Abstract
In 2018, the American Glaucoma Society (AGS), the world’s largest professional society of glaucoma subspecialists, convened a 12-member task force of experts to craft a position statement about microinvasive glaucoma surgery (MIGS). The main objective of this position statement is to provide a succinct overview of these procedures and to address some misconceptions about MIGS. The members of the task force were selected by the AGS Board of Directors and include AGS members with expertise in developing MIGS, teaching MIGS, performing research on use and outcomes of these procedures, and working with the United States Food and Drug Administration (FDA) and other regulatory agencies about developing criteria to evaluate the efficacy and safety of these devices. Each of the sections of the position statement was prepared by subgroups of the task force, and then the material from the various sections was aggregated, and the leader of the task force (J.D.S.) merged the material into a cohesive draft. This draft was shared with the AGS Executive Committee and other members of the AGS Board of Directors for additional input.
Overview and Definition of Microinvasive Glaucoma Surgery
Glaucoma is a group of chronic, often asymptomatic diseases that damage the optic nerve in a characteristic manner. If left untreated or insufficiently treated, glaucoma can lead to irreversible disability, vision loss, and blindness. In the United States, vision loss resulting from glaucoma is a leading cause of disability and blindness in blacks, whites, Latinos, and Asians.1–4 The functional visual loss secondary to glaucoma often is measured by static automated perimetry and is correlated with the degree of structural disc damage measured by optic disc photography and OCT. Key objectives in the management of glaucoma are to maximize health-related quality of life, to preserve remaining visual function, as well as to minimize future vision loss and the risks of treatment necessary to achieve these goals.
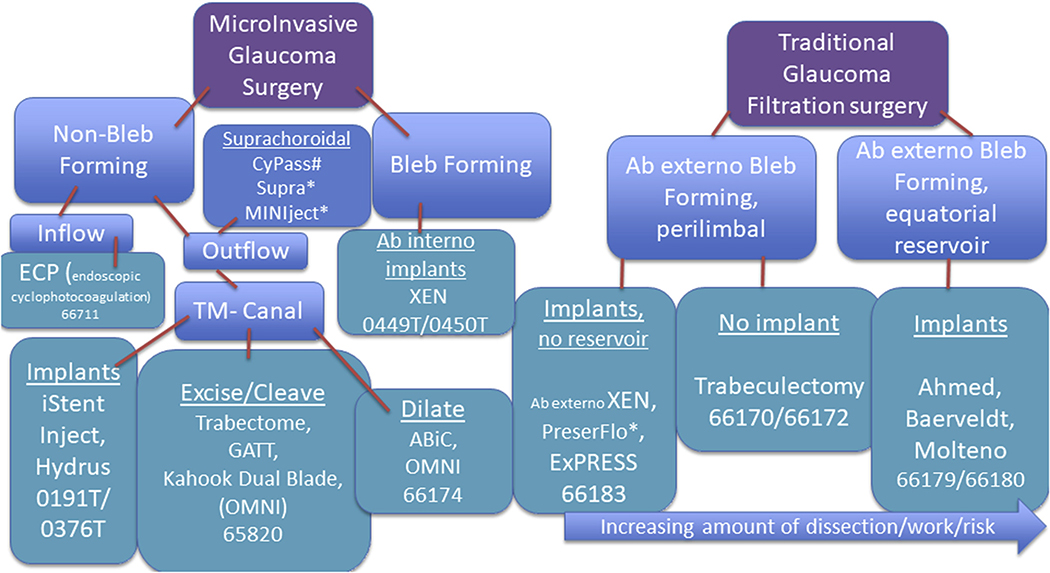
Currently, the only known way of slowing the rate of glaucomatous vision loss is by lowering intraocular pressure (IOP). This can be achieved with medications, laser procedures, or incisional procedures. Medications usually are delivered as eye drops. Although medications can be effective at lowering IOP, they can be expensive and difficult to administer. Nearly two thirds of patients are unable to administer eye drops correctly and more than one half of all patients with glaucoma struggle with medication adherence.5 Self-administration of eye drops can be quite difficult for many patients with glaucoma, and a large proportion of these patients live without a support structure to assist them.6,7 Moreover, a single class of IOP-lowering medication is inadequate to control the IOP in more than 50% of all patients with glaucoma.8 Although it is possible to add additional classes of IOP-lowering medications, complex medication regimens more frequently result in nonadherence, which in turn can lead to disease progression. Traditional incisional glaucoma procedures (i.e., trabeculectomy or aqueous shunt surgery) that create subconjunctival filtration blebs often are reserved for patients with progressive disease who are at high risk of severe vision loss (Fig 1). These procedures can lower IOP substantially and preserve vision, but they themselves can decrease the quality of life and often require a lengthy recovery period, are subject to the vicissitudes of wound healing, and potentially have devastating complications.8–19 In recent years, a push has been made for alternative surgical approaches to lower IOP that reduce the need for glaucoma medications and some of the serious risks associated with traditional glaucoma procedures, such as hypotony (low IOP); choroidal effusion, hemorrhage, or both; tube exposure; bleb leaks; blebitis; and endophthalmitis. Collectively, this new class of procedures is known as microinvasive glaucoma surgery. Microinvasive glaucoma surgery procedures are designed to lower IOP by improving aqueous outflow with minimal disruption to the sclera or conjunctiva with or without an implanted device, or by reducing aqueous production selectively. Outflow enhancement can be accomplished by facilitating access of aqueous humor to Schlemm’s canal (canal based), the suprachoroidal space, or the subconjunctival space. Reducing aqueous production can be accomplished through selective direct laser ablation of the ciliary processes, the structures that produce the aqueous humor.
Figure 1.
Flowchart showing different types of microinvasive and traditional glaucoma surgical options along with their respective Current Procedure Terminology billing codes. These codes are accurate as of January 2020 but may be subject to change. #Withdrawn from market. *Not approved by the United States Food and Drug Administration. ABic = ab interno canaloplasty; GATT = gonioscopy-assisted transluminal trabeculotomy; TM = trabecular meshwork.
Comparison of Microinvasive Glaucoma Surgery with Traditional Incisional Glaucoma Surgeries
All surgical procedures, including those for glaucoma, carry risks of adverse events. These risks must be weighed carefully against the expected benefits of preserving vision and quality of life. Many members of the AGS have supported the development of MIGS enthusiastically, particularly for the treatment of subsets of patients whose risk of vision loss resulting from glaucoma progression may not justify the risk associated with traditional filtration surgery or aqueous shunt implantation. The nontrivial risk of vision loss associated with these traditional glaucoma procedures often outweighs the potential benefit of lowering IOP, discouraging ophthalmologists from recommending these procedures and patients from consenting to them, except in relatively dire circumstances, even when they may help to stabilize the disease.
Unlike traditional glaucoma procedures, canal-based MIGS often can be performed with smaller incisions, akin to those used during modern day cataract surgery. Most of these procedures enhance the physiologic outflow pathways of the eye, which results in a safer lowering of IOP. Patients with ocular hypertension who are at high risk for experiencing vision loss as a result of uncontrolled IOP or with early-stage glaucoma, but who cannot tolerate or afford medical treatment, may be excellent candidates for MIGS procedures. Likewise, patients with moderate- to severe-stage glaucoma whose ocular or medical comorbidities make them less than ideal candidates for traditional glaucoma surgery also may benefit. Fewer postoperative visits make these procedures excellent options for working-age patients and elderly patients who may rely on working-age relatives to bring them to perioperative appointments.
Canal-based MIGS lower IOP by improving outflow through the patient’s natural drainage system, instead of creating a bleb (a subconjunctival reservoir from which aqueous humor can slowly egress, and thereby lower IOP) to capture the aqueous.20 This considerably enhances the safety profile and limits discomfort, which can be an issue with traditional glaucoma procedures. Patients who are deemed not to be good candidates for canal-based MIGS still may be candidates for subconjunctival MIGS, a less invasive form of classic filtration surgery that uses microstents to create a bleb.20,21 Other potential advantages of MIGS over traditional glaucoma procedures include faster recovery, less impact on leisure activities (such as swimming), and reduced risk of damaging other structures in the eye that can necessitate additional ocular surgeries. Although many patients with different types and severities of glaucoma can benefit from MIGS, some patients clearly fare better with traditional glaucoma filtration surgery, so it is important to have all of these procedures available to allow the surgeon and patient together to decide which intervention is most appropriate.
Categorization of Different Microinvasive Glaucoma Surgery Procedures
Most MIGS lower IOP by reducing outflow resistance. To accomplish this, the surgeon may dilate, cleave open, or bypass abnormally resistant tissue that is hindering outflow or may insert a device into an outflow structure or space to enhance the drainage of aqueous. These procedures may be divided broadly into bleb-forming and non–bleb-forming procedures.20 Bleb-forming MIGS are placed through an ab externo (outside of the eye) or ab interno (inside of the eye) approach, whereas all non–bleb-forming MIGS are performed ab interno. These devices may be categorized further based on the anatomic location of device placement or tissue manipulation. This categorization aligns with Current Procedural Terminology coding, as outlined in Figure 1.
Complexity of Microinvasive Glaucoma Surgery Procedures
In general, intraocular surgery is a complex task because it involves working in very small spaces, adjacent to sensitive structures, where missteps can lead to permanent vision loss. The learning curve can be quite long and difficult. Residency and fellowship training form the basis of the acquisition of these skills. Performing MIGS requires an even higher skill level because it requires the use of gonioprism mirrors under an operating microscope to visualize the ocular structures that require treatment, working in even smaller more sensitive spaces within the eye, and requires learning a new proprioceptive skill, namely intraoperative surgical gonioscopic viewing of the angle. This particular skill requires more manual dexterity because the surgeon must use one hand to balance the gonioprism on the cornea to permit visualization of the structures requiring treatment and simultaneously the second hand to carry out the procedure. Although gonioscopy is used to classify and diagnose glaucoma at the slit lamp in all patients with glaucoma, the skills required at the surgical microscope usually are more difficult to master. Those with experience training new surgeons in these techniques have observed that some new surgeons can adapt to these increased demands, but many cannot. It may seem paradoxical that a safer surgical procedure with fewer complications tends to require more manual dexterity involving both hands simultaneously, better anatomic knowledge, and more technical proficiency to perform. However, this is actually relatively common in medicine, as evidenced in the transition from open to laparoscopic surgery and the transition from intracapsular cataract surgery to extracapsular cataract surgery to phacoemulsification.
Preoperative Considerations
As with any surgical procedure, a prerequisite to the success of MIGS involves a thorough preoperative assessment to ensure that the patient is an appropriate candidate for a given procedure, to select which procedure is most likely to succeed for a particular patient, and to be certain that the benefits of the surgery outweigh its risks. There are many nuances of these surgeries that must be considered to achieve optimal results. For example, careful examination of the anterior chamber angle is of paramount importance. If a MIGS procedure is combined with cataract surgery, it is also very important to evaluate for conditions that may increase the complexity of the surgery such as peripheral anterior synechiae, conjunctival scarring, angle visibility, and zonular instability. In addition, determining the site from which to approach a given procedure, for example ab interno or ab externo, requires careful consideration because it may affect the options available for subsequent glaucoma surgery. Another preoperative consideration is the provision of thorough informed consent of the patient. During such a discussion, the surgeon should acknowledge the fact that long-term outcomes for many MIGS procedures are not yet known and there may be unanticipated risks associated with these surgeries.
Postoperative Considerations
The postoperative period after some MIGS procedures generally is much easier on patients than that after traditional glaucoma surgeries. Discomfort from conjunctival sutures is less frequent (because sutures are not needed for many MIGS procedures) and visual recovery tends to be achieved more quickly than with traditional incisional glaucoma procedures. However, careful postoperative care is still required to achieve good outcomes because patients undergoing MIGS are more prone to postoperative complications such as IOP spikes, device movement or erosion, hyphema, and corticosteroid-induced elevation of IOP relative to other patients who undergo cataract surgery alone.22 As such, it is important to educate the patient about the importance of postoperative care to help ensure the success of the procedure.
Considerations for Clinical Trial Design for Microinvasive Glaucoma Surgery
No single ideal therapeutic approach exists that applies to every patient with glaucoma. Glaucoma therapy must be tailored based on weighing the risks and benefits of the various treatment options, taking a patient’s disease state and projected course into careful consideration. Given the variability in the effectiveness, safety profile, patients’ responses, and patients’ perceptions of the relative importance of the various risks and benefits of various therapies, glaucoma care very much remains a mixture of art and science. Maximum tolerated medical therapy and refractory glaucoma are ambiguous terms that may be confusing when integrated into clinical practice guidelines and policy statements. Differences in interpretation of these terms also can result in ambiguity in determining patients who may be eligible for different MIGS procedures. To help provide clarification, the AGS defines the terms as follows.
Maximum tolerated medical therapy is attained as soon as the patient is using successfully the greatest number of topical glaucoma medication classes he or she can tolerate and that add additional IOP reduction. For some patients, this could be as many as 6 or more different classes of glaucoma medications taken daily, and for others, this could be no medications at all. Medication intolerance can be attributed to a variety of reasons, ranging from expense, side effects, and an inability to administer eye drops. As mentioned earlier, nonadherence to glaucoma medications for any of the above reasons can cause permanent vision loss as the disease progresses. In some patients, the clinical presentation is so severe that incisional surgery is the most appropriate initial intervention, even forgoing medical treatment.
Refractory glaucoma is simply glaucoma that is difficult to treat and poorly controlled by current therapy, regardless of the stage of disease. Stage of disease, as defined in the literature and based on the International Classification of Diseases, Tenth Edition, coding system, designates the amount of damage to the visual system from glaucoma at a moment in time. All patients have a risk of progressing to worsening stages of glaucoma damage over time if glaucoma is not controlled. A variety of reasons may explain why a particular patient’s glaucoma may be difficult to treat, ranging from an inability to adhere to the medical management plan properly, inability to instill eye drops, poor responsiveness of the eye to IOP-lowering interventions, systemic side effects, or the presence of scar tissue from prior injury or surgery.
Historically, treatment options have involved topical medications and laser trabeculoplasty for patients with mild to moderate disease. Riskier surgical approaches usually are reserved for those with more advanced glaucoma, those who are at higher risk of losing visual function, or those for whom less invasive options fail. A substantial lack of surgical options exists for patients who are unable or unwilling to take glaucoma medications and for those with mild yet progressive disease in whom medications and laser fail to control IOP sufficiently, but the disease severity level does not justify the inherent risks of traditional incisional glaucoma surgery. The availability of MIGS devices has afforded more therapeutic options to address this large segment of patients with glaucoma. The FDA and the AGS have come together since the development of MIGS for workshops and discussions to advance these innovations to serve patients with glaucoma. Unfortunately, given the absence of an appropriate predicate device, the FDA premarket approval pathway for many implantable MIGS devices has been very expensive, time consuming, and sometimes frustrating for researchers and companies trying to innovate in this area. In the case of ab interno MIGS implants, the devices were required by the FDA to be implanted at the time of concomitant cataract surgery. This in part was to investigate the IOP-lowering effect of cataract surgery alone in a control population, but also as a way to mitigate risk as recipients of the implants received the benefit of cataract surgery. Hence, clinical indications on the labels for the ab interno implants all specify “with cataract surgery.” Another example applies to the bleb-forming MIGS procedures, for which 3 trials are ongoing (with more anticipated soon) of stand-alone MIGS procedures, with the Ahmed glaucoma drainage device (New World Medical, Inc., Fontana, CA), a traditional glaucoma filtration surgery device, as the predicate device. Unfortunately, the study samples for these trials are required to be similar to those used in trials involving the predicate device. As such, these trials must be performed on patients labeled with refractory glaucoma, which previously has been defined by other groups as those in whom at least 1 incisional glaucoma surgery has failed. Yet, the intended population who could benefit most from many MIGS surgeries is actually quite different from those who have undergone incisional glaucoma surgeries already. This study design with the requirement of failed prior surgery can affect the results of the trial greatly and can create a potentially flawed process for evaluating these devices. The AGS recommends that the FDA use the de novo (or other similar or novel) pathway for evaluating glaucoma devices with known (or anticipated) high levels of safety, such as most MIGS procedures. The AGS welcomes the opportunity to work with the FDA to develop appropriate protocols for trials and to work through other logistical considerations to permit new MIGS devices to be evaluated in a fair manner in groups of patients with glaucoma who would align better with the intended target population when such devices are approved for marketing.
The AGS believes that as data accumulate from completed premarket approval pathway studies for various MIGS, greater flexibility with respect to expanding the indication of already approved devices is needed. Furthermore, the introduction of next-generation MIGS devices (for which prior data is undoubtedly useful, particularly with regard to safety) should not be a static process, but instead should permit developers of these devices the flexibility of the 510K pathway for refractory glaucoma. Several different approaches could allow for such flexibility. One option would involve redefining and expanding the criteria for what constitutes refractory glaucoma for the purposes of the regulatory process, in the same way that the AGS defines this condition, as described above. Another option would be to create a parallel 510K pathway with previously approved MIGS devices as a historical predicate, as noted in the aforementioned paragraph. We believe that regulatory authorities should engage the AGS, either via the existing network of experts or via other formal associations, to outline the appropriate clinical trial designs necessary for clearance of the next generation of MIGS devices, as well as for expansion of indications for already approved devices.
In contrast to other regions in the world, regulatory decisions in the United States historically have been separated from those related to reimbursement. The AGS believes that patients and their doctors may be served better if the information gathered by regulatory authorities in the clearance and labelling of innovative products (such as MIGS) were available readily to the authorities that make determinations about reimbursement. This could expedite the availability of these less invasive microincisional devices to patients for whom they would be beneficial. A related issue that often limits patients’ access to these MIGS procedures is that payors may permit patients to undergo these procedures only if they precisely fit the profile of those who received such a procedure during the FDA approval process. For example, because most FDA trials involving MIGS have been performed on patients receiving these devices in combination with cataract surgery, several payors will approve reimbursement of these procedures only in patients who receive them concurrently with cataract surgery, despite the additive IOP-lowering effect of the implants observed in the clinical trials. This can be very problematic, especially for patients who either have no cataract or already have undergone cataract removal, but still may benefit from these MIGS devices as stand-alone procedures.
Conclusions
With the rapidly rising number of patients with glaucoma in the United States and worldwide, it is essential for researchers and industry to continue developing innovative therapeutic options that are effective at lowering IOP, possess a good safety profile, and are well tolerated by patients. The advent and evolution of MIGS has increased the therapeutic options for patients with glaucoma, but considerable room for improvement in the surgical delivery of glaucoma care remains. The AGS supports efforts that facilitate patient access to these procedures and that permit the clinician and patient to decide jointly which intervention(s) along the entire spectrum of care are best for them based on their unique circumstances.
Acknowledgments
Supported by the National Eye Institute, National Institutes of Health grant no. R01-EY026641, Bethesda, Maryland (J.D.S.); and the Lighthouse Guild, New York, NY (J.D.S.).
Footnotes
This manuscript was reviewed and approved by the American Glaucoma Society Board of Directors and does not follow the standard editorial process of Ophthalmology Glaucoma.
Footnotes and Financial Disclosures
Financial Disclosure(s): The author(s) have made the following disclosure(s): R.L.F.: Consultant – Alcon, Beaver Visitech, Inc., BVI Endooptiks, Aerie Pharma; Financial support – Ivantis, Glaukos; Lecturer – Bausch & Lomb.
C.M.: Consultant – Aerie, Alcon, Allergan, Glaukos, Ivantis, New World Medical, Santen, Ocular Therapeutix, Zeiss Meditec.
K.S.: Consultant – Alcon, Allergan, Glaukos, Ivantis, Santen, Sight Sciences.
B. F.: Consultant – Alcon, Ivantis, Sun Pharmaceuticals, Bausch & Lomb, InnFocus, Sight Science, New World Medical, iStar, EyeNovia; Financial support – Alcon, Ivantis, Glaukos, Aerie, InnFocus, Sight Science, iStar, Santen.
B.A.F.: Consultant – Neomedix, Glaukos, Beaver Visitech, BVI Endooptiks; Financial support: Santen, Allergan, iStar; Lecturer – Aerie, Bausch & Lomb.
A.L.R.: Consultant – Google AI.
M.R.B.: Lecturer – Allergan.
M.M.S.: Consultant – Allergan, Glaukos, Katena; Lecturer – Allergan.
J.A.G.: Lecturer – Allergan.
A.S.: Consultant – Allergan, Katena, Ivantis; Lecturer – Glaukos, Allergan.
Contributor Information
Ronald L. Fellman, Dallas, Texas.
Cynthia Mattox, Makawao, Hawaii.
Kuldev Singh, Palo Alto, California.
Brian Flowers, Fort Worth, Texas.
Brian A. Francis, Los Angeles, California.
Alan L. Robin, Ann Arbor, Michigan.
Michelle R. Butler, Dallas, Texas.
Manjool M. Shah, Ann Arbor, Michigan.
JoAnn A. Giaconi, Los Angeles, California.
Arsham Sheybani, St. Louis, Missouri.
Brian J. Song, Ontario, California.
Joshua D. Stein, Ann Arbor, Michigan.
References
- 1.Friedman DS, Wolfs RC, O’Colmain BJ, et al. Prevalence of open-angle glaucoma among adults in the United States. Arch Ophthalmol. 2004;122(4):532–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Quigley HA, West SK, Rodriguez J, et al. The prevalence of glaucoma in a population-based study of Hispanic subjects: Proyecto VER. Arch Ophthalmol. 2001;119(12):1819–1826. [DOI] [PubMed] [Google Scholar]
- 3.Varma R, Wang D, Wu C, et al. Four-year incidence of open-angle glaucoma and ocular hypertension: the Los Angeles Latino Eye Study. Am J Ophthalmol. 2012;154(2):315–325.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stein JD, Kim DS, Niziol LM, et al. Differences in rates of glaucoma among Asian Americans and other racial groups, and among various Asian ethnic groups. Ophthalmology. 2011. June;118(6):1031–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hennessy AL, Katz J, Covert D, et al. A video study of drop instillation in both glaucoma and retina patients with visual impairment. Am J Ophthalmol. 2011;152(6):982–988. [DOI] [PubMed] [Google Scholar]
- 6.Tsai T, Robin AL, Smith JP 3rd. An evaluation of how glaucoma patients use topical medications: a pilot study. Trans Am Ophthalmol Soc. 2007;105:29–33. discussion 33–35. [PMC free article] [PubMed] [Google Scholar]
- 7.The Advanced Glaucoma Intervention Study (AGIS). 3. Baseline characteristics of black and white patients. Ophthalmology. 1998;105(7):1137–1145. [DOI] [PubMed] [Google Scholar]
- 8.Musch DC, Gillespie BW, Niziol LM, et al. Intraocular pressure control and long-term visual field loss in the Collaborative Initial Glaucoma Treatment Study. Ophthalmology. 2011;118: 1766–1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gedde SJ, Heuer DK, Parrish RK 2nd, Tube Versus Trabeculectomy Study Group. Review of results from the Tube Versus Trabeculectomy Study. Curr Opin Ophthalmol. 2010;21(2):123–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fontana H, Nouri-Mahdavi K, Lumba J, et al. Trabeculectomy with mitomycin C: outcomes and risk factors for failure in phakic open-angle glaucoma. Ophthalmology. 2006;113(6): 930–936. [DOI] [PubMed] [Google Scholar]
- 11.Kim HY, Egbert PR, Singh K. Long-term comparison of primary trabeculectomy with 5-fluorouracil versus mitomycin C in West Africa. J Glaucoma. 2008;17(7):578–583. [DOI] [PubMed] [Google Scholar]
- 12.Wong MH, Husain R, Ang BC, et al. The Singapore 5-Fluorouracil Trial: intraocular pressure outcomes at 8 years. Ophthalmology. 2013;120(6):1127–1134. [DOI] [PubMed] [Google Scholar]
- 13.Robin AL, Ramakrishnan R, Krishnadas R, et al. A long-term dose-response study of mitomycin in glaucoma filtration surgery. Arch Ophthalmol. 1997;115(8):969–974. [DOI] [PubMed] [Google Scholar]
- 14.Jampel H Trabeculectomy: more effective at causing cataract surgery than lowering intraocular pressure? Ophthalmology. 2009;116(2):173–174. [DOI] [PubMed] [Google Scholar]
- 15.Wong TT, Khaw PT, Aung T, et al. The Singapore 5-Fluorouracil Trabeculectomy Study: effects on intraocular pressure control and disease progression at 3 years. Ophthalmology. 2009;116(2):175–184. [DOI] [PubMed] [Google Scholar]
- 16.Stein JD, Ruiz D Jr, Belsky D, et al. Longitudinal rates of postoperative adverse outcomes after glaucoma surgery among medicare beneficiaries 1994 to 2005. Ophthalmology. 2008;115(7):1109–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Palanca-Capistrano AM, Hall J, Cantor LB, et al. Long-term outcomes of intraoperative 5-fluorouracil versus intraoperative mitomycin C in primary trabeculectomy surgery. Ophthalmology. 2009;116(2):185–190. [DOI] [PubMed] [Google Scholar]
- 18.Shigeeda T, Tomidokoro A, Chen YN, et al. Long-term follow-up of initial trabeculectomy with mitomycin C for primary open-angle glaucoma in Japanese patients. J Glaucoma. 2006;15(3):195–199. [DOI] [PubMed] [Google Scholar]
- 19.Gedde SJ, Herndon LW, Brandt JD, et al. Postoperative complications in the Tube Versus Trabeculectomy (TVT) study during five years of follow-up. Am J Ophthalmol. 2012;153(5):804–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schehlein EM, Kaleem MA, Swamy R, Saeedi OJ. Microinvasive glaucoma surgery: an evidence-based assessment. Expert Rev Ophthalmol. 2017;12(4):331–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chatzara A, Chronopoulou I, Theodossiadis G, et al. XEN implant for glaucoma treatment: a review of the literature. Semin Ophthalmol. 2019;34(2):93–97. [DOI] [PubMed] [Google Scholar]
- 22.Yook E, Vinod K, Panarelli JF. Complications of microinvasive glaucoma surgery. Curr Opin Ophthalmol. 2018;29(2):147–154. [DOI] [PubMed] [Google Scholar]