∼67%; common lambsquarters, ∼32%; cornflower, ∼83% | c | (39) | |
| dicamba | bis(3-aminopropyl)ammonium | 200 | common amaranth, ∼73%; common lambsquarters, ∼35%; cornflower, ∼44% | c | (39) | |
| dicamba | tebuconazole | 200 | common lambsquarters, 16%; white mustard, 23% | c | (40) | |
| dicamba | propiconazole | 200 | common lambsquarters, 22%; white mustard, 26% | c | (40) | |
| dicamba | (3-aminopropyl)bis(ammonium) | 200 | common amaranth, ∼82%; common lambsquarters, ∼41%; cornflower, ∼39% | c | (39) | |
| dicamba | tetraalkylammonium | 0.002–0.004 mol/L | common lambsquarters, 41.7–45.7%; white mustard, 18.5–27.1% | 200 | common lambsquarters, 62.5–92%; cornflower, 95% | (26) |
| dicamba | tetraalkylammonium | 200 | common lambsquarters, 75–85%; flixweed, 43–75% | 200 | common lambsquarters, ∼97–99%; field pennycress, ∼75–99% | (47) |
| dicamba | alkyl[2-(2-hydroxyethoxy)-ethyl]dimethylammonium | 200 | common lambsquarters, 23.75–68.91%; cornflower, 65.40–84.88%; oilseed rape, 0.00–20.81% | c | (58) | |
| dicamba | alkyl[2-(2-hydroxyethoxy)-ethyl]dimethylammonium | 200 | common amaranth, ∼79%; common lambsquarters, ∼37%; cornflower, ∼27% | c | (39) | |
| dicamba | alkylbis(n-ethoxylated)-methylammonium | c | 200 | common lambsquarters, 56.3–62.5% | (26) | |
| dicamba | alkylbis(n-ethoxylated)-methylammonium | 200 | common amaranth, ∼63%; common lambsquarters, ∼68%; cornflower, ∼52% | c | (39) | |
| dicamba | N-alkylbetainium | 200 | common lambsquarters, ∼90–92%; cornflower, ∼97–99%; rapeseed, ∼50–78% | 200 | common lambsquarters, ∼98%; rapeseed, ∼95% | (45) |
| dicamba | acetylcholine | 200 | rapeseed, −4.46% | c | (62) | |
| dicamba | [2-(methacryloyloxy)-ethyl]trimethylammonium, [2-(acryloyloxy)-ethyl]trimethylammonium | 200 | white mustard efficacy compared to commercial formulation set as 100%, ∼101–112% | 200 | common lambsquarters efficacy compared to commercial formulation set as 100%, ∼80–120% | (63) |
| dicamba | derivatives of phenoxy-2-acetoxyethyldecyl-dimethylammonium | 200 | germination index of cornflower, 5.99–22.29%; shoot length, 12.33–21.75 cm; root length, 9.00–14.89 cm | c | (21) | |
| dicamba | 4,4-dialkylmorpholinium | c | 200 | common lambsquarters, 57.5% | (26) | |
| dicamba | 4,4-dialkylmorpholinium | c | c | (73) | ||
| dicamba | 1,1-dialkylpiperidinium | c | 200 | common lambsquarters, 62.5% | (26) | |
| dicamba | 1-alkyl-4-hydroxy-1-methylpiperidinium | 200 | common amaranth, ∼70–90%; common lambsquarters, ∼54–59%; cornflower, ∼71–82% | c | (39) | |
| dicamba | bis(ammonium) | 200 | common lambsquarters, 32–95%; cornflower, 54%; rapeseed, 54%; white mustard, 9–33% | c | (67) | |
| dicamba | bis(ammonium) derivatives of tebuconazole | 200 | common lambsquarters, 58–85%; rapeseed, 10–38% | c | (68) | |
| 2,4-DP | tetraalkylammonium | 300 | common lambsquarters, ∼20–40%; cornflower, ∼25–77% | c | (57) | |
| 2,4-DP | alkylbis(n-ethoxylated)-methylammonium | 300 | common lambsquarters, ∼17%; cornflower, ∼58% | c | (57) | |
| 2,4-DP | acetylcholine | 400 | rapeseed, 36.28% | c | (62) | |
| 2,4-DP | 1,1-dialkylpiperidinium | 400 | common lambsquarters, 20–90%; cornflower, 20–60%; rapeseed, 20–91% | c | (65) | |
| 4-CPA | derivatives of phenoxy-2-acetoxyethyldecyl-dimethylammonium | 400 | germination index of cornflower, 5.32–19.46%; shoot length, 17.83–45.00 cm; root length, 5.33–14.63 cm | c | (21) | |
| 4-CPA | 1,1-dialkylpyrrolidinium | 400 | common lambsquarters, 53–65% | c | (25) | |
| clopyralid | alkylammonium | c | 50, 100, and 200 | creeping thistle, brachyotus sowthistle, Cephalanoplos setosum, 25.2–77.3% | (27) | |
| clopyralid | derivatives of phenoxy-2-acetoxyethyldecyl-dimethylammonium | germination index of cornflower, 3.16–32.77%; shoot length, 7.50–17.00 cm; root length, 2.71–24.63 cm | c | (21) | ||
| pelargonate | tetraalkylammonium | 2720 | common lambsquarters, ∼18–50%; cornflower, 53%; rapeseed, ∼4–45%; white mustard, 33%; winter wheat, 29% | c | (33) | |
| pelargonate | choline | 2720 | common lambsquarters, approx −28%; rapeseed, approx −4% | c | (33) | |
| pelargonate | alkylbis(n-ethoxylated) methylammonium | 2720 | cornflower, 37%; rapeseed, ∼18%; white mustard, 16%; winter wheat, 5% | c | (33) | |
| pelargonate | N-alkylbetainium | 2720 | common lambsquarters, ∼20% | c | (33) | |
| pelargonate | acetylcholine | 3000 | rapeseed, 24.58% | c | (62) | |
| pelargonate | dialkanoyloxyethyl-dimethylammonium | 2720 | rapeseed, ∼10% | c | (33) | |
| pelargonate | quaternized DABCO | 5440–8160 | common lambsquarters, 36.60–93.48%; rapeseed, 20.70–95.93% | c | (34) | |
| bentazone | 1-alkylpyridinium | c | 1000–3000 | brachyotus sowthistle, common lambsquarters, creeping woodsorrel, ∼30–91% | (24) | |
| bromoxynil | alkylammonium | 90–360 | common amaranth, ∼17–82% | c | (23) | |
| bromoxynil | 1-alkylpyridinium | 90–360 | common amaranth, ∼20–84% | c | (23) | |
| clodinafop | choline | c | complete inhibition of weed grass after 15 days | (8) | ||
| fomesafen | tetraalkylammonium | c | Lack of data | 45–180 | creeping woodsorrel, annual fleabane herb, dandelion, ∼49–88% | (32) |
| glyphosate | tetraalkylammonium | 22.5–720 | cornflower, ∼8–88%; winter wheat, ∼10–84% | 1180 | couchgrass, 94–100% | (31) |
| glyphosate | tetraalkylammonium | 180–360 | common lambsquarters, 83–94%; cornflower, 19–90%; white mustard, 64–89% | 1180 | couchgrass regrowth from rhizome buds, 7–16% | (31) |
| glyphosate | alkylbis(n-ethoxylated) methylammonium | 22.5–720 | cornflower, ∼10–84%; winter wheat, ∼2–64% | 1180 | couchgrass, 99–100% | (31) |
| glyphosate | alkylbis(n-ethoxylated) methylammonium | 180–360 | common lambsquarters, 81–94%; cornflower, 17–88%; white mustard, 75–91% | (31) | ||
| glyphosate | 4,4-dialkylmorpholinium | 22.5–720 | cornflower, ∼10–92%; winter wheat, ∼2–56% | 1180 | couchgrass, 20–99% | (31) |
| glyphosate | 4,4-dialkylmorpholinium | 180–360 | common lambsquarters, 54–68%; cornflower, 21–63%; white mustard, 11–81% | 1180 | couchgrass regrowth from rhizome buds, 26% | (31) |
| iodosulfuron-methyl | acetylcholine | 7.5 | rapeseed, 37.42% | c | (62) | |
| MCPB | 1,1-dialkylpiperidinium | 600 | common lambsquarters, 71–88%; rapeseed, 70–95% | c | (64) | |
| mesotrione | 1-alkylpyridinium | c | 50–200 | common lambsquarters, common amaranth, field bindweed, field sowthistle, ∼20–98% | (38) | |
| metsulfuron-methyl | tetraalkylphosphonium | 4 | common lambsquarters, 93–94%; rapeseed, 44–67% | 8 | chickweed, 100%; field forget-me-not, 100%; field pansy, 93–100%; field poppy, 74%; hemp nettle, 100%; mayweed, 95–100%; persian speedwell, 63%; shepherd’s purse, 96%; wild buckwheat, 97% | (29) |
| metsulfuron-methyl | 4,4-dialkylmorpholinium | 4 | common lambsquarters, 71%; rapeseed, 54% | 8 | chickweed, 100%; field forget-me-not, 96–100%; field pansy, 89–100%; field poppy, 68%; hemp nettle, 100%; mayweed, 89–99%; persian speedwell, 78%; shepherd’s purse, 96%; wild buckwheat, 97% | (29) |
| metsulfuron-methyl | 1,1-dialkylpiperidinium | 4 | common lambsquarters, 21%; rapeseed, 34% | 8 | chickweed, 99–100%; field forget-me-not, 87–100%; field pansy, 90–100%; field poppy, 85%; hemp nettle, 100%; mayweed, 92–100%; persian speedwell, 71%; shepherd’s purse, 94%; wild buckwheat, 95% | (29) |
| metsulfuron-methyl | 1,3-dialkylimidazolium | 4 | common lambsquarters, 67–80%; rapeseed, 26–60% | 8 | chickweed, 100%; field forget-me-not, 92–100%; field pansy, 85–100%; field poppy, 66–76%; hemp nettle, 100%; mayweed, 88–100%; persian speedwel, 66–76%; shepherd’s purse, 95–96%; wild buckwheat, 97% | (29) |
| nicosulfuron | tetraalkylammonium | 0.5–20 mg/L | common amaranth, 37.68–65.11% | c | (30) | |
| nicosulfuron | choline | 0.5–20 mg/L | common amaranth, 46.73–61.73% | c | (30) | |
| nicosulfuron | 1-alkylpyridinium | 0.5–20 mg/L | common amaranth, 59.4–81.43% | 50–200 | common lambsquarters, ∼22–90%; green bristlegrass, ∼23–92% | (30) |
| MCPA/dicamba (oligomeric) | alkylbis(n-ethoxylated) methylammonium | c | 267 (MCPA), 133 (dicamba) | black bindweed, ∼59–92%; common lambsquarters, ∼98–100%; rapeseed, ∼95–98% | (43) | |
| MCPA/dicamba (oligomeric) | alkylbis(n-ethoxylated) methylammonium | c | 300 (MCPA), 100 (dicamba) | black bindweed, ∼62%; common lambsquarters, ∼98–99%; rapeseed, ∼65–100% | (43) | |
| MCPA/glyphosate (oligomeric) | tetraalkylammonium | 360 (glyphosate), 73.9 (MCPA) | common amaranth, 95%; common lambsquarters, 66%; white mustard, 94%; winter wheat, 91% | 360 (glyphosate), 73.9 (MCPA) | cornflower, ∼98–100%; poppy field, ∼86–89%; winter wheat, ∼100% | (42) |
| MCPA/glyphosate (oligomeric) | alkylbis(n-ethoxylated) methylammonium | 360 (glyphosate), 73.9 (MCPA) | common amaranth, 96%; common lambsquarters, 68%; white mustard, 88%; winter wheat, 89% | 360 (glyphosate), 73.9 (MCPA) | cornflower, ∼96–99%; poppy field, ∼90–93%; winter wheat, ∼96–100% | (42) |
| MCPA/dicamba (DSHIL) | alkyl[2-(2-hydroxyethoxy)-ethyl]dimethylammonium | 300 (MCPA), 40 (dicamba) | common lambsquarters, 36.91–53.67%; cornflower, 45.47–85.30%; rapeseed, 6.54–51.63% | c | (58) | |
| MCPA/glyphosate (DSHIL) | tetraalkylammonium | 360 (glyphosate), 73.9 (MCPA) | common amaranth, 91–96%; common lambsquarters, 45–70%; white mustard, 74–89%; winter wheat, 86–89% | c | (42) | |
| MCPA/glyphosate (DSHIL) | alkylbis(n-ethoxylated)-methylammonium | 360 (glyphosate), 73.9 (MCPA) | common amaranth, 94%; common lambsquarters, 48%; white mustard, 88%; winter wheat, 89% | 360 (glyphosate), 73.9 (MCPA) | cornflower, ∼97–99%; poppy field, ∼72–88%; winter wheat, ∼88–97% | (42) |
| dicamba/glyphosate (DSHIL) | tetraalkylammonium | 360 (glyphosate), 83.7 (dicamba) | common amaranth, 96%; common lambsquarters, 68%; white mustard, 90%; winter wheat, 90% | 360 (glyphosate), 73.9 (dicamba) | cornflower, ∼92–96%; poppy field, ∼64–76%; winter wheat, ∼78–92% | (42) |
| dicamba/glyphosate (DSHIL) | tetraalkylphosphonium | 360 (glyphosate), 83.7 (dicamba) | common amaranth, 96%; common lambsquarters, 78%; white mustard, 84%; winter wheat, 93% | 360 (glyphosate), 73.9 (dicamba) | cornflower, ∼98–99%; poppy field, ∼95–99%; winter wheat, ∼90–98% | (42) |
| dicamba/glyphosate (DSHIL) | alkylbis(n-ethoxylated)-methylammonium | 360 (glyphosate), 83.7 (dicamba) | common amaranth, 92%; common lambsquarters, 59%; white mustard, 92%; winter wheat, 89% | 360 (glyphosate), 73.9 (dicamba) | cornflower, ∼87–94%; poppy field, ∼58–85%; winter wheat, ∼89–96% | (42) |
Table does not include salts with melting points greater than 100 °C.
Unless stated otherwise.
Not tested.
On the other hand, it should be noted that some of the biological tests were not environmentally relevant and mainly focused on model cases, which is especially troubling when considering the toxicity and biodegradability of HILs, since these studies were performed rarely (Tables 5 and 6). Moreover, research concerning the impact of these formulations on various organisms was not performed in accordance with any regulatory framework or standard procedures but rather results from individual scientific interests. It is an especially unsettling issue, since this new class of ILs is targeted to be commercialized in the future and hence should be carefully examined.
Table 5. Overview of Biodegradation Studies on HILsa.
| anion | cation | biodegradation | ref |
|---|---|---|---|
| MCPA | tebuconazole | OECD 301 F test: cation, 88 ± 4%; anion, 0 ± 0% | (40) |
| MCPA | propiconazole | OECD 301 F test: cation, 58 ± 3%; anion, 3 ± 0% | (40) |
| MCPA | tetraalkylammonium | BOD5/COD ratio (t = 0–360 min, 0.4 = limit of biodegradability): electrochemical oxidation process, 0.25–0.35; electro-Fenton process, 0.35–0.65 | (49) |
| MCPA | tetraalkylammonium | electrochemically treated, 28–57%; nontreated, 0–8% | (50) |
| MCPA | betainium | OECD 301 F test, 69% | (61) |
| MCPA | N-alkylbetainium | OECD 301 F test, 59–62% | (45) |
| MCPA | carnitinium | OECD 301 F test, 59% | (61) |
| MCPA | acetylcholine | OECD 301 F test, 80% | (62) |
| MCPA | [2-(methacryloyloxy)-ethyl]trimethylammonium, [2-(acryloyloxy)-ethyl]trimethylammonium | OECD 301 F test, 29–37% | (63) |
| MCPA | dialkanoyloxyethyl-dimethylammonium | OECD 301 F test, 63% | (63) |
| 2,4-D | tebuconazole | OECD 301 F test: cation, 94 ± 3%; anion, 0 ± 0% | (40) |
| 2,4-D | propiconazole | OECD 301 F test: cation, 65 ± 3%; anion, 0 ± 1% | (40) |
| 2,4-D | betainium | OECD 301 F test, 87% | (61) |
| 2,4-D | N-alkylbetainium | OECD 301 F test, 72–73% | (45) |
| 2,4-D | carnitinium | OECD 301 F test, 76% | (61) |
| 2,4-D | acetylcholine | OECD 301 F test, 70% | (62) |
| 2,4-D | 4,4-dialkylmorpholinium | OECD 301 F test with microbiota isolated from different environmental niches: river sludge, 9–10%; garden soil, 13–19%; agricultural runoff stream, 18–25%; agricultural soil, 14–24%; waste repository, 20–31% | (73) |
| 2,4-D | 4,4-dialkylmorpholinium | Primary biodegradation for microbiota isolated from different environmental niches: river sludge, cation 52–58%, anion 9–11%; garden soil, cation 74–77%, anion 25–31%; agricultural runoff stream, cation 87–90%, anion 60–61%; agricultural soil, cation 88–92%, anion 60%; waste repository, cation 91–94%, anion 51–55% | (73) |
| MCPP | tebuconazole | OECD 301 F test: cation, 89 ± 4%; anion, 0 ± 1% | (40) |
| MCPP | propiconazole | OECD 301 F test: cation, 68 ± 3%; anion, 0.5 ± 0% | (40) |
| MCPP | betainium | OECD 301 F test, 57% | (61) |
| MCPP | N-alkylbetainium | OECD 301 F test, 51–55% | (45) |
| MCPP | carnitinium | OECD 301 F test, 49% | (61) |
| dicamba | tebuconazole | OECD 301 F test: cation, 100 ± 5%; anion, 44 ± 2% | (40) |
| dicamba | propiconazole | OECD 301 F test: cation, 56 ± 2%; anion, 40 ± 2% | (40) |
| dicamba | N-alkylbetainium | OECD 301 F test, 42–47% | (45) |
| dicamba | acetylcholine | OECD 301 F test, 90% | (62) |
| dicamba | 4,4-dialkylmorpholinium | OECD 301 F test with microbiota isolated from different environmental niches: river sludge, 0%; garden soil, 0–1%; agricultural runoff stream, 1–2%; agricultural soil, 2%; waste repository, 2% | (73) |
| dicamba | 4,4-dialkylmorpholinium | Primary biodegradation for microbiota isolated from different environmental niches: river sludge, cation 38–55%, anion 0%; garden soil, cation 56–77%, anion 0%; agricultural runoff stream, cation 77–81%, anion 32%; agricultural soil, cation 75–79%, anion 29–35%; waste repository, cation 83–86%, anion 34–36% | |
| pelargonate | tetraalkylammonium | OECD 301 F test, 0–83% | (33) |
| pelargonate | alkylbis(n-ethoxylated) methylammonium | OECD 301 F test, 68% | (33) |
| pelargonate | N-alkylbetainium | OECD 301 F test, 85% | (33) |
| pelargonate | dialkanoyloxyethyl-dimethylammonium | OECD 301 F test, 52% | (33) |
| MCPA/dicamba (oligomeric) | alkylbis(n-ethoxylated) methylammonium | OECD 301 F test, 0–6.6% | (43) |
| MCPA/dicamba (oligomeric) | betainium | OECD 301 F test, 65–66% | (61) |
| MCPA/glyphosate (oligomeric) | tetraalkylammonium | OECD 301 F test, 0% | (42) |
| MCPA/glyphosate (oligomeric) | alkylbis(n-ethoxylated) methylammonium | OECD 301 F test, 0% | (42) |
| MCPA/glyphosate (DSHIL) | tetraalkylammonium | OECD 301 F test, 0% | (42) |
| MCPA/glyphosate (DSHIL) | alkylbis(n-ethoxylated) methylammonium | OECD 301 F test, 0% | (42) |
| dicamba/glyphosate (DSHIL) | tetraalkylammonium | OECD 301 F test, 0% | (42) |
| dicamba/glyphosate (DSHIL) | tetraalkylphosphonium | OECD 301 F test, 0% | (42) |
| dicamba/glyphosate (DSHIL) | alkylbis(n-ethoxylated) methylammonium | OECD 301 F test, 0% | (42) |
Table does not include salts with melting points greater than 100 °C.
Table 6. Overview of Toxicity Studies on HILsa.
| anion | cation | toxicity and impact on desired plants | ref |
|---|---|---|---|
| MCPA | tebuconazole | at 10 ppm, fungistatic activity against F. culmorum and M. nivale; no damage toward winter wheat (AI 170 g/ha) | (40) |
| MCPA | propiconazole | at 10 ppm, strong inhibition of F. culmorum, M. nivale, B. cinerea, and S. sclerotiorum mycelia growth; no damage toward winter wheat (AI 170 g/ha) | (40) |
| MCPA | tetraalkylammonium | acute oral LD50 for rats, 300 to >2000 mg/kg; AI 200–500 g/ha, winter wheat 7.88–8.28 t/ha (untreated control, 7.40 t/ha) | (16) |
| MCPA | 2-chloroethyl-trimethylammonium | acute oral LD50 for rats, 300–2000 mg/kg; shortening of wheat stems, increased resistance to lodging | (16) |
| MCPA | 2-chloroethyl-trimethylammonium | growth inhibition of winter wheat, 2–6% | (56) |
| MCPA | betainium | acute oral LD50 for rats, 300–2000 mg/kg | (61) |
| MCPA | N-alkylbetainium | acute oral LD50 for rats, 300–2000 mg/kg | (45) |
| MCPA | carnitinium | acute oral LD50 for rats, 300–2000 mg/kg | (61) |
| MCPA | dialkanoyloxyethyl-dimethylammonium | acute oral toxicity LD50 for rats, >2000 mg/kg; acute toxicity 96 h LD50 for rainbow trout, 7–17 mg/L; acute toxicity 72 h ErC50 for green algae, 1.6–1.9 mg/L; immobilization 48 h EC50 of water flea, 0.2–0.5 mg/L | (63) |
| MCPA | quaternized DABCO | S. aureus MIC (2–138 μM), MBC (2–138 μM); S. epidermidis MIC (<1–138 μM), MBC (<1–138 μM); B. subtilis MIC (4–138 μM), MBC (4–138 μM); E. faecalis MIC (7–1104 μM), MBC (7–1104 μM); M. luteus MIC (<1–18 μM), MBC (<1–35 μM); P. aeruginosa MIC (246–2207 μM), MBC (246 to >2207 μM); S. marcescens MIC (442 to >2207 μM), MBC (885 to >2207 μM); P. vulgaris MIC (28 to >2207 μM), MBC (28 to >2207 μM) M. catarrhalis MIC (<1–138 μM), MBC (<1–138 μM); E. coli MIC (30–2207 μM), MBC (30 to >2207 μM); Rh. rubra MIC (4–1104 μM), MFC (4–2207 μM); C. albicans MIC (4–2207 μM), MFC (4–2207 μM) | (37) |
| MCPA | bis(ammonium) derivatives of tebuconazole | fungicidal activity at 10 ppm, S. sclerotiorum 28.3–38.4%, B. cinerea 39.5–81.5%, F. culmorum 60.5–81.5%, F. oxysporum 50.0–63.8%, Colletotrichum sp. 48.2–62.0%, M. nivale, 11.2–52.9%; fungicidal activity at 100 ppm, S. sclerotiorum 98.2–100.0%, B. cinerea 100.0%, F. culmorum 90.6–100.0%, F. oxysporum 97.8–100.0%, Colletotrichum sp. 95.7%, M. nivale 52.5–68.5%; fungicidal activity at 1000 ppm, S. sclerotiorum 99.3–100.0%, B. cinerea 100.0%, F. culmorum 98.6–100.0%, F. oxysporum 100.0%, Colletotrichum sp. 100.0%, M. nivale 91.3–93.8% | (68) |
| 2,4-D | tebuconazole | at 10 ppm, fungistatic activity against F. culmorum and M. nivale; no damage toward winter wheat (170 g of AI/ha) | (40) |
| 2,4-D | propiconazole | at 10 ppm, strong inhibition of F. culmorum, M. nivale, B. cinerea and S. sclerotiorum mycelia growth; no damage toward winter wheat (AI 170 g/ha) | (40) |
| 2,4-D | tetraalkylammonium | AI 450 g/ha, winter wheat, 4.98–10.50 t/ha (untreated control, 4.84–9.32 t/ha); AI 1200 g/ha, winter wheat, 3.45–7.63 t/ha (untreated control, 3.67–6.83 t/ha); crop injury, 16–38% | (52) |
| 2,4-D | alkylbis(n-ethoxylated)-methylammonium | AI 450 g/ha: winter wheat, 5.78–10.26 t/ha (untreated control, 4.84–9.32 t/ha); AI 1200 g/ha: winter wheat, 3.41–7.86 t/ha (untreated control, 3.67–6.83 t/ha); crop injury, 13–21% | (52) |
| 2,4-D | betainium | acute oral LD50 for rats, 300–2000 mg/kg | (61) |
| 2,4-D | 4,4-dialkylmorpholinium | EC50 of microbiota isolated from different environmental niches: river sludge, 104–148 mg/L, garden soil, 113–184 mg/L, agricultural runoff stream, 195–227 mg/L, agricultural soil, 211–260 mg/L, waste repository, 222–277 mg/L | (73) |
| MCPP | tebuconazole | at 10 ppm, fungistatic activity against F. culmorum and M. nivale; no damage toward winter wheat (AI 170 g/ha) | (40) |
| MCPP | propiconazole | at 10 ppm, inhibition of F. culmorum, M. nivale, B. cinerea, and S. sclerotiorum mycelia growth; no damage toward winter wheat (AI 170 g/ha) | (40) |
| MCPP | tetraalkylammonium | EC50 for P. putida, 0.3–8.0 mM; cis–trans 50%,b 0.1–11.0 mM | (51) |
| MCPP | derivatives of phenoxy-2-acetoxyethyldecyl-dimethylammonium | enzymatic activity (GST, SOD, APX, CAT) and quantitative analysis of nonenzymatic factors (GSH and chlorophyll a and b), mutagenic activity of S. typhimurium | (21) |
| dicamba | tebuconazole | at 10 ppm, fungistatic activity against F. culmorum and M. nivale; no damage toward winter wheat (AI 170 g/ha) | (40) |
| dicamba | propiconazole | at 10 ppm, strong inhibition of F. culmorum, M. nivale, B. cinerea, and S. sclerotiorum mycelia growth; no damage toward winter wheat (AI 170 g/ha) | (40) |
| dicamba | tetraalkylammonium | EC50 for P. putida, 0.1–9.8 mM; cis–trans 50%,b 0.1–15.2 mM | (51) |
| dicamba | 4,4-dialkylmorpholinium | EC50 of microbiota isolated from different environmental niches: river sludge, >500 mg/L; garden soil, >500 mg/L; agricultural runoff stream, >500 mg/L; agricultural soil, >500 mg/L; waste repository, >500 mg/L | (73) |
| dicamba | bis(ammonium) derivatives of tebuconazole | fungicidal activity at 10 ppm, S. sclerotiorum 90.9–98.6%, B. cinerea 31.5–50.4%, F. culmorum 58.7–72.5%, F. oxysporum 59.1–67.4%, Colletotrichum sp. 54.7–63.0%, M. nivale 0.0–46.7%, | (68) |
| fungicidal activity at 100 ppm, S. sclerotiorum 97.8–100.0%, B. cinerea 100.0%, F. culmorum 93.8–100.0%, F. oxysporum 97.8–100.0%, Colletotrichum sp. 95.7–97.8%, M. nivale 63.8–92.0% | |||
| fungicidal activity at 1000 ppm, S. sclerotiorum 98.6–100.0%, B. cinerea 100.0%, F. culmorum 100.0%, F. oxysporum 100.0%, Colletotrichum sp. 100.0%, M. nivale 92.0–95.7% | |||
| clopyralid | derivatives of phenoxy-2-acetoxyethyldecyl-dimethylammonium | enzymatic activity (GST, SOD, APX, CAT) and quantitative analysis of nonenzymatic factors (GSH and chlorophyll a and b), mutagenic activity of S. typhimurium | (21) |
| pelargonate | tetraalkylammonium | S. aureus MIC <0.02–2.0 mM, MBC <0.04–7.8 mM; E. coli MIC 0.1–5.6 mM, MBC <0.04 to >5.6 mM; P. aeruginosa MIC 0.6–5.0 mM, MBC 1.2–7.8 mM; C. albicans MIC <0.04–3.2 mM, MFC <0.04 to >3.2 mM; acute oral toxicity LD50 for rats, 300 to >2000 mg/kg; acute toxicity toward rainbow trout (96 h) LC0 7.27 mg/L, LC50 10.58 mg/L, LC100 16 mg/L; growth inhibition of green algae (72 h) EyC10 0.02 mg/L, EyC20, 0.03 mg/L, EyC50, 0.06 mg/L; immobilization of water flea (48 h) EC0 0.125 mg/L, EC50 0.284 mg/L, EC100 0.5 mg/L | (33) |
| pelargonate | alkylbis(n-ethoxylated) methylammonium | S. aureus MIC <0.03 mM, MBC <0.03 mM; E. coli MIC 1.1 mM, MBC 4.3 mM; P. aeruginosa MIC >4.3 mM, MBC >4.3 mM; C. albicans MIC <0.03 mM, MFC <0.03 mM; acute oral toxicity LD50 for rats, 300–2000 mg/kg | (33) |
| pelargonate | N-alkylbetainium | S. aureus MIC 0.3 mM, MBC 2.6 mM; E. coli MIC 1.3 mM, MBC >5.2 mM; P. aeruginosa MIC 5.2 mM, MBC >5.2 mM; C. albicans MIC 0.6 mM, MFC 0.6 mM | (33) |
| pelargonate | dialkanoyloxyethyl-dimethylammonium | S. aureus MIC 2.8 mM, MBC >2.8 mM; E. coli MIC >2.8 mM, MBC >2.8 mM; P. aeruginosa MIC >2.8 mM, MBC >2.8 mM; C. albicans MIC >2.8 mM; MFC >2.8 mM | (33) |
| pelargonate | quaternized DABCO | feeding-deterrent activity (Tdc): adults granary weevil Td = 110.2–190.0; adults confused flour beetle Td = −19.3–98.4; larvae khapra beetle Td = 44.5–175.2; larvae confused flour beetle Td = −11.0–110.5 | (34) |
| MCPA/dicamba (oligomeric) | alkylbis(n-ethoxylated) methylammonium | next generation sequencing, no significant changes in soil microbial diversity | (43) |
Table does not include salts with melting points greater than 100 °C. AI, active ingredient; LD50, concentration resulting in 50% lethality; ErC50, concentration resulting in 50% reduction in growth; EC50, half maximal effective concentration; MIC, minimum inhibitory concentration; MBC, minimum bactericidal concentration; MFC, minimum fungicidal concentration; EyCx, concentration resulting in x% reduction in yield. S. aureus, Staphylococcus aureus; S. epidermidis, Staphylococcus epidermidis; B. subtilis, Bacillus subtilis; E. faecalis, Enterococcus faecalis; M. luteus, Micrococcus luteus; P. aeruginosa, Pseudomonas aeruginosa; S. marcescens, Serratia marcescens; P. vulgaris, Proteus vulgaris; M. catarrhalis, Moraxella catarrhalis; E. coli, Escherichia coli; Rh. rubra, Rhodotorula rubra; C. albicans, Candida albicans; S. sclerotiorum, Sclerotinia sclerotiorum, B. cinerea, Botrytis cinerea; F. culmorum, Fusarium culmorum; M. nivale, Microdochium nivale; F. oxysporum, Fusarium oxysporum; P. putida, Pseudomonas putida; S. typhimurium, Salmonella typhimurium.
cis/trans 50%, concentration that caused an increase in the trans/cis ratio of unsaturated fatty acids to 50% of the maximum trans/cis level reached at saturating concentrations of the toxicant.
Td, the total coefficient of deterrence (151–200, very good deterrents; 101–150, good deterrents; 51–100, medium deterrents; <50, weak deterrents; negative values, attractants).
4.2.1. Biodegradation Study
In order to further study the fate of HILs in the environment, biodegradation studies are conducted (Table 5), but unfortunately, they are not employed routinely with the use of well-established standard procedures and rather occur as inconsistent and often incomparable tests. Primary biodegradation efficiencies of cation and anion were evaluated for the first time in 201431 by HPLC-MS procedure; further followed by Ławniczak et al.73 and Pęziak-Kowalska et al.49 Subsequently, standard OECD 301 F tests (manometric respirometry) with the use of activated sludge were applied for the assessment of ultimate biodegradability.33,42,45,50,61,62 However, in reality, the majority of herbicides are not going to have contact with activated sludge but rather with microorganisms in soils. This is because activated sludge is typically used in wastewater treatment plants, while potential biodegradation of herbicides in the environment will use significantly different microbiota. This, in turn, may present quite different biodegradation results than the model ones, and hence the scientific significance of OECD tests is rather limited. These tests only indicate general trends and do not provide data regarding the mineralization nature, that is, whether the cation or anion mineralized or both of them.79
As a solution to these drawbacks, some works have already used more environmentally relevant biodegradation tests. For instance, Ławniczak et al.73 evaluated ultimate biodegradation of selected HILs based on a modified OECD 301 F test, using microbiota isolated from particular environmental niches. Furthermore, Czarny et al.85 assessed the biodegradation of HILs after anaerobic digestion, where the batch reactor was inoculated with microbiota originating from an active agricultural biogas plant. On the other hand, Pęziak-Kowalska et al.49 attempted to apply electrochemical removal of herbicide via both direct electrochemical oxidation and electro-Fenton processes. Aside from classical HPLC biodegradation efficiency evaluation, the COD (chemical oxygen demand), BOD5 (biochemical oxygen demand), and TOC (total organic carbon) values were also determined. The study was continued in 2019, when Pęziak-Kowalska et al.50 evaluated the biodegradation of HILs via both a standard respirometric study and BOD5/COD ratio. These reports clearly indicate the incidental nature of research. Standard procedures are still lacking, and hence it is not possible to compare HIL biodegradation under the same conditions. Moreover, the knowledge regarding cation–anion interactions is extremely limited and incomplete, and the impact of these interactions on biodegradation is unknown. This, in turn, might be one of the most important issues, which can be a starting point for future extensive research and understanding of IL behavior in the environment.
4.2.2. Toxicity
After the introduction of any xenobiotic into the ecosystem, it is plausible to expect adverse effects on various organisms. Hence, toxicity studies should be applied in order to assess the possible interactions between HILs and the environment. Unfortunately, to date there are no standards concerning such analyses, and only incidental studies are performed. This problem also applies to classical herbicides available on the market: only the main herbicidal compounds are subjected to toxicity tests, while at the same time adjuvants are proven to be much more toxic than the herbicides themselves.9,10,86 Moreover, the interactions between herbicides and adjuvants are mostly omitted in tests, and hence the nature of toxicity is unknown, whether it is a sum of the toxic effects of all components or the most toxic compound is responsible for overall toxicity.
Nowadays, it is possible to assess the toxicity of HILs as whole compounds and hence to obtain the environmentally relevant outcome for specific formulations. These tests might be divided into analyses on microorganisms or on more developed organisms. The EC50 (half maximal effective concentration) is the most commonly conducted test, with its varieties such as the LD50 (median lethal dose). EC50 tests were applied to green algae (Pseudokirchneriella subcapitata),63 water flea (Daphnia magna),63 and weeds [gallant soldier (Galinsoga parviflora Cav.), common sorrel (Rumex acetosa L.), and white goosefoot (Chenopodium album L.)],87 as well as plants [spring barley (Hordeum vulgare) and common radish (Raphanus sativus L. radicula Pers.)].78 These analyses were also conducted on microbial communities, for example, on microorganisms isolated from particular environmental niches73,85 and specific bacteria (Pseudomonas putida mt-2).51,88 Acute oral toxicity studies (LD50) were conducted on rats16,45,61,63 and rainbow trout.63 Additionally, Piotrowska et al.89 performed toxicity studies on eggs and embryos of zebrafish (Danio rerio) in order to assess the possible adverse effects of HILs on aquatic organisms. The impact of ionic liquids on phospholipid fatty acid (PLFA) composition was examined, showing changes in lipid composition of cellular membranes. The results indicated that the ammonium-based halides affected the membrane fluidity depending on their hydrophobicity, which was proven to be strictly connected with mortality. This, in turn, may be a useful tool for HIL toxicity assessment.
As for the less standard tests, Syguda et al.21 provided phytotoxicity analyses on common wheat (Triticum aestivum) with the use of oxidative stress markers (glutathione S-transferase and glutathione in the oxidized and reduced form). Furthermore, Bałczewski et al.78 investigated the phytotoxicity according to the OECD/OCDE 208 test on spring barley (Hordeum vulgare) and common radish (Raphanus sativus L. radicula Pers.) Additionally, lately analyses such as minimum inhibitory concentration (MIC), minimum bactericidal concentration (MBC),33 or even minimum fungicidal concentration (MFC) were carried out.37
To date, the results of toxicity studies seem to be inconclusive (Table 6). With the absence of standards to be followed, it is impossible to evaluate the actual environmental effects of these formulations. The findings demonstrate the whole range of incomparable results, since despite various procedures applied, different cations and anions are studied in each work. These issues altogether make it impossible to obtain full toxicity assessment just for a single formulation, not to mention dozens. Hence, in order to fully understand the environmental effects of HILs, standardized procedures for toxicity evaluation have to be developed, along with appropriate regulatory frameworks.
4.2.3. Impact of HILs on Microbial Biodiversity
The impact of HILs on the structure of bacterial populations in soil and aqueous environments is an interesting issue. The technique known as 16S rRNA sequencing is widely accepted as a gold standard for purposes of identification and classification of bacteria and archaea.90 Sequencing of the 16S gene allows for employment of NGS (Next Generation Sequencing) techniques, which are well-known for their high throughput (unlike DNA–DNA hybridization or phenotypic tests).91 This is especially important because it is well established that soil-borne bacteria form complex molecular relations with plants, affecting both their growth and well-being. Due to this reason, many attempts have been made to understand these interactions, and potentially employ them to enhance agricultural production.92−99 However, there is little research regarding IL biodegradation and their effects on soil microbiome community diversity in general.
Per contra, research on ammonium- and phosphonium-based ILs by Sydow et al. may be treated as guidepost for further experiments.71,100 Their studies established that ionic liquids are persistent in tested soils71 and change the structure of the microbial community.100 These results showed a significant loss of biodiversity; Shannon’s index (diversity index) decreased from 1.75 to 0.74 and OTUs (operational taxonomic units) decreased from 1399 down to 965. However, the analyses were carried out at sublethal concentrations, and authors suggest that more tests should be applied on environmentally relevant concentrations. Nevertheless, an abundance of hydrocarbon-degrading bacteria such as Sphingomonas was shown to be present in IL-treated soil, which were assumed to degrade ILs or their primary metabolites.100 To date, these types of tests regarding HILs have been conducted only by Ławniczak et al.43 and Czarny et al.85 Ławniczak et al.43 demonstrated that classic herbicides, dicamba and MCPA, are similar or less effective in weed control in comparison to their HIL counterparts. On the other hand, soils treated with dicamba-based HILs were characterized by different microbial community structure compared to soil treated with commercially available herbicides. However, in terms of overall biodiversity, these changes were deemed as statistically insignificant. Additionally, Czarny et al.85 described changes in structure of the anaerobic microbiome of biogas-producing microbial community. However, they were attributed mainly to lack of genes responsible for degradation of 2,4-D and MCPA. Therefore, the changes in structure were considered to happen due to accumulation of herbicides rather than increased toxicity of HILs compared to classic herbicides.
Furthermore, it is important to point out the fact that not a single test (to the best knowledge of the authors) regarding HILs was performed in terms of their toxicity to fungi. Fungi are a very important part of soil microbiome, as they also form complex interactions with both plants and other soil-borne microbes. These relationships, such as enhancing nutrient uptake or increasing plants’ natural immunity, are well-known among the scientific community, and there are numerous attempts to employ fungi in agriculture.101,102
This alteration in the composition of soil microbial communities should be taken into account when introduction of HILs to agriculture is considered.
5. Conclusions and Perspectives for the Future
Herbicidal ionic liquids are specific compounds intended for agricultural use; however the conclusions of this particular review can be expanded to the whole group of ionic liquids. HILs are characterized by well-established synthesis methods, which allow for obtaining both high yield and high purity. Moreover, their tunability also enables the adjustment of hydrophobicity and volatility, which may be beneficial when applied in agriculture. Additionally, the problem associated with the use of hazardous adjuvants is eliminated, which is advantageous when taking into account their overall toxicity. There are, however, still areas that need further development assuming the commercial use of HILs. To date, research is focused mainly on inconsistent and incidental experiments, which seems to be the problem of not only HILs but ionic liquids in general. It is impossible to properly assess their environmental impact based on biological experiments that lack proper standards. Moreover, these studies mainly concern model cases and do not correspond to the actual conditions of their proposed use in agriculture.
Taking into account these drawbacks, future recommendations have been prepared. Although the synthesis and physicochemical tests are well-established, their greatest weakness relates to lack of standardization. All new substances should be thoroughly tested in order to provide at least the most important basic parameters, such as volatilization, solubility in water and surface activity, and adsorption in soil, as well as leaching into groundwater or bioaccumulation. It is worth noting that even though methods regarding the analysis of herbicide volatility that would reflect the field conditions are known, currently there are no reports confirming that these are still valid for HILs.
On the other hand, it of the utmost importance to focus on the biological part of experiments as well.
First of all, the toxicity of HILs should be assessed toward soil bacteria due to their possible contact, since model organisms do not provide information regarding the real effect on soil microcosms. Additionally, the soil–plant interactions need to be studied.
The degradation and mineralization of HILs in soil should be determined to understand their persistence in farmland soil. Additionally, since it has been already confirmed that the biodegradability of anions and cations depends on the test system (aqueous or terrestrial), they need to be considered separately.79 Moreover, as the standard activated sludge procedure is insufficient to fully understand the behavior of ionic liquids in the environment, bacteria isolated from agriculture soils should be used in further experiments.
In order to assess the microbial community response to the presence of this type of xenobiotic, techniques such as NGS should be applied to determine how the whole population of bacteria reacts during field treatments.
Acknowledgments
This review resulted from studies conducted in the frame of OPUS 15 funded by the National Science Centre in Poland, conferred on the basis of decision DEC-2018/29/B/NZ9/01136. Grant title “Bioaugmentation with herbicide degrading bacteria as a potential factor in spreading resistance to herbicides among plants”.
The authors declare the following competing financial interest(s): Robin D. Rogers is a named inventor on related patents and patent applications. The University has licensed related patents and applications.
References
- Oerke E.-C. Crop Losses to Pests. J. Agric. Sci. 2006, 144 (1), 31–43. 10.1017/S0021859605005708. [DOI] [Google Scholar]
- Gavrilescu M. Fate of Pesticides in the Environment and Its Bioremediation. Eng. Life Sci. 2005, 5 (6), 497–526. 10.1002/elsc.200520098. [DOI] [Google Scholar]
- Food and Agriculture Organization of the United Nations. Pesticides Use; 2016. [Google Scholar]
- Kudsk P.; Streibig J. C. Herbicides - A Two-Edged Sword. Weed Res. 2003, 43 (2), 90–102. 10.1046/j.1365-3180.2003.00328.x. [DOI] [Google Scholar]
- United States Environmental Protection Agency . Reregistration Eligibility Decision for Dicamba and Associated Salts; 2006. [Google Scholar]
- Heap I. . The World of Herbicides - According to HRAC Classification on Mode of Action 2010. [Google Scholar]
- Schütte G.; Eckerstorfer M.; Rastelli V.; Reichenbecher W.; Restrepo-Vassalli S.; Ruohonen-Lehto M.; Saucy A.-G. W.; Mertens M.. Herbicide Resistance and Biodiversity: Agronomic and Environmental Aspects of Genetically Modified Herbicide-Resistant Plants. Environ. Sci. Eur. 201729 ( (1), ). 10.1186/s12302-016-0100-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma R. S.; Goswami-Giri A. S. Environment Friendly Herbicidal Compositions. Chem. Sci. Rev. Lett. 2015, 4 (14), 753–757. [Google Scholar]
- Mesnage R.; Bernay B.; Séralini G. E. Ethoxylated Adjuvants of Glyphosate-Based Herbicides Are Active Principles of Human Cell Toxicity. Toxicology 2013, 313 (2–3), 122–128. 10.1016/j.tox.2012.09.006. [DOI] [PubMed] [Google Scholar]
- Defarge N.; Spiroux de Vendômois J.; Séralini G. E. Toxicity of Formulants and Heavy Metals in Glyphosate-Based Herbicides and Other Pesticides. Toxicol. Reports 2018, 5, 156–163. 10.1016/j.toxrep.2017.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesnage R.; Defarge N.; Spiroux de Vendômois J.; Séralini G.-E. Major Pesticides Are More Toxic to Human Cells Than Their Declared Active Principles. BioMed Res. Int. 2014, 2014, 179691. 10.1155/2014/179691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pernak J.; Łęgosz B.; Klejdysz T.; Marcinkowska K.; Rogowski J.; Kurasiak-Popowska D.; Stuper-Szablewska K. Ammonium Bio-Ionic Liquids Based on Camelina Oil as Potential Novel Agrochemicals. RSC Adv. 2018, 8, 28676–28683. 10.1039/C8RA03519A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczmarek D. K.; Kleiber T.; Wenping L.; Niemczak M.; Chrzanowski Ł.; Pernak J. Transformation of Indole-3-Butyric Acid into Ionic Liquids as a Sustainable Strategy Leading to Highly Efficient Plant Growth Stimulators. ACS Sustainable Chem. Eng. 2020, 8, 1591–1598. 10.1021/acssuschemeng.9b06378. [DOI] [Google Scholar]
- Łęgosz B.; Biedziak A.; Klejdysz T.; Pernak J. Quaternary Ammonium Nonanoate-based Ionic Liquids as Chemicals for Crop Protection. Eur. J. Chem. 2016, 7, 217–224. 10.5155/eurjchem.7.2.217-224.1428. [DOI] [Google Scholar]
- Kaczmarek D. K.; Rzemieniecki T.; Marcinkowska K.; Pernak J. Synthesis, Properties and Adjuvant Activity of Docusate-Based Ionic Liquids in Pesticide Formulations. J. Ind. Eng. Chem. 2019, 78, 440–447. 10.1016/j.jiec.2019.05.023. [DOI] [Google Scholar]
- Pernak J.; Syguda A.; Janiszewska D.; Materna K.; Praczyk T. Ionic Liquids with Herbicidal Anions. Tetrahedron 2011, 67 (26), 4838–4844. 10.1016/j.tet.2011.05.016. [DOI] [Google Scholar]
- Hough W. L.; Smiglak M.; Rodríguez H.; Swatloski R. P.; Spear S. K.; Daly D. T.; Pernak J.; Grisel J. E.; Carliss R. D.; Soutullo M. D.; et al. The Third Evolution of Ionic Liquids: Active Pharmaceutical Ingredients. New J. Chem. 2007, 31 (8), 1429–1436. 10.1039/b706677p. [DOI] [Google Scholar]
- Earle M. J.; Esperança J. M. S. S.; Gilea M. A.; Canongia Lopes J. N. C.; Rebelo L. P. N.; Magee J. W.; Seddon K. R.; Widegren J. A. The Distillation and Volatility of Ionic Liquids. Nature 2006, 439 (7078), 831–834. 10.1038/nature04451. [DOI] [PubMed] [Google Scholar]
- Shamshina J. L.; Kelley S. P.; Gurau G.; Rogers R. D. Develop Ionic Liquid Drugs. Nature 2015, 528, 188–189. 10.1038/528188a. [DOI] [PubMed] [Google Scholar]
- Paul B. K.; Moulik S. P.. Ionic Liquid-Based Sufactant Science: Formulation, Characterization and Applications; Bidyut P. K., Moulik S. P., Eds.; Wiley John & Sons Inc, 2015. [Google Scholar]
- Syguda A.; Gielnik A.; Borkowski A.; Woźniak-Karczewska M.; Parus A.; Piechalak A.; Olejnik A.; Marecik R.; Ławniczak Ł.; Chrzanowski Ł. Esterquat Herbicidal Ionic Liquids (HILs) with Two Different Herbicides: Evaluation of Activity and Phytotoxicity. New J. Chem. 2018, 42 (12), 9819–9827. 10.1039/C8NJ01239C. [DOI] [Google Scholar]
- Atta S.; Paul A.; Banerjee R.; Bera M.; Ikbal M.; Dhara D.; Singh N. D. P. Photoresponsive Polymers Based on a Coumarin Moiety for the Controlled Release of Pesticide 2,4-D. RSC Adv. 2015, 5 (121), 99968–99975. 10.1039/C5RA18944F. [DOI] [Google Scholar]
- Tang G.; Liu Y.; Ding G.; Zhang W.; Liang Y.; Fan C.; Dong H.; Yang J.; Kong D.; Cao Y. Ionic Liquids Based on Bromoxynil for Reducing Adverse Impacts on the Environment and Human Health. New J. Chem. 2017, 41 (16), 8650–8655. 10.1039/C7NJ01694H. [DOI] [Google Scholar]
- Wang B.; Ding G.; Zhu J.; Zhang W.; Guo M.; Geng Q.; Guo D.; Cao Y. Development of Novel Ionic Liquids Based on Bentazone. Tetrahedron 2015, 71 (41), 7860–7864. 10.1016/j.tet.2015.08.029. [DOI] [Google Scholar]
- Syguda A.; Marcinkowska K.; Materna K. Pyrrolidinium Herbicidal Ionic Liquids. RSC Adv. 2016, 6 (68), 63136–63142. 10.1039/C6RA12157H. [DOI] [Google Scholar]
- Cojocaru O. A.; Shamshina J. L.; Gurau G.; Syguda A.; Praczyk T.; Pernak J.; Rogers R. D. Ionic Liquid Forms of the Herbicide Dicamba with Increased Efficacy and Reduced Volatility. Green Chem. 2013, 15 (8), 2110–2120. 10.1039/c3gc37143c. [DOI] [Google Scholar]
- Zhu J.; Ding G.; Liu Y.; Wang B.; Zhang W.; Guo M.; Geng Q.; Cao Y. Ionic Liquid Forms of Clopyralid with Increased Efficacy against Weeds and Reduced Leaching from Soils. Chem. Eng. J. 2015, 279, 472–477. 10.1016/j.cej.2015.05.025. [DOI] [Google Scholar]
- Tang G.; Wang B.; Ding G.; Zhang W.; Liang Y.; Fan C.; Dong H.; Yang J.; Kong D.; Cao Y. Developing Ionic Liquid Forms of Picloram with Reduced Negative Effects on the Aquatic Environment. Sci. Total Environ. 2018, 616–617 (2), 128–134. 10.1016/j.scitotenv.2017.10.288. [DOI] [PubMed] [Google Scholar]
- Pernak J.; Niemczak M.; Shamshina J. L.; Gurau G.; Głowacki G.; Praczyk T.; Marcinkowska K.; Rogers R. D. Metsulfuron-Methyl-Based Herbicidal Ionic Liquids. J. Agric. Food Chem. 2015, 63, 3357–3366. 10.1021/jf505782p. [DOI] [PubMed] [Google Scholar]
- Wang W.; Zhu J.; Tang G.; Huo H.; Zhang W.; Liang Y.; Dong H.; Yang J.; Cao Y. Novel Herbicide Ionic Liquids Based on Nicosulfuron with Increased Efficacy. New J. Chem. 2019, 43, 827–833. 10.1039/C8NJ05903A. [DOI] [Google Scholar]
- Pernak J.; Niemczak M.; Giszter R.; Shamshina J. L.; Gurau G.; Cojocaru O. A.; Praczyk T.; Marcinkowska K.; Rogers R. D. Glyphosate-Based Herbicidal Ionic Liquids with Increased Efficacy. ACS Sustainable Chem. Eng. 2014, 2 (12), 2845–2851. 10.1021/sc500612y. [DOI] [Google Scholar]
- Ding G.; Liu Y.; Wang B.; Punyapitak D.; Guo M.; Duan Y.; Li J.; Cao Y. Preparation and Characterization of Fomesafen Ionic Liquids for Reducing the Risk to the Aquatic Environment. New J. Chem. 2014, 38 (11), 5590–5596. 10.1039/C4NJ01186D. [DOI] [Google Scholar]
- Pernak J.; Czerniak K.; Niemczak M.; Ławniczak Ł.; Kaczmarek D. K.; Borkowski A.; Praczyk T. Bioherbicidal Ionic Liquids. ACS Sustainable Chem. Eng. 2018, 6 (2), 2741–2750. 10.1021/acssuschemeng.7b04382. [DOI] [Google Scholar]
- Turguła A.; Stȩsik K.; Materna K.; Klejdysz T.; Praczyk T.; Pernak J. Third-Generation Ionic Liquids with: N-Alkylated 1,4-Diazabicyclo[2.2.2]Octane Cations and Pelargonate Anions. RSC Adv. 2020, 10, 8653–8663. 10.1039/D0RA00766H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwani S. I.; Arif I. A.; Khan H. A.. Modes of Action of Different Classes of Herbicides. In Herbicides: Physiology of Action, and Safety; Price A., Kelton J., Sarunaite L., Eds.; IntechOpen, 2015; pp 165–186. 10.1016/j.colsurfa.2011.12.014. [DOI] [Google Scholar]
- Kervégant M.; Merigot L.; Glaizal M.; Schmitt C.; Tichadou L.; de Haro L. Paraquat Poisonings in France during the European Ban: Experience of the Poison Control Center in Marseille. J. Med. Toxicol. 2013, 9 (2), 144–147. 10.1007/s13181-012-0283-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turguła A.; Materna K.; Gwiazdowska D.; Walkiewicz F.; Marcinkowska K.; Pernak J. Difunctional Ammonium Ionic Liquids with Bicyclic Cations. New J. Chem. 2019, 43 (11), 4477–4488. 10.1039/C8NJ06054A. [DOI] [Google Scholar]
- Wang W.; Liang Y.; Yang J.; Tang G.; Zhou Z.; Tang R.; Dong H.; Li J.; Cao Y. Ionic Liquid Forms of Mesotrione with Enhanced Stability and Reduced Leaching Risk. ACS Sustainable Chem. Eng. 2019, 7 (19), 16620–16628. 10.1021/acssuschemeng.9b03948. [DOI] [Google Scholar]
- Pernak J.; Kaczmarek D. K.; Rzemieniecki T.; Niemczak M.; Chrzanowski L.; Praczyk T. Dicamba-Based Herbicides: Herbicidal Ionic Liquids versus Commercial Forms. J. Agric. Food Chem. 2020, 68, 4588–4594. 10.1021/acs.jafc.0c00632. [DOI] [PubMed] [Google Scholar]
- Pernak J.; Markiewicz B.; Zgoła-Grzeskowiak A.; Chrzanowski; Gwiazdowski R.; Marcinkowska K.; Praczyk T. Ionic Liquids with Dual Pesticidal Function. RSC Adv. 2014, 4 (75), 39751–39754. 10.1039/C4RA04816D. [DOI] [Google Scholar]
- Niu J.; Zhang Z.; Tang J.; Tang G.; Yang J.; Wang W.; Huo H.; Jiang N.; Li J.; Cao Y. Dicationic Ionic Liquids of Herbicide 2,4-Dichlorophenoxyacetic Acid with Reduced Negative Effects on Environment. J. Agric. Food Chem. 2018, 66 (40), 10362–10368. 10.1021/acs.jafc.8b02584. [DOI] [PubMed] [Google Scholar]
- Choudhary H.; Pernak J.; Shamshina J. L.; Niemczak M.; Giszter R.; Chrzanowski Ł.; Praczyk T.; Marcinkowska K.; Cojocaru O. A.; Rogers R. D. Two Herbicides in a Single Compound: Double Salt Herbicidal Ionic Liquids Exemplified with Glyphosate, Dicamba, and MCPA. ACS Sustainable Chem. Eng. 2017, 5 (7), 6261–6273. 10.1021/acssuschemeng.7b01224. [DOI] [Google Scholar]
- Ławniczak Ł.; Syguda A.; Borkowski A.; Cyplik P.; Marcinkowska K.; Wolko Ł.; Praczyk T.; Chrzanowski Ł.; Pernak J. Influence of Oligomeric Herbicidal Ionic Liquids with MCPA and Dicamba Anions on the Community Structure of Autochthonic Bacteria Present in Agricultural Soil. Sci. Total Environ. 2016, 563–564, 247–255. 10.1016/j.scitotenv.2016.04.109. [DOI] [PubMed] [Google Scholar]
- Marcinkowska K.; Praczyk T.; Gawlak M.; Niemczak M.; Pernak J. Efficacy of Herbicidal Ionic Liquids and Choline Salt Based on 2,4-D. Crop Prot. 2017, 98, 85–93. 10.1016/j.cropro.2017.03.011. [DOI] [Google Scholar]
- Niemczak M.; Chrzanowski Ł.; Praczyk T.; Pernak J. Biodegradable Herbicidal Ionic Liquids Based on Synthetic Auxins and Analogues of Betaine. New J. Chem. 2017, 41 (16), 8066–8077. 10.1039/C7NJ01474K. [DOI] [Google Scholar]
- Pernak J.; Syguda A.; Materna K.; Janus E.; Kardasz P.; Praczyk T. 2,4-D Based Herbicidal Ionic Liquids. Tetrahedron 2012, 68 (22), 4267–4273. 10.1016/j.tet.2012.03.065. [DOI] [Google Scholar]
- Pernak J.; Giszter R.; Biedziak A.; Niemczak M.; Olszewski R.; Marcinkowska K.; Praczyk T. Alkyl(C16, C18, C22)Trimethylammonium-Based Herbicidal Ionic Liquids. J. Agric. Food Chem. 2017, 65 (2), 260–269. 10.1021/acs.jafc.6b04528. [DOI] [PubMed] [Google Scholar]
- Pernak J.; Niemczak M.; Materna K.; Marcinkowska K.; Praczyk T. Ionic Liquids as Herbicides and Plant Growth Regulators. Tetrahedron 2013, 69 (23), 4665–4669. 10.1016/j.tet.2013.03.097. [DOI] [Google Scholar]
- Pęziak-Kowalska D.; Fourcade F.; Niemczak M.; Amrane A.; Chrzanowski Ł.; Lota G. Removal of Herbicidal Ionic Liquids by Electrochemical Advanced Oxidation Processes Combined with Biological Treatment. Environ. Technol. 2017, 38 (9), 1093–1099. 10.1080/09593330.2016.1217941. [DOI] [PubMed] [Google Scholar]
- Pęziak-Kowalska D.; Syguda A.; Ławniczak Ł.; Borkowski A.; Fourcade F.; Heipieper H. J.; Lota G.; Chrzanowski Ł. Hybrid Electrochemical and Biological Treatment of Herbicidal Ionic Liquids Comprising the MCPA Anion. Ecotoxicol. Environ. Saf. 2019, 181 (March), 172–179. 10.1016/j.ecoenv.2019.05.084. [DOI] [PubMed] [Google Scholar]
- Piotrowska A.; Syguda A.; Wyrwas B.; Chrzanowski Ł.; Heipieper H. J. Toxicity Evaluation of Selected Ammonium-Based Ionic Liquid Forms with MCPP and Dicamba Moieties on Pseudomonas Putida. Chemosphere 2017, 167, 114–119. 10.1016/j.chemosphere.2016.09.140. [DOI] [PubMed] [Google Scholar]
- Praczyk T.; Kardasz P.; Jakubiak E.; Syguda A.; Materna K.; Pernak J. Herbicidal Ionic Liquids with 2,4-D. Weed Sci. 2012, 60 (02), 189–192. 10.1614/WS-D-11-00171.1. [DOI] [Google Scholar]
- Verma S.; Kasana V. Development of Novel Herbicidal Ionic Liquids. Int. Res. J. Pure Appl. Chem. 2017, 15 (1), 1–8. 10.9734/IRJPAC/2017/36792. [DOI] [Google Scholar]
- Foy C. L.; Pritchard D. W.. Pesticide Formulation and Adjuvant Technology; Foy C. L., Pritchard D. W., Eds.; CRC Press/Taylor & Francis: New York, 1996. [Google Scholar]
- Kordala-Markiewicz R.; Rodak H.; Markiewicz B.; Walkiewicz F.; Sznajdrowska A.; Materna K.; Marcinkowska K.; Praczyk T.; Pernak J. Phenoxy Herbicidal Ammonium Ionic Liquids. Tetrahedron 2014, 70 (32), 4784–4789. 10.1016/j.tet.2014.05.041. [DOI] [Google Scholar]
- Pernak J.; Niemczak M.; Zakrocka K.; Praczyk T. Herbicidal Ionic Liquid with Dual-Function. Tetrahedron 2013, 69 (38), 8132–8136. 10.1016/j.tet.2013.07.053. [DOI] [Google Scholar]
- Niemczak M.; Biedziak A.; Czerniak K.; Marcinkowska K. Preparation and Characterization of New Ionic Liquid Forms of 2,4-DP Herbicide. Tetrahedron 2017, 73 (52), 7315–7325. 10.1016/j.tet.2017.11.032. [DOI] [Google Scholar]
- Niemczak M.; Rzemieniecki T.; Biedziak A.; Marcinkowska K.; Pernak J. Synthesis and Structure–Property Relationships in Herbicidal Ionic Liquids and Their Double Salts. ChemPlusChem 2018, 83 (6), 529–541. 10.1002/cplu.201800251. [DOI] [PubMed] [Google Scholar]
- Pernak J.; Czerniak K.; Biedziak A.; Marcinkowska K.; Praczyk T.; Erfurt K.; Chrobok A. Herbicidal Ionic Liquids Derived from Renewable Sources. RSC Adv. 2016, 6 (58), 52781–52789. 10.1039/C6RA06703D. [DOI] [Google Scholar]
- Giszter R.; Fryder M.; Marcinkowska K.; Sznajdrowska A. Synthesis, Surface Properties and Biological Activity of Long Chain Ammonium Herbicidal Ionic Liquids. J. Braz. Chem. Soc. 2016, 27, 1774–1781. 10.5935/0103-5053.20160058. [DOI] [Google Scholar]
- Pernak J.; Niemczak M.; Chrzanowski Ł.; Ławniczak Ł.; Fochtman P.; Marcinkowska K.; Praczyk T. Betaine and Carnitine Derivatives as Herbicidal Ionic Liquids. Chem. - Eur. J. 2016, 22 (34), 12012–12021. 10.1002/chem.201601952. [DOI] [PubMed] [Google Scholar]
- Czuryszkiewicz D.; Maćkowiak A.; Marcinkowska K.; Borkowski A.; Chrzanowski L.; Pernak J. Herbicidal Ionic Liquids Containing the Acetylcholine Cation. ChemPlusChem 2019, 84, 268. 10.1002/cplu.201800651. [DOI] [PubMed] [Google Scholar]
- Pernak J.; Czerniak K.; Niemczak M.; Chrzanowski Ł.; Ławniczak Ł.; Fochtman P.; Marcinkowska K.; Praczyk T. Herbicidal Ionic Liquids Based on Esterquats. New J. Chem. 2015, 39 (7), 5715–5724. 10.1039/C5NJ00609K. [DOI] [Google Scholar]
- Pernak J.; Niemczak M.; Materna K.; Zelechowski K.; Marcinkowska K.; Praczyk T. Synthesis, Properties and Evaluation of Biological Activity of Herbicidal Ionic Liquids with 4-(4-Chloro-2-Methylphenoxy)Butanoate Anion. RSC Adv. 2016, 6 (9), 7330–7338. 10.1039/C5RA23997D. [DOI] [Google Scholar]
- Niemczak M.; Rzemieniecki T.; Sobiech Ł.; Skrzypczak G.; Praczyk T.; Pernak J. Influence of the Alkyl Chain Length on the Physicochemical Properties and Biological Activity in a Homologous Series of Dichlorprop-Based Herbicidal Ionic Liquids. J. Mol. Liq. 2019, 276, 431–440. 10.1016/j.molliq.2018.12.013. [DOI] [Google Scholar]
- Pernak J.; Luboiński A.; Łacka A.; Praczyk T. Synthesis and Properties of Ionic Liquids Based on Mecoprop. New J. Chem. 2018, 42, 17259–17267. 10.1039/C8NJ03435D. [DOI] [Google Scholar]
- Niemczak M.; Giszter R.; Czerniak K.; Marcinkowska K.; Walkiewicz F. Bis(Ammonium) Ionic Liquids with Herbicidal Anions. RSC Adv. 2015, 5 (20), 15487–15493. 10.1039/C4RA16151C. [DOI] [Google Scholar]
- Czerniak K.; Gwiazdowski R.; Marcinkowska K.; Pernak J. Dicationic Triazolium Fungicidal Ionic Liquids with Herbicidal Properties. Chem. Pap. 2020, 74, 261–271. 10.1007/s11696-019-00875-x. [DOI] [Google Scholar]
- Bakshi K.; Mitra S.; Sharma V. K.; Jayadev M. S. K.; Sakai V. G.; Mukhopadhyay R.; Gupta A.; Ghosh S. K. Imidazolium-Based Ionic Liquids Cause Mammalian Cell Death Due to Modulated Structures and Dynamics of Cellular Membrane. Biochim. Biophys. Acta, Biomembr. 2020, 1862, 183103. 10.1016/j.bbamem.2019.183103. [DOI] [PubMed] [Google Scholar]
- Jordan A.; Gathergood N. Biodegradation of Ionic Liquids - a Critical Review. Chem. Soc. Rev. 2015, 44 (22), 8200–8237. 10.1039/C5CS00444F. [DOI] [PubMed] [Google Scholar]
- Sydow M.; Szczepaniak Z.; Framski G.; Staninska J.; Owsianiak M.; Szulc A.; Piotrowska-Cyplik A.; Zgoła-Grześkowiak A.; Wyrwas B.; Chrzanowski L. Persistence of Selected Ammonium- and Phosphonium-Based Ionic Liquids in Urban Park Soil Microcosms. Int. Biodeterior. Biodegrad. 2015, 103, 91–96. 10.1016/j.ibiod.2015.04.019. [DOI] [Google Scholar]
- Praczyk T.; Zakrocka K.; Wyrzykowska D.; Niemczak M.; Pernak J. Ionic Liquids Based on 2-Chloroethyltrimethylammonium Chloride (CCC) as Plant Growth Regulators. Cent. Eur. J. Chem. 2013, 11 (11), 1816–1821. 10.2478/s11532-013-0309-1. [DOI] [Google Scholar]
- Ławniczak Ł.; Materna K.; Framski G.; Szulc A.; Syguda A. Comparative Study on the Biodegradability of Morpholinium Herbicidal Ionic Liquids. Biodegradation 2015, 26 (4), 327–340. 10.1007/s10532-015-9737-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parus A.; Framski G.; Rypniewski W.; Panasiewicz K.; Szulc P.; Myszka K.; Zgoła-Grześkowiak A.; Ławniczak Ł.; Chrzanowski Ł. Plant Growth Promoting N-Alkyltropinium Bromides Enhance Seed Germination, Biomass Accumulation and Photosynthesis Parameters of Maize (Zea Mays). New J. Chem. 2019, 43 (15), 5805–5812. 10.1039/C8NJ06298F. [DOI] [Google Scholar]
- Parus A.; Wilms W.; Verkhovetska V.; Framski G.; Woźniak-Karczewska M.; Syguda A.; Strzemiecka B.; Borkowski A.; Ławniczak Ł.; Chrzanowski Ł. Transformation of Herbicides into Dual Function Quaternary Tropinium Salts. New J. Chem. 2020, 44 (21), 8869–8877. 10.1039/D0NJ01597K. [DOI] [Google Scholar]
- Kumar R. Approved and Investigational Uses of Modafinil: An Evidence-Based Review. Drugs 2008, 68 (13), 1803–1839. 10.2165/00003495-200868130-00003. [DOI] [PubMed] [Google Scholar]
- Marcinkowska K.; Praczyk T.; Łȩgosz B.; Biedziak A.; Pernak J. Bio-Ionic Liquids as Adjuvants for Sulfonylurea Herbicides. Weed Sci. 2018, 66 (3), 404–414. 10.1017/wsc.2017.85. [DOI] [Google Scholar]
- Bałczewski P.; Biczak R.; Turek M.; Pawłowska B.; Różycka-Sokołowska E.; Marciniak B.; Deska M.; Skalik J. Ammonium 2,2′-Thiodiacetates – Selective and Environmentally Safe Herbicides. Ecotoxicol. Environ. Saf. 2018, 163 (July), 408–416. 10.1016/j.ecoenv.2018.07.093. [DOI] [PubMed] [Google Scholar]
- Wilms W.; Woźniak-Karczewska M.; Niemczak M.; Lisiecki P.; Zgoła-Grześkowiak A.; Ławniczak Ł.; Framski G.; Pernak J.; Owsianiak M.; Vogt C.; et al. Quantifying the Mineralization of 13C-Labeled Cations and Anions Reveals Differences in Microbial Biodegradation of Herbicidal Ionic Liquids between Water and Soil. ACS Sustainable Chem. Eng. 2020, 8, 3412–3426. 10.1021/acssuschemeng.9b07598. [DOI] [Google Scholar]
- Chatel G.; Pereira J. F. B.; Debbeti V.; Wang H.; Rogers R. D. Mixing Ionic Liquids-“simple Mixtures” or “Double Salts”?. Green Chem. 2014, 16 (4), 2051–2083. 10.1039/c3gc41389f. [DOI] [Google Scholar]
- Jones G. T.; Norsworthy J. K.; Barber T.; Gbur E.; Kruger G. R. Off-Target Movement of DGA and BAPMA Dicamba to Sensitive Soybean. Weed Technol. 2019, 33 (1), 51–65. 10.1017/wet.2018.121. [DOI] [Google Scholar]
- Bomgardner M. M. Dicamba Disaster Fuels Controversy. C&EN Glob. Enterp. 2017, 95, 27–29. 10.1021/cen-09533-bus1. [DOI] [Google Scholar]
- Stark A.; Behrend P.; Braun O.; Müller A.; Ranke J.; Ondruschka B.; Jastorff B. Purity Specification Methods for Ionic Liquids. Green Chem. 2008, 10 (11), 1152–1161. 10.1039/b808532c. [DOI] [Google Scholar]
- Vogel D.; Kidwell J.; Tyler J.; Dow M.. Sulfosate (Glyphosate Trimesium): Cotton, Root and Tuber Vegetables, Pistachio, Grain Sorghum, Sweet Corn, Wheat, Fruiting Vegetables (except Cucurbits), Edible-podded Legume Vegetables, Succulent Shelled Pea and Beans, and Dried Shelled Pea and Beans (Exc United States Environ. Prot. Agency, 2001, 1–53.
- Czarny J.; Piotrowska-Cyplik A.; Lewicki A.; Zgoła-Grześkowiak A.; Wolko Ł.; Galant N.; Syguda A.; Cyplik P. The Toxic Effect of Herbicidal Ionic Liquids on Biogas-Producing Microbial Community. Int. J. Environ. Res. Public Health 2019, 16, 916. 10.3390/ijerph16060916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aparecida M.; de Campos Ventura-Camargo B.; Miyuki Hoshina M.. Toxicity of Herbicides: Impact on Aquatic and Soil Biota and Human Health. In Herbicides - Current Research and Case Studies in Use; Price A. J., Kelton J. A., Eds.; Intech, 2013; pp 399–444. 10.5772/55851. [DOI] [Google Scholar]
- Biczak R.; Pawłowska B.; Feder-Kubis J. The Effect of Ionic Liquids with (−)-Menthol Derivative Containing a Chloride Anion to Weed. Ecol. Chem. Eng. S 2017, 24 (4), 637–651. 10.1515/eces-2017-0042. [DOI] [Google Scholar]
- Piotrowska A.; Syguda A.; Chrzanowski Ł.; Heipieper H. J. Toxicity of Synthetic Herbicides Containing 2,4-D and MCPA Moieties towards Pseudomonas Putida Mt-2 and Its Response at the Level of Membrane Fatty Acid Composition. Chemosphere 2016, 144, 107–112. 10.1016/j.chemosphere.2015.08.067. [DOI] [PubMed] [Google Scholar]
- Piotrowska A.; Syguda A.; Wyrwas B.; Chrzanowski L.; Luckenbach T.; Heipieper H. J. Effects of Ammonium-Based Ionic Liquids and 2,4-Dichlorophenol on the Phospholipid Fatty Acid Composition of Zebrafish Embryos. PLoS One 2018, 13 (1), e0190779. 10.1371/journal.pone.0190779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M.; Chun J.. 16S RRNA Gene-Based Identification of Bacteria and Archaea Using the EzTaxon Server, 1st ed.; Elsevier Ltd., 2014; Vol. 41, 10.1016/bs.mim.2014.08.001. [DOI] [Google Scholar]
- Lau S. K. P.; Teng J. L. L.; Ho C. C.; Woo P. C. Y.. Gene Amplification and Sequencing for Bacterial Identification, 1st ed.; Elsevier Ltd., 2015; Vol. 42, 10.1016/bs.mim.2015.04.003. [DOI] [Google Scholar]
- Wu C. H.; Bernard S. M.; Andersen G. L.; Chen W. Developing Microbe-Plant Interactions for Applications in Plant-Growth Promotion and Disease Control, Production of Useful Compounds, Remediation and Carbon Sequestration. Microb. Biotechnol. 2009, 2 (4), 428–440. 10.1111/j.1751-7915.2009.00109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinale F.; Sivasithamparam K.; Ghisalberti E. L.; Marra R.; Woo S. L.; Lorito M. Trichoderma-Plant-Pathogen Interactions. Soil Biol. Biochem. 2008, 40 (1), 1–10. 10.1016/j.soilbio.2007.07.002. [DOI] [Google Scholar]
- Kumar A.; Verma J. P. Does Plant—Microbe Interaction Confer Stress Tolerance in Plants: A Review?. Microbiol. Res. 2018, 207, 41–52. 10.1016/j.micres.2017.11.004. [DOI] [PubMed] [Google Scholar]
- Francis I.; Holsters M.; Vereecke D. The Gram-Positive Side of Plant-Microbe Interactions: Minireview. Environ. Microbiol. 2010, 12 (1), 1–12. 10.1111/j.1462-2920.2009.01989.x. [DOI] [PubMed] [Google Scholar]
- Finkel O. M.; Castrillo G.; Herrera Paredes S.; Salas González I.; Dangl J. L. Understanding and Exploiting Plant Beneficial Microbes. Curr. Opin. Plant Biol. 2017, 38, 155–163. 10.1016/j.pbi.2017.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berendsen R. L.; Pieterse C. M. J.; Bakker P. A. H. M. The Rhizosphere Microbiome and Plant Health. Trends Plant Sci. 2012, 17 (8), 478–486. 10.1016/j.tplants.2012.04.001. [DOI] [PubMed] [Google Scholar]
- Verma M.; Brar S. K.; Tyagi R. D.; Surampalli R. Y.; Valéro J. R. Antagonistic Fungi, Trichoderma Spp.: Panoply of Biological Control. Biochem. Eng. J. 2007, 37 (1), 1–20. 10.1016/j.bej.2007.05.012. [DOI] [Google Scholar]
- Adesemoye A. O.; Kloepper J. W. Plant-Microbes Interactions in Enhanced Fertilizer-Use Efficiency. Appl. Microbiol. Biotechnol. 2009, 85 (1), 1–12. 10.1007/s00253-009-2196-0. [DOI] [PubMed] [Google Scholar]
- Sydow M.; Owsianiak M.; Framski G.; Woźniak-Karczewska M.; Piotrowska-Cyplik A.; Ławniczak Ł.; Szulc A.; Zgoła-Grześkowiak A.; Heipieper H. J.; Chrzanowski Ł. Biodiversity of Soil Bacteria Exposed to Sub-Lethal Concentrations of Phosphonium-Based Ionic Liquids: Effects of Toxicity and Biodegradation. Ecotoxicol. Environ. Saf. 2018, 147, 157–164. 10.1016/j.ecoenv.2017.08.026. [DOI] [PubMed] [Google Scholar]
- Mycorrhizal Fungi: Use in Sustainable Agriculture and Land Restoration; Solaiman Z. M., Abbott L. K., Varma A., Eds.; Springer-Verlag, 2014, 10.1007/978-3-662-45370-4_11. [DOI] [Google Scholar]
- Bhardwaj D.; Ansari M. W.; Sahoo R. K.; Tuteja N. Biofertilizers Function as Key Player in Sustainable Agriculture by Improving Soil Fertility, Plant Tolerance and Crop Productivity. Microb. Cell Fact. 2014, 13, 66. 10.1186/1475-2859-13-66. [DOI] [PMC free article] [PubMed] [Google Scholar]