Abstract
Background:
The high incidence of venous thromboembolism (VTE) following trauma persists in spite of aggressive thromboprophylaxis strategies. Approximately half of VTE patients do not achieve the recommended anti-FXa response to enoxaparin anticoagulation (0.1–0.4 IU/mL), however, research to explain or correct this phenomenon is lacking. We hypothesized that antithrombin III (AT) deficiency is associated with poor enoxaparin responsiveness in trauma patients that develop VTE which can be reversed through supplementation with AT.
Methods and Findings:
A retrospective cohort study was performed on plasma collected from trauma patients who did and did not develop pulmonary embolism (PE) as well as healthy volunteers. AT levels, thrombin generation, and anti-FXa levels were measured in the collected plasma at baseline and in response to supplementation with AT concentrate at 120–200% or plasma (30% volume). A total of 54 PE patients and 46 non-PE patients were enrolled in this study for analysis. Compared to healthy volunteers, trauma patients had lower levels of AT, elevated thrombin generation, and lower anti-FXa levels in response to enoxaparin. Moreover, thrombin generation was higher and responses to enoxaparin were lower in patients who developed PE compared to those who did not develop PE. We found that supplementation with AT, but not plasma, increased AT levels and improved enoxaparin-mediated inhibition of thrombin generation.
Conclusions:
Supplementation with AT may provide a novel adjunct therapy to increase the effectiveness of enoxaparin thromboprophylaxis and reduce the incidence of VTE in the trauma population.
Keywords: trauma, thrombosis, antithrombin, thromboprophylaxis
Introduction
Venous thromboembolism (VTE), the combination of deep vein thrombosis (DVT) and pulmonary embolism (PE), is a prevalent complication in patients with polytraumatic injuries. VTE remains one of the most common preventable causes of in-hospital death in this population, despite extensive prophylactic efforts to mitigate risk [1, 2]. Up to 30% of patients on prophylaxis will experience a VTE while in the hospital, depending largely on patient acuity and degree of surveillance. These events result in aggressive interventions, prolonged hospital stays, and increased medical care costs [2, 3]. Three months after discharge, the rate of VTE in surviving trauma patients remains over 10% [4] and 30% of thromboembolic events in the trauma population overall occur after discharge [5]. These are not clinically insignificant events. In fact, Drake et al. recently showed that 10.8% of preventable or potentially preventable deaths after hospital discharge are due to massive PE [6].
While the exact pathophysiology of VTE in trauma patients is not fully understood, it is most certainly multifactorial and thought to arise secondarily to a combination of risk factors including aberrant coagulation activation, endothelial dysfunction, sustained inflammation, protracted immobility, massive transfusion, and mechanical ventilation [2]. Elevation in thrombin generation following trauma, a strong predictor of VTE in trauma patients [7], has been reported in several studies [7–11]. Thus, after hemorrhage control is achieved, restoring hemostatic homeostasis by limiting thrombin generation becomes critically important for preventing thromboembolic complications in recovering trauma patients.
To address this, the American Academy of Chest Physicians instituted standard of care recommendations for aggressive VTE prophylaxis in trauma patients which includes protocolized anticoagulation [12]. One of the most commonly used prophylactic anticoagulants utilized is enoxaparin (Lovenox), a low molecular weight heparin that can be monitored in-hospital by measuring anti-FXa levels. Enoxaparin works by potentiating the activity of the circulating anticoagulant, antithrombin III (AT). Enoxaparin binds to AT and increases its activity for inhibiting FXa and thrombin, thus downregulating coagulation. Despite increasingly aggressive institutional thromboprophylaxis protocols, 50–70% of trauma patients do not achieve the recommended anti-FXa target range (0.1–0.4 IU/mL) which indicates an altered responsiveness to enoxaparin anticoagulation [13, 14]. Such irresponsiveness has been associated with the risk of VTE. For example, Malinoski et al. showed an increased incidence of DVT among trauma and surgical patients who did not achieve the recommended anti-FXa range [15]. Even escalating doses of heparinoids appear ineffective since the incidence of VTE remains unchanged when enoxaparin doses are adjusted in response to low anti-FXa levels [16]. Sabbagh et al. first introduced the use of fresh frozen plasma (FFP) for the reversal of heparin resistancein the mid 1980’s in patients undergoing cardiopulmonary bypass [17]. However, a recent study of patients undergoing coronary artery bypass graft surgery showed that while administration of FFP had no effect on heparin sensitivity, supplementation with AT significantly improved the response to heparin by reducing the overall heparin requirements with no increased risk of bleeding [18]. Furthermore, data from septic shock patients with acquired AT deficiency demonstrated that AT supplementation safely and effectively improved anticoagulation [19]. These important findings suggest that adjunct AT may provide a novel treatment strategy for safely improving heparin-based thromboprophylaxis [18].
Acquired AT deficiency (AT <80%) is common in trauma patients, affecting approximately 20% on admission [3]. Multiple studies have shown that reduced AT activity in severely injured patients is associated with an increased risk for thrombotic complications [3, 20]. This brings into question the use of AT-dependent thromboprophylaxis without monitoring AT levels [20] and suggests that utilization of AT supplementation could be a potential strategy for reducing the risk of VTE [21]. The aim of this study was to determine if AT deficiency plays a role in altered responses to enoxaparin anticoagulation in critically ill trauma patients. Our hypothesis was that PE patients would have a higher incidence of AT deficiency and reduced responsiveness to enoxaparin. We further hypothesized that the response to enoxaparin in PE patient plasma could be improved by ex vivo supplementation with AT.
Methods
Human Subjects
This study was a single institution, retrospective cohort analysis of prospectively collected data conducted at Memorial Hermann Hospital in Houston, TX, a Level-1 trauma center, and the University of Texas Health Science Center at Houston. Prior approval from the Institutional Review Board was obtained (HSC-GEN-12–0059) for the study to include adult patients (≥16 years of age) admitted between October 2012 and October 2016 who met the criteria for highest-level trauma team activation and for whom consent was obtained within 72 hours of admission from either the patient or a legally authorized representative. A waiver of consent was obtained if a patient was discharged or died within 24 hours. Patients were excluded if they were <16 years of age, pregnant, prisoners, had greater than 20% total body surface area burned, were taking pre-hospital anticoagulants, died within 24 hours, or consent was not obtained. Prospective power analysis estimated that 50 patients per group (with versus without thromboembolic events) would be sufficient to detect a 20% difference in thrombin generation using an alpha of 0.05 and a power of 0.90%.
Patient demographics, vital signs, standard laboratory values, mechanism and severity of injury, and pre-hospital fluid and/or blood product administration were collected upon admission to the emergency department. Outcomes (PE, hospital-, ventilator-, intensive care unit (ICU)-free days, mortality), and 24-hour blood product and fluid administration were collected from patient records. A matched-group design was used to compare patients with and without PEs based on age, gender, injury mechanism and injury severity. After all PE patients were identified, non-PE patients that were admitted during this time frame with similar age, gender, and injury mechanisms and severity to the PE group were selected. Mann-Whitney tests were performed to compare these characteristics between the PE and non-PE groups. If differences approaching p=0.06 were identified, new non-PE patients were selected and the comparison was re-run until the groups were appropriately matched.
PE Categorization
Since our institution routinely monitors for PE symptoms, without screening for DVT, VTE was defined as the development of a PE in this study. If patients present with even mild symptoms related to PE, such as cough or shortness of breath, or asymptomatic pulmonary emboli are identified incidentally during the course of examination for other indications, a standard contrast-enhanced computerized tomography angiogram (CTA) of the chest was performed to establish a diagnosis. All diagnoses of PE were established clinically using radiological criteria, and CTA derived chest images were reviewed by staff radiologists for contrast filling defects in the pulmonary vasculature. A PE diagnosis was then categorized based on location of the filling defect as either central (involvement of the left and/or right main pulmonary artery or one or more lobar arteries), or segmental/sub-segmental (involvement of segmental and/or sub-segmental arteries).
Sample Collection
Patient samples were collected upon hospital admission, prior to clinical administration of enoxaparin. In conjunction with blood collection for standard hospital laboratory tests, an additional 20 mL of blood was obtained for research purposes. Blood was transferred into vacutainer tubes containing 3.2% citrate and after 30 minutes was centrifuged at 3,200 rpm for 20 minutes at room temperature to obtain platelet poor plasma. Plasma samples were then aliquoted and stored at −80° C until analysis. Plasma was also collected from 15 healthy and consented volunteers under a separate protocol (HSC-MS-09–0314). Volunteers were excluded if they were on an anticoagulant or antiplatelet drug, were pregnant, or had a history of cardiovascular disease. Healthy subjects had a median age of 31 (30, 35), were 50% male, and were 63% Caucasian. Fresh frozen plasma (FFP) from five donors was purchased from Gulf Coast Regional Blood Center. A pool of the five donors was used for all ex vivo experimentation.
Plasma Analysis
Citrated plasma samples were thawed immediately prior to use. First, plasma from healthy, uninjured volunteers was used to optimize ex vivo dosing of enoxaparin. The ex vivo dose of enoxaparin needed to achieve 50% inhibition of thrombin generation was found to be 0.13 IU/mL, similar to that previously identified in the literature [22–24]. Given that we spiked plasma with an enoxaparin dose of 0.13 IU/mL, our target anti-FXa level in response to enoxaparin was defined as 0.13 IU/mL. The responses to enoxaparin between healthy uninjured volunteers and trauma patients were compared by treating all plasma samples ex vivo with 0.13 IU/mL of enoxaparin. Trauma patient samples were additionally supplemented with AT using either FFP at a dose of 30% volume or AT concentrate in the form of THROMBATE III®, provided by Grifols, up to 80%, 120%, 150%, 180%, and 200% final concentration based on baseline values. This was done in an effort to improve patient plasma responses to enoxaparin to the levels observed in healthy controls. Similar to our previous work, we chose FFP at a dose of 30% volume to model the effects of transfusing six units of plasma [25]. Calculations were based on the activity (IUs) provided by the manufacturer for a given lot of THROMBATE III® (approximately 500 IUs/10 mL) and the patient’s baseline AT activity levels. AT supplementation was confirmed by measuring AT activity using ACL-TOP. Thrombin generation and anti-FXa levels were measured to assess the response to enoxaparin and supplementation with AT. Healthy volunteer plasma served as an uninjured control for normal AT, anti-FXa, and thrombin generation levels before and after treatment with enoxaparin.
AT activity and anti-FXa levels were determined chromogenically using an ACL-TOP Coagulation Analyzer (Instrumentation Laboratory, Bedford MA). Thrombin generation was measured using a calibrated automated thrombogram (CAT) (Thermo Fisher Scientific, Waltham MA) as previously described [8]. Relevant parameters resulting from CAT analysis include lag time (the time to initiation of thrombin generation; min), peak (the highest amount of thrombin generated; nM), time to peak [ttpeak] (the time it takes to achieve the peak; min), endogenous thrombin potential [ETP] (the total amount of thrombin generated over time; nM), and rate (the velocity of thrombin generation dictated by the slope of the thrombin generation curve; nM/min). Supplemental Figure 1 depicts a representative curve demonstrating these parameters. Both CAT and ACL-TOP assays were conducted simultaneously to avoid further freezing and thawing samples. AT deficiency was defined as AT <80%.
Statistics
All analyses were performed using GraphPad Prism 6 (La Jolla, CA). For multiple comparisons, one-way or two-way ANOVA tests were done with Bonferroni or Sidak corrections, respectively. Mann-Whitney tests were performed where indicated. Data were considered significant at p<0.05.
Results
A consort diagram describing enrollment of the patient cohort is provided in Figure 1. During the study period, 6,089 highest-level trauma activations were received at our institution. Of those, 3,797 met the criteria for inclusion in our study. Plasma samples were available in our biorepository for 2,613 of these patients. A total of 55 patients with PE were identified during the study period. Of these patients, three were excluded for pre-hospital anticoagulant use and seven were excluded due to death from non-survivable traumatic brain injury. This left 100 patients for complete analysis (46 without PE and 54 with PE). The median time to PE diagnosis was hospital day 9 (4, 14). Among those patients who developed a PE, 68.5% were symptomatic and 31.5% were asymptomatic. Most PEs were located in segmental or sub-segmental arteries (67%), with the remainder located centrally (33%). Approximately 85% of PE patients received thrombosis-related interventions that included thrombectomy, tPA infusion, inferior vena cava (IVC) filter placement, unfractionated heparin drip, or another anticoagulant. Only 8 patients (15%) were maintained on enoxaparin alone. Interventions by PE location are provided in Supplemental Table 1. Patient demographics, injury types/severity, and outcomes are summarized in Table 1. There were no apparent differences in demographics or injury type and severity between patients who did versus those who did not develop a PE. However, those who developed a PE received significantly greater volumes of prehospital crystalloids and units of blood products during transport and over the first 24 hours of their hospital stay (all p<0.05). Furthermore, patients who developed a PE had significantly fewer ventilator-, ICU-, and hospital-free days (all p<0.01), although the overall in-hospital mortality rates were the same (8.5% vs 9.3%; p>0.05). Finally, while no differences in the rate of pelvic fractures were observed, patients who developed PE had a significant increase in the rate of lower limb fracture (28% vs 11%; p<0.05).
Figure 1.
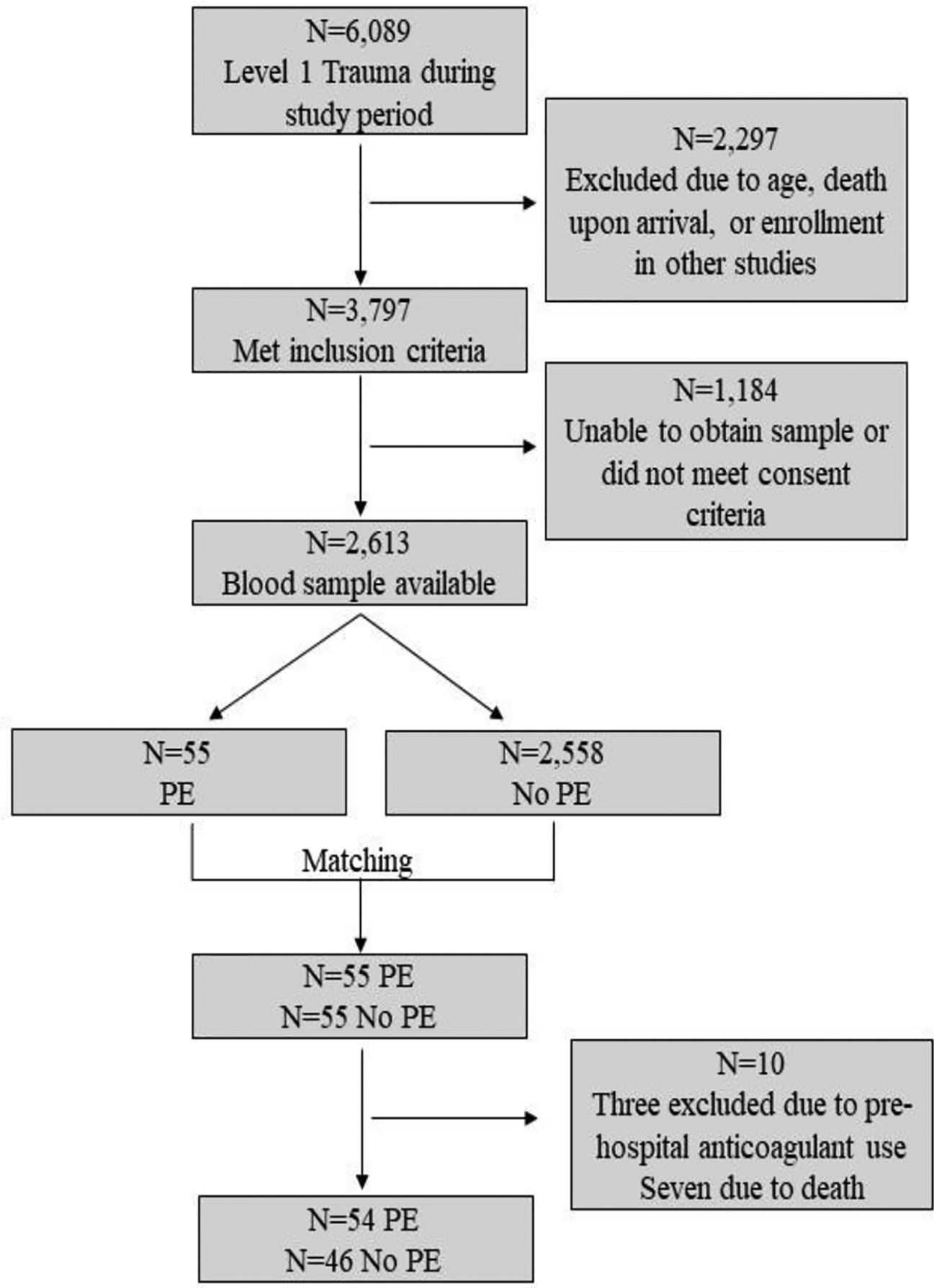
Flow of admitted and enrolled patients. PE = pulmonary embolism.
Table 1.
Patient demographics, injury, resuscitation volumes and outcomes in patients with and without pulmonary embolism (PE). Median and interquartile range (IQR) values are reported. Mann-Whitney tests were performed to determine statistical significance between groups. However, the presence of such fractures did not affect the pertinent outcomes of this study such as AT levels, rate of AT deficiency, thrombin generation, or responsiveness to enoxaparin, with or without AT supplementation. (Supplemental Figure 2 and Supplemental Table 2).
| P-value | ||||
|---|---|---|---|---|
| Demographics | ||||
| Age, years | 46 (33, 54) | 47 (35, 55) | 43 (28, 53) | 0.19 |
| Male, n (%) | 79 (79%) | 42 (78%) | 37 (80%) | 0.75 |
| Injury | ||||
| Blunt, n (%) | 83 (83%) | 47 (87%) | 36 (78%) | 0.24 |
| GCS | 13 (3, 15) | 14 (3, 15) | 8 (3, 15) | 0.24 |
| w-RTS | 6.9 (4.1, 7.8) | 7.1 (4.1, 7.8) | 6.0 (4.1, 7.8) | 0.57 |
| Head AIS | 2 (0, 4) | 0 (0, 3) | 2 (0, 4) | 0.17 |
| ISS | 17 (13, 26) | 19 (13, 26) | 17 (12, 25) | 0.38 |
| Pelvic Fracture, n (%) | 14 (14%) | 8 (15%) | 6 (13%) | 0.80 |
| Lower Limb Fracture, n (%) | 20 (20%) | 15 (28%)* | 5 (11%) | <0.05 |
| Base Excess | −3 (−6, 0) | −3 (−6, 0) | −3 (−6, 0) | 0.98 |
| Resuscitation Volumes | ||||
| Pre-hospital Crystalloid, mL | 0 (0, 220) | 200 (0, 500)* | 0 (0, 0) | <0.01 |
| Pre-hospital RBC, units | 0 (0, 0) | 0 (0, 0)* | 0 (0, 0) | 0.04 |
| Pre-hospital Plasma, units | 0 (0, 0) | 0 (0, 0)* | 0 (0, 0) | <0.01 |
| 24 Hour RBC, units | 0 (0, 2) | 1 (0, 6)* | 0 (0, 0) | <0.01 |
| 24 Hour Plasma, units | 0 (0, 3) | 1 (0, 6)* | 0 (0, 1) | <0.01 |
| 24 Hour Platelets, units | 0 (0, 0) | 0 (0, 6)* | 0 (0, 0) | <0.01 |
| Outcomes | ||||
| Ventilator-free days | 27 (19, 29) | 19 (15, 27)* | 29 (26, 30) | <0.01 |
| ICU-free days | 24 (8, 28) | 17 (1, 25)* | 27 (21, 29) | <0.01 |
| Hospital-free days | 12 (0, 20) | 0 (0, 12)* | 20 (15, 25) | <0.01 |
| 30-Day Mortality, n (%) | 8 (8%) | 5 (9.3%) | 3 (6.5%) | 0.62 |
Effects of enoxaparin on healthy versus trauma patient plasma.
Similar to previous reports (7), baseline thrombin generation was significantly elevated in trauma patients compared to healthy donors as evidenced by shortened lag times, increased peak, reduced time to peak, and increased rates of thrombin generation (Table 2). Trauma patients also displayed significantly lower anti-FXa levels after enoxaparin treatment compared to healthy controls. In response to enoxaparin treatment, all healthy donors achieved a target anti-FXa response of 0.13 IU/mL compared to 59% of trauma patients (p<0.01). Compared to healthy controls, trauma patients demonstrated significantly reduced inhibition of thrombin generation following treatment with enoxaparin as evidenced by reduced peak thrombin inhibition, reduced endogenous thrombin potential (ETP) inhibition, and reduced prolongation of the rate of thrombin production.
Table 2.
Antithrombin III (AT) levels, anti-FXa levels, and thrombin generation values in plasma from healthy donors and trauma patients at baseline and following treatment with enoxaparin (0.13 U/mL). Number with percentage or median and IQR values are reported. Mann-Whitney tests were performed to determine statistical significance between groups.
| P-value | |||
|---|---|---|---|
| Baseline | |||
| Rate AT<80% | 0 | 20 (20%) | |
| AT (%) | 114 (109, 118) | 91 (78, 103)* | <0.01 |
| Lag time (min) | 5.0 (4.3, 5.7) | 4.3 (3.7, 5.0)* | <0.05 |
| Peak (nM) | 258 (153, 304) | 302 (254, 345)* | <0.01 |
| ttPeak (min) | 8.3 (7.0, 9.7) | 6.6 (6.0, 7.4)* | <0.01 |
| ETP (nM) | 1459 (1155, 1649) | 1492 (1237, 1713) | 0.38 |
| Rate (nM/min) | 67.8 (37.2, 107.7) | 129 (106, 156)* | <0.01 |
| Enoxaparin | |||
| Anti FXa levels (IU/mL) | 0.16 (0.14, 0.17) | 0.13 (0.12, 0.16)* | <0.01 |
| Rate Anti FXa>0.13 | 15 (100%) | 59 (59%)* | <0.01 |
| Delta Lagtime (%) | 6.6 (−4.3, 23) | 7.9 (0, 11) | 0.90 |
| Delta Peak (%) | −55 (−66, −36) | −24 (−32, −16)* | <0.01 |
| Delta ttPeak (%) | 17 (3.5, 36) | 12 (7.3, 17) | 0.20 |
| Delta ETP (%) | −43 (−48, −22) | −14 (−20, −9)* | <0.01 |
| Delta Rate (%) | −65 (−82, −57) | −39 (−51, −27)* | <0.01 |
Supplementation of enoxaparin with FFP or AT.
To determine if supplementation with either FFP or AT would increase AT levels and improve the response to enoxaparin, trauma patient plasma was treated ex vivo with enoxaparin (0.13 IU/mL) in addition to either FFP (30% volume) or AT (final concentration of 120–200%) (Figure 2). FFP had a baseline AT level of 98 (96, 102). While treatment with FFP had no effect on AT levels, AT supplementation significantly increased baseline AT levels (limit of detection = 180%). Treatment with FFP did not improve anti-FXa levels from enoxaparin alone; however, AT-supplemented enoxaparin increased anti-FXa levels in a dose dependent manner (Figure 3A: all doses p<0.05). FFP treatment improved the rate of therapeutic response from 59% to 80%; whereas, supplementation with AT to 120% resulted in 92% of trauma patient plasma reaching a therapeutic anti-FXa level. This increased to 100% after AT supplementation was raised to 150% or greater. In addition, while determining whether FFP or AT supplementation could improve the ability of enoxaparin to inhibit thrombin generation, we found that both FFP and AT were able to significantly increase peak thrombin inhibition compared to enoxaparin alone (all p<0.05). The greatest inhibition was observed at AT levels of 150–200% (Figure 3B).
Figure 2: Antithrombin (AT) levels in trauma patient plasma at baseline and following treatment with fresh frozen plasma (FFP) or AT concentrate.
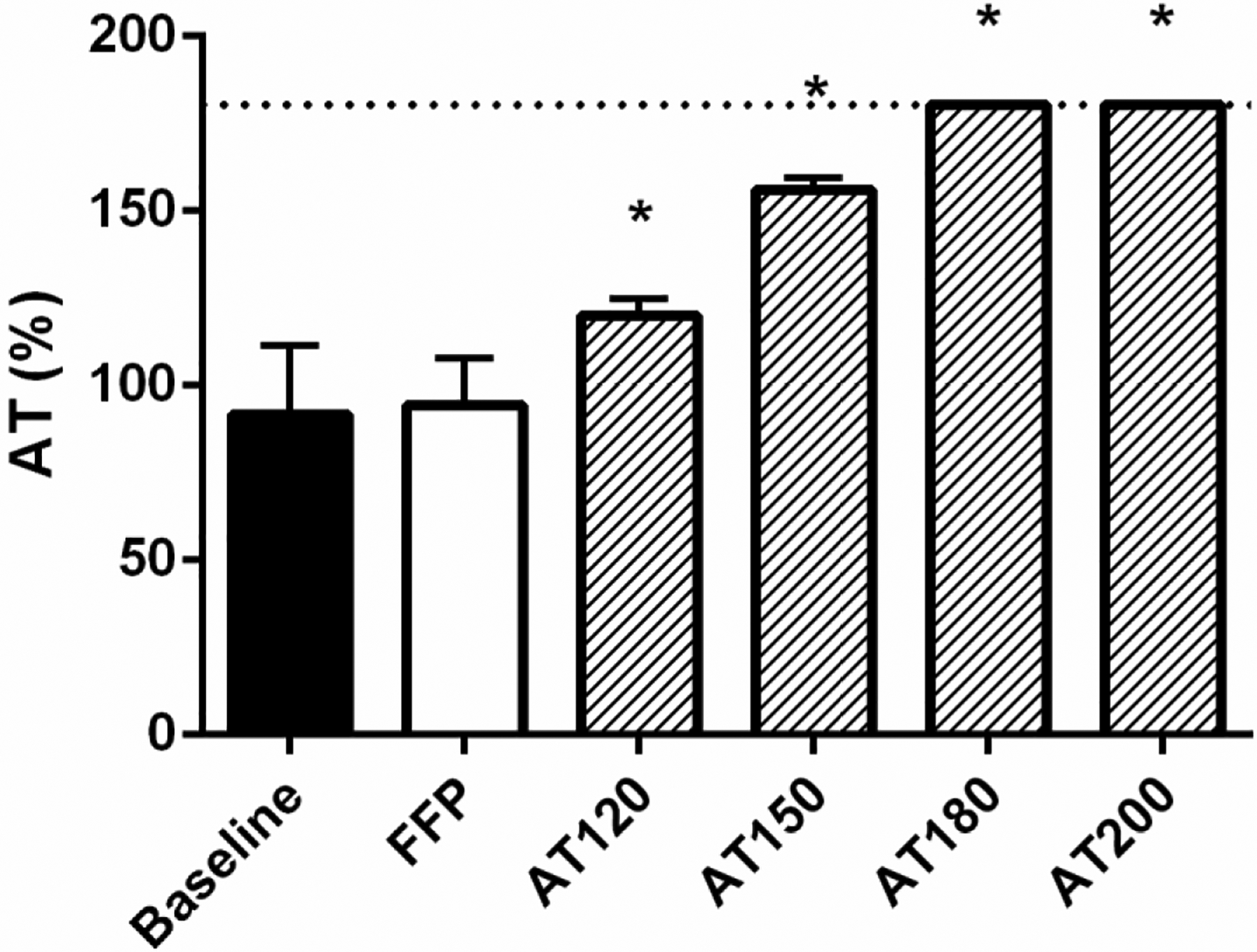
AT is presented as percent activity. FFP treatment was 30% by volume. AT was supplemented to 120, 150, 180, or 200% final concentration. Data are presented as means with standard deviation. The dotted line represents the limits of detection. * denotes p<0.05 compared to baseline following one-way ANOVA analysis with Bonferroni correction.
Figure 3. Levels of Anti-FXa and thrombin following enoxaparin treatment.
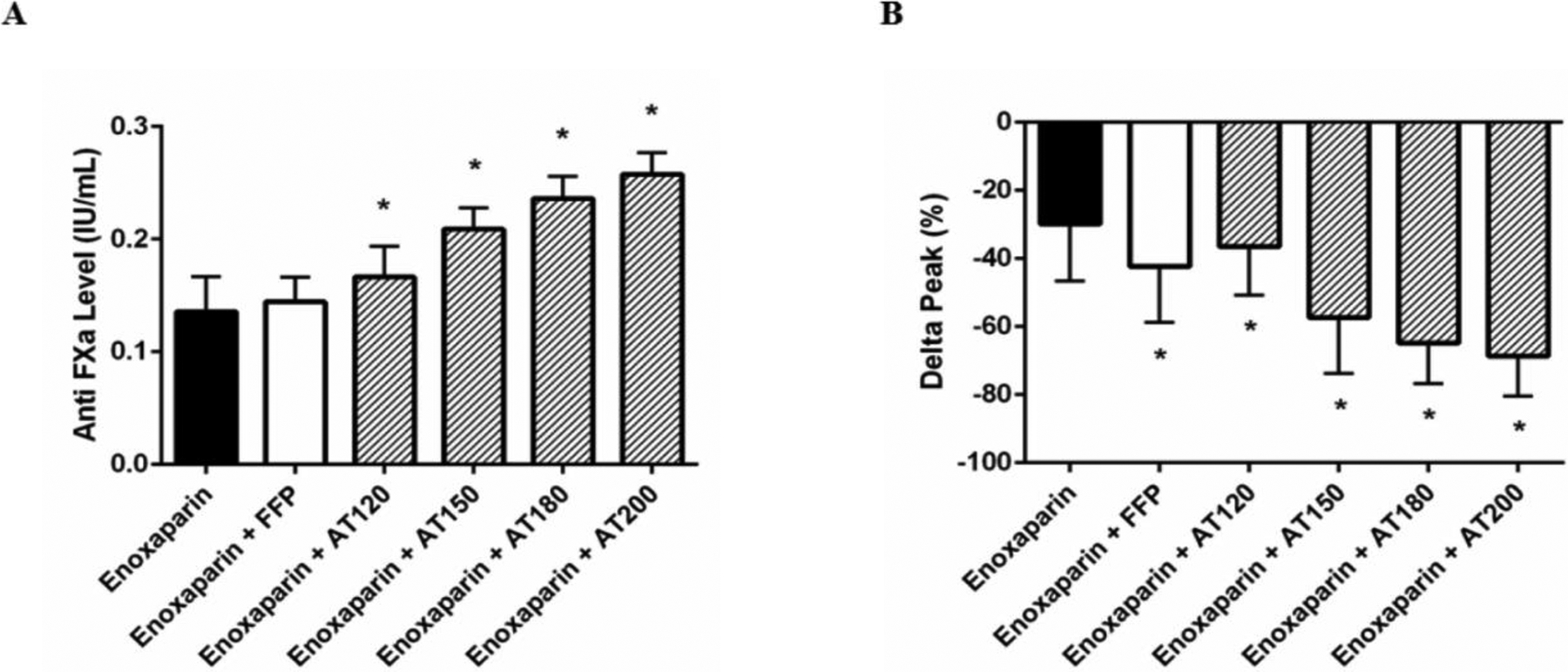
Anti-FXa levels (A) and changes in peak thrombin (B) were measured in trauma patients following treatment of their plasma with enoxaparin in the presence or absence of FFP or AT supplementation. Delta peak represents the percent change of thrombin in treated plasma compared to untreated plasma at baseline. Data are presented as means with standard deviation. * denotes p<0.05 compared to enoxaparin alone following one-way ANOVA analysis with Bonferroni correction.
Effects of enoxaparin in plasma from patients with and without PE.
Baseline AT activity was similar between groups with no difference in the incidence of AT deficiency (Table 3). However, when comparing thrombin generation, patients who developed PE had significantly higher peaks (13%) and faster rates (24%) of thrombin generation at baseline compared to those without a PE. Moreover, patients who developed a PE had a significantly reduced response to enoxaparin ex vivo as measured by both anti-FXa levels and changes in thrombin generation. Anti-FXa levels in response to enoxaparin were significantly higher in plasma from patients who did not develop PE compared to those who did (0.14 vs 0.13 IU/mL; p<0.05). Ex vivo treatment with enoxaparin resulted in a median 30.6% reduction in peak thrombin in patients who did not develop PE; however, only a 20.7% reduction in peak thrombin was detected in those who did develop PE (p<0.01).
Table 3.
AT levels and thrombin generation values in plasma from patients with and without PE at baseline and following treatment with enoxaparin (0.13 U/mL). Number with percentage or median and IQR values are reported. Mann-Whitney tests were performed to determine statistical significance between groups.
| P-value | |||
|---|---|---|---|
| Baseline | |||
| Rate AT<80% | 11 (20.4%) | 9 (19.6%) | 0.92 |
| AT (%) | 90 (77, 99) | 93 (78, 113) | 0.24 |
| Lag time (min) | 4.3 (3.8, 4.8) | 4.2 (3.7, 5.5) | 0.79 |
| Peak (nM) | 322 (272, 351)* | 286 (241, 335) | <0.05 |
| ttPeak (min) | 6.6 (5.8, 7.2) | 6.6 (6.0, 7.8) | 0.58 |
| ETP (nM) | 1524 (1313, 1728) | 1489 (1123, 1689) | 0.12 |
| Rate (nM/min) | 145 (112, 161)* | 118 (94, 150) | <0.05 |
| Enoxaparin | |||
| Anti FXa levels (IU/mL) | 0.13 (0.11, 0.14)* | 0.14 (0.12, 0.17) | <0.05 |
| Rate Anti FXa>0.13 | 24 (44%) | 26 (57%) | 0.23 |
| Delta Lagtime (%) | 7.9 (0, 10.6) | 6.6 (0, 13.1) | 0.74 |
| Delta Peak (%) | −20.7 (−29.7, −15.3)* | −30.6 (−37.8, −18.4) | <0.01 |
| Delta ttPeak (%) | 10.7 (6.4, 15.0)* | 13.0 (9.6, 20.4) | <0.05 |
| Delta ETP (%) | −13.2 (−18.5, −8.8) | −15.6 (−23.3, −11.3) | 0.10 |
| Delta Rate (%) | −34.2 (−43.7, −26.1 * | −45.4 (−55.3, −29.9) | <0.01 |
Supplementation of enoxaparin with AT in PE patient plasma.
In order to determine the effects of supplementation with AT on the ex vivo response to enoxaparin, plasma from both PE and no PE patients was treated with enoxaparin and AT and changes in anti-FXa and thrombin inhibition were observed. AT significantly increased anti-FXa levels and reduced peak thrombin generation at all doses in plasma from all patients tested. PE patient plasma demonstrated lower anti-FXa levels (Figure 4A) and reduced peak thrombin inhibition (Figure 4B) compared to No PE patients with enoxaparin alone; however, these differences in enoxaparin responses were no longer apparent when AT supplementation was 120% or above.
Figure 4. Levels of Anti-FXa and thrombin following enoxaparin treatment of PE vs no PE patients.
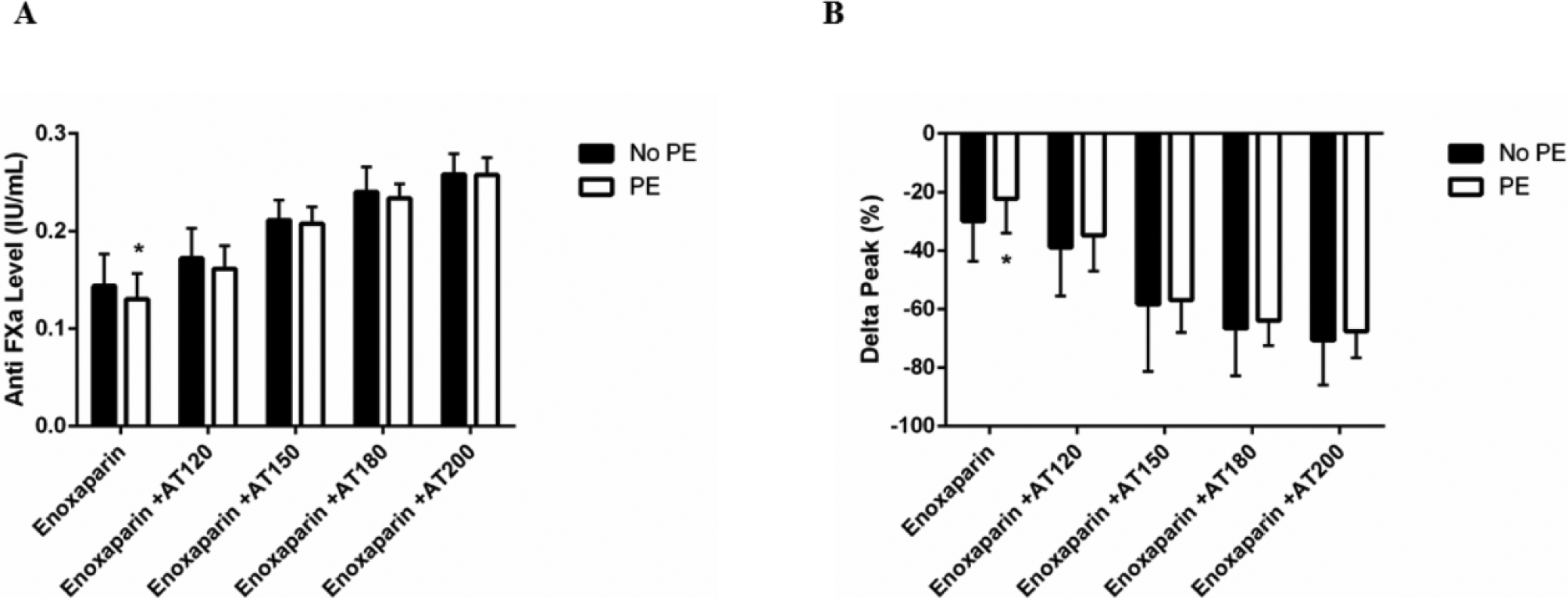
Anti-FXa levels (A) and changes in peak thrombin (B) were measured in plasma from patients who did (white bar) or did not develop a PE (black bar) following treatment with enoxaparin in the presence or absence of FFP or AT supplementation. Data are presented as means with standard deviation. * denotes p<0.05 comparing “PE” and “No PE” patients after two-way ANOVA analysis with Sidak correction.
Discussion
Persistent rates of VTE in the trauma patient population, in spite of aggressive, protocolized thromboprophylaxis, are a significant healthcare concern that necessitates new and evidence-based treatment strategies. AT concentrate is an FDA-approved, clinically available therapeutic used to treat congenital AT deficiency. To date, there is little data to show the potential role of AT supplementation in improving VTE prophylaxis in trauma patients. Here, we demonstrate that plasma samples from severely injured patients, particularly from those who develop PE, exhibit reduced sensitivity to enoxaparin and that supplementation with AT improves enoxaparin-mediated thrombin inhibition ex vivo.
Enoxaparin and other low molecular weight heparin molecules are the most commonly utilized prophylactic agents in critical care patients during recovery. Enoxaparin increases both anti-FXa levels and AT inhibition of thrombin, making it a vital co-factor in preventing VTE. AT deficiency is commonly defined by hematologic standards as <80% activity with normal levels ranging between 80–120%. According to this definition, admission AT deficiency occurred in approximately 20% of trauma patients enrolled in this study which is similar to the rates reported by others [3]. While the majority of patients had AT levels within normal limits, only half of all patient plasma samples achieved a target anti-FXa level of 0.13 IU/mL following treatment with enoxaparin.
We have previously shown that, compared to healthy subjects, trauma patients demonstrate profoundly elevated thrombin generation [8], a known risk factor for VTE [7]. Indeed, marked elevations were detected in this study as well. It is possible that in the setting of such excessive and protracted thrombin generation, circulating levels of AT are not adequate to facilitate prophylactic anticoagulation with enoxaparin. According to our findings, it appears that supraphysiologic levels of ATIII may be necessary to compete with such high levels of thrombin generation. Here, we show that supplementation of trauma patient plasma with AT up to 120% raised enoxaparin effectiveness from 59% to 92% and that increasing AT above 120% optimized enoxaparin responses in terms of both anti-FXa levels and inhibition of thrombin generation. A 100% maximum effectiveness was attained when AT levels were raised to 150%. These findings are in agreement with other reports in the literature. Animal models of sepsis have shown that high doses of AT (~160%) were most beneficial at mitigating hypercoagulability, reducing inflammation, and protecting from organ damage [26]. While maintaining ATIII activity at such high levels may not be feasible for all trauma patients, it may be appropriate to screen patients for potential responsiveness following the routine evaluation of their anti-FXa levels. Those who do not achieve the therapeutic range may be the best candidates for ATIII therapy. This could also provide an effective and safe alternative to hazardous interventions for patients with segmental or sub-segmental thrombi.
Our finding that AT levels were equivalent between patients who did and did not develop a PE was surprising and contrary to our hypothesis. This could be explained by our study design as we only had plasma samples available upon admission rather than at the time of the thrombotic event. Recent work has highlighted the importance of coagulation dynamics over time after severe injury [27]. Although we only measured admission thrombin generation which was higher in PE patients, a highly thrombogenic plasma environment would result from either the same or declining levels of AT over time in the presence of elevated thrombin generation. Owings et al. showed that AT levels tend to fall over time in severely injured patients; and when analyzed prospectively, substantially more patients experience AT deficiency than we observed in this study [20]. They found that 61% of patients had AT levels below the normal level at some point during the first six days of their hospital stay. Furthermore, their analysis concluded that AT was predictive of DVT but not PE. In our study, we only have patients with PE as our institution does not routinely survey for DVTs but does aggressively monitor for PEs. In spite of similar AT levels upon admission, patients who subsequently developed a PE had significantly reduced responses to enoxaparin compared to those who did not, a defect that was corrected through AT supplementation. This finding suggests that while there could be other mechanisms for enoxaparin resistance not evaluated here, modifying AT levels was nonetheless an effective intervention for increasing plasma enoxaparin sensitivity.
While FFP has been used in the past to treat heparin resistance [17, 28], we did not find it to have significant effects here which is in agreement with previous work [18]. Even at 30% volume, equivalent to approximately six units or ~20 ml/kg in a 70 kg adult, FFP had no effect on AT levels or the anti-FXa response to enoxaparin. FFP was, however, able to significantly reduce thrombin generation over enoxaparin alone. This is likely due to the endogenous AT in addition to other circulating anticoagulants present in balanced FFP that affect thrombin production such as tissue factor pathway inhibitor, protein C, protein S and thrombomodulin. Thus, administration of FFP may serve to mitigate thrombin generation by diluting endogenous hypercoagulable plasma with balanced plasma as previously shown [25]; however, our data suggest that it has very little direct effect on improving PE prophylaxis.
This study has several limitations. First, this study was performed in platelet poor plasma and therefore does not account for cellular contributions to thrombin generation or the risk of PE [27]. Second, these experiments were performed ex vivo; thus, we cannot make conclusions concerning clinical administration of AT. Third, plasma samples used in this study were collected upon admission, not at the time of the thromboembolic event. A prospective design in which we sample patients at the time of their thrombosis could allow us to gain greater insights into mechanistic differences in enoxaparin resistance between patients who do and do not develop PE. Also, the absence of PE in the non-PE group was not confirmed by radiologic assessment, therefore some non-PE patients could have had asymptomatic thromboses that were not clinically identified which could have contributed to some similarities in our findings between non-PE and PE groups. Finally, propensity matching was not utilized in this study which could have introduced selection bias into the design.
Conclusions
In summary, trauma is associated with reduced AT levels, increased thrombin generation, and reduced responsiveness to enoxaparin. Thrombin generation was higher and response to enoxaparin lower in patients who developed PE, and ex vivo treatment with AT, but not FFP, resulted in improved enoxaparin-mediated inhibition of thrombin generation. Future studies are needed to determine if supplementation with AT increases the effectiveness of enoxaparin thromboprophylaxis and reduces the incidence of PE in the trauma population which would support its use as a novel adjunct therapy.
Supplementary Material
HIGHLIGHTS:
Acquired AT deficiency is common following severe injury
Only half of trauma patients achieve the recommended anti-FXa levels following enoxaparin
Patients who developed venous thromboembolism demonstrate reduced responsiveness to enoxaparin compared to those who did not
Supplementation with AT optimizes enoxaparin response ex vivo
Supplementation with AT could be used as an effective adjunct therapy to enoxaparin
Acknowledgements
The authors would like to thank Cris Osborn, PhD for his thoughtful feedback and comments on this work and RnA Editing for their editorial assistance.
Funding
This work was supported by a Grifols Investigator Sponsored Research Award to CEW as well as a National Institutes of Health postdoctoral fellowship to JCC (F32HL120549).
ABBREVIATIONS
- AIS
Abbreviated Injury Scale
- ANOVA
Analysis of variance
- AT
Antithrombin III
- CAT
Calibrated automated thrombogram
- CTA
Computerized tomography angiogram
- DVT
Deep vein thrombosis
- ETP
Endogenous thrombin potential
- FFP
Fresh frozen plasma
- FXa
Coagulation Factor Xa
- GCS
Glasgow Coma Scale
- ICU
Intensive care unit
- ISS
Injury Severity Score
- Kg
Kilogram
- PE
Pulmonary embolism
- RBC
Red Blood Cells
- RPM
Revolutions per minute
- ttPeak
Time to Peak
- VTE
Venous thromboembolism
- w-RTS
Weighted Revised Trauma Score
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Ethics approval and consent to participate
This study was performed with the approval of the Institution Review Board at the University of Texas Health Science Center at Houston and in accordance with the Declaration of Helsinki. Consent was obtained within 72 hours of admission from either the patient or legally authorized representative. A waiver of consent was obtained if a patient was discharged or died within 24 hours.
Consent for publications
All authors have read and approved this manuscript.
Disclosure –
The opinions or assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting the views of the Department of the Army or the Department of Defense.
Availability of data and material
Data will be made available from the corresponding author upon reasonable request.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Competing interests
This work was supported by a grant from Grifols. JCC has received speaker honoraria from Grifols.
REFERENCES
- [1].Acosta JA, Yang JC, Winchell RJ, Simons RK, Fortlage DA, Hollingsworth-Fridlund P, Hoyt DB, Lethal injuries and time to death in a level I trauma center, Journal of the American College of Surgeons 186(5) (1998) 528–33. [DOI] [PubMed] [Google Scholar]
- [2].Hamada SR, Espina C, Guedj T, Buaron R, Harrois A, Figueiredo S, Duranteau J, High level of venous thromboembolism in critically ill trauma patients despite early and well-driven thromboprophylaxis protocol, Annals of intensive care 7(1) (2017) 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Connelly CR, Van PY, Hart KD, Louis SG, Fair KA, Erickson AS, Rick EA, Simeon EC, Bulger EM, Arbabi S, Holcomb JB, Moore LJ, Schreiber MA, Thrombelastography-Based Dosing of Enoxaparin for Thromboprophylaxis in Trauma and Surgical Patients: A Randomized Clinical Trial, JAMA surgery 151(10) (2016) e162069. [DOI] [PubMed] [Google Scholar]
- [4].Godat LN, Kobayashi L, Chang DC, Coimbra R, Can we ever stop worrying about venous thromboembolism after trauma?, The journal of trauma and acute care surgery 78(3) (2015) 475–80; discussion 480–1. [DOI] [PubMed] [Google Scholar]
- [5].DeWane MP, Davis KA, Schuster KM, Maung AA, Becher RD, Venous Thromboembolism-Related Readmission in Emergency General Surgery Patients: A Role for Prophylaxis on Discharge?, Journal of the American College of Surgeons (2018). [DOI] [PubMed] [Google Scholar]
- [6].Drake SA, Holcomb JB, Yang Y, Thetford C, Myers L, Brock M, Wolf DA, Cron S, Persse D, McCarthy J, Kao L, Todd SR, Naik-Mathuria BJ, Cox C, Kitagawa R, Sandberg G, Wade CE, Establishing a Regional Trauma Preventable/Potentially Preventable Death Rate, Annals of surgery (2018). [DOI] [PubMed] [Google Scholar]
- [7].Park MS, Spears GM, Bailey KR, Xue A, Ferrara MJ, Headlee A, Dhillon SK, Jenkins DH, Zietlow SP, Harmsen WS, Ashrani AA, Heit JA, Thrombin generation profiles as predictors of symptomatic venous thromboembolism after trauma: A prospective cohort study, The journal of trauma and acute care surgery 83(3) (2017) 381–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Cardenas JC, Rahbar E, Pommerening MJ, Baer LA, Matijevic N, Cotton BA, Holcomb JB, Wade CE, Measuring thrombin generation as a tool for predicting hemostatic potential and transfusion requirements following trauma, The journal of trauma and acute care surgery 77(6) (2014) 839–45. [DOI] [PubMed] [Google Scholar]
- [9].Dunbar NM, Chandler WL, Thrombin generation in trauma patients, Transfusion 49(12) (2009) 2652–60. [DOI] [PubMed] [Google Scholar]
- [10].Wade CE, Baer LA, Cardenas JC, Folkerson LE, Nutall-Aurora K, Cotton BA, Matijevic N, Holcomb JB, Cross JM, Huzar T, Upon admission coagulation and platelet function in patients with thermal and electrical injuries, Burns : journal of the International Society for Burn Injuries 42(8) (2016) 1704–1711. [DOI] [PubMed] [Google Scholar]
- [11].Voils SA, Lemon SJ, Jordan J, Riley P, Frye R, Early thrombin formation capacity in trauma patients and association with venous thromboembolism, Thrombosis research 147 (2016) 13–15. [DOI] [PubMed] [Google Scholar]
- [12].Geerts WH, Bergqvist D, Pineo GF, Heit JA, Samama CM, Lassen MR, Colwell CW, Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition), Chest 133(6 Suppl) (2008) 381S–453S. [DOI] [PubMed] [Google Scholar]
- [13].Karcutskie CA, Dharmaraja A, Patel J, Eidelson SA, Martin AG, Lineen EB, Namias N, Schulman CI, Proctor KG, Relation of antifactor-Xa peak levels and venous thromboembolism after trauma, The journal of trauma and acute care surgery 83(6) (2017) 1102–1107. [DOI] [PubMed] [Google Scholar]
- [14].Costantini TW, Min E, Box K, Tran V, Winfield RD, Fortlage D, Doucet J, Bansal V, Coimbra R, Dose adjusting enoxaparin is necessary to achieve adequate venous thromboembolism prophylaxis in trauma patients, The journal of trauma and acute care surgery 74(1) (2013) 128–33; discussion 134–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Malinoski D, Jafari F, Ewing T, Ardary C, Conniff H, Baje M, Kong A, Lekawa ME, Dolich MO, Cinat ME, Barrios C, Hoyt DB, Standard prophylactic enoxaparin dosing leads to inadequate anti-Xa levels and increased deep venous thrombosis rates in critically ill trauma and surgical patients, The Journal of trauma 68(4) (2010) 874–80. [DOI] [PubMed] [Google Scholar]
- [16].Karcutskie CA, Dharmaraja A, Patel J, Eidelson SA, Padiadpu AB, Martin AG, Lama G, Lineen EB, Namias N, Schulman CI, Proctor KG, Association of Anti-Factor Xa-Guided Dosing of Enoxaparin With Venous Thromboembolism After Trauma, JAMA surgery 153(2) (2018) 144–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Sabbagh AH, Chung GK, Shuttleworth P, Applegate BJ, Gabrhel W, Fresh frozen plasma: a solution to heparin resistance during cardiopulmonary bypass, The Annals of thoracic surgery 37(6) (1984) 466–8. [DOI] [PubMed] [Google Scholar]
- [18].Kanbak M, Oc B, Salman MA, Ocal T, Oc M, Peroperative effects of fresh frozen plasma and antithrombin III on heparin sensitivity and coagulation during nitroglycerine infusion in coronary artery bypass surgery, Blood coagulation & fibrinolysis : an international journal in haemostasis and thrombosis 22(7) (2011) 593–9. [DOI] [PubMed] [Google Scholar]
- [19].du Cheyron D, Bouchet B, Bruel C, Daubin C, Ramakers M, Charbonneau P, Antithrombin supplementation for anticoagulation during continuous hemofiltration in critically ill patients with septic shock: a case-control study, Critical care 10(2) (2006) R45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Owings JT, Bagley M, Gosselin R, Romac D, Disbrow E, Effect of critical injury on plasma antithrombin activity: low antithrombin levels are associated with thromboembolic complications, The Journal of trauma 41(3) (1996) 396–405; discussion 405–6. [DOI] [PubMed] [Google Scholar]
- [21].Owings JT, Gosselin R, Acquired antithrombin deficiency following severe traumatic injury: rationale for study of antithrombin supplementation, Seminars in thrombosis and hemostasis 23 Suppl 1 (1997) 17–24. [PubMed] [Google Scholar]
- [22].Gerotziafas GT, Petropoulou AD, Verdy E, Samama MM, Elalamy I, Effect of the anti-factor Xa and anti-factor IIa activities of low-molecular-weight heparins upon the phases of thrombin generation, J Thromb Haemost 5(5) (2007) 955–62. [DOI] [PubMed] [Google Scholar]
- [23].Ben-Hadj-Khalifa S, Hezard N, Almawi WY, Remy MG, Florent B, Mahjoub T, Nguyen P, Differential coagulation inhibitory effect of fondaparinux, enoxaparin and unfractionated heparin in cell models of thrombin generation, Blood coagulation & fibrinolysis : an international journal in haemostasis and thrombosis 22(5) (2011) 369–73. [DOI] [PubMed] [Google Scholar]
- [24].Cini M, Legnani C, Testa S, Tripodi A, Cosmi B, Palareti G, An in vitro study to investigate the interference of enoxaparin on plasma levels of direct oral factor Xa inhibitors measured by chromogenic assays, Int J Lab Hematol 41(3) (2019) 309–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Cardenas JC, Cap AP, Swartz MD, Huby Mdel P, Baer LA, Matijevic N, Cotton BA, Holcomb JB, Wade CE, Plasma Resuscitation Promotes Coagulation Homeostasis Following Shock-Induced Hypercoagulability, Shock 45(2) (2016) 166–73. [DOI] [PubMed] [Google Scholar]
- [26].Iba T, Kidokoro A, High-dose antithrombin therapy for sepsis: mechanisms of action, Shock 18(5) (2002) 389–94. [DOI] [PubMed] [Google Scholar]
- [27].McCully BH, Connelly CR, Fair KA, Holcomb JB, Fox EE, Wade CE, Bulger EM, Schreiber MA, Group PS, Onset of Coagulation Function Recovery Is Delayed in Severely Injured Trauma Patients with Venous Thromboembolism, Journal of the American College of Surgeons 225(1) (2017) 42–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Spiess BD, Treating heparin resistance with antithrombin or fresh frozen plasma, The Annals of thoracic surgery 85(6) (2008) 2153–60. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


