Abstract
Background
Screening of unvaccinated women remains essential to mitigate the high morbidity/mortality of cervical cancer. Here, we compared visual inspection with acetic acid (VIA), recommended by WHO as the most cost-effective screening approach in LMICs, with HPV-based screening, and usage of p16INK4a/Ki-67 dual stain cytology.
Methods
We prospectively enrolled women participating in a VIA-based cervical cancer screening program in two peri-urban health centers of Kenya. Consenting women had a VIA examination preceded by collection of a liquid-based cytology sample from the cervix stored in PreservCyt medium (Hologic®). Analysis of all samples included a hrHPV DNA test and evaluation of a p16INK4a /Ki-67 (CINtecPLUS®) dual stained slide that was prepared using the ThinPrep® 2000 Processor and evaluated by a pathologist trained in the methodology.
Results
In 701 of a total of 800 women aged 18–64 years, all three investigations were performed and data could be analyzed. The HPV, VIA and dual stain cytology positivity were 33%, 7%, and 2% respectively. The HPV positivity rate of VIA positive cases was 32%. The five most common HPV types were HPV16, 52, 68, 58 and 35. The OR among HIV infected women of an HPV infection, VIA positivity and positive dual stain cytology were 2.6 (95%CI 1.5–4.3), 1.9 (95%CI 0.89–4.4) and 3.4 (95%CI 1.07–10.9) respectively. The sensitivity of VIA to detect a p16INK4a/Ki-67 positive transforming infection was 13% (95%CI 2–38).
Conclusions
Primary HPV testing appears feasible and should be considered as a primary screening test also in LMICs. The poor sensitivity of VIA renders it unsuitable as a triage test for HPV positive women. The utility of p16INK4a/Ki-67 dual stain cytology as a triage test for HPV positive women in LMICs should be further studied.
Keywords: Cervical cancer screening, VIA, HPV, p16INK4a/Ki-67, LMICs, Dual staining, HPV genotype, HIV
Introduction
Cancer of the uterine cervix (cervical cancer) is the leading cancer in women in Sub-Sahara Africa [1]. New cervical cancer cases can be effectively reduced by screening tests that allow for the early detection and subsequent treatment of pre-cancerous lesions [2] and by vaccination against human papillomavirus (HPV) infection [3]. In parallel to the desired rapid scale-up of vaccination programs, screening remains important for both the vaccinated and especially the un-vaccinated population [4, 5].
Historically, the most impressive screening results were achieved in industrialized countries by regular cytological assessment of the cervix using the Pap test [6, 7]. However, this test is not considered suitable for screening in developing countries as it requires highly specialized personnel for evaluation and a reliable infrastructure for regular retesting due to the limited specificity and sensitivity profiles and high rates of equivocal results of the Pap test [8, 9].
Instead, for low-income countries the visual inspection of the cervix after application of acetic acid (VIA) coupled with subsequent management of abnormalities by cryotherapy is recommended, in what is now commonly referred to as “screen and treat” strategy [10–16]. VIA has the advantage of being inexpensive with a limited supply-chain burden and of providing results that are apparent at the time of the examination. However, VIA findings are not reliably reproducible and its accuracy for the identification of precancerous lesions is only moderate [17, 18]. Also, VIA programs have faced significant scale-up challenges [19, 20].
A hrHPV infection is a necessary factor for cervical cancer development [2, 21] and a negative HPV test provides high reassurance against precancerous lesions for at least 5–10 years [22]. The high sensitivity of the HPV DNA test [23–25] comes with the cost of moderate specificity as only a fraction of HPV infections progress to cervical cancer [2, 26].
To eliminate unnecessary follow-up of HPV positive women triage by a more specific test [27] is required. Among the novel, more disease-specific molecular markers of cervical cancer p16 INK4a/Ki-67 dual stain cytology has been most extensively studied. This immunocytochemistry test detects cells that have undergone neoplastic transformation in the course of a persistent HPV infection, a stage in the HPV-mediated cervical carcinogenesis that is also called a transforming infection and that correlates morphologically with a CIN2/3 lesion [28–30]. Its high specificity in the diagnosis of cervical intraepithelial neoplasia of grade 2 or higher (CIN2+) has been demonstrated in large organized screening programs [31] and has been widely approved as a triage test for HPV-infected women.
The objective of the present study was to investigate the overall positivity rates for HPV and p16INK4a /Ki-67 dual stain cytology in a peri-urban screening population of Kenyan women and compare the results with the performance of VIA.
Patients and methods
Study design and description of participants
We carried out a cross-sectional diagnostic study in two representative peri-urban health centers (Huruma and Uasin-Gishu) in Eldoret, Uasin Gishu County, Kenya. Women were recruited at the family planning clinics in both health centers. A total of 800 women were consecutively enrolled in this study between September 2016 to November 2017.
Women aged 18–64 years who were living in the catchment area of one of the recruiting health centers were candidates for the study. Eligibility included past or current sexual activity, an intact uterus, ability to undergo informed consent, an interview procedure, and a pelvic examination. Exclusion criteria included hysterectomy, history of cervical cancer, or current pregnancy. Nurses in the two health facilities identified potential participants who attended the clinic and explained the study in detail. Written informed consent was obtained in Kiswahili or English before study enrollment.
The clinical examination involved a gynecological examination with inspection of the cervix uteri and specimen collection by a trained female nurse in a separate room in the health centers. The study nurses in our two sites were trainer within the regional VIA screening program and thus well experienced in VIA examinations.
A cervical smear sample was collected for HPV DNA testing and p16 INK4a /Ki-67 dual stain cytology in PreservCyt® Solution (Hologic) using the Cervex-Brush® (Rover). The sample was then stored at ambient temperature (no direct sunlight) until tested. Finally, the cervix was evaluated 90 s after the application of 5% acetic acid (VIA), the findings were documented with a digital camera.
Women were informed about the VIA result immediately and told that they would get their laboratory test results within 2 months if they were positive. The relaying of laboratory results to the study participants was done through the established infrastructure by health extension workers who had a mobile phone and knew the women. Treatment was provided at the referral center.
Laboratory testing
The first batch of HPV tests (N = 402) were done at the BIOZeq laboratory, Nairobi, Kenya, using the commercially available Hybrid Capture® 2 HR-HPV test (HC2) by Qiagen. The second batch (N = 383) was tested at AML laboratory, Antwerp, Belgium, using the TaqMan-based qPCR assay (RIATOL, Sonic Healthcare Benelux, Antwerp, Belgium) which detects 17 HPV genotypes and β -globin in seven multiplex reactions. These HPV types include all 12 high-risk types (HPV16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59), three probably high-risk types (HPV53, 66 and 68), one low-risk type (HPV6) and one undetermined risk type (HPV67) [32]. All HC2 positive and a subset of HC2 negatives samples were retested using the TaqMan-based qPCR assay.
All dual stain cytology tests were done at the Department of Applied Tumor Biology, University Hospital Heidelberg (ATB), using CINtec PLUS p16/Ki-67® by Roche MTM Laboratories according to the manufacturer instructions. All slides were evaluated by a specialist pathologist. According to the manufacturer’s instruction, one double-stained cell was sufficient to score the sample as positive.
Data collection
At enrollment information was elicited from participants on socio-demographic status and relevant sexual and reproductive health issues including HIV and ART status.
All study information was collected electronically except consent forms. A paper copy of the informed consent forms was offered to the participants. The consent forms with participants’ signatures and national ID numbers was collected and, at the end of the day, secured in locked file cabinets at the base sites. Labels for laboratory samples were handwritten and contained a computer-generated subject identifier and sample date. No personal identifying information can be derived from the labels.
The electronic database was designed in conjunction with SAP®, Walldorf, and consisted of six user interphases (recruiter, nurse, HPV and dual staining laboratory technician as well as HPV and dual stain cytology evaluator). Data captured by nurse, laboratory technician and gynecologist was pseudonymised and regularly uploaded to the HANAcloud®. Two-level passwords were required to perform computer entry and operate the networking programs.
Statistical analysis
Continuous variables were summarized using mean and standard deviation. Categorical variables were summarized using percentages. Prevalence of HPV positivity, dual stain cytology positivity and of VIA positive results were calculated overall and by age groups. A chi-square test was used to compare proportions. Type-specific HPV prevalence was expressed as the proportion of women positive for a given HPV type among all women tested in the indicated group. Odds ratio and 95% confidence intervals were used to compare the within-group proportions. Logistic regression was used to estimate associations between risk factors and outcome of each of the three screening tests. All analyses were performed with SAS [computer program] Version 9.4. Cary, NC: SAS Institute Inc.. For Kappa agreements GraphPad was used (https://www.graphpad.com/quickcalcs/kappa2/. Accessed 15 December 2019).
Ethics considerations
Study approval was granted from the local review board at Moi Teaching Referral Hospital (MTRH) and Moi University, Eldoret, Kenya, and the Institutional Review Board of the Heidelberg University Hospital, Germany.
Results
Study population
Of the 800 enrolled women, 701 (87.6%) had all three tests (VIA, HPV, and dual stain cytology) available for evaluation The baseline characteristics are summarized in Table 1. The median age (IQR) was 30 [25, 33] years. Sixty-four women reported their HIV status as positive, 625 as negative while 12 did not know their status.
Table 1.
Baseline characteristics of study population
| Variables | |
|---|---|
| Age in years | |
| Age, median (IQR) | 30 (25,36) |
| Age < 30, n (%) | 364 (53) |
| Age 30+, n (%) | 323 (47) |
| Parity, n (%) | |
| 1–4 | 593 (85) |
| More | 64 (9) |
| None | 44 (6) |
| Contraception, n (%) | |
| Contraceptive Injection | 220 (43) |
| Intrauterine Contraceptive Device (IUCD) | 122 (24) |
| Contraceptive Implant | 99 (19) |
| Oral Contraception (OC) | 61 (12) |
| Bilateral Tubal Ligation (BTL) | 8 (2) |
| Condom | 5 (1) |
Positivity rate of screening tests
The overall test positivity of HPV, VIA and dual stain cytology were 32.5%, 7.1%, and 2.3% respectively (Table 2). Table 2 also depicts test positivity broken down in age groups. The relationship between test-positives is depicted as Venn diagram in Fig. 1. All 3 tests were positive in 2 cases, the HPV test was positive in 15 of 16 (93.8%) dual stain positive cases, the VIA was positive in 2 of 16 (12.5%) dual stain positive cases and HPV was positive in 16 of 50 (32%) VIA positive cases. The agreement between HPV status and VIA diagnosis had a Kappa of − 0.002 (95%CI − 0.053 to + 0.049).
Table 2.
Prevalence of positive test results for VIA, p16 INK4a/Ki-67 dual-stained cytology, and human papillomavirus testing *p = 0.059 $non-significant
| VIA positive | Dual-stain cytology positive | HPV positive | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Age group | No. | (%) | No. | (%) | No. | (%) | |||
| all women (N = 701) | 50 | 7,1 | 16 | 2,3 | 228 | 32,6 | |||
| women 18–29 y (N = 378) | 23 | 6,1 | $ | 7 | 1,9 | $ | 137 | 36,2 | * |
| women 30 + y (N = 323) | 27 | 8,4 | 9 | 2,8 | 91 | 28,2 | |||
Fig. 1.
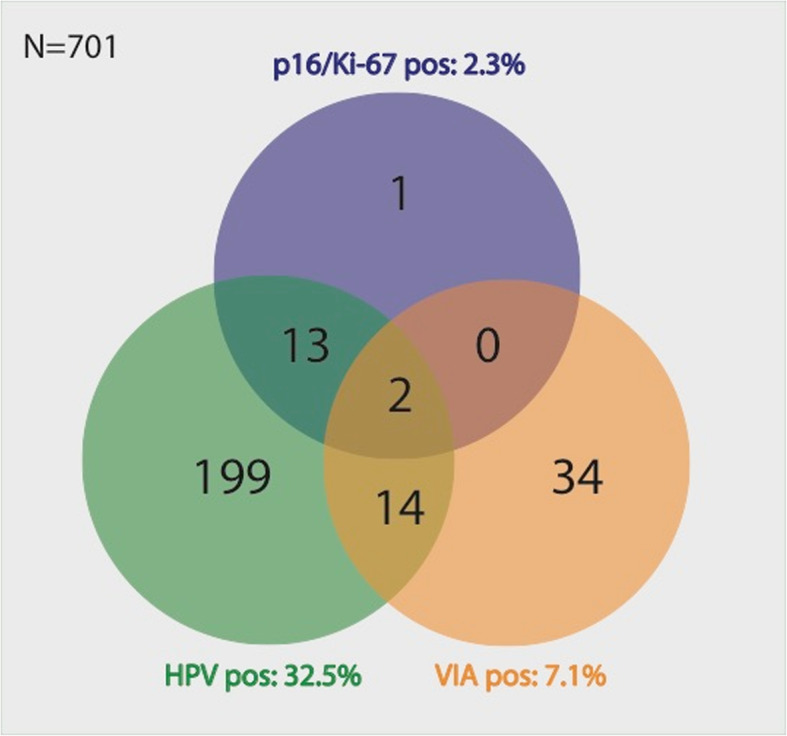
Relationship between test positivities of HPV test, VIA, and dual stain cytology
Risk factors for a positive screening test
In logistic regression models including the risk factors ‘age over 30 years’, ‘HIV-infection’, ‘multipara’, ‘hormonal contraception’ and ‘multiple partners’ a significant association was found between HPV infection and HIV status (p = 0.0002), age (p = 0.023) and multiparity (p = 0.029) and between positive dual stain cytology and HIV status (p = 0.029). No significant association was found between positive VIA and any of the above risk factors. The odds of an HPV infection among HIV positives were 2.6 times higher than among HIV neg (95%CI 1.5–4.3). The odds of a positive VIA among HIV positives were 1.9 times higher than among HIV neg (95%CI 0.89–4.4). The odds of a positive dual stain cytology test among HIV positives were 3.4 times higher than among HIV negatives (95% CI 1.07–10.9).
Frequency and distribution of HPV genotypes
The type-specific hrHPV distribution of the study population is depicted in Fig. 2. The 5 most frequent genotypes were HPV16, 52, 68, 58 and 35.
Fig. 2.
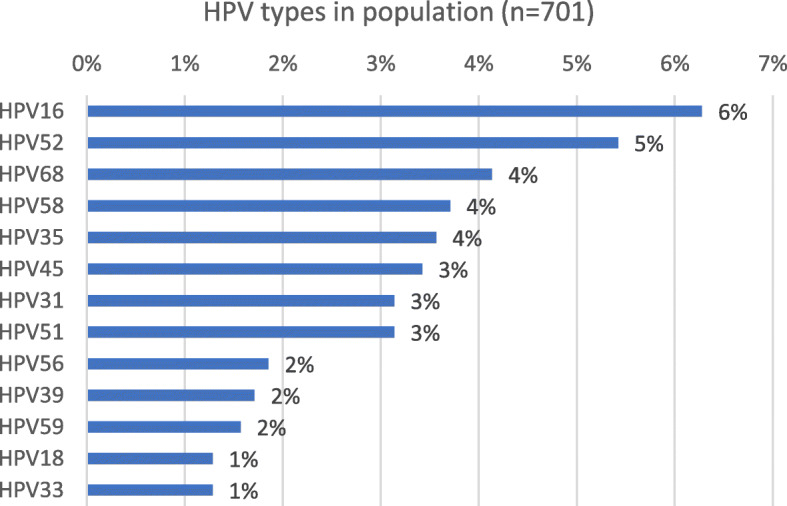
Type-specific HPV frequency in the study population (single and multiple infections, N = 284)
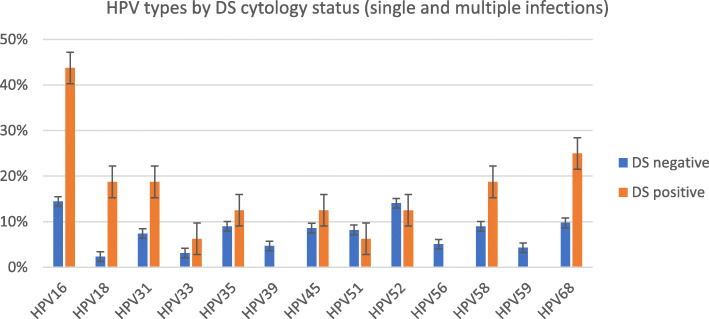
The frequency of hrHPV types among dual stain cytology positive samples is depicted in Fig. 3 and the odds ratio and corresponding 95% confidence intervals for the prevalence of the hrHPV types in dual stain positives compared with those of dual stain negatives are presented in Table 3. The odds ratio for HPV16, 18 in dual stain positive samples were highly significant. The 5 most common hrHPV types among dual stain positive cases were HPV16, 18, 31, 58, 68.
Fig. 3.
Proportion of HPV types among dual stain (DS) cytology positive (N = 15) and negative (N = 686) samples. Error bars represent standard errors
Table 3.
Odds ratio and corresponding 95%CI for HPV types in dual-stain positive compared to dual-stain negative cases and in HIV positive compared to HIV negative cases, (* significant)
| Dual-stain cytology | HIV status | |
|---|---|---|
| HPV 16 | * 13.6 (95%CI 4.8–38.6) | 0,7 (95%CI 0.2–2.3) |
| HPV 18 | * 26.1 (95%CI 5.8–115.9) | 1.2 (95%CI 0.15–9.95) |
| HPV 31 | *8.1 (95%CI 2.1–30.8) | *3 (95%CI 1.1–8.5) |
| HPV 33 | 5,6 (95%CI 0.66–48) | *5.1 (95%CI 1.2–20.8) |
| HPV 35 | 4.1 (95%CI 0.9–19.1) | *5.1 (95%CI 2.1–12.4) |
| HPV 39 | < 0.00 | 3.8 (95%CI 0.99–14.7) |
| HPV 45 | 4.3 (95%CI 0.92–20.1) | 2,7 (95%CI 0.97–7.5) |
| HPV 51 | 2,1 (95%CI 0.3–16.7) | *3 (95%CI 1.1–8.5) |
| HPV 52 | 2.6 (95%CI 0.6–11.18) | *2.4 (95%CI 1.03–5.8) |
| HPV 56 | < 0.00 | < 0.00 |
| HPV 58 | *6.6 (95%CI 1.8–24.9) | 2 (95%CI 0.7–6) |
| HPV 59 | < 0.00 | *4.3 (95%CI 1.1–17.2) |
| HPV 68 | *8 (95%CI 2.7–29.2) | 2.3 (95%CI 0.8–6.3) |
The odds ratio and corresponding 95%CI of hrHPV types in HIV positives as compared with HIV negatives are presented in Table 3. HPV 31, 33, 35, 51, 52, 59, 68 were significantly more frequent in HIV infected women. HIV infected women also harboured more multiple hrHPV infections.
Accuracy of HPV and VIA to detect a transforming HPV infection
The accuracy of VIA and HPV-testing among all women in detecting a p16 INK4a /Ki-67 positive transforming infection are shown in Table 4. HPV had a sensitivity of 0.94 (95%CI: 0.7–0.99) and a specificity of 0.69% (95% CI:0.65–0.72), PPV 0.07 (95% CI:0.06–0.08) and a NPV 0.99 (95% CI: 0.986–0.999). VIA had a sensitivity of 0.13 (95% CI:0.02–0.38), specificity 0.93 (95% CI:0.91–0.95), PPV 0.04 (95% CI:0.001–0.14) and a NPV 0.98 (95% CI,0.97–0.98). No significant difference was found between the age group less than 30 and 30+ years.
Table 4.
Accuracy of HPV and VIA to predict p16 INK4a/Ki-67 dual stain positive infections. PPV = positive predictive value, NPV = negative predictive value
| Test | Sensitivity, % (95% CI) | Specificity, % (95% CI) | PPV, % (95% CI) | NPV, % (95% CI) |
|---|---|---|---|---|
| HPV | 94 (70–100) | 69 (65–72) | 7 (6–8) | 99 (99–100) |
| VIA | 13 (2–40) | 93 (91–95) | 4 (1–14) | 98 (97–98) |
| VIA triage of HPV positives | 13 (1–40) | 93 (89–96) | 12 (3–36) | 93 (92–95) |
Triage of HPV positives using VIA
Sequential testing i.e. VIA on HPV positive cases showed unchanged VIA sensitivity (0.13 (95% CI:0.01–40.5)) and specificity (0.93 (95% CI:0.89–0.96)), the PPV (0.12 (95% CI:0.03–0.36)) performed slightly better but the negative predictive value (0.93 (95% CI:0.92–0.95)) fared worse (Table 4).
Discussion
Main findings
Our study found a high burden of high-risk HPV infections among women attending family planning services in Western Kenya with a more than 2-fold higher burden among HIV infected women. VIA, the current screening test in Kenya and most sub-Saharan African countries (SSA), only poorly correlated with the HPV test, as nearly 2/3 of VIA positive women had no HPV infection and would thus be overtreated.
We used p16INK4a/Ki-67 dual stain cytology in order to differentiate common, and often spontaneously clearing HPV infections from persistent, transforming infections, and their morphological correlate, a CIN2+ lesions. When comparing VIA with dual stain cytology we found that VIA only detected a small fraction of transforming HPV infections and only few among them were HPV 16 and/or 18 infections, which have the highest potential to develop cancer.
A primary HPV testing strategy should be considered also in resource-poor countries. Provided its cost-effectiveness is established, dual stain cytology could be a suitable triage test. The assistance of an electronic data system will greatly facilitate such a multi-contact approach.
Interpretations of results
HPV burden
We found a high overall burden (32.5%) of high-risk HPV infections in our study population, similar to results from another study in our Western Kenya region [34]. A number of other studies done in SSA report lower [33, 35–39] or similarly high [40, 41] HPV burden. All these studies were facility-based with often unknown cervical morbidity and different distribution of risk factors for HPV acquisition, i.e. enrolled populations differed by age distributions and HIV status. A true population HPV prevalence is still lacking for the region. Consistent among all of these studies was the 1.5–2 fold increase of HPV infection among the HIV infected as compared to HIV-negative women- when reported.
HPV genotypes
The five most common genotypes in our study were HPV16, 52, 68, 58, 35. Again, the genotype distribution is influenced by the state of the cervical disease, the geographic region as well as by technical issues including the HPV platform used, assay cut-offs and the selection of hrHPV types included in the analysis. In our study we were able to classify the genotype distribution according to the dual stain cytology findings. A strong association of HPV16, 18 and also HPV31, 58, 68 was found with dual stain cytology positive cases consistent with findings of a meta-analysis that compared normal and HSIL cases in East Africa [42]. In the dual stain negative group HPV16, 52, 35, 58, 68 were most common which is in line with a meta-analysis among African women with normal cytology [43, 44].
Surprisingly we found a high burden of HPV68, a genotype that is uncommon in epidemiological surveys worldwide. In our study the high proportion of HPV68 was found both in the dual stain positive and dual stain negative cases. Two-third of HPV68 was associated with multiple infections and its frequency was more than 2 times higher in HIV infected women. This is consistent with another study in the same region of Kenya where a high HPV68 prevalence among HIV-infected women was found [34]. A study conducted in an isolated rural community in Brazil reported HPV68 as the most prevalent genotype, however it was not present in women with cytological abnormalities [45]. The epidemiological importance of HPV68 needs to be further evaluated, especially as assay-related variation in HPV68 detection has been reported [46].
VIA
The overall VIA positivity in our study population (7.1%), diagnosed by well trained and experienced VIA nurses was similar to reports from India [47], Cameroon [39] and Tanzania [48] but lower than in a meta-analysis of 15 studies in SSA [49] were the pooled estimate of positivity was 17.4% (95&CI 10.4 to 25.6). A high variation in test positivity is observed among VIA studies conducted world wide [49], such discrepancies in VIA positivity is explained by patient characteristics (age, HIV status, precancer prevalence) and inherent procedure issues (high inter-operator variability, unamenable to quality control).
Dual stain cytology
The dual stain cytology positivity of 1.9% in the HIV uninfected population reflects the expected proportion of CIN2+ lesions in similar unscreened populations [49, 50]. Also, the strong association of the highly oncogenic HPV16, 18, 31 with dual stain positivity is well in line with the increased risk of (pre-)cancer associated with these HPV types [51].
All but one dual stain positive samples were also HPV positive. Possible explanation for such rarely found case [52–54] could be a low viral load or an HPV type not included in the common tests.
HIV/HPV co-infection
The detrimental effect of an HIV /HPV co-infection is well known [55, 56]. HIV significantly impacted on the outcome of VIA and dual stain cytology screening. The proportion of dual stain positivity was three times higher among the HIV infected population. The VIA positivity among the HIV infected women was twice as high (12.5%) compared to HIV uninfected women (6.7%). Studies of cohorts with high HIV burden in South Africa and Western Kenya reported high VIA positivity rates ranging from 22 to 55% [13, 57–59].
HIV-infected women in our study carried significantly more single and multiple hrHPV types compared to HIV-negative women. Among the currently available HPV vaccines the nonavalent (9v) vaccine would provide additional protection. Given the substantial number of non-9v hrHPV infections among HIV-infected women found by us and others [60, 61], however, careful post-vaccination followup will be important.
Evaluation of screening technologies
When comparing the studied screening techniques, the poor agreement between HPV status and VIA diagnosis is striking. Only 32% (16/50) of VIA positive cases were hrHPV positive or two third of women would be overtreated based on the VIA test result alone which is consistent with a study from Cameroon where half of all VIA/VILI-DC positive women had no associated hrHPV infection [39].
Our complete dataset gave us the opportunity to evaluate the performance of VIA and HPV testing when using dual stain cytology as a surrogate marker of high-grade cervical lesions. The dual stain biomarker p16INK4a/Ki-67 has a well documented high sensitivity and specificity for identifying the presence of (pre-)cancer [54, 62]. The low sensitivity of VIA in detecting a p16INK4a/Ki-67 positive transforming infections (Table 4) casts serious doubts on the use of VIA as a primary screening test.
As primary HPV testing will likely become the standard of care for cervical cancer screening in both low- and high-resource settings, the search for a suitable triage strategy to avoid overtreatment is crucial [27, 63–65]. The relatively high specificity of VIA suggests that VIA could be used as triage test for HPV positive women [66, 67]. In our study such approach would have slightly improved the positive predictive value of VIA but still be burdened with a low sensitivity.
Dual stain cytology is currently the most extensively studied molecular triage test [31, 68–70], that shows superior performance compared to other disease markers [71, 72]. In centralized laboratories the assay is performed on automated platforms offering high sample throughput. Recent advances in digital imaging and machine learning show promise to also automate the slide evaluation [73]. Although dual stain cytology is unsuitable for self-collected samples [74], it is worthwhile to evaluate the test as part of novel screening algorithms also in LMICS [75–77].
Limitations
There were some limitations to this study. p16INK4a/Ki-67 is a very strong but not a definitive predictor of malignant degeneration (high-grade dysplasia). The gold standard for the diagnosis of high-grade cervical dysplasia is a cervical biopsy. Unfortunately, we could not perform colposcopies and biopsies for case ascertainment. Also, we used two different HPV DNA tests in our study, a nucleic acid hybridization test [78] and a PCR-based (GP5+/6+) test. Both tests, however, were validated in the Valgent study [78]. Finally, the facility-based recruitment, the rural setting and the relatively low sample size preclude generalization of the study findings.
Conclusions
Our findings underscore the superior performance of HPV-based cervical cancer screening over VIA screening in detecting disease at-risk women. When used as triage of HPV positive cases in our high-prevalence setting the positive predictive value of VIA improved, but remained still flawed by an unacceptably low sensitivity. HPV testing should be considered as a primary screening test also in LMICs. P16INK4a/Ki-67 dual stain cytology is an excellent triage test for HPV positive women, its utility for LMICs needs to be further studied.
Acknowledgements
We thank the patients, nurses and other AMPATH Cervical Cancer Screening Program personnel in Kenya who made this study possible. We thank Roche for donating CINtecPLUS® cytology kits and Hologic, Inc. for donating PreservCyt® cytology collection kits. The conduct of the study was supported by the Ladenburg Foundation, New York.
Patient consent for publication
Not required.
Authors’ contributions
All authors read and approved the final manuscript. EOO, EW, HB, MR and MvKD designed the study. EOO, EW, HB, OR, MB, DS, MR and MvKD, analysed and interpreted the data. DS, HS performed and evaluated the HPV testing and dual stain cytology. EOO, KM and EW managed all aspects of the study in Kenya. DVB supervised the performance of laboratory testing. EOO, HB and MvKD drafted the manuscript.
Funding
This work was supported by a grant from the Federal Ministry of Education and Research Germany (BMBF) under a project titled Novel concepts for cervical cancer screening in two rural African regions (01DG13007). The funding body had no role in the design of the study or collection, analysis, and interpretation of data, or in writing the manuscript. Open Access funding enabled and organized by Projekt DEAL.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
Study approval was granted from the local review board at Moi Teaching Referral Hospital (MTRH) and Moi University, Eldoret, Kenya the University of Heidelberg, Germany. Written informed consent was obtained from all participants.
Consent for publication
Not applicable.
Competing interests
None declared.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.International Agency for Research on Cancer (IARC). Kenya-Global Cancer Observatory. Lyon: GLOBOCAN; 2018.
- 2.Schiffman M, Castle PE, Jeronimo J, Rodriguez AC, Wacholder S. Human papillomavirus and cervical cancer. Lancet. 2007;370(9590):890–907. doi: 10.1016/S0140-6736(07)61416-0. [DOI] [PubMed] [Google Scholar]
- 3.Arbyn M, Xu L, Simoens C, Martin-Hirsch PP. Prophylactic vaccination against human papillomaviruses to prevent cervical cancer and its precursors. Cochrane Database Syst Rev. 2018;5:CD009069. doi: 10.1002/14651858.CD009069.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wentzensen N, Klug SJ. Cervical cancer control in the era of HPV vaccination and novel biomarkers. Pathobiology. 2009;76(2):82–89. doi: 10.1159/000201676. [DOI] [PubMed] [Google Scholar]
- 5.Roden RBS, Stern PL. Opportunities and challenges for human papillomavirus vaccination in cancer. Nat Rev Cancer. 2018;18(4):240–254. doi: 10.1038/nrc.2018.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arbyn M, Raifu AO, Weiderpass E, Bray F, Anttila A. Trends of cervical cancer mortality in the member states of the European Union. Eur J Cancer. 2009;45(15):2640–2648. doi: 10.1016/j.ejca.2009.07.018. [DOI] [PubMed] [Google Scholar]
- 7.Kitchener HC, Castle PE, Cox JT. Chapter 7: achievements and limitations of cervical cytology screening. Vaccine. 2006;24(Suppl 3):S3/63–S3/70. doi: 10.1016/j.vaccine.2006.05.113. [DOI] [PubMed] [Google Scholar]
- 8.De Vuyst H, Claeys P, Njiru S, et al. Comparison of pap smear, visual inspection with acetic acid, human papillomavirus DNA-PCR testing and cervicography. Int J Gynecol Obstet. 2005;89(2):120–126. doi: 10.1016/j.ijgo.2005.01.035. [DOI] [PubMed] [Google Scholar]
- 9.Omenge Orang’o E, Liu T, Christoffersen-Deb A, et al. Use of visual inspection with acetic acid, pap smear, or high-risk human papillomavirus testing in women living with HIV/AIDS for posttreatment cervical cancer screening. Aids. 2017;31(2):233–240. doi: 10.1097/QAD.0000000000001327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organisation. WHO guidelines for screening and treatment of precancerous lesions for cervical cancer prevention. 2013. [PubMed]
- 11.Huchko MJ, Sneden J, Sawaya G, et al. Accuracy of visual inspection with acetic acid to detect cervical cancer precursors among HIV-infected women in Kenya. Int J Cancer. 2015;136(2):392–398. doi: 10.1002/ijc.28996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Denny L, Kuhn L, De Souza M, Pollack AE, Dupree W, Wright TC., Jr Screen-and-treat approaches for cervical cancer prevention in low-resource settings: a randomized controlled trial. JAMA. 2005;294(17):2173–2181. doi: 10.1001/jama.294.17.2173. [DOI] [PubMed] [Google Scholar]
- 13.Denny L, Kuhn L, Hu CC, Tsai WY, Wright TC., Jr Human papillomavirus-based cervical cancer prevention: long-term results of a randomized screening trial. J Natl Cancer Inst. 2010;102(20):1557–1567. doi: 10.1093/jnci/djq342. [DOI] [PubMed] [Google Scholar]
- 14.Sauvaget C, Muwonge R, Sankaranarayanan R. Meta-analysis of the effectiveness of cryotherapy in the treatment of cervical intraepithelial neoplasia. Int J Gynaecol Obstet. 2013;120(3):218–223. doi: 10.1016/j.ijgo.2012.10.014. [DOI] [PubMed] [Google Scholar]
- 15.Arbyn M, Sankaranarayanan R, Muwonge R, et al. Pooled analysis of the accuracy of five cervical cancer screening tests assessed in eleven studies in Africa and India. Int J Cancer. 2008;123(1):153–160. doi: 10.1002/ijc.23489. [DOI] [PubMed] [Google Scholar]
- 16.Santesso N, Schünemann H, Blumenthal P, et al. World Health Organization guidelines: use of cryotherapy for cervical intraepithelial neoplasia. Int J Gynecol Obstet. 2012;118(2):97–102. doi: 10.1016/j.ijgo.2012.01.029. [DOI] [PubMed] [Google Scholar]
- 17.Ajenifuja KO, Gage JC, Adepiti AC, et al. A population-based study of visual inspection with acetic acid (VIA) for cervical screening in rural Nigeria. Int J Gynecol Cancer. 2013;23(3):507–512. doi: 10.1097/IGC.0b013e318280f395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mustafa RA, Santesso N, Khatib R, et al. Systematic reviews and meta-analyses of the accuracy of HPV tests, visual inspection with acetic acid, cytology, and colposcopy. Int J Gynaecol Obstet. 2016;132(3):259–265. doi: 10.1016/j.ijgo.2015.07.024. [DOI] [PubMed] [Google Scholar]
- 19.Fokom Domgue J, Valea FA. Is it relevant to keep advocating visual inspection of the cervix with acetic acid for primary cervical Cancer screening in limited-resource settings? J Glob Oncol. 2018;4:1–5. doi: 10.1200/JGO.17.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Silkensen S, Schiffman M, Sahasrabuddhe V, Flanigan J. Is it time to move beyond visual inspection with acetic acid for cervical cancer screening? Glob Health Sci Pract. 2018;6(2):242–246. doi: 10.9745/GHSP-D-18-00206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Walboomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189(1):12–19. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 22.Dijkstra MG, van Zummeren M, Rozendaal L, et al. Safety of extending screening intervals beyond five years in cervical screening programmes with testing for high risk human papillomavirus: 14 year follow-up of population based randomised cohort in the Netherlands. BMJ. 2016;355:i4924. doi: 10.1136/bmj.i4924. [DOI] [PubMed] [Google Scholar]
- 23.Ronco G, Giorgi-Rossi P, Carozzi F, et al. Efficacy of human papillomavirus testing for the detection of invasive cervical cancers and cervical intraepithelial neoplasia: a randomised controlled trial. Lancet Oncol. 2010;11(3):249–257. doi: 10.1016/S1470-2045(09)70360-2. [DOI] [PubMed] [Google Scholar]
- 24.Naucler P, Ryd W, Tornberg S, et al. Human papillomavirus and Papanicolaou tests to screen for cervical cancer. N Engl J Med. 2007;357(16):1589–1597. doi: 10.1056/NEJMoa073204. [DOI] [PubMed] [Google Scholar]
- 25.Mayrand MH, Duarte-Franco E, Rodrigues I, et al. Human papillomavirus DNA versus Papanicolaou screening tests for cervical cancer. N Engl J Med. 2007;357(16):1579–1588. doi: 10.1056/NEJMoa071430. [DOI] [PubMed] [Google Scholar]
- 26.Bulkmans NW, Berkhof J, Bulk S, et al. High-risk HPV type-specific clearance rates in cervical screening. Br J Cancer. 2007;96(9):1419–1424. doi: 10.1038/sj.bjc.6603653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cuschieri K, Ronco G, Lorincz A, et al. Eurogin roadmap 2017: triage strategies for the management of HPV-positive women in cervical screening programs. Int J Cancer. 2018;143(4):735–745. doi: 10.1002/ijc.31261. [DOI] [PubMed] [Google Scholar]
- 28.von Knebel DM, Reuschenbach M, Schmidt D, Bergeron C. Biomarkers for cervical cancer screening: the role of p16(INK4a) to highlight transforming HPV infections. Expert Rev Proteomics. 2012;9(2):149–163. doi: 10.1586/epr.12.13. [DOI] [PubMed] [Google Scholar]
- 29.Wentzensen N, von Knebel Doeberitz M. Biomarkers in cervical cancer screening. Dis Markers. 2007;23(4):315–330. doi: 10.1155/2007/678793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Steenbergen RD, Snijders PJ, Heideman DA, Meijer CJ. Clinical implications of (epi)genetic changes in HPV-induced cervical precancerous lesions. Nat Rev Cancer. 2014;14(6):395–405. doi: 10.1038/nrc3728. [DOI] [PubMed] [Google Scholar]
- 31.Wentzensen N, Clarke MA, Bremer R, et al. Clinical evaluation of human papillomavirus screening with p16/Ki-67 dual stain triage in a large organized cervical Cancer screening program. JAMA Intern Med. 2019;179(7):881–888. doi: 10.1001/jamainternmed.2019.0306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Micalessi IM, Boulet GA, Bogers JJ, Benoy IH, Depuydt CE. High-throughput detection, genotyping and quantification of the human papillomavirus using real-time PCR. Clin Chem Lab Med. 2011;50(4):655–661. doi: 10.1515/cclm.2011.835. [DOI] [PubMed] [Google Scholar]
- 33.Ngabo F, Franceschi S, Baussano I, et al. Human papillomavirus infection in Rwanda at the moment of implementation of a national HPV vaccination programme. BMC Infect Dis. 2016;16:225. doi: 10.1186/s12879-016-1539-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ermel A, Tonui P, Titus M, et al. A cross-sectional analysis of factors associated with detection of oncogenic human papillomavirus in human immunodeficiency virus-infected and uninfected Kenyan women. BMC Infect Dis. 2019;19(1):352. doi: 10.1186/s12879-019-3982-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mutombo AB, Benoy I, Tozin R, Bogers J, Van Geertruyden JP, Jacquemyn Y. Prevalence and distribution of human papillomavirus genotypes among women in Kinshasa, the Democratic Republic of the Congo. J Glob Oncol. 2019;5:1–9. doi: 10.1200/JGO.19.11000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fitzpatrick MB, Dube Mandishora RS, Katzenstein DA, et al. hrHPV prevalence and type distribution in rural Zimbabwe: a community-based self-collection study using near-point-of-care GeneXpert HPV testing. Int J Infect Dis. 2019;82:21–29. doi: 10.1016/j.ijid.2019.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Katanga J, Kjaer SK, Manongi R, et al. Performance of careHPV, hybrid capture 2 and visual inspection with acetic acid for detection of high-grade cervical lesion in Tanzania: a cross-sectional study. PLoS One. 2019;14(6):e0218559. doi: 10.1371/journal.pone.0218559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mchome BL, Kjaer SK, Manongi R, et al. Sex transm infect. 2020. 10.1136/sextrans-2019-054263.
- 39.Cholli P, Bradford L, Manga S, et al. Screening for cervical cancer among HIV-positive and HIV-negative women in Cameroon using simultaneous co-testing with careHPV DNA testing and visual inspection enhanced by digital cervicography: findings of initial screening and one-year follow-up. Gynecol Oncol. 2018;148(1):118–125. doi: 10.1016/j.ygyno.2017.11.002. [DOI] [PubMed] [Google Scholar]
- 40.Mbulawa ZZ, Coetzee D, Williamson AL. Human papillomavirus prevalence in south African women and men according to age and human immunodeficiency virus status. BMC Infect Dis. 2015;15:459. doi: 10.1186/s12879-015-1181-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Castellsagué X, Menéndez C, Loscertales M-P, et al. Human papillomavirus genotypes in rural Mozambique. Lancet. 2001;358(9291):1429–1430. doi: 10.1016/S0140-6736(01)06523-0. [DOI] [PubMed] [Google Scholar]
- 42.Ogembo RK, Gona PN, Seymour AJ, et al. Prevalence of human papillomavirus genotypes among African women with normal cervical cytology and neoplasia: a systematic review and meta-analysis. PLoS One. 2015;10(4):e0122488. doi: 10.1371/journal.pone.0122488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Castellsague X, Klaustermeier J, Carrilho C, et al. Vaccine-related HPV genotypes in women with and without cervical cancer in Mozambique: burden and potential for prevention. Int J Cancer. 2008;122(8):1901–1904. doi: 10.1002/ijc.23292. [DOI] [PubMed] [Google Scholar]
- 44.Bruni L, Diaz M, Castellsague X, Ferrer E, Bosch FX, de Sanjose S. Cervical human papillomavirus prevalence in 5 continents: meta-analysis of 1 million women with normal cytological findings. J Infect Dis. 2010;202(12):1789–1799. doi: 10.1086/657321. [DOI] [PubMed] [Google Scholar]
- 45.Batista JE, Saddi VA, Carvalho KPA, et al. Human papillomavirus genotypes 68 and 58 are the most prevalent genotypes in women from Quilombo communities in the state of Maranhao, Brazil. Int J Infect Dis. 2017;55:51–55. doi: 10.1016/j.ijid.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 46.Jaworek H, Kubanova K, Koudelakova V, Slavkovsky R, Drabek J, Hajduch M. Pitfalls of commercially available HPV tests in HPV68a detection. PLoS One. 2019;14(8):e0220373. doi: 10.1371/journal.pone.0220373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Basu P, Mittal S, Banerjee D, et al. Diagnostic accuracy of VIA and HPV detection as primary and sequential screening tests in a cervical cancer screening demonstration project in India. Int J Cancer. 2015;137(4):859–867. doi: 10.1002/ijc.29458. [DOI] [PubMed] [Google Scholar]
- 48.Ngoma T, Muwonge R, Mwaiselage J, Kawegere J, Bukori P, Sankaranarayanan R. Evaluation of cervical visual inspection screening in Dar Es Salaam, Tanzania. Int J Gynaecol Obstet. 2010;109(2):100–104. doi: 10.1016/j.ijgo.2009.11.025. [DOI] [PubMed] [Google Scholar]
- 49.Fokom-Domgue J, Combescure C, Fokom-Defo V, et al. Performance of alternative strategies for primary cervical cancer screening in sub-Saharan Africa: systematic review and meta-analysis of diagnostic test accuracy studies. BMJ. 2015;351:h3084. doi: 10.1136/bmj.h3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhao S, Zhao X, Hu S, et al. Distribution of high-risk human papillomavirus genotype prevalence and attribution to cervical precancerous lesions in rural North China. Chin J Cancer Res. 2019;31(4):663–672. doi: 10.21147/j.issn.1000-9604.2019.04.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jiang MY, Wu Z, Li T, et al. Performance of HPV genotyping combined with p16/Ki-67 in detection of cervical Precancer and Cancer among HPV-positive Chinese women. Cancer Prev Res (Phila) 2020;13(2):163–172. doi: 10.1158/1940-6207.CAPR-19-0144. [DOI] [PubMed] [Google Scholar]
- 52.Tjalma W. HPV negative cervical cancers and primary HPV screening. Facts Views Vis Obgyn. 2018;10(2):107–113. [PMC free article] [PubMed] [Google Scholar]
- 53.Dona MG, Vocaturo A, Giuliani M, et al. p16/Ki-67 dual staining in cervico-vaginal cytology: correlation with histology, human papillomavirus detection and genotyping in women undergoing colposcopy. Gynecol Oncol. 2012;126(2):198–202. doi: 10.1016/j.ygyno.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 54.Ikenberg H, Bergeron C, Schmidt D, et al. Screening for cervical cancer precursors with p16/Ki-67 dual-stained cytology: results of the PALMS study. J Natl Cancer Inst. 2013;105(20):1550–1557. doi: 10.1093/jnci/djt235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Denny L, Adewole I, Anorlu R, et al. Human papillomavirus prevalence and type distribution in invasive cervical cancer in sub-Saharan Africa. Int J Cancer. 2014;134(6):1389–1398. doi: 10.1002/ijc.28425. [DOI] [PubMed] [Google Scholar]
- 56.Clifford GM, de Vuyst H, Tenet V, Plummer M, Tully S, Franceschi S. Effect of HIV infection on human papillomavirus types causing invasive cervical Cancer in Africa. J Acquir Immune Defic Syndr. 2016;73(3):332–339. doi: 10.1097/QAI.0000000000001113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Huchko MJ, Sneden J, Leslie HH, et al. A comparison of two visual inspection methods for cervical cancer screening among HIV-infected women in Kenya. Bull World Health Organ. 2014;92(3):195–203. doi: 10.2471/BLT.13.122051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mabeya H, Khozaim K, Liu T, et al. Comparison of conventional cervical cytology versus visual inspection with acetic acid among human immunodeficiency virus-infected women in Western Kenya. J Low Genit Tract Dis. 2012;16(2):92–97. doi: 10.1097/LGT.0b013e3182320f0c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Khozaim K, Orang'o E, Christoffersen-Deb A, et al. Successes and challenges of establishing a cervical cancer screening and treatment program in western Kenya. Int J Gynaecol Obstet. 2014;124(1):12–18. doi: 10.1016/j.ijgo.2013.06.035. [DOI] [PubMed] [Google Scholar]
- 60.Menon S, Wusiman A, Boily MC, et al. Epidemiology of HPV genotypes among HIV positive women in Kenya: a systematic review and meta-analysis. PLoS One. 2016;11(10):e0163965. doi: 10.1371/journal.pone.0163965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kojic EM, Conley L, Bush T, et al. Prevalence and incidence of anal and cervical high-risk human papillomavirus (HPV) types covered by current HPV vaccines among HIV-infected women in the SUN study. J Infect Dis. 2018;217(10):1544–1552. doi: 10.1093/infdis/jiy087. [DOI] [PubMed] [Google Scholar]
- 62.Tjalma WAA. Diagnostic performance of dual-staining cytology for cervical cancer screening: a systematic literature review. Eur J Obstet Gynecol Reprod Biol. 2017;210:275–280. doi: 10.1016/j.ejogrb.2017.01.009. [DOI] [PubMed] [Google Scholar]
- 63.Rijkaart DC, Berkhof J, van Kemenade FJ, et al. Evaluation of 14 triage strategies for HPV DNA-positive women in population-based cervical screening. Int J Cancer. 2012;130(3):602–610. doi: 10.1002/ijc.26056. [DOI] [PubMed] [Google Scholar]
- 64.Wentzensen N. Triage of HPV-positive women in cervical cancer screening. Lancet Oncol. 2013;14(2):107–109. doi: 10.1016/S1470-2045(12)70568-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Basu P, Meheus F, Chami Y, Hariprasad R, Zhao F, Sankaranarayanan R. Management algorithms for cervical cancer screening and precancer treatment for resource-limited settings. Int J Gynaecol Obstet. 2017;138(Suppl 1):26–32. doi: 10.1002/ijgo.12183. [DOI] [PubMed] [Google Scholar]
- 66.Bigoni J, Gundar M, Tebeu PM, et al. Cervical cancer screening in sub-Saharan Africa: a randomized trial of VIA versus cytology for triage of HPV-positive women. Int J Cancer. 2015;137(1):127–134. doi: 10.1002/ijc.29353. [DOI] [PubMed] [Google Scholar]
- 67.Muwonge R, Wesley RS, Nene BM, et al. Evaluation of cytology and visual triage of human papillomavirus-positive women in cervical cancer prevention in India. Int J Cancer. 2014;134(12):2902–2909. doi: 10.1002/ijc.28627. [DOI] [PubMed] [Google Scholar]
- 68.Uijterwaal MH, Polman NJ, Witte BI, et al. Triaging HPV-positive women with normal cytology by p16/Ki-67 dual-stained cytology testing: baseline and longitudinal data. Int J Cancer. 2015;136(10):2361–2368. doi: 10.1002/ijc.29290. [DOI] [PubMed] [Google Scholar]
- 69.Wright TC, Jr, Behrens CM, Ranger-Moore J, et al. Triaging HPV-positive women with p16/Ki-67 dual-stained cytology: results from a sub-study nested into the ATHENA trial. Gynecol Oncol. 2017;144(1):51–56. doi: 10.1016/j.ygyno.2016.10.031. [DOI] [PubMed] [Google Scholar]
- 70.Ebisch RMF, Siebers AG, Bosgraaf RP, Massuger LFAG, Bekkers RLM, Melchers WJG. Triage of high-risk HPV positive women in cervical cancer screening. Expert Rev Anticancer Ther. 2016;16(10):1073–1085. doi: 10.1080/14737140.2016.1232166. [DOI] [PubMed] [Google Scholar]
- 71.Rossi PG, Carozzi F, Ronco G, et al. p16/ki67 and E6/E7 mRNA accuracy and prognostic value in triaging HPV DNA-positive women. J Natl Cancer Inst. 2020. 10.1093/jnci/djaa105.
- 72.Zhu Y, Ren C, Yang L, Zhang X, Liu L, Wang Z. Performance of p16/Ki67 immunostaining, HPV E6/E7 mRNA testing, and HPV DNA assay to detect high-grade cervical dysplasia in women with ASCUS. BMC Cancer. 2019;19(1):271. doi: 10.1186/s12885-019-5492-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wentzensen N, Lahrmann B, Clarke MA, et al. Accuracy and efficiency of deep-learning-based automation of dual stain cytology in cervical Cancer screening. J Natl Cancer Inst. 2020;113:1–8. [DOI] [PMC free article] [PubMed]
- 74.Toliman PJ, Phillips S, de Jong S, et al. Evaluation of p16/Ki-67 dual-stain cytology performed on self-collected vaginal and clinician-collected cervical specimens for the detection of cervical pre-cancer. Clin Microbiol Infect. 2019;26(6):748–752. doi: 10.1016/j.cmi.2019.10.020. [DOI] [PubMed] [Google Scholar]
- 75.Ngugi CW, Schmidt D, Wanyoro K, et al. p16(INK4a)/Ki-67 dual stain cytology for cervical cancer screening in Thika district, Kenya. Infect Agent Cancer. 2015;10:25. doi: 10.1186/s13027-015-0020-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jede F, Brandt T, Gedefaw M, et al. Home-based HPV self-sampling assisted by a cloud-based electronic data system: lessons learnt from a pilot community cervical cancer screening campaign in rural Ethiopia. Papillomavirus Res. 2020;9:100198. doi: 10.1016/j.pvr.2020.100198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Termrungruanglert W, Khemapech N, Tantitamit T, Havanond P. Cost effectiveness analysis of HPV primary screening and dual stain cytology triage compared with cervical cytology. J Gynecol Oncol. 2019;30(2):e17. doi: 10.3802/jgo.2019.30.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Arbyn M, Depuydt C, Benoy I, et al. VALGENT: a protocol for clinical validation of human papillomavirus assays. J Clin Virol. 2016;76(Suppl 1):S14–S21. doi: 10.1016/j.jcv.2015.09.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.