Abstract
COVID-19 pandemic revealed several neurological syndromes related to this infection. We describe the clinical, laboratory, and radiological features of eight patients with COVID-19 who developed peripheral facial palsy during infection. In three patients, facial palsy was the first symptom. Nerve damage resulted in mild dysfunction in five patients and moderate in three. SARS-Cov-2 was not detected in CSF by PCR in any of the samples. Seven out of eight patients were treated with steroids and all patients have complete or partial recovery of the symptoms. Peripheral facial palsy should be added to the spectrum of neurological manifestations associated with COVID-19.
Keywords: Bell palsy, COVID-19, Facial nerve
Introduction
The ongoing COVID-19 pandemic has affected millions of people worldwide and revealed several neurological syndromes related to this infection. Anosmia/ageusia, encephalitis, encephalopathy, cerebrovascular complications, myelitis, and Guillain-Barré syndrome, among other neurological complications, occur in a significant proportion of patients (Ellul et al. 2020; Paterson et al. 2020).
Acute facial nerve palsy commonly occurs in clinical practice and is associated with considerable distress due to possible functional and esthetic sequelae (Jowett 2018). There are many potential mechanisms implicated in its occurrence, including viral infections. Herein, we review the clinical and laboratory features of eight patients with COVID-19 who developed peripheral facial palsy during the clinical course of the infection or as its first symptom.
Methods
Case series of eight patients seen from May to July 2020 with a diagnosis of COVID-19 based on positive SARS-CoV-2 RNA RT-qPCR in nasal and oropharyngeal swabs (Biomanguinhos kit (E+P1), FIOCRUZ, Brazil).
Data about the onset of facial palsy, associated clinical conditions, brain imaging, cerebrospinal fluid parameters, treatment, and outcome were recorded. Facial palsy was graded according to the House-Brackmann scale (House and Brackmann 1985). This study was approved by the Local Ethical Committee at INI/FIOCRUZ.
Results
Among the eight patients, seven were women. All had COVID-19 diagnosis based on positive SARS-CoV-2 RNA RT-qPCR in nasal and oropharyngeal swabs. The mean age was 36 years (range 25–50 years). In three patients, facial palsy was the first symptom of COVID-19, while in the remaining five, it appeared from 2 to 10 days after onset of other clinical manifestations. All patients had mild respiratory and systemic COVID-19 symptoms, and none required hospitalization. According to the House-Brackmann grading system, nerve damage resulted in mild (grade 2) dysfunction in five patients and moderate (grade 3) in three (Table 1). The neurological examination disclosed no abnormalities in all but one patient, who had an associated ipsilateral abducent nerve palsy. Deep tendon reflexes were preserved, and no sensory abnormalities were present. Six patients underwent lumbar puncture with normal opening pressure in all cases. CSF analysis showed no inflammatory changes except for a mild protein elevation in one patient (50 mg/dl) (Table 1). SARS-Cov-2 was not detected in CSF by PCR in any of the samples. Imaging (CT scan or MRI) was normal in seven patients. In one patient, MRI showed contrast enhancement in the distal intracanalicular portion in the tympanic and mastoid segments of the left facial nerve (Fig. 1).
Table 1.
Clinical and laboratory manifestations of COVID-19 patients with facial palsy
| Patient | Age | Gendera | Clinical manifestation (House-Brackmann grading scale) | First symptomb | CSFc | Imaging | Treatment | Outcome |
|---|---|---|---|---|---|---|---|---|
| 1 | 43 | F | Right facial palsy (3) | Yes | - | CT scan: normal | Oral steroids | Partial |
| 2 | 25 | F | Right facial palsy (2) | Yes | 5–29–50 | MRI: normal | Oral steroids + acyclovir | Complete |
| 3 | 33 | F | Right facial palsy (3) | Yes | - | - | Oral steroids + acyclovir | Partial |
| 4 | 26 | F | Left facial palsy (2) | No | 4–31–55 | MRI: left facial nerve enhancement | Oral steroids | Complete |
| 5 | 50 | F | Left facial palsy (3) | No | 3–50–56 | CT scan: normal | Oral steroids | Partial |
| 6 | 38 | F | Left facial palsy (2) | No | 1–28–51 | MRI: normal | Supportive | Complete |
| 7 | 39 | F | Right facial palsy (2) | No | 1–32–38 | MRI: normal | Oral steroids | Complete |
| 8 | 34 | M | Left facial palsy (2) | No | 2–33–91 | MRI: normal | Intravenous steroids | Complete |
aGender: F female, M male
bFacial palsy as first COVID-19 symptom/signal
cCerebrospinal fluid analysis: cell count/mm3—protein level mg/dl—glucose level mg/dl
Fig. 1.
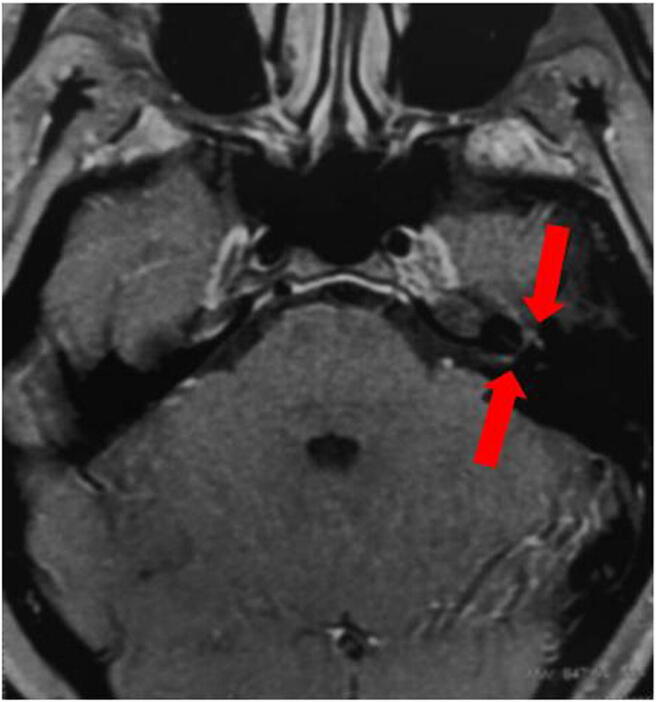
Axial brain MRI (T1/gadolinium) showing contrast enhancement in the distal intracanalicular portion in the tympanic and mastoid segments of the left facial nerve (red arrows)
Six out of seven patients were treated with oral steroids (prednisone 40–60 mg/day for 5–7 days) and one received intravenous methylprednisolone. One patient with mild manifestations received only supportive care (eye lubricant) with complete recovery 2 days later. Two patients received oral acyclovir concomitant to steroids due to possible Herpes simplex virus infection. Complete recovery occurred in five patients, while the other three still had some degree of facial weakness at the last follow-up 30 days after onset of neurological symptoms.
Discussion
Infections such as HSV-1, VZV, and Lyme disease are common causes of facial paralysis (Owusu et al. 2018). The rapid expansion of COVID-19 pandemics led to the development of a growing number of neurological syndromes. Our study shows that peripheral facial palsy can occur during the clinical course of COVID-19 or anticipate other typical manifestations such as fever and respiratory symptoms.
Interestingly, all but one of our patients were women. Idiopathic facial palsy does not have a gender preference (Katusic et al. 1986). Indeed, our sample is too small to assume any conclusion, and the two other cases of isolated facial palsy in association with COVID-19 described by Goh and Casas were men (Casas et al. 2020; Goh et al. 2020).
Most patients in this study had isolated facial palsy with mild or moderate dysfunction and no other neurological findings. Except for the two described above by Goh and Casas (Casas et al. 2020; Goh et al. 2020), in all other studies, facial paralysis in COVID-19 patients occurred unilaterally or bilaterally in association with other manifestations of Guillain-Barré syndrome (Manganotti et al. 2020; Ottaviani et al. 2020; Juliao Caamaño and Alonso Beato 2020; Paybast et al. 2020; Sancho-Saldaña et al. 2020; Bigaut et al. 2020).
CSF basic parameters (cellularity, protein, and glucose levels) are usually normal in patients with idiopathic facial paralysis as observed in our series (Bremell and Hagberg 2011). SARS-CoV2 was not detected in any five cases who underwent lumbar puncture, which is consistent with a recent study that failed to show viral RNA in the CSF of COVID-19 patients with different neurological syndromes (Espíndola et al. 2020).
Possible mechanisms related to nerve damage in idiopathic facial nerve paralysis include ischemia of vasa nervorum and demyelination induced by an inflammatory process (Zhang et al. 2020). Microthrombi and other vascular changes have been consistently reported in several postmortem studies (Silberzahn et al. 1988; Nunes Duarte-Neto et al. 2020) and may be implicated in the development of facial nerve ischemia in COVID-19 patients. Direct viral damage or an autoimmune reaction toward the nerve producing inflammation would be alternative or contributing mechanisms to dysfunction.
Supportive care and oral steroids are the mainstays of treatment (Sullivan et al. 2007). Our patients had complete recovery or significant improvement in few weeks after treatment as the patient reported by Casas et al. (2020), suggesting a good outcome when peripheral facial palsy occurs in association with COVID-19.
In conclusion, peripheral facial palsy should be added to the spectrum of neurological manifestations associated with COVID-19. Most patients had an uncomplicated course with good outcome, and SARS-CoV-2 RNA could not be detected in CSF of any patient.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Bigaut K, Mallaret M, Baloglu S et al (2020) Guillain-Barré syndrome related to SARS-CoV-2 infection. Neurol Neuroimmunol Neuroinflamm 7. 10.1212/NXI.0000000000000785 [DOI] [PMC free article] [PubMed]
- Bremell D, Hagberg L. Clinical characteristics and cerebrospinal fluid parameters in patients with peripheral facial palsy caused by Lyme neuroborreliosis compared with facial palsy of unknown origin (Bell’s palsy) BMC Infect Dis. 2011;11:215. doi: 10.1186/1471-2334-11-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casas E, Barbosa A, Rubio-García E, et al. Isolated peripheral facial paralysis in a patient with COVID-19. Rev Neurol. 2020;71:40–41. doi: 10.33588/rn.7101.2020229. [DOI] [PubMed] [Google Scholar]
- Ellul MA, Benjamin L, Singh B, Lant S, Michael BD, Easton A, Kneen R, Defres S, Sejvar J, Solomon T. Neurological associations of COVID-19. Lancet Neurol. 2020;19:767–783. doi: 10.1016/S1474-4422(20)30221-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espíndola O d M, Siqueira M, Soares CN, et al. Patients with COVID-19 and neurological manifestations show undetectable SARS-CoV-2 RNA levels in the cerebrospinal fluid. Int J Infect Dis. 2020;96:567–569. doi: 10.1016/j.ijid.2020.05.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh Y, Beh DLL, Makmur A, Somani J, Chan ACY. Pearls and oysters: facial nerve palsy as a neurological manifestation of Covid-19 infection. Neurology. 2020;95:364–367. doi: 10.1212/WNL.0000000000009863. [DOI] [PubMed] [Google Scholar]
- House JW, Brackmann DE. Facial nerve grading system. Otolaryngol Head Neck Surg. 1985;93:146–147. doi: 10.1177/019459988509300202. [DOI] [PubMed] [Google Scholar]
- Jowett N. A general approach to facial palsy. Otolaryngol Clin North Am. 2018;51:1019–1031. doi: 10.1016/j.otc.2018.07.002. [DOI] [PubMed] [Google Scholar]
- Juliao Caamaño DS, Alonso Beato R. Facial diplegia, a possible atypical variant of Guillain-Barré syndrome as a rare neurological complication of SARS-CoV-2. J Clin Neurosci. 2020;77:230–232. doi: 10.1016/j.jocn.2020.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katusic SK, Beard CM, Wiederholt WC, Bergstralh EJ, Kurland LT. Incidence, clinical features, and prognosis in Bell’s palsy, Rochester, Minnesota, 1968-1982. Ann Neurol. 1986;20:622–627. doi: 10.1002/ana.410200511. [DOI] [PubMed] [Google Scholar]
- Manganotti P, Bellavita G, D’Acunto L et al (2020) Clinical neurophysiology and cerebrospinal liquor analysis to detect Guillain Barré syndrome and polyneuritis cranialis in COVID-19 patients: a case series. J Med Virol. 10.1002/jmv.26289 [DOI] [PMC free article] [PubMed]
- Nunes Duarte-Neto A, de Almeida Monteiro RA, da Silva LFF, et al. Pulmonary and systemic involvement of COVID-19 assessed by ultrasound-guided minimally invasive autopsy. Histopathology. 2020;77:186–197. doi: 10.1111/his.14160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottaviani D, Boso F, Tranquillini E, Gapeni I, Pedrotti G, Cozzio S, Guarrera GM, Giometto B. Early Guillain-Barré syndrome in coronavirus disease 2019 (COVID-19): a case report from an Italian COVID-hospital. Neurol Sci. 2020;41:1351–1354. doi: 10.1007/s10072-020-04449-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owusu JA, Stewart CM, Boahene K. Facial nerve paralysis. Med Clin North Am. 2018;102:1135–1143. doi: 10.1016/j.mcna.2018.06.011. [DOI] [PubMed] [Google Scholar]
- Paterson RW, Brown RL, Benjamin L, Nortley R, Wiethoff S, Bharucha T, Jayaseelan DL, Kumar G, Raftopoulos RE, Zambreanu L, Vivekanandam V, Khoo A, Geraldes R, Chinthapalli K, Boyd E, Tuzlali H, Price G, Christofi G, Morrow J, McNamara P, McLoughlin B, Lim ST, Mehta PR, Levee V, Keddie S, Yong W, Trip SA, Foulkes AJM, Hotton G, Miller TD, Everitt AD, Carswell C, Davies NWS, Yoong M, Attwell D, Sreedharan J, Silber E, Schott JM, Chandratheva A, Perry RJ, Simister R, Checkley A, Longley N, Farmer SF, Carletti F, Houlihan C, Thom M, Lunn MP, Spillane J, Howard R, Vincent A, Werring DJ, Hoskote C, Jäger HR, Manji H, Zandi MS, the UCL Queen Square National Hospital for Neurology and Neurosurgery COVID-19 Study Group (2020) The emerging spectrum of COVID-19 neurology: clinical, radiological and laboratory findings. Brain. 10.1093/brain/awaa240
- Paybast S, Gorji R, Mavandadi S. Guillain-Barré syndrome as a neurological complication of novel COVID-19 infection: a case report and review of the literature. Neurologist. 2020;25:101–103. doi: 10.1097/NRL.0000000000000291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancho-Saldaña A, Lambea-Gil Á, Liesa JLC, Caballo MRB, Garay MH, Celada DR, Serrano-Ponz M. Guillain-Barré syndrome associated with leptomeningeal enhancement following SARS-CoV-2 infection. Clin Med (Lond) 2020;20:e93–e94. doi: 10.7861/clinmed.2020-0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberzahn P, Bouhamidi R, Zwain I, Gaillard JL, Martin B. Testosterone blood content is regulated by testicular aromatization-conjugation in the stallion. Steroids. 1988;52:353–354. doi: 10.1016/0039-128x(88)90143-2. [DOI] [PubMed] [Google Scholar]
- Sullivan FM, Swan IRC, Donnan PT, Morrison JM, Smith BH, McKinstry B, Davenport RJ, Vale LD, Clarkson JE, Hammersley V, Hayavi S, McAteer A, Stewart K, Daly F. Early treatment with prednisolone or acyclovir in Bell’s palsy. N Engl J Med. 2007;357:1598–1607. doi: 10.1056/NEJMoa072006. [DOI] [PubMed] [Google Scholar]
- Zhang W, Xu L, Luo T, Wu F, Zhao B, Li X. The etiology of Bell’s palsy: a review. J Neurol. 2020;267:1896–1905. doi: 10.1007/s00415-019-09282-4. [DOI] [PMC free article] [PubMed] [Google Scholar]


