Abstract
Background
Although prior studies showed a correlation between environmental manganese (Mn) exposure and neurodevelopmental disorders in children, the results have been inconclusive. There has yet been no consistent biomarker of environmental Mn exposure. Here, we summarized studies that investigated associations between manganese in biomarkers and childhood neurodevelopment and suggest a reliable biomarker.
Methods
We searched PubMed and Web of Science for potentially relevant articles published until December 31th 2019 in English. We also conducted a meta-analysis to quantify the effects of manganese exposure on Intelligence Quotient (IQ) and the correlations of manganese in different indicators.
Results
Of 1754 citations identified, 55 studies with 13,388 subjects were included. Evidence from cohort studies found that higher manganese exposure had a negative effect on neurodevelopment, mostly influencing cognitive and motor skills in children under 6 years of age, as indicated by various metrics. Results from cross-sectional studies revealed that elevated Mn in hair (H-Mn) and drinking water (W-Mn), but not blood (B-Mn) or teeth (T-Mn), were associated with poorer cognitive and behavioral performance in children aged 6–18 years old. Of these cross-sectional studies, most papers reported that the mean of H-Mn was more than 0.55 μg/g. The meta-analysis concerning H-Mn suggested that a 10-fold increase in hair manganese was associated with a decrease of 2.51 points (95% confidence interval (CI), − 4.58, − 0.45) in Full Scale IQ, while the meta-analysis of B-Mn and W-Mn generated no such significant effects. The pooled correlation analysis revealed that H-Mn showed a more consistent correlation with W-Mn than B-Mn. Results regarding sex differences of manganese associations were inconsistent, although the preliminary meta-analysis found that higher W-Mn was associated with better Performance IQ only in boys, at a relatively low water manganese concentrations (most below 50 μg/L).
Conclusions
Higher manganese exposure is adversely associated with childhood neurodevelopment. Hair is the most reliable indicator of manganese exposure for children at 6–18 years of age. Analysis of the publications demonstrated sex differences in neurodevelopment upon manganese exposure, although a clear pattern has not yet been elucidated for this facet of our study.
Keywords: Manganese exposure, Biomarker, Cognitive function, Behavior, Motor
Background
Environmental metal exposure normally occurs in co-exposure to multiple metals, such as lead, cadmium, arsenic, mercury, chromium and manganese. Among these metals, manganese (Mn) is an essential trace element [1], but it is toxic, especially for brain functions, when abnormally deposition occurs in the body [2].
Growing interest has been recently generated to understand environmental manganese exposure in children [3, 4]. Meta-analysis about autism spectrum disorder (ASD) indicated that the mean difference in blood and hair manganese concentrations between ASD and control individuals was not significant [5]. In terms of neurocognitive development, these epidemiological studies had inconsistent conclusions across different biomarkers [6–9], which also left open the question as to whether there exists a useful biomarker for Mn exposure.
Evidence-based studies have also evaluated this association between manganese in hair and childhood IQ [10]. However, no comprehensive meta-analysis has been performed to examine Mn associations between different indicators and neurodevelopment. Thus, to the best of our knowledge, no meta-analysis has been performed regarding the putative correlation between such Mn indicators. Compared with cognition, the impacts of Mn on behavioral and motor development in children have been less evaluated, although Mn-related motor changes, such as in manganism, have been evaluated more extensively in occupational exposures [11, 12]. In addition, the potential for sex difference in the consequences of manganese exposure has also drawn attention, as there may be some differences between males and females in patterns of exposure, gastrointestinal absorption of chemicals, metabolism and detoxification [13].
To address these research gaps, the goal of this systematic review and meta-analysis has been to summarize and quantify the scientific evidence through different biomarkers or sources in order to obtain a clearer understanding of the exposure-response relationship between Mn indicators (biomarkers or environmental samples) and neurodevelopmental outcomes. In addition, we performed meta-analyses to seek a pooled correlation between Mn indicators (hair, blood and drinking water) and, here, suggested a potential biomarker for further epidemiologic studies of the toxic impact of Mn in childhood neurodevelopment. We also performed a preliminary meta-analysis to quantify the sex difference between manganese indicators and intelligence. Our conclusions provide useful suggestions for future public health studies, especially on the consequences of heavy metal exposures, such as Mn, towards human health.
Methods
Search strategy and inclusion criteria
Our study was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) Statement. The completed PRISMA checklist is provided in Additional file 1. This systematic review protocol was registered with PROSPERO (CRD42020182284). Two investigators (authors W.L. and Y.X.) independently conducted a literature search in PubMed and Web of Science for studies published through December 31th 2019 in English, using the following search terms: (“manganese” or “manganism” or “manganese exposure”) and (“children” or “child” or “infant” or “childhood” or “adolescents” or “early life” or “young” or “younger populations”) and (“neurotoxicity” or “neuropsychological effects” or “neurodevelopmental outcomes” or “cognition” or “cognitive” or “intellectual function” or “intellectual impairment” or “intelligence quotient” or “IQ” or “memory” or “attention” or “mental” or “academic performance” or “hyperactivity” or “behavior” or “hyperactive behaviors” or “neurobehavior” or “motor” or “neuromotor”). In addition, the references included in relevant articles were searched for additional eligible publications.
Studies included in this systematic review had to meet the following criteria of being: (1) An original peer reviewed article; (2) A study of populations up to 18 years of age; (3) Manganese exposure was assessed through medicinal biomarkers (i.e. hair and blood) or environmental samples (i.e. drinking water); (4) A study of neurodevelopment derived from manganese exposure, including: cognitive, behavioral and/or motor changes; (5) Potential confounders were adjusted in the mathematical model for the estimated association between Mn indicator and a specific neurological outcome in children.
For inclusion in the meta-analysis, studies had to satisfy the above criteria and had to have measured the effect of manganese exposure on neurodevelopment by regression models, while for correlation analysis, the correlation coefficient (r) was provided. We excluded studies about attention deficit hyperactivity disorder (ADHD), which was reviewed in a recent paper, and the results of which showed higher peripheral manganese concentrations in children diagnosed with ADHD than those in controls [14]. We did not exclude articles published using the same population with different neurodevelopmental assessments [15, 16].
Data extraction and quality assessment
The following information was extracted by two investigators (W.L. and Y.X.) independently using a standardized data collection form: first author, publication year, biomarker, country/study name, study design, sample size, age, sources of manganese exposure, neurological assessments and neurodevelopmental outcomes. For meta-analysis, the regression coefficient (β) with its 95% confidence interval (CI) and correlation coefficient (r) were also extracted. In the event of multiple articles published using the same population when assessing neurodevelopmental outcomes at different ages, and the same data were used in more than one publication, we consistently selected the most informative article, which was usually the most recent publication.
The guideline for Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) was applied to assess the methodological quality of each study by two investigators (W.L. and Y.X.) independently [17]. The STROBE Statement is a checklist of 22 items that was initially developed to evaluate the systematic clarity in communicating research results in observational studies. This checklist has been used in systematic reviews to evaluate the methodological quality of observational studies [10, 18]. Nine items in methods (items 4–12) were selected, which covering the different aspects of methodology in observational studies. The methodological quality was classified by the number of items that the research met. To be more specific, articles that met 0–3 items, 4–6 items and 7–9 items were regarded as low, moderate and high methodological quality, respectively. Any disagreements were resolved by group discussion with a third investigator (Q.L.).
Statistical analysis
A regression coefficient (β) with corresponding 95% CI was used as the common measure of association across studies [6–8, 16, 19–21]. A study that stratified by sex was treated as two separate reports [20]. We used a random-effects model to calculate the summarized β metrics and their corresponding 95% CIs. The meta-analysis was restricted to studies that used the Wechsler scales to evaluate IQ and linear regression models to examine the relationships between manganese exposure and children’s IQ scores. One study exhibited the scores of estimated IQ, vocabulary, block design and digit span, which were subtests from the Wechsler Intelligence Scale [22]. We took the scores of estimated IQ, block design and vocabulary as Full Scale IQ, Performance IQ and Verbal IQ, respectively [23].
Three manganese exposure metrics were included: hair, blood and drinking water. Furthermore, the β metric was estimated through different expressions of manganese concentration: log10, log2, loge or non-transformation. We unified the expression as a log10-transformation to mean that the change in IQ (β) was associated with a 10-fold increase in the manganese exposure indicator, while we did not transform the β in blood, which was transformed using loge consistently.
More specifically, in a linear regression model where the manganese concentration (x) was transformed by logarithm base 2 to correct the skewness of the data distributions, we expressed it into a log10-transformation by the formula log2x * β = log10x * β1 to obtain a new coefficient (β1). The β1 was approximately equal to 3.32 * β. Two studies assessed the effect of manganese exposure with raw manganese concentration. We used the similar formula to transform it into the changes in base log10. Clearly, the β1 was equal to E(x)* β. E(x) was a function about the mean of manganese concentration (x), more specifically, E(x) = x/log10x.
In addition, a meta-analysis of correlation coefficients was also performed. Firstly, the Fisher’s z transformation was used to transform data as below,
| 1 |
| 2 |
| 3 |
Then, we put the Fisher’s z and Standard Error (SE) into RevMan 5.3 using the generic inverse variance random effects model to obtain the summary Fisher’s z. Finally, the formula 3 was used to estimate the summary r [24].
The meta-analysis was performed using Stata version 14 for regression and RevMan 5.3 for correlation. Heterogeneity among studies was estimated using the I2 statistic [25]. A “leave-one-out” sensitivity analysis and subgroup analysis based on the source of exposure were also performed. Publication bias was assessed using Egger’s test with a significant value set to p < 0.10 [26]. Except where noted otherwise, differences with a p-value < 0.05 were considered significant.
Results
A total of 1754 potentially relevant studies were identified through database searches (see Fig. 1). After applying the stringent inclusion and exclusion criteria described in the methods section, 55 original studies encompassing 13,388 children were ultimately included. Fifteen studies reporting 18 outcomes were included in the meta-analysis, with 9 studies for regression and 9 studies for correlation (see Fig. 1).
Fig. 1.
PRISMA flow diagram
The sources of manganese exposure were mainly from industrial activities (i.e. metallurgy and mining) and drinking water (see Tables 1, 2, 3). More studies examined postpartum manganese exposure than prenatal exposure, meanwhile there were also some studies that measured manganese exposure from prenatal to postnatal periods. The concentrations of manganese were more frequently measured in biomarkers (n = 52, i.e. hair, blood and teeth) than environmental samples (n = 21, i.e. drinking water, particulate matter and soil). The associations between manganese in biomarkers and neurodevelopmental outcomes were investigated in 15 cohort studies and 37 cross-sectional studies (see Tables 1, 2). Table 3 presents the associations between manganese in drinking water (W-Mn) and neurodevelopment.
Table 1.
Neurodevelopmental outcomes of manganese exposure mainly prenatal exposure measured in biomarkers from cohort studies
| Author, Year | Age (Years) | Country/ Study Name | Number (Girls/Boys) | Sources | Biomarkers | Neurological Assessments | Associations between Manganese in Biomarkers and Neurodevelopmental Outcomes | Adjustment for Covariates | Study Quality |
|---|---|---|---|---|---|---|---|---|---|
| Chung 2015 [27] | 0.5 | Korea/MOCEH | 232 (124/108) | NA | Maternal blood | BSID-II | An inverted U-shaped: mental and psychomotor development | Maternal age, gestational age, parity, income, breastfeeding status, maternal total calorie intake, residential area, infant sex and birth weight | High |
| Claus Henn 2010 [28] | 1–2 | Mexico | 448# | NA | Blood | BSID-II | An inverted U-shaped: mental development at 1 year of age, 2 years of age: NS | Blood lead, sex, maternal IQ and education, hemoglobin and gestational age | High |
| Claus Henn 2017 [29] | 2 | USA | 224 (91/133) | Mining | Maternal blood, cord blood | BSID-II | Maternal blood: ↓: mental and psychomotor development, cord blood: NS | Maternal age, smoking, gestational period, marital status, parity, income, and prenatal vitamin use | High |
| Freire 2018 [30] | 4–5 | Spain | 302 (86/216) | NA | Placenta | MSCA | Placental Mn: ↓: perceptual-performance function, ↑: memory span and quantitative skills | Child’s sex, psychologist, child age, social class, maternal smoking during pregnancy and pre-pregnancy BMI | High |
| Gunier 2015 [31] | 0.5, 1, 2 | USA/CHAMACOS | 197 (113/84) | NA | Teeth | BSID-II | Postnatal T-Mn: ↓: mental development at 6-months and at 12-months of age | Child’s age, sex, maternal education, IQ, psychometrician, location of assessment, household poverty and HOME score. Postnatal models also adjusted for prenatal Mn | High |
| Lin 2013 [32] | 2 | China/TBPS | 230 (102/128) | NA | Cord blood | CDIIT | Cord blood: ↓: cognition and language | Maternal age, education, fish intake, sex, passive smoking and HOME score | High |
| Mora 2018 [33] | 1 | Costa Rica/ISA | 355 (177/178) | Mancozeb | Maternal blood and hair | BSID-III | Maternal hair: ↓: cognition in girls, maternal blood: NS | Maternal education, parity, gestational period, child age, HOME score and location of assessment | High |
| Takser 2003 [34] | 0.7, 3, 6 | France | 195, 126, 100 (44/56) | NA | * | MSCA | Cord blood: ↓: attention, non-verbal memory, hand skill at 3 years old, the other biomarkers: NS | Child’s sex and mother’s education | Medium |
| Yu 2014 [35] | Newborns | China | 933 (439/494) | NA | Cord serum | NBNA | Cord serum Mn: ↓: fetal neurobehavioral development | Maternal age, education, occupation, incomes, birth weight, passive smoking, gestational age, sex, Pb and Hg | High |
| Yu 2016 [36] | 1 | China/LW birth cohort | 377 (188/189) | NA | Cord serum | GDI | Cord serum Mn: ↓: gross motor and personal-social tasks | Maternal education, income, birth weight, Hg and Fe | High |
| Claus Henn 2018 [37] | 6–16 | Mexico/ELEMENT | 138 (74/64) | Air pollution and diets | Teeth | WRAVMA | NS, stratified by sex, postnatal T-Mn: ↓: visual spatial scores in boys only | Child’s sex, tooth Pb levels, maternal IQ, maternal education and study cohort | High |
| Dion 2018 [20] | 10.5–18 | Canada | 287 (151/136) | Ground water | Hair | WASI | Hair: NS, water Mn increased, Performance IQ scores decreased | Maternal IQ, education and income | High |
| Mora 2015 [38] | 7, 9, 10.5 | USA/CHAMACOS | 248 (140/108) | Mancoze, maneb | Teeth | BASC-2 WISC-IV | Prenatal and postnatal T-Mn: ↓: behavior in boys and girls,↑: motor, memory and cognition in boys | Maternal education, IQ, years in the US, and depression at time of assessment, child’s sex and age, language of maternal interview, HOME score, income and number of children in the home at time of assessment | High |
| Wasserman 2016 [19] | 12.4 ± 0.8 | Bangladesh | 296 | Deep well water with reduced Mn | Blood | WISC-IV | Baseline B-Mn: ↓: working memory, reductions in B-Mn did not translate into improvements in child IQ | Maternal IQ and age, HOME score, child’s school grade, head circumference and plasma ferritin | High |
| Zhou 2019 [39] | 6–8 | China/SMBCS | 296 (126/170) | NA | Cord blood, urine | WISC | Urinary Mn: ↑: Performance IQ in girls | Child sex, maternal age, education, income, inhabitation area and passive smoking | High |
#: 1 year: n = 270 (131/139); 2 years: n = 430 (211/219); *:Maternal blood and hair, cord blood, newborns hair, placenta; ↓: Negative association; ↑: Positive association; NA Not available; NS No significant association. Fe Iron; Hg Mercury; Mn Manganese; Pb Lead. B-Mn Manganese in blood; T-Mn Manganese in teeth. BMI Body mass index; HOME score Home observation for measurement of the environment score; IQ Intelligence Quotient. CHAMACOS The Center for the Health Assessment of Mothers and Children of Salinas study; ELEMENT Early Life Exposures in MExico and NeuroToxicology; ISA Infantes y Salud Ambiental; MOCEH The Mothers and Children’s Environmental Health study; SMBCS Sheyang Mini Birth Cohort Study; TBPS The Taiwan Birth Panel Study
Table 2.
Neurodevelopmental outcomes of manganese exposure measured in biomarkers from cross-sectional studies
| Author, Year | Age (Years) | Country/ Study Name | Number (Girls/Boys) | Sources | Biomarkers | Neurological Assessments | Associations between Manganese in Biomarkers and Neurodevelopmental Outcomes | Adjustment for Covariates | Study Quality |
|---|---|---|---|---|---|---|---|---|---|
| Al-Saleh 2019 [40] | 0.2–1 | Saudi Arabia | 206 (96/110) | NA | Maternal blood and urine, infant urine, breast milk | DDST-II, PEDS | NS | Maternal age and BMI, infant’s age, sex, parity, the location of primary health care centers, maternal education and z score weight for age | Medium |
| Rink 2014 [41] | 1.1–3.7 | Uruguay | 60 (34/26) | NA | Hair | BSID-III | H-Mn: NS | HOME score, age, child Hb, maternal IQ, SES, Pb, marital status, father education and tester | High |
| Bauer 2017 [42] | 10–14 | Italy/PHIME | 142 (79/63) | Fe-Mn alloy plant | Teeth | VRAM | Both low and high prenatal Mn ↓: visuospatial learning and working memory among girls only | Sex, age, SES, videogame use, lead, trial and tooth attrition | High |
| Betancourt 2015 [43] | 11 | Ecuador | 93 (46/47) | Water consumption from the river | Hair | PCM | H-Mn: ↓: IQ | Mother’s literacy | Medium |
| Bhang 2013 [44] | 8–11 | Korea* | 1001 (474/527) | NA | Blood | WASI, ADS, CBCL | B-Mn: ↓: academic performance, such as thinking, reading, calculation, lower Mn ↓: attention | Age, sex, region, children’s IQ, maternal education and age, levels of cotinine and lead | High |
| Bouchard 2007 [45] | 6–15 | Canada | 46 (22/24) | Ground water | Hair | CPRS-R, CTRS-R | H-Mn: ↓: behaviors (teacher-reported hyperactive and oppositional behaviors) | Child’s age, sex and income | High |
| Bouchard 2011 [16] | 6–13 | Canada | 362 (194/168) | Ground water | Hair | WASI | H-Mn: ↓: IQ | Maternal education and IQ, income, home stimulation score, family structure, sex and age of child and IQ testing session, source of water and level of iron in tap water | High |
| Bouchard 2018 [8] | 6–14 | Canada | 259 (132/127) | Ground water | Hair, saliva, toe nail | WISC-IV | NS, possible beneficial effects in boys | Child’s age, maternal IQ and education, income and IQ tester | High |
| Carvalho 2014 [22] | 7–12 | Brazil | 70 (36/34) | Air emissions from Fe-Mn alloy plant | Hair | WISC-III | H-Mn: ↓: estimated IQ, Block Design and Digit Span | Maternal education | High |
| Carvalho 2018 [46] | 7–12 | Brazil | 70 (36/34) | Air emissions from Fe-Mn alloy plant | Hair | NEPSY II | H-Mn: ↓: verbal memory, behaviors (hyperactivity), not motor | Age, sex, SES, mother’s education and mother’s IQ | High |
| Chan 2015 [47] | 11–13 | USA/NICHD | 266 (128/138) | NA | Teeth | DBD | NS | Child’s race, sex, parental education, marital status and SES | High |
| Chiu 2017 [48] | 11–14 | Italy/PHIME | 194 (105/89) | Fe-Mn alloy plant | Teeth | PA, LNMB | Pretnatal T-Mn: ↑: behaviors and motor in boys, T-Mn: ↓: motor (tremor): early postnatal in girls, later postnatal in boys | Children’s age and sex, SES index and tooth attrition | High |
| do Nascimento 2015 [49] | 6–12 | Brazil | 69 (34/35) | Drinking water from well water | Hair, blood | RCPM | H-Mn: ↓: cognitive function, B-Mn: NS | Age, sex and parents’ education | High |
| Ericson 2007 [50] | 3–9 | USA/SECCYD | 27 (16/11) | NA | Teeth | FTT, CBCL | Prenatal T-Mn: ↓: behaviors (hyperactivity) | Pb, mothers’ education, income and child ethnicity | High |
| Frndak 2019 [51] | 6–8 | Uruguay | 345 (155/190) | NA | Hair | CANTAB, W-M | H-Mn: ↑: cognition | Child’s age, sex, Pb, hemoglobin, HOME score, crowding, possessions of wealth and mother’s education | High |
| Haynes 2015 [52] | 7–9 | USA/CARES | 404 (187/217) | Air-borne Mn from Fe-Mn refinery | Hair, blood | WISC-IV | Both low and high Mn: ↓: IQ | Parent IQ | Medium |
| Haynes 2018 [7] | 7–9 | USA/CARES | 106 (65/41) | Air-borne Mn from Industry | Hair, blood | WISC-IV | H-Mn: ↓: IQ, B-Mn: NS | Parent IQ | Medium |
| Hernandez-Bonilla 2011 [53] | 7–11 | Mexico | 172 (84/88) | Air-borne Mn from Mining | Hair, blood | GP, FT, SA | B-Mn: ↓: motor speed and coordination, H-Mn: NS | Pb, Hb, sex, age and maternal education | High |
| Hernandez-Bonilla 2016 [54] | 7–11 | Mexico | 267 (136/131) | Air-borne Mn from mining district | Hair | ROCF | H-Mn: ↓: visuoperception and short-term visual memory | Pb, Hb, child’s age and sex, motor dexterity and mother’s IQ | High |
| Horton 2018 [55] | 8–11 | Mexico/ELEMENT | 133 (69/64) | Air pollution and diets | Teeth | BASC-2 | Prenatal T-Mn: ↑: behaviors, postnatal T-Mn: ↓: behaviors (internalizing problems) | Maternal education and gestational age | High |
| Khan 2011 [56] | 8–11 | Bangladesh | 201 (100/101) | Drinking water from well water | Blood | CBCL | B-Mn: NS | Arsenic, sex, BMI, maternal education and arm circumference | High |
| Kicinski 2015 [57] | 13.6–17 | Belgium | 606 (282/324) | Low-level metal exposure from industrial areas | Blood | FT, CPT, DS | B-Mn: NS | Sex, age, smoking, passive smoking, income, occupation, and maternal education | High |
| Kim 2009 [58] | 8–11 | Korea | 261 (120/141) | NA | Blood | WISC | B-Mn: ↓: IQ | Age, sex, parental education, income, smoking, birth weight and mother’s age | High |
| Lucchini 2012a [59] | 11–14 | Italy | 299 (147/152) | Fe-Mn alloy plant | Hair, blood | WISC | NS | Age, sex, BMI, family size, SES, alcohol consumption, area of residence, Hb, ferritin and parity | High |
| Lucchini 2012b [60] | 11–14 | Italy | 311 (153/158) | Fe-Mn alloy plant | Hair, blood, urine | FT, PA, DPD, LNMB | B-Mn and H-Mn: ↓: motor (tremor), urine, air, water, diet: NS, soil Mn: ↓: tremor intensity | Parity, family size, SES, BMI, maternal education, alcohol intake, smoking, Pb and other metals in air, soil and water. | High |
| Lucchini 2019 [61] | 6–12 | Italy | 299 (161/138) | Industrial emission, with potential contamination of environment | Hair | WISC, CANTAB | H-Mn: ↓: working memory | Sex, age, maternal IQ and cognitive stimulation besides the confounder distance from the point source | High |
| Menezes-Filho 2011 [21] | 6–12 | Brazil | 83 (39/44) | Fe-Mn alloy plant | Hair, blood | WISC-III | H-Mn: ↓: cognition, especially in the verbal domain, B-Mn: NS | Maternal education and nutritional status | Medium |
| Menezes-Filho 2014 [62] | 7–12 | Brazil | 70 (36/34) | Air-borne Mn from Fe-Mn alloy plant | Hair | CBCL | H-Mn: ↓: behaviors (externalizing behaviors), more pronounced in girls | Age, sex and maternal IQ | High |
| Nascimento 2016 [63] | 6–12 | Brazil | 63 (31/32) | Potential contamination from pesticide | Hair, blood | NEUPSILIN-Inf | B-Mn: ↓: visual attention, visual perception and phonological awareness, H-Mn: ↓: working memory | IQ, age, sex and parents’ education | High |
| Oulhote 2014 [15] | 6–13 | Canada | 375 (200/175) | Drinking water from ground water | Hair | WASI, CPT-II, FT, SA | H-Mn: ↓: memory, attention, not hyperactivity, motor: a nonlinear association, ↑: 0.3–0.8 μg/g, ↓: > 10 μg/g, but there were very few observations with such high levels | Child’s sex, age, maternal education and IQ, income, maternal depressive symptoms and tap water lead concentrations. | High |
| Parvez 2011 [64] | 8–11 | Bangladesh | 304 (153/151) | Drinking water from well water | Blood | BOT-2 | B-Mn: NS | Sex, school attendance, head circumference, mother’s intelligence, ferritin, selenium and Pb | High |
| Riojas-Rodríguez 2010 [6] | 7–11 | Mexico | 172 (99/73) | Air-borne Mn from mining district | Hair, blood | WISC | H-Mn: ↓: IQ, B-Mn: NS | Pb, age, sex, nutritional status, maternal education and IQ | High |
| Rugless 2014 [65] | 7–9 | USA/CARES | 55 (35/20) | Air emissions from Fe-Mn refinery | Hair, blood | APS | H-Mn and B-Mn: ↓: postural balance | Sex, height/weight ratio, parent IQ, education, Pb and age | Medium |
| Torrente 2005 [66] | 12–14 | Spain | 100 (61/39) | Industrial emission | Hair | AMP | H-Mn: NS | SES and age | Low |
| Torres-Agustin 2013 [67] | 7–11 | Mexico | 174 (86/88) | Air-borne Mn from mining district | Hair, blood | CAVLT | H-Mn: ↓: long-term memory and learning, B-Mn: NS | Child’s sex, Pb, age, Hb and maternal education | High |
| Wasserman 2006 [9] | 10 | Bangladesh | 142 (72/70) | Drinking water from well water | Blood | WISC-III | B-Mn: NS | Maternal education and IQ, house type, family ownership of a television, child height and head circumference | High |
| Wright 2006 [68] | 11–13 | USA | 31 (16/15) | Mining waste | Hair | WASI | H-Mn: ↓: Full Scale IQ, Verbal IQ | Sex and maternal education | Low |
*: Effects of pollution on neurobehavioral development, and future policies to protect our children; ↓: Negative association; ↑: Positive association; NA Not available; NS No significant association. Fe-Mn Ferro-manganese; Hb Hemoglobin; Mn Manganese; Pb Lead. B-Mn Manganese in blood; H-Mn Manganese in hair; T-Mn Manganese in teeth. BMI Body mass index; HOME score Home observation for measurement of the environment score; IQ Intelligence Quotient; SES Socioeconomic status. CARES Communities Actively Researching Exposure Study; ELEMENT Early Life Exposures in MExico and NeuroToxicology; NICHD National Institute of Child Health and Human Development; PHIME Public Health Impact of Manganese Exposure in susceptible populations, SECCYD Study of Early Child Care and Youth Development
Table 3.
Neurodevelopmental outcomes of manganese exposure measured in drinking water
| Author, Year | Age (Years) | Country/ Study Name | Number (Girls/Boys) | Sources | Neurological Assessments | Associations between Manganese in Drinking Water and Neurodevelopmental Outcomes | Adjustment for Covariates | Study Quality |
|---|---|---|---|---|---|---|---|---|
| Neurodevelopmental outcomes from cohort studies | ||||||||
| Dion 2018 [20] | 10.5–18 | Canada | 287 (151/136) | Ground water | WASI | W-Mn and time-averaged W-Mn: ↓: IQ among girls | Maternal IQ, education and income | High |
| Rahman 2017 [69] | 10 | Bangladesh | 1265 (609/656) | Well water | WISC-IV, SDQ | Prenatal W-Mn: ↑: cognition in girls, W-Mn: ↓: behavior | Maternal IQ, SES, child age, sex, education, height for age, Hb, school type, HOME, tester, number of siblings and arsenic | High |
| Rodrigues 2016 [70] | 1.6–3.3 | Bangladesh | 525 (264/261) | Drinking water from well water | BSID-III | W-Mn: an inverse-U relationship with fine motor function, cognition: NS | Maternal age, maternal education, passive smoking, child’s sex, HOME score, maternal IQ and child’s hematocrit levels | High |
| Neurodevelopmental outcomes from cross-sectional studies | ||||||||
| Bouchard 2011 [16] | 6–13 | Canada | 362 (194/168) | Ground water | WASI | W-Mn and estimated Mn intake from water consumption: ↓: IQ, estimated Mn intake from dietary: NS | Maternal education and IQ, income, home stimulation score, family structure, sex and age of child, IQ testing session, source of water and level of iron in tap water | High |
| Bouchard 2018 [8] | 6–14 | Canada | 259 (132/127) | Drinking water from ground water | WISC-IV | NS, possible beneficial effects in boys | Child’s age, maternal IQ and education, income and IQ tester | High |
| do Nascimento 2015 [49] | 6–12 | Brazil | 69 (34/35) | Drinking water from well water | RCPM | W-Mn: ↓: cognitive function | Age, sex and parents’ education | High |
| Khan 2011 [56] | 8–11 | Bangladesh | 201 (100/101) | Drinking water from well water | CBCL | W-Mn: ↓: behaviors (classroom behavioral problems) | Arsenic, sex, BMI, maternal education and arm circumference | High |
| Khan 2012 [71] | 8–11 | Bangladesh | 840 (444/396) | Well water | AARES | W-Mn > 400 μg/L: ↓: mathematics test | School-grade, parental education and head circumference and controlling for within-teacher correlations in rating the children | High |
| Nascimento 2016 [63] | 6–12 | Brazil | 63 (31/32) | Potential contamination from pesticide | NEUPSILIN-Inf | W-Mn: ↓: written language and executive functions | IQ, age, sex and parents’ education | High |
| Oulhote 2014 [15] | 6–13 | Canada | 375 (200/175) | Drinking water from ground water | WASI, CPT-II, FT, SA | W-Mn: ↓: memory, Mn intake from water:↓: motor function | Child’s sex, age, maternal education and IQ, income, maternal depressive symptoms and tap water lead | High |
| Wasserman 2006 [9] | 10 | Bangladesh | 142 (72/70) | Drinking water from well water | WISC-III | W-Mn: ↓: IQ | Maternal education and IQ, house type, family ownership of a television, child height and head circumference | High |
↓: Negative association; ↑: Positive association; NS No significant association. Hb Hemoglobin; Mn Manganese. W-Mn Manganese in drinking water. BMI Body mass index; HOME score Home observation for measurement of the environment score; IQ Intelligence Quotient; SES Socioeconomic status
The neurological outcomes were assessed more frequently among children between 6 and 18 years of age than children under 6 years old. Amongst children under 6 years old, most studies were cohort studies with the different editions of Bayley Scales of Infant and Toddler Development applied to assess neurodevelopment, and the measurements of manganese mainly reflected prenatal exposures. In the older groups, the well-defined versions of Wechsler Intelligence Scale for Children were used to assess the children’s general cognitive abilities. Specific cognitive functions were assessed through its subtests. For behavioral performance, the variant editions of Conners’ Rating Scale were applied in most studies. For motor coordination, Finger Tapping Test and Luria Nebraska Motor Battery were administered in most studies. Among these studies, the most adjusted confounders in the mathematical model were maternal education, maternal intelligence, child age and sex, which were selected based on established or plausible associations with neurodevelopment. A large percentage (44/55) of included studies was of high quality (see Additional file 2). Except for three studies, all the others described the efforts to address potential sources of bias, such as blinding of exposure status and outcomes assessment, using validated assessment scales and previously trained psychologists.
Manganese in biomarkers and neurodevelopmental outcomes
In children under 6 years of age, evidence from cohort studies in Table 1 revealed that higher manganese exposure had a negative effect on neurodevelopment [29–36], mainly cognitive and motor development. These studies enrolled pregnant women and mainly collected biomarker tissues, such as cord blood, maternal blood and hair, as well as placenta at delivery [29, 30, 32, 34–36]. One study sampled maternal hair and blood at intervals 1–3 times during pregnancy [33]. These biomarkers mentioned above were used to indicate prenatal exposure. The other study collected shed teeth from children beginning at age 7 [31], which provides fine scale temporal profiles of Mn concentrations over the prenatal and early childhood periods. Neurodevelopmental outcomes were assessed by trained psychometricians at follow-up, mainly at 1–2 years of age.
The other two birth cohort studies found an inverted U-shaped association between manganese exposure and cognitive or motor development [27, 28] (see Table 1). Claus Henn et al. (2010) reported that the effect of manganese was apparent for 12-month but diminished for mental development scores at older ages [28], suggesting the possible existence of critical developmental windows. Chung et al. (2015) found a nonlinear dose-response relationship between maternal blood manganese at term and 6-month psychomotor development scores, with a peak point approximately 24–28 μg/L, suggesting adverse neurodevelopmental effects of both low (< 20.0 μg/L) and high (≥ 30.0 μg/L) maternal blood manganese concentrations [27].
The results from cohort studies concerning children over 6 years old were intriguing. Two follow-up studies in Bangladesh and Canada were conducted to evaluate whether changes in drinking water manganese exposure were associated with changes in child intellectual outcomes. In Bangladesh, Wasserman et al. (2016) found that during 2 years of follow-up, the reduction in exposure (indicated by manganese in blood, B-Mn) was not, for the most part, translated into improvements in child IQ. In this cohort, baseline B-Mn was negatively associated with working memory after covariate adjustment [19]. In Quebec (Canada), the result revealed that, for children whose Mn concentrations in their water supply increased between baseline and follow-up, their Performance IQ scores decreased significantly. On the other hand, at follow-up, higher manganese in drinking water was associated with lower Performance IQ in girls, whereas the opposite was observed in boys. Similar trends were observed in hair [20]. Although the results of cohort studies need to be verified, they also suggest the importance of preventing such exposures.
Inconsistent conclusions were drawn from three cohort studies, one measured manganese in cord blood and spot urine [39], the other two sampled dentine of incisors [37, 38] (see Table 1). The birth cohort study in China reported that urinary Mn concentrations, but not cord blood manganese, were positively associated with Performance IQ of school-age children, especially in girls [39]. Mora et al. (2015) found that higher prenatal and early postnatal manganese in teeth (T-Mn) were adversely associated with behavioral outcomes, namely internalizing, externalizing and hyperactivity problems, in children at 7 and 10.5 years. In the sex stratified models, Mora et al.(2015) found that higher prenatal and postnatal T-Mn were associated with better memory abilities at ages 9 and 10.5, and better cognitive and motor outcomes at ages 7 and 10.5 years, among boys only [38]. On the other hand, Claus Henn et al. (2018) found that higher postnatal T-Mn was negatively associated with both Wide Range Assessment of Visual Motor Abilities (WRAVMA) total and visual spatial subtest scores, among boys only [37]. Mn interactions with lead (Pb) were also examined. Mora et al. (2015) reported that higher prenatal T-Mn was associated with poorer visuospatial memory outcomes at 9 years and worse cognitive scores at 7 and 10.5 years in children with higher prenatal blood lead concentrations (≥ 0.8 μg/dL) [38]. And Claus Henn et al. (2018) found that tooth Mn was positively associated with visual spatial and total WRAVMA scores in the second trimester, among children with lower (< median) tooth Pb concentrations, while no significant Mn association was observed at high Pb concentrations [37]. These inconsistent findings may be due to differences in biomarkers (blood and urine vs. teeth) or sources of Mn exposure (Mn-containing fungicides vs. dietary and airborne sources).
Although most cohort studies found adverse association between manganese exposure and neurodevelopment, Mn interactions with sex and other metals, such as Pb, were gaining attention. Among these studies, only six studies described the sources of manganese exposure during pregancy, such as mining, mancozeb and drinking water, the concentration of manganese was only measured in drinking water in two studies [19, 20]. And Dion et al. (2018) reported that Mn in hair (H-Mn) correlated with W-Mn at follow-up (r, 0.48; p < 0.001) and with time-averaged W-Mn (r, 0.43; p < 0.001) [20].
Two out of the 37 cross-sectional studies investigated associations between Mn exposure and developmental scores in infants. Postnatal manganese exposure were measured in breast milk, blood, urine and hair, no significant association was observed [40, 41], with significantly negative association in the unadjusted model [41] (see Table 2).
There were 35 studies concerning children over 6 years old, and these also measured manganese in related biomarkers, such as hair (n = 24) [6–8, 15, 16, 21, 22, 43, 45, 46, 49, 51–54, 59–63, 65–68], blood (n = 17) [6, 7, 9, 21, 44, 49, 52, 53, 56–60, 63–65, 67], teeth (n = 5) [42, 47, 48, 50, 55], saliva, toe nail [8] and urine [60]. One study could be included when using more than one biomarker, as was the case in New Brunswick (Canada), which measured manganese in hair, saliva and toe nail [8].
A central result was that elevated H-Mn was associated with poorer cognitive and behavioral performance in most studies (n = 17), in terms of IQ [6, 7, 16, 21, 22, 43, 49, 52, 68], working memory [61, 63], verbal memory [46], visuoperception and short-term visual memory [54], long-term memory and learning abilities [67], memory and attention [15], hyperactivity behaviors [45, 46], oppositional behaviors [45] and externalizing behavioral problems [62] (see Table 2). Among them, Oulhote et al. (2014) found that there was no significant association between manganese exposure and hyperactivity [15]. In this case, a large percent (13/17) of the studies reported that the mean of manganese in hair exceeded 0.55 μg/g, which was similar to the mean concentration from control groups [6, 54]. Haynes et al. (2015) also found that compared with the middle two quartiles, the lowest quartiles of H-Mn (< 0.21 μg/g) was associated with significantly lower mean perceptual reasoning scores [52]. Similarly, one cross-sectional study revealed a positive association between H-Mn and cognitive function in children aged at 6–8 years, with a low median concentration of Mn in hair (0.82 ng/g) [51]. No significant associations were found in three studies in terms of cognitive functions [8, 59, 66] and behavioral performance [59], with the average of manganese in hair ranged from 0.17 μg/g to 0.3 μg/g. For motor function, two studies showed that elevated H-Mn was associated with tremor intensity [60] and poor postural balance [65], while two articles found no association between H-Mn and motor function [46, 53]. In another publication, Oulhote et al. (2014) found a nonlinear association between H-Mn and motor function, with a slight increase at concentrations between 0.3 and 0.8 μg/g, and an apparent decrease in scores at H-Mn > 10 μg/g, although there were very few observations with such high concentrations [15]. This inconsistency may possibly due to the different levels of manganese exposure and the sensitivity of scales, as the average concentration of H-Mn ranged widely from 0.16 μg/g to 14.6 μg/g [15, 46, 53, 60, 65]. Carvalho et al. (2018) reported poorer cognition and behavior, while no effect on motor in the same exposure population [46] (see Table 2).
In contrast to hair, most (n = 9) reports in Table 2 indicated that B-Mn was non-significantly associated with cognitive and behavioral development [6, 7, 9, 21, 49, 56, 57, 59, 67]. However, two studies did show that elevated B-Mn was associated with poorer cognitive development, when using IQ [58], visual attention, visual perception and phonological awareness [63] as outcome measures. Two publications suggested that both low and high B-Mn were negatively associated with cognitive and behavioral development [44, 52]. In relation to motor development, three studies showed that elevated B-Mn was associated with impairment of motor functions, namely tremor intensity [60], postural balance [65], coordination and motor speed [53]. However, one study indicated no significant association [64] (see Table 2). Among these reports, the mean concentration of manganese in blood was mainly around 10 μg/L, suggesting the relatively homeostatic regulation of blood manganese.
Five publications used teeth as a biomarker [42, 47, 48, 50, 55] (see Table 2). One study found an inverted U-shaped association between prenatal Mn and visuospatial ability in girls, no significant associations were found in postnatal Mn [42]. Ericson et al. (2007) found that higher Mn in teeth was adversely associated with behavioral outcomes [50]. Horton et al.(2018) revealed that prenatal Mn exposure appeared to be protective against behavioral outcomes, yet postnatal Mn appeared as a risk factor for behavioral outcomes [55]. Two of which indicated that there were no significant associations between Mn in deciduous teeth and behavioral [47] and motor development [48]. These results suggested that Mn associations were partly driven by exposure timing and modified by sex. Three studies also found that tooth Mn concentrations were higher in the prenatal than postnatal period [42, 48, 55], indicating a greater demand for manganese in the prenatal period. No significant associations were observed between neurodevelopmental outcomes and Mn in saliva, toe nail [8], and urine [60] (see Table 2).
Manganese in drinking water and neurodevelopmental outcomes
Evidience from cohort studies indicated that elevated W-Mn was associated with lower IQ scores in girls [20] and the increased risk of children’s behavioral problems at 10 years of age [69] (see Table 3). While Rodrigues et al. (2016) also found an inverted U-shaped association between W-Mn and motor development with an inflection point around 400 μg/L [70].
Most (n = 7) cross-sectional studies found that higher W-Mn was associated with poorer cognitive and behavioral function, such as IQ [9, 16, 49], memory [15], written language [63], mathematics scores [71] and the risk of behavioral problems [56]. The mean concentrations of W-Mn were shown to range from 795 to 1387.9 μg/L in three studies conducted in Araihazar, a rural area of Bangladesh [9, 56, 71], which were much higher than W-Mn in Canada, with its arithmetic mean of 98 μg/L reported in two studies [15, 16]. The W-Mn in two studies conducted in Brazil was much lower, with the mean of W-Mn around 20 μg/L in the rural group [49, 63]. By contrast, two reports found no clear association between W-Mn and childhood IQ [8] and behavioral function [15] (see Table 3). Both studies were conducted in Canada [8, 15], one described a situation where W-Mn was low, with approximately half of children’s home tap water with a manganese concentration less than 5 μg/L [8]. For motor function, the association with W-Mn was significant, with a threshold indicating that scores decreased more steeply at concentrations above 180 μg/L, and this research also found that manganese intake from water was negatively associated with motor function [15]. Of note, most studies also measured manganese in hair or blood, the conclusions were mainly consistent with H-Mn [15, 16, 49, 63], whereas the inconsistency was observed in blood [9, 56].
Pooled effect estimates for IQ scores
The details for our meta-analysis were extracted to include three manganese exposure metrics: hair, blood and drinking water, as shown in Additional file 3. Among these studies, seven researches had a cross-sectional design, two cohort studies were treated as cross-sectional studies by using the associations between Mn exposure and concurrent IQ scores at baseline examinations [19] or follow-up examinations [20].
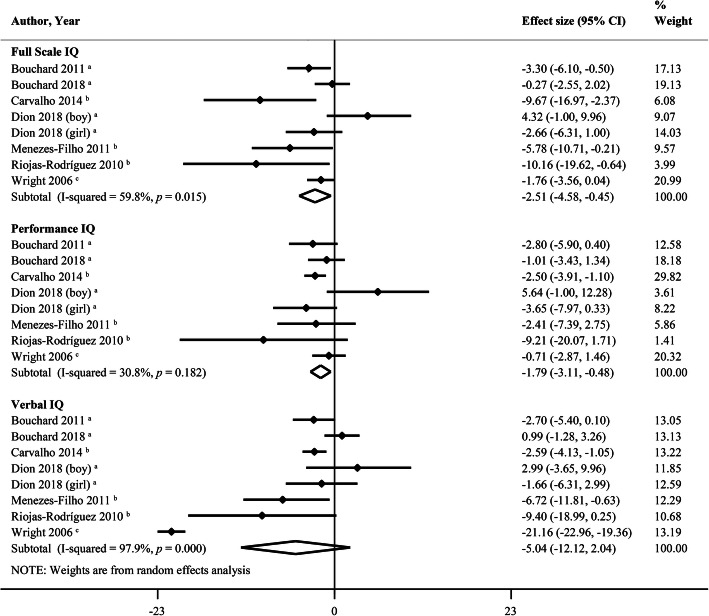
Figure 2 clearly shows that a 10-fold increase in hair manganese is associated with a decrease of 2.51 points (95% CI, − 4.58, − 0.45; I2 = 59.8%) in Full Scale IQ of children aged 6–18 years. Of note, this inverse relationship remained significant when we conducted a sensitivity analysis in which one study was removed at a time (see Additional file 4). The pooled results with respect to Performance IQ were not extremely robust and should be investigated further. Heterogeneity was 59.8% for Full Scale IQ, as a “leave-one-out” analysis revealed that there still existed some heterogeneities. Next, we performed a subgroup analysis based on the source of exposure, revealing a significantly inverse association between H-Mn and IQ scores from airborne manganese exposure [6, 21, 22], but not from waterborne [8, 16, 20] or mining waste manganese exposure [68]. The pooled β for the 10-fold increase in hair manganese from airborne manganese exposure was associated with a decrease of 7.62 points (95% CI, − 11.51, − 3.73; I2 = 0%) for Full Scale IQ. For Performance IQ, the decrease would be 2.60 points (95% CI, − 3.94, − 1.25; I2 = 0%), and 4.56 points decrease (95% CI, − 8.33, − 0.79; I2 = 45.5%) for Verbal IQ. Unexpectedly, the concentrations of H-Mn from airborne manganese exposure were much higher than the others. Therefore, we concluded that both the source of manganese exposure and the concentrations of H-Mn likely account, at least in part, for this relatively high heterogeneity. The results from Begg’s and Egger’s tests did not suggest the existence of publication bias.
Fig. 2.
Forest plots of effect size on intellectual quotient (IQ) by a 10-fold increase in hair manganese. a: waterborne manganese exposure, b: airborne manganese exposure, c: manganese exposure from mining waste
The meta-analysis in drinking water and blood revealed no significant effects (Additional files 5, 6). Of significance, among the reports that used both hair and blood as biomarkers, a large percent (7/11) of which indicated that H-Mn, but not B-Mn, was negatively associated with cognitive development [6, 7, 21, 49, 52, 59, 67] (Table 2).
Different biomarkers in Mn exposure and neurodevelopment
In the elder group, most studies used hair and blood as biomarkers. While teeth was also used as a biomarker in some publications with inconsistent Mn associations. Some researches also measured manganese in environmental samples, such as drinkable water, soil and particles. The correlations between manganese in drinking water and biomarker (hair or blood) or different biomarkers were analyzed in nine studies, as shown in Additional file 7. Among these publications, six studies used Spearman’s rank correlation [7, 9, 49, 56, 63, 67], as the distributions of manganese concentrations in biomarkers and drinking water were considerably skewed. Three studies analyzed the correlation using Pearson correlation tests, among these studies, the concentrations of manganese in indicators were transformed in order to make distributions more symmetrical for Pearson correlation tests [20, 21, 52].
The preliminary meta-analysis was conducted to gain a pooled result of correlations between different manganese indicators (see Additional file 7). The correlation between H-Mn and W-Mn indicated that they did have significance, and the pooled correlation coefficient r was 0.48 (95% CI, 0.40, 0.55). By contrast, the summary correlation between B-Mn and W-Mn, even B-Mn and H-Mn indicated that there had no significance. Although different analytical methods were applied in three studies that analyzed the correlation between H-Mn and W-Mn, the conclusion was consistent [20, 49, 63].
Wasserman et al. (2011) found that blood did not vary predictably across the low and high W-Mn groups, suggesting that blood may not be a good reflection of drinking water Mn exposure [72]. From the airborne manganese exposure, Torres-Agustin et al. (2013) observed a statistically significant difference between the two groups in the median blood Mn concentrations of 8.0 and 9.5 μg/L for non-exposed and exposed children, respectively. Meanwhile, hair Mn concentrations in exposed children were, on average, 20 times higher (median 12.6 and mean 14.2 μg/g) than the nonexposed group (median 0.6 and mean 0.73 μg/g) [67]. These results indicate that hair is more sensitive than blood to reflect environmental manganese exposure.
In infants, one report found that maternal and cord blood manganese concentrations were correlated, though in a nonlinear manner. Here, the median manganese in cord blood was nearly twice the median concentration in maternal blood, unexpectedly, the inverse associations were found between manganese in maternal blood, but not cord blood, and early childhood mental and psychomotor development scores [29]. The other biomarkers (i.e. maternal and infant hair and placenta) used to reflect prenatal Mn exposure did not show the correlation.
Sex specific exposure-response relationships
Evidence from eight cohort studies yielded inconsistent conclusions as to sexual effects (see Table 4). Three of four studies reported a statistically significant sex interaction (p < 0.05), and concluded that girls were more susceptible to manganese exposure than boys in terms of cognition and motor [20, 31, 33]. While Takser et al. (2003) found that in children at 3 years, the hand skill score was negatively associated with cord blood Mn in boys (p = 0.002), but not in girls [34]. Two cohort studies found a positive non-statistically significant association between manganese exposure and cognitive development in girls [69] and cognitive and motor development in boys [38]. Sex interaction p-values in the remaining two reports were not available. Positive association between urinary Mn concentrations and Performance IQ of children was observed, especially in girls [39]. And Claus Henn et al. (2018) found significantly negative associations between T-Mn and visual spatial score, among boys only [37].
Table 4.
Characteristics of the 18 studies that conducted sex-stratified analyses
| Author, Year | Age (Years) | Neurodevelopment | Effect on Boys | Effect on Girls | p-value of Interaction | Manganese Concentrations |
|---|---|---|---|---|---|---|
| Neurodevelopmental outcomes from cohort studies | ||||||
| Claus Henn 2018 [37] | 6–16 | Cognition | ↓ | NS | NA | Teeth: 12ab (n = 138) |
| Dion 2018 [20] | 10.5–18 | Cognition | ↑ (W-Mn) | ↓ (W-Mn) | < 0.01 (W-Mn) | Drinking water: 14.5 μg/Lc (n = 287), hair: 1.4 μg/gc (n = 274) |
| Gunier 2015 [31] | 0.5, 1, 2 | Cognition and motor | NS | ↓ | 0.02 (Cognition), 0.03 (Motor) | Teeth: prenatal: 0.51 ± 0.19bd (n = 197), postnatal: 0.20 ± 0.23bd (n = 193) |
| Mora 2015 [38] | 7, 9, 10.5 | Cognition and motor | ↑ | NS | < 0.1 | Teeth: prenatal: 0.50 ± 0.18bd (n = 248), postnatal: 0.19 ± 0.21bd (n = 244) |
| Mora 2018 [33] | 1 | Cognition | NS | ↓ | 0.01 | Maternal hair: 3.7 ± 5.4 μg/gd (n = 661), maternal blood: 24.4 ± 6.2 μg/Ld (n = 571) |
| Rahman 2017 [69] | 10 | Cognition | NS | ↑ | < 0.081 | Drinking water: 339 μg/La (n = 1265) |
| Takser 2003 [34] | Newborns | Motor | ↓ | NS | 0.03 | Cord blood: 38.5 μg/Lc (n = 222), maternal blood: 20.4 μg/Lc (n = 222), maternal hair: 0.36 μg/gc (n = 173), newborns hair: 0.75 μg/gc (n = 173), placenta: 0.1 μg/gc (n = 200) |
| Zhou 2019 [39] | 6–8 | Cognition | ↑ | NS | NA | Cord blood: 29.29 ± 1.48 μg/L (n = 296), urine: 0.66 ± 3.81 μg/L (n = 207) |
| Neurodevelopmental outcomes from cross-sectional studies | ||||||
| Bauer 2017 [42] | 10–14 | Cognition | NS | ↓ | 0.05 | Teeth: prenatal: 0.42cb (n = 142), postnatal: 0.12cb (n = 142) |
| Bouchard 2011 [16] | 6–13 | Cognition | NS | ↓ | 0.55 (H-Mn), 0.14 (W-Mn) | Hair: 0.7 μg/ga (n = 302), drinking water: 0.8 μg/La (n = 362) |
| Bouchard 2018 [8] | 6–14 | Cognition | NS (Toe nail), ↑ (W-Mn) | ↓ (Toe nail), NS (W-Mn) | 0.028 (Toe nail), 0.015 (W-Mn) | Toe nail: 2.0 μg/gc (n = 258), hair: 0.3 μg/gc (n = 258), saliva: 1.1 μg/Lc (n = 226), drinking water: 5.2/7.3 μg/Lc (boy: 127, girl: 132) |
| Carvalho 2018 [46] | 7–12 | Cognition | ↓ | NS | 0.047 | Hair: 11.5 μg/ga (n = 70) |
| Chiu 2017 [48] | 11–14 | Motor | NS (Prenatal and postnatal), ↓ (Childhood) | ↓ (Prenatal and postnatal), NS (Childhood) | < 0.05 (Prenatal), < 0.01 (Postnatal), 0.01 (Childhood) | Teeth: prenatal: 0.43ab (n = 189), postnatal: 0.13ab (n = 185) |
| Hernandez-Bonilla 2016 [54] | 7–11 | Cognition | NS | ↓ | < 0.15 | Hair: control: 0.55 μg/gc (n = 119), exposed: 5.25 μg/gc (n = 148) |
| Menezes-Filhoet 2014 [62] | 7–12 | Behavior | NS | ↓ | NA | Hair: boys: 12.1 μg/ga (n = 34), girls: 12.4 μg/g a (n = 36) |
| Rink 2014 [41] | 1.1–3.7 | Cognition | ↑ | NS | < 0.05 | Hair: 0.98 ± 0.74 μg/gd (n = 60) |
| Riojas-Rodríguez 2010 [6] | 7–11 | Cognition | NS | ↓ | NA | Control: hair: 0.57 μg/g, blood: 8.22 μg/Lc (n = 93), exposed: hair: 12.13 μg/g, blood: 9.71 μg/Lc (n = 79) |
| Torres-Agustinet 2013 [67] | 7–11 | Cognition | NS | ↓ | NA | Control: hair: 0.6 μg/g, blood: 8.0 μg/La (n = 95), exposed: hair: 12.6 μg/g, blood:9.5 μg/La (n = 79) |
↓: Negative association; ↑: Positive association; NA Not available; NS No significant association. a: Median; b: 55Mn: 43Ca the area under the curve (AUC) × 104; c: Geometric mean; d: Mean ± standard deviation. H-Mn Manganese in hair; W-Mn Manganese in drinking water
Seven cross-sectional studies consistently concluded that girls were more susceptible to manganese exposure than boys with respect to cognition and behavior [6, 8, 16, 42, 54, 62, 67], with non-statistically significant association in three reports [16, 42, 54] and interaction p-values not available in four studies [6, 8, 62, 67]. Three studies reported a statistically significant interaction between manganese exposure and sex (p < 0.05), without a clear pattern [41, 46, 48]. Rink et al. (2014) found that in children aged 14–45 months, H-Mn was negatively associated with the cognitive, receptive language and expressive language scores for girls only in the unadjusted model [41]. While negative association between H-Mn and the free recall after interference score was observed, especially in boys [46]. Chiu et al. (2017) found that higher prenatal Mn was associated with better body stability in boys, with opposite associations in girls. For tremor, on the other hand, higher early postnatal Mn was associated with increased right-hand center frequency in girls, but increased Mn concentration at the later postnatal period was associated with increased center frequency in boys [48].
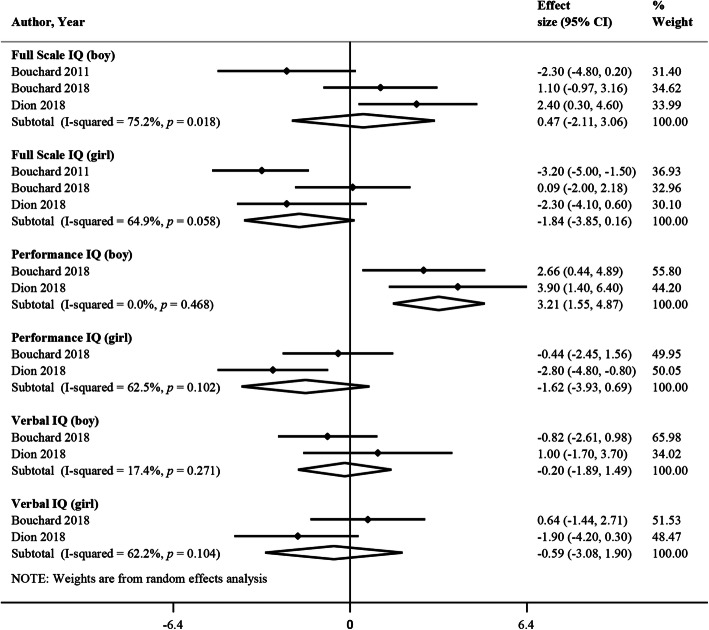
Three studies met the criteria for meta-analysis since they had a similar number of participants, and all adjusted for potential confounders such as maternal nonverbal intelligence, maternal education and family income [8, 16, 20]. Bouchard et al. (2011) only provided the detail of sex-stratified analysis for Full Scale IQ [16], therefore, there were two studies in the meta-analysis for Performance IQ and Verbal IQ. Figure 3 presents that higher W-Mn is associated with better Performance IQ among boys only (change in scores for a 10-fold increase in concentration, β = 3.21; 95% CI, 1.55, 4.87), in these two studies, a large percentage of children were exposed to drinking water manganese under 50 μg/L (the esthetic Canadian guideline concentration for W-Mn) [8, 20]. The meta-analysis on 3 studies concerning childhood IQ and H-Mn, found no significant difference between boys and girls [8, 16, 20] (Additional file 8).
Fig. 3.
Meta-analysis of studies that stratified by sex reporting the effect of a 10-fold increase in drinking water manganese on intellectual quotient (IQ)
Discussion
This systemic review and meta-analysis was based on 55 studies, including 17 cohort studies and 38 cross-sectional studies, with 13,388 participants. Evidence from cohort studies found that higher manganese exposure had a negative effect on neurodevelopment, mainly cognitive and motor skills in children under 6 years of age. In children aged 6–18 years old, results from cross-sectional studies revealed that higher H-Mn and W-Mn, but not B-Mn or T-Mn, were negatively associated with cognitive and behavioral performance. Of these cross-sectional studies, most studies reported that the mean of manganese in hair was more than 0.55 μg/g. The pooled results in H-Mn revealed that a 10-fold increase in H-Mn was associated with a decrease of 2.51 points (95% CI, − 4.58, − 0.45) in Full Scale IQ in children aged 6–18 years old. In the elder group, hair was the most consistent and reliable indicator of manganese exposure. These published data did demonstrate sex differences upon manganese exposure, without a clear pattern, possibly girls were more susceptible to manganese exposure than boys.
It is worth noting that the association between manganese exposure and motor performance was inconsistent in hair, blood and teeth. Only the results from infants [27, 34, 36, 70] and one study from the elder group that measured manganese in drinking water [15] supported the conclusion that higher manganese exposure had a negative effect on motor skills. It is a fact that occupational manganese exposure in adults can cause parkinsonian-like movement disorders [12]. Of note, animal studies reported that the increased brain manganese concentrations, either by Mn exposure or genetic strategies can cause severe motor deficits [73–75]. This consequence of excess Mn appears to be partly due to its interaction with other metals, like iron, operating through the post-transcriptional iron-responsive element driven regulatory mechanisms [76, 77]. Future studies are needed to evaluate the association between manganese exposure and motor performance in children.
Given the emerging evidence associating elevated Mn exposure with neurological impairments in children, it is critical to explore children’s exposure to Mn from the different sources. Evidence from cross-sectional studies indicated that groundwater and industrial emissions from ferromanganese alloy plants and mining were the main sources of environmental manganese exposure. Therefore, children were exposed to manganese mainly by inhaling pollutants from industrial emissions and by drinking water. Compared to cross-sectional studies (Table 2), most birth cohort studies enrolled mother-infant pairs in hospitals or clinics without specific source of manganese exposure. Among these studies, they all measured manganese in biomarkers, which can reflect all sources (i.e. diet, air and water) and routes of exposures [78], although Mn homeostasis differs markedly for dietary uptake and inhalation. Additionally, almost all these studies carried out the analysis based on percentile grouping, with some studies yielding additional indication of a dose-response relation of any shape (i.e. linear or an inverted U). Nevertheless, further research is needed to explore the mechanism with respect to the absorption and distribution of different sources of manganese.
Additionally, there is a particular need for a consistent biomarker to accurately assess children’s exposure to Mn. The concentrations of manganese were frequently measured in hair and blood to reflect internal Mn dose in children aged 6–18 years. Results from water-borne and air-borne manganese exposure indicated that hair was more sensitive than blood to reflect the load of manganese in the body. What is more, hair manganese from airborne manganese exposure was much higher than waterborne manganese exposure and was negatively associated with childhood IQ scores.
From these analyses, hair is the more promising measure of long term Mn exposures when compared to blood (with a half-life of 4 or 39 days due to the different elimination pathways [79]). Many metals are deposited in keratin, a component of hair, and the relatively slow growth rate of hair means that hair represents integrated exposures [80]. The 2 cm of newly grown hair was used for measuring the concentration of manganese in most publications, which reflects the exposure during the 2–4 months before sampling [81]. In addition, teeth also reflects long-term exposures as a slow metabolism and accumulation of Mn occurs in teeth [82]. Among eight studies that used teeth as a biomarker, all of these studies measured manganese in naturally shed deciduous milk teeth, while the teeth type varied among these studies, such as for incisors, canines and molars. In most studies, cumulative Mn exposures were estimated in incisors that were free of obvious defects such as caries and extensive tooth wear, which reflect manganese exposure from 13 to 16 weeks after gestation to approximately 1 year of age [83]. In fact, animal studies showed that H-Mn was significantly correlated with T-Mn. Furthermore, correlation coefficients clearly supported links between H-Mn and cognitive functions, reflected by escape latencies and number of platform crossings, and the correlations were better than those in teeth [84]. Additionally, hair is easier to obtain than teeth. Toe-nail can also be employed as a tissue source to measure of chronic exposure to this metal [8], but this technique has rarely been used when establishing environmental manganese exposures in children. The characteristics of relevant biomarkers are summarized in Table 5 [79, 81–83, 85–99].
Table 5.
Characteristics of relevant biomarkers used in children
| Biomarkers | Characteristics | Advantages | Limitations |
|---|---|---|---|
| Hair | Reflects the exposure during the 2–4 months before sampling [81] | Easy to collect, store and manipulate, non-invasive, most consistent and valid biomarker in pediatric epidemiology [85] | Pigmentation and potential external contamination [86] |
| Blood | With a half-life of 4 or 39 days due to different elimination pathways [79] | Obtained easily and less influence of external contamination [87] | Correlated poorly with exposure [88] |
| Teeth | Reflects the exposure from 13 to 16 weeks after gestation to approximately one year of age [83] | Non-invasive, provides precise exposure information, distinguishes the prenatal and postnatal exposure [82] | Caries and teeth with attrition contained less metal [89], relatively difficult to obtain and measure |
| Saliva and urine | secretes 0.8 to 1.5 L of saliva each day, only a small fraction of Mn eliminates in urine [90] | Non-invasive and easy to collect [90] | Correlated poorly with exposure [88, 91, 92], fairly large variation [93] |
| Toe nail | Reflects an exposure of 7–12 months before sampling [94, 95] | Easy collection, storage and transport [96], correlated with exposure [91] | Difficult to collect sufficient toenail from infants and potential external contamination [97] |
| Cord blood | Reflects an exposure of the last trimester [97] | Correlated with manganese in dentin [98] | Not feasible to obtain at different stages of pregnancy [98] |
| Maternal blood | Mn enters the fetus via an active transport mechanism [99] | Readily sampled [98] | Maternal Mn biomarkers may not accurately reflect Mn levels in fetal tissues [98] |
Mn Manganese
Overall, this review suggests that hair is the most reliable indicator of environmental manganese exposure in children aged 6–18 years old. Traditionally, the main problem of using hair as a biomarker is the potential for external contamination. In response to this, except for a study published in 2007 [45], all other studies that measured manganese in hair had used defined cleaning methodologies to eliminate external contamination. Eastman et al. (2013), for example, developed a hair cleaning methodology to effectively eliminate exogenous metal contamination [86]. This method can substantiate the use of hair as a biomarker of environmental Mn exposure in children. It should be noted that hair dye or other topical treatment could influence the content of manganese in hair [100], although topical hair treatment is unfrequent in children. In spite of this, two studies, now included, also excluded children who reported using hair dye in the preceding 5 months [15, 16]. Further work is needed to determine the utility of hair as a biomarker in preschoolers exposed to manganese. For infants, there appears to be insufficient hair to be analyzed. It is worth noting that teeth provides integrated measures of exposure over the prenatal and early childhood periods of their development, perhaps presenting as a promising biomarker of manganese exposure in infants.
It is always important to accurately determine the safe range of manganese exposure. For this reason, we extracted the reference range or cut-off point used in the reviewed articles (see Additional file 9), while limitations in our data precluded us from directly addressing some aspects of this important issue. In regard to H-Mn, we found that the cut-off point was much higher than the upper limit value of reference range. Accordingly, negative associations between H-Mn and neurodevelopment were observed in two studies that used 2 or 3 μg/g as the cut-off point [43, 45]. And Haynes et al. (2015) found that compared with 0.21–0.75 μg/g, both lower and higher H-Mn were associated with lower IQ scores [52], this may be closer to the possible reference range in children.
It will be critical to consider the timing of Mn exposures, because there may be certain sensitive periods to the effects of environmental manganese exposures in the developing brain. Takser et al. (2003) found that there were negative relationships between cord blood Mn concentrations and several psychomotor sub-scales at age of 3 years, but not at 9 months or 6 years, after adjustment for potential confounders [34].
Some included studies measured prenatal exposure, as indicated by manganese in maternal and cord blood, maternal and infant hair and placenta. Other studies measured manganese in teeth, which reflects prenatal and postnatal exposure (from 13 to 16 weeks after gestation to 1 year of age). Most of the included studies measured postnatal manganese exposure with a cross-sectional design. Hair was the frequently-used biomarker, where that analyzed 2 cm closest to the scalp reflects the exposure during the 2–4 months before sampling [81]. Among these studies, we tend to believe that manganese exposure is continuous, as some cross-sectional studies recruited children who had lived in the same community for a minimum of 3 months or 5 years, to ensure continuous exposure to the same source for this period of time. The follow-up study is warranted to explore the periods of critical vulnerability of environmental manganese exposures.
In this review, information regarding sex differences of manganese exposure from both cohort studies and cross-sectional studies were inconsistent. Perhaps there was a trend showing that girls were more susceptible to manganese exposure than boys. While almost all studies found no significant sex differences for Mn in biomarkers and drinking water, except for four studies without the relevant details available [34, 39, 54, 67]. Given that most studies were not specifically designed to evaluate sex-interactions, therefore, low statistical power may in part explain some of the inconsistency between studies.
Recently a study of single nucleotide polymorphisms in Mn transporter genes SLC30A10 and SLC39A8 also found a sex difference between Mn concentrations and genotypes [101]. The mechanisms behind potential sex differences in Mn toxicity are complicated, possibly due to sex difference in the developing brain [102], possibly related to biological differences in neurochemistry and hormone activity [103]. In addition, data from animal studies had shown that Mn exposure caused sex-dependent neuronal morphological change, and this change was not due to differential Mn accumulation between sexes but due to differences in sensitivity to Mn exposure [104]. All these differences may contribute to sex dimorphism in the associations between Mn exposure and neurodevelopment.
Our study incorporated the following limitations that warrant discussion. Firstly, most studies in this review are cross-sectional studies, so that no causal relationship can be inferred. In addition, a consistent biomarker for infants was not identified, perhaps teeth is the most promising biosample in this case, while being less easy to obtain than hair. Limitations in our data precluded us from identifying the safe range of manganese exposure and the periods of critical vulnerability of environmental manganese exposures. Finally, limited number of studies could be analyzed due to the relative homogeneity, however, we do not believe that this affected our analysis, given the stability of our sensitivity analysis.
Overall, to the best of our knowledge, this is the only comprehensive systemic review and meta-analysis regarding the biomarkers and sources of manganese exposure and cognitive, behavioral and motor functions in children. Outcomes from cohort studies and cross-sectional studies indicated that higher manganese exposures were negatively associated with neurodevelopment in children. In addition, this is the first meta-analysis for correlation between different manganese indicators where our results indicated that H-Mn was more significantly correlated with W-Mn than B-Mn. Therefore, we propose that hair is the most suitable biomarker in future studies.
Conclusions
Higher manganese exposure is negatively associated with childhood neurodevelopment, especially cognitive and motor skills for children under 6 years old and cognitive and behavioral performance for children aged 6–18 years old. In the older group (6–18 years old), hair is the most reliable indicator of manganese exposure. However, evidence demonstrated sex difference upon manganese exposure while a clear pattern is not elucidated. Population based biomonitoring studies with standard cleaning methodologies of hair are warranted in order to set reference ranges of manganese in hair at different ages. Large prospective cohort studies are certainly warranted in order to support these results and identify the underlying biological mechanisms.
Supplementary information
Additional file 1. PRISMA 2009 Checklist
Additional file 2. Evaluation of methodological quality of articles by using checklist in the Strengthening the Reporting of Observational Studies in Epidemiology Statement
Additional file 3. Characteristics of the articles included in the meta-analysis
Additional file 4. Sensitivity analysis was performed to evaluate the stability of the result
Additional file 5. Meta-analysis of studies reporting the effect of a 10-fold increase in drinking water manganese on intellectual quotient (IQ)
Additional file 6. Meta-analysis of studies reporting the effect of a e-fold increase in blood manganese on intellectual quotient (IQ)
Additional file 7. Correlations between manganese in biomarkers and environmental sample
Additional file 8. Meta-analysis of studies that stratified by sex reporting the effect of a 10-fold increase in hair manganese on intellectual quotient (IQ)
Additional file 9. The reference range or cut-off point used in the reviewed articles
Acknowledgments
We thank the members of the Wang and Min Laboratories for helpful discussions, especially Junhao Wang, Hao Wang, Xuexian Fang and Peng An.
Abbreviations
- AARES
The academic achievement records of the elementary schools
- ADS
The attention-deficit/hyperactivity disorder (ADHD) diagnostic system
- AMP
Aptitudes mentales primarias
- APS
Accusway plus system
- BASC-2
Behavior assessment system for children, 2nd edition
- BOT-2
The bruininks-oseretsky test, 2nd edition
- BSID
Bayley scales of infant and toddler development
- CANTAB
Cambridge neuropsychological test automated battery
- CAVLT
The children’s auditory verbal learning test
- CBCL
The standardized child behavior checklist
- CDIIT
The comprehensive developmental inventory for infants and toddlers
- CPRS-R
The revised conners’ rating scale for parents
- CPT-II
Conners’ continuous performance test ii version
- CTRS-R
The revised conners’ rating scale for teachers
- DBD
The disruptive behavior disorders
- DDST-II
Denver developmental screening test II
- DPD
Danish products developments
- DS
Digit span
- FT
Finger tapping
- FTT
The forbidden toy task
- GDI
Gesell developmental inventory
- GP
Grooved pegboard
- LNMB
Luria nebraska motor battery
- MSCA
The McCarthy scales of children’s abilities
- NBNA
Neonatal behavioral neurological assessments
- NEPSY-II
Developmental neuropsychological assessment, second edition
- NEUPSILIN-Inf
The Brazilian child brief neuropsychological assessment battery
- PA
Pursuit aiming
- PCM
The Raven’s progressive color matrices scale
- PEDS
Parents’ evaluation of developmental status
- RCPM
The Raven’s Colored progressive matrices
- ROCF
The rey-osterrieth complex fig.
- SA
Santa ana test
- SDQ
The strengths and difficulties questionnaire
- VRAM
The virtual radial arm maze
- WASI
Wechsler abbreviated scale of intelligence
- WISC
The wechsler intelligence scale for children
- W-M
Woodcock-Muñoz tests of cognitive abilities
- WRAVMA
The wide range assessment of visual motor abilities
Authors’ contributions
W.L., Y.X., F.W. and J.T.R. designed the study; W.L., Y.X.and Q. L identified the studies for inclusion, extracted the data and assessed the quality of the included studies; W.L. and Q.L. conducted the meta-analysis; W.L. and Y.X. wrote the first draft of the manuscript; Y.S. and Z.P. provided critical input for the manuscript; F.W., J.T.R., J.M. and C.M.C. did critical revision of the manuscript to improve and optimally present the key intellectual content and they also supervised this study. All authors have contributed significantly, and all authors are in agreement with respect to the content of the manuscript. The author (s) read and approved the final manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (31530034 and 31930057 to F.W.; 31570791 to J.M.) and the National Key Research and Development Program of China (2018YFA0507802 to F.W.; 2018YFA0507801 to J.M.), and Michael J. FOX Foundation (J.T.R.).
Ethics approval and consent to participate
No human subjects, human material, or human data were involved in this research, which is based on literature review.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Weiwei Liu and Yongjuan Xin contributed equally to this work.
Contributor Information
Weiwei Liu, Email: 1269825530@qq.com.
Yongjuan Xin, Email: yjxinzzu@163.com.
Qianwen Li, Email: lqw9319@163.com.
Yanna Shang, Email: shyn_67@163.com.
Zhiguang Ping, Email: pingzhg@zzu.edu.cn.
Junxia Min, Email: junxiamin@zju.edu.cn.
Catherine M. Cahill, Email: ccahill@helix.mgh.harvard.edu
Jack T. Rogers, Email: jack.rogers@mgh.harvard.edu
Fudi Wang, Email: fwang@zju.edu.cn.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s12940-020-00659-x.
References
- 1.Aschner M, Erikson K. Manganese. Adv Nutr. 2017;8(3):520–521. doi: 10.3945/an.117.015305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Water E, Proal E, Wang V, Medina SM, Schnaas L, Tellez-Rojo MM, et al. Prenatal manganese exposure and intrinsic functional connectivity of emotional brain areas in children. Neurotoxicology. 2018;64:85–93. doi: 10.1016/j.neuro.2017.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leonhard MJ, Chang ET, Loccisano AE, Garry MR. A systematic literature review of epidemiologic studies of developmental manganese exposure and neurodevelopmental outcomes. Toxicology. 2019;420:46–65. doi: 10.1016/j.tox.2019.03.004. [DOI] [PubMed] [Google Scholar]
- 4.Iyare PU. The effects of manganese exposure from drinking water on school-age children: a systematic review. Neurotoxicology. 2019;73:1–7. doi: 10.1016/j.neuro.2019.02.013. [DOI] [PubMed] [Google Scholar]
- 5.Saghazadeh A, Rezaei N. Systematic review and meta-analysis links autism and toxic metals and highlights the impact of country development status: higher blood and erythrocyte levels for mercury and lead, and higher hair antimony, cadmium, lead, and mercury. Prog Neuropsychopharmacol Biol Psychiatry. 2017;79(Pt B):340–368. doi: 10.1016/j.pnpbp.2017.07.011. [DOI] [PubMed] [Google Scholar]
- 6.Riojas-Rodríguez H, Solís-Vivanco R, Schilmann A, Montes S, Rodríguez S, Ríos C, et al. Intellectual function in Mexican children living in a mining area and environmentally exposed to manganese. Environ Health Perspect. 2010;118(10):1465–1470. doi: 10.1289/ehp.0901229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haynes EN, Sucharew H, Hilbert TJ, Kuhnell P, Spencer A, Newman NC, et al. Impact of air manganese on child neurodevelopment in East Liverpool, Ohio. Neurotoxicology. 2018;64:94–102. doi: 10.1016/j.neuro.2017.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bouchard MF, Surette C, Cormier P, Foucher D. Low level exposure to manganese from drinking water and cognition in school-age children. Neurotoxicology. 2018;64:110–117. doi: 10.1016/j.neuro.2017.07.024. [DOI] [PubMed] [Google Scholar]
- 9.Wasserman GA, Liu X, Parvez F, Ahsan H, Levy D, Factor-Litvak P, et al. Water manganese exposure and children's intellectual function in Araihazar, Bangladesh. Environ Health Perspect. 2006;114(1):124–129. doi: 10.1289/ehp.8030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rodriguez-Barranco M, Lacasana M, Aguilar-Garduno C, Alguacil J, Gil F, Gonzalez-Alzaga B, et al. Association of arsenic, cadmium and manganese exposure with neurodevelopment and behavioural disorders in children: a systematic review and meta-analysis. Sci Total Environ. 2013;454–455:562–577. doi: 10.1016/j.scitotenv.2013.03.047. [DOI] [PubMed] [Google Scholar]
- 11.Meyer-Baron M, Knapp G, Schäper M, van Thriel C. Performance alterations associated with occupational exposure to manganese--a meta-analysis. Neurotoxicology. 2009;30(4):487–496. doi: 10.1016/j.neuro.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 12.Kwakye GF, Paoliello MM, Mukhopadhyay S, Bowman AB, Aschner M. Manganese-induced parkinsonism and Parkinson's disease: shared and distinguishable features. Int J Env Res Pub He. 2015;12(7):7519–7540. doi: 10.3390/ijerph120707519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Llop S, Lopez-Espinosa MJ, Rebagliato M, Ballester F. Gender differences in the neurotoxicity of metals in children. Toxicology. 2013;311(1–2):3–12. doi: 10.1016/j.tox.2013.04.015. [DOI] [PubMed] [Google Scholar]
- 14.Shih JH, Zeng BY, Lin PY, Chen TY, Chen YW, Wu CK, et al. Association between peripheral manganese levels and attention-deficit/hyperactivity disorder: a preliminary meta-analysis. Neuropsychiatr Dis Treat. 2018;14:1831–1842. doi: 10.2147/NDT.S165378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oulhote Y, Mergler D, Barbeau B, Bellinger DC, Bouffard T, Brodeur ME, et al. Neurobehavioral function in school-age children exposed to manganese in drinking water. Environ Health Perspect. 2014;122(12):1343–1350. doi: 10.1289/ehp.1307918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bouchard MF, Sauve S, Barbeau B, Legrand M, Brodeur ME, Bouffard T, et al. Intellectual impairment in school-age children exposed to manganese from drinking water. Environ Health Perspect. 2011;119(1):138–143. doi: 10.1289/ehp.1002321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Int J Surg. 2014;12(12):1495–1499. doi: 10.1016/j.ijsu.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 18.Mikó A, Pótó L, Mátrai P, Hegyi P, Füredi N, Garami A, et al. Gender difference in the effects of interleukin-6 on grip strength - a systematic review and meta-analysis. BMC Geriatr. 2018;18(1):107. doi: 10.1186/s12877-018-0798-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wasserman GA, Liu X, Parvez F, Factor-Litvak P, Kline J, Siddique AB, et al. Child intelligence and reductions in water arsenic and manganese: a two-year follow-up study in Bangladesh. Environ Health Perspect. 2016;124(7):1114–1120. doi: 10.1289/ehp.1509974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dion LA, Saint-Amour D, Sauve S, Barbeau B, Mergler D, Bouchard MF. Changes in water manganese levels and longitudinal assessment of intellectual function in children exposed through drinking water. Neurotoxicology. 2018;64:118–125. doi: 10.1016/j.neuro.2017.08.015. [DOI] [PubMed] [Google Scholar]
- 21.Menezes-Filho JA, Novaes Cde O, Moreira JC, Sarcinelli PN, Mergler D. Elevated manganese and cognitive performance in school-aged children and their mothers. Environ Res. 2011;111(1):156–163. doi: 10.1016/j.envres.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carvalho CF, Menezes-Filho JA, de Matos VP, Bessa JR, Coelho-Santos J, Viana GF, et al. Elevated airborne manganese and low executive function in school-aged children in Brazil. Neurotoxicology. 2014;45:301–308. doi: 10.1016/j.neuro.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 23.Wechsler D. Wechsler intelligence scale for children. San Antonio, TX: Psychological Corporation; 1991. [Google Scholar]
- 24.Shadish WR, Haddock CK. Combining estimates of effect size. In: Cooper H, Hedges LV, editors. The Handbook of Research Synthesis. New York: Russell Sage Foundation; 1994. pp. 265–266. [Google Scholar]
- 25.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chung SE, Cheong HK, Ha EH, Kim BN, Ha M, Kim Y, et al. Maternal blood manganese and early neurodevelopment: the mothers and children's environmental health (MOCEH) study. Environ Health Perspect. 2015;123(7):717–722. doi: 10.1289/ehp.1307865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Claus Henn B, Ettinger AS, Schwartz J, Tellez-Rojo MM, Lamadrid-Figueroa H, Hernandez-Avila M, et al. Early postnatal blood manganese levels and children's neurodevelopment. Epidemiology. 2010;21(4):433–439. doi: 10.1097/EDE.0b013e3181df8e52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Claus Henn B, Bellinger DC, Hopkins MR, Coull BA, Ettinger AS, Jim R, et al. Maternal and cord blood manganese concentrations and early childhood neurodevelopment among residents near a mining-impacted superfund site. Environ Health Perspect. 2017;125(6):067020. doi: 10.1289/EHP925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Freire C, Amaya E, Gil F, Fernandez MF, Murcia M, Llop S, et al. Prenatal co-exposure to neurotoxic metals and neurodevelopment in preschool children: the environment and childhood (INMA) project. Sci Total Environ. 2018;621:340–351. doi: 10.1016/j.scitotenv.2017.11.273. [DOI] [PubMed] [Google Scholar]
- 31.Gunier RB, Arora M, Jerrett M, Bradman A, Harley KG, Mora AM, et al. Manganese in teeth and neurodevelopment in young Mexican-American children. Environ Res. 2015;142:688–695. doi: 10.1016/j.envres.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin CC, Chen YC, Su FC, Lin CM, Liao HF, Hwang YH, et al. In utero exposure to environmental lead and manganese and neurodevelopment at 2 years of age. Environ Res. 2013;123:52–57. doi: 10.1016/j.envres.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 33.Mora AM, Cordoba L, Cano JC, Hernandez-Bonilla D, Pardo L, Schnaas L, et al. Prenatal mancozeb exposure, excess manganese, and neurodevelopment at 1 year of age in the infants' environmental health (ISA) study. Environ Health Perspect. 2018;126(5):057007. doi: 10.1289/EHP1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takser L, Mergler D, Hellier G, Sahuquillo J, Huel G. Manganese, monoamine metabolite levels at birth, and child psychomotor development. Neurotoxicology. 2003;24(4–5):667–674. doi: 10.1016/S0161-813X(03)00058-5. [DOI] [PubMed] [Google Scholar]
- 35.Yu XD, Zhang J, Yan CH, Shen XM. Prenatal exposure to manganese at environment relevant level and neonatal neurobehavioral development. Environ Res. 2014;133:232–238. doi: 10.1016/j.envres.2014.04.012. [DOI] [PubMed] [Google Scholar]
- 36.Yu X, Chen L, Wang C, Yang X, Gao Y, Tian Y. The role of cord blood BDNF in infant cognitive impairment induced by low-level prenatal manganese exposure: LW birth cohort, China. Chemosphere. 2016;163:446–451. doi: 10.1016/j.chemosphere.2016.07.095. [DOI] [PubMed] [Google Scholar]
- 37.Claus Henn B, Austin C, Coull BA, Schnaas L, Gennings C, Horton MK, et al. Uncovering neurodevelopmental windows of susceptibility to manganese exposure using dentine microspatial analyses. Environ Res. 2018;161:588–598. doi: 10.1016/j.envres.2017.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mora AM, Arora M, Harley KG, Kogut K, Parra K, Hernandez-Bonilla D, et al. Prenatal and postnatal manganese teeth levels and neurodevelopment at 7, 9, and 10.5 years in the CHAMACOS cohort. Environ Int. 2015;84:39–54. doi: 10.1016/j.envint.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou T, Guo J, Zhang J, Xiao H, Qi X, Wu C, et al. Sex-specific differences in cognitive abilities associated with childhood cadmium and manganese exposures in school-age children: a prospective cohort study. Biol Trace Elem Res. 2019;193(1):89–99. doi: 10.1007/s12011-019-01703-9. [DOI] [PubMed] [Google Scholar]
- 40.Al-Saleh I, Al-Mohawes S, Al-Rouqi R, Elkhatib R. Selenium status in lactating mothers-infants and its potential protective role against the neurotoxicity of methylmercury, lead, manganese, and DDT. Environ Res. 2019;176:108562. doi: 10.1016/j.envres.2019.108562. [DOI] [PubMed] [Google Scholar]
- 41.Rink SM, Ardoino G, Queirolo EI, Cicariello D, Manay N, Kordas K. Associations between hair manganese levels and cognitive, language, and motor development in preschool children from Montevideo, Uruguay. Arch Environ Occup Health. 2014;69(1):46–54. doi: 10.1080/19338244.2012.725229. [DOI] [PubMed] [Google Scholar]
- 42.Bauer JA, Claus Henn B, Austin C, Zoni S, Fedrighi C, Cagna G, et al. Manganese in teeth and neurobehavior: sex-specific windows of susceptibility. Environ Int. 2017;108:299–308. doi: 10.1016/j.envint.2017.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Betancourt O, Tapia M, Mendez I. Decline of general intelligence in children exposed to manganese from mining contamination in puyango river basin, southern Ecuador. Ecohealth. 2015;12(3):453–460. doi: 10.1007/s10393-015-1027-2. [DOI] [PubMed] [Google Scholar]
- 44.Bhang SY, Cho SC, Kim JW, Hong YC, Shin MS, Yoo HJ, et al. Relationship between blood manganese levels and children's attention, cognition, behavior, and academic performance--a nationwide cross-sectional study. Environ Res. 2013;126:9–16. doi: 10.1016/j.envres.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 45.Bouchard M, Laforest F, Vandelac L, Bellinger D, Mergler D. Hair manganese and hyperactive behaviors: pilot study of school-age children exposed through tap water. Environ Health Perspect. 2007;115(1):122–127. doi: 10.1289/ehp.9504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Carvalho CF, Oulhote Y, Martorelli M, Carvalho CO, Menezes-Filho JA, Argollo N, et al. Environmental manganese exposure and associations with memory, executive functions, and hyperactivity in Brazilian children. Neurotoxicology. 2018;69:253–259. doi: 10.1016/j.neuro.2018.02.002. [DOI] [PubMed] [Google Scholar]
- 47.Chan TJ, Gutierrez C, Ogunseitan OA. Metallic burden of deciduous teeth and childhood behavioral deficits. Int J Environ Res Pub He 2015;12(6):6771–87. [DOI] [PMC free article] [PubMed]
- 48.Chiu YM, Claus Henn B, Hsu HL, Pendo MP, Coull BA, Austin C, et al. Sex differences in sensitivity to prenatal and early childhood manganese exposure on neuromotor function in adolescents. Environ Res. 2017;159:458–465. doi: 10.1016/j.envres.2017.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.do Nascimento SN, Barth A, Goethel G, Baierle M, Charao MF, Brucker N, et al. Cognitive deficits and ALA-D-inhibition in children exposed to multiple metals. Environ Res. 2015;136:387–395. doi: 10.1016/j.envres.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 50.Ericson JE, Crinella FM, Clarke-Stewart KA, Allhusen VD, Chan T, Robertson RT. Prenatal manganese levels linked to childhood behavioral disinhibition. Neurotoxicol Teratol. 2007;29(2):181–187. doi: 10.1016/j.ntt.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 51.Frndak S, Barg G, Canfield RL, Quierolo EI, Manay N, Kordas K. Latent subgroups of cognitive performance in lead- and manganese-exposed Uruguayan children: examining behavioral signatures. Neurotoxicology. 2019;73:188–198. doi: 10.1016/j.neuro.2019.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Haynes EN, Sucharew H, Kuhnell P, Alden J, Barnas M, Wright RO, et al. Manganese exposure and neurocognitive outcomes in rural school-age children: the communities actively researching exposure study (Ohio, USA) Environ Health Perspect. 2015;123(10):1066–1071. doi: 10.1289/ehp.1408993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hernandez-Bonilla D, Schilmann A, Montes S, Rodriguez-Agudelo Y, Rodriguez-Dozal S, Solis-Vivanco R, et al. Environmental exposure to manganese and motor function of children in Mexico. Neurotoxicology. 2011;32(5):615–621. doi: 10.1016/j.neuro.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 54.Hernandez-Bonilla D, Escamilla-Nunez C, Mergler D, Rodriguez-Dozal S, Cortez-Lugo M, Montes S, et al. Effects of manganese exposure on visuoperception and visual memory in schoolchildren. Neurotoxicology. 2016;57:230–240. doi: 10.1016/j.neuro.2016.10.006. [DOI] [PubMed] [Google Scholar]
- 55.Horton MK, Hsu L, Claus Henn B, Margolis A, Austin C, Svensson K, et al. Dentine biomarkers of prenatal and early childhood exposure to manganese, zinc and lead and childhood behavior. Environ Int. 2018;121(Pt 1):148–158. doi: 10.1016/j.envint.2018.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Khan K, Factor-Litvak P, Wasserman GA, Liu X, Ahmed E, Parvez F, et al. Manganese exposure from drinking water and children's classroom behavior in Bangladesh. Environ Health Perspect. 2011;119(10):1501–1506. doi: 10.1289/ehp.1003397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kicinski M, Vrijens J, Vermier G, Hond ED, Schoeters G, Nelen V, et al. Neurobehavioral function and low-level metal exposure in adolescents. Int J Hyg Environ Health. 2015;218(1):139–146. doi: 10.1016/j.ijheh.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 58.Kim Y, Kim BN, Hong YC, Shin MS, Yoo HJ, Kim JW, et al. Co-exposure to environmental lead and manganese affects the intelligence of school-aged children. Neurotoxicology. 2009;30(4):564–571. doi: 10.1016/j.neuro.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 59.Lucchini RG, Zoni S, Guazzetti S, Bontempi E, Micheletti S, Broberg K, et al. Inverse association of intellectual function with very low blood lead but not with manganese exposure in Italian adolescents. Environ Res. 2012;118:65–71. doi: 10.1016/j.envres.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lucchini RG, Guazzetti S, Zoni S, Donna F, Peter S, Zacco A, et al. Tremor, olfactory and motor changes in Italian adolescents exposed to historical ferro-manganese emission. Neurotoxicology. 2012;33(4):687–696. doi: 10.1016/j.neuro.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lucchini RG, Guazzetti S, Renzetti S. Neurocognitive impact of metal exposure and social stressors among schoolchildren in Taranto, Italy. Environ Health. 2019;18(1):67. doi: 10.1186/s12940-019-0505-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Menezes-Filho JA, de Carvalho-Vivas CF, Viana GF, Ferreira JR, Nunes LS, Mergler D, et al. Elevated manganese exposure and school-aged children's behavior: a gender-stratified analysis. Neurotoxicology. 2014;45:293–300. doi: 10.1016/j.neuro.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 63.Nascimento S, Baierle M, Goethel G, Barth A, Brucker N, Charao M, et al. Associations among environmental exposure to manganese, neuropsychological performance, oxidative damage and kidney biomarkers in children. Environ Res. 2016;147:32–43. doi: 10.1016/j.envres.2016.01.035. [DOI] [PubMed] [Google Scholar]
- 64.Parvez F, Wasserman GA, Factor-Litvak P, Liu X, Slavkovich V, Siddique AB, et al. Arsenic exposure and motor function among children in Bangladesh. Environ Health Perspect. 2011;119(11):1665–1670. doi: 10.1289/ehp.1103548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rugless F, Bhattacharya A, Succop P, Dietrich KN, Cox C, Alden J, et al. Childhood exposure to manganese and postural instability in children living near a ferromanganese refinery in southeastern Ohio. Neurotoxicol Teratol. 2014;41:71–79. doi: 10.1016/j.ntt.2013.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Torrente M, Colomina MT, Domingo JL. Metal concentrations in hair and cognitive assessment in an adolescent population. Biol Trace Elem Res. 2005;104(3):215–221. doi: 10.1385/BTER:104:3:215. [DOI] [PubMed] [Google Scholar]
- 67.Torres-Agustin R, Rodriguez-Agudelo Y, Schilmann A, Solis-Vivanco R, Montes S, Riojas-Rodriguez H, et al. Effect of environmental manganese exposure on verbal learning and memory in Mexican children. Environ Res. 2013;121:39–44. doi: 10.1016/j.envres.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 68.Wright RO, Amarasiriwardena C, Woolf AD, Jim R, Bellinger DC. Neuropsychological correlates of hair arsenic, manganese, and cadmium levels in school-age children residing near a hazardous waste site. Neurotoxicology. 2006;27(2):210–216. doi: 10.1016/j.neuro.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 69.Rahman SM, Kippler M, Tofail F, Bolte S, Hamadani JD, Vahter M. Manganese in drinking water and cognitive abilities and behavior at 10 years of age: a prospective cohort study. Environ Health Perspect. 2017;125(5):057003. doi: 10.1289/EHP631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rodrigues EG, Bellinger DC, Valeri L, Hasan MO, Quamruzzaman Q, Golam M, et al. Neurodevelopmental outcomes among 2- to 3-year-old children in Bangladesh with elevated blood lead and exposure to arsenic and manganese in drinking water. Environ Health. 2016;15:44. doi: 10.1186/s12940-016-0127-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Khan K, Wasserman GA, Liu X, Ahmed E, Parvez F, Slavkovich V, et al. Manganese exposure from drinking water and children's academic achievement. Neurotoxicology. 2012;33(1):91–97. doi: 10.1016/j.neuro.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wasserman GA, Liu X, Parvez F, Factor-Litvak P, Ahsan H, Levy D, et al. Arsenic and manganese exposure and children's intellectual function. Neurotoxicology. 2011;32(4):450–457. doi: 10.1016/j.neuro.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tuschl K, Meyer E, Valdivia LE, Zhao N, Dadswell C, Abdul-Sada A, et al. Mutations in SLC39A14 disrupt manganese homeostasis and cause childhood-onset parkinsonism-dystonia. Nat Commun. 2016;7:11601. doi: 10.1038/ncomms11601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Xin Y, Gao H, Wang J, Qiang Y, Imam MU, Li Y, et al. Manganese transporter slc39a14 deficiency revealed its key role in maintaining manganese homeostasis in mice. Cell Discov. 2017;3:17025. doi: 10.1038/celldisc.2017.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xia Z, Wei J, Li Y, Wang J, Li W, Wang K, et al. Zebrafish slc30a10 deficiency revealed a novel compensatory mechanism of Atp2c1 in maintaining manganese homeostasis. PLoS Genet. 2017;13(7):e1006892. doi: 10.1371/journal.pgen.1006892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rogers JT, Xia N, Wong A, Bakshi R, Cahill CM. Targeting the iron-response elements of the mRNAs for the Alzheimer's amyloid precursor protein and ferritin to treat acute lead and manganese neurotoxicity. Int J Mol Sci. 2019;20(4):994. doi: 10.3390/ijms20040994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Venkataramani V, Doeppner TR, Willkommen D, Cahill CM, Xin Y, Ye G, et al. Manganese causes neurotoxic iron accumulation via translational repression of amyloid precursor protein and H-ferritin. J Neurochemistry. 2018;147(6):831–848. doi: 10.1111/jnc.14580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Aprea MC. Environmental and biological monitoring in the estimation of absorbed doses of pesticides. Toxicol Lett. 2012;210(2):110–118. doi: 10.1016/j.toxlet.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 79.Mahoney JP, Small WJ. Studies on manganese. 3. The biological half-life of radiomanganese in man and factors which affect this half-life. J Clin Invest. 1968;47(3):643–653. doi: 10.1172/JCI105760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kordas K, Queirolo EI, Ettinger AS, Wright RO, Stoltzfus RJ. Prevalence and predictors of exposure to multiple metals in preschool children from Montevideo, Uruguay. Sci Total Environ. 2010;408(20):4488–4494. doi: 10.1016/j.scitotenv.2010.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Robbins CR. Chemical and physical behavior of human hair. 4. New York: Springer-Verlag; 2002. [Google Scholar]
- 82.Arora M, Hare D, Austin C, Smith DR, Doble P. Spatial distribution of manganese in enamel and coronal dentine of human primary teeth. Sci Total Environ. 2011;409(7):1315–1319. doi: 10.1016/j.scitotenv.2010.12.018. [DOI] [PubMed] [Google Scholar]
- 83.Hillson S. Dental anthropology. New York: Cambridge University Press; 1996. [Google Scholar]
- 84.Liang G, Zhang L, Ma S, Lv Y, Qin H, Huang X, et al. Manganese accumulation in hair and teeth as a biomarker of manganese exposure and neurotoxicity in rats. Environ Sci Pollut Res Int. 2016;23(12):12265–12271. doi: 10.1007/s11356-016-6420-z. [DOI] [PubMed] [Google Scholar]
- 85.Coetzee DJ, McGovern PM, Rao R, Harnack LJ, Georgieff MK, Stepanov I. Measuring the impact of manganese exposure on children's neurodevelopment: advances and research gaps in biomarker-based approaches. Environ Health. 2016;15(1):91. doi: 10.1186/s12940-016-0174-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Eastman RR, Jursa TP, Benedetti C, Lucchini RG, Smith DR. Hair as a biomarker of environmental manganese exposure. Environ Sci Technol. 2013;47(3):1629–1637. doi: 10.1021/es3035297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ward EJ, Edmondson DA, Nour MM, Snyder S, Rosenthal FS, Dydak U. Toenail manganese: a sensitive and specific biomarker of exposure to manganese in career welders. Ann Work Expos Heal. 2017;62(1):101–111. doi: 10.1093/annweh/wxx091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Smith D, Gwiazda R, Bowler R, Roels H, Park R, Taicher C, et al. Biomarkers of Mn exposure in humans. Am J Ind Med. 2007;50(11):801–811. doi: 10.1002/ajim.20506. [DOI] [PubMed] [Google Scholar]
- 89.Niedzielska K, Struzak-Wysokińska M, Wujec Z. Analysis of correlations between the content of various elements in hard tissues of milk teeth with and without caries. Czas Stomatol. 1990;43(6):316–322. [PubMed] [Google Scholar]
- 90.Zheng W, Fu SX, Dydak U, Cowan DM. Biomarkers of manganese intoxication. Neurotoxicology. 2011;32(1):1–8. doi: 10.1016/j.neuro.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lucas EL, Bertrand P, Guazzetti S, Donna F, Peli M, Jursa TP, et al. Impact of ferromanganese alloy plants on household dust manganese levels: implications for childhood exposure. Environ Res. 2015;138:279–290. doi: 10.1016/j.envres.2015.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ntihabose R, Surette C, Foucher D, Clarisse O, Bouchard MF. Assessment of saliva, hair and toenails as biomarkers of low level exposure to manganese from drinking water in children. Neurotoxicology. 2018;64:126–133. doi: 10.1016/j.neuro.2017.08.011. [DOI] [PubMed] [Google Scholar]
- 93.Wang D, Du X, Zheng W. Alteration of saliva and serum concentrations of manganese, copper, zinc, cadmium and lead among career welders. Toxicol Lett. 2008;176(1):40–47. doi: 10.1016/j.toxlet.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Laohaudomchok W, Lin X, Herrick RF, Fang SC, Cavallari JM, Christiani DC, et al. Toenail, blood, and urine as biomarkers of manganese exposure. J Occup Environ Med. 2011;53(5):506–510. doi: 10.1097/JOM.0b013e31821854da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yaemsiri S, Hou N, Slining MM, He K. Growth rate of human fingernails and toenails in healthy American young adults. J Eur Acad Dermatol Venereol. 2010;24(4):420–423. doi: 10.1111/j.1468-3083.2009.03426.x. [DOI] [PubMed] [Google Scholar]
- 96.He K. Trace elements in nails as biomarkers in clinical research. Eur J Clin Investig. 2011;41(1):98–102. doi: 10.1111/j.1365-2362.2010.02373.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rodrigues EG, Kile M, Dobson C, Amarasiriwardena C, Quamruzzaman Q, Rahman M, et al. Maternal-infant biomarkers of prenatal exposure to arsenic and manganese. J Expo Sci Environ Epidemiol. 2015;25(6):639–648. doi: 10.1038/jes.2015.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Arora M, Bradman A, Austin C, Vedar M, Holland N, Eskenazi B, et al. Determining fetal manganese exposure from mantle dentine of deciduous teeth. Environ Sci Technol. 2012;46(9):5118–5125. doi: 10.1021/es203569f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yoon M, Nong A, Clewell HJ, 3rd, Taylor MD, Dorman DC, Andersen ME. Evaluating placental transfer and tissue concentrations of manganese in the pregnant rat and fetuses after inhalation exposures with a PBPK model. Toxicol Sci. 2009;112(1):44–58. doi: 10.1093/toxsci/kfp198. [DOI] [PubMed] [Google Scholar]
- 100.Sky-Peck HH. Distribution of trace elements in human hair. Clin Physiol Biochem. 1990;8(2):70–80. [PubMed] [Google Scholar]
- 101.Wahlberg K, Arora M, Curtin A, Curtin P, Wright RO, Smith DR, et al. Polymorphisms in manganese transporters show developmental stage and sex specific associations with manganese concentrations in primary teeth. Neurotoxicology. 2018;64:103–109. doi: 10.1016/j.neuro.2017.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kaczkurkin AN, Raznahan A, Satterthwaite TD. Sex differences in the developing brain: insights from multimodal neuroimaging. Neuropsychopharmacology. 2019;44(1):71–85. doi: 10.1038/s41386-018-0111-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ngun TC, Ghahramani N, Sánchez FJ, Bocklandt S, Vilain E. The genetics of sex differences in brain and behavior. Front Neuroendocrinol. 2011;32(2):227–246. doi: 10.1016/j.yfrne.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Madison JL, Wegrzynowicz M, Aschner M, Bowman AB. Gender and manganese exposure interactions on mouse striatal neuron morphology. Neurotoxicology. 2011;32(6):896–906. doi: 10.1016/j.neuro.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. PRISMA 2009 Checklist
Additional file 2. Evaluation of methodological quality of articles by using checklist in the Strengthening the Reporting of Observational Studies in Epidemiology Statement
Additional file 3. Characteristics of the articles included in the meta-analysis
Additional file 4. Sensitivity analysis was performed to evaluate the stability of the result
Additional file 5. Meta-analysis of studies reporting the effect of a 10-fold increase in drinking water manganese on intellectual quotient (IQ)
Additional file 6. Meta-analysis of studies reporting the effect of a e-fold increase in blood manganese on intellectual quotient (IQ)
Additional file 7. Correlations between manganese in biomarkers and environmental sample
Additional file 8. Meta-analysis of studies that stratified by sex reporting the effect of a 10-fold increase in hair manganese on intellectual quotient (IQ)
Additional file 9. The reference range or cut-off point used in the reviewed articles