Abstract
The Western Pacific Region (WPR) established a goal to decrease chronic hepatitis B virus (HBV) infection among children to <1% and to achieve ≥95% hepatitis B vaccine birth dose (HepB-BD) and ≥95% three-dose (HepB3) coverage by 2017. In 2016, we conducted a national serosurvey in the Solomon Islands among 6–7-year-old school children to assess progress towards the control goal and immunity to measles, rubella, tetanus and diphtheria. Eighty schools were selected systematically proportional to their 6–7-year-old population; all 6–7-year-olds were enrolled. We collected basic demographic information and vaccination history. Children were tested for HBV surface antigen (HBsAg) using a rapid test, and for immunity to measles, rubella, tetanus, and diphtheria using a multiplex bead assay. In total, 1,249 out of 1,492 children (84%) were enrolled, among whom 1,169 (94%) underwent HBsAg testing and 1,156 (93%) provided dried blood spots. Almost 80% (n = 982) of enrolled children had vaccination cards, among whom 59% (n= 584) received a timely HepB-BD (within 24 hours of birth), 95% (n= 932) received HepB3, and >90% received vaccines for diphtheria, tetanus, and measles (rubella vaccine was not available at the time). HBsAg prevalence was 3.1% (95% confidence interval (CI): 2.0%–4.9%), with 55% of identified cases from one province. Among 982 children with vaccination cards, HBsAg prevalence was higher among children who had not received a timely HepB-BD and at least two HepB doses compared to those who had (4% vs. 2%). Of 1,156 tested children, immunoprotection estimates were 99% (95% CI: 98%–99%) for measles, 99% (95% CI: 97%–100%) for rubella, 85% (95% CI: 83%–87%) for tetanus, and 51% (95% CI: 47%–55%) for diphtheria. Improving timely HepB-BD coverage and maintaining high HepB3 coverage could help Solomon Islands reach the regional HBV control goal. Low immunity to tetanus and diphtheria suggests the need to introduce booster doses to ensure long-term protection.
Keywords: Hepatitis B, Vaccination, Hepatitis B surface antigen, Seroepidemiological studies, Immunity, Immunization, Vaccine preventable diseases, Solomon Islands
1. Introduction
Chronic hepatitis B virus (HBV) infection is the leading cause of liver cancer worldwide [1]. Infection during early childhood, especially vertical transmission from mother to child at birth, is strongly associated with progression to chronic infection [2]. Furthermore, children are more likely to become infected if the mother has a high viral load or is HBV e-antigen (HBeAg; a marker for infectiousness) positive [3]. The World Health Organization (WHO) recommends children receive a timely hepatitis B vaccine (HepB) birth dose (HepB-BD), defined as vaccine receipt within 24 hours of birth, followed by at least two additional doses of HepB to prevent perinatal and early childhood HBV infection [3]. The WHO Western Pacific Region (WPR) had a target to reduce chronic HBV infection prevalence to <1% among children ≥5 years of age and to achieve coverage of HepB-BD and 3 doses of HepB (HepB3) of at least 95% by 2017 [4,5]. The Global Health Sector Strategy on Viral Hepatitis, endorsed in 2016, set targets of a 30% reduction in incidence (equivalent to 1% HBsAg prevalence among children) by 2020 and a 90% reduction in incidence (equivalent to 0.1% HBsAg prevalence among children) by 2030 [6]. Verification of achievement of these targets is primarily based on estimating the HBsAg prevalence among children ≥5 years old born after the start of a nationwide infant vaccination program through a nationally representative serosurvey [7].
The WHO WPR has also set a target of measles and rubella elimination, defined as the absence of endemic measles or rubella virus transmission in a defined geographical area for ≥12 months, in the presence of a well performing surveillance system [8,9]. As part of the elimination strategy, countries are recommended to achieve and maintain at least 95% vaccine coverage with 2 doses of measles-containing vaccine (MCV) in each birth cohort [10,11]. The neonatal tetanus (NT) elimination (defined as <1 NT case per 1000 live births in every district per year) initiative was launched at the World Health Assembly in 1989 to reduce NT as a public health problem in all countries [12]. Immunization is a key component of NT elimination and WHO recommends 6 doses of tetanus toxoid be administered, including a primary series of 3 doses administered at 6, 10 and 14 weeks of age, and 3 booster doses administered at 12–23 months, 4–7 years, and 9–15 years of age to ensure long-term protection [13]. To ensure long-term protection against diphtheria, WHO recommends diphtheria vaccine be given in combination with tetanus toxoid following the same schedule [13,14]. Assessment of levels of seroprotection against vaccine preventable diseases in specific birth cohorts can provide additional evidence to support vaccine coverage estimates, to track progress towards achievement and maintenance of regional and global control and elimination goals, and to document immunity gaps in order to guide vaccination activities.
The Solomon Islands is located in the WPR and consists of almost 1,000 islands totaling 30,400 square kilometers of land area within 1.5 million square kilometers of sea [15]. The country has 10 provinces: Central, Choiseul, Isabel, Guadalcanal, Honiara City Council (the capital), Malaita, Makira, Rennel and Bellona, Temotu, and Western [16]. In 2018, the population was estimated at around 623,000 persons, with 38% under 15 years of age [17]. The proportion of health facility deliveries has remained stable at 85% over the last 15 years, but is higher in urban areas (95%) and among the wealthy (96%; range by wealth index quintile: 69%–96%) [18]. The Solomon Islands introduced HepB and HepB-BD into the routine immunization program in 1990 and 2001, respectively, and since 2005 has recommended that children receive a monovalent timely HepB-BD and three doses of pentavalent vaccine (containing diphtheria, tetanus, pertussis, hepatitis B, and H.influenzae type b vaccines) at 6, 10 and 14 weeks of age. They also recommend children receive Bacille Calmette Guerin (BCG; tuberculosis vaccine) at birth, oral polio vaccine (OPV) at 6, 10, and 14 weeks of age, and 2 doses of measles and rubella vaccine (MR) at 12 and 18 months of age (rubella vaccine was introduced in 2013 and the second MR dose was introduced at the end of 2018). MR doses were also given during supplementary immunization activities (SIAs) conducted in 2012 (target age group: 12–59 months) and in response to a measles outbreak in 2014 (target age group: 6 months to 30 years). The Solomon Islands additionally recommend tetanus toxoid (TT) booster doses at 12–23 months and at 4–7 years, but currently do not recommend diphtheria booster doses [19]. In 2018, WHO and UNICEF estimates of national immunization coverage (WUENIC) were 85% for 3 doses of HepB and 67% for the timely HepB-BD [20]. Estimates for the other routine infant vaccines were: BCG-83%, DTP3–85%, OPV3–85% and MR1–93% [20]. For 2008–2010 (the birth years for the cohort of children included in the survey), WUENIC estimates were: HepB3–89%–90%, HepB-BD-80%–62%, BCG-92%–91%, DTP3–85%–83%, OPV3–88%–84% and measles containing vaccine dose 1 (MCV1)-71%–74%. Solomon Islands was validated to have achieved NT elimination pre-2000, however it is important for the country to monitor maintenance of elimination [21].
In the Solomon Islands, serosurveys were used to estimate chronic HBV infection prevalence (indicated by HBV surface antigen (HBsAg) prevalence) at 21% among all ages in 2007, and 13% among 0–9-year-olds that attended the national reference hospital in 1994 [22,23]. To assess progress made by the Solomon Islands towards the regional HBV control goal and immunity to measles, rubella, tetanus and diphtheria, we conducted a national school-based serosurvey among children aged 6–7 years old in the Solomon Islands. These children would have been eligible to receive measles, tetanus, and diphtheria vaccines through the routine immunization schedule and rubella vaccine only through SIAs.
2. Materials and methods
2.1. Survey population and design
In 2016, we conducted a national cross-sectional school-based 2-stage cluster survey among 6–7-year-old children (born during 2008–2010) in the Solomon Islands. Schools were selected systematically proportional to estimated population size from Ministry of Education lists, which were ordered by province and number of registered students aged 6–7 years. Schools with <5 registered students aged 6–7 years were excluded a priori. All children aged 6–7 years that attended the selected schools were eligible to participate. School teachers obtained written parental consent for each child to provide a blood sample via a finger-prick. Surveyors collected basic demographic information and vaccination history for each eligible child; SIA dose coverage was collected if recorded on the vaccination card. For children with parental consent, finger-prick blood was collected for the HBsAg rapid test (Alere Determine™) and dried blood spots (DBS) were collected on filter paper with six circular extensions (TropBio Pty Ltd, Queensland, Australia) to test for antibodies to measles, rubella, diphtheria, and tetanus. DBS were dried in-situ and then individually sealed in zip-lock bags with desiccant prior to being shipped to the US Centers for Disease Control and Prevention, Atlanta, GA, (US CDC) for testing. Antibody results and questionnaires were linked with a unique identification number. Surveyors attempted to locate any missing vaccination information in the local clinic’s immunization register matching on name, village, and date of birth. If an eligible child was not present during the first school visit, surveyors returned to the school on another day before classifying the child as missing.
2.2. Sample size
The sample size was calculated based on an estimated HBsAg prevalence among school children aged 6–7 years old of 1% with a precision of ±0.5%, design effect of 1.2, and an alpha of 0.05 (simple asymptotic formula). The sample size was adjusted for a finite population correction factor (N/(n0 + N-1) where n0 is the sample size and N is the size of the target population of ~ 32,000), resulting in a minimum sample size of 1,728 children. Adjusting for 15% non-response, the minimum sample size needed was 2,033 children. Of the 1,150 public and private schools in the Solomon Islands, 80 (7%) schools had <5 registered students aged 6–7 years and were therefore excluded (<1.3% of the target population of students). Of the remaining 1,070 schools, 80 schools were selected based on the average class size to meet the target sample size.
2.3. HBsAg rapid test
Following manufacturer’s instructions, whole blood (50 μL) was applied to the sample portal of the Alere Determine™ HBsAg rapid test strip (sensitivity: 95%–100%, specificity: 96%–100%) followed by 1 drop of kit buffer. After 15 min, surveyors visually interpreted the test as positive, negative, or invalid. For invalid results, if the child assented, surveyors collected a second blood sample and repeated the test. A second invalid result was not repeated and was given a final determination of invalid. The Solomon Islands Ministry of Health and Medical Services (MHMS) contacted the family of all children that tested HBsAg positive to inform them of the test result and to schedule a repeat HBsAg rapid test, if the family was willing.
2.4. Multiplex bead assay (MBA)
Diphtheria toxoid (List Biological Laboratories, CA), tetanus toxoid (Massachusetts Biological Laboratories, MA), whole rubella virus and recombinant measles virus nucleoprotein (MV-N) (Meridian Life Sciences, TN) [24] were purchased from commercial sources. Prior to use, MV-N was run on an anion exchange column to decrease non-specific reactivity [25]. Antigens were coupled covalently to SeroMap microspheres as previously described [25–27]. All bead preparation and MBA testing were conducted at the US CDC.
The detection of IgG antibody to measles, rubella, tetanus, and diphtheria using the MBA platform has been previously validated against gold standard assays [28–31]. Briefly, DBS eluates at a 1:320 dilution were incubated with all bead-antigen sets (2500/antigen/well) for 1.5 hours [32]. Total IgG was detected with 50 ng of biotinylated mouse anti-human IgG (Southern Biotech, Birmingham, AL) and 20 ng of biotinylated mouse anti-human IgG4 (Southern Biotech) for 45 minutes, followed by incubation with 250 ng phycoerythrin-labelled streptavidin (Invitrogen, South San Francisco, CA) for 30 minutes. Wells were incubated with PBS containing 0.5% BSA, 0.02% sodium azide and 0.05% Tween 20 for 30 minutes to remove any loosely bound antibodies. Beads were washed, resuspended in PBS and read on a BioPlex 200 instrument (Bio-Rad, Hercules, CA) equipped with BioPlex Manager 6.0 software (Bio-Rad). The median fluorescence intensity (MFI) with the background from the blank well subtracted out (MFI-BG) was recorded for each sample.
The MFI-BG cutoffs indicative of immunoprotection for tetanus, diphtheria, and rubella were calculated using dilution series of WHO international reference sera: tetanus (TE-3, National Institute for Biological Standards and Controls (NIBSC), UK), diphtheria (10/262; NIBSC, UK), rubella (67/182; NIBSC, UK). For tetanus, MBA values ≥102 MFI-BG were equivalent to ≥0.01 IU/mL, MBA values ≥1459 MFI-BG were equivalent to ≥0.1 IU/mL, and MBA values ≥20833 MFI-BG were equivalent to ≥1 IU/mL. Antibody levels ≥0.01 IU/mL were considered to be indicative of minimal immunoprotection and antibody levels ≥1 IU/mL were considered to be indicative of long term protection against tetanus [33,34]. For diphtheria, MBA values ≥883 MFI-BG were equivalent to ≥0.01 IU/mL and MBA values ≥15141 MFI-BG were equivalent to ≥0.1 IU/mL. Antibody levels ≥0.01 IU/mL were considered to be indicative of minimal immunoprotection against diphtheria [35]. MBA values of ≥3771 MFI-BG equivalent to ≥10 IU/mL were considered immunoprotective for rubella [36]. For measles (MV-N), the NIBSC standard 97/648 was used to extrapolate an immunoprotective measles cutoff of MFI-BG of ≥53 as described previously [37]. This cutoff is based on work from Coughlin et al (in preparation) to define an IgG-based cutoff for MV-N in the multiplex bead assay using receiver operating characteristic curve analysis with 140 sera characterized by the gold standard plaque reduction neutralization test [25,38]. MBA values ≥53 MFI-BG were considered immunoprotective for measles.
2.5. Data analysis
A descriptive summary of participants’ sex, age, and place of birth was completed. Vaccine history was checked for potential data entry errors: vaccines administered before the date of birth, missing doses, or doses out of order; and any identified data entry errors were corrected, where possible. Invalid dates of vaccination with respect to date of birth were set to missing during the cleaning process (n = 56). Receipt of each vaccine and timeliness of receipt were calculated for each participant with a vaccination card. Timeliness was defined as within 24 hours of birth for HepB-BD (interpreted as date of birth plus day after date of birth), within 4 weeks for BCG, and within 12–15 months for MCV1; for pentavalent and oral polio vaccines, it was defined as within 6–8 weeks for dose 1, within 10–12 weeks for dose 2, and within 14–16 weeks for dose 3. We used time to event analysis methods for receipt of each vaccine with a censoring time of 2 years of age and plotted the reverse event probability (1-event probability) to track vaccine receipt over time for children with documented dates on vaccination cards, and known date of birth (R v3.5.1). Rao-Scott second order Chi-square test was used to determine associations with birth day of the week and receipt of timely HepB-BD. Due to the high proportion of cumulative missing data (>34%) and since most vaccination history was only available for children with vaccination cards, vaccination receipt was not weighted and is only presented as among enrolled children with vaccination cards. Hepatitis B seroprevalence, the proportion of children with minimal immunoprotection to measles, rubella, tetanus, and diphtheria, and Wilson 95% confidence intervals (CI) were calculated using survey methodology accounting for the survey design. Sampling weights were based on the probability of selection for a given child adjusted for response rates in surveyed schools within each province. For the analysis of seroprevalence and the proportion of children with minimal immunoprotection to specified vaccine preventable diseases, additional nonresponse adjustments at the province level were included to account for children without a test result. The distribution of children with tetanus antibody levels <0.01 IU/mL, 0.01–0.09 IU/mL, 0.1–0.99 IU/mL, ≥1 IU/mL and diphtheria antibody levels <0.01 IU/mL, 0.01–0.09 IU/mL, ≥0.1 IU/mL and the Wilson 95% confidence intervals are presented. Rao-Scott second order Chi-square test was used to determine associations with diphtheria and tetanus immunoprotection and documented 3-dose pentavalent vaccine receipt. All data analyses were conducted using SAS v9.4.
2.6. Ethics
The survey protocol was determined not to be human-subjects research according to the US CDC Human Subjects research protection procedures and was approved by the National Health Research and Ethics Committee of the MHMS and the Ethics Review Committee at the WHO Regional Office for the Western Pacific.
3. Results
3.1. Characteristics of study population
Of 80 selected schools, one had no eligible children, one could not be contacted, and two that were listed separately but in the same location (kindergarten and primary) were erroneously merged during data collection, resulting in 77 schools being included in the analysis. From these schools, 1,492 children were eligible to participate based on age (6–7 years) at the time of the survey visit and 1,249 (84%) had parental consent to participate. Participation varied by province (median: 95%, interquartile range (IQR): 90% to 98%; Table 1), and by school (median: 100%; IQR: 82% to 100%), with 22 schools having < 90% participation. The province of Honiara had the lowest participation at 46% and included the school with the lowest participation (29%). Among the 1,249 enrolled children, 51% were male, 53% were 6 years old, and 99% were born in the Solomon Islands (Table 1). In total, 1,169 (94%) of enrolled children underwent HBsAg testing and 1,156 (93%) provided DBS for measles, rubella, tetanus and diphtheria testing.
Table 1.
Distribution and characteristics of all eligible and enrolled school children aged 6–7 years from selected schools that participated in a national school-based hepatitis B serosurvey — Solomon Islands, 2016.
| Eligible children |
Enrolled children |
% Participation* | ||||
|---|---|---|---|---|---|---|
| No. | % | No. | % | |||
| Overall | 1492 | 100 | 1249 | 100 | 84 | |
| Province | Central | 78 | 5 | 78 | 6 | 100 |
| Choiseul | 79 | 5 | 72 | 6 | 91 | |
| Guadalcanal | 217 | 15 | 205 | 16 | 94 | |
| Honiara City Council | 253 | 17 | 117 | 9 | 46 | |
| Isabel | 105 | 7 | 101 | 8 | 96 | |
| Makira | 150 | 10 | 143 | 12 | 95 | |
| Malaita | 387 | 26 | 343 | 27 | 89 | |
| Rennel & Bellona | 8 | 1 | 8 | 1 | 100 | |
| Temotu | 49 | 3 | 46 | 4 | 94 | |
| Western | 166 | 11 | 136 | 11 | 82 | |
| Sex | Male | 734 | 49 | 636 | 51 | 87 |
| Female | 712 | 48 | 599 | 48 | 84 | |
| Unknown | 46 | 3 | 14 | 1 | 30 | |
| Age | 6 years | 768 | 51 | 664 | 53 | 86 |
| 7 years | 708 | 48 | 585 | 47 | 83 | |
| Unknown | 16 | 1 | 0 | 0 | 0 | |
| Born in the Solomon Islands | Yes | 1327 | 89 | 1218 | 98 | 92 |
| No | 19 | 1 | 16 | 1 | 84 | |
| Unknown | 146 | 10 | 15 | 1 | 10 | |
No. - number; % - percent;
Children had written parental consent to participate in the survey (No. enrolled/no. eligible).
3.2. Vaccination receipt and timeliness
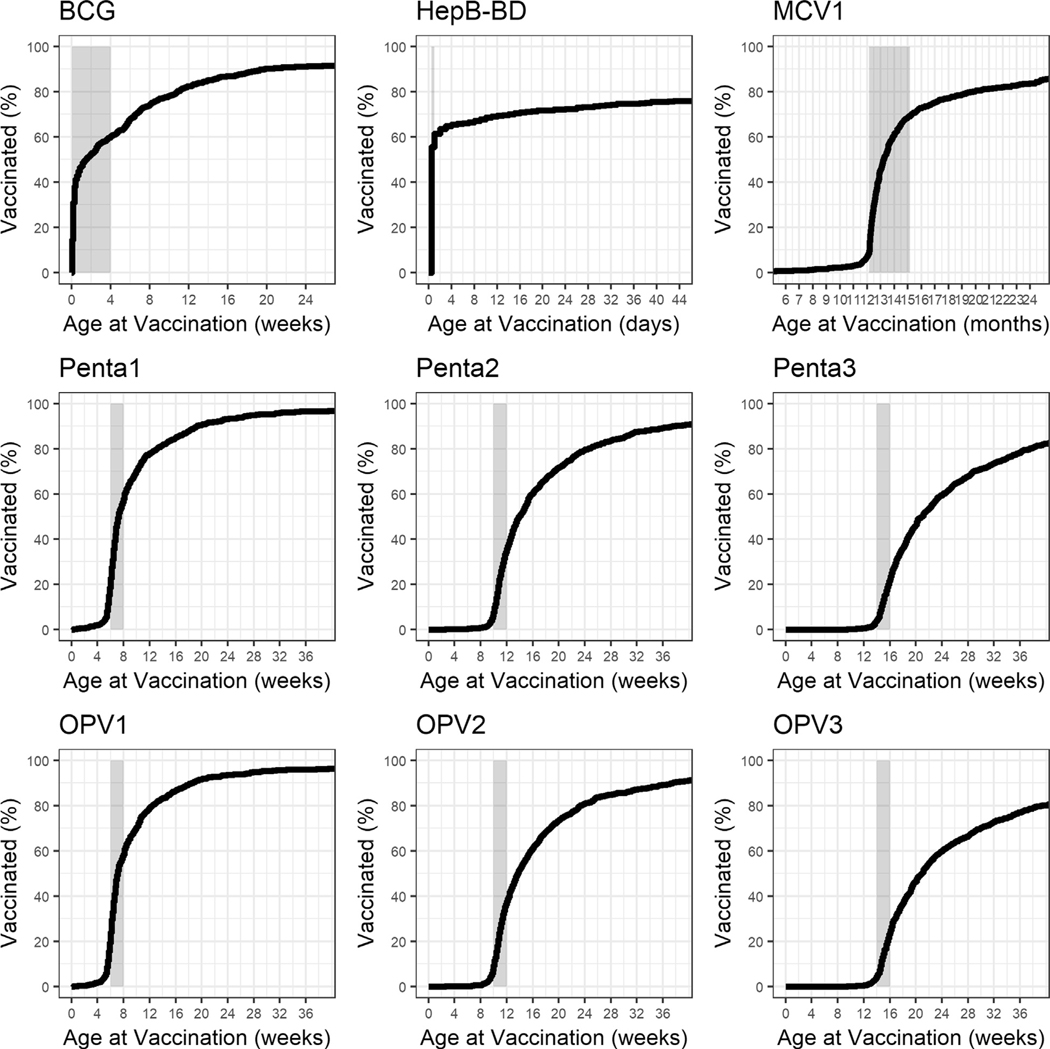
Documented vaccination history was available for 982 (79%) of 1,249 enrolled children with 97% (n = 949/982) from the child’s vaccination book and 3% (n = 33/982) from the local clinic immunization register. Total HepB-BD receipt among enrolled children with vaccination cards was 80% (n = 789/982), with 59% (n = 584/982) of these children receiving a timely HepB-BD (Fig. 1, Table 2). HepB-BD receipt within 7 days of birth was 64% (n = 631/982). Among enrolled children with vaccination cards, receipt of timely HepB-BD did not vary if the child was born during the weekend or working week (p = 0.113). Over 95% of children with vaccination cards had received pentavalent vaccine doses 1–3, with a 4% dropout between doses 1 and 3, and 59% (n = 580/982) had received a timely HepB-BD and at least 2 doses of pentavalent vaccine (Fig. 1, Table 2). Among children with vaccination cards, 93% (n = 912/982) had received a BCG vaccine, over 94% had received OPV doses 1–3, and 90% (n = 881/982) had received MCV1 (Fig. 1, Table 2). Only 3 participants had a recorded TT dose. Receipt of the 2012 MR SIA dose was recorded for 61% of children with a vaccination card (n = 603/982).
Fig. 1.
Receipt and timeliness of routine childhood vaccines among school children aged 6–7 years with vaccination cards—Solomon Islands, 2016. The window for timely vaccination is indicated in the shaded area on each graph. BCG – Bacille Calmette Guerin (tuberculosis vaccine; timely vaccination defined as within 4 weeks of age); HepB-BD – Hepatitis B vaccine birth dose (timely vaccination defined as within 24 hours of birth); OPV – oral polio virus vaccine (timely vaccination defined as within 6–8 weeks for dose 1, within 10–12 weeks for dose 2, and within 14–16 weeks for dose 3); Penta – pentavalent vaccine, which includes diphtheria, tetanus, pertussis, hepatitis B, and H. influenzae type b vaccines (timely vaccination defined as within wihin 6–8 weeks for dose 1, within 10–12 weeks for dose 2, and within 14–16 weeks for dose 3); MCV1 – measles containing vaccine dose 1 (timely vaccination defined as within 12–15 months); % – percent.
Table 2.
Routine vaccination among school children aged 6–7 years with vaccination cards — Solomon Islands, 2016.
| Vaccination history | No. (n = 982) | % | |
|---|---|---|---|
| Received any HepB-BD | Yes | 789 | 80 |
| Age (days) when received HepB-BD | ≤1 day (timely) | 584 | 59 |
| 2–7 days | 47 | 5 | |
| 8–42 days | 158 | 16 | |
| No receipt | 193 | 20 | |
| No. of doses of pentavalent vaccine received | 0 doses | 10 | 1 |
| 1 dose | 10 | 1 | |
| 2 doses | 30 | 3 | |
| 3 doses | 932 | 95 | |
| Received at least 2 doses of pentavalent vaccine and | no HepB-BD | 150 | 15 |
| non-timely | 201 | 20 | |
| HepB-BD timely HepB-BD | 580 | 59 | |
| Received any BCG | Yes | 913 | 93 |
| No. of doses of OPV received | 0 doses | 15 | 1 |
| 1 dose | 11 | 1 | |
| 2 doses | 35 | 4 | |
| 3 doses | 921 | 94 | |
| Received any MCV1 | Yes | 882 | 90 |
No. - number, % - percent, HepB-BD – hepatitis B vaccine birth dose, BCG – Bacille Calmette Guerin (tuberculosis vaccine); OPV – oral polio virus vaccine; MCV1 – measles containing vaccine dose 1. Pentavalent vaccine includes diphtheria, tetanus, pertussis, hepatitis B, and H.influenzae type b vaccines. Timely HepB-BD is defined as administration of the vaccine within 24 h of birth.
Where documented vaccine history was available, pentavalent vaccine doses 1–3 were received on time by 39% (n = 387/982), 30% (n = 293/982), and 19% (n = 184/982) of children, respectively (Fig. 1). The median time of receipt for pentavalent and OPV doses 1–3 was 7 weeks, 14 weeks, and 21 weeks of age, respectively. For pentavalent vaccine dose 1, 18% (n = 181/982) of children received the vaccine before 6 weeks of age (range 2–41 days, with 22% [n = 40] before 5 weeks of age). The median time of receipt for BCG was 10 days of age and 59% (n = 581/982) of children received a timely vaccination; 39% (n = 384/982), 30% (n = 297/982), and 19% (n = 197/982) of children received timely OPV doses 1–3, respectively; median time for receipt of MCV1 was 13 months of age and 60% (n = 59/982) received timely MCV1 dose (Fig. 1). Overall, 42% (n = 415/982) of children with vaccination cards were fully vaccinated by 15 months of age (defined as having received a timely HepB-BD, BCG, 3 doses of pentavalent vaccine and OPV vaccine, and a dose of MCV).
3.3. HBsAg prevalence
HBsAg prevalence among school children aged 6–7 years old in the Solomon Islands was estimated at 3.1% (95% CI: 2.0%–4.9%). HBsAg prevalence did not vary by age (prevalence ratio [PR] 1.1 [95% CI: 0.6%–2.0%]) or by sex (PR 1.3 [95% CI: 0.6%–2.5%]). Of the 40 HBsAg positive children, 22 (55%) were identified in Guadalcanal province (22 HBsAg-positive out of 190 children tested [12%]), with the remaining cases identified in Isabel, Makira, Malaita, and Western provinces. The cases in Guadalcanal were identified in 9 out of the 12 schools enrolled from the province. Vaccination history was available for 31 (78%) of the 40 children. Of 31 HBsAg positive children with vaccination information, 23 (74%) had received any HepB-BD, 15 (48%) received a timely HepB-BD, and 14 (45%) received a timely HepB-BD and at least 2 doses of pentavalent vaccine. HBsAg prevalence was 4% (n = 17/381) among children who had not received a timely HepB-BD and at least 2 doses of pentavalent vaccine compared to 2% (n = 14/562) among those who had.
3.4. Immunoprotection to measles, rubella, tetanus and diphtheria
Antibody data were available for 1,156 (93%) of 1,249 enrolled children. Minimal protective immunity among school children aged 6–7 years old in the Solomon Islands was estimated at 99% (95% CI: 98%–99%) for measles, 99% (95% CI: 97%–100%) for rubella, 85% (95% CI: 83%–87%) for tetanus, and 51% (95% CI: 47%–55%) for diphtheria (Table 3). Seventeen percent (12%–23%) of children had tetanus antibody levels ≥1 IU/mL, the threshold considered to be indicative of long-term immunoprotection. The distribution of diphtheria and tetanus antibody levels among participating children is shown in Table 3. Among children with vaccination cards, 93% (n = 124/134) with tetanus antibody levels below 0.01 IU/mL had documented receipt of 3 doses of pentavalent vaccine compared to 95% (n = 760/797) of children with antibody levels ≥0.01 IU/mL. Similarly, 94% (n = 419/445) of children with diphtheria antibody levels below 0.01 IU/mL had documented receipt of 3 doses of pentavalent vaccine compared to 96% (n = 465/486) of children with antibody levels ≥0.01 IU/mL.
Table 3.
Weighted prevalence of hepatitis B surface antigen (HBsAg) and seroprotection against measles, rubella, diphtheria, and tetanus among children 6–7 years old — Solomon Islands, 2016.
| Hepatitis B | n | % (95% CI) |
|---|---|---|
| HBsAg positive | 40 | 3.1 (2.0–4.9) |
| Seroprotection | n | % (95%CI) |
| Measles (≥53 MFI-bg) | 1143 | 99 (98–99) |
| Rubella (≥10 IU/mL) | 1144 | 99 (97–100) |
| Diphtheria Immunity Range | ||
| Seroprotected (≥0.01 IU/mL) | 599 | 51 (47–55) |
| <0.01 IU/mL | 557 | 49 (45–53) |
| 0.01–0.09 IU/mL | 475 | 40 (36–44) |
| ≥0.1 IU/mL | 124 | 11 (9–13) |
| Tetanus Immunity Range | ||
| Seroprotected (≥0.01 IU/mL) | 987 | 85 (83–87) |
| <0.01 IU/mL | 169 | 15 (13–17) |
| 0.01–0.09 IU/mL | 559 | 48 (43–53) |
| 0.1–0.99 IU/mL | 233 | 20 (18–22) |
| ≥1 IU/mL | 195 | 17 (12–23) |
Wilson 95% confidence limits
4. Discussion
The weighted HBsAg prevalence, indicative of chronic HBV infection burden, among children aged 6–7 years old who were enrolled in school in the Solomon Islands was 3.1% (95% CI: 2.0%–4.9%). This is much lower than previous estimates (13%) among children prior to HepB introduction [23]. However, it also indicates that the Solomon Islands have yet to reach the 2017 regional hepatitis B control goal of <1% HBsAg prevalence among children ≥5 years of age and the 2020 global target of ≤1% HBsAg prevalence among children [6,39]. The high proportion of cases identified in Guadalcanal province is suggestive of potential programmatic issues that may need further investigation.
Among children who had documented vaccination, children who had received a timely HepB-BD and at least 2 doses of pentavalent vaccine had a lower prevalence of HBsAg (2%) compared to those who had not received those doses (4%), highlighting the importance of achieving high coverage with the timely HepB-BD and additional HepB doses to prevent mother-to-child transmission of HBV and subsequent progression to a chronic HBV infection. However, HBsAg prevalence among children who had received a timely HepB-BD and at least 2 doses of pentavalent vaccine was also above the regional control goal of 1%. As HepB is sensitive to cold, any exposures to freezing during transport, such as to frozen cool packs, might have rendered the vaccine less potent [40]. In the Solomon Islands, freeze indicators and sufficient historical temperature monitoring data were not available, so we were unable to review this information. Also, infants born to mothers with high HBV DNA titers at the time of the child’s birth may become infected with HBV despite having received the HepB-BD [41]. In Hubei Province, China, 17% of children born to HBsAg/HBeAg-positive mothers (HBV DNA titers ≥6log10 copies/mL) became infected despite receiving a timely HepB-BD [41]. In the Solomon Islands, there are limited data on maternal HBV DNA viral titers. Since timely HepB-BD coverage is low, the Solomon Islands could prioritize improving and maintaining at least 95% timely birth dose and 95% HepB3 coverage in order to reduce HBsAg prevalence among children, while taking efforts to ensure the quality of the vaccine, such as by using freeze monitors during vaccine transport [5].
Among children with vaccination cards, only 59% had received a timely HepB-BD. Improving timely HepB-BD coverage could be achieved by storing monovalent HepB outside the cold chain (OCC) as was shown in a recent pilot study in the Solomon Islands [42]. Solomon Islands is currently scaling up this successful OCC pilot to improve timely HepB-BD coverage in at least two provinces among health facilities that lack any cold chain capacity and among home deliveries. Other timely HepB-BD improvement options include increasing awareness of healthcare workers, increasing awareness of pregnant women during antenatal visits, improving collaboration between EPI and maternal child health, increasing health facility deliveries and conducting outreach visits to babies born at home [43,44].
Documented coverage with a single dose of measles vaccine was moderately high, but insufficient to provide protective immunity, and timeliness of vaccine receipt was reasonable but could be improved. Rubella vaccine was not part of the routine immunization schedule when the children enrolled in the survey were eligible for the Expanded Program for Immunization. Thus the high measles and rubella immunity found in this cohort must reflect SIA doses (2012 - targeted children aged 12–59 months with 102% coverage; 2014 - targeted 6 months to 30 year olds with 93% coverage) and exposure to natural disease [19,45,46]. Although only 61% of children with vaccination cards had documented receipt of the MR SIA dose, SIA doses may not have been recorded if a child’s vaccination card was not presented at the time. As the routine measles vaccine coverage was lower than 95%, the Solomon Islands could prioritize vaccination activities to increase coverage to 95% for both doses of measles and rubella vaccines to maintain high population immunity against these diseases and to reach the regional elimination goal [9].
Pentavalent coverage was very high, but again timeliness was an issue especially for the third dose. For tetanus, despite the low report of the tetanus booster dose in the vaccination cards, we found that 85% of children had antibody levels above the immunoprotective cutoff threshold of 0.01 IU/mL. Since the estimated duration of protection following 3 doses of tetanus-containing vaccine is around 5 years and tetanus immunity is only induced by vaccination, it is likely that some of the enrolled children received the scheduled TT booster doses at 12–23 months and 4–7 years, although we were unable to confirm this because of a lack of booster dose documentation in the vaccination cards [34]. For diphtheria, around half of the children had protective antibody levels, reflecting protection provided by the 3-dose primary series only and a decline in antibody titers since vaccination during infancy. The Solomon Islands does not currently provide diphtheria booster doses but plans to introduce diphtheria vaccine for 6–7 year-olds as a combined Td dose in 2020, as recommended by WHO [47]. Our data suggest that introducing diphtheria booster doses during childhood and adolescence and improving Td coverage would be helpful to maintain protection against both diseases throughout adolescence and adulthood [14]. Given the low tetanus and diphtheria immunity among the children enrolled in the survey, there is a need for catch-up vaccination with diphtheria and tetanus containing vaccines for children aged >7 years old and adolescents.
This survey might not have been nationally representative, as school enrollment for 6–7-year-olds was approximately 85%–90% and survey participation was relatively low at 78% (n = 1169/1492). We had high nonresponse in Honiara schools, perhaps because these schools had a higher student-to-teacher ratio and fewer teachers available to support the survey. The low participation of students in Honiara may have affected the representativeness of the estimates. Since we conducted the survey at schools, we didn’t have an opportunity to ask the child’s parent about location of birth and thus couldn’t assess whether location of birth impacted HepB-BD receipt or timeless of receipt. Furthermore, the Alere Determine™ rapid test can have lower sensitivity in field settings as it has been shown to be poor at identifying low HBsAg concentrations (<4 IU/mL) and is not able to detect certain HBsAg phenotypes or specific escape mutants [48–50]. This may have resulted in some false negative results in our survey. Enrolled participants had high vaccination card retention (80%), but as we had no information on the vaccination status of those participants that did not provide their vaccination card and thus only present vaccination history among card carrying children, the presented coverage is likely an over-estimate. Additionally, we were unable to determine if there was any potential selection bias introduced as only 10% of non-enrolled eligible participants provided any vaccination history information compared to 79% of enrolled participants. The high proportion of missing vaccination history information (almost 40%) also affected our ability to draw conclusions about the impact of hepatitis B vaccine receipt upon HBsAg prevalence.
5. Conclusions
Timeliness of HepB-BD coverage needs to be improved for the Solomon Islands to reach the 2017 WPR HBV control goal of <1% HBsAg prevalence among children. To reach the regional measles and rubella elimination goals, Solomon Islands would also need to improve measles and rubella vaccination coverage to 95% for both doses. Timeliness of administration of BCG, pentavalent vaccine, and OPV was identified as a weakness and should be reviewed to inform the development of solutions appropriate to the Solomon Islands setting. Diphtheria antibody levels suggest immunity in this age group is low. Therefore, adding diphtheria to the tetanus vaccine booster doses in the routine immunization program could help maintain protection throughout adolescence and adulthood.
Acknowledgements
We would like to thank the schoolteachers from the selected schools for volunteering a considerable amount of their time to support the survey. We also like to thank the provincial EPI officers for supporting and coordinating the teams within their province and the national EPI team for their tireless work; Fiona K. Laeta from the Solomon Islands Ministry of Education for obtaining the student lists, liaising with the selected schools and for supporting data collection; Philip Okia from the Solomon Islands national EPI team for his help with the training and preparatory work; Lana Childs from the Global Immunization Division at US CDC for helping with data entry; and Gavin Grant from the Global Immunization Division at US CDC for support with producing Fig. 1. Jeff Priest (Waterborne Disease Prevention Branch of US CDC) validated and optimized the tetanus, diphtheria, rubella and measles antigens for use in the MBA. Melissa Coughlin of (Viral Vaccine Preventable Diseases Branch US CDC) helped defined the MFI-BG immunoprotection threshold for MV-N.
Funding
This work was supported by the US CDC through a cooperative agreement with the WHO Office for the Western Pacific Region.
Abbreviations:
- HBV
hepatitis B virus
- HBsAg
hepatitis B virus surface antigen
- HepB
hepatitis B vaccine
- HepB-BD
hepatitis B vaccine birth dose
- HepB3
three doses of hepatitis B vaccine
- WHO
World Health Organization
- WUENIC
WHO and UNICEF estimates of national immunization coverage
- EPI
Expanded Program on Immunization
- WPR
Western Pacific Region
- MBA
multiplex bead assay
- US CDC
United States Centers for Disease Control and Prevention
- MHMS
Ministry of Health and Medical Services
- CI
confidence interval
- BCG
Bacille Calmette Guerin
- DTP
diphtheria, tetanus and pertussis vaccines
- Penta
diphtheria, tetanus, pertussis, hepatitis B, and H.influenzae type b vaccines
- OPV
oral polio virus vaccine
- MCV
measles containing vaccine
- DBS
dried blood spot
- MV-N
measles virus nucleoprotein
- SIA
supplemental immunization activity
- BSA
bovine serum albumin
- PBS
phosphate buffered saline
- PBST
phosphate buffered saline tween
- MFI
median fluorescence intensity
- MFI-BG
median fluorescence intensity minus background
- IU
international units
- IQR
interquartile range
- PR
prevalence ratio
- HBeAg
hepatitis B virus envelope antigen
- NT
neonatal tetanus
- MR
measles and rubella vaccine
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Disclaimer
The findings and conclusions in this paper are those of the authors and do not necessarily reflect the position of the US CDC.
Conflicts of interest
None.
References
- [1].Lok AS, McMahon BJ, and Practice Guidelines Committee American Association for the Study of Liver Diseases, Chronic hepatitis B. Hepatology, 2001. 34(6): p. 1225–41. [DOI] [PubMed] [Google Scholar]
- [2].Fattovich G, Bortolotti F, Donato F. Natural history of chronic hepatitis B: special emphasis on disease progression and prognostic factors. J Hepatol 2008;48(2):335–52. [DOI] [PubMed] [Google Scholar]
- [3].World Health Organization, Hepatitis B vaccines: WHO position paper - July 2017. Weekly Epidemiological Record, 2017. 92: p. 369–392. [Google Scholar]
- [4].World Health Organization, Hepatitis B control through vaccination: setting the target. 2015; Available from: https://apps.who.int/iris/bitstream/handle/10665/249167/WPR_RC066_Res1_2015_en.pdf;jsessionid=D32B657D3E45834C3DF52824CBA3DC9D?sequence=1.
- [5].World Health Organization, Regional action plan for viral hepatitis in the Western Pacific 2016–2020. 2016; Available from: http://iris.wpro.who.int/handle/10665.1/13141.
- [6].World Health Organization, Global health sector strategy on viral hepatitis 2016–2021: towards ending viral hepatitis 2016; Available from: http://apps.who.int/iris/bitstream/10665/246177/1/WHO-HIV-2016.06-eng.pdf?ua=1.
- [7].World Health Organization, Guidelines for certification of achievement of hepatitis B control goal in the Western Pacific Region 2007; Available from: http://www.wpro.who.int/immunization/documents/HepBControlCertifGuidelines/en/.
- [8].World Health Organization, Elimination of measles and acceleration of rubella control. 2012; Available from: http://www.wpro.who.int/about/regional_committee/63/resolutions/WPR_RC63_R5_Measles_elimination_03Oct.pdf.
- [9].World Health Organization, Regional strategy and plan of action for measles and rubella elimination in the Western Pacific. 2018; Available from: https://iris.wpro.who.int/bitstream/handle/10665.1/14227/9789290618515-eng.pdf.
- [10].World Health Organization, Measles vaccines: WHO position paper - April 2017. Weekly Epidemiological Record, 2017. 92(17): p. 205–228.28459148 [Google Scholar]
- [11].World Health Organization, Rubella vaccines: WHO position paper. Weekly Epidemiological Record, 2011. 86(29): p. 301–316. [PubMed] [Google Scholar]
- [12].World Health Organization, Protecting all against tetanus: guide to sustaining maternal and neonatal tetanus elimination (MNTE) and broadening tetanus protection for all populations. 2019; Available from: https://www.who.int/immunization/diseases/tetanus/Protecting_All_Against_Tetanus_final_draftV4_23Jan_web.pdf?ua=1.
- [13].World Health Organization, Tetanus vaccines: WHO position paper - February 2017. World Health Organization Weekly Epidemiological Record, 2017. 92 (6): p. 53–7628185446 [Google Scholar]
- [14].World Health Organization, Diphtheria vaccine: WHO position paper – August 2017. Weekly Epidemiological Record, 2017. 92(31): p. 417–436.28776357 [Google Scholar]
- [15].Solomon Islands National Statistics Office, Demographic and health survey 2006–2007. 2009. [Google Scholar]
- [16].Solomon Islands National Statistics Office, Report on 2009 population and housing census: basic tables and census description. 2011. [Google Scholar]
- [17].United Nations Population Division, World population prospects. [Accessed March 21, 2019]; Available from: https://population.un.org/wpp/DataQuery/.
- [18].UNICEF, Maternal and newborn health coverage dataset. 2019. [Accessed: 04/ 27/2020]; Available from: https://data.unicef.org/resources/dataset/deliverycare/.
- [19].World Health Organization, EPI country profile: Solomon Islands, 2014. 2014; Available from: http://www.wpro.who.int/immunization/documents/epi_country_poster_2014_sol.pdf.
- [20].World Health Organization, WHO and UNICEF estimates time series for Solomon Islands (SLB). [Accessed: 27/04/2020]; Available from: http://apps.who.int/immunization_monitoring/globalsummary/estimates?c=SLB
- [21].World Health Organization, Maternal and neonatal tetanus (MNT) elimination: progress towards global MNT elimination; Available from: https://www.who.int/immunization/diseases/MNTE_initiative/en/index4.html.
- [22].World Health Organization, Western Pacific regional plan for hepatitis B control through immunization. 2007; Available from: http://www.wpro.who.int/immunization/documents/docs/POA_HepB.pdf.
- [23].Furusyo N et al. Markedly high seroprevalence of hepatitis B virus infection in comparison to hepatitis C virus and human T lymphotropic virus type-1 infections in selected Solomon Islands populations. Am J Trop Med Hyg 1999;61(1):85–91. [DOI] [PubMed] [Google Scholar]
- [24].Hummel KB et al. Baculovirus expression of the nucleoprotein gene of measles virus and utility of the recombinant protein in diagnostic enzyme immunoassays. J Clin Microbiol 1992;30(11):2874–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Njenga SM et al. Integrated cross-sectional multiplex serosurveillance of IgG antibody responses to parasitic diseases and vaccines in coastal Kenya. Am J Trop Med Hyg 2020;102(1):164–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Cooley GM et al. Evaluation of multiplex-based antibody testing for use in large-scale surveillance for yaws: a comparative study. J Clin Microbiol 2016;54(5):1321–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Priest JW et al. Integration of multiplex bead assays for parasitic diseases into a national, population-based serosurvey of women 15–39 years of age in Cambodia. PLoS Negl Trop Dis 2016;10(5):e0004699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Binnicker MJ, Jespersen DJ, Rollins LO. Evaluation of the Bio-Rad BioPlex measles, mumps, rubella, and varicella-zoster virus IgG multiplex bead immunoassay. Clin Vaccine Immunol 2011;18(9):1524–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Pickering JW et al. Comparison of a multiplex flow cytometric assay with enzyme-linked immunosorbent assay for quantitation of antibodies to tetanus, diphtheria, and Haemophilus influenzae Type b. Clin Diagn Lab Immunol 2002;9(4):872–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Scobie HM et al. Tetanus immunity among women aged 15 to 39 Years in Cambodia: a national population-based serosurvey, 2012. Clin Vaccine Immunol 2016;23(7):546–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].von Hunolstein C et al. Relevance and criticality in an external quality assessment for the determination of diphtheria antitoxin. J Immunol Clin Res 2014;2(2):1022–33. [Google Scholar]
- [32].Won KY et al. Multiplex serologic assessment of schistosomiasis in western Kenya: antibody responses in preschool aged children as a measure of reduced transmission. Am J Trop Med Hyg 2017;96(6):1460–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Kristiansen M, Aggerbeck H, Heron I. Improved ELISA for determination of anti-diphtheria and/or anti-tetanus antitoxin antibodies in sera. APMIS 1997;105(11):843–53. [DOI] [PubMed] [Google Scholar]
- [34].Borrow R, Balmer P, and Roper MH, The immunological basis for immunization series Module 3:Tetanus update 2006. 2007; Available from: http://apps.who.int/iris/bitstream/10665/43687/1/9789241595551_eng.pdf.
- [35].Scheifele DW and Ochnio JJ, The immunological basis for immunization series module 2: Diphtheria update 2009. 2009; Available from: http://apps.who.int/iris/bitstream/10665/44094/1/9789241597869_eng.pdf.
- [36].Skendzel LP. Rubella immunity. Defining the level of protective antibody. Am J Clin Pathol 1996;106(2):170–4. [DOI] [PubMed] [Google Scholar]
- [37].Ondigo BN et al. Impact of mothers’ schistosomiasis status during gestation on children’s IgG antibody responses to routine vaccines 2 years later and anti-schistosome and anti-malarial responses by neonates in western Kenya. Front Immunol 2018;9:1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Bentley M, Christian P, Cohen BJ, and Heath A, Report of a collaborative study to assess the suitability of a replacement for the 2nd international standard for anti-measles serum. 2006; Available from: http://apps.who.int/iris/bitstream/10665/70612/1/WHO_BS_06.2031_eng.pdf.
- [39].World Health Organization, Measles elimination, hepatitis B control and poliomyelitis eradication. 2005. WPR/RC56.R8; Available from: http://www2.wpro.who.int/rcm/en/archives/rc56/rc_resolutions/wpr_rc56_r08.htm. [Google Scholar]
- [40].World Health Organization, Temperature sensitivity of vaccines. 2006; Available from: http://apps.who.int/iris/bitstream/10665/69387/1/WHO_IVB_06.10_eng.pdf. [Google Scholar]
- [41].Zhang L et al. Effects of hepatitis B immunization on prevention of mother-toinfant transmission of hepatitis B virus and on the immune response of infants towards hepatitis B vaccine. Vaccine 2014;32(46):6091–7. [DOI] [PubMed] [Google Scholar]
- [42].Breakwell L et al. Evaluation of storing hepatitis B vaccine outside the cold chain in the Solomon Islands: identifying opportunities and barriers to implementation. Vaccine 2017;35(21):2770–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].World Health Organization, Preventing perinatal hepatitis B virus transmission: a guide for introducing and strengthening hepatitis B birth dose vaccination. 2015; Available from:https://apps.who.int/iris/bitstream/handle/10665/208278/9789241509831_eng.pdf;sequence=1 .
- [44].World Health Organization, Consultation on improving and monitoring hepatitis B birth dose vaccination. 2012; Available from: http://www.wpro.who.int/hepatitis/consultation_hepb_birthdose_june2012_mtgrpt.pdf.
- [45].World Health Organization, Measles outbreak, Solomon Islands. Health Situation Report; 2014; Available from: http://reliefweb.int/report/solomon-islands/measles-outbreak-solomon-islands-healthsituation-report-no-7. [Google Scholar]
- [46].World Health Organization, Solomon Islands country profile - measles elimination. 2014; Available from: http://www.wpro.who.int/immunization/documents/measles_country_profile_nov2014_sol.pdf.
- [47].World Health Organization, Replacement of TT with Td vaccine for dual protection. 2018; Available from: https://www.who.int/immunization/diseases/tetanus/WHO_UNICEF_Joint_communique_on_TT_to_Td_Replacement_Final28June2018.pdf?ua=1.
- [48].World Health Organization, Hepatitis B surface antigen assays: operational characteristics (phase 1). 2001; Available from: http://www.who.int/diagnostics_laboratory/evaluations/en/hep_B_rep1.pdf.
- [49].Bottero J et al. Performance of rapid tests for detection of HBsAg and anti-HBsAb in a large cohort. France. J Hepatol 2013;58(3):473–87. [DOI] [PubMed] [Google Scholar]
- [50].Lin YH et al. Evaluation of a new hepatitis B virus surface antigen rapid test with improved sensitivity. J Clin Microbiol 2008;46(10):3319–24. [DOI] [PMC free article] [PubMed] [Google Scholar]