Abstract
Mast cells and eosinophils are innate immune cells involved in both acute and chronic inflammatory responses. Siglecs are a family of cell surface receptors that share sialic acid binding activity. Over the past 20 years, our knowledge of the expression and function of Siglecs on cells of the immune system and others has greatly expanded, as has our understanding of their signaling, ligands, and possible roles in disease pathophysiology. Because of this, Siglecs have garnered interest as potential drug targets using strategies ranging from biologics to ligand-directed nanoparticles. This mini-review will highlight the state of our knowledge regarding human eosinophil and mast cell Siglecs, their biology, what they recognize, tools developed for in vitro and preclinical experimentation, and the status of ongoing efforts to develop drugs that engage eosinophil and mast cell Siglecs for potential therapeutic benefit.
Keywords: AK002, antolimab, eosinophils, ligands, mast cells, Siglec, signaling
1 |. INTRODUCTION
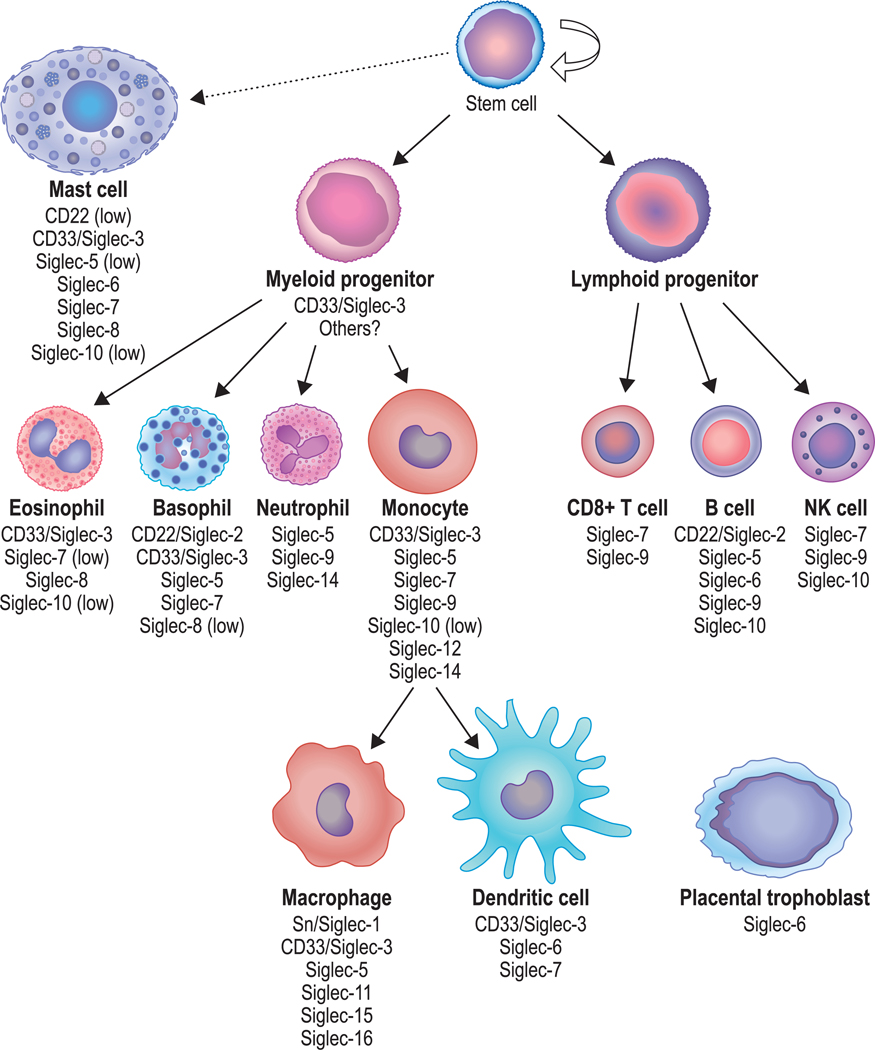
Siglec, or sialic acid-binding immunoglobulin-like lectin, is a term that was adopted in 19981 to describe a group of receptors within the sub-family of I-type (immunoglobulin-type) lectins.2,3 These are single-pass cell surface receptors whose N terminus consists of a sialic acid binding lectin domain and whose C-terminal cytoplasmic region typically, but not uniformly, contains conserved signaling domains. At the time of the adoption of this new nomenclature, there were 5 known Siglecs: sialoadhesin (Siglec-1), CD22 (Siglec-2), CD33 (Siglec-3), and 2 myelin-associated proteins (Siglec-4a and Siglec-4b). Within a decade, the number of mammalian Siglecs expanded dramatically, as did our understanding of the function and expression of these molecules on leukocytes and other cells that express them, including myelinated cells and placental trophoblasts.4,5 One of the conspicuous aspects of Siglecs is their restricted pattern of cell surface expression (Fig. 1), a character-istic that, along with their specificity for differing sialoside ligands, pro-vides each Siglec with its own distinct biology. This review will focus on the subset of Siglecs expressed on mast cells and eosinophils, cells associated with a range of functions and disease pathology.6,7
FIGURE 1.
Patterns of surface expression of Siglecs on human mast cells, eosinophils and other cells. Artwork by Jacqueline Schaffer. Sn, sialoadhesin
2 |. EOSINOPHIL SIGLEC BIOLOGY
Several Siglec family members are known to be expressed by human eosinophils, including Siglec-3/CD33, Siglec-7, −8, and −10 (Fig. 1).8–13 Transcriptional profiling of human circulating eosinophils in patients with severe asthma, parasitic disease, aspergillosis, and dermatologic disease as well as healthy controls shows that SIGLEC7, SIGLEC8, and SIGLEC10 mRNA levels are the highest of the Siglecs detected by RNA hybridization using Agilent microarrays (CD33 was not included, for example), with SIGLEC8 and SIGLEC10 levels approximately equiva-lent and exceeding those of SIGLEC7.14 These findings were consistent with unbiased proteomic profiling of purified human eosinophils.13 Once on the cell surface, levels of eosinophil Siglecs remain relatively stable in blood and tissue compartments as well as disease states,15–18 although minor changes in surface expression of eosinophil Siglec-7 and Siglec-8 have been reported under some in vitro and clinical situations.9,19 Soluble forms of Siglec-7 and Siglec-8, typically in the range of low nanogram per milliliter concentrations, have been detected in serum. For Siglec-7, but not for Siglec-8, these levels cor-relate with absolute eosinophil counts in blood, while higher levels of soluble levels of Siglec-8 was reported in the serum of severe asthmatics compared to healthy controls.9,15,20 Regarding other examples of studies of soluble forms of Siglecs that could originate from mast cells and/or eosinophils, soluble Siglec-5 levels in saliva were found to be elevated in Sjögren’s syndrome compared to controls21 and blood levels of soluble CD22 were elevated in hairy cell leukemia.22 In general, however, the biological, pharmacologic, and functional significance of soluble forms of Siglecs remains uncertain and unlikely to be clinically relevant for Siglec-targeting therapeutics due to the low concentrations found in serum.
Regarding structure and function, the most extensively studied among eosinophil Siglecs is Siglec-8, which was discovered by sequencing of a cDNA library from eosinophils isolated from a patient with hypereosinophilic syndrome.10,11 The form of Siglec-8 that was originally identified possessed a short cytoplasmic domain lacking any known signaling motif; however, a variant generated by alternative exon usage included a longer cytoplasmic domain containing an ITIM and a cytoplasmic motif similar to an immunoreceptor tyrosine-based switch motif (ITSM).23 These forms have come to be known as the short form of Siglec-8 versus the full-length form, the long form, or just Siglec-8, respectively. Eosinophils have been found to express both variants at the mRNA level and by western blotting, although the long form appeared to be more abundantly and consistently expressed in these cells, suggesting that Siglec-8 is mainly found as the long form.24,25
Siglec-8 is expressed relatively late in eosinophil development, reaching peak expression levels after the secondary granule protein MBP1.18 Consistent with this, Siglec-8 is not detected on eosinophil or eosinophil-like cell lines that are thought to be poorly differentiated such as EoL-1 or AML14.3D10 cells, nor on EoL-3 or HL-60 cells, even after treatment with sodium butyrate to promote eosinophilic differentiation.11,18 Indeed, the transcription factor Olig2, expressed late in eosinophil development, appears to play a role in promoting SIGLEC8 transcription.26 Nevertheless, certain eosinophil cell lines, such as EoL-1 or AML14.3D10, express Olig2 but not Siglec-8, demonstrating that Olig2 expression by itself is insufficient to permit Siglec-8 expression.
Structures that engage Siglec-8 have been used to study its function, which also depends on the activation state of the eosinophil. For example, the effect of Ab ligation of Siglec-8 on human eosinophils depends on the extent of cross-linking or the priming status of the cell. There has been no effect detected when Siglec-8 is ligated with an mAb alone in vitro on non-cytokine-primed eosinophils; however, the addition of a secondary Ab to enhance cross-linking or the Ab engagement of Siglec-8 on eosinophils primed with IL-5, GM-CSF, or IL-33 leads to the death of the cell.25,27 This form of cell death involves reactive oxygen species production but appears to be caspase-dependent in the absence of priming and caspase-independent in its presence.28,29 The mode of cell death induced in cytokine-primed eosinophils via Siglec-8 engagement and the precise mechanism underlying the actions of IL-5, GM-CSF, or IL-33 in this context have not yet been fully char-acterized. Ab or artificial ligand binding of the receptor on cytokine-primed eosinophils leads to a signaling pathway involving PI3K, Rac1, and MEK1/2; the induction of β2-integrin-dependent adhesion; and the generation of reactive oxygen species via NADPH oxidase, eventually culminating in cell death.30–32 Ab ligation of Siglec-8 also induces receptor endocytosis in a tyrosine kinase-, protein kinase C-, and actin rearrangement-dependent manner that is influenced by the ITIM but not the ITSM-like motif.33
Regarding Siglec-7, human eosinophils in the blood and in nasal polyp tissue express Siglec-7, albeit at levels well below those found on natural killer (NK) cells.9,34,35 Ab ligation of Siglec-7 on eosinophils leads to rapid phosphorylation of SHP-1 and trends toward reductions in the GM-CSF-induced phosphorylation of ERK1/2 and p38.9 This receptor is up-regulated on eosinophils upon overnight stimulation with IL-5 or GM-CSF. Unlike Siglec-8, Ab ligation of Siglec-7 does not lead to cytokine-primed eosinophil cell death but instead partially inhibits the degranulation, cytokine release, and CD69 surface up-regulation that accompanies GM-CSF stimulation.9
CD33 (or Siglec-3) has been found to be expressed on hematopoietic stem cells, where it remains at moderate levels on immature eosinophils then levels decline over time such that mature eosinophils express only low levels.36,37 CD33 acts to inhibit cellular activation on other cell types through the phosphatases SHP-1 and SHP-238; however, its function on human eosinophils is unknown. Similarly, while multiple isoforms of Siglec-10 are expressed by human peripheral blood eosinophils,12,13,39 there are no published reports examining Siglec-10 function on these cells.
3 |. MAST CELL SIGLEC BIOLOGY
Human mast cells express Siglec-2/CD22, Siglec-3/CD33, and Siglec-5, −6, −7, −8, and −10, with levels of Siglec-2, −5, and −10 being low.11,18,40–43 Compared to the study of Siglec function on eosinophils, less is known about their biology on mast cells. In general, the preclinical study of mast cell Siglec function has yielded 1 main functional theme involving inhibitory biology mediated via the canonical effects of the cytoplasmic ITIM, such as studies targeting Siglec-7 or Siglec-8, where inhibition of allergic secretory responses has been demonstrated.16,43,44 Siglec-6, among the most prominently expressed Siglecs on mast cells,40,41 appears to mediate more modest inhibition of secretion.45 Additional studies showed that CD33 targeting with liposomal nanoparticles, by co-engagement of FcɛRI, profoundly protects from anaphylaxis, both in humanized mouse models and primary human mast cells.41 Interestingly, this paper and others report the detection of low levels of CD22 mRNA and/or surface expression on mast cells40,41 or none at all.46 Regardless, to date there are no published data to support any function of CD22 on mast cells, nor any reports of anti-CD22 mAb (e.g., inotuzumab ozogamicin) depleting mast cells in patients with B cell disorders. Similarly, while low levels of Siglec-5 and Siglec-10 mRNA and surface expression have been detected in or on some mast cells or mast cell lines,40,42,45 their biology on these cells is unknown, although it has been shown that when Siglec-5 is transfected into rat basophilic leukemia cells, it functions as a phosphatase-dependent inhibitor of FcɛRI-mediated secretion.47 Finally, whenever inhibitory biology has been observed, the ITIM domain and phosphatases have been implicated.41,44,48 This concept is not unique to mast cells, as there are many other examples of similar inhibitory biology with other Siglecs and other cells, including B cells, T cells, MΦs, and NK cells.49–56 Finally, most Siglecs undergo endocytosis following engagement,57 and this process has been exploited to kill malignant mast cells in vitro by targeting Siglec-8 with an Ab-toxin conjugate.33
4 |. NOVEL CELLULAR AND OTHER PRECLINICAL TOOLS TO STUDY SIGLEC BIOLOGY
4.1 |. Cell lines and transfectants
Efforts to study Siglec-8 biology by modulating receptor expression or mutating key residues of the receptor have been hampered by the lack of an eosinophilic cell line that expresses Siglec-8 at the cell surface, including those treated with sodium butyrate to induce further eosinophilic maturation.11,18 However, other cell lines (e.g., COS-1, HEK293T, and CHO cells) have been transfected with full-length SIGLEC8 or versions in which the ITIM or ITSM-like tyrosine residues have been converted to phenylalanine residues, permitting studies of ligand binding,10,11,30,58 the roles of these cytoplasmic signaling motifs in receptor endocytosis,33 and the inhibition of mediator release.44 In contrast, at least some human mast cell lines express lower levels of Siglec-8 at the cell surface, including certain lines or passages of HMC-1.2, LAD2 cells, and LUVA cells,18,33 while cell-surface Siglec-8 is either absent or low in HMC-1.1 cells.18,40 Whether any of the eosinophilic cell lines express other Siglec family members typically found in eosinophils has not yet been reported. Numerous cell lines that are not eosinophil-or mast cell-like have been found to express some of these Siglecs, however, and could be used to examine ligand binding and, potentially, receptor signaling and function. For example, the monocytic cell line U937 and placental cells express relatively high levels of Siglec-6, and HEL erythroblast-like cells, monocytic THP-1 cells, and Daudi B lymphoblast-like cells also express the receptor.59,60 Additionally, cell lines have been transfected to study these Siglecs. For instance, the inhibitory role of Siglec-5 has been studied in transfected monocytic THP-1 cells,61 and the role of Siglec-7 in inducing a non-apoptotic form of cell death has been explored using monocytic U937 cells transfected with SIGLEC7 or various forms of the gene in which portions have been deleted or replaced with corresponding regions of the SIGLEC9 gene, although the moderate levels of Siglec-7 expressed by untransfected U937 cells are apparently unable to exert the same effect.62
4.2 |. Siglec ligand biology
By definition, Siglecs recognize sialic acid, but only recently has this sialic acid dependency been better defined. One approach that revolutionized the field was the development of printed glycan arrays that provided unbiased, high-throughput screening of sialoside ligands and their α2,3, α2,6, and α2,8 binding specifici-ties, including efforts by the Consortium for Functional Glycomics (http://www.functionalglycomics.org) and others.63–65 Typically performed as an ELISA-like screen, Siglec-Ig fusion proteins were employed to look for carbohydrate binding on a chip or slide. As a result, most Siglec-specific binding characteristics have now been at least partially characterized,3,4,66–68 although Siglec-6 remains some-what of an enigma in this regard.60 For example, screens for potential glycan ligands of Siglec-8 have revealed highly selective binding to 2 related molecules: 6′-sulfo-sLex (NeuAcα2–3[6-O-sulfo]Galβ1–4[Fucα1–3]GlcNAc) and 6′-sulfated sialyl N-acetyl-D-lactosamine (NeuAcα2–3[6-O-sulfo]Galβ1–4GlcNAc), identical except for the presence or absence of the fucose.66,69 None of the other hundreds of glycans included in the array appreciably interacted with Siglec-8, including unsulfated sLex or 6-sulfo-sLex in which the sulfate is at the 6-position of the N-acetyl-glucosamine rather than the galactose. Similarly, neither of these Siglec-8 ligands binds well to other human Siglecs. A structural analysis of the interaction between 6′sulfo-sLex and Siglec-8 has revealed that the sulfate at the 6-position of the galactose stabilizes binding through its interactions with R56 and Q59 of Siglec-8, whereas the fucose is positioned away from the binding pocket of Siglec-8 and is thus largely expendable for binding.70 It remains uncertain whether these exact glycan interactions represent those that occur physiologically in humans. In contrast, Siglec-7 pref-erentially binds to α2,8-linked polysialic acid–containing structures, but also binds to branched α2,6-linked sialylated molecules and the α2,3-linked Lewisx structure sulfated at the 6 or 6′ position (see http://www.functionalglycomics.org).71
Because Siglec-F, the functionally convergent paralog in the mouse, appears to play an important role in eosinophilic lung inflammation58,72–74 much of the initial search for physiological Siglec-8 ligands has focused on these tissues. In the upper airway, a subpopulation of cells in the submucosal glands of the inferior turbinate and uncinate produce a sialoglycan that binds to Siglec-8-Fc, and this staining is increased in tissues from patients with chronic rhinosinusitis (CRS) with or without nasal polyposis relative to healthy control tissues.75 Siglec-8 ligands are similarly found in submucosal glands of the trachea and bronchi, but sialidase-sensitive Siglec-8-Fc binding is also prominent in tracheal ring cartilage.76 Siglec-8 ligands produced by tracheal gland cells appear to be high-molecular-weight O-linked sialoglycoproteins. Affinity purification, enzymatic treatment, and Siglec-8-Fc blotting revealed that Siglec-8 binds to sialylated keratin sulfate chains of the proteoglycan aggrecan.77 No staining of airway epithelium was noted in either location, and there was no Siglec-8 ligand detected on lung epithelium or in lung parenchyma. In addition, no Siglec-8 ligands were detected in mouse trachea, although Siglec-F bound to epithelium, connective tissue, submucosal glands, and cartilage in human tissue, so there are some endogenous tissue ligands for Siglec-F that are not recognized by Siglec-8.
Additionally, knowledge of Siglec-glycan binding specificities has facilitated the development of Siglec-specific binding agents, including multivalent glycans decorated on structures such as polyacrylamide, as well as the development of glycomimetics that can be displayed either alone or in tandem with co-ligands to provide cellular targeting specificity to eosinophil and mast cell Siglecs such as CD33 and Siglec-8.30,41,78
4.3 |. Animal models including genetically altered mice
Evolutionarily speaking, many of the Siglecs found on human mast cells and eosinophils are not conserved in lower species, such as Siglec-6, −7, −8, and −10.18,79–81 In contrast, Siglec-2/CD22 is conserved in mice, as is a related form of Siglec-3/CD33.3 Siglec-F, while not a true ortholog of Siglec-8, is highly expressed on mouse eosinophils58 but not on mouse mast cells, which only express 1 Siglec, namely CD33.41 Because in vitro examination of Siglec biology can only provide so much information, to determine how the presence or targeting of particular Siglecs alter biology under physiologically relevant conditions, mice have been genetically engineered to lack particular receptors or ligands, express human Siglecs not normally found in the mouse, or accommodate the development of Siglec-expressing human cells from stem cell precursors.
Mouse Siglec-E, -F, and -G have been studied in part due to their functional similarities with human Siglecs-9, −8, and −10, respectively, whether truly orthologous or not. Mice in which these receptors have been knocked out have demonstrated, for example, roles for Siglec-E in impeding neutrophil migration by altering β2-integrin signaling and reducing platelet activation82–84; Siglec-F in reducing and resolving eosinophilic lung inflammation74; and Siglec-G in promoting cell turnover and inhibiting BCR signaling in B1a cells.85 These receptors achieved these effects by interacting with their physiological ligands (or, conceivably, through basal signaling).
Enzymes involved in sialylation and sulfation of glycans have similarly been deleted from mice to establish the importance of these sialoside glycan ligands for Siglecs in the context of inflammation. Deficiency of the sialyltransferase St3gal3, which is necessary for the production of ligands for Siglec-F, was found to amplify eosinophilic lung inflammation.69,73 Ligand binding preferences of Siglec-8 and Siglec-F are similar and have substantial overlap; however, unlike Siglec-8, Siglec-F binds to multi-antennary glycans lacking sulfation.67 This is important because deletion in mice of the 2 enzymes known to sulfate galactose at the 6 position, keratin sulfate galactose 6-O-sulfotransferase (KSGal6ST) and chondroitin 6-O-sulfotransferase 1, did not impact Siglec-F ligand abundance in the lung,86 and KSGal6ST deficiency did not affect eosinophilic lung inflammation after OVA sensitization and challenge.87
The differences between Siglec-F in the mouse and Siglec-8 in humans go well beyond ligand binding and include cellular expression patterns, signaling and endocytic pathways, and mode and extent of cell death induction, which preclude the use of Siglec-F as a reliable stand-in for Siglec-8. To study Siglec-8 in animal models of disease, new mouse strains have been generated that express human Siglec-8 in the eosinophil compartment (SIGLEC8Eo),88 mast cell compartment (Mcpt5-Siglec-8 and Cpa3-Siglec-8),89 or in eosinophils, mast cells, and basophils (Siglec-8 transgenic).90 The former strains, which rely on cell-specific or cell-selective Cre expression to remove a STOP cassette, allow for the discrimination between the effects of Siglec-8 on each cell population, while the Siglec-8 transgenic mouse, which bears the human SIGLEC8 gene, including the putative promoter and regulatory elements, most accurately mimics Siglec-8 expression in humans. Anti-Siglec-8 mAb administration to the latter mice diminishes the number of eosinophils and mast cells in GI tissues in models of eosinophilic gastroenteritis,90 while Siglec-8 ligation induces the cell death of eosinophils isolated from the peripheral blood of SIGLEC8Eo mice in vitro.88 A similar Cre-dependent strategy was employed to express human CD33 in mouse mast cells, which revealed that CD33 recruitment to Ag-aggregated IgE receptor on these cells was effective in blocking FcɛRI signaling and anaphylaxis.41
If for any reason it turns out that expressing human Siglecs on mouse eosinophils or mast cells for in vivo studies is insufficiently rep-resentative, genetically modified mouse strains can be used to allow the development and use of human eosinophils or mast cells for this purpose. NSG-SGM3 mice expressing human SCF, GM-CSF, and IL-3 engrafted with human thymus, liver, and hematopoietic stem cells have been used to permit human mast cell development to examine the inhibitory function of CD33 or Siglec-8 by demonstrating that nanoparticle and/or Ab targeting protected these humanized mice from passive systemic anaphylaxis.16,41
4.4 |. Clinical status of Siglec therapeutics that target mast cells and eosinophils
Siglecs are attractive therapeutic targets because of their selective surface expression, immunomodulatory function, and endocytic properties.91 The majority of Siglec-targeting approaches have been focused on Ab-based strategies toward CD22 and CD33.92 Dating back to the late 1980s, the high expression of CD22 and CD33 on lymphoma and leukemia cells led to the development of both naked and cargo-conjugated Abs for oncologic diseases.92,93 Despite the noted expression of CD22 on mast cells and CD33 on mast cells and eosinophils, the activity of these anti-Siglec Abs against these cell types has been minimal or not reported in any clinical setting.
More recently, Siglec-8 has emerged as a therapeutic target based on its selective expression and immunomodulatory function on mast cells and eosinophils.94 One of the first clues of this potential came from studies of commercial preparations of pooled human IgG Abs (IVIg) where naturally occurring anti-Siglec-8 Abs were detected. This led to the theory that such anti-Siglec-8 Abs could be responsible for some of the therapeutic benefits that can be seen when IVIg, especially at high doses, is given as a treatment for eosinophil-and mast cell-related disorders.95,96 These and other clinically relevant characteristics led to the development of a humanized anti-Siglec-8 mAb, antolimab (AK002). Antolimab is a humanized non-fucosylated IgG1 anti-Siglec-8 Ab that depletes blood eosinophils by Ab-dependent cellular cytotoxicity (ADCC), induces apoptosis of tissue eosinophils, and broadly inhibits mast cells.16 In a randomized, double-blind, placebo-controlled, phase 1 study in healthy volunteers, antolimab completely depleted blood eosinophils by the first post-dosing time point (1 h) in all doses tested (0.001–1.0 mg/kg).97 Antolimab has also demonstrated clinical activity in multiple mast cell-and eosinophil-driven diseases, including multiple forms of chronic urticaria, severe allergic conjunctivitis, and indolent systemic mastocytosis (Table 1).98,99
TABLE 1.
Antolimab (AK002) completed clinical trials
| Disease | Study design | Primary Findings |
|---|---|---|
| Eosinophilic Gastritis and/or Gastroenteritis | Phase 2 randomized, double-blind, placebo-controlled (NCT03496571) | • Met all primary and secondary endpoints including significant histologic and symptomatic improvements in EG/EN patients treated with antolimab vs placebo |
| • Strong histologic and symptomatic proof of concept in concomitant EoE | ||
| Chronic Spontaneous Urticaria (omalizumab-naïve & omalizumab-refractory); Chronic Inducible Urticarias (cholinergic & symptomatic dermographism) | Phase 2a open-label (NCT03436797) | • High response rates in multiple forms of antihistamine-resistant chronic urticaria, including omalizumab-refractory and inducible urticaria |
| Severe Allergic Conjunctivitis (atopic keratoconjunctivitis, vernal keratoconjunctivitis, and perennial allergic conjunctivitis) | Phase 1b open-label (NCT03379311) | • Substantial reduction of patient-reported ocular symptoms and physician-assessed signs and symptoms |
| • Improvements observed in comorbid atopic dermatitis, asthma, and rhinitis | ||
| Indolent Systemic Mastocytosis | Phase 1b open-label (NCT02808793) | • Substantial symptom and quality-of-life improvement |
Most recently, antolimab safety and efficacy was evaluated in a randomized, double-blind, placebo-controlled phase 2 study in adults with active eosinophilic gastritis (EG) and/or gastroenteritis (EN) (ENIGMA study).100 Biopsy-confirmed EG/EN patients with moderate to severe symptoms were randomized to receive low dose antolimab (n = 19; 0.3–1.0 mg/kg), high dose antolimab (n = 20; 0.3–3.0 mg/kg), or placebo (n = 20). In this study, antolimab-treated subjects demonstrated a 95% reduction in stomach and duodenal tissue eosinophils compared to a 10% increase in the placebo group. There were significant reductions in EG/EN symptom scores in the treated group compared to placebo. In addition, antolimab treatment resulted in histologic and symptomatic improvements in patients with concomitant eosinophilic esophagitis. These data demonstrate the clinical utility of targeting Siglec-8 on mast cells and eosinophils with an Ab and support the continued evaluation of antolimab in mast cell and eosinophil-driven diseases.
5 |. CONCLUDING REMARKS
Siglecs expressed on the surface of various subsets of immune cells function primarily as inhibitory receptors. While their endogenous and potentially exogenous ligands are still being defined, the ability of Abs and other ligands to suppress functional responses among many cell types, including mast cells and eosinophils, is now well established both in vitro and in vivo. Prior efforts to develop Ab-based therapies against CD22 (Siglec-2) and CD33 (Siglec-3) are now being expanded to include Siglec-8 and other Siglecs. It is anticipated that advances in pharmacology will expand treatment options for diseases involving Siglecs and the cells that express them or their ligands.
ACKNOWLEDGMENTS
This work was supported in part by grants from National Institute of Allergy and Infectious Diseases (U19AI136443 to B.S.B. and U19AI070535 subaward 107905120 to J.A.O.). We also thank Jacqueline Schaffer for generating artwork used in this review.
Abbreviations:
- ADCC
Ab-dependent cellular cytotoxicity
- EG
eosinophilic gastritis
- EN
eosinophilic gastroenteritis
- Siglec
sialic acid-binding immunoglobulin-like lectin
Footnotes
DISCLOSURE
J.A.O. has nothing to disclose. A.C. and B.A.Y. are employees of and/or own stock and/or stock options from Allakos, Inc. B.S.B. receives remuneration for serving on the scientific advisory board of Allakos, Inc. and owns stock in Allakos. He receives publication-related royalty payments from Elsevier and UpToDate®. He is a co-inventor on existing Siglec-8–related patents and thus may be entitled to a share of royalties received by Johns Hopkins University during development and potential sales of such products. Dr. Bochner is also a co-founder of Allakos, which makes him subject to certain restrictions under University policy. The terms of this arrangement are being managed by Johns Hopkins University and Northwestern University in accordance with their conflict of interest policies.
REFERENCES
- 1.Crocker PR, Clark EA, Filbin M, et al. Siglecs: a family of sialic-acid binding lectins. Glycobiology. 1998;8:v. [DOI] [PubMed] [Google Scholar]
- 2.Varki A, Angata T. Siglecs – the major sub-family of I-type lectins. Glycobiology. 2006;16:1R–27R. [DOI] [PubMed] [Google Scholar]
- 3.Varki A, Schnaar RL, Crocker PR. I-type lectins. In: Varki A, Cummings R D, Esko J D, Stanley P, Hart G W, Aebi M, Darvill A G, Kinoshita T, Packer N H, Prestegard J H, Schnaar R L, Seeberger P H, eds. Essentials of Glycobiology. 3rd ed. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2017. [Google Scholar]
- 4.Macauley MS, Crocker PR, Paulson JC. Siglec-mediated regulation of immune cell function in disease. Nat Rev Immunol. 2014;14:653–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bochner BS, Zimmermann N. Role of siglecs and related glycan-binding proteins in immune responses and immunoregulation. J Allergy Clin Immunol. 2015;135:598–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robida PA, Puzzovio PG, Pahima H, et al. Human eosinophils and mast cells: birds of a feather flock together. Immunol Rev. 2018;282: 151–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klion AD, Ackerman SJ, Bochner BS. Contributions of eosinophil to human health and disease. Ann Rev Pathol. 2020. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shen ZJ, Hu J, Esnault S, et al. RNA Seq profiling reveals a novel expression pattern of TGF-beta target genes in human blood eosinophils. Immunol Lett. 2015;167:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Legrand F, Landolina N, Zaffran I, et al. Siglec-7 on peripheral blood eosinophils: surface expression and function. Allergy. 2019;74: 1257–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Floyd H, Ni J, Cornish AL, et al. Siglec-8: a novel eosinophil-specific member of the immunoglobulin superfamily. J Biol Chem. 2000;275:861–866. [DOI] [PubMed] [Google Scholar]
- 11.Kikly KK, Bochner BS, Freeman SD, et al. Identification of SAF-2, a novel siglec expressed on eosinophils, mast cells, and basophils. J Allergy Clin Immunol. 2000;105:1093–1100. [DOI] [PubMed] [Google Scholar]
- 12.Munday J, Kerr S, Ni J, et al. Identification, characterization and leucocyte expression of Siglec-10, a novel human sialic acid-binding receptor. Biochem J. 2001;355:489–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilkerson EM, Johansson MW, Hebert AS, et al. The peripheral blood eosinophil proteome. J Proteome Res. 2016;15:1524–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barnig C, Alsaleh G, Jung N, et al. Circulating human eosinophils share a similar transcriptional profile in asthma and other hypereosinophilic disorders. PLoS One. 2015;10:e0141740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Legrand F, Cao Y, Wechsler JB, et al. Sialic acid-binding immunoglobulin-like lectin (Siglec) 8 in patients with eosinophilic dis-orders: receptor expression and targeting using chimeric antibodies. J Allergy Clin Immunol. 2019;143:2227–2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Youngblood BA, Brock EC, Leung J, et al. AK002, a humanized sialic acid-binding immunoglobulin-like lectin-8 antibody that induces antibody-dependent cell-mediated cytotoxicity against human eosinophils and inhibits mast cell-mediated anaphylaxis in mice. Int Arch Allergy Immunol. 2019;180:91–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johansson MW, Kelly EA, Nguyen CL, et al. Characterization of Siglec-8 expression on lavage cells after segmental lung allergen challenge. Int Arch Allergy Immunol. 2018;177:16–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hudson SA, Herrmann H, Du J, et al. Developmental, malignancy-related, and cross-species analysis of eosinophil, mast cell, and basophil Siglec-8 expression. J Clin Immunol. 2011;31:1045–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arakawa S, Suzukawa M, Ohshima N, et al. Expression of Siglec-8 is regulated by interleukin-5, and serum levels of soluble Siglec-8 may predict responsiveness of severe eosinophilic asthma to mepolizumab. Allergol Int. 2018;67S:S41–S44. [DOI] [PubMed] [Google Scholar]
- 20.Na HJ, Hamilton RG, Klion AD, et al. Biomarkers of eosinophil involvement in allergic and eosinophilic diseases: review of phenotypic and serum markers including a novel assay to quantify levels of soluble Siglec-8. J Immunol Methods. 2012;383:39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee J, Lee J, Baek S, et al. Soluble Siglec-5 is a novel salivary biomarker for primary Sjogren’s syndrome. J Autoimmun. 2019;100:114–119. [DOI] [PubMed] [Google Scholar]
- 22.Matsushita K, Margulies I, Onda M, et al. Soluble CD22 as a tumor marker for hairy cell leukemia. Blood. 2008;112:2272–2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Foussias G, Yousef GM, Diamandis EP. Molecular characterization of a Siglec-8 variant containing cytoplasmic tyrosine-based motifs, and mapping of the Siglec-8 gene. Biochem Biophys Res Commun. 2000;278:775–781. [DOI] [PubMed] [Google Scholar]
- 24.Aizawa H, Plitt J, Bochner BS. Human eosinophils express two Siglec-8 splice variants. J Allergy Clin Immunol. 2002;109:176. [DOI] [PubMed] [Google Scholar]
- 25.Nutku E, Aizawa H, Hudson SA, et al. Ligation of Siglec-8: a selective mechanism for induction of human eosinophil apoptosis. Blood. 2003;101:5014–5020. [DOI] [PubMed] [Google Scholar]
- 26.Hwang SM, Uhm TG, Lee SK, et al. Olig2 is expressed late in human eosinophil development and controls Siglec-8 expression. J Leukoc Biol. 2016;100:711–723. [DOI] [PubMed] [Google Scholar]
- 27.Na HJ, Hudson SA, Bochner BS. IL-33 enhances Siglec-8 mediated apoptosis of human eosinophils. Cytokine. 2012;57:169–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nutku E, Hudson SA, Bochner BS. Mechanism of Siglec-8-induced human eosinophil apoptosis: role of caspases and mitochondrial injury. Biochem Biophys Res Commun. 2005;336:918–924. [DOI] [PubMed] [Google Scholar]
- 29.Nutku-Bilir E, Hudson SA, Bochner BS. Interleukin-5 priming of human eosinophils alters Siglec-8 mediated apoptosis pathways. Am J Respir Cell Mol Biol. 2008;38:121–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hudson SA, Bovin N, Schnaar RL, et al. Eosinophil-selective binding and pro-apoptotic effect in vitro of a synthetic Siglec-8 ligand, polymeric 6’-sulfated sialyl Lewis X. J Pharmacol Exp Ther. 2009;330: 608–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carroll DJ, O’Sullivan JA, Nix DB, et al. Sialic acid-binding immunoglobulin-like lectin 8 (Siglec-8) is an activating receptor mediating beta2-integrin-dependent function in human eosinophils. J Allergy Clin Immunol. 2018;141:2196–2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kano G, Almanan M, Bochner BS, et al. Mechanism of Siglec-8-mediated cell death in IL-5-activated eosinophils: role for reactive oxygen species-enhanced MEK/ERK activation. J Allergy Clin Immunol. 2013;132:437–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O’Sullivan JA, Carroll DJ, Cao Y, et al. Leveraging Siglec-8 endocytic mechanisms to kill human eosinophils and malignant mast cells. J Allergy Clin Immunol. 2018;141:1774–1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Munitz A, Bachelet I, Eliashar R, et al. The inhibitory receptor IRp60 (CD300a) suppresses the effects of IL-5, GM-CSF, and eotaxin on human peripheral blood eosinophils. Blood. 2006;107: 1996–2003. [DOI] [PubMed] [Google Scholar]
- 35.Nicoll G, Ni J, Liu D, et al. Identification and characterization of a novel Siglec, Siglec-7, expressed by human natural killer cells and mono-cytes. J Biol Chem. 1999;274:34089–34095. [DOI] [PubMed] [Google Scholar]
- 36.Gorczyca W, Sun ZY, Cronin W, et al. Immunophenotypic pattern of myeloid populations by flow cytometry analysis. Methods Cell Biol. 2011;103:221–266. [DOI] [PubMed] [Google Scholar]
- 37.Wood B. Multicolor immunophenotyping: human immune system hematopoiesis. Methods in Cell Biology. 2004;75:559–576. [DOI] [PubMed] [Google Scholar]
- 38.Paul SP, Taylor LS, Stansbury EK, et al. Myeloid specific human CD33 is an inhibitory receptor with differential ITIM function in recruiting the phosphatases SHP-1 and SHP-2. Blood. 2000;96:483–490. [PubMed] [Google Scholar]
- 39.Whitney G, Wang S, Chang H, et al. A new siglec family member, Siglec-10, is expressed in cells of the immune system and has signaling properties similar to CD33. Eur J Biochem. 2001;268:6083–6096. [DOI] [PubMed] [Google Scholar]
- 40.Yokoi H, Myers A, Matsumoto K, et al. Alteration and acquisition of Siglecs during in vitro maturation of CD34+ progenitors into human mast cells. Allergy. 2006;61:769–776. [DOI] [PubMed] [Google Scholar]
- 41.Duan S, Koziol-White CJ. CD33 recruitment inhibits IgE-mediated anaphylaxis and desensitizes mast cells to allergen. J Clin Invest. 2019;129:1387–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ghannadan M, Hauswirth AW, Schernthaner GH, et al. Detection of novel CD antigens on the surface of human mast cells and basophils. Int Arch Allergy Immunol. 2002;127:299–307. [DOI] [PubMed] [Google Scholar]
- 43.Mizrahi S, Gibbs BF, Karra L, et al. Siglec-7 is an inhibitory receptor on human mast cells and basophils. J Allergy Clin Immunol. 2014;134:230–233. [DOI] [PubMed] [Google Scholar]
- 44.Yokoi H, Choi OH, Hubbard W, et al. Inhibition of FcɛRI-dependent mediator release and calcium flux from human mast cells by sialic acid-binding immunoglobulin-like lectin 8 engagement. J Allergy Clin Immunol. 2008;121:499–505. [DOI] [PubMed] [Google Scholar]
- 45.Yu Y, Blokhuis BRJ, Diks MAP, et al. Functional inhibitory Siglec-6 is upregulated in human colorectal cancer-associated mast cells. Front Immunol. 2018;9:2138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Valent P, Majdic O, Maurer D, et al. Further characterization of surface membrane structures expressed on human basophils and mast cells. Int Arch Allergy Appl Immunol. 1990;91:198–203. [DOI] [PubMed] [Google Scholar]
- 47.Avril T, Freeman SD, Attrill H, et al. Siglec-5 (CD170) can mediate inhibitory signaling in the absence of immunoreceptor tyrosine-based inhibitory motif phosphorylation. J Biol Chem. 2005;280:19843–19851. [DOI] [PubMed] [Google Scholar]
- 48.Stefanski AL, Renecle MD, Rumer KK, et al. Siglec-6 phosphorylation at intracellular tyrosine residues leads to the recruitment of SHP-2 phosphatase. Reproduct Sci. 2014;21:388A. [Google Scholar]
- 49.Ikehara Y, Ikehara SK, Paulson JC. Negative regulation of T cell receptor signaling by Siglec-7 (p70/AIRM) and Siglec-9. J Biol Chem. 2004;279:43117–43125. [DOI] [PubMed] [Google Scholar]
- 50.Kardava L, Moir S, Wang W, et al. Attenuation of HIV-associated human B cell exhaustion by siRNA downregulation of inhibitory receptors. J Clin Invest. 2011;121:2614–2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jandus C, Boligan KF, Chijioke O, et al. Interactions between Siglec-7/9 receptors and ligands influence NK cell-dependent tumor immunosurveillance. J Clin Invest. 2014;124: 1810–1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kawasaki N, Vela JL, Nycholat CM, et al. Targeted delivery of lipid antigen to macrophages via the CD169/sialoadhesin endocytic pathway induces robust invariant natural killer T cell activation. Proc Natl Acad Sci USA. 2013;110:7826–7831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Macauley MS, Pfrengle F, Rademacher C, et al. Antigenic liposomes displaying CD22 ligands induce antigen-specific B cell apoptosis. J Clin Invest. 2013;123:3074–3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Spence S, Greene MK, Fay F, et al. Targeting Siglecs with a sialic acid-decorated nanoparticle abrogates inflammation. Sci Transl Med. 2015;7:303ra140. [DOI] [PubMed] [Google Scholar]
- 55.Pang L, Macauley MS, Arlian BM, et al. Encapsulating an immuno-suppressant enhances tolerance induction by siglec-engaging tolero-genic liposomes. Chembiochem. 2017;18:1226–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Orgel KA, Duan S, Wright BL, et al. Exploiting CD22 on antigen-specific B cells to prevent allergy to the major peanut allergen Ara h 2 J Allergy Clin Immunol. 2017;139:366–369.e362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.O’Reilly MK, Paulson JC. Siglecs as targets for therapy in immune-cell-mediated disease. Trends Pharmacol Sci. 2009;30: 240–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tateno H, Crocker PR, Paulson JC. Mouse Siglec-F and human Siglec-8 are functionally convergent paralogs that are selectively expressed on eosinophils and recognize 6’-sulfo-sialyl Lewis X as a preferred glycan ligand. Glycobiology. 2005;15:1125–1135. [DOI] [PubMed] [Google Scholar]
- 59.Brinkman-Van der Linden EC, Hurtado-Ziola N, Hayakawa T, et al. Human-specific expression of Siglec-6 in the placenta. Glycobiology. 2007;17:922–931. [DOI] [PubMed] [Google Scholar]
- 60.Patel N, Brinkman-Van der Linden EC, Altmann SW, et al. OB-BP1/Siglec-6. a leptin- and sialic acid-binding protein of the immunoglobulin superfamily. J Biol Chem. 1999;274:22729–22738. [DOI] [PubMed] [Google Scholar]
- 61.Ali SR, Fong JJ, Carlin AF, et al. Siglec-5 and Siglec-14 are poly-morphic paired receptors that modulate neutrophil and amnion signaling responses to group B Streptococcus. J Exp Med. 2014;211: 1231–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mitsuki M, Nara K, Yamaji T, et al. Siglec-7 mediates nonapop-totic cell death independently of its immunoreceptor tyrosine-based inhibitory motifs in monocytic cell line U937. Glycobiology. 2010;20:395–402. [DOI] [PubMed] [Google Scholar]
- 63.Blixt O, Head S, Mondala T, et al. Printed covalent glycan array for ligand profiling of diverse glycan binding proteins. Proc Natl Acad Sci USA. 2004;101:17033–17038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cummings RD, Pierce JM. The challenge and promise of glycomics. Chem Biol. 2014;21:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McQuillan AM, Byrd-Leotis L, Heimburg-Molinaro J, et al. Natural and synthetic sialylated glycan microarrays and their applications. Front Mol Biosci. 2019;6:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bochner BS, Alvarez RA, Mehta P, et al. Glycan array screening reveals a candidate ligand for Siglec-8. J Biol Chem. 2005;280: 4307–4312. [DOI] [PubMed] [Google Scholar]
- 67.Kiwamoto T, Katoh T, Evans CM, et al. Endogenous airway mucins carry glycans that bind Siglec-F and induce eosinophil apoptosis. J Allergy Clin Immunol. 2015;135:1329–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Varki A. Biological roles of glycans. Glycobiology. 2017;27:3–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kiwamoto T, Brummet ME, Wu F, et al. Mice deficient in the St3gal3 gene product 2,3 sialyltransferase (ST3Gal-III) exhibit enhanced allergic eosinophilic airway inflammation. J Allergy Clin Immunol. 2014;133:240–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Propster JM, Yang F, Rabbani S, et al. Structural basis for sulfation-dependent self-glycan recognition by the human immune-inhibitory receptor Siglec-8. Proc Natl Acad Sci USA. 2016;113: E4170–4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yamaji T, Teranishi T, Alphey MS, et al. A small region of the natural killer cell receptor, Siglec-7, is responsible for its preferred binding to alpha 2,8-disialyl and branched alpha 2,6-sialyl residues. A compari-son with Siglec-9. J Biol Chem. 2002;277:6324–6332. [DOI] [PubMed] [Google Scholar]
- 72.Cho JY, Song DJ, Pham A, et al. Chronic OVA allergen challenged Siglec-F deficient mice have increased mucus, remodeling, and epithelial Siglec-F ligands which are up-regulated by IL-4 and IL-13. Respir Res. 2010;11:154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Suzukawa M, Miller M, Rosenthal P, et al. Sialyltransferase ST3Gal-III regulates Siglec-F ligand formation and eosinophilic lung inflammation in mice. J Immunol. 2013;190:5939–5948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang M, Angata T, Cho JY, et al. Defining the in vivo function of Siglec-F, a CD33-related Siglec expressed on mouse eosinophils. Blood. 2007;109:4280–4287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jia Y, Yu H, Fernandes SM, et al. Expression of ligands for Siglec-8 and Siglec-9 in human airways and airway cells. J Allergy Clin Immunol. 2015;135:799–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yu H, Gonzalez-Gil A, Wei Y, et al. Siglec-8 and Siglec-9 binding specificities and endogenous airway ligand distributions and properties. Glycobiology. 2017;27:657–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gonzalez-Gil A, Porell RN, Fernandes SM, et al. Editor’s choice: sialylated keratan sulfate proteoglycans are Siglec-8 ligands in human airways. Glycobiology. 2018;28:786–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nycholat CM, Duan S, Knuplez E, et al. A sulfonamide sialoside analogue for targeting Siglec-8 and -F on immune cells. J Am Chem Soc. 2019;141:14032–14037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Angata T, Margulies EH, Green ED, et al. Large-scale sequencing of the CD33-related Siglec gene cluster in five mammalian species reveals rapid evolution by multiple mechanisms. Proc Natl Acad Sci USA. 2004;101:13251–13256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cao H, Crocker PR. Evolution of CD33-related siglecs: regulating host immune functions and escaping pathogen exploitation. Immunology. 2011;132:18–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Varki A. Colloquium paper: uniquely human evolution of sialic acid genetics and biology. Proc Natl Acad Sci USA. 2010;107(Suppl 2): 8939–8946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.McMillan SJ, Sharma RS, McKenzie EJ, et al. Siglec-E is a negative regulator of acute pulmonary neutrophil inflammation and suppresses CD11b β2-integrin-dependent signaling. Blood. 2013;121: 2084–2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.McMillan SJ, Sharma RS, Richards HE, et al. Siglec-E promotes β2-integrin-dependent NADPH oxidase activation to suppress neutrophil recruitment to the lung. J Biol Chem. 2014;289:20370–20376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Uchiyama S, Sun J, Fukahori K, et al. Dual actions of group B Streptococcus capsular sialic acid provide resistance to platelet-mediated antimicrobial killing. Proc Natl Acad Sci USA. 2019;116:7465–7470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hoffmann A, Kerr S, Jellusova J, et al. Siglec-G is a B1 cell-inhibitory receptor that controls expansion and calcium signaling of the B1 cell population. Nat Immunol. 2007;8:695–704. [DOI] [PubMed] [Google Scholar]
- 86.Patnode ML, Cheng CW, Chou CC, et al. Galactose 6-O-sulfotransferases are not required for the generation of Siglec-F ligands in leukocytes or lung tissue. J Biol Chem. 2013;288: 26533–26545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kumagai T, Kiwamoto T, Brummet ME, et al. Airway glycomic and allergic inflammatory consequences resulting from keratan sulfate galactose 6-O-sulfotransferase (CHST1) deficiency. Glycobiology. 2018;28:406–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.O’Sullivan JA, Wei Y, Carroll DJ, et al. Frontline science: characterization of a novel mouse strain expressing human Siglec-8 only on eosinophils. J Leukoc Biol. 2018;104:11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wei Y, Chhiba KD, Zhang F, et al. Mast cell-specific expression of human Siglec-8 in conditional knock-in mice. Int J Mol Sci. 2018;20:E19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Youngblood BA, Brock EC, Leung J, et al. Siglec-8 antibody reduces eosinophils and mast cells in a transgenic mouse model of eosinophilic gastroenteritis. JCI Insight. 2019;4:e126219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jandus C, Simon HU, von Gunten S. Targeting siglecs–a novel pharmacological strategy for immuno- and glycotherapy. Biochem Pharmacol. 2011;82:323–332. [DOI] [PubMed] [Google Scholar]
- 92.Angata T, Nycholat CM, Macauley MS. Therapeutic targeting of siglecs using antibody- and glycan-based approaches. Trends Pharmacol Sci. 2015;36:645–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Walter RB. Investigational CD33-targeted therapeutics for acute myeloid leukemia. Expert Opin Investig Drugs. 2018;27:339–348. [DOI] [PubMed] [Google Scholar]
- 94.Bochner BS. “Siglec”ting the allergic response for therapeutic targeting. Glycobiology. 2016;26:546–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.von Gunten S, Vogel M, Schaub A, et al. Intravenous immunoglobulin preparations contain anti-Siglec-8 autoantibodies. J Allergy Clin Immunol. 2007;119:1005–1011. [DOI] [PubMed] [Google Scholar]
- 96.von Gunten S, Simon HU. Natural anti-Siglec autoantibodies mediate potential immunoregulatory mechanisms: implications for the clinical use of intravenous immunoglobulins (IVIg). Autoimmun Rev. 2008;7:453–456. [DOI] [PubMed] [Google Scholar]
- 97.Rasmussen HS, Chang AT, Tomasevic N, et al. A randomized, double-blind, placebo-controlled, ascending dose phase 1 study of AK002, a novel Siglec-8 selective monoclonal antibody, in healthy subjects. J Allergy Clin Immunol. 2018;141:AB403. (abstr). [Google Scholar]
- 98.Altrichter S, Staubach P, Pasha M, et al. Efficacy and safety data of AK002, an anti-Siglec-8 monoclonal antibody, in patients with multiple forms of uncontrolled chronic urticaria (CU): results from an open- label phase 2a study. Allergy. 2019;74:120. (abstr). [Google Scholar]
- 99.Siebenhaar F, Bonnekoh H, Hawro T, et al. Safety and efficacy data of AK002, an anti-Siglec-8 monoclonal antibody, in patients with indolent systemic mastocytosis (ISM): results from a first-in-human, open-label phase 1 study. Allergy. 2019;74:910–911. (abstr).30515838 [Google Scholar]
- 100.Dellon E, Peterson K, Murray J, et al. Efficacy and safety of AK002 in adult patients with active eosinophilic gastritis and eosinophilic enteritis: primary results from a randomized, double-blind placebo-controlled phase 2 trial (ENIGMA study). Am J Gas-troenterol. 2019;44(Suppl):36. [Google Scholar]